Page 1
[ CARE AND USE MANUAL ]
Quantitative Peptide Retention Standard
TABLE OF CONTENTS:
I. INTRODUCTION
II. STORAGE AND STABILITY
III. RECONSTITUTION OF THE PEPTIDE
RETENTION STANDARD
IV. EXAMPLE OF USING THE PEPTIDE
RETENTION STANDARD ON THE
ACQUIT Y U PLC
V. ORDERING INFORMATION
I. INT RO DUC TION
This standard was specifically designed and formulated as a simple peptide
mixture that provides a broad retention window for a variety for a variety of
peptide separation applications. It is an ideal reference material since the
peptides were designed to be stable and to not be prone to modification such
as oxidation.
This standard complements it’s qualitative counterpart, the MassPREP™
Peptide Standard which offers a versatility of various peptides but it not
quantitative. Each peptide in the Quantitative Peptide Retention Standard
is quantitated via amino acid analysis to provide greater accuracy to each
analysis, has been verified by mass identification and is highly pure (more
than 95% pure). Each standard is provided in a Waters TruView™ Maximum
Recovery Vial which simplifies the sample preparation process and allows
for direct injection once solubilized.
The standard consists of four neutral peptides with a single A280
chromophore and varying hydrophobicities and is formulated such that
the peptide components are present in equal abundance. See Table 1 for
specific peptide information.
Table 1: Peptide Mixture Components and Example Data Generated
from Figure 1
Peptide Retention Standard
Peak Area
Peptide MWmono RT (min)* (A
DGYGK
DTVGYGVK 837.4232 10.5 2572 25
DFVGYGVK 883.4440 13 2801 27
DFVGYGVKDFVGVGVK
280 nm chromophore (Y) highlighted
* See Figure 1 for retention time examples.
Applications of the standard can include, but are not limited to: evaluation
of columns and LC systems dedicated to peptide analysis as well as
monitoring performance and recovery across romatographic gradients.
538.2387
1684.8 825
2.6 2446 24
16.4 2437 24
) % Area
280
Page 2

[ CARE AND USE MANUAL ]
II. STORAGE AND STABILITY
Lyophilized peptides generally have excellent stabilities, often showing little or no degradation after a few years at -20 °C. Long-term
storage (> 1 year) should be at -80 °C desiccated, medium-term storage (1–12 months) should be at -20 °C desiccated, short-term
storage (< 1 month) may be at 4 °C. Once reconstituted the solution should be used immediately to avoid degradation of peptides which
would compromise the benefit of the standard having been quantitatively formulated.
III. RECONSTITUTION OF THE QUANTITATIVE PEPTIDE RETENTION STANDARD
This standard contains 20 nmoles of each peptide lyophilized. Reconstitute with 133.3 µL of either 0.1% aqueous formic acid or 0.1%
aqueous trifluoroacetic acid to produce a sample concentration of 150 pmol/µL (per peptide). Suggested injection volumes and sample
loads are outlined below.
Table 2: Recommended Mass Load for LC Column Evaluation
Reconstitution Volume Column ID Injection Volume Quantity of Peptide Loaded (each species)
133.3 µ L 4.6 mm 19.2 µL 2.9 nmol
133.3 µ L 2.1 mm 4.0 µL 600 pmol
133.3 µ L 1.0 mm 0.9 µL 140 pmol
IV. EXAMPLE OF USING THE QUANTITATIVE PEPTIDE RETENTION STANDARD
Below is a reference chromatogram with conditions to help provide an example of using the Quantitative Peptide Retention Standard.
Please note retention times are dependent on LC conditions, column and LC system. If the column size is different, the gradient/
injection volume can be scaled accordingly
System: ACQUITY UPLC® H-Class Bio
Column: ACQUITY UPLC CSH130 C18, 130Å, 1.7 µm, 2.1 x 150 mm
Mobile Phase A: Water with 0.1% FA (v/v)
Mobile Phase B: ACN with 0.1% FA (v/v)
Gradient: 0.5% B for 1 min, then to 50% B over 30 min
Flow Rate: 0.3 mL/min
Column Temp. 40 °C
Detector: UV @ 280 nm , 10 Hz
Software: Monoisotopic masses calculated with MassLynx® 4.1
2
Quantitative Peptide Retention Standard
Page 3
[ CARE AND USE MANUAL ]
0.12
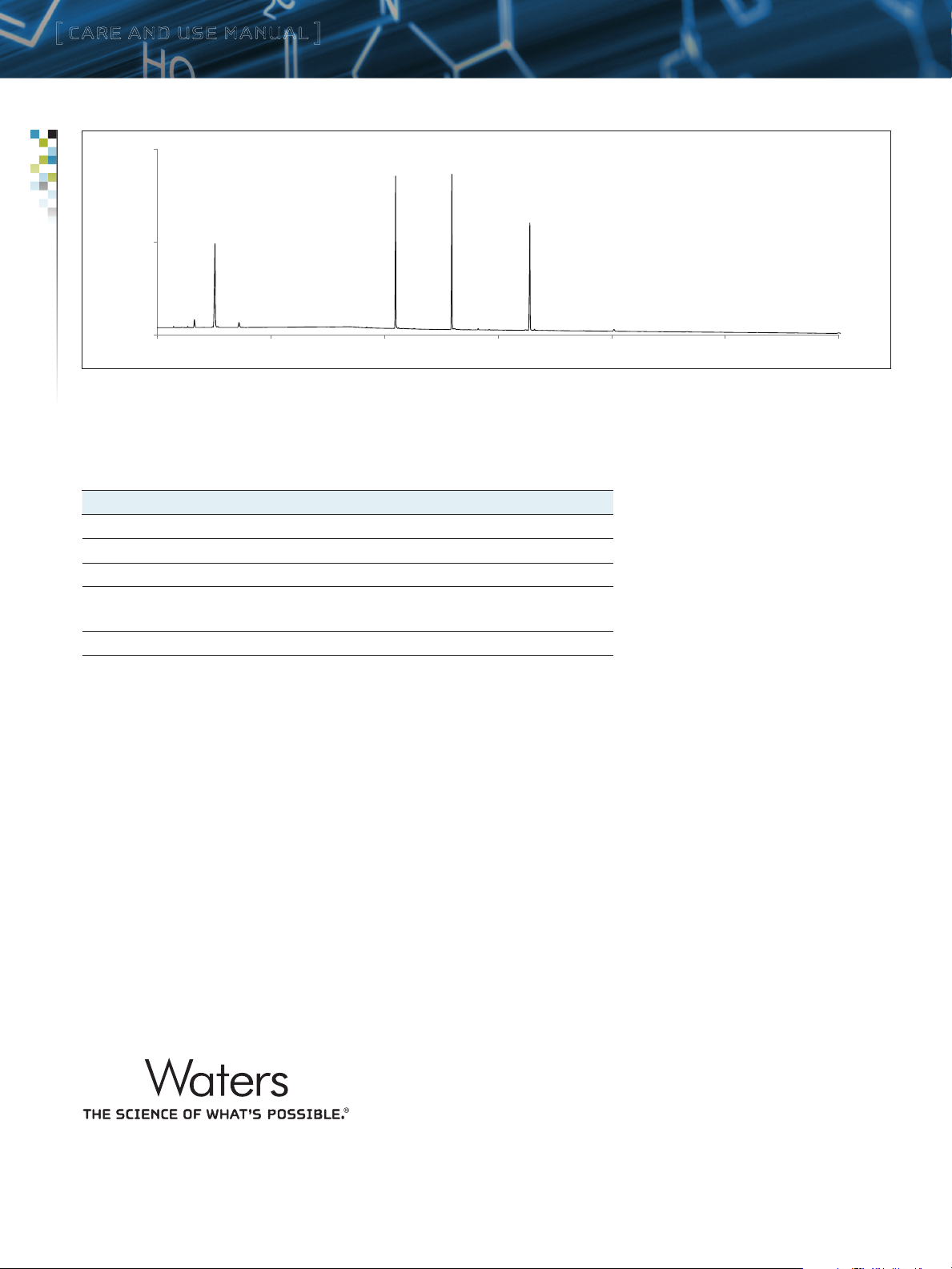
DTVGYGVK
DFVGYGVK
DFVGYGVKDFVGVGVK
DGYGK
280
0.06
A
0
0510 15 20 25 30 min
Figure 1: UV chromatogram and measurements representative of the Quantitative Peptide Retention Standard as obtained with an ACQUITY U PLC CSH130 C18 column.
V. ORDERING INFORMATION
Description Qty Part Number
Quantitative Peptide Retention Standard 1 186006555
MassPREP Peptide Mix 1 186002337
MassPREP Peptide Mix 5 186002338
LCGC Certified Clear Glass, 12 x 32 mm, Screw Neck,
Max Recovery Vial
ACQUITY UPLC CSH130 C
, 130Å, 1.7 µm, 2.1 x 150 mm 1 186006938
18
100 186000327C
Waters, The Science of W hat’s Possible, ACQUITY UPLC, and MassLynx are registered trademarks
of Waters Corporation. MassPREP and TruView are trademarks of Waters Corporation.
©2014 Waters Corporation. Produced in the U.S.A. June 2014
720004786EN VW-PDF
Waters Corporation
34 Maple Street
Milford, MA 01757 U.S.A.
T: 1 508 478 2000
F: 1 508 872 1990
www.waters.com
 Loading...
Loading...