Page 1
[ Care and Use ManUal ]
ACQUItY UPLC® ost C
18
*ost: oLIgonUCLeotIde sePArAtIon teChnoLogY
Thank you for choosing an ACQUITY UPLC® OST C18 column designed
®
specifically for use on Waters ACQUITY UPLC
tion of detritylated oligonucleotides on Waters second generation of
™
hybrid-silica BEH Technology
particles are based on the well estab-
lished method on ion-pair, reversed-phase chromatography. ACQUITY
®
OST C18 columns are available in several configurations to
UPLC
address different application needs. Your ACQUITY UPLC
packing material is manufactured in a cGMP, ISO 9002 certified plant
using ultra pure reagents. Each batch of ACQUITY UPLC
material is chromatographically tested with acidic, basic and neutral
analytes and the results are held to narrow specification ranges to
assure excellent, reproducible performance. In addition, every column
is individually tested and the associated Performance Test Chromatogram and Certificate of Acceptance information is available through
™
the attached eCord
intelligent chip technology.
Note: Optimum performance of ACQUITY UPLC
best assured using an appropriately configured Waters ACQUITY UPLC
System (e.g., See Section II, g). Consequently, use of ACQUITY UPLC
columns on conventional HPLC systems is not recommended.
OST C
18
System. The separa-
®
OST C
®
OST C18
®
OST C18 columns is
18
Contents
I. GETTING STARTED
a. Column Connectors
b. Column Installation
c. Column Equilibration
d. eCord™ Installation
e. Initial Column Efficiency Determination
II. COLUMN USE
a. Sample Preparation
b. Recommended Mobile Phases
c. Recommended Injector Wash Solvents
d. pH Range
e. Pressure
f. Temperature
g. Mixer Options
®
h. Flow Rate
®
III. COLUMN CLEANING, REGENERATING AND STORAGE
a. Cleaning and Regeneration
b. Storage
IV. ECORD™ INTELLIGENT CHIP TECHNOLOGY
a. Introduction
b. Installation
V. ADDITIONAL INFORMATION
a. Band Spreading Minimization
Page 2

[ Care and Use ManUal ]
I. GETTING STARTED
Each ACQUITY UPLC® OST C18 column comes with Certificate of
Analysis and a Performance Test Chromatogram embedded within
™
the eCord
the batch of packing material contained in the ACQUITY UPLC
C
18
particles, analysis of bonded particles, and chromatographic results
and conditions. The Performance Test Chromatogram is specific to
each individual column and contains such information as gel batch
number, column serial number, USP plate count, USP tailing factor,
capacity factor, and chromatographic conditions. These data should
be stored for future reference.
a. Column Connectors
The ACQUITY UPLC
compression screws that have been designed to meet stringent
tolerance levels and minimize extra column volumes. Optimized
column inlet tubing (part # 430001084) is supplied with the
ACQUITY UPLC
clearly marked with a blue shrink tube marker. Insert the opposite
end of the tubing into the ACQUITY UPLC
compression fitting using two 5/16-inch wrenches. For information
on the correct column outlet tubing, please refer to the relevant
detector section in the ACQUITY UPLC
(part # 71500082502).
b. Column Installation
1. Purge the pumping system of any buffer-containing mobile
phases and connect the inlet end of the column to the
injector outlet.
2. Flush column with 100% organic mobile phase
(acetonitrile with TEAA or methanol for TEA-HFIP ion-pairing
method) by setting the pump flow rate to 0.1 mL/min and
increase the flow rate to 0.5 mL/min over 5 minutes.
3. When the mobile phase is flowing freely from the column
outlet, stop the flow and attach the column outlet to the
detector. This prevents entry of air into the detection system
and gives more rapid baseline equilibration.
intelligent chip. The Certificate of Analysis is specific to
®
OST
column and includes the gel batch number, analysis of unbonded
®
system utilizes tubing and gold plated
®
system. The inject valve end of the tubing is
®
column and tighten the
®
System Operator’s Guide
c. Column Equilibration
®
ACQUITY UPLC
OST C18 columns are shipped in 100% acetonitrile.
It is important to ensure mobile phase compatibility before changing
to a different mobile phase system. Equilibrate the column with a
minimum of 10 column volumes of the mobile phase to be used for
the oligonucleotide separation.
Note: When mobile phase additives are present in low concentrations
(e.g., TEAA or TEA-HFIP ion-pairing reagents), 100 to 200 column
®
volumes may be required for complete ACQUITY UPLC
OST C18
column equilibration.
Table 1. Empty Column Volumes in mL (multiply by 10 for flush solvent volumes)
Column Length (mm) Internal Diameter 2.1 mm
50 0.2
100 0.4
150 0.5
™
d. eCord
Installation
The eCord™ button should be attached to the side of the column heater
™
module. The eCord
button is magnetized and does not require specific
orientation.
e. Initial Column Efficiency Determination
1. Perform an efficiency test on the column before using it.
Waters recommends using a suitable solute mixture, as found in
the “Performance Test Chromatogram”, to analyze the column
upon receipt.
2. Determine the number of theoretical plates (N) and use this
value for periodic comparisons.
3. Repeat the test at predetermined intervals to track column
performance over time. Slight variations may be obtained
®
on two different UPLC
systems due to the quality of the
connections, operating environment, system electronics,
reagent quality, column condition, and operator technique.
4. Gradually increase the flow rate as described in step 2.
5. Once a steady backpressure and baseline at 260 nm have been
achieved, proceed to the next section.
2
Page 3
[ Care and Use ManUal ]
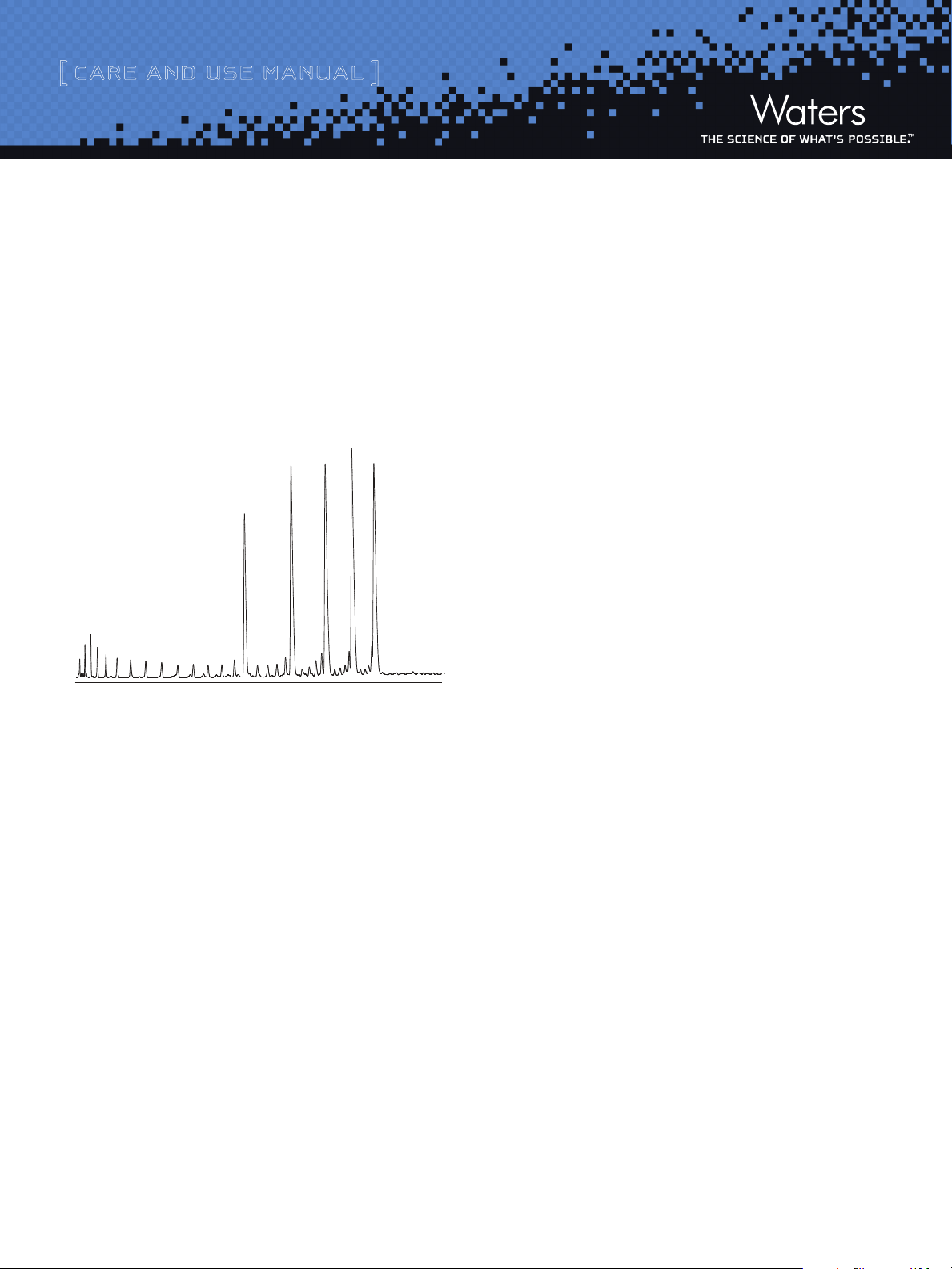
0 minutes 28
ACQUITY UPLC OST C18 column performance can be tested with
the MassPREP OST Standard (P/N 186004135), a quality controlled
synthetic oligonucleotide sample consisting of 15, 20, 25, 30 and
35 mer deoxythymidine. Approximately 0.1 nmol of each oligonucleotide was injected onto the ACQUITY UPLC OST C18 column.
Refer to P/N 715001677 for more information on sample prep for
the MassPREP OST Standard. Smaller peaks eluting prior to the main
peaks are failure, by-product sequences from the synthesis.
30
20
15
25
35
II. COLUMN USE
To ensure the continued high performance of your ACQUITY UPLC®
columns, follow these guidelines:
OST C
18
a. Sample Preparation
1. Dissolve the detritylated synthetic oligonucleotide sample in
Mobile Phase A (e.g., 0.1 M TEAA). For example, a 0.05 - 0.2 µmole
scale synthesis can be prepared in 0.1 mL of 0.1 M TEAA.
Proportionately larger or smaller volumes of 0.1M TEAA is required
when dissolving samples from different scale syntheses. Due to
the nature of gradient separations, relatively large volumes of sample
(in low organic strength eluent) can be injected and concentrated onto
the head of the column before beginning the gradient elution program.
2. Samples must be completely in solution and free of
particulates. Remove all particles from the sample
(Controlled Pore Glass Synthesis Support, etc.), which may
block the inlet column frit, increase the operating pressure,
and shorten the column life time. Sample contamination with
high concentration of salts and/or detergents may also interfere
with analysis.
Figure 1: Separation using the MassPREP OST Standard on ACQUITY
UPLC OST C18
UPLC® System: Waters ACQUITY U PLC® System with installed 425 µL mixer
(see Section II, g), PDA Detector with standard UV cell
Sample Injected: Approximately 0.1 nmol of Mass PREP OS T Standard (P/N 186004135)
diluted in 0.1 M TE AA
Column: Waters ACQUIT Y UPLC® OST C18 column, 1.7µm (2.1 x 50 mm)
Mobile P hases: A: 0.1 M TEAA,
B: Acetonitrile / 0.1M TEAA, 20/80, v/v
Flow rate: 0.2 mL/min
Column Temp.: 60 ˚C
Gradient delay: 0.45 mL
Gradient: 40 to 59.5% B in 26 minutes (8-11.9% acetonitrile,
0.15% acetonitrile per minute)
Detection: 260 nm, 5 scans per second
3. To remove particulates the sample may be filtered with a
0.2 μm membrane. Be sure that the selected membrane is
compatible and does not dissolve with the selected mobile
phase diluent. Contact the membrane manufacturer with
solvent compatibility questions. An alternative method of
particulate removal involves centrifugation for 20 minutes
at 8,000 rpm, followed by the transfer of the supernatant liquid
to an appropriate vial.
b. Recommended Mobile Phases
The most common ion-pair mobile phase for synthetic oligonucleotide separations is based on Triethylammonium Acetate (TEAA).
This mobile phase can be prepared by titrating Glacial Acetic Acid
aqueous solution with Triethylamine (TEA).
Note: To maximize column life, it is ESSENTIAL that all prepared OST
mobile phases be filtered through a solvent compatible, 0.2 µm mem-
brane and contained in bottles that are clean and particulate free.
TEAA
1 L of 0.1 M TEAA may be prepared as follows:
1) Perform work in a hood.
3
Page 4

[ Care and Use ManUal ]
2) Add 5.6 mL of glacial Acetic Acid into 950 mL of water and
mix well.
3) Slowly add 13.86 mL of TEA.
4) The pH should be adjusted to pH 7 +/- 0.5 by careful addition
of Acetic Acid.
5) Adjust final volume to 1 L with water.
Alternatively, premixed TEAA can be used [e.g., Sigma 1 M TEAA
(part # 90357)]. Mix 100 mL with 900 mL of water to prepare 1 L of
0.1 M TEAA mobile phase.
Alternative ion-pairing reagents are recommended for improved separation of phosphorothioates or when performing LC-MS analyses. An
ion-pairing mobile phase based on Triethylamine (TEA) and Hexafluoroisopropanol (HFIP) as the buffering acid produces an efficient eluent
system for improved separations involving these application types
.
As indicated below, two ion-pairing systems are useful.
For routine detritylated oligonucleotide applications, aqueous buffer
consisting of 8.6 mM TEA and 100 mM HFIP is effective. For applications such as those involving the separation of G-rich oligonucleotides, it is advisable to use aqueous buffer consisting of 15 mM TEA
and 400 mM HFIP (pH 7.9).
TEA-HFIP System 1
1L of 8.6 mM TEA / 100 mM HFIP is prepared as follows:
1) Perform work in a hood
2) Add 10.4 mL of HFIP (16.8 g) into 988.4 g of water
and mix well.
3) Slowly add 1.2 mL of TEA.
4) The pH is approximately 8.3 +/- 0.1.
TEA-HFIP System 2
1 L of 15 mM TEA / 400 mM HFIP is prepared as follows:
1) Perform work in a hood
c. Recommended Injector Wash Solvents
®
Between analyses, the ACQUITY UPLC
system injector and seals
can and should be washed with two separate solvents. A 90%
water/10% acetonitrile mixture is the recommended strong solvent
injector wash solution for the TEAA ion-pairing based method.
A 90% water/10% methanol mixture is the recommended strong
solvent injector wash solution for the TEA-HFIP based method.
0.20 μm membrane filtered, LC grade Water is the recommended
weak wash solvent solution for all ACQUITY OST separation methods.
Note: Do not use OST Separation mobile phases A and B for the
respective weak and strong injector wash solvents especially with TEA-
HFIP ion pairing systems due to seal incompatibility issues with HFIP.
d. pH Range
®
The recommended operating pH range for ACQUITY UPLC
OST C18
columns is 1 to 12.
e. Pressure
®
ACQUITY UPLC
OST C18 columns can tolerate pressures of up to
15,000 psi (1034 bar or 103 Mpa).
f. Temperature
Temperatures between 20 ˚C – 65 ˚C are recommended for operating
®
ACQUITY UPLC
OST C18 columns in order to enhance selectivity,
lower solvent viscosity and increase mass transfer rates.
Note: Operating at elevated pH, temperature, and/or pressure may
potentially result in shortened column life.
®
g. ACQUITY UPLC
The standard Waters ACQUITY UPLC
Mixer Options
®
system is equipped with 50 µL
in-line mobile phase mixer. For demanding biopolymer separations
(e.g., peptide mapping), use of a shallow gradients (e.g., 0.15%
Mobile Phase B change per min) is required. In these situations, it is
recommended that the organic solvent concentration in Mobile Phase
B be reduced by “premixing” with a measured amount of mobile
phase A (e.g., mobile phase A= 0.1 M TEAA and mobile phase B=
Acetonitrile / 0.1M TEAA, 20/80, v/v).
2) Add 41.56 mL (67.17 g) of HFIP into 956.36 g of water
and mix well.
3) Slowly add 2.08 mL (1.52 g) of TEA.
4) The pH of final buffer is approximately 7.9 +/- 0.1.
Use of a 425 µL mixer (Part # 205000403) specifically designed
®
for shallow UPLC
gradient separations is recommended when the
solvent premixing technique (detailed above) is not used and when
mobile phase B contains either 100% acetonitrile (for TEAA ion-pairing method) or 100% methanol (for TEA-HFIP ion-pairing method).
In addition, the Solvent Deliver System Outlet Tube Assembly (Part
4
Page 5

[ Care and Use ManUal ]
Number 430001486) is required for 425 µL mixer installation onto
®
a standard ACQUITY UPLC
System.
Note: The 425 µL mixer introduces an additional delay volume to
gradient separations. For ultra-fast OST analyses, the smaller
50 µL mixer should be used with the described premixed mobile
phase technique.
h. Flow Rate
The recommended flow rate for oligonucleotide separations performed
®
on a 2.1 x 50 mm ACQUITY UPLC
OST C18 column is 0.2 mL/minute.
When faster flow rates are desired for separations, use of the 425 µL
mixer with installed Outlet Tubing Assembly is recommended.
III. COLUMN CLEANING, REGENERATING AND STORAGE
a. Cleaning and Regeneration
Changes in peak shape, peak splitting, shoulders on the peak, shifts
in retention, change in resolution or increasing backpressure may
indicate contamination of the column. Flushing with approximately
20 column volumes of 0.2 μm membrane filtered, neat organic
solvent (e.g., acetonitrile with the TEAA method of methanol with the
TEA/HFIP protocol) is usually sufficient to remove the contaminant.
If the neat organic solvent flushing procedure does not solve the
problem, purge the column with 20 column volumes of OST mobile
phase A followed by 20 column volumes of either 7M guanidine
hydrochloride or 7M urea. Be sure to flush column with an additional
20 column volumes of 0.2 μm membrane filtered, LC Grade Water
prior to reuse of OST mobile phases. If the column performance is
poor after regenerating and cleaning, call your local Waters office for
additional support.
IV. ECORD™ INTELLIGENT CHIP TECHNOLOGY
a. Introduction
™
The eCord
history of a column’s performance throughout its lifetime. The eCord
is permanently attached to the column to assure that the column’s
performance history is maintained in the event that the column is
moved from one instrument to another.
At the time of manufacture, tracking and quality control information
will be downloaded to the eCord™. Storing this information on the
chip will eliminate the need for a paper Certificate of Analysis.
Once the user installs the column, the software will automatically
download key parameters into a column history file stored on the
chip. The eCord™ provides a solution to easily track the history of
column usage.
intelligent chip is a new technology that will provide the
Waters eCord™- intelligent chip
™
b. Storage
For periods longer than four days at room temperature, store the
column in 100% acetonitrile. Immediately after use at elevated
temperature and/or pH, store column in 100% acetonitrile for
the best column lifetime. Do not store column in highly aqueous
(<20% organic) mobile phase, as this may promote bacterial growth.
Completely seal column to avoid evaporation and drying out of the
packed bed.
eCord™ inserted into side of
column heater
5
Page 6

[ Care and Use ManUal ]
b. Installation
Install the column into the column heater. Plug the eCord™ into the
side of the column heater. Once the eCord™ is inserted into the column heater the identification and overall column usage information
®
will be available in Empower
and MassLynx® software allowing the
user to access column information on their desktop.
c. Manufacturing Information
The eCord™ chip provides the user with an overview of the bulk
material QC test results.
The eCord™ chip will automatically capture column use data. The top
of the screen identifies the column including chemistry type, column
dimensions and serial number. The overall column usage information
includes the total number of samples, total number of injections,
total sample sets, date of first injection, date of last injection,
maximum pressure and temperature. The information also details
the column history by sample set including date started, sample set
name, user name, system name, number of injections in the sample
set, number of samples in the sample set, maximum pressure and
temperature in the sample set and if the column met basic system
suitability requirements.
V. ADDITIONAL INFORMATION
a. Band Spreading Minimization
®
Waters ACQUITY UPLC
column band spreading. If desirable, the mass spectrometer can be
connected either directly or in series with UV (PDA) detector. The connecting tubing internal diameter should be 100 µm or less in order
to preserve the achieved separation. Length of the tubing should also
be kept to a minimum.
System is designed to have a minimal post
The eCord™ chip provides the user with QC test conditions and results on
the column run by the manufacturer. The information includes mobile
phases, running conditions and analytes used to test the columns. In
addition the QC results and acceptance is placed onto the column.
d. Customer Use Information
Detritylated synthetic oligonucleotide separations are almost
exclusively performed using gradient elution techniques. As such,
the effect of pre-column sample band broadening can be minimized
by allowing the sample to bind to the column before beginning the
actual separation gradient. However, proper connection from the
®
ACQUITY UPLC
OST C18 column outlet to the detector is critical in
order to minimize the deleterious effect of post-column sample band
spreading. Use of appropriate internal diameter tubing (e.g., 0.005
inch PEEK™ tubing for UV detector applications) is recommended.
6
Page 7
[ Care and Use ManUal ]
Table 2. Ordering Information
Waters Part Number Column Name Pore Size Particle Size Dimension
®
186003949 *AC QUIT Y UPLC
186003950 *ACQUIT Y UPLC
186003951 *Custom ACQUITY UPLC
OST C
®
OST C
®
OST C
18
18
18
186004135 MassPREP OST Standard - - -
135Å 1.7 micron 2.1 x 50 mm
135Å 1.7 micron 2.1 x 100 mm
- - -
• Note: Optimum performance of ACQUITY UPLC
®
System. Consequently, use of ACQUITY UPLC
OST C18 columns on conventional HPLC systems is not recommended.
®
OST C18 columns is best assured using an appropriately configured Waters ACQUITY UPLC®
Waters Corporation. Waters, ACQUITY UPLC, BEH Technology,
UPLC, eCord, Empower, MassLynx, and The Science of W hat’s
Possible are trademarks of Waters Corporation. PEEK is a
trademark of Victrex plc. Produced in the U.S.A.
©2013 Waters Corporation. Produced in the U.S.A.
February 2013 715001464 Rev C VW-P DF
Waters Corporation
34 Maple Street
Milford, MA 01757 U.S.A.
T: 1 508 478 2000
F: 1 508 872 1990
www.waters.com
 Loading...
Loading...