Page 1

[ CARE AND USE MANUAL ]
MetID STANDARD MIX
CONTENTS
I. INTRODUCTION
II. STORAGE & STABILITY
III. RECONSTITUTION OF THE STANDARD
SOLUTION FOR TESTING
IV. EXAMPLE OF USING OMEPRAZOLE STANDARD MIX
V. ORDERING INFORMATION
VI. REFERENCES
I. INTRODUCTION
Omeprazole Metabolite Mix is used to test and demonstrate the
Waters UNIFI Application Solution for Metabolite Identification.
The standard was designed to both test and confirm LC and MS
performance but also to test and demonstrate the UNIFI system
solution. Omeprazole is a commonly prescribed medication used to treat
acid reflux causing heartburn (known clinically as gastroesophageal
reflux disease, GERD). When the compound is dosed in vivo in human,
two of the commonly detected metabolites which are formed in
human are 5-hydroxy omeprazole and omeprazole sulfone.
O
N
H
S
O
Omeprazole
O
N
H
S
O
5-Hydroxy Omeprazole
N
N
OH
O
Figure 1. Structures of Omeprazole, 5-Hydroxy Omeprazole, and Omeprazole Sulfone
N
N
O
N
OO
H
S
O
Omeprazole Sulfone
N
N
O
MetID Standard Mix 1
Using Waters ACQUIT Y UPLC® BEH column chemistry, the generic
gradient method can be used to analyze and effectively separate the
standard into its parent and 2 authentic metabolite standard components.
The standard mix stock solution comprises Omeprazole, 5-hydroxy
omeprazole and omeprazole sulfone at concentrations of 10, 1 and
0.1 µg/mL respectively.
II. Storage and Stability.
The lyophilized powder is shipped at ambient temperatures, in brown
amber vials in foil packaging. It is highly recommended that upon
receipt, the standard should be stored frozen and thawed before use.
Once reconstituted the sample should be used immediately for
best results. Omeprazole has been reported to be unstable in the
presence of continuous exposure to UV. Care should be taken to prepare
samples and standard curves out of direct light whenever possible. If
Page 2
[ CARE AND USE MANUAL ]
moderate precautions are taken and the samples, once reconstituted,
are stored properly at -10°C (and analyzed within an autosampler
set to 10°C or lower) the standard is typically stable for at least one
week. Beyond this time for performance testing and evaluation, fresh
re-preparation of the standard is recommended.
III. Reconstitution of the standard solution for testing
1. To the vial of Lyophilized Waters MetID Standard Mix, add 1 mL
of Methanol.
2. Recap and then agitate the vial contents (e.g. via shaking or
ultrasonic bath), to ensure the lyophilized powder dissolves in
the methanol.
3. This standard can be further diluted as per the UNIFI
Metabolite Identification Test Protocol, which documents
formal standard curve, PDA and UNIFI system performance
1
testing.
IV. Example of Using Omeprazole Standard Mix
V. ORDERING INFORMATION
To order these products, contact your nearest subsidiary, or visit
www. waters.com and click on Order Center.
Description Part number
MetID Standard Mix 186006550
Formic Acid 186006691
VI. References
1
Metabolite Identific ation Application Solution Quick Start P/N 715003759.
2
Maton, P.N., Ome prazole Review Article, N. Engl J Med 1991; 324:965-975.
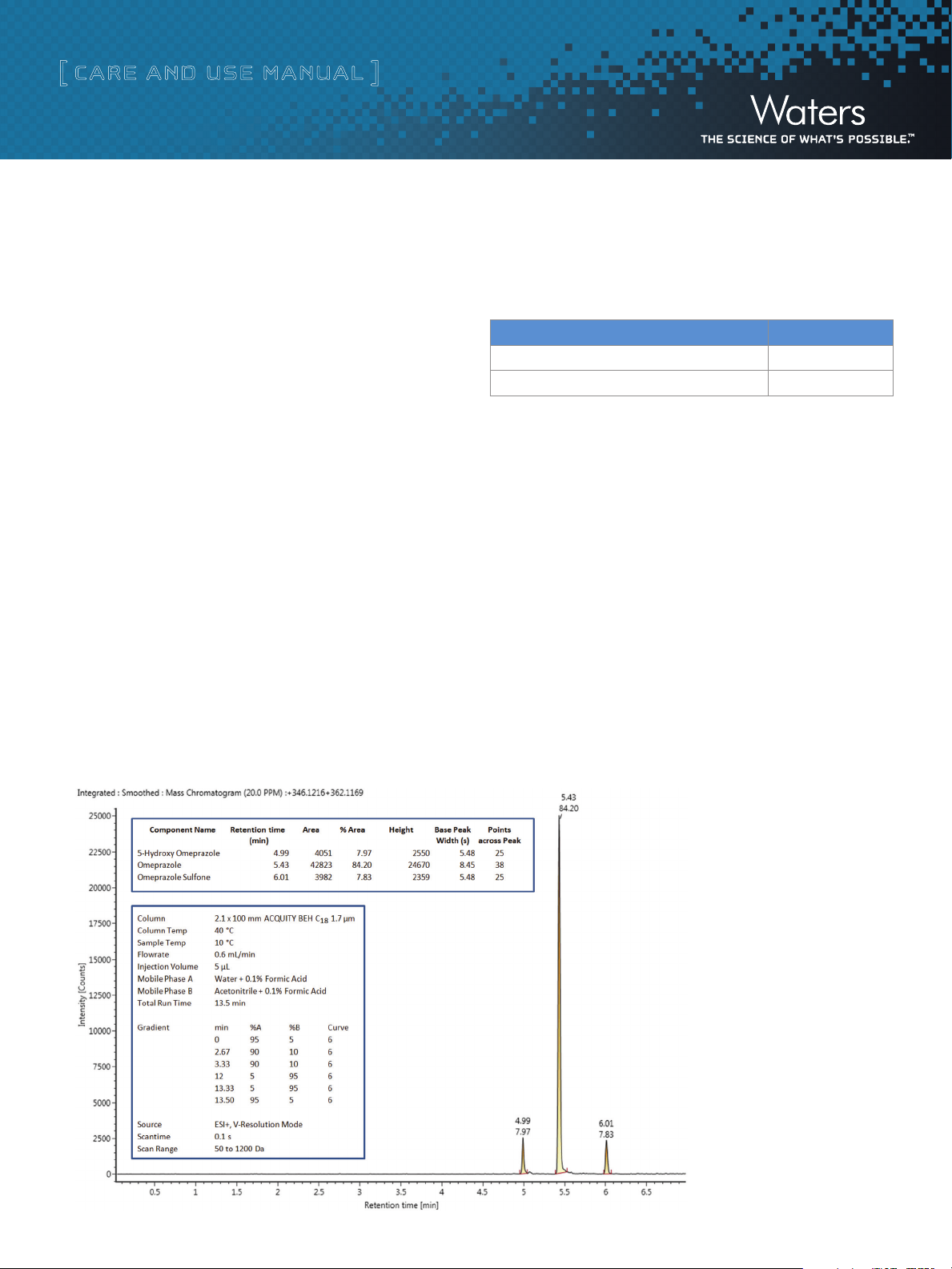
Shown in figure 2 is a representative chromatogram under standard
chromatography conditions.
Figure 2. Omeprazole Standard Mix (33.3 ng/mL) extracted
ion chromatogram acquired on an ACQUITY U PLC I-Class
coupled to a Xevo® G2-S QTof Mass Spectrometer System
and processed using UNIFI Scientific Information System.
MetID Standard Mix 2
Page 3

[ CARE AND USE MANUAL ]
Austria and European Export
(Central South Eastern Europe, CIS
and Middle East) 43 1 877 18 07
Australia 61 2 9933 1777
Belgium 32 2 726 1000
Brazil 55 11 4134 3788
Canada 1 800 252 4752
China 86 21 6156 2666
Czech Republic 420 2 617 11384
Denmark 45 46 59 8080
Finland 358 9 5659 6288
France 33 1 30 48 72 00
Germany 49 6196 400 600
Hong Kong 852 2964 1800
Hungary 36 1 350 5086
Norway 47 6 384 6050
Poland 48 22 101 5900
Puerto Rico 1 787 747 8445
Russia/CIS 7 495 727 4490/ 290 9737
Singapore 65 6593 7100
Spain 34 93 600 9300
Sweden 46 8 555 115 00
Switzerland 41 56 676 7000
Taiwan 886 2 2501 9928
United Kingdom 44 208 238 6100
All other countries:
Waters Corporation U.S.A.
1 508 478 2000
1 800 252 4752
www.waters.com
India 91 80 2837 1900
Ireland 353 1 448 1500
Italy 39 02 265 0983
Japan 81 3 3471 7191
Korea 82 2 6300 4800
Mexico 52 55 52 00 1860
The Netherlands 31 76 508 7200
©2012 Waters Corporation. Waters, ACQUITY U PLC and Xevo
are registered trademarks of Waters Corporation. The Science of
What's Possible is a trademark of Waters Corporation. All ot her
trademarks are the property of their res pective owners.
November 2012 720004501EN Rev A IH-PDF
Waters Corporation
34 Maple Street
Milford, MA 01757 U.S.A.
T: 1 508 478 2000
F: 1 508 872 1990
www.waters.com
3
 Loading...
Loading...