Page 1

Waters Xevo G2 QTof
Operator’s Overview and Maintenance Guide
Revision B
Copyright © Waters Corporation 20102011
All rights reserved
Page 2

Copyright notice
© 2010–2011 WATERS CORPORATION. PRINTED IN THE UNITED
STATES OF AMERICA AND IN IRELAND. ALL RIGHTS RESERVED. THIS
DOCUMENT OR PARTS THEREOF MAY NOT BE REPRODUCED IN ANY
FORM WITHOUT THE WRITTEN PERMISSION OF THE PUBLISHER.
The information in this document is subject to change without notice and
should not be construed as a commitment by Waters Corporation. Waters
Corporation assumes no responsibility for any errors that may appear in this
document. This document is believed to be complete and accurate at the time
of publication. In no event shall Waters Corporation be liable for incidental or
consequential damages in connection with, or arising from, its use.
Trademarks
ACQUITY, ACQUITY UPLC, Connections INSIGHT, ESCi, UPLC, and
Waters are registered trademarks of Waters Corporation. IntelliStart,
LockSpray, MassLynx, NanoFlow, NanoLockSpray, T-Wave, THE SCIENCE
OF WHAT'S POSSIBLE., UNIFI, Xevo, and ZSpray are trademarks of Waters
Corporation.
GELoader is a registered trademark of Eppendorf-Netheler-Hinz GmbH.
PEEK is a trademark of Victrex plc.
POZIDRIV is a registered trademark of Phillips Screw Company, Inc.
Swagelok is a registered trademark of Swagelok Company.
Super Flangeless and SealTight are trademarks of Upchurch Scientific, Inc.
TaperTip is a trademark of New Objective, Inc.
Teflon and Viton are registered trademarks of E. I. du Pont de Nemours and
Company.
Valco is a trademark of Valco Instruments, Inc.
Xylan is a registered trademark of Whitford Corporation.
Other trademarks or registered trademarks are the sole property of their
respective owners.
ii
Page 3

Customer comments
Waters’ Technical Communications department invites you to tell us of any
errors you encounter in this document or to suggest ideas for otherwise
improving it. Please help us better understand what you expect from our
documentation so that we can continuously improve its accuracy and
usability.
We seriously consider every customer comment we receive. You can reach us
at tech_comm@waters.com.
Contacting Waters
Contact Waters® with enhancement requests or technical questions regarding
the use, transportation, removal, or disposal of any Waters product. You can
reach us via the Internet, telephone, or conventional mail.
Waters’ contact information:
Contacting medium Information
Internet The Waters Web site includes contact
information for Waters locations worldwide.
Visit www.waters.com.
Telephone From the USA or Canada, phone 800
252-HPLC, or fax 508 872-1990.
For other locations worldwide, phone and fax
numbers appear on the Waters Web site.
Conventional mail Waters Corporation
34 Maple Street
Milford, MA 01757
USA
iii
Page 4
Safety considerations
Some reagents and samples used with Waters instruments and devices can
pose chemical, biological, and radiological hazards. You must know the
potentially hazardous effects of all substances you work with. Always follow
Good Laboratory Practice, and consult your organization’s safety
representative for guidance.
Considerations specific to the Xevo G2 QTof
Solvent-leakage hazard
The source exhaust system is designed to be robust and leak-tight. Waters
recommends you perform a hazard analysis, assuming a maximum leak into
the laboratory atmosphere of 10% LC eluate.
Warning:
• To confirm the integrity of the source exhaust system, renew
the source O-rings at intervals not exceeding one year.
• To avoid chemical degradation of the source O-rings, which can
withstand exposure only to certain solvents (see “Solvents used
to prepare mobile phases” on page C-3), determine whether any
solvents you use that are not listed are chemically compatible
with the composition of the O-rings.
Flammable-solvents hazard
Warning: To prevent the ignition of accumulated solvent vapors inside
the source, maintain a continuous flow of nitrogen through the source
whenever significant amounts of flammable solvents are used during
the instrument’s operation.
Never let the nitrogen supply pressure fall below 690 kPa (6.9 bar, 100 psi)
during analyses that require flammable solvents. Connect to the LC output
with a gas-fail connector to stop the LC solvent if the nitrogen supply fails.
iv
Page 5

Glass-breakage hazard
Source ion block assembly
Warning: To avoid injuries from broken glass, falling objects, or
exposure to toxic substances, never place containers on top of the
instrument or on its front covers.
High-temperature hazard
Warning: To avoid burn injuries, do not touch the source ion block
assembly when operating or servicing the instrument.
Xevo G2 QTof high-temperature hazard:
v
Page 6

Hazards associated with removing an instrument from service
Warning: To avoid personal contamination with
biohazardous or toxic materials, wear chemical-resistant
gloves during all phases of instrument decontamination.
Warning: To avoid puncture injuries, handle syringes, fused silica lines,
and borosilicate tips with care.
When you remove the instrument from use to repair or dispose of it, you must
decontaminate all of its vacuum areas. These are the areas in which you can
expect to encounter the highest levels of contamination:
• Source interior
• Waste tubing
• Exhaust system
• Rotary pump oil (where applicable)
The need to decontaminate other vacuum areas of the instrument depends on
the kinds of samples the instrument analyzed and their levels of
concentration. Do not dispose of the instrument or return it to Waters for
repair until the authority responsible for approving its removal from the
premises specifies the extent of decontamination required and the level of
residual contamination permissible. Management must also prescribe the
method of decontamination to be used and the appropriate protection for
personnel undertaking the decontamination process.
You must handle items such as syringes, fused silica lines, and borosilicate
tips used to carry sample into the source area in accordance with laboratory
procedures for contaminated vessels and sharps. To avoid contamination by
carcinogenic, toxic, or biohazardous substances, you must wear
chemical-resistant gloves when handling or disposing of used oil.
Safety advisories
Consult Appendix A for a comprehensive list of warning and caution
advisories.
vi
Page 7

Operating this instrument
When operating this instrument, follow standard quality control procedures
and the guidelines presented in this section.
Applicable symbols
Symbol Definition
Manufacturer
Authorized representative of the European
Community
Confirms that a manufactured product complies
with all applicable European Community
directives
Australia C-Tick EMC compliant
Confirms that a manufactured product complies
with all applicable United States and Canadian
safety requirements
Consult instructions for use
Audience and purpose
This guide is for operators of varying levels of experience. It gives an overview
of the instrument, and explains how to prepare it, change its modes of
operation, and maintain it.
Intended use
Waters designed the orthogonal-acceleration, time-of-flight Xevo™ G2 QTof
for use as a research tool to deliver authenticated mass measurement. The
Xevo G2 QTof is for research use only and is not intended for use in diagnostic
applications.
vii
Page 8

Calibrating
To calibrate LC systems, follow acceptable calibration methods using at least
five standards to generate a standard curve. The concentration range for
standards should cover the entire range of QC samples, typical specimens,
and atypical specimens.
When calibrating mass spectrometers, consult the calibration section of the
operator’s guide for the instrument you are calibrating. In cases where an
overview and maintenance guide, not operator’s guide, accompanies the
instrument, consult the instrument’s online Help system for calibration
instructions.
Quality control
Routinely run three QC samples that represent subnormal, normal, and
above-normal levels of a compound. Ensure that QC sample results fall within
an acceptable range, and evaluate precision from day to day and run to run.
Data collected when QC samples are out of range might not be valid. Do not
report these data until you are certain that the instrument performs
satisfactorily.
ISM classification
ISM Classification: ISM Group 1 Class A
This classification has been assigned in accordance with CISPR 11 Industrial
Scientific and Medical (ISM) instruments requirements. Group 1 products
apply to intentionally generated and/or used conductively coupled
radio-frequency energy that is necessary for the internal functioning of the
equipment. Class A products are suitable for use in commercial, (that is,
nonresidential) locations and can be directly connected to a low-voltage,
power-supply network.
viii
Page 9

EC authorized representative
Waters Corporation (Micromass UK Ltd.)
Floats Road
Wythenshawe
Manchester M23 9LZ
United Kingdom
Telephone: +44-161-946-2400
Fax: +44-161-946-2480
Contact: Quality manager
ix
Page 10

x
Page 11

Table of Contents
Copyright notice ................................................................................................... ii
Trademarks ............................................................................................................ ii
Customer comments ............................................................................................ iii
Contacting Waters ............................................................................................... iii
Safety considerations .......................................................................................... iv
Considerations specific to the Xevo G2 QTof..................................................... iv
Safety advisories................................................................................................. vi
Operating this instrument ................................................................................ vii
Applicable symbols ........................................................................................... vii
Audience and purpose....................................................................................... vii
Intended use...................................................................................................... vii
Calibrating ....................................................................................................... viii
Quality control ................................................................................................. viii
ISM classification .............................................................................................. viii
ISM Classification: ISM Group 1 Class A ...................................................... viii
EC authorized representative ........................................................................... ix
1 Waters Xevo G2 QTof Overview .......................................................... 1-1
Waters Xevo G2 QTof ........................................................................................ 1-2
IntelliStart technology..................................................................................... 1-2
ACQUITY and nanoACQUITY Xevo G2 QTof UPLC/MS systems............... 1-3
Software and data system ............................................................................... 1-6
Instrument Console ......................................................................................... 1-6
LockSpray source and ionization modes .................................................... 1-6
Electrospray ionization (ESI).......................................................................... 1-7
Atmospheric pressure chemical ionization (APCI) ........................................ 1-8
Combined electrospray and atmospheric pressure chemical
ionization (ESCi)........................................................................................ 1-9
Table of Contents xi
Page 12

NanoLockSpray source and ionization modes .......................................... 1-9
Combined APPI/APCI source ....................................................................... 1-11
IntelliStart Fluidics system .......................................................................... 1-11
IntelliStart Fluidics system physical layout ................................................ 1-12
System operation ........................................................................................... 1-13
Ion optics ........................................................................................................... 1-14
Leak sensors ..................................................................................................... 1-15
Vacuum system ................................................................................................ 1-15
2 Preparing the Mass Spectrometer for Operation ........................... 2-1
Starting the mass spectrometer .................................................................... 2-2
Verifying the instrument’s state of readiness ................................................ 2-3
Monitoring the mass spectrometer LEDs....................................................... 2-3
Calibration ....................................................................................................... 2-3
Flow rates for the Xevo G2 QTof system ........................................................ 2-4
Preparing the IntelliStart Fluidics system ................................................. 2-4
Installing the reservoir bottles........................................................................ 2-4
Adjusting the solvent delivery tube positions ................................................ 2-7
Purging the pump ............................................................................................ 2-8
Rebooting the mass spectrometer ................................................................. 2-8
Leaving the mass spectrometer ready for operation ............................... 2-8
Emergency shutdown of the mass spectrometer ....................................... 2-9
3 Configuring the LockSpray Source ................................................... 3-1
Configuring the LockSpray source ............................................................... 3-2
Configuring for ESI mode ............................................................................... 3-2
Installing the ESI probe .................................................................................. 3-2
Removing the ESI probe.................................................................................. 3-6
Installing the small-bore capillary option .................................................. 3-7
xii Table of Contents
Page 13

Configuring for APCI mode .......................................................................... 3-13
Installing the APCI probe ............................................................................. 3-13
Removing the APCI probe ............................................................................. 3-17
Configuring for ESCi mode .......................................................................... 3-18
Optimizing the ESI probe for ESCi operation.............................................. 3-18
4 Configuring the NanoLockSpray Source ......................................... 4-1
Overview of the NanoLockSpray source ..................................................... 4-2
Sample sprayer ................................................................................................ 4-3
Lock-spray sprayer .......................................................................................... 4-3
NanoFlow gas supply....................................................................................... 4-4
Purge gas.......................................................................................................... 4-4
Sprayer platform adjuster assembly............................................................... 4-4
Configuring the NanoLockSpray source ..................................................... 4-4
Source type selection ....................................................................................... 4-5
Advancing and retracting the sprayer platform ....................................... 4-6
Adjusting the sprayer tip position ................................................................ 4-7
Setting up the camera ...................................................................................... 4-8
Optional glass capillary sprayer ................................................................... 4-9
Plumbing the backpressure gas ...................................................................... 4-9
Installing the glass capillary sprayer ........................................................... 4-10
Fitting and loading the glass capillary......................................................... 4-11
5 Maintenance Procedures ..................................................................... 5-1
Maintenance schedule ..................................................................................... 5-3
Spare parts ......................................................................................................... 5-4
Troubleshooting with Connections INSIGHT ............................................ 5-5
Safety and handling ......................................................................................... 5-6
Preparing the instrument for working on the source ............................. 5-7
Table of Contents xiii
Page 14

Removal and refitting of the source enclosure .......................................... 5-7
Removing the source enclosure from the instrument.................................... 5-7
Fitting the source enclosure to the instrument............................................ 5-10
Installing and removing the corona pin .................................................... 5-11
Installing the corona pin in the source......................................................... 5-11
Removing the corona pin from the source .................................................... 5-13
Operating the source isolation valve ......................................................... 5-14
Removing O-rings and seals ......................................................................... 5-17
Cleaning the instrument case ...................................................................... 5-17
Emptying the nitrogen exhaust trap bottle .............................................. 5-18
Inspecting the Varian roughing pump oil level ....................................... 5-20
Adding oil to the Varian roughing pump .................................................. 5-21
Replacing the Varian roughing pump’s oil and oil mist filter ............. 5-23
Emptying the roughing pump’s oil................................................................ 5-23
Replacing the oil mist filter........................................................................... 5-24
To fill the pump with oil ................................................................................ 5-26
Cleaning the source components ................................................................ 5-28
Cleaning the sampling cone assembly ....................................................... 5-29
Removing the sampling cone assembly from the source ............................. 5-29
Disassembling the sampling cone assembly................................................. 5-31
Cleaning the sample cone and cone gas nozzle ............................................ 5-34
Assembling the sampling cone assembly...................................................... 5-36
Fitting the sampling cone assembly to the source ....................................... 5-36
Cleaning the extraction cone ....................................................................... 5-38
Removing the ion block assembly from the source assembly...................... 5-38
Removing the extraction cone from the ion block ........................................ 5-41
Cleaning the extraction cone......................................................................... 5-42
Fitting the extraction cone to the ion block.................................................. 5-44
Fitting the ion block assembly to the source assembly................................ 5-45
xiv Table of Contents
Page 15

Cleaning the ion block assembly ................................................................. 5-46
Disassembling the source ion block assembly.............................................. 5-46
Cleaning the ion block components .............................................................. 5-53
Assembling the source ion block assembly................................................... 5-55
Cleaning the source hexapole assembly .................................................... 5-57
Removing the ion block assembly, ion block support, and hexapole
from the source assembly ........................................................................ 5-57
Cleaning the hexapole assembly................................................................... 5-59
Fitting the hexapole assembly, PEEK ion block support, and ion
block assembly to the source assembly................................................... 5-61
Replacing the ESI probe tip and gasket .................................................... 5-63
Removing the ESI probe tip and gasket ....................................................... 5-63
Fitting the ESI probe tip and gasket............................................................ 5-65
Replacing the ESI probe sample capillary ............................................... 5-66
Removing the existing capillary.................................................................... 5-66
Installing the new capillary .......................................................................... 5-71
Cleaning the APCI probe tip ........................................................................ 5-75
Replacing the APCI probe sample capillary ............................................ 5-75
Removing the existing capillary.................................................................... 5-75
Installing the new capillary .......................................................................... 5-78
Replacing the reference probe capillary (LockSpray source) ............. 5-82
Removing the existing capillary.................................................................... 5-82
Installing the new capillary .......................................................................... 5-84
Replacing the reference probe capillary (NanoLockSpray source) ... 5-85
Removing the reference probe from the NanoLockSpray source ................ 5-85
Installing the new TaperTip and capillary................................................... 5-88
Cleaning or replacing the corona pin ........................................................ 5-90
Replacing the APCI probe heater ............................................................... 5-91
Removing the APCI probe heater ................................................................. 5-91
Fitting the new APCI probe heater .............................................................. 5-93
Replacing the ion block source heater ...................................................... 5-94
Table of Contents xv
Page 16

Replacing the LockSpray source’s assembly seals ................................. 5-98
Removing the probe adjuster assembly probe and source
enclosure seals ......................................................................................... 5-98
Fitting the new source enclosure and probe adjuster assembly
probe seals.............................................................................................. 5-100
Replacing the mass spectrometer’s air filters ........................................ 5-102
Replacing the air filter inside the front door.............................................. 5-102
Replacing the air filters on the sides of the instrument............................ 5-104
Replacing the IntelliStart Fluidics tubing ............................................. 5-106
Removing the IntelliStart Fluidics tubing ................................................. 5-107
Plumbing the IntelliStart Fluidics lock-spray system............................... 5-108
Plumbing the IntelliStart Fluidics sample delivery system...................... 5-116
A Safety Advisories .................................................................................. A-1
Warning symbols ............................................................................................... A-2
Task-specific hazard warnings........................................................................ A-2
Specific warnings ............................................................................................. A-3
Caution symbol .................................................................................................. A-5
Warnings that apply to all Waters instruments ......................................... A-6
Electrical and handling symbols ................................................................. A-11
Electrical symbols .......................................................................................... A-11
Handling symbols .......................................................................................... A-12
B External Connections .......................................................................... B-1
Mass spectrometer external wiring and vacuum connections ............. B-2
Connecting the Varian oil-filled roughing pump ..................................... B-3
Making the electrical connections to the Varian oil-filled
roughing pump........................................................................................... B-6
Connecting the Edwards oil-free roughing pump ................................... B-7
Making the electrical connections to the Edwards oil-free
roughing pump......................................................................................... B-10
Connecting to the nitrogen gas supply ..................................................... B-10
xvi Table of Contents
Page 17

Connecting to the collision cell gas supply ............................................. B-12
Connecting the nitrogen exhaust line ...................................................... B-12
Connecting the liquid waste line ............................................................... B-15
Connecting the EPC ...................................................................................... B-18
Connecting the workstation (systems with no ACQUITY LC) ............ B-19
Connecting Ethernet cables (systems with ACQUITY LC) .................. B-20
Input/output signal connectors .................................................................. B-20
Signal connections ......................................................................................... B-23
Connecting to the electricity source ......................................................... B-26
Connecting the NanoLockSpray source camera .................................... B-26
Installing the camera driver software .......................................................... B-27
C Materials of Construction and Compatible Solvents ................... C-1
Preventing contamination ............................................................................. C-2
Items exposed to solvent ................................................................................ C-2
Solvents used to prepare mobile phases .................................................... C-3
Index ..................................................................................................... Index-1
Table of Contents xvii
Page 18

xviii Table of Contents
Page 19

1 Waters Xevo G2 QTof Overview
This chapter describes the instrument, including its controls, sources
and IntelliStart™ Fluidics system.
Contents:
Topic Page
Waters Xevo G2 QTof 1-2
LockSpray source and ionization modes 1-6
NanoLockSpray source and ionization modes 1-9
Combined APPI/APCI source 1-11
IntelliStart Fluidics system 1-11
Ion optics 1-14
Leak sensors 1-15
Vacuum system 1-15
1-1
Page 20

Waters Xevo G2 QTof
The Xevo™ G2 QTof Mass Spectrometry (MS) system is a hybrid, quadrupole,
orthogonal acceleration, time-of-flight (oaTOF) mass spectrometer operated by
®
Waters
Either of the following high-performance, ZSpray™, dual-orthogonal, API
sources is fitted as standard equipment:
• LockSpray™ electrospray ionization/atmospheric pressure chemical
• NanoLockSpray™ ESI source, see “NanoLockSpray source and
You can also use the optional combined APPI/APCI source with the
Xevo G2 QTof (see the Waters APPI Source Operator’s Guide Supplement).
For the instrument’s specifications, see the Waters Xevo G2 QTof Site
Preparation Guide.
IntelliStart technology
informatics software.
ionization/combined electrospray ionization and atmospheric pressure
chemical ionization (ESI/APCI/ESCi
and ionization modes” on page 1-6.
ionization modes” on page 1-9.
®
) source, see “LockSpray source
IntelliStart technology monitors instrument performance and reports when it
is ready for use.
The console software automatically mass calibrates the mass spectrometer
and displays performance readbacks to enable simplified setup of the system
for use in routine analytical and open access applications.
1
The IntelliStart Fluidics
system is built into the mass spectrometer. It
delivers sample directly to the MS probe from the LC column or from three
integral reservoirs. The reservoirs can also deliver sample through direct or
combined infusion so that you can optimize instrument performance at
analytical flow rates. An additional reservoir contains solvent for the
automated flushing of the solvent delivery system.
1. In this document, the term “fluidics” is used to describe plumbing components and fluid
pathways within and between instruments and devices.
1-2 Waters Xevo G2 QTof Overview
Page 21

ACQUITY and nanoACQUITY Xevo G2 QTof UPLC/MS systems
The Waters Xevo G2 QTof is compatible with the ACQUITY UPLC® and
®
nanoACQUITY UPLC
the documentation relevant to your LC system.
The ACQUITY
®
Xevo G2 QTof UPLC®/MS system includes an ACQUITY
UPLC system and the Waters Xevo G2 QTof fitted with the LockSpray
ESI/APCI/ESCi source.
The nanoACQUITY Xevo G2 QTof UPLC/MS system includes a
nanoACQUITY UPLC system and the Waters Xevo G2 QTof fitted with the
NanoLockSpray source.
ACQUITY UPLC system
The ACQUITY UPLC system includes a binary solvent manager, sample
manager, column heater, sample organizer, detectors, and a specialized
ACQUITY UPLC column. Watersinformatics software controls the system.
For further information, see the ACQUITY UPLC System Operator’s Guide or
Controlling Contamination in UPLC/MS and HPLC/MS Systems (part
number 715001307). You can find the latter document on
http://www.waters.com; click Services and Support > Support.
systems. If you are not using either system, refer to
Waters Xevo G2 QTof 1-3
Page 22

Waters ACQUITY Xevo G2 QTof UPLC/MS system:
Sample organizer (optional)
Solvent tray
Column heater
Xevo G2 QTof
Sample manager
Binary solvent manager
Access door to the fluidics pump
High voltage
connector for the
ESI probe
Probe
Source interface
sliding door
LockSpray source
enclosure
Access door to the
fluidics valves
1-4 Waters Xevo G2 QTof Overview
Page 23

nanoACQUITY UPLC system
Solvent tray
Column heater
Xevo G2 QTof
Sample
manager
Access door to the fluidics pump
Access door to the fluidics valve
Source interface
sliding door
NanoLockSpray
source enclosure
Binary
solvent
manager
The nanoACQUITY UPLC system includes a binary solvent manager,
auxiliary solvent manager, sample manager, column heater, sample
organizer, detectors, and a specialized nanoACQUITY UPLC column. Waters
informatics software controls the system.
For further information, see the nanoACQUITY UPLC System Operator’s
Guide or Controlling Contamination in UPLC/MS and HPLC/MS Systems
(part number 715001307). You can find the latter document on
http://www.waters.com; click Services and Support > Support.
Waters nanoACQUITY Xevo G2 QTof UPLC/MS system:
Waters Xevo G2 QTof 1-5
Page 24

Software and data system
Waters informatics software controls the mass spectrometer. The software
acquires, analyzes, manages, and distributes data from mass spectrometry,
ultraviolet (UV), evaporative light scattering, and other sources.
Waters informatics software enables these major operations:
• Configuring the instrument.
• Creating LC and MS methods that define operating parameters for a
run.
• Tuning and mass calibrating the mass spectrometer.
• Running samples.
• Monitoring sample runs.
• Acquiring data.
• Processing data.
•Reviewing data.
• Printing data.
See the online Help for more information
Instrument Console
The Instrument Console is an area within the Waters informatics software in
which you configure settings, monitor performance, run diagnostic tests, and
maintain the mass spectrometer. The instrument console functions
independently of the data and does not recognize or control the data software.
See the online Help for details.
LockSpray source and ionization modes
The LockSpray source uses lock-mass correction to acquire exact mass data.
The analyte is introduced into the source through a probe. A reference flow,
containing a compound of known mass, flows through a separate ESI probe.
An oscillating baffle allows the sprays to be analyzed as two separate data
functions. The lock-mass correction calculated from the reference data is then
applied to the analyte data set.
You can use the LockSpray source with the ESI, APCI, and ESCi ionization
modes. See Chapter 3 “Configuring the LockSpray Source”.
1-6 Waters Xevo G2 QTof Overview
Page 25

Xevo G2 QTof fitted with LockSpray source:
Electrospray ionization (ESI)
In electrospray ionization (ESI), a strong electrical charge is applied to the
eluent as it emerges from a nebulizer. The droplets that compose the resultant
aerosol undergo a reduction in size (solvent evaporation). As solvent continues
to evaporate, the charge density increases until the droplet surfaces eject ions
(ion evaporation). The ions can be singly or multiply charged.
To operate the LockSpray source in ESI mode, you fit the source enclosure
with an ESI probe.
LockSpray source and ionization modes 1-7
Page 26

The standard ESI probe capillary accommodates flow rates of up to 2 mL/min
APCI probe
Sample cone
Corona pin
making it suitable for LC applications in the range 100 µL/min to 2 mL/min.
To reduce peak broadening for lower-flow-rate LC applications, such as 1-mm
UPLC columns, use the optional, small-bore capillary, which can
accommodate a maximum flow rate of 200 µL/min.
Atmospheric pressure chemical ionization (APCI)
Atmospheric pressure chemical ionization (APCI) produces singly-charged
protonated or deprotonated molecules for a broad range of nonvolatile
analytes.
To operate the LockSpray source in APCI mode, you fit the source enclosure
with a corona pin and an APCI probe. Mobile phase from the LC column
enters the probe, where it is pneumatically converted to an aerosol, rapidly
heated, and vaporized or gasified at the probe tip.
APCI mode:
Hot gas from the APCI probe passes between the sample cone and the corona
pin. Mobile phase molecules rapidly react with ions generated by the corona
discharge to produce stable reagent ions. Analyte molecules introduced into
the mobile phase react with the reagent ions at atmospheric pressure and
typically become protonated (in the positive ion mode) or deprotonated (in the
negative ion mode). The sample and reagent ions then pass through the
sample cone and into the mass spectrometer.
1-8 Waters Xevo G2 QTof Overview
Page 27

Combined electrospray and atmospheric pressure chemical ionization (ESCi)
In combined electrospray and atmospheric pressure chemical ionization
©
(ESCi
pin, to allow alternating acquisition of ESI and APCI ionization data,
facilitating high-throughput processing and wider compound coverage.
) mode, the standard ESI probe is used in conjunction with a corona
NanoLockSpray source and ionization modes
The NanoLockSpray source allows electrospray ionization performed in the
flow rate range of 5 to 1000 nL/min. For a given sample concentration, the ion
currents for similar experiments approximate those in normal flow rate
electrospray. However, because sample consumption is greatly reduced, the
sensitivity gains are significant when similar scan parameters are used.
Lock-mass correction with the NanoLockSpray source works as the LockSpray
source does in electrospray ionization mode.
The NanoLockSpray source enclosure consists of a sprayer—universal,
borosilicate glass capillary, or CE (see below)—mounted on a ZSpray,
three-axis manipulator.
A light within the source provides illumination for the spray, which you can
observe using the video camera mounted on the corner of the source housing.
See Chapter 4 “Configuring the NanoLockSpray Source”.
NanoLockSpray source and ionization modes 1-9
Page 28

Xevo G2 QTof fitted with NanoLockSpray source:
The following options are available for the spraying capillary:
• Universal NanoFlow™ nebulizer sprayer.
This option, for flow injection or coupling to nanoACQUITY, uses a
pump to regulate the flow rate as low as 100 nL/min.
• Borosilicate glass capillary NanoFlow (nanovials).
This option uses metal-coated glass capillaries, which allow the lowest
flow rates. Usable for one sample only, they must then be discarded.
• NanoFlow capillary electrophoresis (CE) sprayer.
This option uses a make-up liquid at the CE capillary tip, which allows a
stable electrospray to occur. The make-up flow rate is less than
1µL/min.
1-10 Waters Xevo G2 QTof Overview
Page 29

Combined APPI/APCI source
Atmospheric pressure photoionization (APPI) uses photons generated by a
discharge UV lamp (~10.2 eV) to produce sample ions from vaporized LC
eluent. Direct photoionization of the sample molecule occurs when the photon
energy exceeds the ionization potential of the sample molecule.
The optional dual-mode (APPI/APCI) ionization source comprises an APPI
source enclosure, which is used in conjunction with a standard APCI probe.
You can operate the source in APPI or dual-mode, which switches rapidly
between ionization modes, facilitating high-throughput analyses.
For further details, see the Waters APPI Source Operator’s Guide Supplement.
IntelliStart Fluidics system
The IntelliStart Fluidics system is built into the instrument; it controls how
sample is delivered to the source.
For standard flow applications, the system delivers sample directly to the
mass spectrometer source in one of three ways:
• From the LC column.
• From three integral reservoir bottles. Use standard reservoir bottles
(30 mL) for instrument setup and calibration. Use low-volume vials
(1.5 mL) to infuse smaller volumes.
Tip: The reservoir bottles can also deliver sample through direct or
combined infusion to enable optimization at analytical flow rates.
• From a wash reservoir, which contains solvent for automated flushing of
the instrument’s solvent delivery system.
For nanoACQUITY, the valves and pumps that make up the IntelliStart
Fluidics system introduce dead volume, which can cause unacceptable peak
broadening. For this reason, the nanoACQUITY is plumbed directly to the
NanoFlow sprayer using a suitable short piece of silica tubing.
For reference flows for both the LockSpray and NanoLockSpray source, the
IntelliStart Fluidics system delivers lock mass solution from reservoir bottle B
or, for extended operating hours, from a separate, external bottle of lock mass
solution.
Combined APPI/APCI source 1-11
Page 30

IntelliStart Fluidics system physical layout
A
B
C
AA
BB
C
AA
BB
C
Water sWater s
A
A
B
B
C
C
Lock-spray selector valve
Sample selector
valve
Divert valve
Sample pump
Lock-spray pump
Sample reservoir bottles (A, B and C)
Tube guides Flow sensor
Grounded union
Access doors
The IntelliStart Fluidics system comprises the components shown in the
following figure.
IntelliStart Fluidics system components:
1-12 Waters Xevo G2 QTof Overview
Page 31

The IntelliStart Fluidics system consists of these components:
• A sample delivery system, with a pump, sample selector valve, and
diverter valve used for LC and probe connections.
• A lock-spray system, with a pump capable of ultra-low flow rates, a
lock-spray selector valve, flow sensor, and grounded union. The
grounded union protects the flow sensor from probe voltages. The flow
sensor regulates flow down to the very low volumes required by the
NanoLockSpray source.
• Three, shared, 30-mL sample reservoir bottles: A, B, and C.
• Plumbing for shared wash and waste bottles.
The sample reservoirs are mounted on the instrument’s front panel. When you
select a solvent from the Instrument Console, a light-emitting diode (LED)
illuminates the appropriate reservoir. You can simultaneously illuminate all
three reservoirs or extinguish the LEDs for light-sensitive samples.
Recommendation: Use reservoir A for the sample solution, reservoir B for the
lock-spray solution, and reservoir C for the calibrant solution.
The wash reservoir and (optionally) the reservoirs containing the lock-mass
reference solutions are external to the instrument; typically they are bottles
on the LC system. The waste reservoir is normally a bottle stored under the
instrument bench.
During normal operation the instrument access doors should be kept closed.
System operation
You configure the IntelliStart Fluidics system using the console software
where you can edit the parameters, frequency, and extent of the automation.
See the mass spectrometer’s online Help for further details on operating the
IntelliStart Fluidics system.
During auto-calibration, the software automatically controls lock mass and
sample delivery.
IntelliStart Fluidics system 1-13
Page 32

Ion optics
Sample sprayer
Pusher Detector
DRE lens
Quadrupole
Hexapole
Lock-spray sprayer
Transfer lenses
T-Wave collision cell
Sample cone
Isolation valve
Reflectron
Flight tube
The mass spectrometer’s ion optics operate as follows:
1. Samples from the LC or instrument’s solvent delivery system are
introduced at atmospheric pressure into the ionization source.
2. The ions pass through the sample cone into the vacuum system.
3. The ions pass through the hexapole to the quadrupole, where they are
filtered according to their mass-to-charge ratio.
4. The mass-separated ions pass into the T-Wave™ collision cell, where
they can undergo collision-induced dissociation (CID).
5. The ions then pass into the time-of-flight (ToF) analyzer. A high voltage
pulse orthogonally accelerates the ions up the flight tube, where a
reflectron reflects them back again towards the detector. Ions of
different mass-to-charge ratios arrive at the detector at different times,
hence a mass spectrum can be created.
6. The signal from the detector is amplified, digitized, and sent to the
software.
Ion optics overview:
1-14 Waters Xevo G2 QTof Overview
Page 33

Leak sensors
Leak sensors in the instrument’s drip trays continuously monitor for liquid
leaks. A leak sensor stops system flow when its optical sensor detects about
1.5 mL of accumulated leaked liquid in its surrounding reservoir. At the same
time, the Instrument Console displays an error message alerting you that a
leak has developed. See Waters ACQUITY UPLC Leak Sensor maintenance
instructions (part number 71500082506) for complete details.
Vacuum system
An external roughing pump and three internal turbomolecular pumps
maintain the instrument’s vacuum.
Protective interlocks guard against vacuum leaks and electrical or vacuum
pump failure. The system monitors the turbomolecular pump speeds and
continuously measures vacuum pressure with built-in gauges. The gauges
also serve as switches, stopping operation when vacuum loss is sensed.
A vacuum isolation valve isolates the source from the mass analyzer, allowing
the sample cone to be cleaned without venting the instrument.
Leak sensors 1-15
Page 34

1-16 Waters Xevo G2 QTof Overview
Page 35

2 Preparing the Mass
Spectrometer for Operation
This chapter explains how to start up and shut down the mass
spectrometer.
Contents:
Topic Page
Starting the mass spectrometer 2-2
Preparing the IntelliStart Fluidics system 2-4
Rebooting the mass spectrometer 2-8
Leaving the mass spectrometer ready for operation 2-8
Emergency shutdown of the mass spectrometer 2-9
2-1
Page 36

Starting the mass spectrometer
The Waters Xevo G2 QTof is compatible with the ACQUITY UPLC and
nanoACQUITY UPLC systems. If you are not using either system, refer to the
documentation relevant to your LC system (see “Software and data system” on
page 1-6).
Caution: Using incompatible solvents can cause severe damage to the
instrument. For more details, refer to the following sources:
• Appendix C, “Materials of Construction and Compatible Solvents”,
for mass spectrometer solvent information.
• Appendix C of the ACQUITY UPLC System Operator’s Guide for
solvent compatibility with ACQUITY devices.
Requirement: Power-on the instrument server or workstation PC first, to
ensure that it obtains the IP addresses of the system instruments.
See the mass spectrometer’s online Help for details.
To start the mass spectrometer:
Warning: To avoid ignition of flammable solvents, never let the nitrogen
supply pressure fall below 690 kPa (6.9 bar, 100 psi).
1. On the rear panel, ensure the nitrogen supply is connected to the
instrument’s nitrogen inlet connection (see the figure on page B-2).
Requirement: The nitrogen must be dry and oil-free, with a purity of at
least 95%. Regulate the supply at 600 to 690 kPa (6.0 to 6.9 bar, 90 to
100 psi).
2. Ensure that the collision gas supply is connected to the instrument’s
collision cell gas inlet.
Requirement: The collision gas is argon; it must be dry and of high
purity (99.997%). Regulate the supply at 50 kPa (0.5 bar, 7 psi).
3. Power-on the instrument server or workstation PC.
4. Switch on the Xevo G2 QTof at the power outlet.
5. Press the power switches of the ACQUITY instruments.
Result: Each system component runs a series of startup tests.
2-2 Preparing the Mass Spectrometer for Operation
Page 37

6. Allow 4 minutes for the embedded PC to initialize.
Tip: The power and status LEDs change as follows:
• During initialization, the binary solvent manager’s and sample
manager’s status LED flashes green.
• After the instruments are successfully powered-on, all power LEDs
show steady green. The binary solvent manager’s flow LED, the
sample manager’s run LED, and the mass spectrometer’s status
LED remain unlit.
7. Start the software and monitor the instrument console for messages and
LED indications.
8. Click Operate.
Result: When the mass spectrometer is in good operating condition, the
software indicates “Ready” in the instrument console.
Verifying the instrument’s state of readiness
When the mass spectrometer is in good operating condition, the power and
status LEDs show constant green. You can view any error messages in console
software.
Monitoring the mass spectrometer LEDs
Light-emitting diodes on the mass spectrometer indicate its operational
status.
Power LED – The power LED, below the mass spectrometer’s source, indicates
when the mass spectrometer is powered-on or powered-off.
Status LED – The status LED, on the right-hand side of the power LED,
indicates the operating condition. See the mass spectrometer’s online Help for
details on the status LED indications.
Calibration
Calibrate the mass spectrometer prior to use, see the mass spectrometer’s
online Help.
Starting the mass spectrometer 2-3
Page 38

Flow rates for the Xevo G2 QTof system
The Xevo G2 QTof system can run at high flow rates. To optimize desolvation,
and thus sensitivity, run the system at appropriate gas flows and desolvation
temperatures.
Flow rate versus temperature and gas flow:
Flow rate
(mL/min)
0.000 to 0.020 100 200 800
0.020 to 0.100 120 350 800
0.101 to 0.300 120 450 800
0.301 to 0.500 150 500 1000
>0.500 150 0 1200
Source
temperature (°C)
Desolvation
temperature (°C)
Preparing the IntelliStart Fluidics system
For additional information, see “Connecting the liquid waste line” on
page B-15.
Warning: To avoid injuries from broken glass, falling objects, or
exposure to toxic substances, never place containers on top of the
instrument or on its front covers.
Installing the reservoir bottles
Use standard reservoir bottles (30-mL) for instrument setup and calibration.
Use the Low-volume Adaptor Kit (included) to infuse smaller volumes. The
low-volume vials have a volume of 1.5 mL.
Desolvation gas
flow (L/h)
Required materials
Chemical-resistant, powder-free gloves
2-4 Preparing the Mass Spectrometer for Operation
Page 39
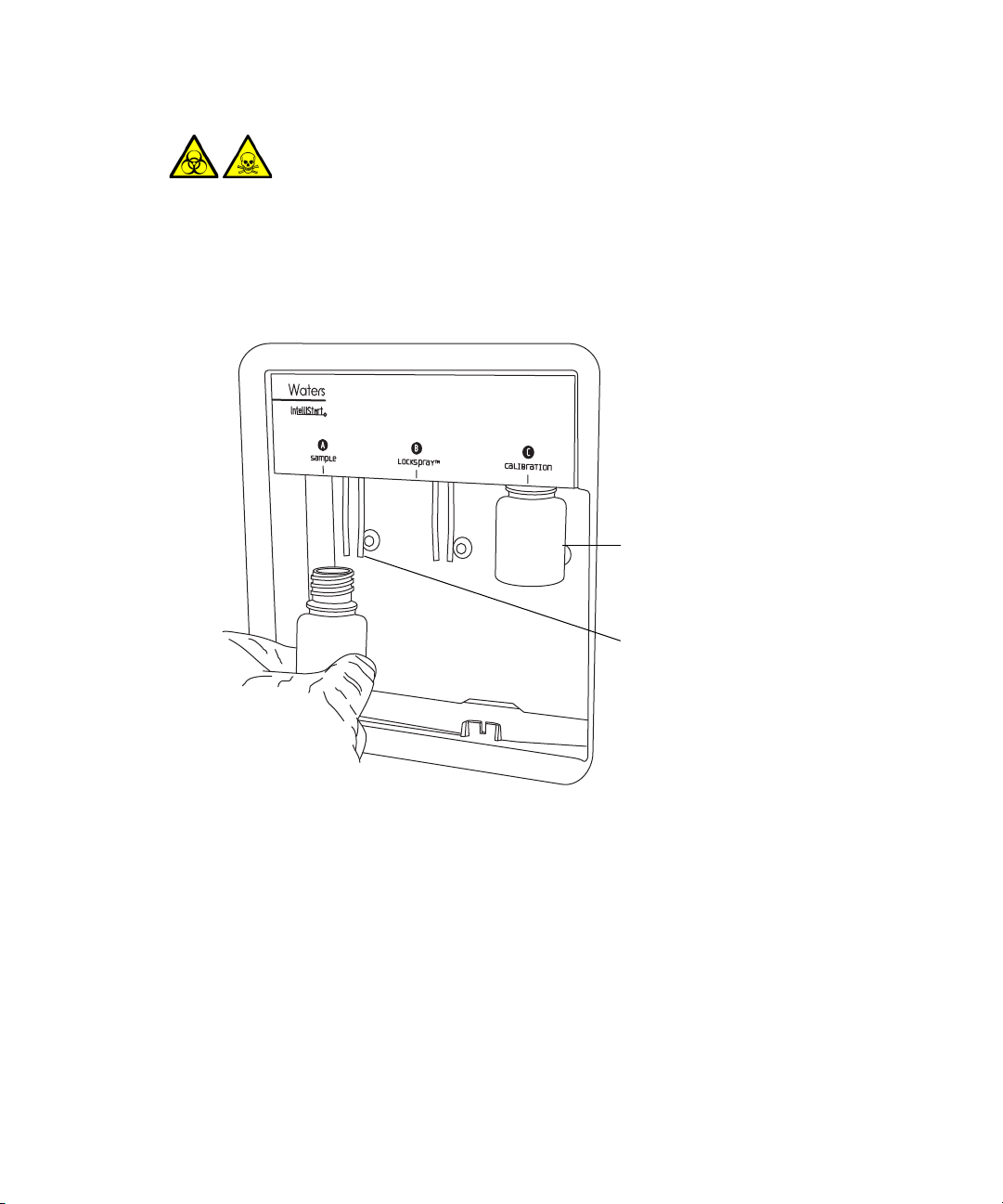
To install the reservoir bottles:
Reservoir bottle
Solvent delivery tube
Warning: The reservoir bottles can be contaminated with
biohazardous and/or toxic materials. Always wear
chemical-resistant, powder-free gloves while performing this
procedure.
1. Remove the reservoir bottle caps.
2. Screw the reservoir bottles onto the mass spectrometer, as shown below.
3. For each reservoir bottle, ensure that the ends of the solvent delivery
tubes are positioned so that they are close to, but do not touch, the
bottom of the bottle (see page 2-7).
Preparing the IntelliStart Fluidics system 2-5
Page 40

To install the low-volume vials:
Low-volume vial
Low-volume adaptor
Solvent delivery tube
Warning: The reservoir bottles can be contaminated with
biohazardous and/or toxic materials. Always wear
chemical-resistant, powder-free gloves while performing this
procedure.
1. If a standard reservoir bottle is fitted, remove it.
2. Screw the low-volume adaptors into the manifold and finger-tighten
them.
Warning: Low-volume glass vials are fragile and can shatter,
cutting fingers. Take care when screwing them in, and never use
force.
3. Screw the low-volume vials into the adaptors.
4. For each low-volume vial, ensure that the ends of the solvent delivery
tubes are positioned so that they are close to, but do not touch, the
bottom of the vial (see page 2-7).
2-6 Preparing the Mass Spectrometer for Operation
Page 41
Adjusting the solvent delivery tube positions
Finger-tight fitting
Solvent delivery tube
For correct operation of the IntelliStart Fluidics system, you must adjust each
solvent delivery tube so that its end is close to, but does not, touch, the bottom
of the reservoir bottle or low volume vial.
To adjust the position of a solvent delivery tube:
1. Open the access door to the fluidics pump (see the figure on page 1-4).
2. Loosen the finger-tight fitting for the solvent delivery tube you are
adjusting.
3. Move the solvent delivery tube so that its end is close to, but does not
touch, the bottom of the reservoir bottle or low volume vial.
4. Tighten the finger-tight fitting.
5. Close the access door.
Preparing the IntelliStart Fluidics system 2-7
Page 42

Purging the pump
Whenever you replace a solution bottle, purge the pump with the solution that
you are going to use next. See the mass spectrometer’s online Help for details.
Requirement: Ensure that the end of the tubing is fully submerged in the
solvent in the wash reservoir.
Tip: Depending on the solutions used, the system can require more than one
purge cycle to minimize carryover.
Rebooting the mass spectrometer
The reset button on the external EPC reboots the mass spectrometer.
Reboot the mass spectrometer when either of these conditions applies:
• The console software fails to initialize or connect.
• Immediately following a software upgrade.
Leaving the mass spectrometer ready for operation
When you are not using the instrument, stop the LC flow and put the
instrument in Standby mode, to conserve energy and reduce nitrogen
consumption.
Tip: After you return the instrument to Operate mode, the LockSpray source’s
temperature requires as much as 30 minutes to stabilize at the relatively high
temperatures needed for UPLC operation.
2-8 Preparing the Mass Spectrometer for Operation
Page 43

Emergency shutdown of the mass spectrometer
To shut down the mass spectrometer in an emergency:
Warning: To isolate the instrument from the electrical supply,
disconnect the power cable from the instrument’s rear panel.
Caution: Data can be lost during an emergency shutdown.
1. Switch off the power at the electrical outlet.
Result: The instrument turns off and vents.
2. Disconnect the power cable from the instrument’s rear panel.
Emergency shutdown of the mass spectrometer 2-9
Page 44

2-10 Preparing the Mass Spectrometer for Operation
Page 45

3 Configuring the LockSpray
Source
This chapter explains how to configure the LockSpray source for the
following ionization modes:
• ESI (electrospray ionization)
• APCI (atmospheric pressure ionization)
• ESCi (combined electrospray and atmospheric pressure ionization)
Contents:
Topic Page
Configuring the LockSpray source 3-2 Configuring for ESI mode 3-2 Installing the small-bore capillary option 3-7 Configuring for APCI mode 3-13 Configuring for ESCi mode 3-18
3-1
Page 46

Configuring the LockSpray source
The following table summarizes how you configure the LockSpray source for
the various ionization modes.
Configuring the LockSpray source:
Ionization mode Probe type Corona pin fitted?
ESI ESI No
APCI APCI Yes
ESCi ESI Yes
Configuring for ESI mode
To operate in ESI mode, you must fit the ESI probe to the LockSpray source
enclosure. If you intend using the small-bore capillary option, fit the capillary
to the probe first (see page 3-7).
For more information on using ESI mode, see the Xevo G2 QTof system online
Help.
Installing the ESI probe
Required materials
• Chemical-resistant, powder-free gloves
• Sharp knife or PEEK™ tubing cutter
3-2 Configuring the LockSpray Source
Page 47

To install the ESI probe:
TP03129
Location hole of the probe adjuster assembly
Probe location dowel
Probe label
Warning: The LC system connections, ESI probe, and source can
be contaminated with biohazardous and/or toxic materials. Always
wear chemical-resistant, powder-free gloves while performing this
procedure.
Warning: To avoid electric shock, ensure that the instrument is
prepared for working on the source before commencing this procedure.
1. Prepare the instrument for working on the source (see page 5-7).
Warning: The ESI probe tip is sharp. To avoid puncture wounds,
handle the probe with care.
2. Remove the protective sleeve, if fitted, from the ESI probe tip.
3. With the probe label facing you, carefully slide the ESI probe into the
hole in the probe adjuster assembly, ensuring that the probe location
dowel aligns with the location hole in the probe adjuster assembly.
Configuring for ESI mode 3-3
Page 48

ESI probe, mounted on the LockSpray source enclosure:
TP03128
ESI probe cable
ESI probe
Vernier probe adjuster
Probe locking ring
Source window
High voltage connector
Source enclosure
release
Vertical probe
adjuster
Caution: To avoid nitrogen leakage, fully tighten the probe locking
ring.
3-4 Configuring the LockSpray Source
4. Tighten the probe locking ring to secure the probe in place.
Tip: An automatic pressure test is performed when the probe is correctly
seated in position.
5. Connect the ESI probe’s cable to the high voltage connector.
6. Open the access door to the IntelliStart Fluidics system (see the figure
on page 1-12).
Warning: To avoid electric shock, do not use stainless steel tubing
to connect the diverter valve to the ESI probe; use the PEEK
tubing supplied with the instrument.
7. Using a long “finger-tight” fitting, connect 0.004-inch ID (or greater)
tubing, from port 2 (the top port) of the diverter valve to the ESI probe,
where you use a PEEK, “finger-tight” nut and ferrule to connect to the
union.
Page 49
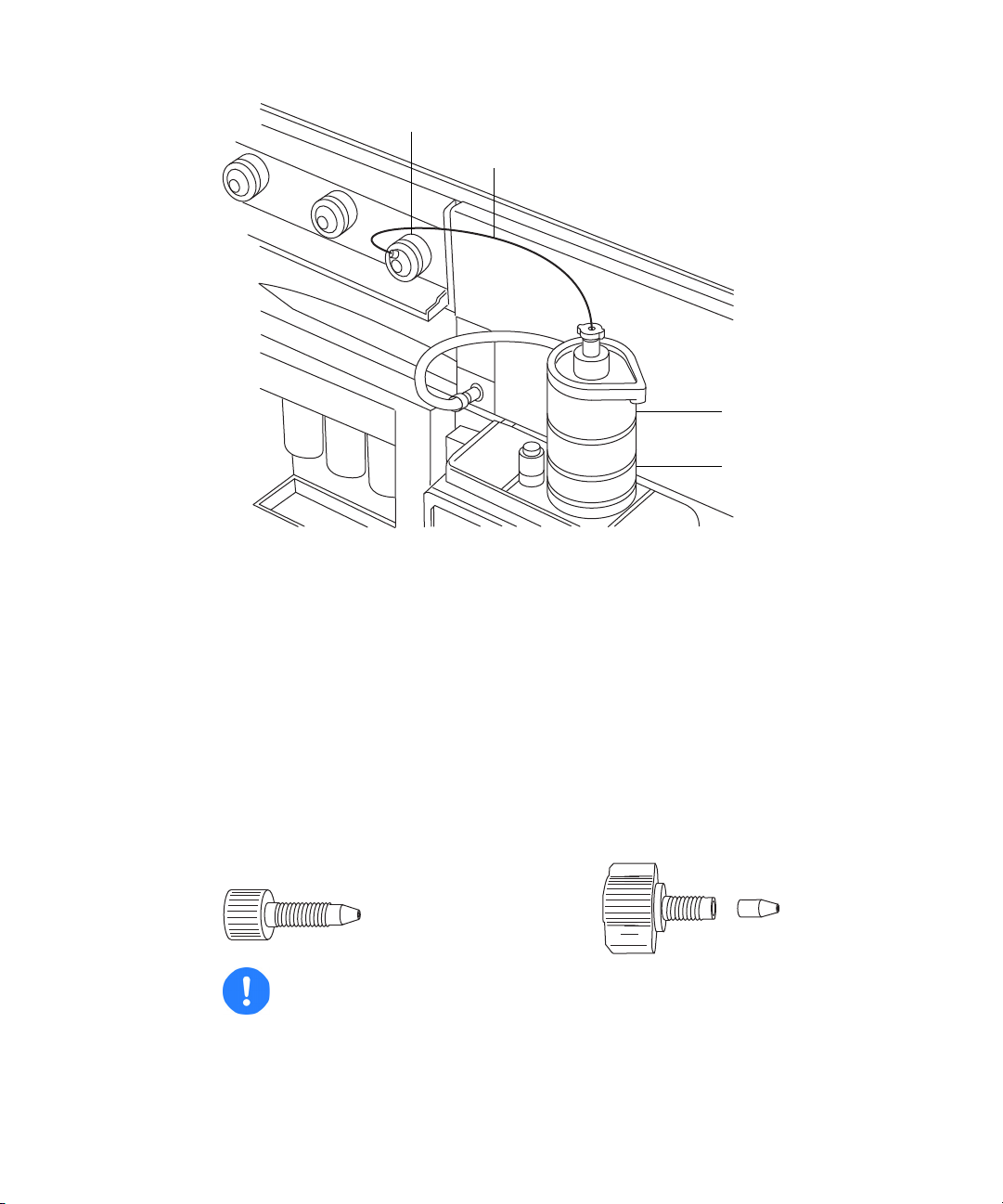
Recommendation: To reduce peak broadening, use 0.004-inch ID tubing
ESI probe
Diverter valve
Tubing connection
Probe adjuster
assembly
for sample flow rates 1.2 mL/min; use 0.005-inch ID tubing for sample
flow rates >1.2 mL/min.
Requirements:
• If you are replacing the tubing supplied with the instrument, you
must minimize the length of the tube connecting the diverter valve
to the ESI probe. Doing so minimizes delays and dispersion.
• When cutting the tubing to length, cut it squarely (that is,
perpendicular to its horizontal axis).
Long “finger-tight” fitting and PEEK, “finger-tight” nut and ferrule:
Caution: Ensure that the tubing does not become trapped when
closing the access door to the IntelliStart Fluidics system.
8. Close the access door to the IntelliStart Fluidics system.
Configuring for ESI mode 3-5
Page 50

Removing the ESI probe
Required materials
Chemical-resistant, powder-free gloves
To remove the ESI probe:
Warning: The LC system connections, ESI probe, and source can
be contaminated with biohazardous and/or toxic materials. Always
wear chemical-resistant, powder-free gloves while performing this
procedure.
Warning: To avoid electric shock, ensure that the instrument is
prepared for working on the source before commencing this procedure.
1. Prepare the instrument for working on the source (see page 5-7).
2. Disconnect the fluidics tubing from the ESI probe.
3. Disconnect the ESI probe’s cable from the high voltage connector.
4. Unscrew the probe locking ring.
Warning: The ESI probe tip is sharp. To avoid puncture wounds,
handle the probe with care.
5. Carefully remove the ESI probe from the probe adjuster assembly.
6. If available, fit the protective sleeve to the ESI probe tip.
3-6 Configuring the LockSpray Source
Page 51

Installing the small-bore capillary option
Use the small-bore capillary option with 300-µm UPLC columns in systems
running at typical flow rates of 10 µL/min. The materials necessary to install
the option are supplied in kit form.
Caution: To avoid damage from excessive pressure, do not exceed flow
rates of 50 µL/min through the ESI probe when the small-bore
capillary is fitted.
Required materials
• Chemical-resistant, powder-free gloves
• Combined 2.5-mm Allen wrench and cone extraction tool
• 10-mm wrench
•8-mm wrench
• 7-mm wrenches (2)
•LC pump
• HPLC-grade (or better) 1:1 acetonitrile/water
• Sharp knife or PEEK tubing cutter
• From the small-bore capillary kit
– capillary
– small-bore, UNF coupler (slide port)
– collar nut (thumb nut)
– PTFE liner tubing
– conductive sleeve
– two ferrules (1/16-inch)
• Metal gasket for the probe tip
• Red PEEK tubing
Installing the small-bore capillary option 3-7
Page 52

To install the capillary:
TP02671
PEEK “finger tight” nut and ferrule
PEEK tubing
PEEK union
Warning: The probe and source components can be contaminated
with biohazardous and/or toxic materials. Always wear
chemical-resistant, powder-free gloves while performing this
procedure.
Warning: The ESI probe tip is sharp. To avoid puncture wounds, handle
the probe with care.
1. Remove the existing capillary (see page 5-66).
2. Use the sharp knife or PEEK tubing cutter to cut an approximately
60-cm (24-inches) length of red, PEEK tubing.
Requirement: Cut the tubing squarely (that is, perpendicular to its
horizontal axis).
3. Insert one end of the red, PEEK tubing in the probe inlet connector, and
screw the connector, finger-tight, into the PEEK “finger tight” nut and
ferrule.
Rationale: Doing so ensures a minimum dead volume when fitting the
capillary.
3-8 Configuring the LockSpray Source
Page 53

4. Using the needle-nose pliers, slide the UNF coupler, PTFE liner sleeve,
Ferrule
UNF coupler
PTFE liner sleeve
Ferrule
Locknut
Conductive sleeve
Collar nut
and a ferrule onto the capillary.
5. Insert the capillary in the PEEK union, and ensure that it is fully
seated.
6. Screw the UNF coupling into the PEEK union, finger-tight only.
7. Gently tug on the capillary, testing to ensure that it stays in place.
8. Using the 7-mm wrench for the locknut and the 8-mm wrench for the
PEEK union, tighten the locknut against the PEEK union until the
union can no longer be twisted.
9. Using the needle-nose pliers, slide another 1/16-inch ferrule over the
capillary, and seat it in the UNF coupler over the exposed end of the
PTFE liner sleeve.
10. Slide a new conductive sleeve and the collar nut over the capillary.
11. Using two 7-mm wrenches, tighten the collar nut to the UNF coupling.
Installing the small-bore capillary option 3-9
Page 54

Warning: To avoid eye injury from high-pressure liquid jet spray,
UNF coupling locating pin
Probe assembly locating slot
wear safety goggles when performing the leak test.
12. Test for leaks by attaching the free end of the PEEK tubing to an LC
pump and pumping mobile phase through it, at 200 µL/min.
• If leakage occurs, disassemble and remake the connection, and then
repeat the leak test.
• If the back pressure on the LC pump is unacceptably high, replace
the capillary, and repeat the leak test.
13. When no leakage occurs and the LC pump back pressure is normal,
disconnect the PEEK tubing from the LC pump.
14. Remove the PEEK, “finger-tight” nut and ferrule and red PEEK tubing
from the PEEK union.
15. Carefully thread the capillary through the probe assembly.
16. Carefully push the PEEK union/UNF coupling assembly and capillary
into the probe assembly so that the locating pin on the UNF coupling
engages in the locating slot at the head of the probe assembly.
17. Fit the nebulizer adjuster knob to the PEEK union/UNF coupling
assembly.
18. Finger tighten the nebulizer adjuster knob onto the probe assembly.
3-10 Configuring the LockSpray Source
Page 55
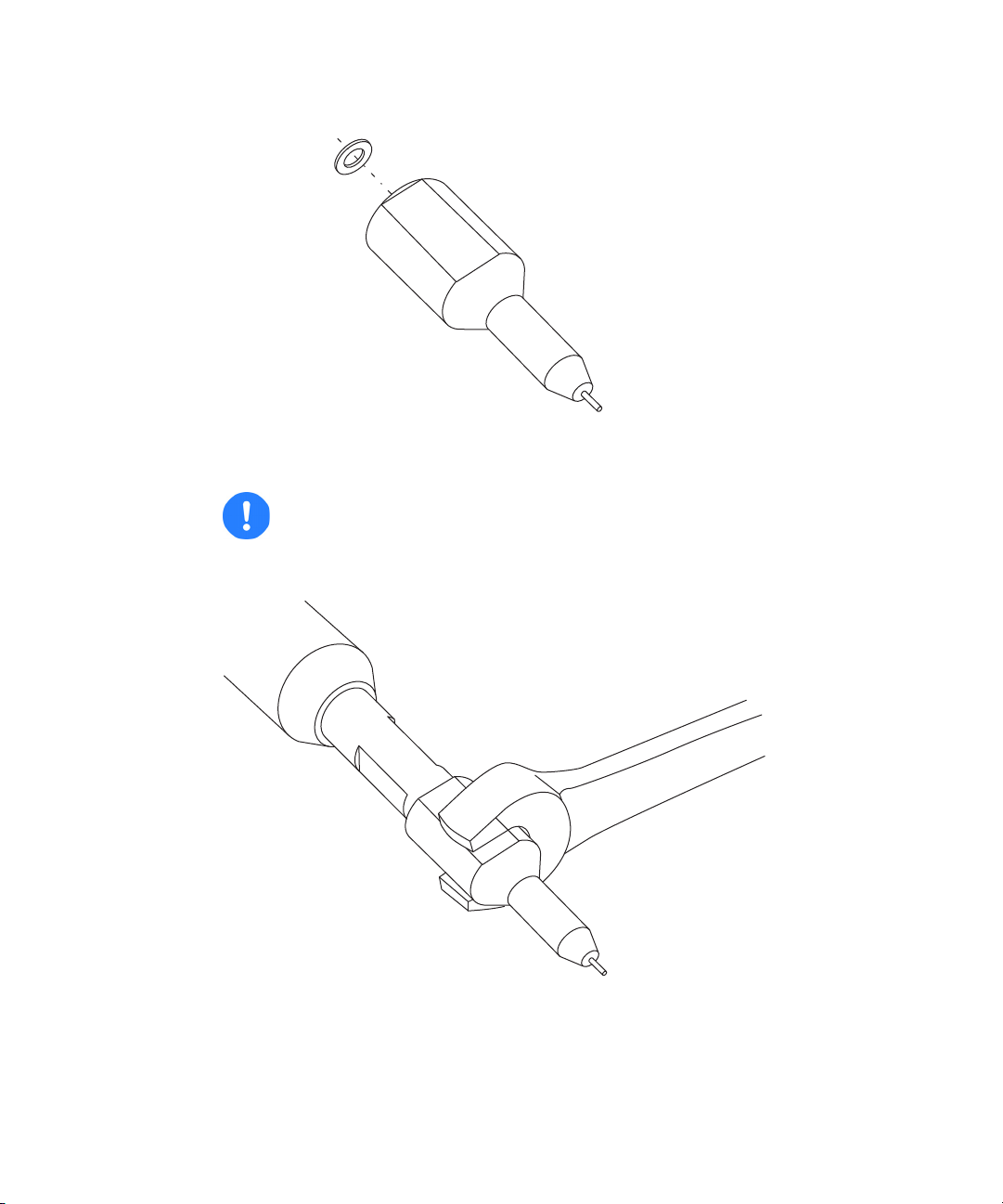
19. Fit a new, metal gasket to the probe tip.
Metal gasket
Probe tip
10-mm wrench
20. Fit the probe tip over the capillary, and screw the tip onto the probe
assembly.
Caution: To avoid gas leakage, fully tighten the probe tip.
21. Use the 10-mm wrench to tighten the probe tip.
Installing the small-bore capillary option 3-11
Page 56

22. Using the nebulizer adjuster knob, adjust the capillary so that it
End-cover
Nebulizer adjuster knob
Gasket
protrudes by approximately 0.5 mm from the end of the probe tip.
Tip: During normal operation, the adjuster knob relies on gas pressure
to retract the capillary. To retract the capillary without gas connected,
invert the probe and use gravity.
23. Fit the end-cover and gasket to the probe assembly.
3-12 Configuring the LockSpray Source
Page 57

24. Use the combined 2.5-mm Allen wrench to fit and tighten the 3 probe
End-cover retaining screws
end-cover retaining screws.
25. Fit the ESI probe to the source (see page 3-2).
Configuring for APCI mode
To operate in APCI mode, you must fit the APCI probe and corona pin to the
LockSpray source enclosure.
For more information on using APCI mode, see the Xevo G2 QTof system
online Help.
Installing the APCI probe
Required materials
• Chemical-resistant, powder-free gloves
• Sharp knife or PEEK tubing cutter
Configuring for APCI mode 3-13
Page 58

To install the APCI probe:
TP03129
APCI probe location dowel
Location hole in the probe
adjuster assembly
Probe label
Warning: The LC system connections, APCI probe, and source can
be contaminated with biohazardous and/or toxic materials. Always
wear chemical-resistant, powder-free gloves while performing this
procedure.
Warning: To avoid electric shock, ensure that the instrument is
prepared for working on the source before commencing this procedure.
1. Prepare the instrument for working on the source (see page 5-7).
2. With the probe label facing you, carefully slide the APCI probe into the
hole in the probe adjuster assembly, ensuring that the probe location
dowel aligns with the location hole in the probe adjuster assembly.
3-14 Configuring the LockSpray Source
Page 59

3. Tighten the probe locking ring to secure the probe in place.
TP03128
APCI probe
Vernier probe adjuster
Probe locking ring
Source window
Source enclosure
release
Vertical probe
adjuster
Tip: An automatic pressure test is performed when the probe is correctly
seated in position.
APCI probe mounted on the source enclosure:
4. Open the access door to the IntelliStart Fluidics system (see the figure
page 1-12).
Warning: To avoid electric shock, do not use stainless steel tubing
to connect the diverter valve to the APCI probe; use the PEEK
tubing supplied with the instrument.
5. Using a long “finger-tight” fitting, connect 0.004-inch ID (or greater)
tubing, from port 2 (the top port) of the diverter valve to the APCI probe,
where you use a PEEK, “finger-tight” nut and ferrule to connect to the
union.
Configuring for APCI mode 3-15
Page 60

Recommendation: To reduce peak broadening, use 0.004-inch ID tubing
APCI probe
Diverter valve
Tubing connection
Probe adjuster
assembly
for sample flow rates 1.2 mL/min; use 0.005-inch ID tubing for sample
flow rates >1.2 mL/min.
Requirements:
• If you are replacing the tubing supplied with the instrument, you
must minimize the length of the tube connecting the diverter valve
to the ESI probe. Doing so minimizes delays and dispersion.
• When cutting the tubing to length, cut it squarely (that is,
perpendicular to its horizontal axis).
Long “finger-tight” fitting and PEEK, “finger-tight” nut and ferrule:
3-16 Configuring the LockSpray Source
Page 61

Caution: Ensure that the tubing does not become trapped when
closing the access door to the IntelliStart Fluidics system.
6. Close the access door to the IntelliStart Fluidics system.
7. Install the corona pin in the source (see page 5-11).
Removing the APCI probe
Required materials
Chemical-resistant, powder-free gloves
To remove the APCI probe:
Warning: The LC system connections, APCI probe, and source can
be contaminated with biohazardous and/or toxic materials. Always
wear chemical-resistant, powder-free gloves while performing this
procedure.
Warning: To avoid electric shock, ensure that the instrument is
prepared for working on the source before commencing this procedure.
1. Prepare the instrument for working on the source (see page 5-7).
2. Remove the corona pin from the source (see page 5-13).
3. Disconnect the diverter valve tubing from the APCI probe.
4. Unscrew the probe locking ring.
5. Carefully remove the probe from the probe adjuster assembly.
Configuring for APCI mode 3-17
Page 62

Configuring for ESCi mode
To operate in ESCi mode, you must fit an ESI probe and corona pin to the
LockSpray source enclosure.
The system, with the ESI probe installed and corona discharge pin fitted, can
alternate between ESI and ESCi modes, facilitating data acquisition in ESI
and ESCi modes in parallel. For more information on using dual ESI and
ESCi modes, see the Xevo G2 QTof system online Help.
See “Installing the ESI probe” on page 3-2, “Installing the corona pin in the
source” on page 5-11, and “Combined electrospray and atmospheric pressure
chemical ionization (ESCi)” on page 1-9.
Optimizing the ESI probe for ESCi operation
See the mass spectrometer’s online Help for details on how to optimize the ESI
probe for ESCi operation.
3-18 Configuring the LockSpray Source
Page 63

4 Configuring the NanoLockSpray
Source
The Waters NanoLockSpray dual, electrospray, ion source enables the
optimized co-introduction of sample and lock mass compound directly
into the ion source. This feature provides authenticated, exact-mass
measurement in MS mode at low flow rates.
Contents:
Topic Page
Overview of the NanoLockSpray source 4-2 Configuring the NanoLockSpray source 4-4 Source type selection 4-5 Advancing and retracting the sprayer platform 4-6 Adjusting the sprayer tip position 4-7 Setting up the camera 4-8 Optional glass capillary sprayer 4-9
4-1
Page 64

Overview of the NanoLockSpray source
Sprayer platform adjuster assembly
Thumbscrew
Thumbscrew (on left-hand
side of sprayer platform)
Sprayer safety cover
Z-position adjuster
Y-position adjuster
X-position adjuster
Lock mass inlet
Camera
Camera focussing
ring
NanoLockSpray source:
The NanoLockSpray source enclosure holds two nanospray probes positioned
orthogonally with respect to one another. The sample flows through one probe
and the lock-mass reference through the other. A motorized baffle rotates to
admit spray from either probe to the sampling cone.
4-2 Configuring the NanoLockSpray Source
Page 65

Schematic of the NanoLockSpray source:
Sample inlet
Sample cone
Lock-spray inlet
Baffle
Spray indexing permits acquiring sample and lock-spray data in separate
data channels, and the baffle design ensures negligible cross talk between the
two sprays. Data from the lock-spray are used to calculate a correction factor
for the mass scale calibration, which is then applied to the sample data to
provide exact mass information.
Sample sprayer
The NanoLockSpray source can be used with different NanoFlow sprayers.
For sprayer-specific instructions on how to set up these sprayers, see page 4-4.
Lock-spray sprayer
The lock-spray sprayer runs from the instrument's IntelliStart Fluidics
system. Fitted with a 500-µL pump, the lock spray operates at 0.5 µL/min.
You must choose a concentration for the lock-spray solution that produces a
suitable ion intensity.
For maintenance information, see page 5-85.
Overview of the NanoLockSpray source 4-3
Page 66

NanoFlow gas supply
The nebulizer gas supply pressure for the sample sprayer is electronically
controlled from 0 to 2 bar. The optimum pressure is sprayer-dependent, but it
usually lies between 0.3 and 1.0 bar.
Purge gas
Purge gas, typically flowing at 100 L/hour, provides a positive pressure in the
source enclosure, reducing the chemical background interference caused by
contaminants in the laboratory air. You can adjust this flow in the software
(see the mass spectrometer’s online Help for details).
Sprayer platform adjuster assembly
The sprayer platform adjuster assembly allows precise X-, Y-, and
Z-positioning of the sprayer tip. You can also withdraw the sprayer from the
source to allow access to the sprayer tip.
Using the two thumbscrews on the base of the adjuster assembly, you can
move the platform in and out of the source (see page 4-6).
Configuring the NanoLockSpray source
The Universal NanoFlow sprayer is installed as standard equipment on the
NanoLockSpray source. For installation and maintenance details, see the
Waters Universal NanoFlow Sprayer Installation and Maintenance Guide
(part number 71500110107).
Caution: Connect the UPLC column directly to the universal sprayer.
Do not connect via the diverter valve. The analyte section of the
IntelliStart Fluidics system is not designed for use at the flow rates
used in NanoFlow ESI.
The following table summarizes how you configure the NanoLockSpray source
for the various ionization modes.
Tip: A corona pin is not used with the NanoLockSpray source.
4-4 Configuring the NanoLockSpray Source
Page 67

Warning: To avoid electrical shock when working with capillary
electrophoresis (CE) equipment, connect a CE interlock from the rear
of the instrument to the CE apparatus (see page B-20).
NanoLockSpray source configuration:
Sprayer type Used for
Universal NanoFlow nebulizer
sprayer
Borosilicate glass capillary
NanoFlow. For further details see
page 4-9.
NanoFlow capillary electrophoresis
(CE) sprayer. For further details, see
Capillary Electrophoresis/Capillary
Electrochromatography Sprayer
User's Guide (part number 6666522).
Source type selection
To select the source:
From the Tune window, click Source > NanoFlow.
Flow injection or for coupling to
nanoACQUITY with regulated flow
rates down to 100 nL/min.
Extremely low flow rates (less than
100 nL/min).
Stable electrospray by means of a
make-up liquid at the CE capillary
tip. The make-up flow rate is less
than 1 µL/min.
Source type selection 4-5
Page 68

Advancing and retracting the sprayer platform
To move the sprayer platform out of the source:
Warning: To avoid electrical shock, ensure the safety cover is in place
over the sprayer.
1. Confirm that the sprayer’s safety cover is installed (see the figure on
page 4-2).
2. Unscrew the thumbscrew on the front of the sprayer platform.
3. Pull out the side thumbscrew and withdraw the sprayer platform from
the source.
4. Release the side thumbscrew, locking the platform in the withdrawn
position.
To move the sprayer platform into the source:
Warning: To avoid electrical shock, ensure the safety cover is in place
over the sprayer.
1. Confirm that the sprayer’s safety cover is installed (see the figure on
page 4-2).
2. Pull out the side thumbscrew, and push the sprayer platform into the
source.
3. Release the side thumbscrew, locking the platform in position.
4. Tighten the front thumbscrew, securing the adjuster assembly rigidly to
the source.
4-6 Configuring the NanoLockSpray Source
Page 69

Adjusting the sprayer tip position
Alignment marker
Arrow
To adjust the tip position:
1. Adjust the X, Y, and Z controls on the adjuster assembly to move the
sprayer tip close to the sampling cone and baffle.
2. Adjust the height of the sprayer so that its tip is level with the center of
the baffle.
Tip: To align the Universal Sprayer with the centre of the sampling
cone, adjust the platform Z control until the arrow is in the centre of the
alignment marker.
Sprayer platform showing arrow and alignment marker:
3. Adjust the horizontal position of the sprayer so that the tip points
toward the left-hand side of the baffle.
Tips:
• If you observe an electrical discharge between the sprayer tip and
baffle, move the tip farther from the baffle, or reduce the capillary
voltage. Note, however, that the capillary voltage must be high
enough to maintain a good spray.
• Fine tune the position of the sprayer while acquiring a spectrum of a
standard compound. Small adjustments to the sprayer position can
make large differences to the source sensitivity.
Adjusting the sprayer tip position 4-7
Page 70

Setting up the camera
Sample cone
Baffle
Sample spray
To set up the camera:
1. In the instrument console, ensure that the NanoFlow source is selected.
2. Open the camera control, see the online help for details.
Camera Control view of sprayers and sample cone:
3. Rotate the camera’s focusing ring to focus on the sample sprayer (see the
figure on page 4-2).
4-8 Configuring the NanoLockSpray Source
Page 71

Optional glass capillary sprayer
The glass capillary sprayer is designed for use with metal-coated borosilicate
glass capillaries. They allow extremely low flow rates (less than 100 nL/min).
The glass capillaries are used for one sample only and must then be discarded.
To use the glass capillary sprayer, complete the following procedures:
• Plumb the backpressure gas.
• Install the glass capillary sprayer.
• Fit and load the glass capillary.
• Optimize the sprayer.
Plumbing the backpressure gas
The electronic pressure controller can control pressure only when gas is
flowing. However, the glass capillary requires a backpressure without a flow.
To resolve this situation, the backpressure gas is plumbed from the source to
the glass capillary sprayer through an in-line tee-piece. The tee-piece permits
the gas flow to bleed to atmosphere while the selected pressure is maintained
at the back of the capillary.
Required materials
• Chemical-resistant, powder-free gloves
• Glass capillary option spares kit, which includes these items:
– PTFE tubing, 1/16-inch OD, 0.006-inch ID, 530 mm
– PTFE tubing, 1/16-inch OD, 0.023-inch ID, 120 mm
– Tee piece and nuts, 1/16-inch “finger-tight”, 0.5-mm bore
– SealTight™ nut and ferrule
• Sharp knife or PEEK tubing cutter
To plumb the backpressure gas:
1. Use the sharp knife or PEEK tubing cutter to cut a 30-mm length from
the 0.006-inch ID PTFE tubing to leave a 500-mm length, and retain
both pieces.
Optional glass capillary sprayer 4-9
Page 72

2. Thread the indicated length of PTFE tubing through each of the
0.023-inch ID, 120-mm tubing
Pressure
controller
Sprayer
0.006-inch ID, 500-mm tubing
0.006-inch ID, 30-mm tubing
“finger-tight” nuts, and tighten them into the tee piece in the
arrangement as shown.
3. Using the SealTight nut and ferrule, connect the 0.023-inch ID PTFE
tubing to the pressure controller connection on the base of the source.
4. Using the nut and ferrule at the sprayer assembly, connect the
0.006-inch ID PTFE tubing to the sprayer.
Installing the glass capillary sprayer
Required materials
Chemical-resistant, powder-free gloves
To install the glass capillary sprayer:
Warning: The source components can be contaminated with
biohazardous and/or toxic materials. Always wear
f
chemical-resistant, powder-free gloves while performing this
procedure.
1. Prepare the instrument for working on the source (see page 5-7).
Warning: The probe and source can be hot. To avoid burn injuries,
take great care while working with these components.
2. Ensure the instrument is in Source Standby mode and that the status
indicator is yellow.
4-10 Configuring the NanoLockSpray Source
Page 73

3. Retract the sprayer platform adjuster assembly from the source (see
PTFE backpressure gas tubing
Blue conductive elastomerFerrule
Glass capillary
Knurled nutUnion
page 4-6).
4. Remove the sprayer’s safety cover.
5. Place the sprayer (with gas line fitted) on the platform, and secure it
with the thumbscrew.
6. Refit the safety cover.
Fitting and loading the glass capillary
Required materials
• Chemical-resistant, powder-free gloves
• Fused silica syringe needle or a GELoader
To fit and load the glass capillary:
Warning: The source components can be contaminated with
biohazardous and/or toxic materials. Always wear
f
chemical-resistant, powder-free gloves while performing this
procedure.
®
tip
1. Unscrew the union at the front of the sprayer, and remove the sprayer’s
front section.
Optional glass capillary sprayer 4-11
Page 74

Warning: To avoid injury with a sliver of glass
Union
Knurled nut
Glass capillary
Elastomer
Union Knurled nut
Glass capillary
7 mm
contaminated with toxic samples, do not touch the
sharp end of the capillary.
Caution: The capillaries are extremely fragile. Handle them with
great care from their square-cut ends. Touching their sharp end
can render the needle inoperable.
2. Carefully remove the capillary from its case by lifting it vertically while
pressing down on the foam with two fingers.
3. Over the square-cut end of the capillary, pass first the knurled nut, then
approximately 5 mm of conductive elastomer, and finally the union.
4. Finger tighten the nut onto the union.
4-12 Configuring the NanoLockSpray Source
5. Ensure that the tip of the glass capillary protrudes about 7 mm from the
front of the knurled nut, as measured from the end of the nut to the
shoulder of the glass capillary, and then full tighten the nut onto the
union.
Page 75

6. Load sample into the capillary using either a fused silica syringe needle
or a GELoader tip.
Tip: Shake the loaded capillary to move the liquid to the tip of the
sprayer.
7. With the sprayer mounted on the adjuster platform, screw the union
back into the assembly; finger-tight is sufficient.
8. In the console software, ensure the capillary parameter is set to 0 V.
9. Push the sprayer platform into the source (see page 4-6).
To optimize the glass capillary sprayer:
Warning: To avoid eye injuries, always wear eye protection when cutting
fused silica.
Warning: To avoid injury from trace chemicals adhering to the probe,
always wear chemical-resistant, powder-free gloves.
1. Set the NanoFlow gas pressure to 0.3 bar and the cone gas to 40 L/h.
2. Ascertain that sample is flowing by observing a droplet on the tip.
Tip: If you cannot observe a droplet, increase the pressure briefly, up to
a maximum of 1.5 bar, and then return the pressure to 0.3 bar.
3. If you observe a droplet, continue the procedure at step 9.
Requirement: If you do not observe a droplet, in the console software,
ensure the capillary parameter is set to 0 V, and follow step 4 through
step 8.
4. Move the sprayer back and to the left-hand side until the tip aligns with
the groove on the cone.
Tip: Aligning is best done while viewing from the front of the source.
Optional glass capillary sprayer 4-13
Page 76

5. While watching the camera image, carefully move the tip forward
Groove
Glass capillary
toward the groove, until it touches, and a small piece of the glass
capillary shears off.
6. Return the sprayer to its previous position.
7. Ascertain that sample is flowing by observing a droplet on the tip.
Tip: If you cannot observe a droplet, increase the pressure briefly, up to
a maximum of 1.5 bar, and then return the pressure to 0.3 bar.
8. If you observe a droplet, continue the procedure at step 9.
Requirement: If you do not observe a droplet, repeat step 4 through
step 8.
If, after you repeat step 4 through step 8, you still cannot observe a
droplet, discard the glass capillary and fit a new one (see page 4-11).
9. Set the capillary voltage slider to between 1.0 and 3.0 kV.
10. With an ion beam now visible on the peak display, optimize the sprayer
position and capillary voltage for maximum signal intensity.
Tip: The spray optimizes between 1.0 and 3.0 kV. To stop the sprayer,
set the capillary voltage to 0.
4-14 Configuring the NanoLockSpray Source
Page 77

5 Maintenance Procedures
This chapter provides the maintenance guidelines and procedures
necessary to maintain the instrument’s performance.
Keep to a maintenance schedule, and perform maintenance as required
and described in this chapter.
Contents:
Topic Page
Maintenance schedule 5-3
Spare parts 5-4
Troubleshooting with Connections INSIGHT 5-5
Safety and handling 5-6
Preparing the instrument for working on the source 5-7
Removal and refitting of the source enclosure 5-7
Installing and removing the corona pin 5-11
Operating the source isolation valve 5-14
Removing O-rings and seals 5-17
Cleaning the instrument case 5-17
Emptying the nitrogen exhaust trap bottle 5-18
Inspecting the Varian roughing pump oil level 5-20
Adding oil to the Varian roughing pump 5-21
Replacing the Varian roughing pump’s oil and oil mist filter 5-23
Cleaning the source components 5-28
Cleaning the sampling cone assembly 5-29
Cleaning the extraction cone 5-38
Cleaning the ion block assembly 5-46
Cleaning the source hexapole assembly 5-57
Replacing the ESI probe tip and gasket 5-63
5-1
Page 78

Contents: (Continued)
Topic Page
Replacing the ESI probe sample capillary 5-66
Cleaning the APCI probe tip 5-75
Replacing the APCI probe sample capillary 5-75
Replacing the reference probe capillary (LockSpray source) 5-82
Replacing the reference probe capillary (NanoLockSpray
source) 5-85
Cleaning or replacing the corona pin 5-90
Replacing the APCI probe heater 5-91
Replacing the ion block source heater 5-94
Replacing the LockSpray source’s assembly seals 5-98
Replacing the mass spectrometer’s air filters 5-102
Replacing the IntelliStart Fluidics tubing 5-106
5-2 Maintenance Procedures
Page 79

Maintenance schedule
The following table lists periodic maintenance schedules that ensure optimum
instrument performance.
Maintenance schedule:
Procedure Frequency For information...
Clean the instrument case. As required. See page 5-17.
Empty the nitrogen exhaust
trap bottle.
Inspect and adjust the
roughing pump’s oil level.
Replace the roughing pump’s
oil and oil mist filter.
Replace the oil-free (scroll)
pump’s seals.
Check daily, empty as
required.
Weekly. See page 5-20.
Annually. See page 5-23.
Annually. See Edwards
See page 5-18.
document XDS35i
Instruction Manual
A730-01-880.
Clean the source components. When sensitivity
decreases to
unacceptable levels.
Replace the ESI probe tip. When sensitivity
decreases to
unacceptable levels.
Replace the ESI probe
capillary.
Clean the APCI probe tip.
(Options using the APCI
probe only.)
Replace the APCI probe
capillary.
When sensitivity
decreases to
unacceptable levels or
sample flow is
inconsistent.
When sensitivity
decreases to
unacceptable levels.
When sensitivity
decreases to
unacceptable levels or
sample flow is
inconsistent.
See page 5-28.
See page 5-63.
See page 5-66.
See page 5-75.
See page 5-75.
Maintenance schedule 5-3
Page 80

Maintenance schedule: (Continued)
Procedure Frequency For information...
Replace the reference probe
capillary.
Clean or replace the corona
pin (APCI and ESCi modes).
Replace the APCI probe
heater.
Replace the ion block heater
cartridge.
Replace the source assembly
seals.
Replace the mass
spectrometer air filters.
Replace the IntelliStart
Fluidics tubing.
Annually. See page 5-82.
When the corona pin is
corroded or black, or
the sensitivity
decreases to
unacceptable levels.
If the heater fails to
heat the probe.
If the heater fails to
heat the ion block.
Annually. See page 5-98.
Annually. See page 5-102.
In the event of
blockage in the tubing
connections between
the IntelliStart
Fluidics system
components.
See page 5-90.
See page 5-91.
See page 5-94.
See page 5-106.
Spare parts
Waters recommends that you replace only the parts mentioned in this
document. For spare parts details, see the Waters Quality Parts Locator on
the Waters Web site’s Services/Support page.
5-4 Maintenance Procedures
Page 81

Troubleshooting with Connections INSIGHT
Connections INSIGHT® is an “intelligent” device management (IDM) Web
service that enables Waters to provide proactive service and support for the
ACQUITY UPLC system. To use Connections INSIGHT, you must install its
service agent software on your MassLynx™ workstation. In a client/server
system, the service agent must also be installed on the computer from which
you control the system. The service agent software automatically and securely
captures and sends information about the support needs of your system
directly to Waters.
If you encounter a performance issue when using the Instrument Console, you
can manually submit a Connections INSIGHT request to Waters customer
support. Alternatively, you can use Remote Desktop, a real-time collaboration
option that controls the two-way connection with the ACQUITY UPLC system
by enabling the Connections INSIGHT iAssist service level.
Consult these sources for more information about Connections INSIGHT and
Connections INSIGHT iAssist:
• http://www.waters.com
• Connections INSIGHT Installation Guide (part number 715001399)
• Connections INSIGHT User's Guide (part number 715001400)
• Your sales representative
• Your local Waters subsidiary
• Waters Customer Support
To submit a Connections INSIGHT request:
1. Select Troubleshoot > Submit Connections INSIGHT request.
2. In the Connections INSIGHT Request dialog box, type your name,
telephone number, e-mail address, and a description of the problem.
3. Click Submit, and allow approximately 5 minutes to save the service
profile.
Result: A .zip file containing your Connections INSIGHT profile is
forwarded to Waters customer support for review. Saving a service
profile or plot file from the Instrument Console can require as much as
150 MB of file space.
Troubleshooting with Connections INSIGHT 5-5
Page 82
Safety and handling
Bear in mind the following safety considerations when performing
maintenance procedures:
Warning: The instrument components can be contaminated with
biohazardous and/or toxic materials. Always wear
chemical-resistant, powder-free gloves while handling the
components.
Warning: To prevent injury, always observe Good Laboratory Practice
when handling solvents, changing tubing, or operating the instrument.
Know the physical and chemical properties of the solvents used (see the
Material Safety Data Sheets for the solvents in use).
Warning: To avoid electric shock,
• do not remove the instrument’s panels. There are no user-serviceable
items inside the instrument.
• ensure that the instrument is in Standby mode before commencing
any maintenance.
Warning: The probe and source can be hot. To avoid burn injuries, take
great care while working with these components.
Warning: To avoid puncture wounds, take great care while working with
the source enclosure open if one or both of these conditions apply:
• An ESI probe is fitted (the probe tip is sharp).
• A corona pin is fitted (the pin tip is sharp).
See Appendix A for safety advisory information.
5-6 Maintenance Procedures
Page 83

Preparing the instrument for working on the source
For safety reasons, you must follow the procedure described below before
working on the source (for example, when changing the probe, installing or
removing the corona pin, operating the source isolation valve, and when
maintaining the source).
To prepare the instrument for working on the source:
1. In the instrument console, stop the LC flow or, if column flow is
required, divert the LC flow to waste.
2. In the instrument console, select source standby and confirm that the
operate indicator is not illuminated.
3. Set the source temperature to 30 °C.
Warning: To avoid burn injuries, do not touch metal surfaces that
exceed 70 °C.
4. Wait for the source temperature, desolvation temperature or APCI probe
temperature to cool.
5. Ensure that the API desolvation gas flow is stopped.
Removal and refitting of the source enclosure
You must first remove the LockSpray or NanoLockSpray source enclosure
from the instrument to perform certain maintenance procedures, or if you are
fitting the optional dual-mode APPI/APCI source to the instrument.
The following procedures apply to both the standard and optional source
enclosures.
Removing the source enclosure from the instrument
Required materials
Chemical-resistant, powder-free gloves
Preparing the instrument for working on the source 5-7
Page 84

To remove the source enclosure:
Warning: The source components can be contaminated with
biohazardous and/or toxic materials. Always wear
chemical-resistant, powder-free gloves while performing this
procedure.
1. Prepare the instrument for working on the source (see page 5-7).
Warning: The probe and source can be hot. To avoid burn injuries,
take great care while working with these components.
2. Remove the probe from the source:
• If you are removing an ESI probe, see page 3-6.
• If you are removing an APCI probe, see page 3-17.
3. Slide open the instrument’s source interface door (see the figure on
page 1-4).
4. Disconnect the probe adjuster and options cables from the instrument’s
connectors.
Warning: The corona pin tip is sharp. To avoid puncture wounds,
take great care while working with the source enclosure open if a
corona pin is fitted.
Caution: If you are removing a NanoLockSpray source enclosure,
to avoid damage to the sample inlet, you must move the sprayer
platform out of the source enclosure before you open the source
enclosure (see page 4-6).
5. Pull the source enclosure release (located at the bottom, right-hand side)
outwards, and swing open the enclosure.
5-8 Maintenance Procedures
Page 85

6. Using both hands, grasp the source enclosure, and lift it vertically off the
TP03164
Supporting stud
Source enclosure
Cable storage positions
two supporting studs on the source adaptor housing.
7. Store the cables neatly by plugging them into the cable-storage positions
on the rear of the source enclosure.
Removal and refitting of the source enclosure 5-9
Page 86

Fitting the source enclosure to the instrument
Required materials
Chemical-resistant, powder-free gloves
To fit the source enclosure to the instrument:
Warning: The source components can be contaminated with
biohazardous and/or toxic materials. Always wear
chemical-resistant, powder-free gloves while performing this
procedure.
Warning: To avoid puncture wounds, take great care while fitting the
source enclosure to the source if a corona pin is fitted (the pin tip is
sharp).
1. Using both hands, fit the source enclosure to the two supporting studs
on the source adaptor housing.
Caution: If you are fitting a NanoLockSpray source enclosure, to
avoid damage to the sample inlet, you must move the sprayer
platform out of the source enclosure before you close the source
enclosure (see page 4-6).
2. Close the source enclosure.
3. Connect the probe adjuster and options cables to the instrument’s
connectors.
Tip: The cables and connectors are color coded; the blue-sleeved cable
connects to the blue connector and the yellow-sleeved cable to the yellow
connector.
4. Slide closed the instrument’s source interface door.
5-10 Maintenance Procedures
Page 87

Installing and removing the corona pin
For APCI, ESCi, and dual-mode APPI/APCI operation, you must fit a corona
pin to the source.
Installing the corona pin in the source
Required materials
Chemical-resistant, powder-free gloves
To install the corona pin in the source:
Warning: The LC system connections, ESI probe, and source can
be contaminated with biohazardous and/or toxic materials. Always
wear chemical-resistant, powder-free gloves while performing this
procedure.
Warning: To avoid electric shock, ensure that the instrument is
prepared for working on the source when commencing this procedure.
1. Prepare the instrument for working on the source (see page 5-7).
Warning: The source can be hot. To avoid burn injuries, take great
care while working with the source enclosure open.
Warning: The ESI probe tip is sharp. To avoid puncture wounds,
take great care while working with the source enclosure open if an
ESI probe is fitted.
2. Pull the source enclosure release (located at the bottom, right-hand side)
outwards, and swing open the enclosure.
3. Remove the blanking plug from the corona pin mounting contact.
Tip: Store the blanking plug in a safe location.
Installing and removing the corona pin 5-11
Page 88

Corona pin mounting contact:
TP03130
Corona pin mounting
contact blanking plug
TP03130
Corona pin
Sample cone orifice
Warning: The corona pin tip is sharp. To avoid puncture wounds,
handle it with care.
4. Fit the corona pin to the corona pin mounting contact, ensuring that the
corona pin is securely mounted and that its tip aligns with the sample
cone orifice.
Corona pin:
5-12 Maintenance Procedures
Page 89
5. Close the source enclosure.
6. Look through the source window, and use the vernier probe adjuster (see
page 3-4) to position the ESI probe tip so that it is pointing,
approximately, midway between the tips of the sample cone and corona
pin.
Removing the corona pin from the source
Required materials
Chemical-resistant, powder-free gloves
To remove the corona pin from the source:
Warning: The LC system connections, ESI probe, and source can
be contaminated with biohazardous and/or toxic materials. Always
wear chemical-resistant, powder-free gloves while performing this
procedure.
Warning: To avoid electric shock, ensure that the instrument is
prepared for working on the source when commencing this procedure.
1. Prepare the instrument for working on the source (see page 5-7).
Warning: The source can be hot. To avoid burn injuries, take great
care while working with the instrument’s source enclosure open.
Warning: The ESI probe tip is sharp. To avoid puncture wounds,
take great care while working with the source enclosure open if an
ESI probe is fitted.
2. Pull the source enclosure release (located at the bottom, right-hand side)
outwards, and swing open the enclosure.
3. Remove the corona pin from its mounting contact (see the figure on
page 5-12).
Tip: Store the corona pin in a safe location.
4. Fit the blanking plug to the corona pin mounting contact (see the figure
on page 5-12).
5. Close the source enclosure.
Installing and removing the corona pin 5-13
Page 90

Operating the source isolation valve
You must close the source isolation valve to isolate the source from the
instrument vacuum system for certain maintenance procedures.
Required materials
Chemical-resistant, powder-free gloves
To close the source isolation valve before starting a maintenance
procedure:
Warning: The source components can be contaminated with
biohazardous and/or toxic materials. Always wear
chemical-resistant, powder-free gloves while performing this
procedure.
Warning: To avoid electric shock, ensure that the instrument is suitably
prepared before commencing this procedure.
1. Prepare the instrument for working on the source (see page 5-7).
Warning: The source can be hot. To avoid burn injuries, take great
care while working with the instrument’s source enclosure open.
Warning: To avoid puncture wounds, take great care while
working with the source enclosure open if one or both of these
conditions apply:
• An ESI probe is fitted (the probe tip is sharp).
• A corona pin is fitted (the pin tip is sharp).
2. Pull the source enclosure release (located at the bottom, right-hand side)
outwards, and swing open the enclosure.
5-14 Maintenance Procedures
Page 91

3. Close the source isolation valve by moving its handle counterclockwise,
TP03130
Isolation valve
handle in closed
position
to the vertical position.
Operating the source isolation valve 5-15
Page 92

To open the source isolation valve after completing a maintenance
TP03130
Isolation valve
handle in open
position
procedure:
Warning: The source components can be contaminated with
biohazardous and/or toxic materials. Always wear
chemical-resistant, powder-free gloves while performing this
procedure.
Warning: To avoid puncture wounds, take great care while working with
the source enclosure open if one or both of these conditions apply:
• An ESI probe is fitted (the probe tip is sharp).
• A corona pin is fitted (the pin tip is sharp).
1. Open the source isolation valve by moving its handle clockwise to the
horizontal position.
2. Close the source enclosure.
5-16 Maintenance Procedures
Page 93

Removing O-rings and seals
Too l 1
Tool 2
When performing certain maintenance procedures, you must remove O-rings
or seals from instrument components. An O-ring removal kit is provided with
the instrument.
O-ring removal kit:
To remove an O-ring:
Caution: When removing an O-ring or seal from a component, be careful
not to scratch the component with the removal tool.
Use the tools as aids to pull the O-ring or seal from its groove.
Tip: If the O-ring or seal is not going to be reused, you can use the forked end
of tool 1 to impale the O-ring or seal and aid removal.
Cleaning the instrument case
Caution: Do not use abrasives or solvents to clean the instrument’s case.
Use a soft cloth, dampened with water, to clean the outside surfaces of the
mass spectrometer.
Removing O-rings and seals 5-17
Page 94
Emptying the nitrogen exhaust trap bottle
TP03164
From instrument
exhaust connection
To laboratory
exhaust port
Cap
Nitrogen exhaust
trap bottle
From instrument
pilot valve port
Inspect the nitrogen exhaust trap bottle in the instrument exhaust line daily,
and empty it before it is more than approximately 10% full.
Nitrogen exhaust trap bottle:
5-18 Maintenance Procedures
Page 95
Required materials
Chemical-resistant, powder-free gloves
To empty the nitrogen exhaust trap bottle:
1. In the Instrument Console, click Stop Flow .
2. Pull the source enclosure release (located at the bottom, right-hand side)
outwards, and swing open the enclosure.
Warning: The waste liquid in the nitrogen exhaust trap
bottle comprises ACQUITY UPLC solvents and analytes.
Always wear chemical-resistant, powder-free gloves while
handling it.
3. Unscrew and remove the nitrogen exhaust trap bottle from the cap and
associated fittings.
Warning: The waste liquid can be contaminated with
biohazardous and/or toxic materials. Dispose of it according
to local environmental regulations.
4. Dispose of the waste liquid in accordance with local environmental
regulations.
5. Fit and tighten the nitrogen exhaust trap bottle to the cap.
6. Secure the nitrogen exhaust trap bottle in the upright position.
7. Close the source enclosure.
Tip: An automatic pressure test will now be performed.
8. In the Instrument Console, click Start Flow .
Emptying the nitrogen exhaust trap bottle 5-19
Page 96

Inspecting the Varian roughing pump oil level
Maximum oil level
Minimum oil level
Note: This procedure is not required for an Edwards oil-free roughing pump
Caution: To ensure correct operation of the roughing pump, do not
operate the pump with the oil level outside the range shown by the oil
level sight glass minimum and maximum oil level indicators.
Requirement: You must determine the oil level while the roughing pump is
running.
The roughing pump oil level appears in the pump’s oil-level sight glass.
Inspect the oil level weekly, while the pump is running. If the oil level is
significantly below the maximum oil level indicated by the oil level sight glass,
add oil to the pump (see page 5-21).
Sight glass for the roughing pump oil level:
5-20 Maintenance Procedures
Page 97

Adding oil to the Varian roughing pump
TP03159
Oil filler plug
Oil level sight glass
Oil drain plug
Note: This procedure is not required for an Edwards oil-free roughing pump.
If, on checking the pump’s oil level, you find it low, you must add oil (see
page 5-20).
Varian roughing pump:
Required materials
• Chemical-resistant, powder-free gloves
• Funnel
• Varian GP45 oil
Adding oil to the Varian roughing pump 5-21
Page 98

To add oil to the roughing pump:
1. Vent and shut down the mass spectrometer (see the mass spectrometer’s
online Help for details).
Warning: To avoid personal injury, as well as damage to the
roughing pumps and mass spectrometer, disconnect the power
cords for the mass spectrometer and roughing pumps from the
main power source.
2. Disconnect the power cords for the mass spectrometer and roughing
pump from the main power source.
3. Allow the oil to settle in the pump.
Warning: The pump oil can be contaminated with analyte
accumulated during normal operation. Always wear
chemical-resistant, powder-free gloves when adding or
replacing oil.
Warning: To avoid burn injuries, take great care while working
with the roughing pump: it can be hot.
4. Unscrew and remove the roughing pump’s oil filler plug.
Caution: To maintain pump performance,
• use only Varian GP45 oil.
• do not operate the pump with the oil level above the oil level
sight glass maximum oil level indicator.
5. Using the funnel, slowly add Varian GP45 oil into the oil filler aperture
until the oil reaches the oil level sight glass maximum oil level indicator.
Caution: To avoid oil leakage, when fitting the oil filler plug to the
roughing pump,
• ensure that the plug is not cross-threaded.
• do not over-tighten the plug.
6. Fit and tighten the roughing pump’s oil filler plug.
5-22 Maintenance Procedures
Page 99

7. Connect the power cords for the mass spectrometer and roughing pump
to the main power source.
8. Start the mass spectrometer (see page 2-2).
Tips: After you add oil to the pump, the following situations can occur:
• The oil level drops slightly during the first month of operation.
• The oil changes color (darkens) over time.
• After running the pump for 12 to 48 hours, a few drops of oil
sometimes appear near the filler plug. Excess oil around the lip of
the filler plug will run down and drips off the pump once the pump
reaches operating temperature.
• When the pump begins to run at normal operating temperature,
spilled oil becomes slightly odorous.
Replacing the Varian roughing pump’s oil and oil mist filter
Replace the roughing pump oil and oil mist filter annually.
Note: This procedure is not required for an Edwards oil-free roughing pump.
Emptying the roughing pump’s oil
Required materials
• Chemical-resistant, powder-free gloves
• 8-mm Allen wrench
• Container to catch used oil
• Lint-free cloth
To empty the roughing pump’s oil:
1. Vent and shut down the mass spectrometer. See the mass spectrometer’s
online Help for details.
2. Disconnect the power cords for the mass spectrometer and roughing
pump from the main power source.
Replacing the Varian roughing pump’s oil and oil mist filter 5-23
Page 100

3. Allow the roughing pump to cool.
Warning: The roughing pump oil can be contaminated with
analyte accumulated during normal operation. Always wear
chemical-resistant, powder-free gloves when adding or
replacing oil.
Warning: To avoid burn injuries, take great care while working
with the roughing pump: it can be hot.
4. Place the container for catching used oil under the pump’s drain plug
(see the figure on page 5-21).
5. Unscrew and remove the oil filler plug.
6. Use the 8-mm Allen wrench to remove the oil drain plug.
7. Tip the pump toward the drain plug aperture and allow the oil to drain
completely into the container.
Warning: The roughing pump oil can be contaminated with
biohazardous and/or toxic materials. Dispose of it according
to local environmental regulations.
8. Dispose of the roughing pump oil in accordance with local environmental
regulations.
9. Use the lint-free cloth to remove loose particulate from the magnetic tip
of the oil drain plug.
Replacing the oil mist filter
Required materials
• Chemical-resistant, powder-free gloves
• 3-mm Allen wrench
• New oil mist filter
5-24 Maintenance Procedures
 Loading...
Loading...