Page 1

[ Care and Use ManUal ]
SUGAR-PAK I COLUMN
Contents
I. IntRoDUCtIon
II. PRePARAtIon FoR oPeRAtIon
a. Column Installation
b. Mobile-Phase Requirements
c. Equilibration
III. CARe AnD Use
a. Prec autions
b. Storage Considerations (more than 72 hours without use)
c. Starting Up After a Shutdown
d. Overnight Shutdown (less than 72 hours without use)
e. Starting Up After an Overnight Shutdown
IV. sAMPLe PRePARAtIon
a. Pre-Injection Filtration
b. In-Line Filtration
c. Chemical Cleanup of Samples
d. Pre-Injection Cleanup of Proteins and Lipids
e. In-Line Cleanup of Proteins and Lipids
f. Cleanup of Polysacchacrides
g. Removal of Salts and Acids
h. In-Line Cleanup for Samples Not Prone to Hydrolysis
I. IntRoDUC tIon
The Waters Sugar-Pak™ Column is used for the analysis of sugar
products and process streams in beet, cane, and starch hydrolysis
processing plants; and for incoming quality inspection of commercial
sugar products. Glucose, fructose, maltose, and maltotriose can be
separated from the higher oligomers found in typical corn syrups. The
course of alcoholic fermentations can be followed by monitoring the
reduction of fermentable sugars and the production of alcohol.
The column can be used to separate many monosaccharides,
polyols, and alcohols. Disaccharides and larger sugars are also
separated, mainly according to molecular weight. Many other
useful separations can also be accomplished (e.g., fruit juices,
non-nutritive sweeteners containing sorbitol or mannitol, cell, and
cell hydrolyzates, etc.) where a variety of monosaccharides or sugar
alcohols require separation and analysis.
The 300 x 6.5 mm (ID) Sugar-Pak I Column is packed with a
microparticulate cation-exchange gel in calcium form. To obtain
results comparable to Waters performance specifications, certain
procedures regarding storage, handling, and operation should be
followed carefully, and as explained in this manual.
V. CoLUMn ReGeneRAtIon PRoCeDURe
VI. test ConDItIons
VII. tRoUBLesHootInG
VIII. oRDeRInG InFoRMAtIon
Sugar-Pak I Columns 1
Page 2
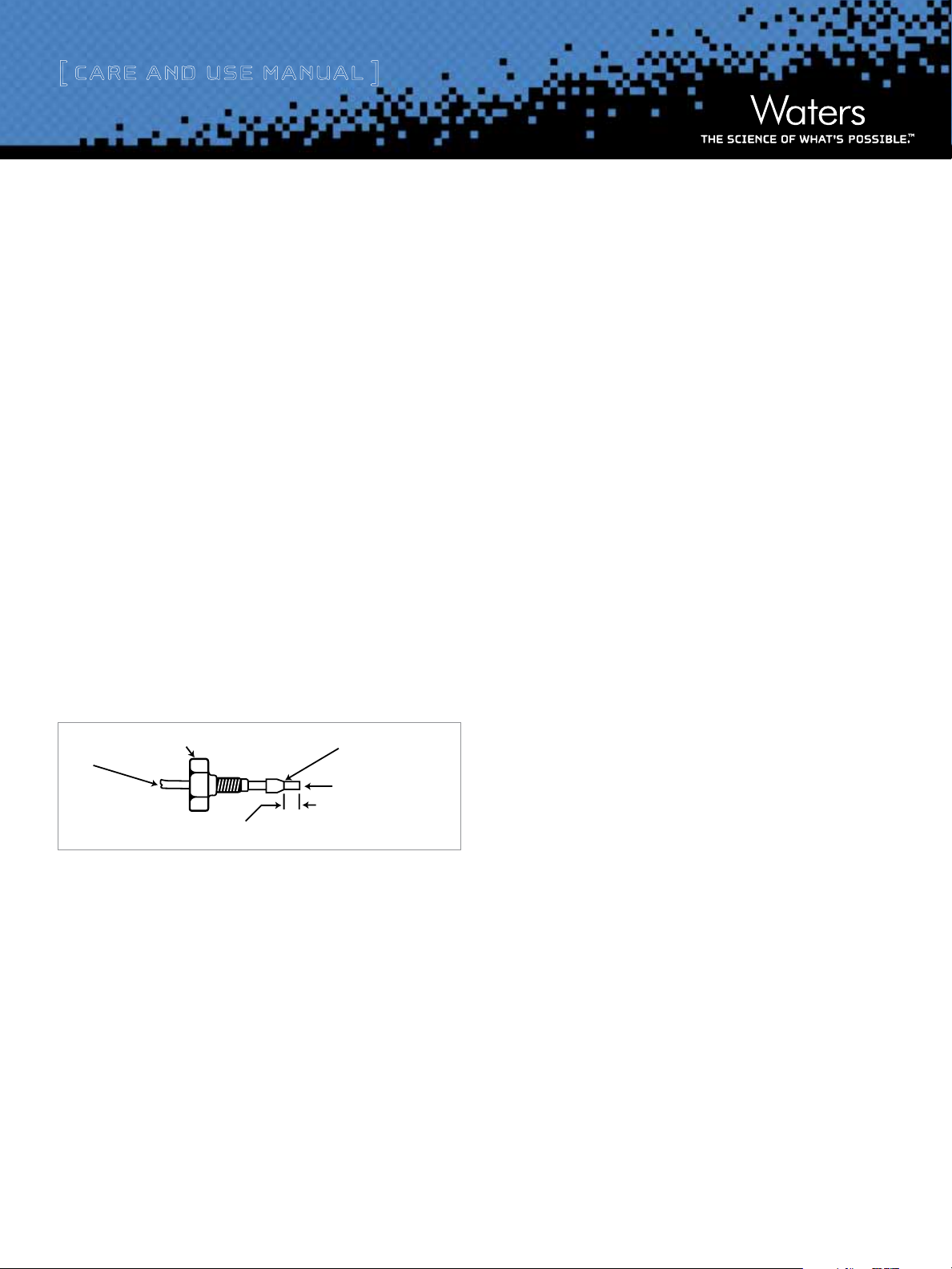
[ Care and Use ManUal ]
COMPRESSION SCREW OR NUT
II. PRePARAtIon FoR oPeRAtIon
a. Column Installation
Remove the end plugs from the steel column with a 5/16-inch open-
end wrench and save them for storage when the column is removed
from the system. The column outlet is indicated by an arrow on the
label (showing the direction solvent should flow). Tighten the fittings
to turn past finger tight. DO NOT OVERTIGHTEN – this will damage the
fitting seat. A properly prepared and assembled compression fitting in
good condition is all that is required.
If tube cutting is required to connect a new column or to improve the
end connections on your existing fittings, follow these steps:
1. Use a file with a cutting edge (such as the file included in the
Startup Tool Kit, P/N WAT096146) to scribe the circumference of
the tube at the desired break.
2. Grasp the tube on both sides of the scribe mark with cloth-
covered or smooth-faced pliers (to prevent marring the tube
surface) and gently work the tube back-and-forth until it
separates.
FERRULE
TUBE
END MUST BE STRAIGHT
AND SMOOTH TO ACHIEVE
MAXIMUM COLUMN EFFICIENCY
b. Mobile-Phase Requirements
This column is packed with a calcium-loaded resin. Hydrogen or
other cations can replace the calcium and cause inversion on the
column of sugars such as sucrose, which are prone to inversion.
It is recommended that a small amount of calcium be used in the
mobile phase to maintain the equilibrium and prevent inversion.
The recommended mobile phase is deionized, bacteria-free water
containing approximately 0.0001 M calcium EDTA* (50 mg/L).
* Calcium EDTA has several common names:
- Calcium di-sodium (ethylene dinitrilo) tetra-acetic acid
- Calcium di-sodium ethylene diamine tetraacetate
- Calcium di-sodium edentate
Water should be deionized to greater than 2 megohms resistivity.
It is essential that the water used is free of polyvalent cations,
particularly transition and heavy metals (e.g., iron).
Remove bacteria and other particulates from the water just
before use by vacuum filtration. The Solvent Clarification Kit is
recommended for solvent filtration (110 V, P/N WAT085113;
240 V, P/N WAT085122) using PES filter membranes (P/N
WAT200538).
Although the mobile phase will be partially degassed by vacuum
filtration, it is highly desirable that it be thoroughly degassed by
the following procedure:
CRTITICAL DISTANCE TO BE DETERMINED BY
EACH APPLICATION (UNION, COLUMN FITTING, ETC.)
Figure 1. Ferrule and compression assembly.
3. Inspect the tube at the break for burrs. File the outer edges at an
angle to the tube opening. Do not file flat across the open tube
as this might cause plugging or uneven flow delivery. Assemble
as shown.
4. Slide the compression fitting over the tube, followed by the
ferrule (large end of the taper first).
5. Seat the ferrule by tightly mating the assembly to the fitting seat
in which it will be used. An improperly positioned ferrule can
form unwanted dead volume which could result in unintentional
sample mixing.
Note: Attach a union in place of the column and flush the lines
free of microparticulates before attaching the column.
Sugar-Pak I Columns 2
1. Place the mobile phase in an Erlenmeyer flask on a
stirrer/hot plate.
2. Cover the mouth of the flask with aluminum foil to minimize
evaporation.
3. Bring the mobile phase to its boiling point for a few
minutes just before use, but maintain the temperature
between 70-90 °C during use. This practice ensures that
gases (especially C0
) do not redissolve in the mobile
2
phase. It also prevents the growth of microorganisms.
4. Keep the mobile-phase reservoir clean and covered and supply
freshly prepared mobile phase every 24 hours.
If the system is to be used continually for more than one
day, place the mobile phase in an Erlenmeyer flask on a
stirrer/hot plate. Cover the mouth of the flask with aluminum
Page 3

[ Care and Use ManUal ]
foil to minimize evaporation. Bring the mobile phase to
its boiling point for a few minutes just before use, but
maintain the temperature between 70-90 °C during use.
This practice ensures that gases (especially C0
redissolve in the mobile phase. It also prevents the growth of
microorganisms.
Keep the mobile phase reservoir clean and covered and supply
freshly prepared mobile phase every 24 hours.
) do not
2
c. Equilibration
To ensure that a new column is fully equilibrated with calcium prior to
analysis, the following procedure is recommended.
1. Install the column with the normal direction of flow reversed
(arrow on label pointing to column inlet tubing). A backflush
valve may also be used.
2. Back flush the column with at least 100 mL of 0.001 M calcium
EDTA solution at 90 °C using a flow rate of 0.5 mL/min. A
concentration of 500 mg/L of calcium EDTA approximates
0.001 M.
3. Reverse the flow direction again (back to the normal direction)
and flush the column with the stable baseline indicating that the
column is ready for use.
III. CARe AnD Use
Liquid chromatography columns have a finite life influenced by their
care and use, number of injections, sample and solvent cleanliness,
frequency of solvent changeover, and handling and storage procedures
(among other factors). If a change is observed in the:
retention of a particular compound,
resolution between two compounds, or
peak shape
take immediate steps to determine the reason for the changes. Until you
have made this determination, you must not rely upon the results of any
separation using the column. Follow generally accepted procedures for
quality control and methods development when using these columns.
Note: Before running the first analysis on your new column, perform
the test sample separation given in the test conditions section.
Before using your new Sugar-Pak I column for the first time, it is
recommended that the following procedure be observed:
1. Connect the column (refer to Section II a)
2. Set the flow rate at 0.2 mL/min until the column temperature
reaches 70 °C.
3. Once the column temperature reaches 70 °C, increase the
flow rate to 0.6 mL/min in steps of 0.1 mL/min. Wait for the
backpressure to stabilize between each 0.1 mL/min increase.
a. Precautions
Normal recommended pressure should not exceed: 2000 psi.
Maximum flow rate: 0.6 mL/min (at or above 70 °C).
DO NOT use calcium chloride, calcium nitrate, or any other acidic
calcium salt in the mobile phase, as this can corrode the column
and damage the packing.
Maximum mobile-phase organic content is 5% V/V. Small amounts
of acetonitrile, ethanol, methanol, and isopropanol in the sample
will not effect column performance.
Column temperatures should not exceed 95 °C. Generally, high
temperatures provide greater resolution, but little improvement
occurs between 90 °C and 95 °C. Temperatures below 70 °C do not
provide adequate resolution of many sugars due to the separation
of anomers. For quantification of ethanol, a column temperature of
75 °C should be used.
Column temperature changes can be rapid without adverse
effects. If the column temperature is below 60 °C, a maximum
flow rate of 0.2 mL/min should be used. Higher flow rates may
produce excessive backpressure due to the higher viscosity of
water at lower temperatures.
Reverse flow direction through the column (after every 5 liters
of mobile phase). This procedure will prolong column life
significantly. Flow rate changes should be made slowly and not
exceed 0.5 mL/min
change the flow rate in steps of 0.1 mL/min and wait until the
backpressure stabilizes before the next step change.
2
(0.5 mL/min per minute). It is advisable to
Sugar-Pak I Columns 3
Page 4

[ Care and Use ManUal ]
The pressure limit setting on the solvent delivery system should
be set to a few hundred psi above the normal working pressure
to avoid any excessive pressure buildup while the instrument is
unattended.
If sugars not prone to inversion are analyzed (e.g., hydrolyzates),
it is not essential to use calcium EDTA in the mobile phase. Pure
water can be used but it should be filtered and degassed as
recommended. Column regeneration and flow direction changes
should be carried out regularly for optimum column performance.
b. Storage Considerations (more than 72 hours without use)
Turn off the column heater and stop the pump.
When the column is cool, remove it from the system and replace the
endcaps originally supplied with the column. T he column should not
be allowed to dry out during storage. T he normal mobile phase is
suitable as a storage solvent in the column. Refrigerate the column
at 5 °C (not frozen) to minimize any microbial growth.
The end fittings in the system should be joined by a union after the
column is removed. Flush the entire liquid chromatography system
first with water, then methanol, and store it in methanol.
c. Starting Up After a Shutdown
e. Starting Up After an Overnight Shutdown
Turn on the column heater and start the mobile phase flowing at no
more than 0.1 to 0.2 mL/min. Continue at this flow rate for at least
20 minutes after the column has reached its working temperature
before setting normal operating conditions for analysis.
IV. Sample Preparation
a. Pre-Injection Filtration
Filter all samples through a 0.45 µm membrane filter just before
injection to remove particulates. Suitable replacement filters are
provided in the Waters Aqueous Sample Clarification Kit (P/N
WAT026865). Suitable disposable filters with a larger surface area
are also available (HV Filter Unit, P/N WAT085996).
b. In-Line Filtration
If a Guard-Pak™ Pre-Column module is being used, disposable
pre-column area filter inserts (P/N WAT032472) for use instead of
Guard-Pak Pre-Column inserts. If guard columns are not being used,
it is advisable to use an in-line pre-column filter (P/N WAT084560)
immediately before the column to protect it from any particulate
matter in the samples or mobile phase.
Note: Make sure all methanol is flushed from the system with water
before the column is reinstalled.
When starting up a system containing a cold column, turn on the
column heater and start the mobile phase flowing at no more than
0.2 mL /min. Allow the instrument to stabilize under these conditions
for at least 20 minutes after the column reaches working temperature
before running the system under usual working conditions.
d. Overnight Shutdown (less than 72 hours without use)
The column may be left in the system with the flow rate reduced to
0.2 mL/min. Recirculate the solvent by placing the effluent line in the
supply flask. If it is undesirable to leave the column heater on, shut off
the pump and the column heater and allow them to cool down.
Sugar-Pak I Columns 4
c. Chemical Cleanup of Samples
Substances such as proteins, lipids, polysaccharides, salts and acids,
which could contaminate your column, should be removed from samples
using the suggested techniques outlined in sections d through h.
d. Pre-Injection Cleanup of Proteins and Lipids
Proteins, or lipids such as fats and oils, can contaminate the column
and cannot be removed by washing with organic solvents. If samples
are contaminated with large amounts of lipid, the excess can be
removed by liquid-liquid extraction into a solvent such as chloroform.
Residual lipophilic compounds can be removed using a Sep-Pak
Cartridge (P/N WAT051910) as explained in the following procedure:
1. Fill a 10 mL syringe with 5 mL of methanol and slowly flush
the methanol through the cartridge to condition the
Sep-Pak C
packing.
18
®
C
18
Page 5

[ Care and Use ManUal ]
2. Wash the methanol from the cartridge with two 5 mL portions of
pure water.
3. Blow excess water from the cartridge with the syringe.
4. Slowly pass the sample through the Sep-Pak C
Cartridge.
18
a. Reject the first 2 mL.
b. Collect the next few milliliters for subsequent analysis.
e. In-Line Cleanup of Proteins and Lipids
Protect your column from small amounts of lipophilic material by
using the Waters Guard-Pak Holder (P/N WAT080040) and Guard-
Inserts (P/N WAT085824). As the Guard-Pak Insert becomes
Pak C
18
contaminated with lipophilic sample components, it can be simply
replaced and the old one discarded.
f. Cleanup of Polysacchacrides
Note: Since C18-guard columns will adsorb some neutral polysaccharides,
they should not be used for the analysis of such samples.
Neutral polysaccharides will pass through the Sugar-Pak I Column.
For some samples, such as starch hydrolyzates, the analysis of such
polysaccharides is desirable and the use of guard columns containing
resins (as described under “Removal of Salts and Acids”) is
recommended. These resins remove most residual proteins and lipids,
but allow neutral polysaccharides to pass through.
g. Removal of Salts and Acids
a. Reject the first 5 mL.
b. Collect the next few milliliters of sample for injection.
Make sure this procedure is suitable for quantitative recovery for your
particular samples.
h. In-Line Cleanup for Samples Not Prone to Hydrolysis
Use a Waters Guard Column (P/N WAT084550) between the column
and the injector to remove salts and acids from the sample. Pack the
+
guard column with a mixture of strong cation resins (in the H
-
and strong anion resins (in the OH
form) with a maximum particle
size of 200/400 mesh. These resins are available from a number of
sources (e.g., CG120 and CG400 resins made by Rohm and Haas), but
have to be converted into the appropriate ionic forms just before use.
In particular, the anion-exchange resins are unstable in the OH
during prolonged storage. Repack the guard column with fresh resin
when necessary.
Note: Do not use a guard column containing the mixed bed resin for
sucrose analysis, as this will cause severe inversion of the sucrose (or
other sugars prone to inversion). Only use the mixed bed resin in the
guard column when 100% pure water is used as the mobile phase.
Any calcium EDTA in the mobile phase will saturate the column. Any
other type of guard column that exceeds the bed volume of the Waters
guard column (about 0.2 mL) is not recommended for use with Sugar-
Pak I Columns. The increased bed volume will cause excessive band
spreading and loss of resolution.
form)
-
form
Salts and organic acids present in crude samples can co-elute with
sugars and interfere with the analysis. Typically, salts of strong acids
such as sodium chloride, elute near the void volume. Salts of weak
acids, such as lactate and acetate, elute later.
For some samples of low MW sugars, Waters Sep-Pak Alumina
Type A Cartridges (P/N WAT051800) are suitable for removing
salts and acids.
The recommended procedure is as follows:
1. Pass 5 mL of pure water through the Sep-Pak Alumina Type A
Cartridge.
2. Place 10 mL (maximum) of sample in the syringe and slowly
pass the sample through the cartridge.
Sugar-Pak I Columns 5
V. CoLUMn ReGeneRAtIon PRoCeDURe
The need to regenerate your Sugar-Pak I Column will vary according
to the relative purity of your samples. The cruder the samples
(particularly those containing heavy or transition metals or organic
acids), the more frequent regeneration will be required due to
inversion of the column.
You can test for inversion by injecting sucrose. If a tail is produced on
the sucrose peak, the column should be retreated as follows:
Make up 500 mL of regeneration solution (500 mg calcium EDTA/L)
and allow it to run through the column overnight at 0.5 mL/min at
90 °C. Make sure the direction of flow is reversed before starting
regeneration and returned to normal following regeneration.
Page 6

[ Care and Use ManUal ]
5.80 DP3 Maltoriose
When flow is reversed it is important to maintain solvent temperature
in order to ensure proper cleaning. Using cold solvent will slow the
cleaning process.
VI. test ConDItIons
Every column is thoroughly tested and passes strict manufacturing
specifications. It is recommended that you perform a test sample
injection, like the one below, prior to your first analysis. Record the
results along with all relevant instrument settings. These results
can then be used for comparison throughout the lifetime of the
column to monitor equipment performance, sample variation, and
column condition.
6.82 DP2 Maltose
4.22
9.80 Glucose
the mobile phase at 0.1 mL/min. Allow 20 minutes after the column
has reached its working temperature before bringing the column up to
its working flow rate.
Refer to column regeneration and other cleanup methods explained in
this manual for actual procedures. Remember to switch your column
to its original flow direction and allow proper warm-up time before
continuing with further analyses.
Caution: The resin in Sugar-Pak I Columns is tightly packed and elastic.
If the column end frits are removed, the packing may expand out of the
column. Thus, opening the end fittings of the column to inspect or clean
the end frits (in an ultrasonic bath) should be done only as a last resort.
Table 1. Typical Column Problems and Solutions
Problem Cause Solution
Excess
pressure buildup
In-line filters
plugged with
particulates
Replace the in-line filter, or shut off the
pump. When inlet pressure has dropped to
zero, disconnect the in-line filter from the
column and backflush the prefilter ONLY
with mobile phase at 9 ml/min until the
particulates are backflushed out.
Test Sample:
Flow Rate:
Temp.:
Mobile Phase:
Figure 2: A typical chromatogram of sugar standards analyzed with a Sugar-Pak I Column.
Note the high resolution of glucose, maltose and maltotriose. Glucose should elute in less
than 10 minutes. Maximum backpressure should be less than 2000 psi.
42 DE Corn Syrup
0.5 mL/min
90 ˚C
Deionized water
VII. TROUBLESHOOTING
The Sugar-Pak I Column is tightly packed with a resin in a swollen
form. If good sample preparation is practiced, most problems that
arise will relate to the resin packing. The resin packing is delicate and
can be forced into the end frits of the column by pump pulsations due
to faulty check valves or by sudden or excessive pressure buildup.
Should pressure buildup occur, check for trapped particulates in the
in-line filter or guard column.
If this is not the problem, it is possible that the resin has partially
blocked the outlet frit. Should this occur, shut down the system.
Reverse the direction of flow through the column and begin pumping
Fluctuating
backpressure
Peak “tailing”
on sucrose
Band
broadening
“Spurious”
peaks, not
due to sugars
Leaking mobile
phase
Variable
elution times
Column inlet
frit plugged with
particulates
Exit frit plugged
with resin
Gas in solvent Check degassing procedure.
Faulty pump
operation
+
ions or heavy
H
metals (e.g, Fe)
on column
Void in column Reverse column flow direction. This may
Elution of salts
and/or acids
Fittings in bad
state of repair
Variations in
flow rate or
temperature
Always use an in-line filter or guard
column to prevent this. Reverse column
to try to wash out particulates at normal
flow rate.
Reverse flow of mobile phase. Check pump
and system operation before proceeding.
Inspect pump check valves and carry
out “Ramp Test” (consult the operator's
manual for your pump).
Follow column regeneration procedure
using 500 mg calcium EDTA per liter as
described.
allow the packing bed to reform, thus
improving peak shape.
Check sample cleanup procedures.
Tighten fittings properly. DO NOT
OVERTIGHTEN. Replace worn fittings.
Check system for faults, leaks,
especially pulsations from pump.
Sugar-Pak I Columns 6
Page 7

[ Care and Use ManUal ]
VIII. oRDeRInG InFoRMAtIon
Description Part Number
Compression Screws and Ferrules, 5/pk WAT025604
Guard-Pak Holder , 1/pk WAT088141
Guard-Pak Inserts, 10/pk WAT085824
Inline Pre-Column Filter Kit WAT084560
LC/GC Certified Vials, 12 x 32 mm, 100/pk 186000272C
Solvent Clarification Kit, 110V WAT085113
Solvent Clarification Kit, 220V WAT085112
47mm PES Filters, 0.45 μm, 100/pk WAT200538
Austria and European Export (Central South Eastern Europe, CIS and Middle East) 43 1 877 18 07, Australia 61 2 9933 1777, Belgium 32 2 726 1000, Brazil 55 11 4134 3788,
Canada 1 800 252 4752, China 86 21 6156 2666, Czech Republic 420 2 617 11384, Denmark 45 46 59 8080, Finland 358 9 5659 6288, France 33 1 30 48 72 00,
Germany 49 6196 400 600, Hong Kong 852 2964 1800, Hungary 36 1 350 5086, India and India Subcontinent 91 80 2837 1900, Ireland 353 1 448 1500, Italy 39 02 265 0983,
Japan 81 3 3471 7191, Korea 82 2 6300 4800, Mexico 52 55 52 00 1860, The Netherlands 31 76 508 7200, Norway 47 6 384 6050, Poland 48 22 833 4400, Puerto Rico 1 787 747 8445,
Russia/CIS 7 495 727 4490/ 290 9737, Singapore 65 6593 7100, Spain 34 93 600 9300, Sweden 46 8 555 115 00, Switzerland 41 56 676 7000, Taiwan 886 2 2501 9928,
United Kingdom 44 208 238 6100, All other countries: Waters Corporation U.S.A. 1 508 478 2000/1 800 252 4752
© 2012 Waters Corporation. Waters is a registered trademark
of Waters Corporation. Sugar-Pak, Guard-Pak, Sep-Pak, and
The Science of What’s Possible are trademarks of Waters
Corporation. All other trademarks are the property of their
respective owners.
Waters Corporation
34 Maple Street
Milford, MA 01757 U.S.A.
T: 1 508 478 2000
F: 1 508 872 1990
May 2012 WAT085454 Rev C V W-PDF
Sugar-Pak I Columns 7
www.waters.com
 Loading...
Loading...