Page 1
[ CARE AND USE MANUAL ]
XBridge Protein BEH SEC Columns and Standards
CONTENTS
I. INTRODUCTION
II. SYSTEM CONSIDERATIONS FOR
SEC SEPARATIONS
a. Getting Started
b. Column Installation
c. Column Equilibration
d. Useful Functional Tests for Benchmarking LC System
and XBridge Protein BEH SEC Column
III. COLUMN SPECIFICATIONS AND USE
a. SEC Eluent and Needle Wash Preparation
b. Sample Preparation
c. Column Specification
IV. TROUBLESHOOTING
V. COLUMN CLEANING, REGENERATION
AND STORAGE
a. Cleaning and Regeneration
b. Storage
I. INTRODUCTION
Waters family of XBridge® Protein BEH SEC 200Å and 450Å,
3.5 µm Columns was developed to complement the existing line of
UPLC®-based SEC offerings for use where traditional HPLC-based
instrumentation and methods are employed for peptide or protein
size-exclusion chromatography (SEC). These new HPLC-based, SEC
chemistries are based on the same Waters Ethylene Bridged Hybrid
(BEH)-based particle technology and diol-bonded surface coating
as used in our successful line of UPLC-based SEC columns. This
process offers chromatographers the option and ability to easily
transfer methods based on lab instrumentation and component
resolution or sample throughput needs.
All of Waters BEH-based SEC columns are manufactured in a cGMP,
ISO 9001 certified plant using stringent manufacturing protocols
and ultra pure reagents. Each batch of manufactured material
undergoes a series of standard QC measurements (e.g., particle
and pore size distribution) followed by an application specific
test using appropriate peptide and protein test mixture. A packed
column efficiency test is then performed on every batch approved,
packed SEC column to further help ensure reproducible batch to
batch and column to column performance for use in research or in a
demanding validated method.
Page 2
[ CARE AND USE MANUAL ]
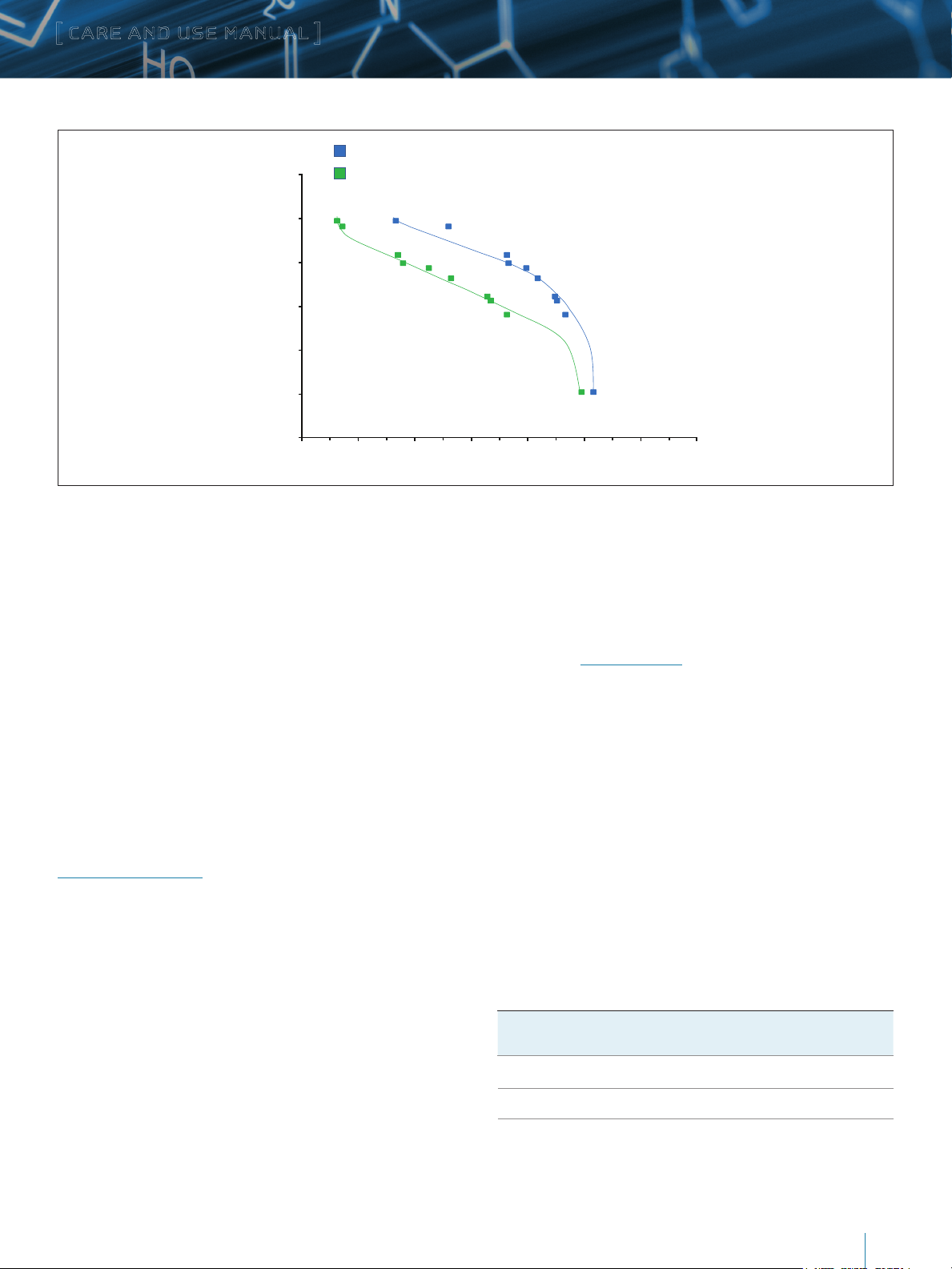
Uracil (112 Da)
Aprotinin (6.5 KDa)
RNAse A (14 KDa)
Myoglobin (17 KDa)
Ovalbumin (44 KDa)
Conalbumin (75 KDa)
Amyloglucosidase (97 KDa)
IgG (150 KDa)
Thyroglobulin (669 KDa)
IgM (900 KDa)
10
100
1,000
10,000
100,000
1,000,000
10,000,000
0.3 0.4 0.5 0.6 0.7 0.8 0.9 1
Protein Molecular Weight (KDa)
Normalized Retention Volume (Vr/VC)
XBridge Protein BEH SEC 450Å, 3.5 µm, 100K – 1,500K Daltons
XBridge Protein BEH SEC 200Å, 3.5 µm, 10K – 450K Daltons
Figure 1: Calibration Curves on XBridge Protein BEH SEC 200Å and 450Å Columns
II. SYSTEM CONSIDERATIONS FOR
SEC SEPARATION
a. Getting Started
In order to obtain the best performance from your Waters XBridge
Protein BEH SEC Column, it is important that your LC system be
properly configured. It is recommended that only pre-cut tubing is
used, and that the ID of all connecting tubing is 0.005” or less for
optimal chromatographic performance.
Size-exclusion chromatography may require modifications to
an existing LC system. Please refer to “Size-Exclusion and IonExchange Chromatography of Proteins using the ACQUITY UPLC
System” (P/N 715002147A that can be obtained at
www.waters.com/chemcu) for examples of LC System components
that can affect SEC results.
The sample loop used may affect the performance of your
separation. Optimally, select the smallest volume sample loop that
is required for the application. Sample loops larger than 20 µL are
not recommended.
b. Column Installation
1. Prior to placing the column on the system, purge the solvent
delivery system of any organic or water-immiscible mobile
phases. When connecting the column, orient it in the proper
direction as noted by the arrow on the column inlet side which
indicates the correct direction of solvent flow.
2. Flush column with 100% aqueous buffer, by pumping at a flow
rate of 0.2 mL/min.
3. Ensure that the mobile phase is flowing freely from the column
outlet. Attach the column outlet to the detector using .004”
ID tubing (P/N 430001562). Monitor the system pressure to
ensure the column is within its pressure limitations.
4. Gradually increase the flow rate, by not more than 0.1 mL/min
at a time, as described in Step 2.
5. Once the system pressure has stabilized, ensure that there are
no leaks at either the column inlet or outlet.
c. Column Equilibration
XBridge Protein BEH SEC Columns are shipped in 20% methanol
in water. It is important to ensure mobile-phase compatibility
before changing to a different mobile-phase system. Equilibrate
the column with a minimum of 10 column volumes of the buffer to
be used (refer to Table 1 for column volumes).
Column Dimension Approximate Volume
7.8 x 150 mm 7 mL
7.8 x 300 mm 14 m L
Table 1. Empty Column Volumes in mL (multiply by 10 for flush solvent volume)
XBridge Protein BEH SEC Columns and Standards
2
Page 3

[ CARE AND USE MANUAL ]
d. Useful Functional Tests for Benchmarking LC System and
XBridge Protein BEH SEC Column
Waters recommends performing a benchmarking test upon
receipt of your column and throughout the lifetime usage. By
using a separation of common proteins with an appropriate
method, you can:
Verify the performance of the column upon receipt.
Monitor the condition of the columns for extended use.
Troubleshoot separation difficulties that may arise.
Waters BEH200 SEC Protein Standard Mix (P/N 186006518)
and BEH450 SEC Protein Standard Mix (P/N 186006842) were
specifically designed for this purpose with carefully chosen
proteins and/or peptides to provide a good representation of the
intended application.
The information below details how to successfully prepare and
use the included BEH SEC Protein Standard for benchmarking or
troubleshooting purposes. Figures 2 and 3 provide separation
conditions and results you should expect to obtain.
Buffer Preparation
Chemicals:
Sodium phosphate monobasic, monohydrate
Sodium phosphate dibasic, anhydrous
HPLC-grade water
1
0.036
0.024
AU
0.012
0.000
0.00 1.00 2.00 3.00 4.00 5.00 6.00 7.00 8.00 9.00 10.00
1.) Thyroglobulin, 2.) IgG FU, 3.) BSA, 4.) Myoglobin, 5.) Uracil
Figure 2: Protein Mixture separation on XBridge Protein BEH SEC 200,
3.5 µm 7.8 x 150
4
3
2
5
Minutes
Analyte pl MW
1. Thyroglobulin, 3 mg/mL 4.6 669,000
2. IgG, 2 mg/mL 6.7 150,000
3. BSA, 5 mg/mL 4.6 66,400
4. Myoglobin, 2 mg/mL 6.8, 7.2 17,000
5. Uracil, 0.1 mg/mL N/A 112
Table 2: BEH200 SEC Test Mix
Conditions:
Instrument: ACQUITY TUV with Tunable UV detector
Column: XBridge Protein BEH SEC, 200Å, 3.5 μm,
7.8 x 150 mm
Preparation of 100 mM sodium phosphate buffer (500 mL):
1. Weigh out 500 ± 0.02 g of water into a 500 mL beaker.
2. Weigh out 3.55 ± 0.02 g of sodium phosphate dibasic and add
to the 500 mL beaker.
3. Weigh out 3.45 ± 0.02 g of sodium phosphate monobasic and
add to the 500 mL beaker.
4. Stir for a minimum of 30 minutes then filter the solution
through a 0.2 µm filter.
5. Take the pH and record the value for reference purposes
(approximate pH: 6.8).
Sample: BEH200 SEC Protein Standard Mix
(P/N 186006518)
Mobile phase: 100 mM sodium phosphate, pH 6.8
Weak needle wash: 100% Milli-Q
®
water
Strong needle wash: 100% Milli-Q water
Seal wash: 90/10 water/methanol
Injection type: Full loop
Injection volume: 2 µL
Flow rate: 0.86 mL/min
Column temp.: Ambient
Detection: UV @ 280 nM
XBridge Protein BEH SEC Columns and Standards
3
Page 4

[ CARE AND USE MANUAL ]
5
0.030
3
0.020
AU
0.010
1
0.000
0.00 1.00 2.00 3.00 4.00 5.00 6.00 7.00 8.00 9.00 10.00
1.) Thyroglobulin dimer, 2.) Thyroglobulin, 3.) IgG FU, 4.) BSA, 5.) Myoglobin, 6.) Uracil
Figure 3: Protein Mixture separation on XBridge Protein BEH SEC 450,
3.5 µm 7.8 x 150 mm
4
6
2
Minutes
Analyte pl MW
1. Thyroglobulin, 0.1 mg/mL 4.6 669,000
2. Thyroglobulin, Approx 3 mg/mL 4.6 669,000
3. IgG, 2 mg/mL 6.7 150,000
4. BSA, 5 mg/mL 4.6 66,400
5. Myoglobin, 2 mg/mL 6.8, 7.2 17,000
6. Uracil, 0.1 mg/mL N/A 112
Table 3: BEH450 SEC Test Mix
Conditions:
Instrument: ACQUITY TUV with Tunable UV detector
Column: XBridge Protein BEH SEC, 450Å, 3.5 μm,
7.8 x 150 mm
III. COLUMN SPECIFICATIONS AND USE
To ensure the continued high performance of XBridge Protein BEH
SEC, 3.5 µm Columns, follow these guidelines:
a. SEC Eluent and Needle Wash Preparation
Use HPLC-grade buffers, water, and organic solvents
when possible.
Filter solutions through a compatible 0.2 µm or smaller pore size
filter. The use of a sterile filtration apparatus is recommended
for buffers capable of supporting microbial growth.
Solutions that are susceptible to microbial growth should be
replaced at regular intervals to avoid column contamination.
Do NOT refill partially full SEC eluent bottles with new eluent.
Rather, when required use new bottle containing freshly
prepared SEC eluent.
Select solvent inlet filters that are compatible with solutions
used, and clean or replace filters regularly when using
solutions that are susceptible to microbial growth.
b. Sample Preparation
Ensure that samples are free of particulates before injecting
onto the SEC column. If samples appear cloudy or turbid,
they should not be injected, as this could lead to column
pressure increases. Sample preparation such as filtration or
centrifugation may be used, if appropriate.
If the sample is not dissolved in the mobile phase, ensure that
the sample, solvent and mobile phases are miscible in order to
avoid sample and/or buffer precipitation.
Sample: BEH450 SEC Protein Standard Mix
(P/N 186006842)
Mobile phase: 100 mM sodium phosphate, pH 6.8
Weak needle wash: 100% Milli-Q water
Strong needle wash: 100% Milli-Q water
Seal wash: 90/10 water/methanol
Injection type: Full loop
Injection volume: 2 µL
Flow rate: 0.86 mL/min
Column temp.: Ambient
Detection: UV @ 280 nM
c. Column Specifications
Shipping solvent: 20% methanol in water
Recommended maximum flow rate and backpressure:
XBridge Protein BEH SEC 200, 7.8 x 150 mm:
4 mL/min/2,600 psi
XBridge Protein BEH SEC 200, 7.8 x 300 mm:
2.7 mL/min/3,200 psi
XBridge Protein BEH SEC 450, 7.8 x 150mm:
4mL/min / 2600psi
XBridge Protein BEH SEC 450, 7.8 x 300mm:
2.7 mL/min / 3200psi
XBridge Protein BEH SEC Columns and Standards
4
Page 5

[ CARE AND USE MANUAL ]
Mass load: < 300 μg for a 7.8 x 150 mm
Volume load: < 60 μL for 7.8 x 150 mm
Recommended pH range: 2 to 8. The column lifetime will vary
depending upon the operating temperature as well as the type
and concentration of buffer used.
Recommended salt conc.: less than or equal to 0.5 M
Recommended organic conc.: < 20% acetonitrile
(Caution: Many proteins are insoluble at elevated organic
concentrations. Prior to chromatography, test to ensure the sample
does not precipitate at the organic concentration to be used for
the chromatography. Also, if column is run under denaturing
conditions (greater than 10% organic), subsequent column
performance under 100% aqueous conditions may be affected.)
Recommended temperature: 4–60 °C. Reduce flow rate when
operating at low temperatures (e.g. 10 °C) to avoid excessive
column pressure.
Recommended storage: For overnight storage, continuously
flush the column with the mobile phase at 10–20% of the
maximum recommended flow rate. Store the column in the
HPLC-grade water when it will be used within 24 hrs or in 20%
methanol for long term storage.
Note: Working at extremes of pressure, pH and/or temperature may
result in shorter column lifetimes.
IV. TROUBLESHOOTING
The first step in systematic troubleshooting is comparison of the
column performance in its current state to the performance when
it was functioning properly. The functional tests with the protein
mixture may reveal subtle changes in surface chemistry that affect
the application.
There are several common symptoms of change in the column.
1. An increase in pressure is often associated with decreased
performance in the application. The first step in diagnosis is
to ensure that the elevated pressure resides in the column
rather than somewhere else in the system. This is determined
by monitoring pressure of the system as each connection
is broken from the outlet end to the inlet. If the system is
occluded, the blockage should be identified and removed. If the
pressure increase resides in the column, it is helpful to know
whether the problem was associated with a single injection or
whether it occurred over a series of injections. If the pressure
gradually built up, it is likely that the column can be cleaned
as described in Section VI. If a single sample caused the
pressure increase, it likely reflects particulates or insoluble
components, such as lipids or higher order aggregates.
Cleaning is still an option, but using the more aggressive
options. If samples appear cloudy or turbid, they should not
be injected, as this will lead to pressure increases. Sample
preparation such as filtration or centrifugation may be used,
but one should first check whether this impacts the results.
2. Loss of resolution and increased peak tailing can be caused
by microbial contamination. It is important to follow
good standard laboratory practices to prevent microbial
contamination. This includes changing buffer bottles
frequently, using high purity water, using a sterile filtration
apparatus, and storing system and column under recommended
conditions. If microbial contamination has occurred, cleaning
the column will have no effect on performance. When changing
the flow rate, ramp it at a rate of 0.1 mL/min and avoid
immediate flow-rate increases greater than 0.1 mL/min.
3. Increased peak tailing can be caused by failure of a tubing
connector or a build-up of material on the column inlet frit.
Before proceeding with diagnostic or corrective measures,
check all connections that the mobile phases have been
correctly prepared and the correct method has been selected.
Then repeat the protein standard test. If the proteins shows
increased peak tailing, it is likely that there is significant
build-up of material on the column inlet and the column will
require replacement.
4. Carryover is defined as the appearance of the constituents
of one sample in the next analysis. In size-exclusion
chromatography carryover is typically due to system
components or improper wash solvents. Run a blank injection.
If the protein peaks only appear when an injection is made,
they likely originate from system component or inadequate
wash solvents. Adsoprtion in the system components most
likely occurs in the loop or needle. In these instances the
component may need to be changed.
Note: Useful general information on column troubleshooting
problems may be found in HPLC Columns Theory, Technology
and Practice, U.D. Neue (Wiley-VCH, 1997), the Waters HPLC
Troubleshooting Guide (Literature code 720000181EN), and the
Waters website, www.waters.com.
XBridge Protein BEH SEC Columns and Standards
5
Page 6

[ CARE AND USE MANUAL ]
V. COLUMN CLEANING, REGENERATION
AND STORAGE
a. Cleaning and Regeneration
Changes in peak shape, such as increased tailing, shoulders on
the peak, shifts in retention, change in resolution, ghost peaks,
or increased backpressure may indicate contamination of the
column. Choose a cleaning option that may be expected to
dissolve the suspected contaminant.
It may be useful to conduct cleaning procedures at one-half
the flow rate typically used with that column. In this way the
possibility of high pressure events is reduced.
Recommended cleaning solvents:
a. A concentrated salt solution at low pH (e.g. 0.5 M Na SO, pH 2.7).
b. A low concentration of methanol (e.g., 20%) in HPLC-grade water.
c. Use of ionic detergents and other surfactants should be
avoided if the SEC column is to be subsequently used to
analyze native proteins.
b. Storage
For overnight storage, continuously flush the column with the
mobile phase at 20% of the maximum recommended flow rate.
Store the column in the HPLC-grade water when it will be used
within 24 hours or in 20% methanol for long term storage.
Note: Working at extremes of pressure, pH and/or temperature may
result in shorter column lifetime.
Note: Choose a cleaning solvent based on sample properties, e.g.
use (a) to remove basic protein and (b) to remove hydrophobic
proteins. Chaotrophic agents can solvate strongly adsorbed
proteins via hydrogen bond disruption.
As a last resort, flow reversal or back flushing can be tried
at a low flow rate (e.g., 0.1 mL/min). However, this approach
may further damage the column or only provide short-lived
improvement in performance.
Waters, The Science of W hat’s Possible, XBridge and UPLC are registered trademarks of Waters Corporation.
All other trademarks are the property of their respective owners.
©2014 Waters Corporation. Produced in the U.S.A. October 2014 720005206EN AW-PDF
Waters Corporation
34 Maple Street
Milford, MA 01757 U.S.A.
T: 1 508 478 2000
F: 1 508 872 1990
www.waters.com
 Loading...
Loading...