Page 1

[ Care and Use ManUal ]
WATERS nanoACQUITY COLUMNS
(FOR USE WITH 10,000 P.S.I SYSTEMS)
I. INTRODUCTION
Waters nanoACQUIT Y™ columns are manufactured to exacting
specifications, providing outstanding peak symmetry for maximum
sensitivity and accurate quantitation. As the columns are frit-
ted at both ends, they are able to withstand pressure changes
during injection and thus provide superior longevity. In order to
achieve maximum performance, it is important to ensure that proper
connections are made to minimize peak tailing and poor efficiency.
Each nanoACQUITY column is individually tested to ensure that
it passes stringent quality control. Compared to standard flow
chromatography, successful day-to-day performance of nanoflow
systems can require extra attention to detail. This document
provides several essential recommendations for the successful use of
nanoACQUITY columns.
CONTENTS
I. PREPARING ELUENTS
II. PREPARING SAMPLE
III. PREPARING AND CONNECTING 75, 100
AND 150 µM nanoACQUITY COLUMNS
IV. PREPARING AND CONNECTING 300 µM nanoACQUITY
CAPILLARY COLUMNS
V. CARE WHEN STOPPING FLOW TO COLUMN
VI. HOW TO DIAGNOSE AND ADDRESS ABNORMALLY
HIGH BACKPRESSURE AND LEAKS
VII. CHECKING AND CLEANING A FOULED EMITTER
VIII. LONG TERM, NANOACQUITY COLUMN STORAGE
IX. ORDERING INFORMATION
Note:GlovesshouldbewornforALLoperations
detailedinthisdocument
nanoACQUITYSystem ExampleofananoACQUITYcolumn.Availablein75,100,and150µm
nanoACQUITY UPLC Column Care and Use 1
Page 2
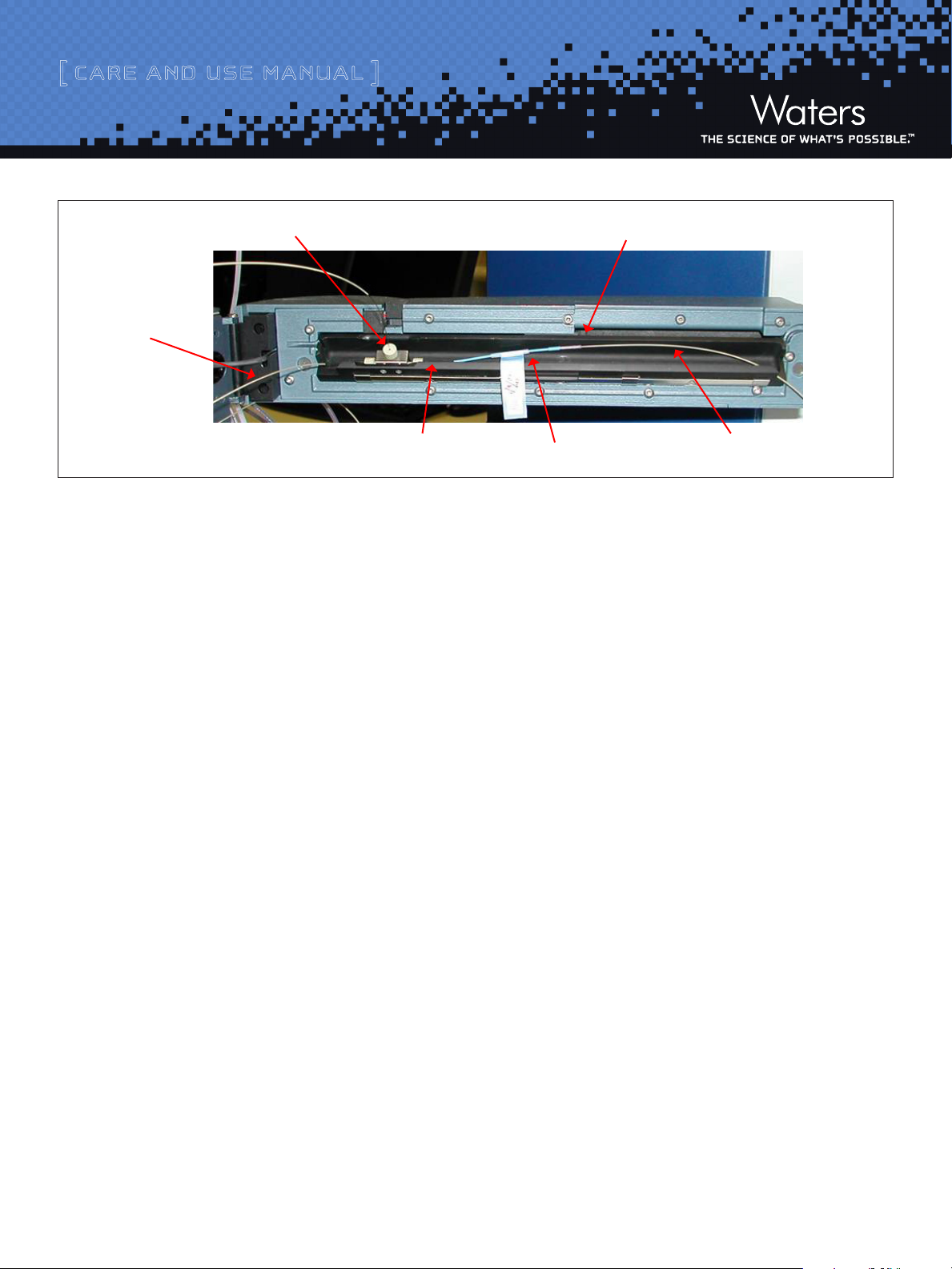
[ Care and Use ManUal ]
Nano-Teewithmagneticmount
Tubingto
HTMvalve
Trappingcolumn
outlettubing
I. PREPARING ELUENTS
Do not filter solvents. Use MS grade solvents directly from the bottle
A solvent: 100% water with 0.1% formic acid
B solvent: 100% acetonitrile or methanol with 0.1% formic acid
Seal wash solvent: water, may contain small amount of
organic (no acid)
Weak needle wash for peptides: 3% acetonitrile with 0.1% formic acid
Note:
Good lab practices: no solvent bottles to go through the dishwasher!
Wear gloves when handling solvent lines and hardware
If system is contaminated with poor quality solvents,
flush with appropriate solvent
Many report successful use of solution containing 25% water,
25% acetonitrile, 25% methanol, 25% IPA and 0.1% formic acid
For PEG contaminated, try IPA
DO NOT USE strong bases, as they can strip fused silica
Where to source solvents:
In North America: Fisher Optima or Burdick & Jackson
In Europe: Biosolve (Netherlands) provide very good solvents.
They offer smaller bottles (0.5 L) as well as "UPLC" grade solvents.
Innercompartment(swiveledopen)
Analyticalcolumn
Tubingto
MSsource
II. PREPARING SAMPLE
Samples for expression analysis must contain tryptic peptides derived
from proteins of interest at suitable concentrations
Samples may contain buffers and residual reagents from approved
digestion procedure
The sample must not contain other reagents, denaturants, detergents,
lipids, and must be free of particulates.
III. P REPAR ING A ND C ONN EC TING 75, 100 O R
150 µM nanoACQUITY COLUMNS
A: PREPARATION
Carefully remove your column from bag.
The gold ferrule on the inlet of the column is placed snugly on the
tubing at the factory. T he ferrule is lightly secured to reduce the risk of
it being lost as the column is removed from the packaging. The ferrule
is purposely set high on the tubing but will properly seat itself in the
correct position upon tightening.
A small piece of Teflon® tubing is present on the outlet end of the
column for those who want to connect the device to a UV or PDA
detector rather than a mass spectrometer.
Note: The column is attached to a transfer tube with the use of a
zero dead volume union. T his is hidden beneath the heat shrink
tubing. Care must be taken NOT to excerpt force on the ends of the
nano column (e.g., when removing teflon tubing at column outlet)
that could cause this joint to separate.
nanoACQUITY UPLC Column Care and Use 2
Page 3

[ Care and Use ManUal ]
Wrong Correct
Carefully grab the teflon tubing with one hand and the BARE FUSED
SILICA capillary, not the PEEK tubing, with the other hand, to remove
the Teflon sleeve. Again, be careful NOT to put unnecessary force
when removing the teflon tubing which could cause the fragile joint
between transfer tube and packed capillary to separate.
B: CONNECTION
Connecting and proper tightening of 75, 100, or 150 µm,
nanoACQUITY columns to 10 K UPLC system
Flow mobile phase only in the direction indicated by the arrow on the
column label. Connect the column with the direction of the flow arrow
on the label pointing to the detector or Mass Spectrometer.
1. For a column never installed on a nanoACQUIT Y system
Turn nut till snug then an additional 1/2 turn
2. For a previously installed and removed column from
a nanoACQUIT Y system
Hand tight plus 1/8 turn
“Hand Tight” is when the ferrule bottoms in the M-detail fitting. The
ferrule is pre-staked higher than necessary on the tube, so the ferrule
must be seated by hand (hand tight) or lightly with a wrench, then
an additional turn. Note: In virtually every case, gentle wrenching is
required to get to the “hand tight” state before the final half turn.
Taking “hand tight” or “finger tight” too literally can result in under
tightening/leaks.
Note: DO NOT over tighten, the leak rate will increase and the ferrule
may get stuck in the port
IV. PREPARING AND CONNECTING
300 µM nanoACQUITY CAPILLARY COLUMNS
• Carefullyremovecolumnfrombox
• ConnectthecolumninlettotheinjectorutilizingaValcostyle
compression screw
• ConnectthecolumnoutlettothedetectororMassSpectrometer
using a Valco style compression screw.
V. CARE WHEN STOPPING FLOW TO COLUMN
• DonotstoptheflowtoyournanoACQUITYcolumnsuddenly.Itis
critical that the flow be slowly lowered via the nanoACQUITY system
controller to prevent column damage.
VI. HOW TO DIAGNOSE AND ADDRESS ABNORMALLY
HIGH BACKPRESSURE AND LEAKS
The inlet or the head of a chromatography column experiences the
largest amount of pressure in a LC system. The nanoACQUITY column
inlet is designed to operate at pressures up to 10 K psi.
The fluidic pressure drops across the column at the outlet of the column
should only experience atmospheric pressure. The outlet connection
which connects the column to the transfer capillary is designed to
handle a maximum pressure of 800 psi.
With a higher than normal system pressure, a small leak might be
detected at the outlet of a nanoACQUITY column (see below).
Ferrule Nut
nanoACQUITY UPLC Column Care and Use 3
Leakatcolumnoutlet
Column Union Transfertubing
Page 4

[ Care and Use ManUal ]
Problem
If a clog or a blockage is created after the nanoACQUIT Y column
(e.g., at emitter) the pressure at the outlet of the column may rise
The increase in pressure at the column outlet may create a temporary
leak between the column and the transfer tubing
The most common source of post column clogs is a blocked
nanospray emitter
Note: Set high pressure limit on the nanoACQUITY system to approxi-
matively 1000 psi above maximum normal operating pressure.
Diagnosis 1
The pressure traces in the console window show an increase in
pressure during the analytical gradient (red arrows) between two
injections (highlighted by the red squares).
Diagnosis 2
This shows the increase in system pressure must originate after the
trap column.
Notice the pressure during the trapping portions of the analysis did
not increase between injections (red arrows).
nanoACQUITY UPLC Column Care and Use 4
Representative diagram of nanoACQUITY system with
attached nano trap and nano analytical column
Page 5

[ Care and Use ManUal ]
Diagnosis 3
A clogged emitter may still produce an ion signal
The charged emitter may act as an APCI needle and ionize the gas in
the source producing ions for the mass spectrometer (below).
Test for a clogged emitter
If the system pressure drops more than 200 psi the emitter is clogged
Remove the column transfer tubing from the universal sprayer (or other
nanospray source)
Note: In normal use, stop the flow and wait for the pressure to drop
near zero before breaking the connection at the union to minimize
the chance of dislodging potential particulates in the union and hav-
ing them be swept into (and clog) the emitter. If one needs to discon-
nect to relieve presser, it is much better to disconnect the outlet end,
as shown here, rather than the inlet.
The sprayer should be retracted to shut off the high voltage before
touching the sprayer.
Should your nanoACQUITY column be replaced?
Excessive pressure at the column outlet may create a small leak.
If the column outlet only experiences excess pressure for a short period
of time the column should still be acceptable for use since the union
between packed column and transfer tube can “recover.”
If the column outlet experiences pressure over an extended period
of time (over night) the union between the column and the transfer
tubing may be permanently compromised. Any non-reversible open
space created between the column and transfer line may lead to
band broadening (wider peaks).
VII. CHECKING AND CLEANING A FOULED EMITTER
Examine the emitter
Examining a clogged nanospray emitter under a microscope may allow
the user to determine the source of the clog
Fused silica particles: These particles will be transparent and have sharp,
fractured edges. Fused silica particles can be created when a column and
an emitter are joined with too much force (over tightened union)
Organic material: The material will appear brown in color (bottom).
Organic material can derive from a dirty sample or solvents.
Clean Emitter
GLOVES MUST BE WORN to prevent contamination with undesired
biological material.
nanoACQUITY UPLC Column Care and Use 5
Emitter clogged with organic residue
ExaminingtheUnion(PartNumber700002843,availableonwaters.com)
Page 6

[ Care and Use ManUal ]
1. Remove the clogged emitter and the column from the union on the
universal sprayer
2. Remove the PEEK nuts on either end
3. Loosen the screw on the sliding portion of the sprayer and remove
the union
5. Even if the union does not appear to be clogged it should be cleaned to
remove any small particulates
Cleaning the Union (Method #1)
As the union is 100% stainless steel, 20% nitric acid in water can be
used to clean it. This aggressive procedure helps ensure that the union
becomes free of even the strongest contaminations. In addition, it
provides excellent electric contact for electrospray ionization.
Wash the outside of the outlet capillary and fitting with water/acetron-
itrile (50/50) before reconnecting to union.
Note: Do not mix nitric acid with organic acids, as they will react and
produce high volumes of CO2, which could cause the explosions
of gas tight bottles.
Note: Again for demonstration purposes only, these photos show
fittings and tubing being handled with bare fingers, which is known
to introduce contamination in the system. It is therefore critical that
customers ALWAYS use powder-free, clean room and compatible
gloves when changing tubing or handling fittings.
4. Hold the union up to a light (above). Light should shine through an
unclogged union. If the union is completely clogged the obstruction
must be removed.
To order the Union: Waters PN 700002843
To order Peek Fitting: Waters PN 700002842.
Cleaning the Union (Method #2)
The union can be cleaned by sonication in a solution of IPA/water for at
least 15 minutes
After sonication, remove any residual solvent by flushing the union
with air or nitrogen
After cleaning, examine the union with a microscope and ensure that
any particulate matter has been removed. While looking at the union
be sure the internal threads and opening have not been scored or dam-
aged in any way
If the obstruction within the union cannot be removed or it appears
damaged please replace the union.
Note: In many cases it is best to simply REPLACE the union whenever
a new emitter is used.
nanoACQUITY UPLC Column Care and Use 6
Page 7

[ Care and Use ManUal ]
Replace the Emitter
Place the pin plug at the back side of the union. T his will mark the
center of the union (right)
Thread the replacement emitter (back end first) through the front of the
sprayer and into the union
Tighten the PEEK nut around the union carefully (do not over-tighten)
Remove the pin plug and replace it with the transfer line from the
nanoACQUITY column (again, do not over-tighten the fitting).
About poorly cut fittings
DO NOT cut or modify the emitter or column tubing in any way.
To reduce the occurrence of clogging
Make sure the union between the column and emitter is clean
Do not over tighten the fittings on the union (finger tight only)
Make sure the UPLC solvents are clean
Keep a flow on the tip even when the instrument is not in use (as low as
200 nL/min)
Try using a TaperTip instead of a Pulled Tip Emitter if clogging persists
TaperTip Emitter
A TaperTip (P/N 186003932) is offered as an alternative to a
PicoTip and is the default emitter in the universal sprayer kit. (July,
2007). The TaperTip has an ID of 20 µm but unlike the PicoTip the ID
remains unchanged thought out the emitter. This allows the emitter
to be implemented for higher flow rates. Furthermore, the TaperTip
may experience a lower frequency of particle clogging.
All tubing and columns supplied by Waters is pre-cut and polished.
Improperly cut tubing can lead to "shedding" and introduction of
fused silica particles downstream of nanoACQUITY 75, 100, or
150um I.D. column. This consequently can cause undesired system
backpressure increases.
Note: Compared to the PicoTip Emitter, Waters TaperTip Emitter does
not produce as stable a spray at 250-400 nL/min, which is the flow
rate range most customers use with 75 µm or 100 µm ID columns.
As indicated above, use of the TaperTip Emitter is the suggested
when a more robust device is preferred.
Column ID Optimal Flow Rate (nL/min) Emitter Type
75 µm 300 PicoTip
100 µm 400-500 PicoTip / TaperTip
150 µm 1000 TaperTip
300 µm 4000 ESI Source required
nanoACQUITY UPLC Column Care and Use 7
Page 8

[ Care and Use ManUal ]
What flow rate can I used with each type of emitter?
The flow rate of an LC method should be determined by the optimal
flow rate for the particular column. The optimal flow rate is directly
related to the column ID
A PicoTip is ideally suited for nanospray applications while the TaperTip
is suited for microspray applications (>1 µl/min)
Waters currently does not condone the use of a nanospray source for
the 300 µm column format.
VIII. LONG TERM, NANOACQUITY COLUMN STORAGE
When not used, it is recommended to maintain flow through the
nanoACQUITY column using 100% eluent B to maximize bed stability
and minimize potential bed drying
If prolonged column storage required or if the column needs to be
removed from system, it is recommended that the nanoACQUITY
column is flushed with 100% organic solvent containing no TFA
or FA (e.g. simply store in 100% acetonitrile).
XI. ORDERING INFORMATION
Description
nanoACQUITY UPLC Columns (10,000 psi)
Symmetry C
Symmetry C
Symmetry C
Symmetry C
Symmetry C
Symmetry C
Symmetry C
Symmetry C
Atlantis dC
Atlantis dC
Atlantis dC
Atlantis dC
Atlantis dC
Atlantis dC
Atlantis dC
Atlantis dC
18
18
18
18
18
18
18
18
18
18
18
18
18
18
18
18
Peptide Separation Technology nanoACQUITY UPLC
Columns (10,000 psi)
nanoACQUITY UP LC BEH130 C18 75 µm 100 mm 1.7 µm 186003542
nanoACQUITY UP LC BEH130 C18 75 µm 150 mm 1.7 µm 186003543
nanoACQUITY UP LC BEH130 C18 75 µm 200 mm 1.7 µm 186003544
nanoACQUITY UP LC BEH130 C18 75 µm 250 mm 1.7 µm 186003545
nanoACQUITY UP LC BEH130 C18 100 µm 100 mm 1.7 µm 186003546
nanoACQUITY UP LC BEH130 C18 150 µm 100 mm 1.7 µm 186003550
nanoACQUITY UP LC BEH300 C
nanoACQUITY UP LC BEH300 C18 100 µm 100 mm 1.7 µm 186003811
nanoACQUITY UP LC BEH300 C18 150 µm 100 mm 1.7 µm 186003812
nanoACQUITY UP LC BEH300 C18 75 µm 150 mm 1.7 µm 186003813
nanoACQUITY UP LC BEH300 C18 75 µm 200 mm 1.7 µm 186003814
nanoACQUITY UP LC BEH300 C18 75 µm 250 mm 1.7 µm 186003815
Inner
Diameter Length
Particle
Size
75 µm 100 mm 3.5 µm 186003491
75 µm 150 mm 3.5 µm 186003492
100 µm 100 mm 3.5 µm 186003493
100 µm 150 mm 3.5 µm 186003494
150 µm 100 mm 3.5 µm 186003495
150 µm 150 mm 3.5 µm 186003496
300 µm 100 mm 3.5 µm 186003497
300 µm 150 mm 3.5 µm 186003498
75 µm 100 mm 3 µm 186003499
75 µm 150 mm 3 µm 186003500
100 µm 100 mm 3 µm 186003501
100 µm 150 mm 3 µm 186003502
150 µm 100 mm 3 µm 186003503
150 µm 150 mm 3 µm 186003504
300 µm 100 mm 3 µm 186003505
300 µm 150 mm 3 µm 186003506
75 µm 100 mm 1.7 µm 186003810
18
Part No.
nanoACQUITY UPLC Column Care and Use 8
Protein Separation Technology nanoACQUITY UPLC
Columns (10,000 psi)
nanoACQUITY UP LC BEH300 C4 75 µm 100 mm 1.7 µm 186004639
nanoACQUITY UP LC BEH300 C4100 µm 100 mm 1.7 µm 186004640
nanoACQUITY UP LC BEH300 C4150 µm 100 mm 1.7 µm 186004641
For use with nanoACQUITY UPLC systems rated to 10,000 psi only.Not for use with nanoACQUIT Y UPLC systems rated to 5,000 psi.
Page 9

[ Care and Use ManUal ]
Sales Offices
Austria and European Export
(Central South Eastern Europe, CIS
and Middle East) 43 1 877 18 07
Australia 61 2 9933 1777
Belgium 32 2 726 1000
Brazil 55 11 5094-3788
Canada 1 800 252 4752 x2205
China 86 21 6879 5888
CIS/Russia +497 727 4490/290 9737
Czech Republic 420 2 617 1 1384
Denmark 45 46 59 8080
Finland 09 5659 6288
France 33 1 30 48 72 00
Germany 49 6196 400600
Hong Kong 852 29 64 1800
The Netherlands 31 76 508 7200
Norway 47 6 384 60 50
Poland 48 22 833 4400
Puerto Rico 1 787 747 8445
Singapore 65 6593 7100
Spain 34 93 600 9300
Sweden 46 8 555 11 500
Switzerland 41 56 676 70 00
Taiwan 886 2 2543 1898
United Kingdom 44 208 238 6100
All other countries:
Waters Corporation U.S.A.
1 508 478 2000
1 800 252 4752
www.waters.com
Hungary 36 1 350 5086
India and India Subcontinent
91 80 2837 1900
Ireland 353 1 448 1500
Italy 39 02 265 0983
Japan 81 3 3471 7094
Korea 82 2 6300 4800
Mexico 52 55 5524 7636
©2009 Waters Corporation, ACQUITY, UP LC, Waters,
The Science of What’s Possible, Symmetry, Atlantis and
NanoACQUITY are trademarks of Waters Corporation.
PEEK is a trademark of Victrex plc.
October 2009 715001354 Rev C VW-IH-P DF
Waters Corporation
34 Maple Street
Milford, MA 01757 U.S.A.
T: 1 508 478 2000
F: 1 508 872 1990
www.waters.com
nanoACQUITY UPLC Column Care and Use 9
 Loading...
Loading...