Page 1

[ CARE AND USE MANUAL ]
Enzymate BEH Pepsin Column
CONTENTS
I. INTRODUCTION
II. HOW WATERS HDX TECHNOLOGY WORKS
III. GETTING STARTED
a. Column Connection
b. Column Installation and Equilibration
c. Digesting/Trapping Flow Rate
d. Quenc hing
e. Protein Injection
f. Trapping Time
g. pH and Solvent Compatibility
h. Temperature
i. Column Washing/Cleaning
j. Column Storage
IV. CHECKING FOR ENZYMATIC ACTIVITY
V. REFERENCES
I. INTRODUCTION
Thank you for choosing the Waters Enzymate™ BEH Pepsin Column.
Pepsin is immobilized onto rugged 5 μm BEH (Ethylene Bridged
Hybrid) particles which are packed into low dispersion 2.1 x 30 mm
stainless steel column hardware containing 0.5 μm porosity inlet
and outlet frits. This is the same column hardware used in our
eX tended P erformance [XP] Column line. This online, flow-through
digestion column reproducibly digests intact proteins into peptides
after incubating with H2O/D2O during HDX experiments. Benefits
of online protein digestion include reduced sample preparation
time, reproducible digests, minimized back-exchange, and limited
enzyme in solution.
The Enzymate BEH Pepsin Column is housed in a temperature-
controlled compartment to ensure optimal performance and
reproducibility. Once the peptic peptides elute from the pepsin
column, they are focused on a trap column. The peptides are then
separated at 0 ˚C on an ACQUITY UPLC® BEH C18 Column and
detected by high resolution mass spectrometry.
The Enzymate BEH Pepsin Column was designed and tested
specifically for use on the ACQUITY UPLC M-Class System with
HDX Technology and the nanoACQUITY UPLC® System with HDX
Technology. Waters cannot support the use of the Enzymate BEH
Pepsin Column on any other LC system.
Page 2
[ CARE AND USE MANUAL ]
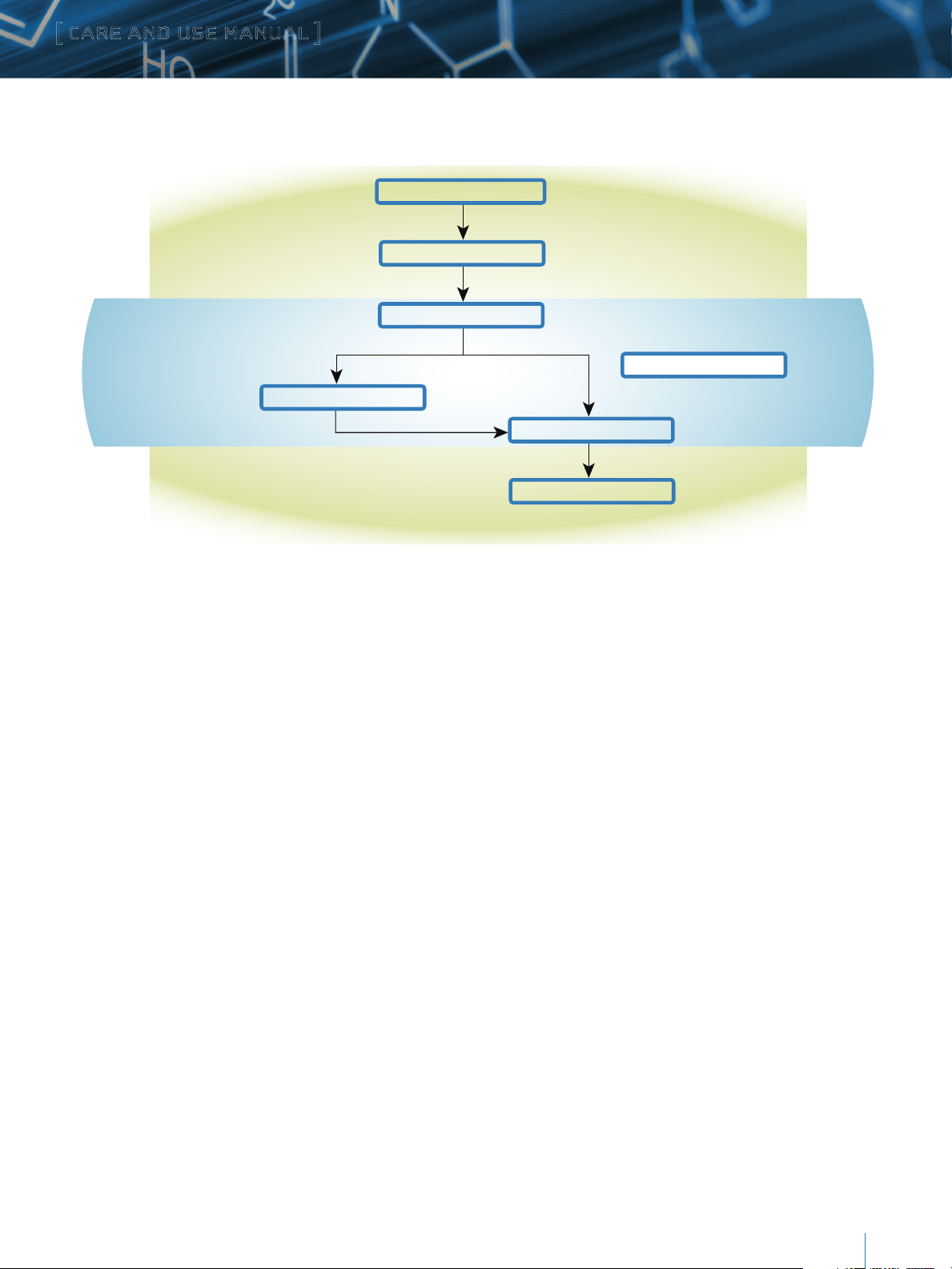
2
Protein in HO, pH 7.0, 20 °C
Protein Labeling
Labeling Quenched
Enzymate BEH Pepsin Column
UPLC Separation
Add excess D2O
Reduce pH to 2.5
ESI MS Analysis
Global analysisLocal analysis
Maintained at
10–20 ˚C
HDX Manager
II. HOW WATERS HDX TECHNOLOGY WORKS
Figure 1. How Hydrogen Deuterium Exchange (HDX) works in the ACQUITY UPLC M-Class System with HDX Technology.
Waters offers a complete HDX MS system for protein higher-order
structure analysis, such as protein conformational dynamics,
protein-drug binding, protein-protein interactions including
6. The peptides are then trapped on a VanGuard™ Pre-Column.
7. The trapped peptides are then eluted from the trap column,
and separated on an ACQUITY UPLC Column at 0 °C.
epitope mapping, and protein aggregation. This integrated system
includes automated sample preparation with a LEAP system,
on-line pepsin digestion using the Enzymate BEH Pepsin Column,
III. GETTING STARTED
UPLC, and QTof MS instrumentations for peptide separation
and characterization with automated data processing and
interpretation by DynamX.
a. Column Connection
The Enzymate BEH Pepsin Column uses eXtended Performance
[XP] or ACQUITY UPLC Column-style hardware. Therefore, the
ACQUIT Y UPLC M-Class with HDX Technology workflow:
1. Deuterium oxide (D2O) is added to the protein sample which is
solubilized in a physiological buffer (e.g., 100 mM phosphate
buffer, pH 7.0).
2. The exchange reaction proceeds until a defined end point or
multiple time points for rate determinations.
3. The exchange reaction is stopped by reducing the pH to 2.5
and decreasing the temperature to 0 °C.
4. Sample is injected into the injection port of the HDX Manager
which is maintained at 0 °C.
internal geometry of the Enzymate BEH Pepsin Column end nuts
is the same as eX tended Performance [XP] or ACQUITY UPLC
Columns. This geometry is very similar to “Parker-style” depths
which is the LC industry standard. It is important, however, to
reset the fitting every time a new Enzymate BEH Pepsin Column is
installed to ensure no voids or leaks occur.
Use 1/16” PEEK tubing (0.005” internal diameter) for the
Enzymate BEH Pepsin Column inlet and outlet tubing, hand cut
to the appropriate lengths (e.g., part number WAT022995).
Alternatively, 1/16” steel tubing of similar dimensions and
lengths may also be used.
5. Proteins are digested into peptides on the Enzymate BEH
Pepsin column maintained at 10–20 °C.
Enzymate BEH Pepsin Column
2
Page 3

[ CARE AND USE MANUAL ]
W
W
b. Column Installation and Equilibration
Install the Enzymate BEH Pepsin Column in the temperature controlled compartment located on the left side of the HDX Manager
(maintained between 10 and 20 °C). Connect it between port 6 of the Injection Valve and port 3 of the Trap Valve as indicated in Figures 2a
and 2b.
Trap ValveInjection Valve
Sample
aste
2
3
4
1
6
5
Enzymate BEH
Pepsin Column
Waste
Auxiliary Solvent
Manager (ASM)
Figure 2a. Protein digestion in trapping mode (sample already injected and is in loop).
Sample
2
3
1
6
Enzymate BEH
Pepsin Column
Waste
3
4
Binary Solvent
Manager (BSM)
Trap ValveInjection Valve
3
Trap
Trap
12
ACQUITY UPLC® Column
6
5
12
To MS
ACQUITY UPLC® Column
6
To MS
aste
4
5
Auxiliary Solvent
Manager (ASM)
Figure 2b. After proteins are digested on the Enzymate BEH Pepsin Column, the valve is switched and the peptic peptides are separated using reversed-phase UPLC.
The trap column used is a VanGuard 2.1 x 5 mm Pre-Column containing 1.7 μm ACQUITY UPLC BEH C
reversed-phase separation occurs on an ACQUITY UPLC Column containing 1.7 μm ACQUITY UPLC BEH C
or 1.0 x 50 mm, part number 1860 02344).
4
5
Binary Solvent
Manager (BSM)
packing material (part number 186003975). The analytical
18
packing material (1.0 x 100 mm, part number 1860 02346
18
1. Set the temperature of the Enzymate BEH Pepsin Column to between 10–20 °C. Higher temperatures aid digestion and lower
temperatures minimize back-exchange.
2. Equilibrate the Enzymate BEH Pepsin Column using 0.1% formic acid in water, pH 2.5 for at least twenty minutes (20 column volumes) at
a flow rate of 100 μL/min before performing protein digestions.
Enzymate BEH Pepsin Column
3
Page 4

[ CARE AND USE MANUAL ]
c. Digesting/Trapping Flow Rate
The recommended starting digestion flow rate is at 100 μL/min
using 0.1% formic acid (pH 2.5) in water. Decreasing the digestion
flow rate increases the contact time between the proteins and
the immobilized pepsin enzyme. This can increase digestion
efficiency but will result in longer digestion times. Increasing
the flow rate decreases the contact time between the proteins
and the immobilized pepsin enzyme and can decrease digestion
efficiency.
d. Quenching
A recommended quenching solution is 100 mM potassium
phosphate, pH 2.50 dissolved in water (50 mM K2HPO4 and 50 mM
in KH2PO4 adjusted to exactly pH 2.50 with concentrated HCl).
e. Protein Injection
Typical protein buffers include Tris (tris(hydroxymethyl)
aminomethane), HEPES (4-(2-hydroxyethyl)-1-piperazineethanesulfonic
acid), or PBS (phosphate buffered saline). Between 10 and 50 pmol
of protein can be injected on column.
f. Trapping Time
The digested peptides can be trapped and desalted on the trap
column for several minutes (and for as little as thirty seconds)
depending on the buffer concentration before eluting to the
ACQUITY UPLC Column. Increasing the trapping time by decreasing
the flow rate can increase digestion efficiency but may cause
back-exchange (i.e., deuterium for hydrogen).
g. pH and Solvent Compatibility
The Enzymate BEH Pepsin Column is NOT
compatible with organic solvents, detergents, or
high pH. Therefore, DO NOT expose the Enzymate
BEH Pepsin Column to high concentrations of
organic solvents, detergents, or high pH solvents (pH > 4.5).
The Enzymate BEH Pepsin Column can tolerate a very low
concentration of organic solvent (≤ 5%) which can be injected
during a column cleaning procedure as described in section i.
h. Temperature
The Enzymate BEH Pepsin Column is maintained at a digestion
temperature of between 10–20 °C. Increasing digestion
temperature may increase digestion efficiency but may also
promote back-exchange (i.e., deuterium for hydrogen).
i. Column Washing/Cleaning
Under normal operating conditions, the digestion buffer (0.1%
formic acid in water, pH 2.5) should be sufficient to remove
residual proteins and/or peptides from the Enzymate BEH Pepsin
Column and the trap column, thereby reducing injection-to-
injection carryover. However, if carryover is observed, prepare
a column cleaning solution of 1.5 M guanidine hydrochloride/
acetonitrile/formic acid (95.2/4/0.8). Inject 100 μL of this
cleaning solution and allow it to flow through the Enzymate
BEH Pepsin Column and the trap column. Inject a blank solution
to ensure no injection-to-injection carryover is observed. If
carryover is observed, repeat the cleaning solution injection
protocol until no injection-to-injection carryover is observed,
j. Column Storage
Store the Enzymate BEH Pepsin Column at 4 °C in 0.1% formic
acid in water when not in use. Make certain that the column end
caps are secured tightly in place to ensure that the Enzymate BEH
Pepsin Column does not dry out.
IV. CHECKING FOR ENZYMATIC ACTIVITY
The Enzymate BEH Pepsin Column should be checked periodically
to ensure acceptable enzymatic activity. Waters recommends
checking the enzymatic activity by injecting a standard
protein (e.g., Waters HDX Phosphorylase B Check Standard, PN
1860 06930) onto the Enzymate BEH Pepsin Column, followed
by LC-MS analysis to detect the peptides from the on-column
proteolysis. Acceptable performance criteria for the Enzymate
BEH Pepsin Column activity can be subjective. In general, if the
Phosphorylase B sequence coverage from the LC-MS analysis
is ≥ 75%, the Enzymate BEH Pepsin Column has maintained its
optimum enzymatic activity.
Enzymate BEH Pepsin Column
4
Page 5

[ CARE AND USE MANUAL ]
V. REFERENCES
1. Zhang, Z., and Smith, D. L., Determination of amide hydrogen exchange by mass spectrometry: a new tool for
protein structure elucidation, Protein Science, 1993, 2, 522-531.
2. Wales, T. E., and Engen, J. R., Hydrogen exchange mass spectrometry for the analysis of protein dynamics,
Mass Spectrometry Reviews, 2006, 25, 158-170.
3. Jacob, R.E., and Engen, J.R., Are we out of the quicksand?, American Society of Mass Spectrometry, 2012,
23, 1003 -1010.
To learn more about Hydrogen Deuterium Exchange with Mass Spectrometry,
please visit www.waters.com/HDX
Waters, The Science of W hat’s Possible, ACQUITY U PLC, nanoACQUITY UPLC, and UPLC are registered trademarks of Waters Corporation.
Enzymate and VanGuard are trademarks of Waters Corporation.
©2014 Waters Corporation. Produced in the U.S.A. April 2014 720005019EN KP-P DF
Waters Corporation
34 Maple Street
Milford, MA 01757 U.S.A.
T: 1 508 478 2000
F: 1 508 872 1990
www.waters.com
 Loading...
Loading...