Page 1

[ Care and Use ManUal ]
Gen-PAk fAX CoLUMns
Contents
I. IntRoDUCtIon
II. InstALLAtIon
III. GeneRAL UsAGe ConsIDeRAtIons
IV. GeneRAL MetHoD DeVeLoPMent GUIDeLInes
V. eXAMPLe UsInG HAe III DIGesteD DnA
VI. tRoUBLsHootInG
Waters Gen-Pak™ FAX columns offer the highest resolution available in
anion- exchange HPLC of nucleicacids. The Gen-Pak FAX column
contains a weak anion exchanger based on DEAE functionalized non-porous
resin. It contains 2.5 µm particles and is well suited for analytical and
micro-preparative applications.
I. IntRoDUCtIon
This manual covers the use of the Waters Gen-Pak FAX column. The
Gen-Pak FAX column is a 4.6 x 100 mm steel column containing
a p o l y m e r - b a s e d h i g h - p e r f o r m a n c e a n i o n - e x c h a n g e p a c k i n g . T h e
c o l u m n i s d e s i g n e d t o p e r f o r m h i g h - r e s o l u t i o n a n a l y s i s a n d
p u r i f i c a t i o n o n v a r i o u s n u c l e i c a c i d s p e c i e s ( u p t o a t l e a s t
5,000 base pairs) such as DNA restriction
reaction (PCR) products, plasmids and synthetic oligonucleotides.
fragments, polymerase chain
Gen-Pak FAX Columns 1
Page 2
[ Care and Use ManUal ]
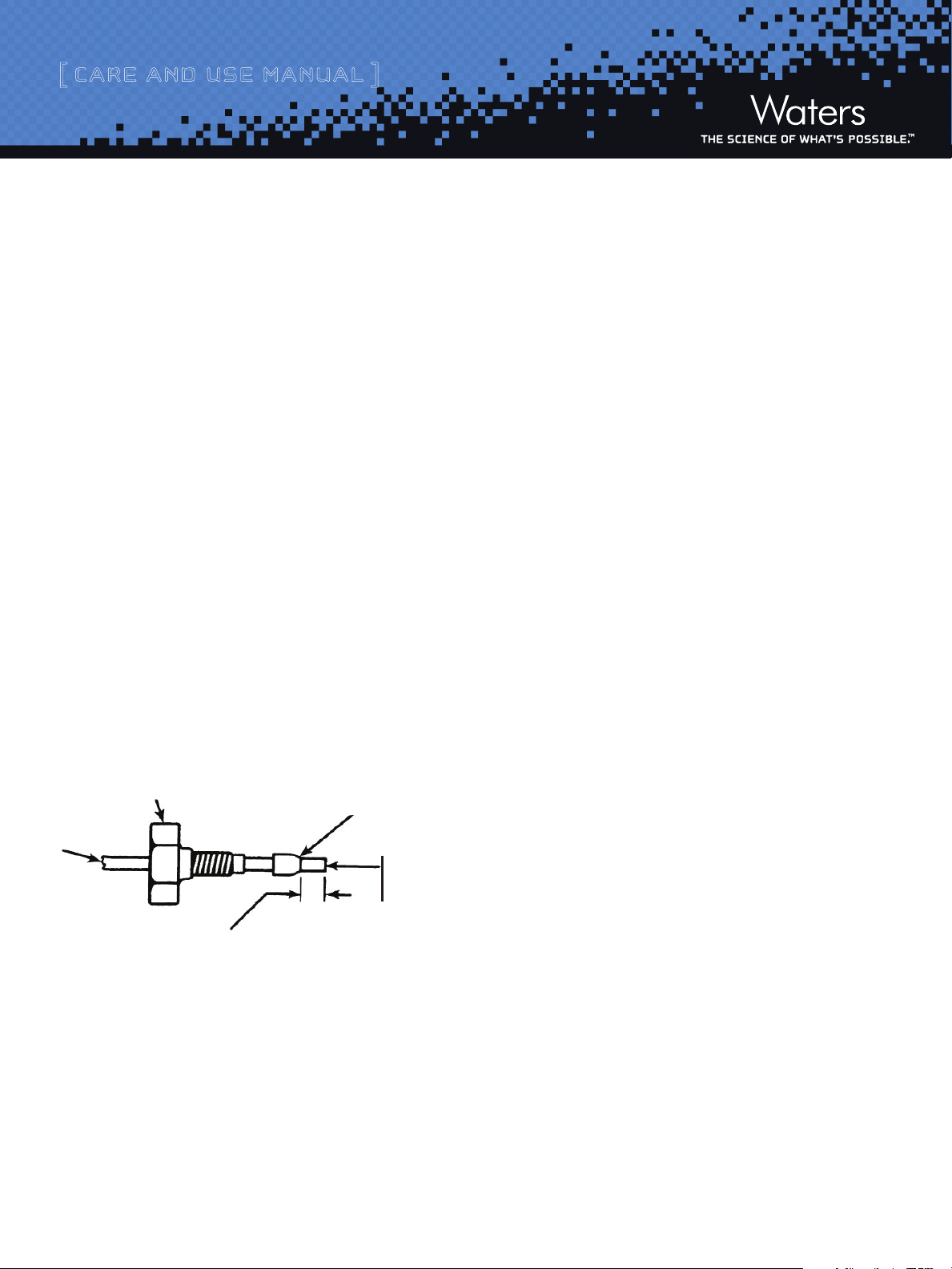
Tube
Critical distance to be determined by each
application (union, column fitting, etc.)
Compression screw or nut
Ferrule
End must be straight and smooth
to achieve maximum column
efficiency
II. InstALLAtIon
a. Column
Before you attach a new column, attach a union in place of the column and
flush the HPLC system free of previously used solvent.
Note: To achieve optimum performance from each column, use tubing of
0.010 inch i.d. or smaller to connect the
column to the detector.
Procedure to install the column:
1. Remove the end plugs from the column. (Be sure to save the end
plugs for use when the column is removed from the system for
storage.) The column outlet is indicated by an arrow on the label
showing the direction solvent should flow.
2. Finger-tighten the tubing compression screws and then
wrench-tighten them 1/4 to 1/2 turn.
Note: Do not over-tighten; this damages the connection.
3. Make sure that the compression fitting is in good condition and
properly prepared as shown in Figure 1. Because fittings may
vary, it is important to verify that the tubing in your system
bottoms in the column end nuts.
injector to the column and the
3. Slide the compression screw, followed by the ferrule (large end
of the taper first) over the tube. Be certain to bottom the tube in
the fitting seat to assure a leak-free connection.
c. Testing
To validate system and column performance, test each new column
with a standard sample. T his provides a basis for detecting system
component changes and troubleshooting.
Suggested samples include:
• Hae Ill digest of ØX 174 RF DNA
• BstN I digest of pBR322 DNA
III. GeneRAL UsAGe ConsIDeRAtIons
This section presents guidelines that can extend the life of your
column and help you achieve the best possible chromatographic
results.
a. Samples
When preparing and using samples:
• Centrifuge or filter the sample before injection.
Figure 1: Ferrule and Compression Screw Assembly
b. Tubing
Follow the next three steps to cut tubing to connect a new steel
column or to improve the end connections on existing fittings:
1. Using a three-cornered file with a cutting edge, scribe the
circumference of the tubing at the desired break.
2. Grasp the tubing on both sides of the scribe mark with
cloth-covered pliers (to prevent marring the tube surface) and
gently work the tube back and forth until it separates.
Gen-Pak FAX Columns 2
• Do not inject samples containing microparticulates.
b. Solvents
When preparing and using solvents:
• Use pure buffer salts.
• Use of high quality reagents, water, and solvents is critical in
preparing chromatography eluents. Fouling of the Gen-Pak FAX
resin, leading to a loss in retention and / or separation efficiency,
occurs faster on this column chemistry due to the small surface
area of the non-porous resin particles. As such, all prepared HPLC
eluents should be filtered through a solvent compatible 0.45 µm or
0.22 µm depth filter.
• Solvents should be degassed (vacuum filtration, sonication or
helium sparged) prior to use.
• Use of organic solvents other than methanol or acetonitrile at
greater than 10% in water is not recommended.
Page 3
[ Care and Use ManUal ]
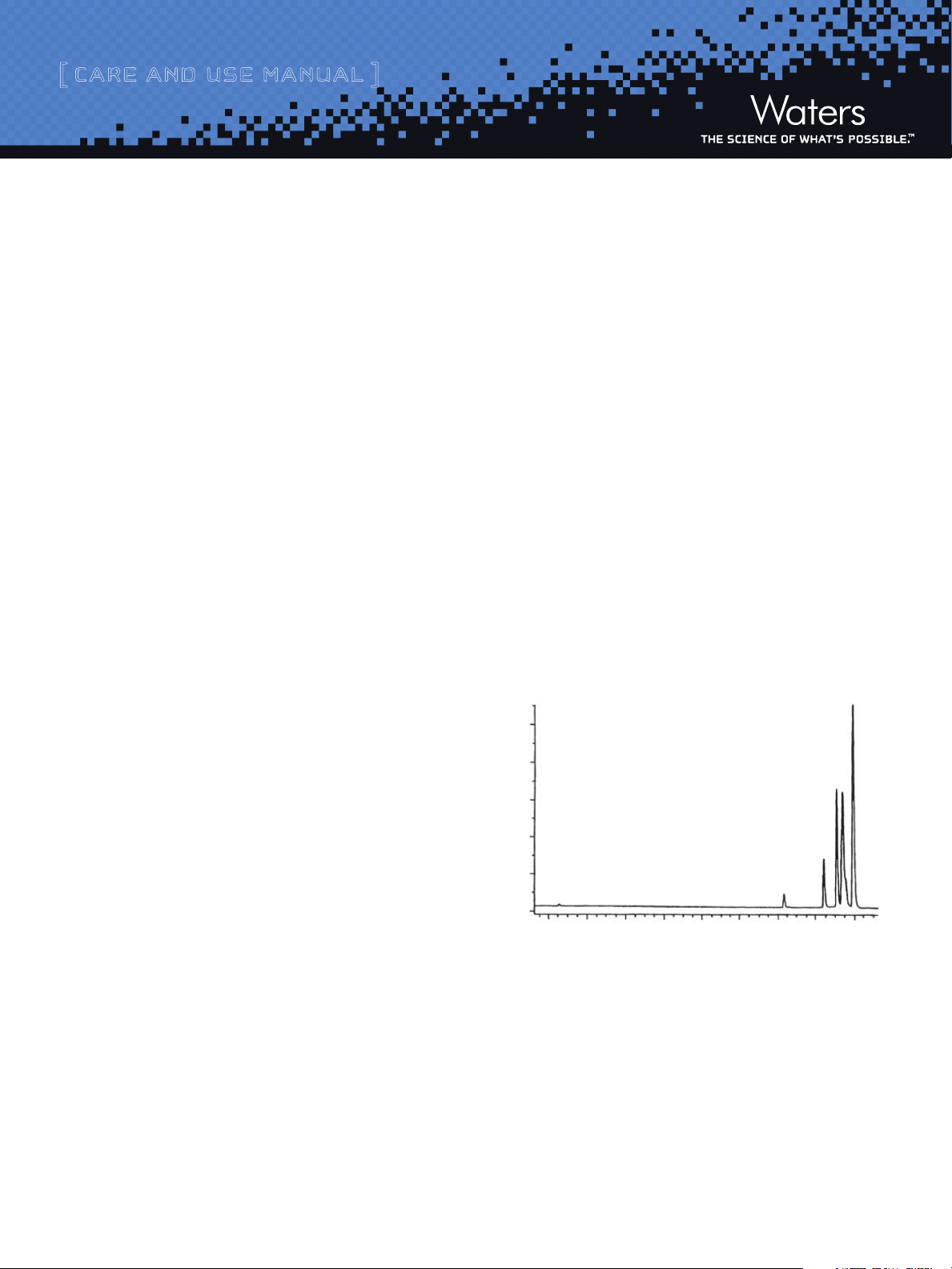
Column: Gen-Pak FAX™ (4.6 x 100 mm)
Buffer A: 25 mM Tris/Cl, 1 mM EDTA, pH 8.0
Buffer B: 25 mM Tris/Cl, 1 mM EDTA, 1.0 M NaCl, pH 8.0
Gradient: 30 to 100% B in 30 min., linear
Flow: 0.75 mL/min
Temperature: 30˚C
0.30
0
18
35
Minutes
121 bp
382 bp
1060 bp
929 bp
1857 bp
Column: Gen-Pak FAX (4.6 x 100 mm)
Buffer A: 25 mM Tris/Cl, 1 mM EDTA, pH 8.0
Buffer B: 25 mM Tris/Cl, 1 mM EDTA, 1.0 M NaCl, pH 8.0
Gradient: 30 to 100% B in 30 min., linear
Flow: 0.75 mL/min
Temperature: 30˚C
Absorbance 260 nm
0.30
0
18
35
Minutes
121 bp
382 bp
1060 bp
929 bp
1857 bp
c. Operating Pressure
Do not excee d 4,000 psi op erating pre ssu re or ab out
1 mL/min at 25 °C.
d. pH range
Stay within a pH range of about 1.5 to 12 (do not use concentrated
acids or bases).
e. Flow
Make flow rate changes in a gradual manner (less than 1 mL/min) to
avoid column voiding. Never reverse flow in the column.
f. Cleaning
Clean the column between nucleic acid injections with 3 to 5 mL of
with 3 to 5 mL of 20 - 40% acetic acid.
g. Storage
When you store a column for less than 24 hours, you typically do
not need to follow special storage procedures. However, be sure
that the column never dries out; this can degrade chromatographic
performance.
a. Separating Double Stranded DNA Fragments
DNA fragments are usually isolated using gel electrophoresis.
Although resolution is good, the technique has limited mass capacity,
often gives low yields of extracted fragments, and is time consuming.
The Gen-Pak FAX column is a useful alternative for the
r a p i d p u r i f i c a t i o n a n d a n a l y s i s o f s u c h n u c l e i c a c i d s p e c i e s .
Separations are often accomplished in about 30 minutes. Recoveries
of biologically active material directly from the column are usually
greater than 95%. Direct UV monitoring of the column effluent
provides subnanogram sensitivity without the need for indirect
visualization via ethdium bromide staining or autoradiography.
Depending upon sample complexity, as much as 50 to 100 μg
of DNA can be separated in a single run. Since the separation is
based primarily upon the overall charge of each fragment, smaller
fragments elute prior to larger ones using an ionic strength gradient
as shown in Figure 2.
Figure 2: Separation of 3.0 μg BstN I Digest of pBR322 DNA
For longer term storage, follow the procedure described below:
1. Flush the column with approximately 25 mL of Milli-Q
remove salts.
2. Flush the column with approximately 4 mL of a mixture of 10
percent methanol and 90% Milli-Q water.
3. Disconnect the column.
4. Screw end plugs firmly in place and return the column to its box.
5. Store the column at 4 °C.
Note: Before reusing the column, flush 4 with 25 mL of Milli-Q water
to remove any methanol prior to introducing buffers.
IV. GeneRAL MetHoD DeVeLoPMent GUIDeLInes
This section discusses the use of Waters Gen-Pak FAX columns for
purifying DNA restriction fragments, polymerase chain reaction (PCR)
products, plasmids, and synthetic oligonucleotides.
®
water to
b. Buffers
The preferred chromatographic buffer is 25 mM Tris/Cl. 1mM EDTA,
pH 8.0. The recommended buffers for DNA fragmet separations are:
• Buffer A: 25 mM Tris/Cl, 1 mM EDTA, pH 8.0
• Buffer B: 25 mM Tris/Cl, mM EDTA, 1.0 M NaCl, pH 8.0
Gen-Pak FAX Columns 3
Page 4

[ Care and Use ManUal ]
The inclusion of EDTA in the buffers is recommended to protect the
DNA from nucleases during and after the chromatography1. Filter and
vacuum degas the buffers before use.
Useful chromatography can be obtained with sodium phosphate
buffer, but the phosphate coprecipitates with the DNA in the common
ethanol-precipitation procedures.
c. Equilibration and flow
Rigorous control of column equilibration is essential for predictable
and reproducible chromatography. Before injection, equlibrate the
column with 10 column volumes of buffer at initial chromatographic
conditions. The column can be used at flow rates from below 0.5 to
1 mL/min, but 0.75 mL/min is recommended for general use.
d. Temperature Effects
The conformation and intermolecular hydrogen bonding of DNA
fragments, and therefore the Gen-Pak FAX separation of those
fragments, are affected by temperature. At higher temperatures, the
structure of DNA is more open or relaxed so more ionic groups can
interact with the column. In general, higher ionic strength is required
as temperature is elevated.
Since conformation is dependent on base sequence, variations in
temperature over the range of 30 to 60 °C can be used to optimize a
particular separation. Where elevated temperatures are not required,
the column should still be operated at a controlled temperature,
typically 5 °C above ambient, to ensure reproducibility.
e. Detection
The separation of subnanogram quantities of DNA can be monitored
at 260 nm, provided that the buffers are pure and free of UV
a b s o r b i n g c o n t a m i n a n t s . A s s e s s b l a n k g r a d i e n t s o n a p r o p e r l y
equilibrated column prior to performing actual sample
chromatography.
f. Sample Size
D e p e n d i n g u p o n t h e s i z e d i f f e r e n c e s a m o n g t h e f r a g m e n t s t o b e
isolated, as much as 50 to 100 μg of DNA can be applied to the
column. Injection volume is essentially unlimited as long as the ionic
strength of the sample is at least 0.1 M less than that required to
elute the fragment of interest. For injection volumes above 100 μL,
the sample should be at pH 8.
Excellent recoveries, in most cases more than 95 percent, of
biologically active material have been obtained purifying samples
containing fragments of 5000 base pairs and less.
g. Sample Treatments
DNA fragments generated by restriction enzyme digestion or
from the polymerase chain reaction may be extracted with phenol/
chloroform prior to chromatorgraphy. However, the reaction may be
injected directly since most proteins are completely unretained under
conditions used for the separation (i.e., 0.30 M NaCl).
In either case perform these steps before injection:
1. Remove particulate material from samples by centrifugation or
filtration through a 0.45 µm filter.
2. Heat treated samples at 37 °C for 10 min prior to injection to
disrupt any reannealed termini of the DNA fragments.
h. Gradients
Typical DNA fragments elute from the Gen-Pak FAX column between
0.4 and 0.75 M NaCl at 30°C. Equilibrate the column to 0.1 M below
the ionic strength required to elute the smallest fragment of interest.
Useful gradients are usually 5 to 10 mM/min at 0.75 mL/min.
i. Column Washing
Upon completion of each separation, wash the column with a small
volume of 20 - 40% acetic acid to maintain its chromatographic
characteristics as well as to eliminate sample carry-over from injection to injection.
Procedure
After the last fragment elutes, perform the following procedure:
1. Flush the column with 5 mL of buffer B.
2. Pump or inject 3 to 5 mL of 20 - 40% acetic acid onto the
column. This may be performed with the column at 60 °C.
3. Flush the column with about 5 mL of buffer B.
4. Reequilibrate the column for the next sample injection.
Note: Washing the column with sodium hydroxide does not perform as
well as the above acetic acid procedure and is not recommended.
Gen-Pak FAX Columns 4
Page 5

[ Care and Use ManUal ]
Absorbance at 260 nm
Minutes
0
10 35
1
2
3
4
5-7
8-11
0.2
Column: Gen-PakôFAX (4.6 mm x 100 mm)
Buffer A: 25 mM Tris/Cl, 1mM EDTA, pH 8.0
Buffer B: 25 mM Tris/Cl, 1 mM EDTA, 1.0 M NaCl, pH 8.0
Gradient: 30 to 100% B in 30 min, linear
Flow: 0.75 mL/min
Temperature: 30˚C
V. eXAMPLe UsInG HAe III PLAsMID DIGesteD DnA
This section shows an example of how to develop a method using
Gen-Pak FAX columns. The example in this chapter can serve as a a
model for adapting this technique to the requirements of a particular
sample.
The example describes the evolution of the separation of a restriction
enzyme digest, the Hae III digest of ØX 174 RF DNA. This DNA sample
contains 11 fragments ranging in size from 72 to 1353 base pairs.
Table 1: Number of Base Pairs in Each Fragment
Fragment Base Pairs
1 72
2 118
3 194
4 234
5 271
6 281
7 310
8 603
9 872
10 1078
11 135 3
a. Initial Gradient
Chromatography is initally performed using a relatively steep
ionic strength gradient. As Figure 3 shows, these conditions do not
completely resolve the 234, 271, 281, and 310 (peaks 4-7) nor the
603, 872, 1078, and 1353 (peaks 8-11) base pair fragments.
Figure 3: Initial Separation uf 2 μg of Hae III Digest of ØX 174 RF
DNA at 30°C using a Steep Salt Gradient
b. Gradient Optimization
Resolution can often be improved by systematic adjustment of
gradIent conditions. In the current example, examination of the inital
chromatorgram indicates a more shallow gradient is required.
c. Calculating NaCl Concentration
It is possible to calculate the approximate NaCl concentration
required to elute the first (72bp) and last ( 1353 bp) restriction
fragments from the data obtained in the inital chromatography.
First, determine the system delay volume from the point of gradient
formation to the detector cell.
d. Calculating System Delay Volume
To calculate the system delay volume from point of gradient
formation to detector cell:
1. Remove the column from the system, insert a union, and set the
detector to 260 nm.
2. Flush the pump and solvent lines with eluent A ( Milli-Q water)
3. Start the flow of eluent B (0.01% acetone in Milli-Q water)
4. Monitor the detector output until it rises to the new, higher value
of about 0.032 absorbance units if a cm detector cell is used.
The system delay volume corresponds to the volume that has flowed
from the start of eluent B to the midpoint of the absorbance rise plus
the excluded column volume of 0.6 mL.
e. Using a More Shallow Gradient
The system delay volume for this instrument was 7.5 mL,
corresponding to 10 minutes at 0.75 mL/minute.
Under inital conditions, elution occurs as follows:
Gen-Pak FAX Columns 5
• The 72 base-pair fragment elutes at 22 minutes, reflecting
the NaCl concentration at 12 minutes in the gradient table of
0.56 M NaCl
• The 1353 base-pair fragment elutes at 0.65 M NaCl
Figure 4 shows the results of changing to a more shallow NaCl
gradient, from 0.54 to 0.67 NaCl, over the same 30 minute time
interval. As this figure shows, the change significantly imrproved the
resolution.
Page 6

[ Care and Use ManUal ]
Absorbance at 260 nm
0.2
0
10
1
2
3
4
5-7
8
9
10
11
35
Column: Gen-Pak
FAX (4.6 x 100 mm)
Buffer A: 25 mM Tris/Cl, 1 mM EDTA, pH 8.0
Buffer B: 25, mM Tris/Cl, 1mM EDTA, 1.0 M NaCl, pH 8.0
Gradient: Load at 30% B to 54% B in 0.01 min.
Then 54 to 67% in 30 min., linear.
Flow:
Temperature:
Minutes
0.75 mL/min
30˚C
Absorbance at 260 nm
1
2
3
4
5-7
8
9
10
11
0
0.2
Column: Gen-Pak
ô
FAX (4.6 x 100 mm)
Buffer A: 25 mM Tris/Cl, 1 mM EDTA, pH 8.0
Buffer B: 25 mM Tris/Cl, 1mM EDTA, 1.0 M NaCl, pH 8.0
Gradient: Load at 30% B to 54% B in 0.1 min.
Then 64 to 77% in 30 min., linear.
Flow: 0.75 mL/min
Temperature:
Minutes
60˚C
35
10
Figure 4: Separation of 2 μg of Hae III Digest of ØX 174 RF DNA at
30 ˚C using a Shallow Salt Gradient
f. Temperature Optimization
Ad ju st ment s of gra di ent s lo pe m ay no t pro du ce a de qu ate
r e s o l u t i o n o f D N A f r a g m e n t s i n e v e r y c a s e . H o w e v e r , c h a n g e s i n t h e
t e m p e r a t u r e a t w h i c h t h e s e p a r a t i o n i s p e r f o r m e d c a n a l t e r t h e
relative retention of the fragments. As Figure 5 shows,
chromatography of the Hae III digest of ØX 174 at 60 °C yields
slightly less resolution of the 72 and 118 base-pair fragments.
Since it is not possible to predict the best separation temperature for
a particular sample, it is often useful to compare separations at two
temperatures, such as 30 °C and 60 °C. Although increased NaCl
concentration is required to elute DNA fragments from the column at
elevated temperatures, chromatography at such temperatures does
not shorten the column life nor affect the biological activity of the
collected samples.
Figure 5: Separation of 2 μg of Hae III Digest of ØX 174 RF DNA at
60 °C using a Shallow Gradient
VI. tRoUBLsHootInG
Liquid chromatography columns have a finite life that is directly
related to the care and use they recieve. Column life is influenced
by the number of injections, cleanliness of sample and solvent,
frequency of solvent changeover, and procedures for handling and
storage.
If you observe a change in the following areas, take immediate steps
to determine the reason for the changes.
• Retention of a particular compound
• Resolution between two compounds
• Peak shape
Until the cause of the change is determined, you should question the
validity of the results of any separation using the column
a. Pressure Buildup/Loss of Resolution
With continued use, it is possible that excessive pressure buildup
(more than 4,000 psi at 1.0 mL/min at 25°C) or loss of resolution
may be experienced. These may both be caused by the buildup of
particulates from samples or eluents on the inlet filter. Filtering
samples and eluents greatly extends inlet filter and column life.
b. Possible Corrective Actions
If pressure buildup or loss of resolution occurs, any of the following
corrective actions, listed in order preference, may help:
• Perform a 20 - 40% acetic acid wash as described in Column
Washing Section. The length of the wash can be extended to
10 to 15 minutes.
• R e p l a c e t h e i n l e t f i l t e r w i t h a n e w o n e ( W a t e r s P a r t N u m b e r
WA T015 715)
Gen-Pak FAX Columns 6
Page 7

[ Care and Use ManUal ]
Sales Offices:
Austria and European Export
(Central South Eastern Europe,
CIS and Middle East) 431 877 18 07
Australia 2 9933 1777
Belgium 32 2 726 1000
Brazil 55 11 5094 3788
Canada 800 252 4752
China 8621 6495 6999
CIS/Russia +7 495 3367000
Czech Republic 42 02 617 11384
Denmark 45 46 59 8080
Finland +358 9 5659 6288
France (33) 1 30 48 72 00
Germany 49 6196 400600
Hong Kong 852 29 64 1800
Hungary 36 1 350 5086
India and India Subcontinent
91 80 2 837 1900
Ireland 353 1 448 1500
Italy 39 02 274 211
Japan (81) 3 3471 7191
Korea (82) 2 820 2700
Mexico 5255 5200 1860
The Netherlands +31 (0)76-50 87 200
Norway 47 63 84 60 50
Poland (48) 22 833 4400
Puerto Rico 787 747 8445
Singapore 65 6273 1221
Spain 34 93 600 93 00
Sweden 46 8 555 11500
Switzerland 41 56 676 7000
Taiwan 886 2 2543 1898
United Kingdom 44 208 238 6100
©2008 Waters Corporation. Waters, The Science of W hat’s
Possible, and Gen-Pak are trademarks of Waters Corporation.
Milli-Q is a trademark of Millipore Corporation.
October 2009 WAT015493 Rev 6 VW-P DF
Gen-Pak FAX Columns 7
Waters Corporation
34 Maple Street
Milford, MA 01757 U.S.A.
T: 1 508 478 2000
F: 1 508 872 1990
www.waters.com
 Loading...
Loading...