Page 1

nanoACQUITY UPLC
System
Quick Start Guide
71500097503/Revision D
CORPORATION
Copyright © Waters Corporation 2006.
All rights reserved.
Page 2

Copyright notice
© 2006 WATERS CORPORATION. PRINTED IN THE UNITED STATES OF
AMERICA AND IRELAND. ALL RIGHTS RESERVED. THIS DOCUMENT
OR PARTS THEREOF MAY NOT BE REPRODUCED IN ANY FORM
WITHOUT THE WRITTEN PERMISSION OF THE PUBLISHER.
The information in this document is subject to change without notice and
should not be construed as a commitment by Waters Corporation. Waters
Corporat io n assumes no re spo n sibility fo r an y errors that may app ea r in t h is
document. This document is believed to be complete and accurate at the time
of publicatio n. In no eve n t s h all Wate rs Corpor ation be liable fo r in ciden t al or
consequential damages in connection with, or arising from, the use of this
document.
Trademarks
Millennium and Waters are registered trademarks, and ACQUITY UPLC,
Atlantis, BEH Technology, Empower, MassLynx, MassPREP, nanoACQUITY
UPLC, and Symmetry are trademarks of Waters Corporation.
CTC PAL is a trademark of CTC Analytics AG.
Optima is a registered trademark of Fisher Scientific Company, L.L.C.
Teflon is a registered trademark of E.I. duPont de Nemours and Company.
Windows is a registered trademark of Microsoft Corporation.
Other trademarks or registered trademarks are the sole property of their
respective owners.
Page 3

Customer comments
Please contact us if you hav e questi ons, s ug gestions for improvements, or f ind
errors in this document. Your comments will help us improve the quality,
accuracy, and organization of our documentation.
You can reach us at tech_comm@waters.com.
Waters Corporation
34 Maple Street
Milford, MA 01757
USA
iii
Page 4

Operating this device
When operating this device, adhere to standard quality control procedures
and the following equipment guidelines.
Attention: Changes or modifications to this unit not expressly approved by
the party responsible f or compliance c ould void t he user’s a uthority to operate
the equipment.
Important: Toute modification sur cette unité n’ayant pas été expressément
approuvée par l’autorité responsable de la conformité à la réglementation
peut annuler le droit de l’utilisateur à exploiter l’équipement.
Achtung: Jedwede Änderungen oder Modifikationen an dem Gerät ohne die
ausdrückliche Genehmigung der für die ordnungsgemäße Funktionstüchtigkeit verantwortlichen Personen kann zum Entzug der
Bedienungsbefugnis des Systems führen.
Avvertenza: eventuali modifiche o alterazioni apportate a questa unità e
non espressamente approvate da un ente responsabile per la conformità
annulleranno l’autorità dell’utente ad operare l’apparecchiatura.
Atencion: cualquier cambio o modificación efectuado en esta unidad que no
haya sido expresamente aprobado por la parte responsable del cumplimiento
puede anular la autorización del usuario para utilizar el equipo.
iv
Page 5

Caution: Use caution when working with any polymer tubing under
pressure:
• Always wear eye protection when near pressurized polymer tubing.
• Ext in guish a ll n earby flames.
• Do not use tubing that has been severely stressed or kinked.
• Do not use nonmetallic tubing with tetrahydrofuran (THF) or concentrated
nitric or sulfuric acids.
• Be aware that methylene chloride and dimethyl sulfoxide cause
nonmetallic tubing to swell, which greatly reduces the rupture pressure of
the tubing.
Attention: Manipulez les tubes en polymère sous pression avec precaution:
• Portez systématiquement des lunettes de protection lorsque vous vous
trouvez à prox im ité de tub e s e n polymère pressu ri sé s.
• Eteignez toute flamme se trouvant à proximité de l’instrument.
• Evitez d'utiliser des tubes sévèrement déformés ou endommagés.
• Evitez d'utiliser des tubes non métalliques avec du tétrahydrofurane
(THF) ou de l'acide sulfurique ou nitrique concentré.
• Sachez que le chlorure de méthylène et le diméthylesulfoxyde entraînent le
gonflement des tuyaux non métalliques, ce qui réduit considérablement
leur pression de rupt u re.
Vorsicht: Bei der Arbeit mit Polymerschläuchen unter Druck ist besondere
Vorsicht ange bracht:
• In der Nähe von unter Druck stehenden Polymerschläuchen stets
Schutzbrille tragen.
• Alle offenen Flammen in der Nähe löschen.
• Keine Schläuche verwenden, die stark geknickt oder überbeansprucht
sind.
• Nicht metallische Sch läu che nicht fü r Tetrahydrofura n (TH F) oder
konzentrierte Salpeter- oder Schwefelsäure verwenden.
• Durch Methylenchlorid und Dimethylsulfoxid können nichtmetallische
Schläuche quellen; dadurch wird der Berstdruck des Schlauches erheblich
reduziert.
v
Page 6

Attenzione: prestare attenzione durante l’utilizzo dei tubi di polimero
pressurizzati:
• Indossare sempre occhiali da lavoro protett ivi nei p ressi di tubi di polimero
pressurizzati.
• Estinguere ogni fonte di ignizione circostante.
• Non utilizzare tubi soggetti che hanno subito sollecitazioni eccessive o son
stati incurva ti.
• Non utilizzare tubi non metallici con tetraidrofurano (THF) o acido
solforico o nitrico concentrato.
• Tenere presente che il cloruro di metilene e il dimetilsolfossido provocano
rigonfiamento nei tubi non metallici, riducendo notevolmente la resi stenza
alla rottura dei tubi stessi.
Advertencia: se recomienda precaución cuando se trabaje con tubos de
polímero sometidos a presión:
• El usuario deberá protegerse siempre los ojos cuando trabaje cerca de
tubos de polímero sometidos a presión.
• Si hubiera alguna llama las proximidades.
• No se deb e tra ba ja r co n t u bo s que se h ay an do bl ado o sometido a al ta s
presiones.
• Es necesario utilizar tubos de metal cuando se trabaje con
tetrahidrofurano (THF) o ácidos nítrico o sulfúrico concentrados.
• Hay que tener en cuenta que el cloruro de metileno y el sulfóxido de
dimetilo dilatan los tubos no metálicos, lo que reduce la presión de ruptura
de los tubos.
vi
Page 7

vii
Page 8

Caution: The user shall be made aware that if the equipment is used in a
manner not specified by the manufacturer, the protection provided by the
equipment may be impaired.
Attention: L’utilisateur doit être informé que si le matériel est utilisé d’une
façon non spécifiée par le fabricant, la protection assurée par le matériel
risque d’être défectueuses.
Vorsicht: Der Benutzer wird darauf aufmerksam gemacht, dass bei unsachgemäßer Verwenddung des Gerätes unter Umständen nicht ordnungsgemäß
funktionieren.
Attenzione: l’utente deve essere al corrente del fatto che, se l’apparecchiatura viene usta in un modo specificato dal produttore, la protezione fornita
dall’a pparecchiat u ra potrà essere invalidata.
Advertencia: el usuario deberá saber que si el equipo se utiliza de forma
distinta a la espe c ifi cada por el fabr ica nt e , las medidas de prote cción del
equipo podrían ser insuficientes.
viii
Page 9

Caution: To protect against fire hazard, repl ace f us es wi th those of the s a me
type and ra t ing .
Attention: Remplacez toujours les fus ib les pa r d’autres du mêm e type et de
la même puissance afin d’éviter tout risque d’incendie.
Vorsicht: Zum Schutz gegen Feuergefahr die Sicherungen nur mit
Sicherungen des gleichen Typs und Nennwertes ersetzen.
Attenzione: per una buona protezione contro i rischi di incendio, sostituire i
fusib ili con altri dello s tes so tipo e amperaggio.
Advertencia: sustituya los fusibles por otros del mismo tipo y características
para evitar el ri es go de incendio.
ix
Page 10

Caution: To avoid possible electrical shock, disconnect the powe r cord befo re
servicing the instrument.
Attention: Afin d’éviter toute possibilité de commotion électrique,
débra n ch e z le co rdon d’al imentat io n de la prise avant d’effectuer la maint e nance de l’instrument.
Vorsicht: Zur Vermeidung von Stromschlägen sollte das Gerät vor der
Wartung vom Netz getrennt werden.
Attenzione: per evitare il rischio di scossa elettrica, scollegare il cavo di
alimentazione prima di svolgere la manutenzione dello strumento.
Precaución: para evitar descargas eléctricas, desenchufe el cable de alimentación de l in st ru mento antes de re alizar cualquier reparación.
x
Page 11
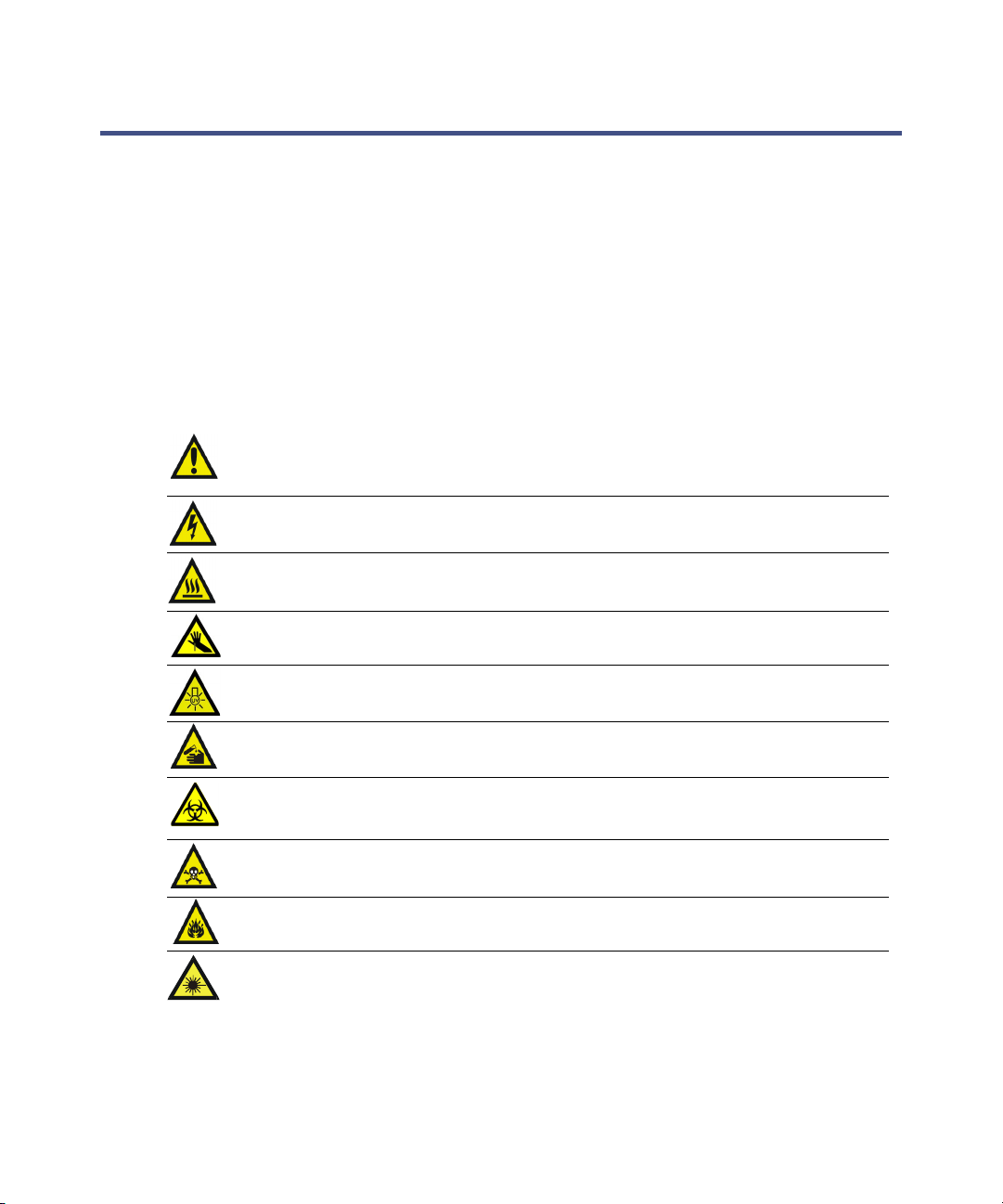
Observing safety precautions
Observe all safety precautions while servicing, repairing, installing, and
operating the instrument. Failing to do so violates safety standards and
intende d u se of the ins tr ument. Waters C o rpo ration assumes n o liabilit y fo r
failure to comply with these precautions.
Precautions can be of these two types:
• Warnings that indicate risk of injury or death
• Cautions that indicate risk of damage to the system or equipment
Accompanying the word “Warning” or “Caution,” these are the safety
precaution symbols you might encounter on instruments and/or in documents :
Warning: Indicates a potential health or safety hazard. Refer to the
manual.
Warning: Indicates hazardous voltages can ex ist .
Warning: Indicates hot surfaces or high temperatures can exist.
Warning: Indicates danger from needle-stick punctures.
Warning: Indicates danger fro m ultraviolet ra diation.
Warning: Indicates danger from corrosive substances.
Warning: Indicates danger from contaminatio n by a biolog ical agent.
Warning: Indicates danger from toxic substances.
Warning: Indicates danger from flammable substances.
Warning: Indicates danger from laser radiat ion .
xi
Page 12

Using Waters equipment
In addition to warning symbols, you might encounter the following symbols and
labels on p ackagi n g, in s t ru ments , an d / o r in documen ts.
Direct current
Alternating current
Protective conductor terminal
Frame or chassis terminal
Fuse
Electrical power on
l
Electrical power off
xii
Keep upright
Keep dry
Fragile, handle contents with care
Page 13

Safety and electromagnetic equipment compatibility
United States – FCC rules
This device complies with Part 15 of the FCC Rules. Operation is subject to
the following two conditions: (1) this device may not cause harmful
interfer e nc e , a n d (2) this device must ac ce pt an y interfere n ce re ceived,
including interference that may cause undesired operation.
Changes or modifications to this unit not expressly approved by the party
responsible for compliance could void the user’s authority to operate the
equipment.
Rationale: This equipment has been tested and found to comply with the
limits for a Class B digital device, pursuant to Part 15 of the FCC Rules.
These limits are designed to provide reasonable protection against harmful
inter fe ren ce in a res i den tia l inst al lat io n. This equi pme nt ge ner at es, uses, and
can radiate radio frequency energy and, if not installed and used in
accordance with the instructions, may cause harmful interference to radio
communications. However, there is no guarantee that interference will not
occur in a particular installation. If this equipment does cause harmful
interference to radio or t e le vision reception, whic h can be determined b y
turning the equipment off and on, the user is encouraged to try to correct the
interference by one or more of the following measures:
• Reorient or relocate the receiving antenna.
• Increase the separation between the equipment and receiver.
• Connect the equipment into an outlet on a circuit different from that to
which the receiver is connected.
• Consult the dealer or an experienced radio TV technician for help.
Shielded cables must be used with this unit to ensure compliance with the
Class B FCC limits.
United States – safety requirements
Waters products meet the safety requirements for laboratory instruments set
forth by the Occupational Safety and Health Administration (OSHA). All
products are evaluated b y an OSHA-appr oved, Na tionally Recognized Testing
Laboratory (NRTL) to ensure they meet applicable safety standards. NRTLs
perform safety testing on instruments to ensure the safety of the operator.
xiii
Page 14

Waters pr o du ct s carry a safe ty la be l from an NRTL to show co mplianc e . T h e
particular safety standard with which Waters complies is UL 61010A-1:
Electrical equipment for laboratory use; Part 1: General Requirements.
Canada – spectrum management
This Class B digital apparatus complies with Canadian ICES-003.
Cet appareil numérique de la classe B est conforme à la norme NMB-003.
Waters products meet the safety requirements for laboratory instruments set
forth by the Standards Council of Canada. All products are evaluated by an
approved laboratory to meet Canada’s safety requirements. Waters
instrume n t s carry a safety label from an approved testi n g laborato ry to show
compliance. The particular Canadian safety standard with which Waters
complies is CAN/CAS-C22.2 No. 1010.1: Safety requirements for electrical
equipmen t fo r measurem ent, control and labo ratory us e, Part 1: Gene ral
Requirements.
Europe – safety and electromagnetic compatibility
Waters products have been tested to meet the safety and electromagnetic
requirements of the European community. Display of the CE mark indicates
compliance to these requirements. The safety requirements are set forth via
the standard EN61010: Safety requirements for electrical equipment for
measurement, con t ro l , a n d laboratory use – Part 1: Ge n e ral require ments.
The EMC requirements are supported in the standard EN61326: Electrical
equipment for the measurement, control, and laboratory use – EMC
requirements. Compliance to the EN61010 standard ensures the safety of the
operator from any hazardous situations that could have been caused by the
instrument. Adherence to the EMC standard guarantees that the instrument
will not cause interference to adjacent electronic products nor will other
electronic units interfere with its operation.
Australia – emissions requirements
Australian aut h o riti e s re qu ir e tha t in st ru ments do not excee d specified
radiat io n limits. The se radiation limits are given in the standard A S/NZS
2064: Limits and methods of measurement of electronic disturbance
charac teristics o f in d u strial, sc ie n t ific and medical (ISM) radio frequ en cy
equipment. Conformance to this standard is shown by displaying the
Australian C-tick mark.
xiv
Page 15

nanoACQUITY UPLC system information
Intended use
Use the Waters® nanoACQUIT Y UPLC system for analyzing compounds an d
introducing separated sample components into a mass spectrometer.
The Waters nanoACQUITY UPLC system is not intended for use in
diagnostic procedures.
When you develop methods, follow the “Protocol for the Adoption of Analytical
Methods in the Clinical Chemistry Laboratory,” American Journal of Medical
Technology, 44, 1, pages 30–37 (1978). Th is protocol covers good operati n g
procedures and techniques necessary to validate system and method
performance.
Biological hazard
When you analyze physiological fluids, take all necessary precautions and
treat all s pe cime ns a s pot en tia lly i nf ect iou s. P rec aut io ns are out li ned in “C DC
Guidelines on Specimen Handling,” CDC – NIH Manual, 1984.
Calibration
Follow acce ptable met h o ds o f calibratio n with pu re st an dards to calibrate
methods. Use a minimum of five standards to generate a standard curve. The
concentration range should cover the entire range of quality-control samples,
typica l sp e cimens , and atyp ical spec imens.
Quality control
It is recommended that you routinely run three quality-control samples.
Quality-control samples should represent subnormal, normal, and
above-no rmal leve ls of a compou n d . E n su re t h at qu ality-con t ro l sample
results are within an acceptable range, and evaluate precision from day to day
and run to run. Data collected when quality-control samples are out of range
might not be valid. Do not report this data until you ensure that
chromatographic system performance is acceptable.
xv
Page 16

xvi
Page 17

Table of Contents
Operating this device .......................................................................................... iv
Observing safety precautions ........................................................................... xi
Using Waters equipment ................................................................................... xii
Safety and electromagnetic equipment compatibility ............................. xiii
United States – FCC rules.............................................................................. xiii
United States – safety requirements.............................................................. xiii
Canada – spectrum management.................................................................... xiv
Europe – safety and electromagnetic compatibility........................................ xiv
Australia – emissions requirements................................................................ xiv
nanoACQUITY UPLC system information .................................................... xv
Intended use....................................................................................................... xv
Biological hazard................................................................................................ xv
Calibration ......................................................................................................... xv
Quality control................................................................................................... xv
1 System Overview .................................................................................... 1-1
Instruments and components ........................................................................ 1-1
nanoACQUITY operating modes ................................................................... 1-3
Direct injection ................................................................................................. 1-4
Single-pump trapping...................................................................................... 1-4
Two-pump trapping......................................................................................... 1-5
Two-dimensional liquid chromatography (2D-LC)......................................... 1-5
nanoACQUITY binary solvent manager ...................................................... 1-6
How the binary solvent manager works......................................................... 1-6
Flow control modules....................................................................................... 1-6
Auxiliary solvent manager ............................................................................. 1-7
Flow control module......................................................................................... 1-7
Table of Contents xvii
Page 18

Sample manager ................................................................................................ 1-7
Sample consumption guidelines...................................................................... 1-8
Heating and trapping module ........................................................................ 1-9
TUV optical detector ...................................................................................... 1-10
Mass detectors ................................................................................................. 1-10
Q-Tof Premier™............................................................................................. 1-10
LCT Premier™............................................................................................... 1-11
MassLynx data system ................................................................................... 1-11
nanoACQUITY UPLC Console ..................................................................... 1-11
2 Preparing System Hardware ............................................................... 2-1
Powering-on the system .................................................................................. 2-1
Monitoring startup tests ................................................................................. 2-2
Monitoring the LEDs of system instruments ............................................. 2-2
Power LED....................................................................................................... 2-2
Status LEDs..................................................................................................... 2-3
Preparing the auxiliary solvent manager ................................................... 2-4
Priming the seal wash..................................................................................... 2-5
Preparing the binary solvent manager ....................................................... 2-8
Priming the seal wash..................................................................................... 2-8
Priming the binary solvent manager............................................................ 2-10
Preparing the sample manager ................................................................... 2-12
Selecting weak and strong wash solvents.................................................... 2-12
Priming the sample manager........................................................................ 2-14
Washing the sample manager needle........................................................... 2-15
Characterizing the needle seal...................................................................... 2-17
Characterizing the needle and sample loop volumes................................... 2-18
Loading sample plates in the sample manager............................................ 2-19
Preparing the detector .................................................................................. 2-21
Preparing the TUV detector.......................................................................... 2-21
Preparing the mass spectrometer................................................................. 2-22
xviii Table of Contents
Page 19

Conditioning the column .............................................................................. 2-23
Shutting down the system ............................................................................ 2-24
Between analyses........................................................................................... 2-24
Overnight or weekends.................................................................................. 2-24
More than 72 hours (long-term).................................................................... 2-25
3 Configuring System Software ............................................................. 3-1
Configuring MassLynx ..................................................................................... 3-1
Starting the nanoACQUITY UPLC Console from MassLynx .................. 3-5
Configuring events ........................................................................................... 3-6
4 Verifying System Operation ......................................... ....................... 4-1
Required materials ........................................................................................... 4-1
Preparing the mobile phases ......................................................................... 4-2
Preparing the sample ....................................................................................... 4-3
Preparing the system ....................................................................................... 4-4
Creating the test methods ............................................................................... 4-6
Creating the instrument method.................................................................... 4-6
Performing the test ........................................................................................ 4-10
Table of Contents xix
Page 20

xx Table of Contents
Page 21

1 System Overview
Contents:
Topic Page
Instruments and components 1-1
nanoACQUITY operating modes 1-3
nanoACQUITY binary solvent manager 1-6
Auxili ary solve n t manage r 1-7
Sample manager 1-7
Heating and t rapping modu le 1-9
TUV optical detector 1-10
Mass detectors 1-10
MassLynx data system 1-11
nanoACQUITY UPLC Console 1-11
Instruments and components
The Wate rs® nanoACQUITY UPLC™ system is designed for capillary-tonano-scale sep ara t ion s. Its se n si tivi t y, re so lut ion, and reprod ucibility well
suit it for biomark e r discov e ry and pr ot e o mics applicatio n s , in cluding protein
identification and characterization.
The system consists of these modules and components:
• Bina ry solvent manager with flow control mod u les
• Auxiliary solvent manager (for NanoLockSpray™ lock-mass addition
and two-pump trapping ) with flow control module
• Sample manager and heating and trapping module, which holds the
analytical column
1-1
Page 22

• Waters Ma ssLynx™ ch romatography an d mass spectrometry software
• nanoACQUITY UPLC Console, which provides control, status, and
advan ce d diagnos ti c in fo rmatio n in a grap h ical display
Waters nanoACQUITY UPLC system:
Solvent tray
Heating and
trapping module
(HTM)
Column heater
(“away” position)
Sample manager
Binary solvent
manager (BSM)
Auxiliary solvent
manager (ASM)
The system is optimized for high-resolution separations at precise nanoflow
rates. With clo sed loop co n tro l, t h ose rates rang e be t we e n 0. 20 and
5.00 µL/min. With open loop control and nanoACQUITY UPLC columns of
internal diameters ranging from 75 µm to 1 mm, the nanoflow rates can
extend to 100 µL/min. The column hardware and the matched outlet tubing
can withst an d up to 69,000 kPa (690 bar, 10,000 psi). The column dimen s io n s
allow optimal MS-co mpatible fl ow rates, and matched outl et tubing minimizes
the effect of extra-column volume.
1-2 System Overview
Flow control modules
Page 23

Smaller-column diameters require lower flow rates, which can encourage
extra-column bandspreading. The system counters this with precise injection
volume capacity and gradient formation, optional detector flow cell volume,
and low-volume connection tubing.
Waters offers nanoA C QUITY UP LC co lumns p acked with 1.7-µm, bridge d,
ethane-silicon (BEH), hybrid particles as well as conventional reversed phase
packing materials, typically 3- to 5-micron particle sizes. Compared with
traditio nal HPLC columns, nan oACQU ITY UPLC colum ns delive r s u perior
resolut ion and sensiti vity in th e same r un time, or gr e ater sensitiv it y and
faster run times with equivalent resolution.
nanoACQUITY operating modes
The nanoACQUITY system can operate in direct i njec ti on mode or any of four
trapping m o de s. Trapping im pro v e s system perfor ma n ce in se veral ways:
• Removes salts
•Cleans samples
• Concen t rat e s larger sam p l e vo lu mes
• Decre as e s sample lo ading time
Each mode requires a different configuration of solvent managers and
columns:
• Direct injection mode uses the binary solvent manager with an
analytical column.
• Single-pump trapping uses the binary solvent manager with a trap
column and an analytical column.
• Two-pump trapping uses the auxiliary solvent manager and
nanoACQUITY binary solvent manager with a trap column and an
analytical column.
• Onlin e t wo -dimensiona l liquid ch ro matogr ap h y (2D-L C ) t rapping, w it h
salt plugs, use s the auxiliary solv e n t manager an d bi n ary solven t
manage r with an ion ex ch ange column, trap colum n , and analyt i c al
column.
• Off-line 2D-LC trapping requires the CTC PAL MALDI Spotter
Microfraction Collector.
1-3
Page 24

For typical operating conditions, use the following table as a starting point.
nanoACQUITY UPLC typical operating conditions:
Analytical
Mode
Direct 75 0.20—0.40 n/a n/a 1—5
Direct 100 0.40—0.60 n/a n/a 1—5
Direct 150 0.80—1.20 n/a n/a 1—10
Direct 300 4.00—5.00 n/a n/a 1—10
1-pump
trapping
1-pump
trapping
2-pump
trapping
2D-LC 75 0.20—0.40 180 × 20 3.00—5.00 5—10
Column ID
(µm)
75 0.20—0.40 180 × 20 4.00 1—5
100 0.40—0.60 180 × 20 4.00 1—5
100 0.40—0.60 180 × 20 4.00 (u p to
Analytical
Flow Rate
(µL/min)
Trap
Column
(ID, µm ×
length, mm)
Trapping
Flow Rate
(µL/min)
50)
Injection
Volume (µL)
Direct injection
The direct in j ec t io n mode is us e d to in j e ct sample dire ctly onto an an al ytical
column. The trap valve stays in the “elute” position, which routes all flow
through the analytical column. The sample manager post-injection mode can
be programmed in either of two ways:
1—5
• To keep the sample loop in the flow path throughout the run (most
common).
• To remove the loop from the flow path after time that the user enters.
Single-pump trapping
At nL/min flow rates, samples larger than 2 µL ar e sl o w to load o n t o a
small-bore column. Using single-pump trapping improves sample loading time
by loading samp le s o n t o a s e p arate trap column a t a higher flo w rate whil e
excess solvent, salts, and impurities elute to waste. After loading, the trap
column is connected to the flow path, and a gradient elutes the sample from
the trap co lu mn onto the anal yt ical column, usually at a sl o we r rate.
1-4 System Overview
Page 25

Two-pump trapping
With tw o -p u mp tra pp ing, the s ample manager loads t h e sample in t o th e
sample lo op. A dedicat ed trappi ng pump in the auxi liary solv e n t manager
(pump A) then loads the sample onto the trapping column. With the HTM
(heating and trapping module) valve in the waste position, unwanted solutes
flush through the trapping column and elute to waste while the column
retains the analytes. When trapping is finished, the valve then closes the
waste pathway and opens the flow path. Gradient elution proceeds as the
binary solvent manager pumps solvents through the trapping and analytical
columns and out to the detector or mass spectrometer.
Two-dimensional liquid chromatography (2D-LC)
2D-LC can se parate and ch aracteriz e com plex biological comp o u n ds, for
example, as an adjunct to protein digestion and polyacrylamide gel
electrophoresis (PAGE) techniques.
Pump A of the auxiliary solvent manager serves as a sample loading pump,
injecting the sample onto an ion exchange column. Salt plugs of increasing
strength are prepared separately and injected from vials in the sample
manager, to elute various portions of the sample off the ion exchange column
and onto a trap column. At that point, separation and analysis proceeds as in
two-pump trapping.
Two-dimensional liquid chromatography can increase the amount of
information from complex proteomics samples, reducing their complexity and
dynamic range prior to mass spectrometry (MS) analysis.
• To further improve the identification and increase the sequence
coverage of high and low abundanc e proteins from complex samples, the
system can perform fully automated online 2D-LC separations of
complex protein digest s amples u s in g an ion e xch ange column in t h e
first dimension and a reversed phase UPLC (nanoACQUITY columns
with BEH Technology™) in the second dimension.
• For some of the most complex proteomics samples, off-line 2D-LC
separation using the CTC PAL MALDI Spotter/Fraction Collector is
available t o minimize band broa de n in g an d thus preserve high p e ak
capacity na n os cal e an d ca pil lary separat ion s. To minimize sample
complexity an d dyn amic ran ge, the CTC PAL™ microf raction colle ction
platform first collec ts d isc re t e fr actions from a gradie n t separation ont o
MALDI targets or into microtiter plates for LC/electrospray ionization
(ESI) analysis, followed by MALDI or LC/ESI MS/MS analysis.
1-5
Page 26

nanoACQUITY binary solvent manager
The nanoACQUITY binary solvent manager is a high-pressure pump that
moves solvent through the system. I t prov ides st eady (p ulse- f r ee) solvent flow
at flow ra t e s rangi n g from 0.20 to 5.00 µL/min (under closed loop control) or to
100.0 µL/min (under open loop control) at 69,000 kPa (690 bar, 10,000 psi).
The solvent manager can generate high-pressure gradients with minimal
gradient delay.
How the binary solvent manager works
Each of the solvent manager’s two independent pump systems, pump A (left)
and pump B (right), contains two linear-drive actuators. Each left and right
actuator pair compri ses a sin gle recipr oc at ing serial pu mp that delivers
precise flow of a single solvent. The two pump systems combine their two
solvents at a mixing tee. From there, the solvent mixture flows to the sample
manager.
To crea t e gradients and mixtu re s, the chromat ography sof t ware contro ls t h e
two solvents’ mixing ratio by varying the flow of pump A relative to that of
pump B. A pressure transducer in each pump head relays pressure data to the
solvent manager, whose firmware measures pump head pressures during the
pumping cycle. Thus the solvent manager adjusts the precompression to
ensure consistent solvent delivery and minimize pump-induced detector
baseline disturbances.
Flow control modules
The flow co n trol mod u le s allow t h e b in ary solve n t manager to pe rform
nano-flow chromatography by monitoring the solvent flow in each solvent
channel . F lo w contro l is calibrat e d fo r water, ace t o n it rile, and m eth anol.
The flow control modules’ two mass flow sensors measure the flow from each
pump separately, b efore they ar e mixed. T he binary solvent manager moni tors
the output and adjusts the solvent flow accordingly for precise flow from 0.20
to 5.00 µL/min. Solvents are mixed in the flow control module tee outlet.
1-6 System Overview
Page 27

Auxiliary solvent manager
The nanoACQUITY auxiliary solvent manager incorporates two isocratic,
high-pressure pumps that move solvent through the system and provide
steady (pulse -free) solvent flow.
• Pump A is for sa mple loading .
• Pump B is for NanoLock S pray lock-mass addit ion, two -pu mp trapping ,
and 2D online separation.
In the NanoLockSpray lock-mass addition mode, the pump operates at flow
rates from 0.10 to 5.00 µL/min.
Flow control module
The flow control m odule allows th e a u xiliary s o lvent man ager to pe rform
nano-flow chromatography by monitoring the solvent flow in the solvent
channel . F lo w contro l is calibrat e d fo r water, ace t o n it rile, and m eth anol.
The flow control module’s mass flow se nso r measures the f l ow fr om t he p ump.
The auxiliary solvent manager monitors the output and adjusts the solvent
flow acco rdingly for precise flo w from 0.2 0 t o 5 .0 0 µL/min.
Sample manager
The sample manager injects the samples it draws from microtiter plates, or
vials, into t h e ch ro matograph i c flow stream . In maximum t h ro u ghput mode ,
the sam ple manag e r can perform an inj e ct io n in approximately 45 seconds ,
including a si n gle def au lt wash, or it can do so in less than 60 seconds,
including a du a l de fault wash.
The sample manager accepts standard footprint plates, 5.03 ±0.02 inch ×
3.365 ±0.02 inch, that conform to SBS/ANSI-compliant plates (maximum
height of 2.2 in ch e s, including covers ).The sample manager can maint ain
samples at any temperature between 4 and 40 °C (39 and 104 °F) in ambient
conditio n s o f 25 °C (77 °F) or le ss.
1-7
Page 28

Sample consumption guidelines
Sample consumption varies depending on your system configuration and
injectio n mode.
Sample consumption guidelines
System
Configuration
Loop: 2 µL
Needle: 15 µL
Loop: 5 µL
Needle: 15 µL
Injection Mode
Requested
Sample Size
(µL)
Sample
Consumed
(µL)
Maximum
Injection
Volume (µL)
a
Partial loop 1 1 1.9
Part ial loop with
Not applicable Not applicable Not applicable
needle overfill
Full loop
Auto 242
1 × Overfill222
2 × Overfill242
3 × Overfill262
Part ial lo o p 2 2 4.9
Partial loop with
263.8
needle overfill
Full loop
Auto 5 10 5
1 × Overfill555
2 × Overfill 5 10 5
3 × Overfill 5 15 5
Loop: 10 µL
Needle: 15 µL
Part ial lo o p 5 5 9.5
Partial loop with
needle overfill
Full loop
Auto 10 20 10
1 × Overfill 10 10 10
2 × Overfill 10 20 10
3 × Overfill 10 30 10
1-8 System Overview
597.5
Page 29

Sample consumption guidelines (Continued)
System
Configuration
Loop: 20 µL
Needle: 15 µL
Requested
Injection Mode
Sample Size
(µL)
Part ial lo o p 5 5 19
Partial loop with
5915
Sample
Consumed
(µL)
Maximum
Injection
Volume (µL)
needle overfill
Full loop
Auto 10 40 20
1 × Overfill 10 20 20
2 × Overfill 10 40 20
3 × Overfill 10 60 20
a. Maximum injecti on volume is approxi mately 0.1 µL less th an actua l s ample l oop vol ume. Sampl e
loop volumes are nominal value only; volumes vary slightly from loop to loop.
Heating and trapping module
The heating an d t ra pping module is attached to the sample mana g er an d
serves as its top cover . Th e heating and tr apping module’s column t ray can
accommodate a nano tee and any analytical Waters column up to 300 microns
internal diameter and 250 mm length. The column compartment heats to
temperat u r e s from 5 °C (9 °F) above ambient to 65 °C (149 °F).
a
To reduce dispersion associate d with dead volum e an d minimize the leng t h of
tubing between system modules, the column tray swings outward to any
position between 0° and 180°. In the 0° “home” position, the column tray is
directly above the sample manager and can b e connected to a n optional optical
detector. In the 180° “away” position, the analytical column can be plumbed
into a mass spectrometer (located on the system’s right).
1-9
Page 30

You can select several types of columns for the nanoACQUITY system.
• Analytical columns can have an I.D. of 75 to 300 µm and a l ength o f 10 to
25 cm. A nanoACQUITY BEH C18 column has particles of 1.7 µm.
Alternative particle sizes are available with Symmetry
(3.5 µm) or Atl an t is® dC18 columns (3.0 µm).
• The nanoACQUITY UPLC trap column is 180 µm I.D. × 2 cm long with
Symmetry C18, 5 µm particl e s.
• An ion exchange column of 180 µm I.D. × 2.4 cm length with SCX
material can be used in online 2D-LC techniques.
TUV optical detector
The optional TUV (tunable ultraviolet) optical detector can be used as the
system’s sole detector or in conjunction with a mass spectrometer. A
two-channel, ultraviolet/visible (UV/Vis) absorbance detector, the TUV
detector operates from 190 to 700 nm. Its li ght-guidi ng flow cel l is int ended for
high sensitivity chromatography with high peak capacity. The detector,
controlled by MassLynx software for LC/MS applications, operates as an
integral part of the system.
®
C18 columns
Mass detectors
The nanoACQUITY UPLC™ system acts as a mass spectrometry inlet for
nanoflow rate applications such as proteomics. When coupled with a mass
spectrometer, the system provides sensitive, robust, and reproducible LC/MS
and LC/MS/MS analyses. The system can be configured with an optional
Q-Tof Premier or LCT Premier mass detector.
Q-Tof Premier™
The Waters Q-Tof Premier mass spectrometer is an exact-mass API/MALDI
MS/MS plat form for th e ph armaceut ical, biot ec h n o lo gy, and life science
industries. Designed in conjunction with the nanoACQUITY UPLC system,
the Q-Tof Premier quantifies, identifies, and characterizes compounds from
simple o r co m plex mixtu res.
1-10 System Overview
Page 31

LCT Premier™
The Waters LCT Premier is a benchtop mass spectrometer that uses a high
resolution, orthogonal acceleration (oa), time-of-flight (ToF) design to enable
automated exact mass measurements. The instrument provides information
on elemental composition, structural characteristics (through the use of
in-sour ce collisio n -indu ce d dissocia t io n ), and spec ificity for identifying
compounds in complex matrices or from a database search.
MassLynx data system
The system run s under MassL y nx so ft ware control . MassLynx is a m ass
spectrome t ry application that acquires, a n alyzes, ma n ages, and distributes
UV and mass spectrometry dat a. It o ffe rs intellige n t in st ru ment con t ro l an d
can acquire nominal mass, exact mass, MS/MS, and exact mass MS/MS data.
See also: MassLynx Getting Started Guide and MassLynx Help.
nanoACQUITY UPLC Console
The nanoACQUITY UPLC Console is a software application that replaces the
keypads and small display screens traditionally found on the front of system
hardware. As such, it provides a conv enient way to conf igure setti ngs, monitor
performance, run diagnostic tests, and maintain the system and its modules.
From the software’s Web-like interface, you can quickly navigate to visual
representations of each system module and its components. You can also
navigate to interactive diagrams, which show module interconnections and
provide diagnostic tools for troubleshooting problems.
The nanoACQUITY UPLC Console includes controls for configuring,
monitoring, maintaining, and managing system components. For example,
graphical status indicators monitor and report the real-time use of
components like the detector lamp. The status indicators let you configure
usage thresholds that, when reached, display messages and change the status
indicator’s color. These alerts can help you schedule routine maintenance
before problems occur.
General categories are logically grouped in the menus. The sub-menus,
windows, system tree, and task buttons provide access to system and module
information and functionality.
1-11
Page 32

1-12 System Overview
Page 33

2 Preparing System Hardware
Contents:
Topic Page
Powering-on the system 2-1
Monitoring startup tests 2-2
Monitoring the LEDs of system instruments 2-2
Prepar in g th e au x iliary sol ven t manag e r 2-4
Preparing the binary solvent manager 2-8
Preparing the sample manager 2-12
Preparing the detector 2-21
Conditioning the column 2-23
Shutting down the system 2-24
Powering-on the system
Powering-o n th e syst e m entails starting each system module, the
nanoACQUITY workstation, and the MassLynx software.
1. Press the power switch on the top, left-hand side of each module’s door.
Each modul e be ep s three times and ru ns a series of startup tes ts . F ull
initialization usually requires about seven minutes.
2-1
Page 34

The powe r an d stat u s LEDs chan g e as follows:
• Each LED shows red for a few seconds, except for t h e pow e r LE Ds.
• During initialization, these LEDs flash green:
– Auxiliary solvent manager’s flow LED
– Binary solv e n t manager’ s flow LED
– Sample manager’s run LED
– Detector’s lamp LED
• After the modules are successfully powered-on, each one’s power
LED shows steady green. The auxiliary solvent manager’s and
binary solvent manager’s flow LEDs and the sample manager’s run
LED are unlit. The detector’s lamp LED shows steady green.
2. Power-on the workstation. You can monitor the nanoACQUITY UPLC
Console for m e ssag e s an d visual signa ls.
3. Sta rt M assLynx.
Monitoring startup tests
These startup tests run when you power-on the workstation.
•CPU board
• Memory (RAM and ROM)
• External communication system (Ethernet)
•Clock
Monitoring the LEDs of system instruments
Light emitting diodes (LEDs) on each system module indicate the module’s
state of functioning. The LEDs are specifi c to each module, s o the sign if ica nce
of their various colors and modes can differ from one module to another.
Power LED
The power LED, on the left side of a module’s front panel, indicates when the
module is pow e re d-o n o r powered-off .
2-2 Preparing System Hardware
Page 35

Status LEDs
Flow LED (Auxiliary and binary solvent managers)
The flow LED, to the right of the power LED on the front panels of the
auxiliar y and binary solv ent managers, indicate s the flow status.
Run LED (Sample manager)
The run LED, to the right of the power LED on the sample manager’s front
panel, indicates the run status.
Lamp LED (Detector)
The lamp LED, to the right of the power LED on the detector’s front panel,
indica tes the lamp st atus.
Status LED indications:
LED mode and color Description
Unlit Indicates the module is currently idle.
Constant green Auxiliary and binary solvent managers—
Indicates the solvent manager is operating
normally and solvent is flowing.
Sample manager—Indicates the sample manager
is operating normally, attempting to complete
any outstan ding samples or di agnostic requ e st s.
When sa mple and diagnostic requests are
finished, the LED reverts to the unlit mode.
Detector—Indicates the detector lamp is on and
is operating normally.
Flashing green Sample manager—Indicates the system is
waitin g for at least one module to bec om e
operable. Detector lamp warm-up and column
temperature equilibration times typically cause
such a delay.
Detector—Indicates the detector is initializing or
calibrating.
2-3
Page 36

Status LED indications: (Continued)
LED mode and color Description
Flashing red Indicates an error has stopped the module. Look
at the nanoACQUITY UPLC Console for
information on the error that caused the failure.
Constant red Indicates a module failure that prevents further
operation. Power-off the module, and then
power-on. If the LED is still constant red, contact
your Wat e rs service repre sentative .
Preparing the auxiliary solvent manager
For optimal performance of the nanoACQUITY UPLC system , you must
prepare the solvent manager for operation. Preparing the auxiliary solvent
manager includes
• pr imi ng th e seal wash.
• pri ming the au xiliar y so lvent manager.
Warning: Observe safe laboratory pra ct ic e s wh e n yo u han dle solv e n ts .
See the Material Safety Data Sheets for the solvents you use.
Caution: To prevent salts from precipitating in the system, introduce an
intermediate solvent, such as water, when changing from buffers to
high-organic-content solvents. For details, see the nanoACQUITY UPLC
System Opera tor’s Gui d e.
2-4 Preparing System Hardware
Page 37

Requirements:
• To maintain the efficiency of the auxiliary solvent manager, and to
obtain acc u rat e , re pro d u cible chro matograms, use onl y HPLC-gr ade (o r
higher) quality solvents, water, and additives. For details, see the
nanoACQ U I TY UPLC System Operato r’s Guide.
• Solvent A must be aqueous and solvent B must be organic (acetonitrile
or methanol). The flow control module supports only the solve nts shown
in the Solvent drop-down list of the solvent manager’s instrument
method di al og bo x .
• The nanoACQUITY UPLC System should not be run with high pH
mobile phases. Alkaline solut ions s uc h as ammonium hydrox ide ( pH 10)
can etch glass solvent bottles and the silica capillary tubing, resulting in
an elevated chemical background being detected by mass spectrometry.
For high sensitivity applications, Waters recommends MS-grade eluents. In
our labora tories, Wate rs h as had succes s with Baker water and Fish e r
Optima™ acetonitrile. It is important to flush the system with the appropriate
solvents before passing eluent into the column, optical detector, and/or mass
spectrometer.
Your system is configured with the degassers removed from the fluidic
pathway (by passed). Only the we a k and st ro n g wash solvents ar e de ga sse d.
Priming the seal wash
Prime the seal wash in the auxiliary solvent manager to lubricate the
plungers and flush away solvent and/or any precipitated salts that have
seeped past the plunger seals from the high-pressure side of the piston
chambers.
Prime the plunger seal wash under these conditions:
• After usin g buffere d mo bile phase
• When the solvent manager has been inactive for a few hours or longer
• When the solvent manager is dry
• When troubleshooting a low-pressure error
Caution: To avoid damag e t o the so len o id v al ve se ats and seals in the so lve n t
path, do not use a nonvolatile buffer as the weak wash or strong wash solvent.
Rule: To prevent contamination, do not recycle seal wash.
2-5
Page 38

Recommendations:
• Seal wash should contain no more than 10% organic solvent.
• Before priming the plunger seals, ensure the solvent reservoir contains
sufficient solv e n t fo r pri mi n g and use.
Required materials
• Tubing adapter (startup kit)
• 30-mL syringe (startup kit)
• Seal-wash so lut ion
• Powder-free nitrile gloves
To prime the seal wash:
1. Ensu re t h e sea l-w ash waste line is in a su i t able waste container. Never
recycle seal wash.
Caution: To prevent contamination, wear powder-free nitrile gloves
when handling the solvent filter. Skin oils can contaminate the filter.
2. If the sy st em is dry
a. remove the seal-wash inlet tube from the solvent reservoir, and
disconnect the inlet filter.
b. con n ect the t u bing adapte r t o the sy ringe.
c. fill the syringe with seal-wash solution, and then connect the
syringe assem bly to the seal-wash inlet tub e.
3. In the nanoACQUITY UPLC Console, select Auxiliary Solvent Manager
from the system tree.
4. Click Control > Prime seal wash, and then click Start.
5. If you connected a syringe, push on the syringe plunger to force
seal-wash so lve n t thr oug h the sy st e m.
6. Click C o n t ro l > Prime seal wash wh e n t h e sea l-wash solven t flows from
the seal-wash tube to stop the priming process.
7. Remove the syringe and adapter, reconnect the filter, and place the
seal-wash in let tube int o t h e seal-wash sol ven t re se rvoir.
2-6 Preparing System Hardware
Page 39

Priming the auxiliary solvent manager
Priming, a timed operation, replaces solvent in the path from the reservoir to
the auxiliary s o lvent man ager. During pr iming, th e vent valve move s to Vent
position to ensure minimal backpressure.
Prime the auxiliary solvent manager when performing these tasks:
• Changing reservoirs or solvents
• Preparing a new system or auxiliary solvent manager for use
• Running the system after it has been idle for more than four hours
To prime the auxiliary solvent manager:
1. In the nanoACQUITY UPLC Console, select Auxiliary Solvent Manager
from the system tree.
2. Click Control > Prime A/B Solvents.
Prime A/B Solvents dialog box:
3. Sel e ct so lv e n t B, an d then sele ct B 1.
4. In th e Time box, sp ec ify the num be r o f minutes fro m 0.10 thr ough
999.99. T h e default val u e i s 1. 0 minute.
Recommendation: 2.0 minutes.
5. Cli ck S t art .
6. When finished, repeat as needed for B1 and for all solvents in use.
2-7
Page 40

Preparing the binary solvent manager
For optimal performance of the nanoACQUITY UPLC system , you must
prepare the solvent manager for operation. Preparing the solvent manager
includes these tasks:
•Priming the seal wash
• Priming the binary solvent manager
Warning: Observe safe laboratory pra ct ic e s wh e n yo u han dle solv e n ts .
See the Material Safety Data Sheets for the solvents you use.
Requirements:
• To maintain the efficiency of the binary solvent manager, and to obtain
accurate, reproducible chromatograms, use only HPLC-grade (or higher)
quality s o lvents, w ater, and additive s. For details, see the
nanoACQ U I TY UPLC System Operato r’s Guide.
• Solvent A must be aqueous and solvent B must be organic (acetonitrile
or methanol).
• The flow control module supports only the solvents shown in the Solvent
drop-down list of the solvent manager’s instrument method dialog box.
Priming the seal wash
Prime the seal wash in the binary solvent manager to lubricate the plungers
and flush away solvent and/or any precipitat ed salts that have seeped past the
plunger seals fro m the high-p re ssu re si de of the piston cham be rs.
Prime the plunger seal wash under these conditions:
• After usin g buffere d mo bile phase
• When the binary solvent manager has been inactive for a few hours or
longer
• When the binary solvent manager is dry
• When troubleshooting a low-pressure error
Caution: To avoid damag e t o the so len o id v al ve se ats and seals in the so lve n t
path, do not use a nonvolatile buffer as the weak wash or strong wash solvent.
Rule: To prevent contamination, do not recycle seal wash.
2-8 Preparing System Hardware
Page 41

Recommendations:
• Seal wash should contain no more than 10% organic solvent.
• Before priming the plunger seals, ensure the solvent reservoir contains
sufficient solv e n t fo r pri mi n g and use.
Required materials
• Tubing adapter (startup kit)
• 30-mL syringe (startup kit)
• Seal-wash so lut ion
• Powder-free nitrile gloves
To prime the seal wash:
1. Ensu re th e sea l-w ash waste line is in a suitab le waste container. Never
recycle seal wash.
Caution: To prevent contamination, wear powder-free nitrile gloves
when handling the solvent filter. Skin oils can contaminate the filter.
2. If the sy st em is dry
a. remove the seal-wash inlet tube from the solvent reservoir, and
disconnect the inlet filter.
b. con n ect the t u bing adapte r t o the sy ringe.
c. fill the syringe with seal-wash solution, and then connect the
syringe assem bly to the seal-wash inlet tub e.
3. In the nanoACQUITY UPLC Console, select Binary Solvent Manager
from the system tree.
4. Click Control > Prime seal wash, and then click Start to begin the
seal-wash priming process.
5. If you connected a priming syringe, push on the syringe plunger to force
seal-wash so lve n t thr oug h the sy st e m.
6. When the seal-wash solvent flows from the seal-wash waste tube, click
Control > Prime seal wash to stop the priming process.
7. Remove the syringe and adapter, reconnect the filter, and place the
seal-wash in let tube int o t h e seal-wash sol ven t re se rvoir.
2-9
Page 42

Priming the binary solvent manager
Priming, a timed operation, replaces solvent in the path from the reservoirs to
the vent valve, not including the flow control module. During priming, the
vent valve moves to Vent position to ensure minimal backpressure. The flow
rate dur in g priming is 8 m L/ min tota l (4 mL/min each fo r pu mps A and B).
Prime the binary solvent manager when performing these tasks:
• Changing reservoirs or solvents
• Preparing a new system or binary solvent manager for use
• Running the system after it has been idle for more than four hours
Caution: To prevent salts from precipitating in the system, introduce an
intermediate solvent, such as water, when changing from buffers to
high-organic-content solvents. For details, see the nanoACQUITY UPLC
System Opera tor’s Gui d e.
Recommendations:
• Whenev e r y ou ch ange solve n t s, alw ays purge and autozero t h e flo w
control module (see the nanoACQUIT Y UP LC S ys tem Ope ra tor ’s Gu ide ).
• Ensure the solvent reservoirs contain enough solvent for adequate
priming and use, and ensure the waste container has suffic ient capa city
for used solvent. The priming flow rate is 4 mL/min for each pump, or
8 mL/min t ot al, so priming bot h solve n ts f or 5 minutes requir e s
approximately 20 mL of each solvent.
To prime the binary solvent manager:
1. In the nanoACQUITY UPLC Console, select Binary Solvent Manager
from the system tree.
2. Click Control > Prime A/B Solvents.
2-10 Preparing System Hardware
Page 43

Prime A/B Solvents dialog box:
3. Select solvent A1 or B1.
4. In the Time (min) box, specify the number of minutes from 0.10 through
999.99. T h e default val u e i s 1. 0 minute.
Recommendation: 2.0 minutes.
5. Cli ck S t art .
6. When finished, repeat as needed for B1 and for all solvents in use.
2-11
Page 44

Preparing the sample manager
Prepare the sample manager for operation after you prepare the solvent
managers. Preparing the sample manager involves these steps:
• Priming the sample and w ash syringes
• Characterizing the seal
• Characterizing the needle and sample loop volumes
• Loading sample plates
Caution: To avoi d s o lvent sp ills and m aintain p roper le ak drain a ge, alw ays
close the sample manager fluidics tray before operating the system.
Tip: For venting purposes, the sample manager and sample organize r fans are
always operating.
Warning: To avoid electric shock, power-off and unplug the sample
manager before performing any maintenance operation such as
replacing fu se s.
Selecting weak and strong wash solvents
Tip: For best performance, follow these guidelines when selecting wash
solvents. Otherwise, performance can be reduced, specifically Area/Height
RSD and Lin e arity. Th is do e s n o t m e an that all o ther solv ent combi n ations
are prohibited. Other combinations can be run with lower performance
expectations or by manipulating default injection parameters.
Use a weak wash solvent based on the sample and mobil e phase chemistr ies of
your appl ication, making sure all solution s and buffers are misc ible and
soluble.
Caution: The nanoACQUITY UPLC System should not be run with high pH
mobile phases. Alkaline solutions such as ammonium hydroxide (pH 10) can
etch glass sol ven t bo ttl e s an d th e sil ica capillary t u bi n g, re su lt i n g in an
elevated chemical background being detected by mass spectrometry.
2-12 Preparing System Hardware
Page 45

Suggested wash solvents:
• Strong wash solv e nt —50 to 100% acetonitrile/water or
methanol/acetonitrile (with 0.1% formic acid)
• Weak wash solven t—100% water, or 0 to 25% acetonitrile or methanol
(with 0.1% formic acid); initial conditions of the gradient or isocratic
conditions. High sample concentrations can require other weak wash
solvents. For best results, weak wash solvent should be equivalent to the
– mobile phase composition (for isocratic separations).
– initial gradient condition (for gradient separations).
Caution: To avoid damag e t o the so len o id v al ve se ats and seals in the so lve n t
path, do not use a nonvolatile buffer as the weak wash or strong wash solvent.
Tip: For best performance, the weak wash solvent should be similar or
identi cal to you r isocratic or initial grad ie nt solv e n t co n dition s, excludi ng
buffers. Do n ot us e sa lt bu ffers in wash solv e n t s.
Wash solvent effects:
Property Effect
Organi c spe cies A s a genera l principle, st ro n g a n d we ak solvent s sh o u ld
include the same organic species. This might not always
be pra cticable , es pe cially in t h e case of “s ti ck y ” samples.
You can, however, use a 100% organic strong wash
solvent.
Solvent
composition
The weak wash solvent should reflect as closely as
possible the same composition as the initial gradient
mobile phase.
pH Adjust the pH of strong and weak solvents for best peak
shape and carryov e r pe rformance .
Concentration of
strong solvent
Strong so lv e nt sho u ld be no stronge r t han t h e
concentration needed to reduce carryover to an acceptable
level.
Solubili t y of
analyt e an d
sample
The matrix must be soluble in both the weak and strong
wash solven t s.
Caution: Proteins (in plasma, fo r example) are not soluble
in solvents with less than 40% organic content.
Preparing the sample manager 2-13
Page 46

Wash solvent effects: (Continued)
Property Effect
Sample diluent The weak wash solvent can contact the sample, so match
the weak wash solvent and sample matrix as closely as
possible.
To offset adverse effects on peak shape caused by the
matr ix’s comp o s ition, a djust the weak wash composition
when us in g the module in partial loop mode.
Wash volume
ratio (weak to
strong)
Cycle times Higher viscosity wash solvents lengthen wash cycles. For
Within a method, this should be about 3:1, weak wash to
strong wash, su ffi cie nt t o ensure the weak was h flus he s
the strong from the needle and sample loop.
high-throughput work (cycle times <
times accordingly to accommodate the longer wash cycles.
Priming the sample manager
During the priming process the sample needle fills with solvent, the solvent
changes, and/or air is purged from the lines. You prime the sample needle
and/or sample syringe to accomplish these tasks:
• Prepare the sample manager for operation
• Rinse the internal needle, the ext ernal pierci ng needle, and the injection
port
• Remove bubbles from the lines
Guidelines: Ensure that the pr iming solv ent is c orrec tl y compos ed and that it
is high in qu ality an d miscib le with th e o ther solve n t s . U s e filters in all
solvent reservoirs. Ensure the volumes of solvents are sufficient for priming.
Caution: Allow the priming sequence to finish. Stopping the priming sequence
can leave strong solvent in the needle, which can affect the chromatography.
1 min), adj u s t cycle
Requirement: The sample manager must be primed before you attempt to
characterize the seal.
2-14 Preparing System Hardware
Page 47

To prime the sample manager:
1. In the nanoACQUITY UPLC Console, select Sample Manager from the
system tree.
2. Click Control > Prime syringes.
Alternative: Right-click in the MassLynx sample manager control panel,
and then click Prime syringes.
Prime Syringes dialog box:
3. Sele ct S ample syringe and wash syrin ges .
4. Type the number of primes in the “Number of cycles” text box. The
default value is 1.
Recommendation: Waters recomme n ds 5 t o 7 cycles wh e n yo u are
changing so lvents.
5. Cli ck OK.
Tip: Each prime takes approximately 2 to 4 minutes.
6. When the system status is Idle, priming is finished. Click Close.
Washing the sample manager needle
Washing the needle is an optional procedure that flushes strong and/or weak
wash solvent through the needle and injection port. Washing the sample
manager n e e d le re moves cont aminants fro m its inner and outer surfaces an d
from the external piercing needle and injection port. You can also perform a
needle wash to ascertain proper flow throug h the waste tub ing and to c onfir m
that the needle wash system is primed and properly operating.
Preparing the sample manager 2-15
Page 48

Tip: Priming the system washes the sample needle, so whenever you prime
the system, you can omit this procedure.
Recommendations:
• Do not use buffered solvents as wash solvents.
• Match the types of organic species in the wash solvents to those in the
chromato graphic a ppl ication.
Example: If the weak wash solvent is 30% acetonitrile and 70% water,
the strong wash solvent should contain a greater concentration of
acetonitrile in water.
• To ensure t hat the st ron g wash solvent is complete ly remo v e d, the
system washes the needle with 200 µL of weak wash solvent after you
use strong wash solvent. You can increase, but not decrease, the default
value of 200 µL.
• Do not in t errupt th e priming sequen ce; wait u ntil it fin ishes.
Before you begin, ensure that the solvents are compatible with your
applicati o n, t h at their volume s are sufficien t , an d t h at t h e w ast e rese rvoir is
large enough to contain the waste solvent.
To wash the sample manager needle:
1. In the nanoACQUITY UPLC Console, select Sample Manager from the
system tree.
2. Click Control > Wash Needle.
Wash Needle dialog box:
3. In the Strong Wash box, specify the volume for the strong wash solvent.
The default value is 0.0 µL. To omit strong wash solvent enter 0 in the
Strong Wash box, or leave it blank.
2-16 Preparing System Hardware
Page 49

Tip: Using both a we ak a nd s tr ong wash so lve nt inc r ease s t he w ash ti me
and solvent consumption because the system must be fully cleansed of
the strong solvent before starting the next injection.
4. In the Weak Wash box, specify the volume for the weak wash solvent.
The default value is 200.0 µL.
Caution: If you do not use a sufficient quantity of weak wash solvent,
the strong wash solvent ca n co n t act th e sample and co nta minate it.
5. Cli ck OK. The ne edle wash be g in s .
6. When washing is complete, the status returns to Idle. Click Close.
To stop a needle wash routine before it finishes:
From the sample manager information window, click Control > Reset SM.
Characterizing the needle seal
The seal calibration procedure fi nds the position at which the needle obtai ns a
seal within the wash station bl o ck. Th e sample man ag e r mu st be primed
before starting this procedure.
Requirements:
• Perform this procedure before calibrating the needle and sample loop
volumes.
• Perform this procedure after priming the sample manager or after you
replace and/or adjust these items:
• The needle
• Any part of the needle assembly
• The needle (Z) or piercing needle (Zp) flags (home and top-of-plate)
• A home or top-of-plate sensor
•The seal mechanism
• The NVRam battery on the CPU2000
Preparing the sample manager 2-17
Page 50

To characterize the needle seal:
1. In the nanoACQUITY UPLC Console, select Sample Manager from the
system tree.
2. Cli ck M aintain > Ch aracteriz e > Ne e dle seal.
3. In the Chara ct e rize Need le Seal dialo g box, click Start. T h e calibrat e
seal operation begins, and the sample manager status displays
“Calibrating seal.”
4. Wh e n calibration e n ds, the sample manager stat u s displays “Idle.”
5. Click Re su lts to vi ew t h e nee dl e seal characterization results.
6. Click Close.
Characterizing the needle and sample loop volumes
Whenever you replace the sample loop and/or the sample needle, you must
instruct the system to characterize the volume of the replacement parts. Do
this regardless of whether the sizes of the replacement parts are nominally
the same as those of the original parts or differ from them. Also perform this
procedure when the composition of the weak wash solvent changes.
• Characterizing the loop volume compares the loop’s nominal volume to
its meas u red volu me.
• Characterizing the needle volume compares the needle’s nominal
volume, 15.0 µL, to its measured volume.
Tip: C h aracteri z in g th e system volume is cr it ical to acce p t able sample
manager pe rformance.
Requirements:
• Before characterizing the vol umes, prime the sample manager, and then
characterize the seal.
• Perform a method setup (MassLynx) with any method that has the same
air gap and sample draw r ate t h at you will be using.
Tip: This procedure takes approximately 15 minutes.
To characterize the needle and sample loop volumes:
1. In the nanoACQUITY UPLC Console, select Sample Manager from the
system tree.
2-18 Preparing System Hardware
Page 51

2. Click Maintain > Characterize > Needle and loop volumes.
3. In th e C h aracteriz e Nee dle and L oop Volumes dialog box , click Start.
4. Click Results.
• If the needle fails the test, check all fittings for leaks. Also, check
the needle to see if it is bent, broken, or blocked.
• If the sample loop fails the test, the syringe draw (aspiration) rate
used for the test might be too high. Also examine all fittings for
leaks, or determine whether the loop is blocked or leaking.
• Check the config uration and make s ure the needle a nd loop s izes are
correct.
5. Click Close.
Loading sample plates in the sample manager
The sample manager holds up to two ANSI/SBS plates, which you load
through the front door. The left plate is refer red to a s position 1, the ri ght one
as position 2.
The nanoACQUITY UPLC Sample Manager (SM) supports ANSI sample
plates and vial holders only. The Sample Manager (SM) does not support vial
plates with cap mats or vials without pre-slit septa.
To load a sample plate:
1. Open the sample manager door.
2. Squeeze the tray button as you pull the tray toward you.
3. Load the plate onto the tray so that position A,1 is at the right-rear
corner, and the forward edge of the plate is behind the spring inside the
front of the carrier.
Preparing the sample manager 2-19
Page 52

Positioning the sample plate:
Sample plate
A-1 well
position
Button
TP02389
Plate tray
4. Slide the tray into the sample manager until it clicks into place.
5. Close the sample compartment door. A mechanism on the door ensures
the plates are positioned correctly when the door closes.
Caution: The plates must be positioned correctly to avoid damaging the
sample n e e d le .
2-20 Preparing System Hardware
Page 53

Preparing the detector
If your system includes a TUV detector or mass spectrometer, prepare it for
operation by following the procedures in this section.
See also: the documentation included with your mass spectrometer.
Preparing the TUV detector
Preparing the TUV detector involves two steps: starting and verifying.
Starting the detector
Use only thoroughly degassed HPLC-grade solvents. Gas in the mobile phase
can form bubbles in the flow cell, causing the detector to fail the power-up
diagnostic tests.
To start the TUV detector:
1. Ensure the detector flo w cell is fi lled with transparent solvent ( methanol
or water) that it is free of air bubbles, and that the door is closed firmly.
Tip: The detector might not initialize correctly if the flow cell contains
air.
2. Press the power switch on the detector door. The detector beeps three
times and runs a series of startup tests while the lam p L E D blinks.
Initialization requires approximately 2 minutes, and lamp warm-up
requires approximately 3 m in u t e s.
3. When the lamp LED shows constant green, start MassLynx. You can
monitor the nanoACQUITY UPLC Console for messages and visual
signals. For best re su l t s, all ow 30 minutes for the baseline to stabi li ze .
Tip: The absorbance value appears in the nanoACQUITY UPLC Console
and also in the MassLynx Inlet Editor window. If the detector is in dual
wavelength mode, two absorbance values appear.
Absorbance values have a resolution of 0.0001 AU.
When the lamp is extinguished, “Lamp Off” appears instead of
absorban ce values.
4. Configure the detector according to the instructions in the MassLynx
Online H elp.
Preparing the detector 2-21
Page 54

Recording sample and reference energies
After you install the detector or perform maintenance operations, like
changing the lamp or flow cell, complete the procedures in this section to
verify that the detector optics and electronics work properly.
To record sample and reference energies:
1. Ensure that the detector is connected to the workstation.
2. Flush the system tubing with filtered, degassed, HPLC-grade methanol
or acetonitrile.
3. Pump mobil e ph ase fo r 15 minut e s or mo re at 2 µL/min.
Caution: The maximum pressure drop across the flow cell is 1,000 psi. If
the solvent is viscous (methanol/water, for example), you might need to
decrease the maximum flow rate to prevent breaking the cell.
4. Ensure the detector cell is filled with solvent and free of air bubbles.
Tip: The detector might not initialize correctly if air is present in the
cell.
5. When both LEDs show constant green, initialization is complete.
6. Start t h e MassLynx so ftware.
7. To determine baseline v alues on the detector for fut ure re ferenc e, and to
monitor lamp aging (for decreased lamp output energy), record the
baseline sample and reference energies.
Requirement: Perform this procedure each time you change the lamp.
8. Set the wavelength to 230 nm.
9. Flush the flow cell for 15 minutes or more with HPLC-gra de methanol a t
2 µL/min.
10. Record the sample and reference energies at 230 nm.
Preparing the mass spectrometer
Prepare the optional mass spectrometer for operation after you prepare the
sample manager. For specific steps, consult the mass spectrometer’s
accompanying documentation.
To configur e Ma ssL yn x , see MassLynx Online Help.
2-22 Preparing System Hardware
Page 55

Conditioning the column
Conditioning the column involves running a solvent gradient through it
without injecting samples or running the Events table. The run time for
conditioning the column should equal the gradient table run time.
Tips:
• Flush new columns with high organic (up to 85%) for 15 to 20 minutes at
your typical analytical flow rate.
• To ensure best performance, flush with higher organic at the end of each
run.
Caution: To prevent damage to the detector flow cell, ensure that the waste
solvent does not flow through the detector during this procedure. After
installing a new column, flush solvent through it—typically 10
column-volumes—and out to waste before connecting the column to the
detector.
To condition the column:
1. Remove the column inlet line from the detector, and place the line’s end
in a small waste cont ainer.
2. In MassLynx, open the Sample Set window, and select an inlet method
that includes the chromatographic conditions you want to use.
3. In the Samples table, add an inlet prerun field.
a. In the R u n Samples page , sel ect Sa mples > Form at > C u st om i z e.
b. In the Custom Field Display window, select Inlet prerun.
c. To save the column as part of the window, save the sample set
format.
4. Select method setup in the Sample Set window as a pre-inlet method,
and select a method for the inlet file (these methods can be the same).
5. Run the sample set line. The system runs the condition column method
and then runs the separation method.
Preparing the detector 2-23
Page 56

Shutting down the system
Caution: Buffers left in the system can precipitate, damaging module
components.
You might want to shut down the system
• between analyses
•overnight
• for a weekend
• for 72 hours or more
Tip: Set system shu t down paramete rs in the MassLynx Shutdow n Ed ito r.
Consult the MassLynx Online Help for more information.
Between analyses
To shut down the system between analyses:
1. Between analyses, continue to pump the initial mobile phase mixture
through the column to maintain the column equilibrium necessary for
good retention-time reproducibility.
2. If a few hours will pass before the next injection, slow the flow rate in
the interim to a few tenths of a µL/min to conserve solvent.
Tip: Ensure that Auto-Shutdown for your shutdown method is
deactivated.
3. Keep the detector operating, and the heating and trapping module at
operating temperature, during this period.
Overnight or weekends
To shut down the system overnight or over a weekend:
1. Flush the col u mn with a 1:1 m ixture of me t h an ol/acet o nit rile. This
keeps the column bed in an active, wetted state.
Requirement: If you are using buffers, you must first flush the column
with a high-water-content mobile phase (90% water). Then stop the
pump flow.
2. Power-off the detector to lengthen lamp life.
2-24 Preparing System Hardware
Page 57

3. The heating and trapping module can operate overnight but should be
shut down over the weekend.
Warning: To prevent injury, set the power switch to off, and then
unplug its power cord from the AC outlet to completely interrupt power
to a system module.
The power switch o n ea ch sy st em module co n t ro ls the basic
operational stat e of that module. Neverthel es s, a p or ti on of the module
remains powered-on after the module is switched off.
More than 72 hours (long-term)
To shut down the system long-term or indefinitely:
1. Follow the preceding procedure for overnight or weekend shutdown.
2. After flushing the column and letting it cool to ambient temperature,
disconnect the inlet and outlet tubes, and join them with a union.
Recommendation: You s hould flush high-ionic b uf f ers not only from the
column but also from the system. Flushing the column with
methanol/acetonitr ile lea ves tha t solv ent in the flui d lines of the sol vent
manager, sample manager, and detector, eliminating the risk of solute
precipitation.
3. Pump water through the system for 10 to 20 minutes at 10 µL/min,
followed by iso propyl alcohol for anot her 10 to 20 minutes. Then turn the
pump o ff, le aving iso propyl alcohol in the fluid lines.
Caution: If any system modules are to be used for another type of
analys is, ensure t h at the liquids pumped ini t ially thr ough the system
are miscible with methanol, water, methanol/acetonitrile, or isopropyl
alcohol. Likewise, before restarting the system, ensure that any residual
material not miscible with the initial methanol/water mobile phase has
been flushed thoroughly from the system with an appropriate
intermediate solvent.
Shutting down the system 2-25
Page 58

2-26 Preparing System Hardware
Page 59

3 Configuring System Software
Contents:
Topic Page
Configuring MassLynx 3-1
Starting the nanoACQUITY UPLC Console from MassLynx 3-5
Configuring events 3-6
Configuring MassLynx
Requirements:
• MassLyn x software must be inst alled.
• You must be assigned administrator privileges to configure the
Instrument Control Option Pack (ICOP).
You must star t the application an d se lect sy st em modules to con fig ure
MassLynx:
To start MassLynx:
1. Sele ct S ta rt > All Programs > MassLyn x > M a ssLynx V4.1.
Alternative: You can also double-click the MassLynx desktop shortcut.
If MassLynx S e curity is not enable d, Ma ssL yn x st art s a n d the
MassLynx window appears. If MassLynx Security is enabled, the
MassLy n x L o gin dialog box appears.
2. In the MassLynx Login dialog box, type your user name and password,
and select you r domain.
3. Cli ck OK.
3-1
Page 60

To select modules in the nanoACQUITY system:
1. In the MassLynx window, click Inlet Method.
Inlet Method window:
2. Select Tools > Instrument Configuration.
3-2 Configuring System Software
Page 61

Inlet Configuration window:
3. Click Configure.
4. In the Welcome screen of the Inlet Configuration wizard, click Next.
5. In th e Se l e ct Pu mp dialog box, select Waters A cquity, and t h en click
Next.
6. In the Select Auto Sampler dialog box, select Waters Acquity, and then
click Nex t .
7. In the Select Detector dialog box, select Waters Acquity TUV as the
detector if present, and then click Next.
8. Click Next > Finish > Finish.
To install the Instrument Control Option Pack:
1. Click OK to start the Instrument Control Option Pack installation.
2. Ensure that “Install new instrument software, or upgrade existing
installation(s)” is sele ct ed, and then click N e xt.
3-3
Page 62

Instrument Control Option Pack dialog box (Select window):
3. Select ACQUITY Binary Sol vent Manager, ACQUITY Sample Ma nager ,
and ACQUITY TUV Detector, and then click Next. A progress bar
appears at the bo t t o m o f t h e dial o g bo x.
Requirement: You must select ACQUITY TUV Detector even if your
system does not include a TUV detector. If you do not do so, the control
will be blank when you open the nanoACQUITY UPLC Console.
4. In the Results screen of the Instrument Control Option Pack dialog box,
click Finish.
Result: The Inlet Method window appears.
3-4 Configuring System Software
Page 63

Starting the nanoACQUITY UPLC Console from MassLynx
The nanoACQUITY UPLC Console is a software application that replaces the
keypads and small display screens traditionally found on the fronts of system
instruments. It provides a convenient way to configure settings, monitor
performan ce, ru n dia gn ost ic tests, and mai n tain the system.
To start the nanoACQUITY UPLC Console from MassLynx:
1. In the MassLynx window, click Inlet Method.
2. Click the ACQUITY Additional Status tab.
ACQUITY Additional Status tab:
3. Click Display console .
Result: The nanoACQUITY UPLC Console window appears.
Starting the nanoACQUITY UPLC Console from MassLynx 3-5
Page 64

Configuring events
Besides making the signal connections, you must configure the events in
MassLynx.
To configure events:
1. In the MassLynx window, click Inlet Method.
2. Select Tools > Instrument Configuration.
3. Click Events & Triggering, and then click Next.
4. Select the check boxes that correspond to the event in and event out
connections you made to the mass spectrometer.
Example: If you co n n e ct e d t h e in ject st art terminals on the sa mple
manager co n n e ct o r t o the n u mb e r 1 E vent I/P con n e ct o r o n the ma ss
spectrometer, select box 1 in the Event In section.
Choose Events dialog box:
5. Click Next. In the Configuration Successful window, click Finish.
3-6 Configuring System Software
Page 65

4 Verifying System Operation
Contents:
Topic Page
Required materials 4-1
Preparing the mobile phases 4-2
Preparing the sample 4-3
Preparing the system 4-4
Creating the test methods 4-6
Performing the test 4-10
This chapter explains how to run a gradient performance test to v erify that
the system is operating properly. The sample you use to verify the system is
included in the system startup kit.
Before you begin this procedure, the system must be set up and configured as
described in the nanoACQUITY UPLC System Operator’s Guide.
Required materials
Make sure these materials are on hand before you begin the verific ation test:
•MS-grade water
• aceton itrile (W aters re co mmend s Fisher Sc ientific’s Optima
• MS-grade formic acid
• MassPREP™ Peptide Standard (system startup kit)
• nanoACQUITY BEH C18 analytical column (1.7 µm , 10 0 µm × 100 mm)
• nanoACQUITY Symmetry C18 trap colum n (5 µm, 180 µm × 20 mm)
• powder-free nitrile gloves
®
brand)
4-1
Page 66

Requirement: To prevent contamination, always use powder-free nitrile
gloves when handling com ponents of the nanoACQUITY system.
Caution: Never change directly between immiscible eluents or between
buffered solutions and organic eluents. Immiscible eluents form emulsions in
the flow path. Combini ng buffered solutions and organic eluents can result in
salt precipitation in the gradient proportioning valves, pump heads, check
valves, or other parts of the system. Confirm that all fluids in the system are
miscible with acetonitrile.
Preparing the mobile phases
The verification test requires two mobile phases: 0.1% formic acid/water for
solvent A and 0.1% formic acid/acetonitrile for solvent B. T he test also
requires w ea k wash and str ong wash.
Requirement: All solvents must be HPLC-grade or better.
To prepare solvent A, 0.1% formic acid/water:
1. Measure 100 mL of HPLC-grade water in a 100-mL graduated cylinder.
2. Carefully transfer the water to a 250-mL reservoir bottle.
3. Pipe t t e 1 00 µL of formic acid int o the re se rvoir bottle.
4. Cap the reservoir bottle and mix well.
5. La be l the re se rvoir bottl e a s 0.1% formic acid/wate r.
6. Submerge lines A1, Seal Wash, Weak Wash, and Strong Wash in the
solvent A reservoir bottle with 0.1% formic acid/water.
7. Place the solvent A reservoir bottle in the solvent tray.
To prepare solvent B, 0.1% formic acid/acetonitrile:
1. Me asure 100 mL of acetonitrile in a 100-mL graduated cylinde r.
2. Carefully t ransfer t h e a ce t o n it rile to a 250-mL reservoir bottle.
3. Pipe t t e 1 00 µL of formic acid int o the re se rvoir bottle.
4. Cap the reservoir bottle and mix well.
5. La be l the re servoir bo ttle as 0.1% formic acid/ acetonitr ile.
4-2 Verifying System Operation
Page 67

6. Submerge the B1 line in the solvent B reservoir bottle with 0.1% formic
acid/acetonitrile.
7. Place the solvent B reservoir bottle in the solvent tray.
Preparing the sample
The verification test uses the MassPREP Peptide Standard to verify that your
system is operati ng correct ly. The mixt ure incl udes nine pepti des with a broad
range of polarities and isoelectric points. In addition, there is a void volume
marker. The following ta ble lists the components of the peptide mixture, in
elution order, and their molecular weights.
MassPREP peptides mixture components:
Peptide
MW,
monoisotopic
(g/mol)
M + 2H M + 3H
Allantoin (V
RASG-1 (not trapped) 1000.4938
Angiot e n s i n fr ag. 1-7 898.4661 450.2401 300.4957
Bradykinin 1059.5613 530.7877 354.1941
Angiot e n s in I I 1045.5345 523.7743 349.5185
Angiotensin I 1295.6775 648.8458 432.8995
Renin substrate 1757.9253 879.9697 586.9821
Enolase T35 1871.9604 936.9872 624.9938
Enolase T37 2827.2806 1414.6473 943.4339
Melitin 2845.7381 1423.8761 949.5864
Tip: Because Enolase T35, Enolase T37, and Melitin are weaker ionizers in
ESI, they might not b e detected.
The peptide mixture c ontains approximately 1.5 µg (~1 nmole) of each peptide.
When reconstituted in 1 m L of solvent (95:5 water/acetonitrile with 0.1%
formic a cid), the fi n al s ample co n ce ntration is ~1 pmole/µL.
marker) 158.0440
0
4-3
Page 68

To prepare the sample:
1. Pipet te 1.0 mL (0.95 mL of 0. 1% fo r mic acid/wate r you mi xed in the
previous se ct ion and 0.050 mL of 0.1% formi c acid/acet onitrile mo bi le
phase) into the vial containing the peptides mixture.
2. Cap the sample via l, an d v o rt e x-m ix the sample for 10 seconds.
3. Pipette 1.0 mL of 0.1% formic acid/water in t o an e mp t y vial, and then
cap the vial. The mobile phase blank will be injected from this vial.
4. Place the sample vials in the v ial plate, no ting the vial p ositions, a nd put
the plate in position 2 of the sample manager.
The peptides solution is stable for one week when stored at 8 ºC. You can
extend stabilit y by freezi n g t h e sample at -20 ºC. However, fo r best res ults,
you should run the ver ification test immediately after preparing the sample.
Caution: The sample degrades rapidly when contaminated with
endopeptidase or exopeptidase enzymes.
Preparing the system
Warning: Always observ e s afe laborato ry practices w hen you use this
equipment and when you work with solvents and test solutions. Know
the chemical and physical properties of the solvents and test solutions
you use. See the Material Safety Data Sheet for each solvent and test
solution in use.
Recommendation: Flush the system with the appropriate solvents before
passing eluent into the column, optical detector, and/or mass spectrometer.
To prepare the system for verification:
1. Connect and install the trap column.
2. Install the analytical column in the heating and trapping module, and
then connect the column outlet to a suitable waste container.
3. In the nanoACQUITY UPLC Console, perform these tasks if you have
not already done so:
a. Prime the A1 and B1 solvent lines for 5 minutes.
b. Prime the seal wash pump for one minute.
4-4 Verifying System Operation
Page 69

c. Prime the sample and wa sh syringes 20 cycles.
d. Charact e ri z e the system volu me.
Tip: C h aracteri z in g th e system volume is cr it ical to acce p t able sample
manager pe rformance.
4. If either the trap or analytical column are new, flush them as follows.
Duration
(minutes)
Flow rate
(µL/min)
%B solvent
Trap valve
position
20 10 85 Trapping
20 0.4 85 Analytical
10 0.4 50 Analytical
10 0.4 1 Analytical
5. Connect the analytical column outlet to the mass spectrometer inlet.
Exception: If your system includes a TUV detector, connect the column
outlet to the TUV flow cell inlet, a nd then connect the TUV flow cell
outlet to the mass spectrometer inlet.
6. Equilibrate the system wi th initial s t arting con dition s o f t h e gradient
(99% A, 1% B) until the detector baseline is stable.
Tip: To mainta in p ro p e r drainage and leak cont ro l in the nan o A C Q UITY
UPLC system, the SM Fluidics tray must be fully cl osed during routine
operation.
4-5
Page 70

Creating the test methods
Follow the steps below to create the methods, setting the parameter values to
match those reflected in the accompanying screen representations. T his
method is designed as a rapid test procedure implementing a 150-µm ID
column at high flow rate.
Creating the instrument method
To create the instrument method:
1. Create an instrument method with thes e binary solvent manager
parameters.
Exception: If you use the 75 µm ID × 100 mm column, in the gradient
table, enter 0.300 µL/min as the flow rate.
Binary solvent manager instrument parameters (General tab):
4-6 Verifying System Operation
Page 71

Binary solvent manager instrument parameters (Trapping tab):
20.00
1.00
15.000
99.0
1.0
Tip: The instrument Comment field is limited to 500 characters. This ensures
correct spacing when generating reports.
Recommended trapping parameters (with a 5-microliter loop):
•Flow rate: 15 µL/min
• %A: 99.0, %B: 1.0
• Sample loading time (duration): 1 minute
• High pressu re limit: 5000 psi
• The system uses the binary solvent manager as the trapping pump for
one minute of the gradient performance test.
Creating the test methods 4-7
Page 72

2. Set t h e s e parameter s in the samp le manager in st rument me t h o d.
Sample manager instrument parameters:
4-8 Verifying System Operation
Page 73

3. If your system includes a TUV detector, set these parameters in the
TUV detector instrument method.
4. Save the in strument method .
5. Create a sample list composed of six 1-µL injections of the peptides
mixture and one 1-µL i njection of the blank mobile phase.
Tip: The typical column back pressure at 0.400 µL/min is approximately
2300 psi.
Creating the test methods 4-9
Page 74

Performing the test
When the system is prepared and the test methods are created, you are ready
to perform the test.
To perform the test:
1. At th e MassLy nx main pa ge, sel ect Ru n > Sta r t.
2. Verify the settings for the test, and then click OK.
3. Verify these options, afterward cli cking OK for operations, quanti fy, and
LIMS expor t .
4. When the sample list is complete, enter the appropriate results in the
table, below.
Retention Time Reproducibility (Six injections):
Peak Component
Peak Retention Time (six
injections)
123456
Mean
Value
RSD
1 Allantoin (V
0
marker)
2RASG-1
3 Angiotensin frag.
1-7
4Bradykinin
5 Angiotensin II
6 Angiotensin I
7Renin substrate
8Enolase T35
9Enolase T37
10 Melitin
4-10 Verifying System Operation
Page 75

5. Review the gradient performance report. The system verification test
result is “passing” when these conditions are realized:
• The peaks are symmetrical, integrated, and identified correctly.
(Compare the chromatogram on the report to the sample
chromatogram, below, to determine this.)
• The peak retention times show a relative standard deviation (RSD)
of less than 15.0 seconds (0.25 minutes). Use the table you
completed to determine this.
Sample system verification test chromatogram (MS detection):
Angiotension frag 1-7
Angiotensin I
Angiotensin II
Bradykinin
Example: This is a representative chromatogram. The results from your
system can v ary slightly.
Performing the test 4-11
Page 76

4-12 Verifying System Operation
 Loading...
Loading...