Page 1

[ CARE AND USE MANUAL ]
PROTEIN-PAK SEC COLUMNS
I. INTRODUCTION
This Care and Use Manual contains information pertaining to the use of
the following Protein-Pak™ SEC Columns:
Protein-Pak 60 7.8 mm (i.d.) x 30 cm, Steel (P/N
WAT085250)
Recommended for use with biopolymers such as peptides, polypeptides,
steroids and small proteins. T he pore size distribution provides
excellent separation in the following molecular weight ranges: Native
Globular 1000-20,000 and Random Coil 600 - 8000. Shipped in
100 % m ethanol .
Protein-Pak 125 7.8 mm (i.d.) x 30 cm, Steel (P/N
WAT 084601)
Especially suited for separation of biopolymers such as proteins and
enzymes in the following molecular weight ranges: Native Globular
2000 - 80,000 and Random Coil 1000 - 30,000. Shipped in
100 % m ethanol .
Protein-Pak 300 SW 7.8 mm (i.d.) x 30 cm, Steel (P/N
WAT080013)
Protein-Pak 200 SW 8.0 mm (i.d.) x 30 cm, Glass (P/N
WAT 01178 6)
Protein-Pak 300 SW 8.0 mm (i.d.) x 30 cm, Glass (P/N
WAT011787)
CONTENTS
I. INTRODUCTION
II. PREPARATION FOR OPERATION
a. Steel Column Installation
b. Glass Column Installation
c. Solvent Requirements
d. Buffers
e. Protein Denaturants
f. Equilibration
g. Column Purge Volumes
III. CARE AND USE
a. Sample Preparation and Filtration
b. Precautions
c. Storage Consideratons
IV. TROUBLESHOOTING
a. Service and Applications Information
b. Column Efficency
c. Test Conditions
The columns of choice for use with larger biological active molecules
in the molecular weight range of Native Globular 10,000 - 400,000
and Random Coil 2000 - 150,000. Shipped in 0.05% sodium azide in
water solution.
Protein-Pak SEC Columns 1
Page 2
[ CARE AND USE MANUAL ]
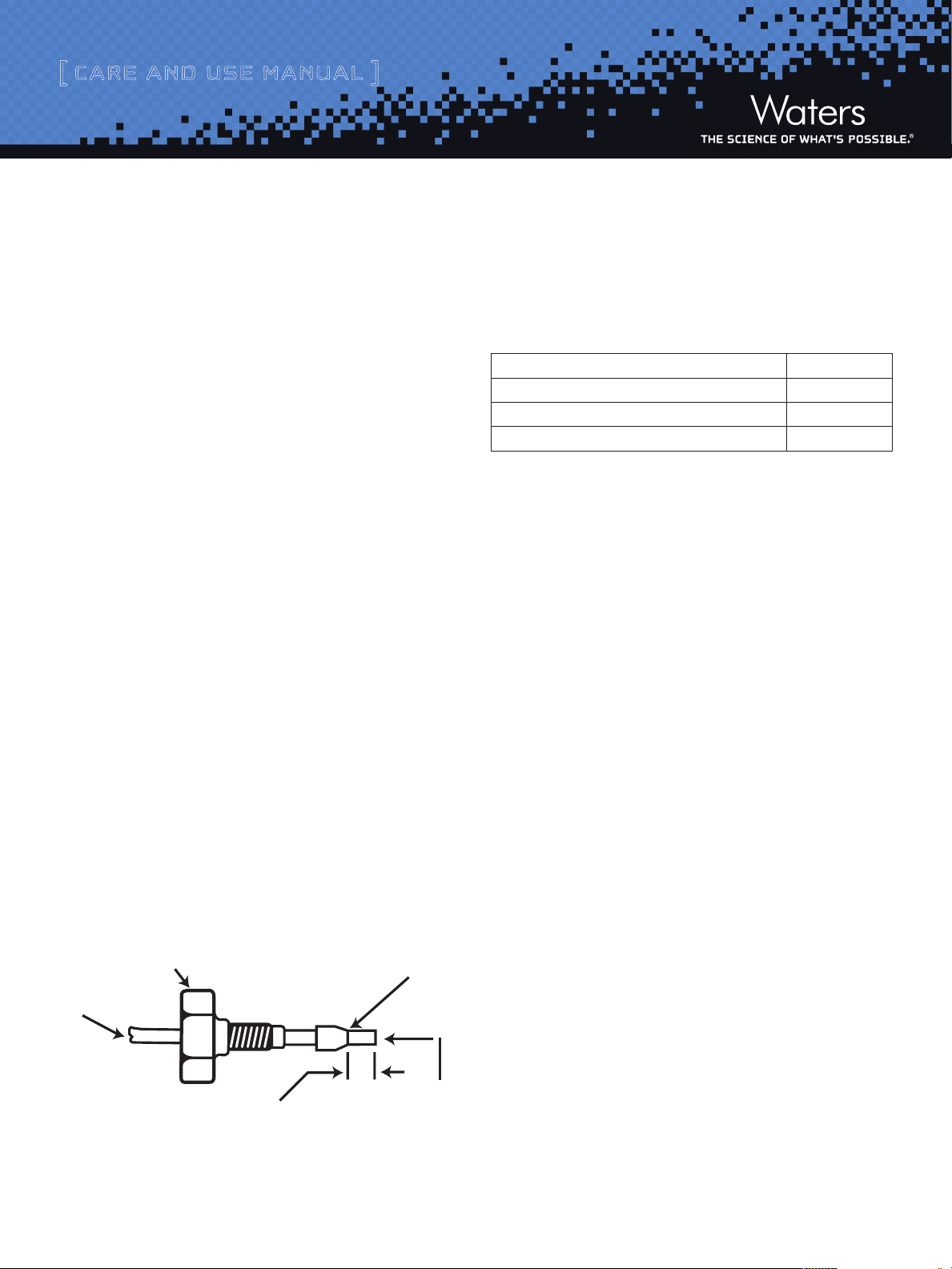
maximum column eciency
Critical Distance to be determined by each
II. PREPARATION FOR OPERATION
a. Steel Column Installation
Remove the end plugs from your colum with a 5/18” wrench and
save them for storage when the column is removed from the system.
The column outlet is indicated by an arrow on the label (showing the
direction solvent should flow). Tighten the fittings 1/4-to-1/2 turn.
DO NOT OVERTIGHTEN – THIS WILL DAMAGE THE FITTING SEAT.
A properly prepared and assembled compression fitting in good
conditions is all that is required.
Follow the next four steps of this procedure if tubing cutting is required
to connect a new steel column or to improve the end connections on your
existing fittings.
1. Using a file with a cutting edg, escribe the circumference of the tubing at
the desired break.
2. Grasp the tubing on both sides of the scribe mark with cloth covered pliers
(to prevent marring the tube surface), and gently work the tube back and
forth until it separates.
3. File the ends smooth and assemble as shown.
Note: Proper positioning of the fetrrule in the fitting seat will prevent
formation of unwanted dead volume which could result in sample mixing.
4. Slide the compression fitting, followed by the ferrule (large end of the
taper first) over the tube. Be certain to bottom the tube in the fitting seat
for which its use is intended to assure a leak-free connection.
Note: Attach a union in place of the column and flush the lines free of
microparticulates before attaching the column.
Figure 1: Ferrule and Compression Screw Assembly
Compression screw or nut
Tube
application (union, column tting, etc.)
End must be straight
and smooth to achieve
Ferrule
b. Glass Column Installation
Waters Protein-Pak 200 or 300 SW glass columns can be connected to
any HPLC or medium-pressure system remembering not to exceed 300 psi
backpressure with the installed glass SEC columns. T he following parts can
be used to adapt these columns to any of our standard Waters systems:
WAZ TO 1/4 UNF Adapter WAT089472
Compression Screw 1/8 o.d. 1/4 - 28 WAT08 8467
Reverse Ferrule 1/8 o.d. WAT088466
Tube Cut 0.125 x 0.009 x 120 WAT 088431
c. Solvent Requirements
Protein-Pak 60 and 125 columns are shipped containing an organic
solvent compatible with water-miscible solvents (such as methanol,
acetonitrile, etc.) The Protein-Pak 200 and 300 SW columns are shipped
in water containing 0.05% sodium azide, added as a bacteriostat. If
an immiscible solvent is to be used for analysis, make the changeover
gradually using at least one itermediate solvent (similar to changing over
solvents during analysis). When changing the organic concentration in
the mobile phase to reduce the flow rate. When adding organic solvents
to aqueous buffer solutions avoid salt precipitation.
d. Buffers
Protein-Pak columns are stable in normal salt buffer solutions such as
sodium sulfate, ammonium acetate, ammonium formate, phosphate
buffers, tris acetate and acetate buffers. Ionic strength equivalent to 0.1 M
minimizes the ionic interaction between the protein sample and the silica
stationary phase. Generally the salt concentrations in aqueous solutions
should be maintained below 0.5 M.
e. Protein Denaturants
Aqueous solutions of SDS, guanidine hydrochloride and urea are
compatible with the Protein-Pak columns although there is a tendency
to display shorter column life when compared to non-detergent
aqeuous solutions. We recommend dedicating columns used with these
solutions rather than changing back to other aqueous solutions with the
same column.
Protein-Pak SEC Columns 2
Page 3

[ CARE AND USE MANUAL ]
f. Equilibration
A necessary step to sucessful use of your new column is the inital
solvation (or wetting) of the packing. Purge the column with 5 - 10
column volumes of the stronger eluting component of the mobile phase
alone before a a final purge with the actual mobile phase. Equlibration
between the mobile phase and packing is established when a stable
baseline can be produced. If your result is unsatisfactory repeat the
equilibration process.
g. Column Purge Volumes
Column Size (mm x cm) Recommended Volumes (mL)
7.5 x 30 100
7.8 x 30 200
II. CARE AND USE
Liquid chromatography columns have a finite life influenced by their
care and use, number of injections, sample and solvent cleanliness,
frequency of solvent changeover and handling and storage procedures
among other factors. If a change is observed in the :
• Retention of a particular compound
• Resolution between two compounds
• Peak shape
Take immediate steps to determine the reason for the changes, and until
the determination is made, the results of any separations using the
column must not be relied upon. Follow generally accepted procedures
for quality control and methods development when using these columns.
Important Note: Before running the first analysis on your new column perform the test sample separation given in the test conditions section.
a. Sample Preparation and Filtration
Use HPLC grade solvents, filtered to remove microparticulate matter
above the 0.45 μm. This reduces the problem of plugged filters and
preserves column life. Vacuum filtration or sonification may be used to
remove dissolved gasses which could affect your solvent delivery system.
Always filter prepared samples to prevent excessive pressure buildup due
to particlate matter. Adequate sample cleanup (using Sep-Pak
designed for this purpose) prevents alteration of the column chemistry by
strongly adsorbing or precipitatin sample components.
®
cartridges
b. Precautions
Normal recommended pressure should not exceed 3500 psi for the
Protein-Pak SEC steel columns and no greater than 300 psi for glass SEC
columns. Maximum flow rate should not exceed: 1 mL/min
– For all silica-based packing materials, stay within a pH range of 2-8 (i.e.
avoid using concentrated acids or bases). For maximum column life stay
within the pH range: 3.5 - 6.5.
– Use Sentry™ Universal Guard Column Holder (WAT046910) and Protein-
Pak 125 Sentry Guard 2/pkg. (186000926) to protect your column from
contaiminants and extend column life.
– Try to dedicate more columns to specific applications. Constant switching
of samples and solvents will cause a more rapid column contamination
and loss of resolution.
– Filter all aqueous buffers. Avoid using turbid or cloudy buffers. Be sure that
any solutions containing buffers, salts, etc. are compatible with the wetted
surfaces of the column and equipment.
– Protect column from vibration, mechanical shock, and rapid changes in
pressure. Column packings are based on a highly porous and delicate silica
gel alignment. Any thermal, physical or chemical shock (such as changing
solvents rapidly or at high flow rates) can cause the particles to shift and
may result in a loss of efficiency.
Protein-Pak SEC Columns 3
®
– When using water, distill or treat with a Milli-Q
De-ionized water is not acceptable because it contains organi compounds
which alter column selectivity.
or equivalent system.
Page 4

[ CARE AND USE MANUAL ]
INJECT
lue of
– Avoid precipitation. DO NOT inject sample directly into the mobile
phase whenever possible. Dissolve (or dilute) samples in an appropri
ate volume of the mobile phase first. If other solvents must be used,
be sure that no precipitation occurs when they are injected into the
mobile phase.
– Protect the column from rapid changes in solvent composition. DO NOT
change the flow rate faster than 0.5 mL/min. increments.
-
C. STORAGE CONSIDERATONS
– DO NOT store column in water alone for more than three days, this practive
will promote microbial contaimination. Store this series of columns in a
0.05% sodium azide in water solution.
– DO NOT allow buffers or other potentially harmful materials to remain in
the system overnight when not being used. Flush and replace with 0.05%
sodium azide in water solution.
– Return the column to its box with the end caps firmly in place for
storage. Allowing steel columns to dry out can result in poor chro
matographic performance.
-
Problem Cause Solution
Excess pressure
buildup
Loss of
resolution, broad
peaks, low plate
counts
• Filters plugge
with Particulates
• Sample precipitates
on column (sample
not soluble in mobile
phase)
• Filters partially
plugged
• Contaminated
colum, insufficent
equilibration
• Column collapse
and void formation
• Clean in an ultrasonic
bath or replace.
• Always alter mobile
phases and samples.
• Slowly purge with a
strong mobile phase that
is both appropriate to
dis-solve the contaminate
and compatible with the
column.
• Replace or clean inlet
and outlet filters in an
ultrasonic bath.
b. Column Efficency
IV. TROUBLESHOOTING
a. Service and Applications Information
The Waters staff of trained and experienced Service Specialists
provides maintenance for Waters instruments on preventative and/or
corrective levels. Contact Waters Office at 1-800-252 HPLC or your
local Waters Representative for answers to specific chromatography
questions in methods development, applications, quality control, and
service related matters.
Waters measures the efficiency of the Protein-Pak 60 and 125
columns by using the 5 sigma method. Plate count as an expression of
effieciency, is determined by measuring the peak width at 4.4% of the
peak height method.
Figure 2: Half Height Method
N= Column Efficiency
V
= Elution time (min)
e
W 1/2= Half width va
h
sample peak (min) taken at
half height (1/2 h)
N = 5.54 x
V
e
W½
2
e
½h
Protein-Pak SEC Columns 4
Page 5

[ CARE AND USE MANUAL ]
INJECT
m)
Figure 3: 5 Sigma Method
N = Column efficiency
Vr = Elution volume (mL)
or distance from INJET (m
W 1/2= Volume at 4.4%
of peak height (mL) or peak
width at 4.4% (mm)
N = 25 x
V
r
W
2
c. Test Conditions
Columns are thoroughly testing in our quality control laboratories for adherence to our specifications. Since slight variations in your results will
occur depending on the equipment used, test your sample makeup and equipment settings and condition, perform the test sample run given here for
your new column and record the results (retention time and the settings used) before attempting the first analysis. Use these results for comparison
throughout the life of your column.
Detectors should run at 254 nm or 280 nm [UV ]; otherwise, use intstrument settings that produce an acceptable test peak [this may mean using a
lower sensitivity on UV detectors than normally practiced for a specific analysis].
Note: Be sure to record results and instrument settings (and configurations) to allow exact reproduction and comparison in the future.
Key
Mobile Phase Test Sample Peak
1 100% Methanol A 1,2 Dichlorobenzene
2 0.05% Sodium Azide B Ethylene glycol in water
Column name Part Number Mobile Phase Optimal Flow Rate
(mL /min)
Protein-Pak 60
7.8 mm x 30 cm
Prot e in -P a k 125
7.8 mm x 30 cm
Protein-Pak 300 SW
7.8 mm (i.d.) x 30 cm, Steel
Protein-Pak 200 SW
8.0 mm (i.d.) x 30 cm, Glass
Protein-Pak 300 SW
8.0 mm (i.d.) x 30 cm, Glass
WAT085250 1 0.5 A
WAT 0 8 4 6 01 1 0.5 A
WAT080013 2 0.5 B
WAT 01178 6 2 0.5 B
WAT 01178 7 2 0.5 B
Test Sample Peak
Protein-Pak SEC Columns 5
Page 6

[ CARE AND USE MANUAL ]
Sales Offices
Austria 43 1 877 18 07
Australia 61 2 9933 1777
Belgium and Luxembourg 32 2 726 1000
Brazil 55 11 4134 3788
Canada 1 800 252 4752
China 86 21 6156 2666
Czech Republic 420 2 617 11384
Denmark 45 46 59 8080
Finland 358 9 5659 6288
France 33 1 30 48 72 00
Germany 49 6196 400 600
Hong Kong 852 2964 1800
Hungary 36 1 350 5086
India 91 80 2837 1900
Ireland 353 1 448 1500
Israel 9723 3731391
Italy 39 02 265 0983
Japan 81 3 3471 7191
The Netherlands 31 76 508 7200
Norway 47 6 384 6050
Poland 48 22 101 5900
Portugal 351 21 893 61 77
Puerto Rico 1 787 747 8445
Russia/CIS 7 495 727 4490 / 290 9737
Singapore 65 6593 7100
Spain 34 93 600 9300
Sweden 46 8 555 115 00
Switzerland 41 56 676 7000
Taiwan 886 2 2501 9928
UK 44 208 238 6100
US 1 800 252 4752
Waters Corporation
34 Maple Street
Milford, MA 01757 U.S.A.
T: 508 478 2000
F: 508 872 1990
www.waters.com
Korea 82 2 6300 4800
Mexico 52 55 52 00 1860
©2013 Waters Corporation. Waters, The Science of W hat’s
Possible and Sep-Pak are registered trademarks of Waters
Corporation. Protein-Pak is a trademark of Waters Corporation.
All other trademarks are property of their respective owners.
September 2013 WAT020052 Rev G VW-PDF
Protein-Pak SEC Columns 6
Waters Corporation
34 Maple Street
Milford, MA 01757 U.S.A.
T: 1 508 478 2000
F: 1 508 872 1990
www.waters.com
 Loading...
Loading...