Page 1

[ CARE AND USE MANUAL ]
iKey Separation Device
CONTENTS
I. INTRODUCTION
II. iKEY FEATURES
a. Analytical iKey
b. Accessory iKeys
c. iKey Component Description
III. CONNECTING THE iKEY TO THE
ionKEY SOURCE
IV. iKEY USAGE
a. Sample Preparation
b. Mobile Phases
c. pH Range
d. Pressure
e. Temperature
V. REMOVING THE iKEY FROM THE
ionKEY/MS SYSTEM
VI. CARING FOR THE iKEY
a. Cleaning and Regeneration
b. Storage
c. Good Practices
I. INTRODUCTION
The Waters iKey™ Separation Device is a high-pressure,
microfluidic device that provides UPLC® separations with
the ionKey/MS™ System. The iKey eliminates manual fitting
connections and minimizes the potential for manual variability
and extra-column dispersion.
The iKey contains a ceramic substrate produced using a modified,
co-fired ceramic manufacturing process which contains an
analytical separation channel packed with stationary phase, an
electrospray emitter, a column heater, and an integrated memory
device. The integrated memory maintains the number of injections,
maximum backpressure, and number of sample sets run. A circuit
in the iKey senses and controls temperature as well.
The iKey is manufactured to exacting specifications, providing
outstanding peak symmetry for maximum sensitivity and
accurate quantitation. Each iKey is individually tested to
ensure that it passes stringent quality control requirements.
Compared to conventional chromatographic systems, successful
day-to-day performance of the ionKey/MS System requires
certain considerations. This document provides several essential
recommendations for the successful use of the iKey. The iKey
is the only analytical consumable device compatible with
ionKey/MS System.
VII. TROUBLESHOOTING iKEY PERFORMANCE
VIII. ORDERING INFORMATION
Page 2
[ CARE AND USE MANUAL ]
II. iKEY FEATURES
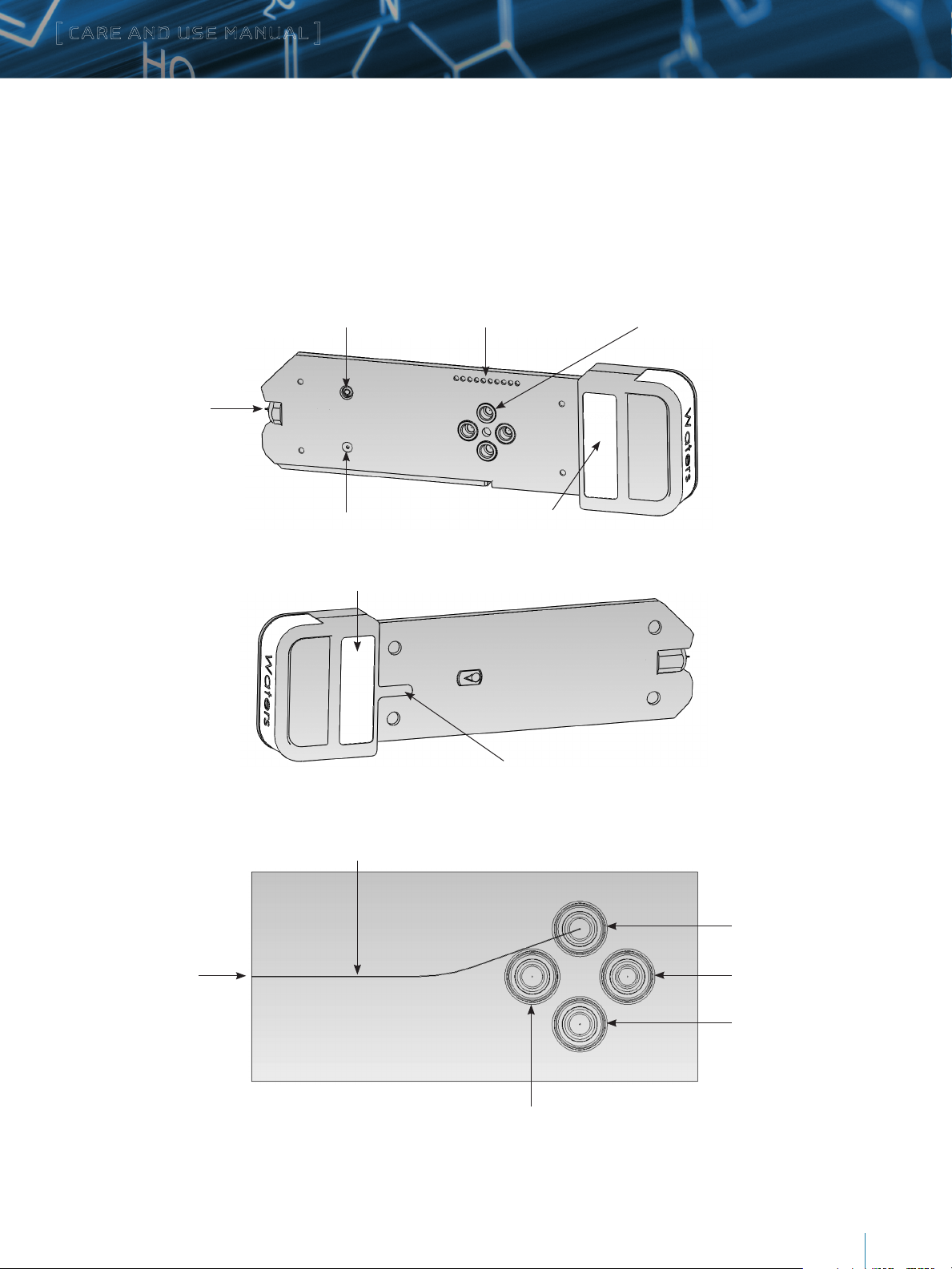
a. Analytical iKey
This iKey has a 150 µm x 50 mm or 150 μm x 100 mm analytical separation channel available with a variety of packing materials.
An electrospray emitter is located at the end of the channel. A sheath is provided to protect the iKey and its emitter from damage
during handling.
Left side of the iKey
Electrospray connection contact Electronic connections Fluidic ports (4)
Electrospray
emitter tip
Right side of the iKey
Fluidic path diagram
Emitter connection port
NanoFlow gas port
Notes label
Flow channel to emitter (50 mm shown, 100 mm iKey also available)
Identification label
Orientation rib
Injection inlet port
Auxiliary port (inactive)
Infusion port (inactive)
Outlet port (inactive)
iKey
2
Page 3
[ CARE AND USE MANUAL ]
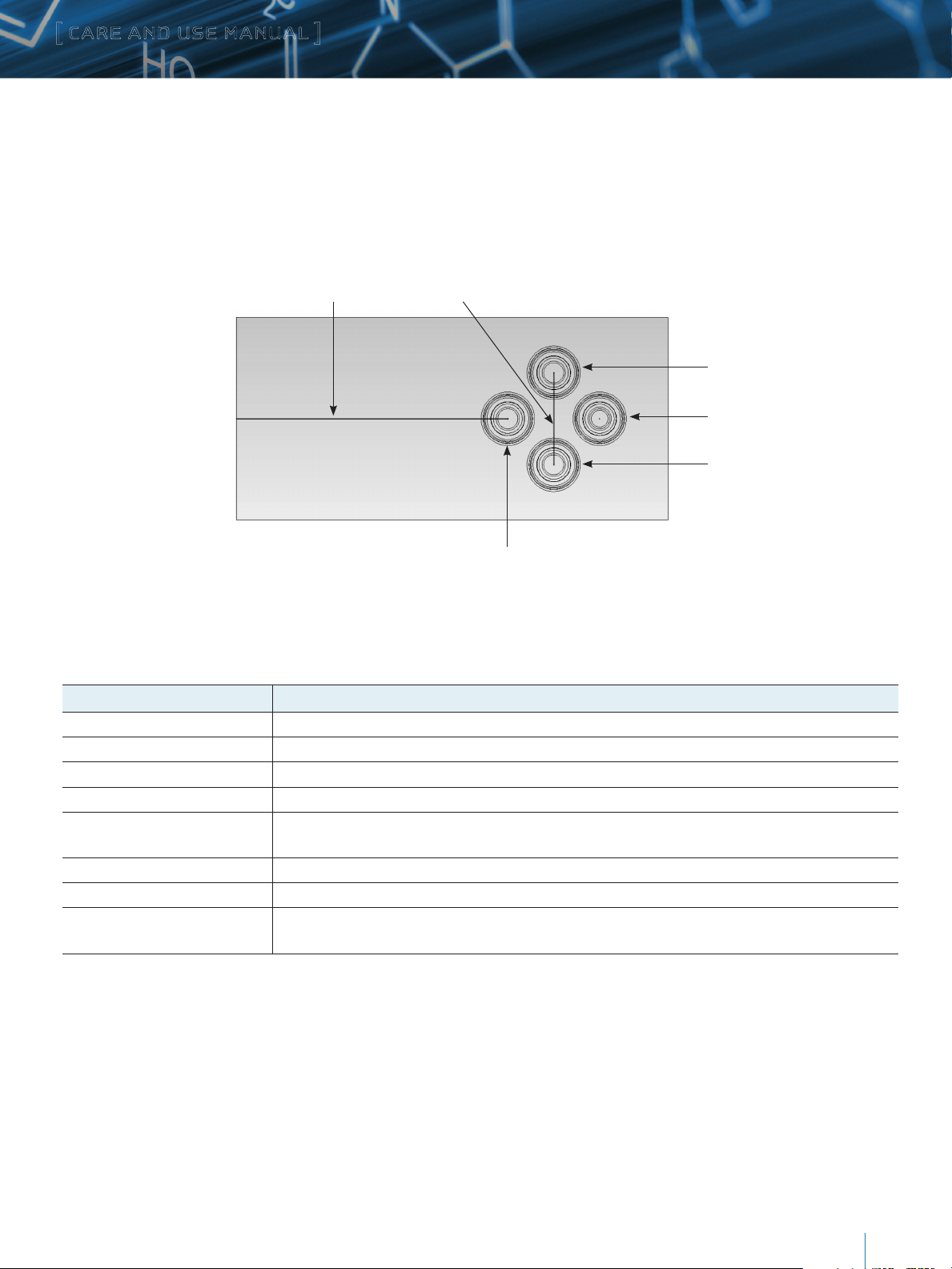
b. Accessory iKeys
Flow Injection iKey: This iKey has no packing material. It enables one to inject a sample from the sample manager directly to the emitter
on the iKey without any chromatographic separation. Flow path is identical to the 50 mm Analytical iKey.
Infusion iKey: This iKey enables calibrant infusion from the ionKey/MS fluidics. Additionally, it can also be used to divert flow to waste
for solvent changeovers and idle flow conditions, when liquid flow into the source is not desired.
Flow channel to emitter Flow channel to waste
Injection inlet port
Auxiliary port (inactive)
Outlet port to waste
Infusion port
Diagnostic iKey: This iKey has no flow channels. It provides a seal at the inlet fitting of the ionKey™ source for testing the fluidic integrity
of the system.
c. iKey Component Description
Component Description
Auxiliary fluidic port Used for alternative system arrangements.
Infusion port Connects to the MS fluidics.
Electronic connections Electronic connections for the heater and EEPROM device.
Electrospray connection contact Electrospray potential is applied to the iKey emitter through a conductive pad.
Electrospray emitter tip The emitter is housed within the iKey. Electrospray occurs when liquid within the emitter is charged
by a high-voltage connection.
Identification label Identifies the specifications for the iKey.
Injection inlet port Receives the injections and mobile phase flow from the system.
NanoFlow gas inlet Connects to a supply of nitrogen from the mass spectrometer. The gas pressure is set in the mass
spectrometer’s tune window.
iKey
3
Page 4
[ CARE AND USE MANUAL ]
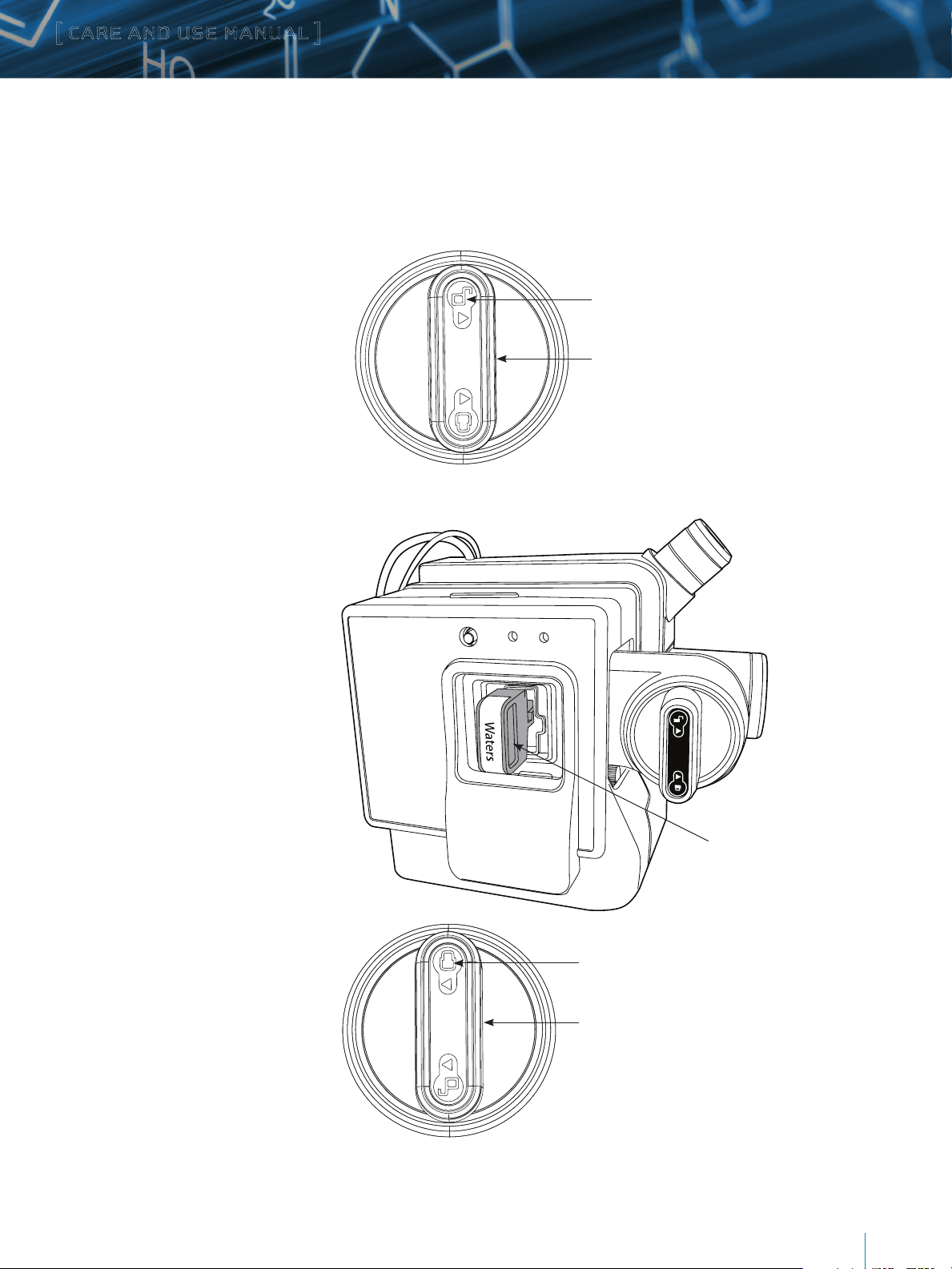
III. CONNECTING THE iKEY TO THE ionKEY SOURCE
These procedures outline the steps necessary to connect your iKey into the ionKey source. Connecting your iKey properly is essential
for operation.
To insert the iKey in the ionKey source:
1. Ensure the lever is vertical, and the unlock
indicator is in the 12 o’clock position.
2. Remove the iKey from the protective sheath.
Unlock indicator in 12 o’clock position
Lever in vertical position
3. Insert the iKey fully in the ionKey
source’s docking port with sufficient
force until it reaches a hard stop and
the base of the iKey handle is nearly
flush with the face plate on the source.
The green STATUS LED on the source
will flash indicating that the iKey is
inserted but not clamped.
4. Rotate the lever counterclockwise
180 degrees until the lock indicator
is in the 12 o’clock position. Doing so
locks the iKey in place and engages the
fluid, gas, and electronic connections.
Once clamped, the high-voltage
electrospray ionization (ESI) is
connected for electrospray, and
pressurization and temperature
control are active.
STOP F LOW
STATU S PRESSURE
iKey
Lock indicator in 12 o’clock position
Lever in vertical position
iKey
4
Page 5

[ CARE AND USE MANUAL ]
IV. iKEY USAGE
To ensure the continued high performance of your iKey Separation Device, please follow these guidelines:
a. Sample Preparation
1) Samples containing hundreds of nanograms of undigested protein (and higher) per injection have been shown to limit or shorten
iKey lifetimes.
Therefore, it is essential that proper sample preparation protocols are followed. For example, below are examples of recommended
protocols for preparing plasma:
Shaded green = Readily adaptable to ionKey/MS Shaded purple = Requires more careful consideration
Sample Preparation
Protein precipitation
>2:1 AC N: H2O
Liquid/liquid extraction
Digestion Proteins are broken into smaller pieces that no longer present an issue.
Affinity
Solid-phase extraction
Filter Filters will not remove protein that is in solution.
Centrifugation Centrifugation will not remove protein that’s in solution, unless it’s been crashed with PPT.
Dilution It depends on the dilution ratio, and the diluent. In general small aqueous dilutions will not remove protein.
This method effectively removes the majority of protein from solution, pay attention to final organic content.
This method effectively extracts the analyte leaving the protein behind, pay attention to final
organic content.
Small amounts of specific proteins are extracted from the sample for analysis This can be an effective way
of preparing samples by removing residual protein.
This is an issue when the analyte has similar properties to proteins. E.g. The methods used to isolate
larger peptides often leave significant protein content in the final extract. If solid-phase extraction is the
sole sample preparation technique, multidimensional chromatography (i.e., trap and back-flush elution)
is required.
Sample Type
Urine
Bile
Microsomes
Plasma
Organic Extracts High amounts of organic affect the performance of any column, this effect is greater with small dimensions.
2) The use of multidimensional chromatography, specifically a trap and back-flush elution strategy, can provide further sample cleanup
and facilitate injections of higher organic strength injection solvents without experiencing analyte breakthrough. This multidimensional
chromatographic strategy is required when solid-phase extraction is the sole sample preparation technique (i.e., no additional
sample preparation technique is used to remove large amounts of undigested protein).
3) It is preferable to prepare the sample in the initial mobile phase conditions or a weaker solvent for the best peak shape and sensitivity.
4) If the sample is not prepared in the mobile phase, ensure that the sample, solvent, and mobile phases are miscible in order to avoid
sample and/or buffer precipitation.
Typically low protein content and does not require organic additive to crash proteins. Dilution and
centrifugation is recommended.
Typically low protein content and does not require organic additive to crash proteins. Dilution and
centrifugation is recommended.
Microsomal incubations contain proteins. Pay close attention to sample preparation to ensure that
minimal protein remains in the final sample extract.
Plasma typically contains high concentrations of protein. Pay close attention to sample preparation
to ensure that minimal protein remains in the final sample extract. Injecting a large amount of undigested
proteins onto the iKey will result in compromised performance and shorter lifetimes.
iKey
5
Page 6

[ CARE AND USE MANUAL ]
5) Consider filtering samples with a 0.2 µm membrane to remove particulates. If the sample is dissolved in a solvent that contains an
organic modifier (e.g., acetonitrile, methanol, etc.) ensure that the membrane/filter material is compatible with the solvents in use.
Alternatively, centrifuge the sample for 20 minutes at 8000 RPM, followed by the transfer of the supernatant to an appropriate
Waters TruView LCMS Certified Vial could be considered.
6) Below are two examples of commonly used sample preparation protocols:
Protein precipitation (PPT)*
a. Add acetonitrile to plasma at a ratio of 2:1 (acetonitrile: plasma).
b. Vortex (mix) for one minute.
c. Centrifuge at 5000 relative centrifugal force (RCF) for five minutes.
d. Remove supernatant.
e. Perform solid-phase extraction (SPE) cleanup (optional).*
f. Pipette into Waters TruView™ LCMS Certified Vial (e.g., part number 186005663CV).
Liquid/Liquid Extraction (LLE)*
1. Add hexane to plasma at a ratio of 10:1 (hexane:plasma).
2. Vortex (mix) for one minute.
3. Centrifuge at 5000 relative centrifugal force (RCF) for five minutes.
4. Remove supernatant and evaporate to dryness.
5. Reconstitute (e.g., initial gradient conditions solvents).
6. Centrifuge at 5000 relative centrifugal force (RCF) for five minutes (optional).
7. Perform solid phase extraction (SPE) cleanup (optional).*
8. Remove supernatant and pipet into Waters TruView LCMS Certified Vial (e.g., part number 186005663CV).
*Additional sample cleanup steps using Oasis
sensitivity, and reduced matrix effects.
®
or Sep-Pak® SPE devices can provide cleaner samples, improved selectivity, higher
iKey
6
Page 7

[ CARE AND USE MANUAL ]
b. Mobile Phases
To maintain and ensure maximum iKey performance, use high quality chromatography solvents. Use only filtered and degassed mobile
phases. Filter all aqueous buffers prior to use through a 0.2 μm filter. Solvents containing suspended particulate materials will generally
clog the iKey ceramic substrate (analogous to clogging the inlet frit of an LC column). This may result in higher backpressure or distorted
peak shape. A table of recommended buffers is listed below:
Additive/Buffer pKa Buffer Range
Acetic Acid 4.76 Volatile Yes
Formic Acid 3.75 Volatile Yes
Acetate
(NH4CH2COOH)
Formate
(NH4COOH)
Additionally, the iKey lifetime will vary depending upon the operative temperature and pressure as well the type and concentration of
additive/buffer used.
4.76 3.76 – 5.76 Volatile Yes
3.75 2.75 – 4.75 Volatile Yes
Vola t ility (±1
pH unit)
Used for
Mass Spec
Comments
Maximum buffering obtained when used with
ammonium acetate salt. Used in 0.1–1.0% range.
Maximum buffering obtained when used with
ammonium formate salt. Used in 0.1–1.0% range.
Used in the 1–10 mM range. Note that sodium or
potassium salts are not volatile.
Used in the 1–10 mM range. Note that sodium or
potassium salts are not volatile.
c. pH Range
The recommended operating range for iKey is 1 to 7.
d. Pressure
iKeys can be operated at pressures up to 10000 psi (690 bar). Infusion and flow injection iKeys can be operated at at pressures up to
8000 psi (550 bar).
e. Temperature
Temperatures can be used to enhance selectivity, lower solvent viscosity, and increase mass transfer rates. When operating near the upper
pH limit for iKey, lower temperatures are recommended for higher iKey lifetime. iKey temperature limits are shown below:
Component Description
Ethylene bridged hybrid (BEH) 90 °C
Charged surface hybrid (CSH) 80 °C
High strength silica (HSS) 45 °C
Note: Working in combinations of extreme pH, temperature and pressure may result in reduced iKey lifetime.
iKey
7
Page 8

[ CARE AND USE MANUAL ]
V. REMOVING THE iKEY FROM THE ionKEY SOURCE
CAUTION: Opening the locking lever on the ionKey system without first stopping flow and decompressing the fluidic pressure
can damage the iKey.
1. Stop the solvent flow and decompress the fluidic pressure on the iKey using one of these methods.
a. Press the STOP FLOW button on the front of the source.
b. In the Console, select Sample Manager from the system tree, and then click > Maintain > Remove iKey.
2. Make sure the mobile phase flow is completely stopped and that the system pressure has decompressed below 100 psi. If the solvent
is flowing, the PRESSURE LED is steady green. The PRESSURE LED blinks green as the system is decompressing. Wait until the light is
turned off completely. If an ikey is removed without first decompressing the system, a “Removed under pressure” error message will
appear, indicating potential damage to the iKey.
3. Rotate the lever clockwise 180 degrees to the vertical position with the unlock indicator in the 12 o’clock position.
Unlock indicator in 12 o’clock position
Lever in vertical position
4. Pull the iKey from the docking port.
5. Place the iKey in the protective sheath.
WARNING: The electrospray emitter at the front end of the iKey contains a very small metal tip. Like a needle, it can pierce skin.
WARNING: If the iKey had been run at high temperature, the metal rings near the fluidic ports on the side may be hot.
Avoid touching them.
VI. CARING FOR THE iKEY
a. Cleaning and Regeneration
Changes in peak shape, peak splitting, shouldering peaks, shifts in retention, change in resolution or increasing backpressure may
indicate iKey contamination. Flush with a neat organic solvent to remove the non-polar contaminant(s), taking care not to precipitate any
buffered mobile phase components. If this flushing procedure does not solve the problem, purge the iKey with the following cleaning and
regeneration procedures.
Use a cleaning routine that matches the properties of the samples and stationary phase type and will solubilize the suspected contaminate.
Flush with 20 iKey volumes of solvent at an intermediate temperature of 40 °C. Return to the initial mobile phase conditions by reversing
the sequence.
Purge the iKey with a sequence of progressively more non-polar solvents (e.g., water, methanol, acetonitrile, isopropanol, etc.). If iKey
performance has not improved after regeneration/cleaning procedures, contact your local Waters representative for additional support.
If iKey performance has not improved after regeneration/cleaning procedures, contact your local Waters representative for
additional support.
iKey
8
Page 9

[ CARE AND USE MANUAL ]
b. Storage
For periods longer than four days, store the reversed-phase iKey in 100% acetonitrile. Do not store iKeys in buffered eluents. If the mobile
phase contained a buffer salt, flush the reversed-phase iKey with 10 iKey volumes of HPLC grade water followed by 10 iKey volumes of
acetonitrile. Failure to perform this intermediate step could result in precipitation of the buffer salt in the column when 100% acetonitrile
is introduced. Completely seal the iKey to avoid solvent evaporation and drying out of the chromatographic bed.
Note: If an iKey has been run with a formate-containing mobile phase (e.g., ammonium formate, formic acid, etc.) and is purged with 100%
acetonitrile, slightly longer equilibration times may be necessary when the iKey is re-installed and re-wetted with that same formate-
containing mobile phase.
VII. TROUBLESHOOTING iKEY PERFORMANCE
The table below lists symptoms, causes, and solutions for troubleshooting the iKey.
Symptom Cause Solution
Excessive baseline noise The emitter tip has residue on it Rinse the emitter tip in isopropanol.
Poor LC quality such as
retention time shift, or peak
width variance
The iKey does not fit into the
ionKey source’s docking port
Fluid leak
The iKey is upside down
Use the Fluidic Integrity Test within the Console
software in combination with the Diagnostic iKey
to find and identify leaking components.
Remove and orient the iKey so that the four ports
are on the left side.
a. Tips for Maximizing iKey Lifetimes
To maximize iKey lifetime, pay close attention to:
Water quality (including water purification systems);
Solvent quality;
Mobile phase preparation, storage and age;
Sample, buffer and mobile phase solubilities;
Sample quality and preparation.
When problems arise, systematically troubleshoot potential causes one variable at a time in a systematic fashion.
Always remember to:
Discourage bacterial growth by minimizing the use of 100% aqueous mobile phases where possible;
Discard and re-prepare aqueous mobile phase every 24 to 48 hours (if 100% aqueous mobile phase is required);
Routinely maintain your water purification system to ensure it is functioning properly;
Only use ultra-pure water (18 MOhm-cm) and highest quality solvent possible;
Consider improving sample preparation (e.g., solid-phase extraction, filtration, centrifugation, etc.) when possible.
Avoid when possible:
100% aqueous mobile phases;
HPLC-grade bottled water;
“Topping off” your mobile phases.
iKey
9
Page 10

[ CARE AND USE MANUAL ]
Don’t assume the iKey is to blame:
Investigate cause of iKey failure;
Monitor backpressure;
Examine mobile phase age, bacterial contamination, mobile phase precipitation, etc.;
Examine sample quality;
Examine injection solvent strength.
Do not prepare excessive amounts of mobile phase:
To reduce the chances of mobile phase contamination or degradation, prepare enough mobile phase to last for 3–4 days.
Alternatively, store excess bulk quantities in a refrigerated environment.
b. Troubleshooting Questions
What is the age of your 100% aqueous mobile phase?
Is the mobile phase filtered through a 0.2 µm membrane?
Was the mobile phase prepared fresh or topped off?
Is the water source of adequate quality?
When was the last time the water system was serviced or was the bottle of water unopened?
Is bacterial growth a possibility?
If a neat standard is prepared in the initial mobile phase conditions and injected, are the problems still observed?
If the sample is additionally filtered/purified (i.e., SPE, filtration, etc.) is the problem still observed?
Has the quality of the samples changed over time?
c. Good Practices
Do not operate at pressures that exceed 10000 psi (690 bar).
Do not apply electrospray potential to the emitter for an extended period of time without mobile phase flow.
Do not immerse in liquid.
Do not touch any part of the iKey other than the handle.
Do not freeze.
Flush with acetonitrile and store at room temperature when not in use.
Use the iKey sheath to protect device when not in use.
Do not bend or pull the capillary connection tubing at the ionKey source coupling.
Avoid excess voltage (>4.0 kV) which can erode emitter over time.
iKey
10
Page 11

[ CARE AND USE MANUAL ]
VIII. ORDERING INFORMATION
Product Description Part Number
iKey, Peptide BEH C
iKey, BEH C
18
iKey, Peptide CSH C
iKey, CSH C
iKey, BEH C
18
18
iKey, Peptide BEH C
iKey, BEH C
18
iKey, Infusion 186007049
iKey, Diagnostic 186007050
iKey, Injection 186007051
* QC tested with a Peptide Standard Mix.
,
130Å, 1.7 µm, 150 µm x 50 mm* 186006764
18
,
130Å, 1.7 µm, 150 µm x 50 mm 186007256
,
130Å, 1.7 µm, 150 µm x 50 mm* 186007257
18
,
130Å, 1.7 µm, 150 µm x 50 mm 186007244
,
300Å, 1.7 µm, 150 µm x 50 mm 186006969
,
130Å, 1.7 µm, 150 µm x 100 mm* 186006766
18
,
130Å, 1.7 µm, 150 µm x 100 mm 186007258
Please go to at www.waters.com/ikey for a complete list of iKey Separation Devices.
Waters, The Science of W hat’s Possible , UPLC, Oasis, and Sep-Pak are registered trademarks of Waters Corporation.
TruView, iKey, ionKey/MS, and ionKey are trademarks of Waters Corporation.
©2014 Waters Corporation. Produced in the U.S.A. March 2014 20004897EN TC-PDF
Waters Corporation
34 Maple Street
Milford, MA 01757 U.S.A.
T: 1 508 478 2000
F: 1 508 872 1990
www.waters.com
 Loading...
Loading...