Page 1
[ CARE AND USE MANUAL ]
ACQUITY UPLC BEH SEC COLUMNS AND STANDARDS
CONTENTS
I. INTRODUCTION
II. CONFIGURING AN ACQUITY UPLC SYSTEM FOR USE
IN SEC PROTEIN SEPARATIONS
a. Calibrators
III. GETTING STARTED
a. eCord™ Installation
b. Column Connectors
c. Column Installation
d. Column Equilibration
e. Useful Functional Tests for Benchmarking a New Column
IV. COLUMN SPECIFICATIONS AND USE
a. SEC Eluent and Needle Wash Preparation
b. Sample Preparation
c. Column Specification
V. TROUBLESHOOTING
VI. COLUMN CLEANING, REGENERATION AND STORAGE
a. Cleaning and Regeneration
b. Storage
I. INTRODUCTION
Thank you for choosing a Waters ACQUITY UPLC
column, and/or standard mix that are integral parts of Waters
ACQUITY UPLC SEC System Solution. The BEH125 SEC offering is
best suited for the analysis of peptides and proteins in the molecular
weight range from 1,000–80,000 Daltons that include insulin and
its aggregates. The BEH200 SEC column was designed to characterize
proteins ranging in molecular weight range from 10,000–450,000
Daltons that include monoclonal IgG monomers from aggregates,
while our BEH450 SEC is best suited for the analyses of proteins
and conjugates that range from 100,000–1.5 million Daltons
(Fig 1). All of these SEC chemistries are based on Waters Ethylene
Bridged Hybrid (BEH)-based particle technology and diol-bonded
surface that provide a stable chemistry with minimal non-desired
secondary interactions for proteins and peptides. The columns are
manufactured in a cGMP, ISO 9001 certified plant using ultra
pure reagents. Each batch of Protein Separation Technology SEC
packing material has been tested and qualified using a protein
test mixture (that is also available for purchase), and the results
are held to narrow specification ranges to ensure reproducible
performance. Every column is also individually tested for efficiency.
A Performance Test Chromatogram along with a Certification of
Analysis are available with each column.
®
BEH SEC guard,
VII. INTRODUCING eCord INTELLIGENT CHIP TECHNOLOGY
a. Introduction
b. Installation
c. Manufacturing Information
d. Column Use Information
VIII. ORDERING INFORMATION
ACQUITY UPLC BEH SEC Columns
1
Page 2
[ CARE AND USE MANUAL ]
II. CONFIGURING AN ACQUITY UPLC SYSTEM FOR USE IN
SEC PROTEIN SEPARATIONS
a. Calibrators
In order to obtain the best performance from your SEC column, it is
important that your ACQUITY UPLC system is properly configured. It
is recommended that only pre-cut tubing is used, and that the ID of
all connecting tubing is 0.005” or less for optimal chromatographic
performance.
Size-exclusion chromatography may require modifications to an
existing ACQUITY UPLC system. Please refer to “Size-Exclusion and
Ion-Exchange Chromatography of Proteins using the ACQUITY UPLC
System” (P/N 715002147A) for specific recommendations that can
be obtained at www.waters.com/chemcu
The sample loop used may affect the performance of your separation.
Optimally, select the smallest volume sample loop that is required
for the application. Sample loops larger than 20 µL with the ACQUITY
UPLC BEH SEC column are not recommended.
III. GETTING STARTED
A Performance Test Chromatogram and a Certification of Analysis
(COA) are available for each shipped ACQUITY UPLC BEH SEC column.
The COA is specific to each batch of packing material and includes the
batch number and chromatographic separation obtained using defined
protein standards. This document is available upon request at
www.waters.com/chemcu
1,000,000
100,000
10,000
MW
1000
100
IgM (900 kDa)
Ovalbumin (44 kDa)
Myoglobin (17 kDa)
RNase A (13.7 kDa)
Blue Dextran
Conalbumin (75 kDa)
Aprotinin (6.5 kDa)
Angiotensin frag 1-7 (899 Da)
Thyroglobulin (669 kDa)
IgG (150 kDa)
Amylglucosidase (97 kDa)
Allantoin (158 Da)
Uracil (112 Da)
ACQUITY UPLC BEH125 SEC,
1.7 µm: 1K–80K Daltons
ACQUITY UPLC BEH200 SEC,
1.7 µm: 10K–450K Daltons
ACQUITY UPLC BEH450 SEC,
2.5 µm: 100K–1500K Daltons
1
0.15
0.10
0.05
0.00
0.12
0.08
0.04
0.00
Thyroglobulin dimer
(Approx. 1.4 million Da ltons)
0.10
0.08
0.06
AU AU AU
0.04
0.02
0.00
2 3 4 5 6 7 8 min
4 5
2
3
1
6
4
2
3
1
7
5
6
2
3
ACQUITY U PLC
BEH125 SEC
ACQUITY U PLC
BEH200 SEC
7
ACQUITY U PLC
BEH450 SEC
Note: Stds 4 and 6 above
were not included in the
5
BEH450 Column test mix
7
Compounds:
1. Thyroglobulin (670K)
2. BSA (66K)
3. Ovalbumin (44K)
4. Carbonic Anhydrase (29K)
Columns
Column
Configuration
ACQUITY U PLC BEH125, 1.7 µm
ACQUITY U PLC BEH200 1.7 µm
5. Myoglobin (17K)
6. Angiotensin Frag 1–7 (899)
7. Uracil (112)
ACQUITY U PLC BEH450, 2.5 µm
4.6 x 150 mm
Mobile Phase 100 mM Sodium Phosphate Buffer, pH 6.8
Weak
Needle Wash
Strong
Needle Wash
100% Milli-Q® Water
100% Milli-Q Water
Seal wash 90/10 water/methanol
Thyroglobulin 0.3 mg/mL
Samples:
Diluted in
mobile phase
BSA 0.3 mg/mL
Ovalbumin 0.3 mg/mL
Carbonic Anhydrase 0.3 mg/mL
Myoglobin 0.3 mg/mL
Angiotensin Frag. 1–7 0.1 mg/mL
Uracil 0.1 mg/mL
Thyroglobulin 3 mg/mL
BSA 5 mg/mL
Ovalbumin 3 mg/mL
Myoglobin 2 mg/mL
Uracil 0.1 mg/mL
Injection Vol. 2 µL, Full Loop
Flow Rate 0.3 mL/min
Column Temp. Ambient
Detection
Wavelength
UV @ 220 nm UV @ 280 nm
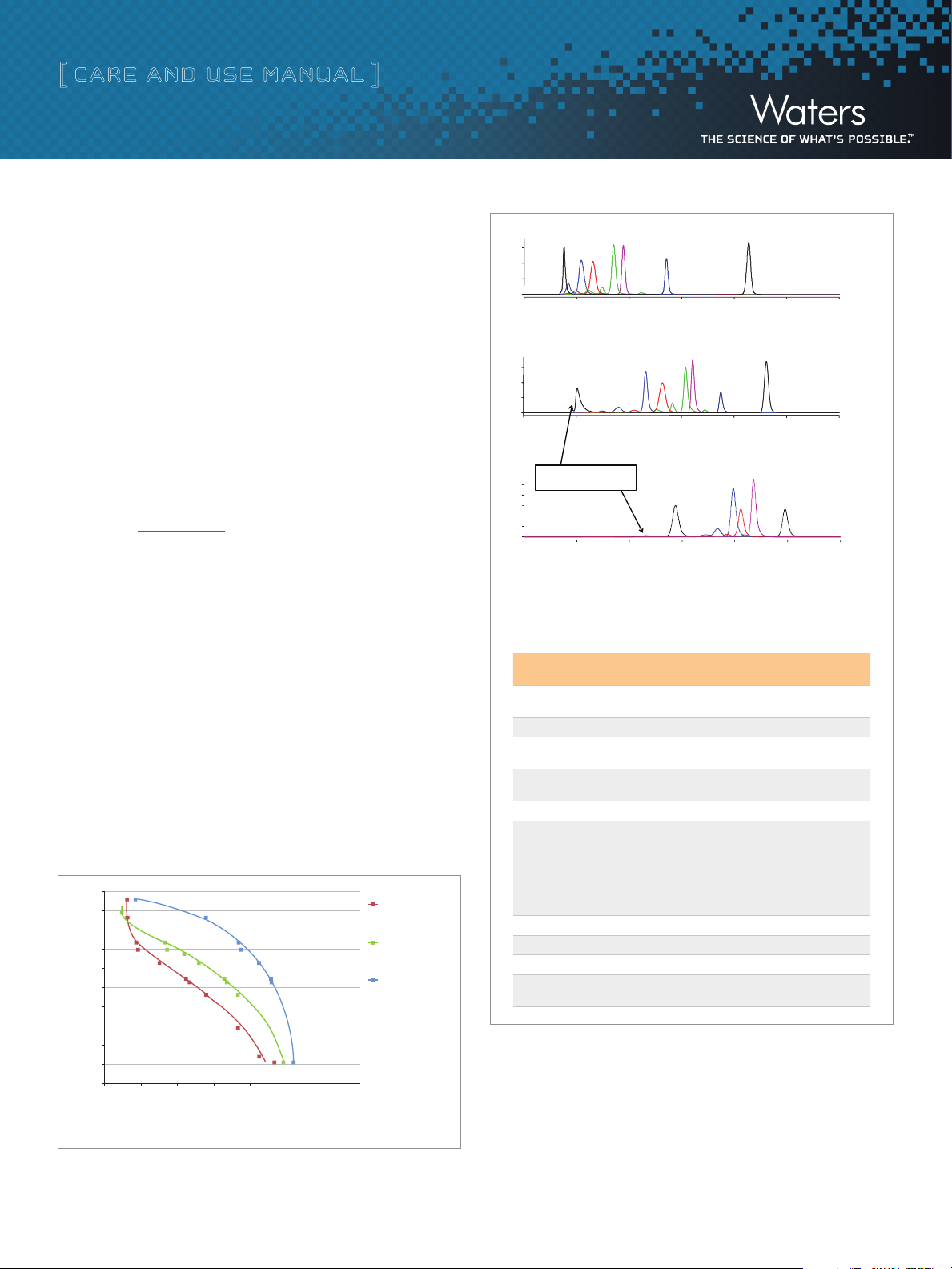
Figure 2. Separation of Protein and Peptide Standards on ACQUITY UPLC
BEH125, BEH200, and BEH450 SEC Columns
0.4 1.00.80.6
Normalized Retention Volume (Vr/VC)
Protein standards; T: 30°C; Mobile phase: 100mM sodium phosphate, pH=6.8
Figure 1. Calibration Curves on ACQUITY UPLC BEH125, BEH200, and
BEH450 SEC Columns
ACQUITY UPLC BEH SEC Columns
a. eCord Installation
The eCord button should be attached to the side of the ACQUITY UPLC
column heater module. The eCord button is magnetized and does not
require specific orientation.
2
Page 3

[ CARE AND USE MANUAL ]
b. Column Connectors
It is is recommended to use the 150 mm column stabilizer
(P/N 205000365) to connect the ACQUITY UPLC BEH SEC column
to the ACQUITY UPLC system. A reusable fitting is supplied with the
ACQUITY UPLC column stabilizer.
c. Column Installation
1. Prior to placing the column on the system, purge the solvent
delivery system of any organic or water-immiscible mobile phases.
When connecting the column, orient it in the proper direction as
noted by the arrow on the column inlet side which indicates the
correct direction of solvent flow.
2. Flush column with 100% aqueous buffer, by pumping at a flow
rate of 0.2 mL/min.
3. Ensure that the mobile phase is flowing freely from the column
outlet. Attach the column outlet to the detector using .004” ID
tubing (P/N 430001562). Monitor the system pressure to
ensure the column is within its pressure limitations.
e. Useful Functional Tests for Benchmarking a New Column
Waters recommends performing a benchmarking test upon receipt of
your column and throughout the lifetime usage. By using a separation
of common proteins with an appropriate method, you can:
Verify the performance of the column upon receipt.
Monitor the condition of the columns for extended use.
Troubleshoot separation difficulties that may arise.
The BEH125 SEC Protein Standard Mix (P/N 186006519), BEH200
SEC Protein Standard Mix (P/N 186006518), and BEH 450 SEC
Protein Standard Mix (P/N 1860 06 842) were specifically designed
for this purpose with carefully chosen proteins and/or peptides
to provide a good representation of the intended application.
Figure 3 is an example of the Performance Test Chromatogram using
Part Number 18600 6519, Figure 4 is an example of the Performance
Test Chromatogram using Part Number 186006518. Figure 5 is an
example of the Performance Test Chromatogram using Part Number
186006842. These are typical results obtained in a waters laboratory
using 4.6 x 150 mm columns on an ACQUITY UPLC system.
4. Gradually increase the flow rate, by not more than 0.1mL/min at a
time, as described in Step 2.
5. Once the system pressure has stabilized, ensure that there are no
leaks at either the column inlet or outlet.
d. Column Equilibration
ACQUITY UPLC BEH SEC columns are shipped in 20% methanol
in water. It is important to ensure mobile-phase compatibility before
changing to a different mobile-phase system. Equilibrate the column
with a minimum of 10 column volumes of the buffer to be used
(refer to Table 1 for column volumes).
Table 1. Empty Column Volumes in mL (multiply by 10 for flush
solvent volume)
Column Dimension Approximate Volume
4.6 x 150 mm 2.5 mL
4.6 x 300 mm 5.0 mL
Buffer Preparation for Standard:
Chemicals:
- Sodium Phosphate Monobasic, Monohydrate
- Sodium Phosphate Dibasic, Anhydrous
- Milli-Q Water
Preparation of 100mM Sodium Phosphate Buffer (500 mL)
- Weigh out 500 ± 0.02 grams of H2O into a 500 mL beaker.
- Weigh out 3.55 ± 0.02 grams of Sodium Phosphate dibasic and
add to the 500 mL beaker.
- Weigh out 3.45 ± 0.02 grams of Sodium Phosphate monobasic
and add to the 500 mL beaker.
- Stir for a minimum of 30 minutes then filter the solution
through a 0.2 µm Millipore GV filter.
- Take the pH and record the value for reference purposes
(approximate pH: 6.8).
ACQUITY UPLC BEH SEC Columns
3
Page 4
[ CARE AND USE MANUAL ]
0.09
0.06
0.03
0.00
0.0 1.0 2.0 3.0 4.0 5.0 6.0 7.0 8.0 9.0 10.0 min
3
2
1
4
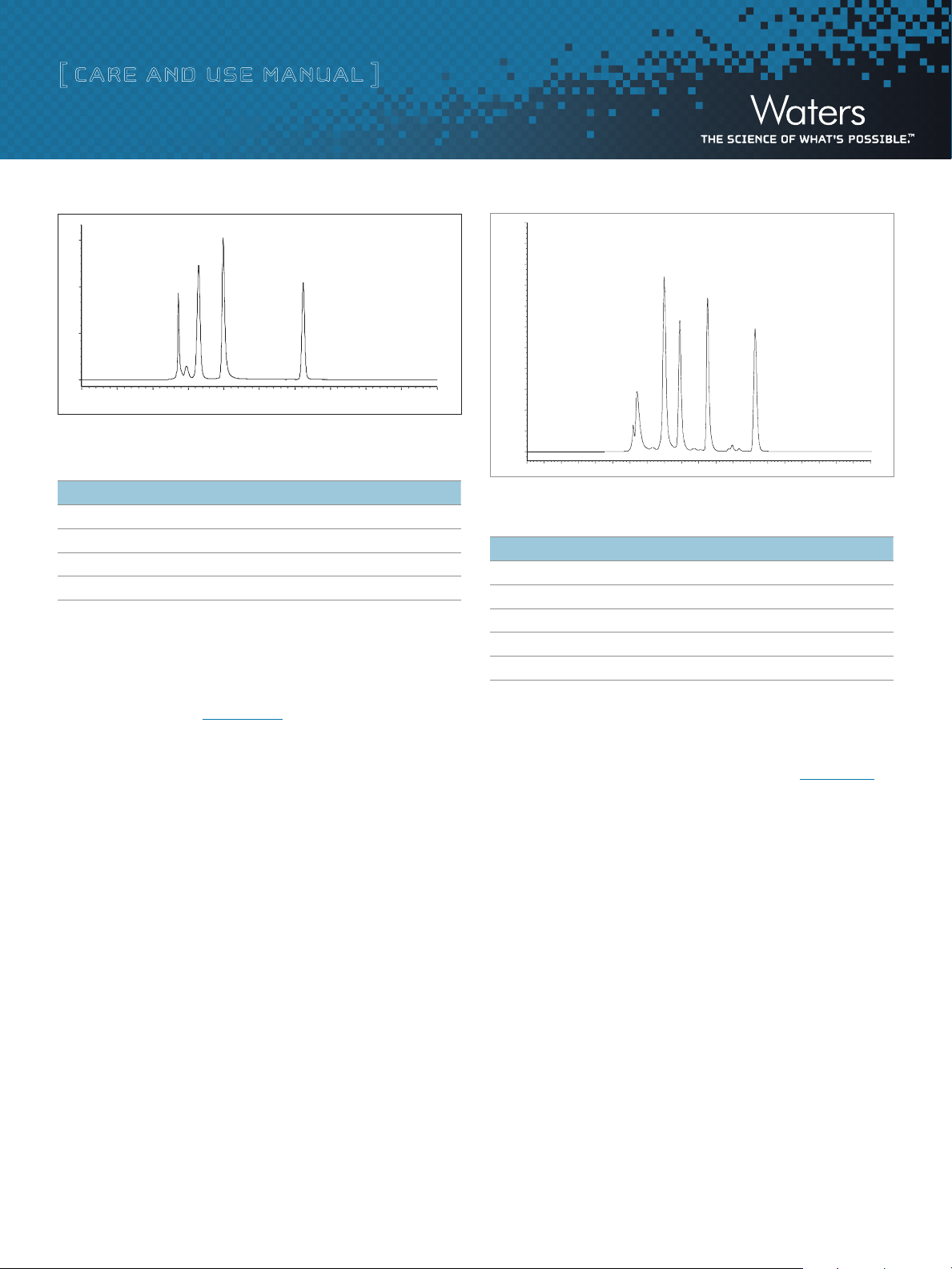
Figure 3. Protein mixture separation on an ACQUITY UPLC BEH125 SEC, 1.7 µm column.
Table 2. BEH125 SEC Test Mix
Analyte pI MW
1. Thyroglobulin, 0.1 mg/mL 4.6 669,000
2. Ovalbumin, 0.3 mg/mL 4.5 44,200
3. Ribonuclease A, 0.3 mg/mL 9.6 13,700
4. Uracil, 0.05 mg/mL N/A 112
Instrument: ACQUITY UPLC System with Tunable UV (TUV) detector
Column: ACQUITY UPLC BEH125 SEC, 4.6 x150 mm, 1.7 µm
Sample: BEH125 SEC Protein Standard Mix
(P/N 186006519)
BEH125 SEC Protein Standard Preparation:
Dissolve the BEH125 SEC Protein Standard in
500 μL of 100 mM sodium phosphate buffer pH
6.8. Once solubilized, do not freeze sample,
store at 2–8 °C for no more than a week.
Mobile Phase: 100 mM sodium phosphate, pH 6.8
Weak Needle Wash: 100% Milli-Q water
0.22
0.20
0.18
0.16
0.14
0.12
0.10
AU
0.08
0.06
0.04
0.02
0.00
0.0 0.5 1.0 1.5 2.0 2.5 3.0 3.5 4.0 4.5 5.0 5.5 6.0 6.5 7.0 7.5 8.0 8.5 9.0 9.5
2
4
3
1
5
10 min
Figure 4. Protein mixture separation on an ACQUITY UPLC BEH200 SEC, 1.7 µm column.
Table 3. BEH200 SEC Test Mix
Analyte pI MW
1. Thyroglobulin, 3 mg/mL 4.6 669,000
2. IgG, 2 mg/mL 6.7 150,000
3. BSA, 5 mg/m
L
4.6 66,400
4. Myoglobin, 2 mg/mL 6.8, 7.2 17,000
5. Uracil, 0.1 mg/mL N/A 112
Instrument: ACQUITY UPLC with Tunable UV (TUV) detector
Column: ACQUITY UPLC BEH200 SEC, 4.6 x 150 mm, 1.7 µm
Sample: BEH200 SEC Protein Standard (P/N 186006518)
BEH200 SEC Protein Standard Preparation:
Dissolve the BEH200 SEC Protein Standard in
500 µL of 100 mM sodium phosphate buffer
pH 6.8. Once solubilized, do not freeze sample,
store at 2–8 °C for no more than a week.
Strong Needle Wash: 100% Milli-Q water
Seal Wash: 90/10 water/methanol
Injection Type: Full loop
Injection Volume: 2 μL
Flow Rate: 0.3 mL/min
Column Temp.: 30 °C
Detection: UV @ 220 nm
ACQUITY UPLC BEH SEC Columns
Mobile Phase: 100 mM sodium phosphate, pH 6.8
Weak Needle Wash: 100% Milli-Q water
Strong Needle Wash: 100% Milli-Q water
Seal Wash: 90/10 water/methanol
Injection Type: Full loop
Injection Volume: 2 µL
Flow Rate: 0.3 mL/min
Column Temp.: 30 ˚C
Detection: UV @ 280 nm
4
Page 5

[ CARE AND USE MANUAL ]
IV. COLUMN SPECIFICATIONS AND USE
0.12
0.08
0.04
0.00
0.01.0 2.03.0 4.05.0 6.07.0 8.09.0 10.0 min
Figure 5. Protein Mixture on an ACQUITY UPLC BEH450 SEC, 2.5 µm column.
3
5
4
2
1
6
Table 4. BEH450 SEC Test Mix
Analyte pI MW
1. Thyroglobulin Dimer 4.6 1.4 million
2. Thyroglobulin, Approx 3 mg/mL 4.6 669,000
3. IgG, 2 mg/mL 6.7 150,000
4. BSA, 5 mg/mL 4.6 66,400
5. Myoglobin, 2 mg/mL 6.8, 7.2 17,000
6. Uracil, 0.1 mg/mL N/A 112
Instrument: ACQUITY UPLC System with Tunable UV (TUV) detector
Column: ACQUITY UPLC BEH450 SEC, 4.6 x150 mm, 2.5 µm
Sample: BEH450 SEC Protein Standard Mix
(P/N 186006842)
BEH450 SEC Protein Standard Preparation:
Dissolve the BEH450 SEC Protein Standard in
500 μL of 100 mM sodium phosphate buffer pH
6.8. Once solubilized, do not freeze sample,
store at 2–8 °C for no more than a week.
Mobile Phase: 100 mM sodium phosphate, pH 6.8
To ensure the continued high performance of ACQUITY UPLC BEH SEC,
1.7 µm columns, follow these guidelines:
a. SEC Eluent and Needle Wash Preparation
Use HPLC-grade buffers, water, and organic solvents when possible.
Filter solutions through a compatible 0.2 µm or smaller pore size
filter. The use of a sterile filtration apparatus is recommended
for buffers capable of supporting microbial growth.
Solutions that are susceptible to microbial growth should be
replaced at regular intervals to avoid column contamination.
Do NOT refill partially full SEC Eluent bottles with new Eluent.
Rather, when required use new bottle containing freshly prepared
SEC Eluent.
Select solvent inlet filters that are compatible with solutions
used, and clean or replace filters regularly when using
solutions that are susceptible to microbial growth.
b. Sample Preparation
1. Ensure that samples are free of particulates before injecting onto
the SEC column. If samples appear cloudy or turbid, they should
not be injected, as this could lead to column pressure increases.
Sample preparation such as filtration or centrifugation may be
used, if appropriate.
2. If the sample is not dissolved in the mobile phase, ensure that
the sample, solvent and mobile phases are miscible in order to
avoid sample and/or buffer precipitation.
Weak Needle Wash: 100% Milli-Q water
Strong Needle Wash: 100% Milli-Q water
Seal Wash: 90/10 water/methanol
Injection Type: Full loop
Injection Volume: 2 μL
Flow Rate: 0.3 mL/min
Column Temp.: 30 °C
Detection: UV @ 280 nm
ACQUITY UPLC BEH SEC Columns
c. Column Specifications
Shipping Solvent: 20% methanol in water
Recommended Operating Parameters
Recommended Flow Rate Range for maximum column lifetime:
0.1–0.4 mL/min
Recommended Maximum Operating Column Backpressure:
BEH125—10,000 psi for both 150 and 300 mm
BEH200—7,000 psi for both 150 and 300 mm
BEH450—4,500 psi for 150 and 7,500 psi for 300 mm
Mass Load: < 100 μg for a 4.6 x 150 mm
5
Page 6

[ CARE AND USE MANUAL ]
Volume Load: < 20 μL for 4.6 x 150 mm
Recommended pH Range: 2 to 8. The column lifetime will vary
depending upon the operating temperature as well as the type
and concentration of buffer used.
Recommended Salt Conc.: less than or equal to 0.5 M
Recommended Organic Conc.: < 20% acetonitrile (Caution: Many
proteins are insoluble at elevated organic concentrations. Prior to
chromatography, test to ensure the sample does not precipitate at
the organic concentration to be used for the chromatography. Also,
if column is run under denaturing conditions (greater than 10%
organic), subsequent column performance under 100% aqueous
conditions may be affected.)
Recommended Temperature: 4–60 °C. Reduce flow rate when
operating at low temperatures (e.g. 10 °C) to avoid excessive
column pressure.
Recommended Storage:
For overnight storage, continuously flush the column with the mobile
phase at 10–20% of the maximum recommended flow rate. Store
the column in the HPLC grade water when it will be used within
24 hrs or in 10–20% methanol for long term storage.
Note: Working at extremes of pressure, pH and/or temperature may
result in shorter column lifetimes.
V. TROUBLESHOOTING
The first step in systematic troubleshooting is comparison of the column
performance in its current state to the performance when it was
functioning properly. The functional tests with the protein mixture may
reveal subtle changes in surface chemistry that affect the application.
There are several common symptoms of change in the column.
1. An increase in pressure is often associated with decreased
performance in the application. The first step in diagnosis is to
ensure that the elevated pressure resides in the column rather than
somewhere else in the system. This is determined by monitoring
pressure of the system as each connection is broken from the outlet
end to the inlet. If the system is occluded, the blockage should be
identified and removed. If the pressure increase resides in the
column, it is helpful to know whether the problem was associated
with a single injection or whether it occurred over a series
of injections. If the pressure gradually built up, it is likely
that the column can be cleaned as described in Section VI.
If a single sample caused the pressure increase, it likely reflects
particulates or insoluble components, such as lipids or higher order
aggregates. Cleaning is still an option, but using the more
aggressive options. If samples appear cloudy or turbid, they should
not be injected, as this will lead to pressure increases. Sample
preparation such as filtration or centrifugation may be used, but one
should first check whether this impacts the results.
2. Loss of resolution and increased peak tailing can be caused by
microbial contamination. It is important to follow good standard
laboratory practices to prevent microbial contamination,
This includes changing buffer bottles frequently, using high purity
water, using a sterile filtration apparatus, and storing system and
column under recommended conditions. If microbial contamination
has occurred, cleaning the column will have no effect on
performance. When changing the flow rate, ramp it at a rate of
0.1 mL/min and avoid immediate flow-rate increases greater than
0.1 mL/min.
3. Increased peak tailing can be caused by failure of a tubing
connector or a build-up of material on the column inlet frit.
Before proceeding with diagnostic or corrective measures, check
all connections that the mobile phases have been correctly
prepared and the correct method has been selected. Then repeat
the protein standard test. If the proteins shows increased peak
tailing, it is likely that there is significant build-up of material
on the column inlet and the column will require replacement.
4. Carryover is defined as the appearance of the constituents of one
sample in the next analysis. In size-exclusion chromatography
carryover is typically due to system components or improper
wash solvents. Run a blank injection. If the protein peaks only
appear when an injection is made, they likely originate from
system component or inadequate wash solvents. Adsoprtion in
the system components most likely occurs in the loop or needle.
In these instances the component may need to be changed.
Note: Useful general information on column troubleshooting problems may
be found in HPLC Columns Theory, Technology and Practice, U.D. Neue,
(Wiley-VCH, 1997), the Waters HPLC Troubleshooting Guide (Literature code
720000181EN), and the Waters website, www.waters.com
ACQUITY UPLC BEH SEC Columns
6
Page 7
[ CARE AND USE MANUAL ]
VI. COLUMN CLEANING, REGENERATION AND STORAGE
a. Cleaning and Regeneration
Changes in peak shape, such as increased tailing, shoulders on
the peak, shifts in retention, change in resolution, ghost peaks, or
increased backpressure may indicate contamination of the column.
Choose a cleaning option that may be expected to dissolve the
suspected contaminant.
1. It may be useful to conduct cleaning procedures at one-half
the flow rate typically used with that column. In this way the
possibility of high pressure events is reduced.
2. Recommended cleaning solvents:
a. A concentrated salt solution at low pH
(e.g. 0.5 M Na2SO4, pH 2.7).
b. A low concentration of methanol (e.g., 20%) in HPLC
grade water.
c. Use of ionic detergents and other surfactants should be
avoided if the SEC column is to be subsequently used to
analyze native proteins.
b. Storage
For overnight storage, continuously flush the column with the mobile
phase at 10–20% of the maximum recommended flow rate. Store
the column in the HPLC grade water when it will be used within 24 hrs
or in 20% methanol for long term storage.
Note: Working at extremes of pressure, pH and/or temperature may
result in shorter column lifetimes.
VII. INTRODUCING eCord INTELLIGENT CHIP TECHNOLOGY
a. Introduction
The eCord Intelligent Chip is a technology that provides the history
of a column’s performance throughout its lifetime. The eCord
is permanently attached to the column to assure that the column’s
performance history is maintained in the event that the column
is moved from one instrument to another.
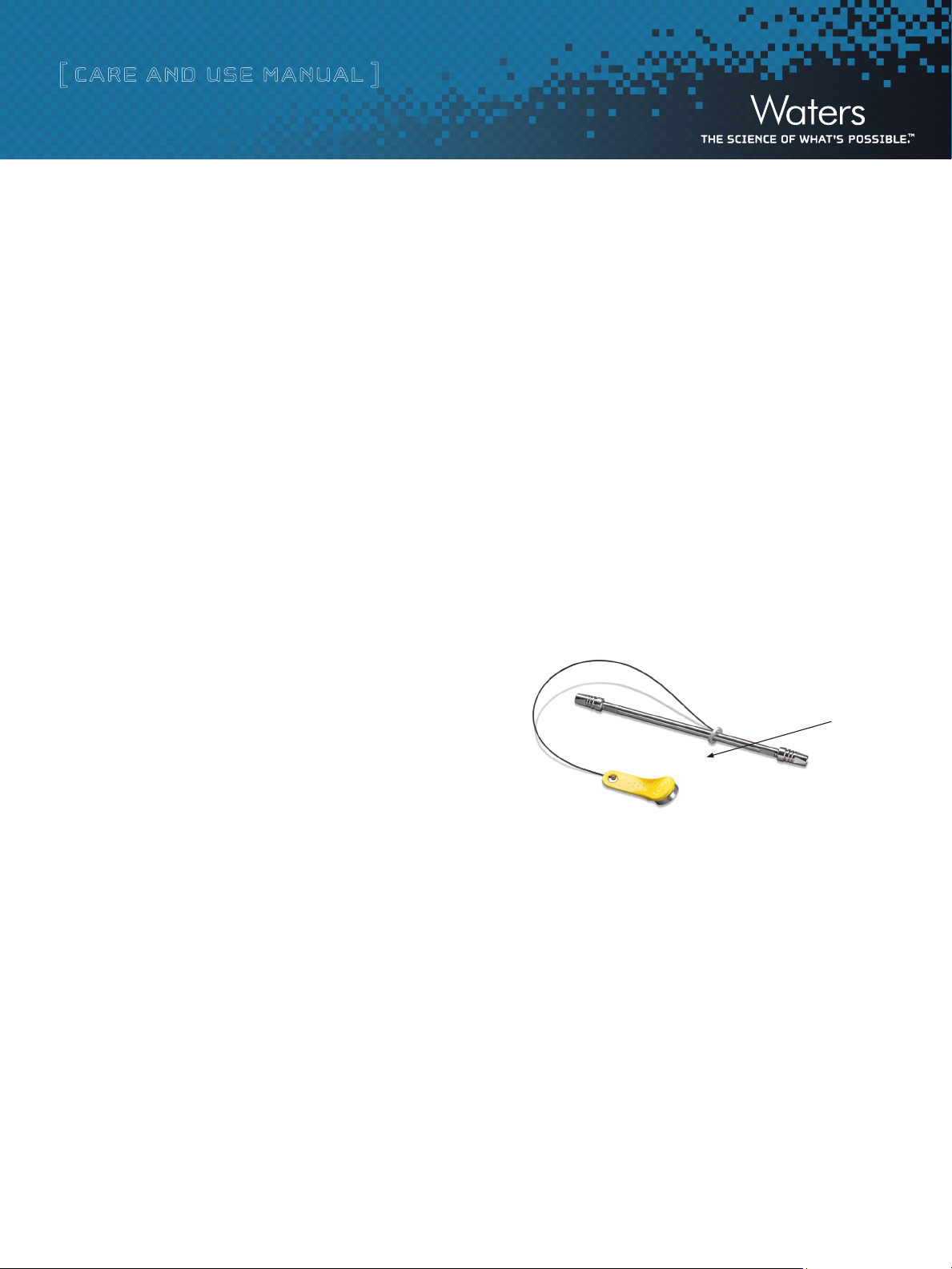
Waters eCord -
intelligent chip
Note: Choose a cleaning solvent based on sample properties, e.g. use
(a) to remove basic protein and (b) to remove hydrophobic proteins.
Chaotrophic agents can solvate strongly adsorbed proteins via hydrogen
bond disruption.
3. As a last resort, flow reversal or back flushing can be tried
at a low flow rate (e.g., 0.1 mL/min). However, this approach
may further damage the column or only provide short-lived
improvement in performance.
Figure 6. Waters eCord Intelligent Chip
At the time of manufacture, tracking and quality control information
will be downloaded to the eCord. Storing this information on the chip
will eliminate the need for a paper Certificate of Analysis. Once the
user installs the column, the software will automatically download
key parameters into a column history file stored on the chip. In this
manual, we explain how the eCord will provide a solution for easily
tracking the history of the columns, reduce the frustration of paper-
work trails, and give customers the reassurance that a well-performing
column is installed onto their instruments.
ACQUITY UPLC BEH SEC Columns
7
Page 8
[ CARE AND USE MANUAL ]
d. Column Use Information
b.
eCord inserted into
side of column heater
Figure 7. eCord Inserted into Side of Column Heater
Installation
Install the column into the column heater. Plug the eCord into the
side of the column heater. Once the eCord is inserted into the column
heater the identification and overall column usage information will be
available in Empower® and MassLynx® software allowing the user to
access column information on their desktop.
c. Manufacturing Information
The eCord chip provides the
user with an overview of the
bulk material QC test results.
The eCord chip provides the customer with column use data. The top
of the screen identifies the column including chemistry type, column
dimensions and serial number. The overall column usage information
includes the total number of samples, total number of injections, total
sample sets, date of first injection, date of last injection, maximum
pressure and temperature. The information also details the column
history by sample set including date started, sample set name, user
name, system name, number of injections in the sample set, number of
samples in the sample set, maximum pressure and temperature in the
sample set and if the column met basic system suitability requirements.
Up to 50 sample sets can be stored on the eCord chip.
ACQUITY UPLC BEH SEC Columns
The eCord chip provides the
user with QC test conditions
and results on the column
run by the manufacturer. The
information includes mobile
phases, running conditions
and analytes used to test
the columns. In addition the
QC results and acceptance is
placed onto the column.
8
Page 9

[ CARE AND USE MANUAL ]
VIII. ORDERING INFORMATION
Description Particle Size Dimensions Part No.
ACQUITY UPLC BEH125 SEC column 1.7 μm
ACQUITY UPLC BEH125 SEC column 1.7 μm
ACQUITY UPLC BEH125 SEC guard column kit 1.7 μm
ACQUITY UPLC BEH200 SEC column 1.7 μm
ACQUITY UPLC BEH200 SEC column 1.7 μm
ACQUITY UPLC BEH200 SEC guard column kit 1.7 μm
4.6 x 150 mm
4.6 x 300 mm
4.6 x 30 mm
4.6 x 150 mm
4.6 x 300 mm
4.6 x 30 mm
ACQUITY UPLC BEH450 SEC column 2.5 μm 4.6 x 150 mm
ACQUITY UPLC BEH450 SEC column 2.5 μm 4.6 x 300 mm
ACQUITY UPLC BEH450 SEC guard column kit
2.5 μm 4.6 x 30 mm
BEH125 SEC Protein Standard Mix
BEH200 SEC Protein Standard Mix
BEH450 SEC Protein Standard Mix
ELSD outlet tubing (0.004” id x 6” length)
0.005 x 1.75” SEC UPLC Connection Tubing, 2/pk
Notes: Size-exclusion chromatography may require modifications to an existing ACQUITY UPLC system. Please refer to “Size-Exclusion
and Ion-Exchange C hromatography of Proteins using the ACQUITY UPLC System”, (715002147) or “Size-Exclusion and Ion-Exchange
Chromatography of Proteins using the ACQUITY UPLC H-Class System”, (715002909) for specific recommendations.
186006505
186006506
186006504
186005225
186005226
186005793
176002996
176002997
186006850
186006519
186006518
186006842
430001562
186006613
Austria and European Export (Central South Eastern Europe, CIS and Middle East) 43 1 877 18 07, Australia 61 2 9933 1777, Belgium 32 2 726 1000, Brazil 55 11 4134 3788,
Canada 1 800 252 4752, China 86 21 6156 2666, Czech Republic 420 2 617 11384, Denmark 45 46 59 8080, Finland 358 9 5659 6288, France 33 1 30 48 72 00,
Germany 49 6196 400 600, Hong Kong 852 2964 1800, Hungary 36 1 350 5086, India and India Subcontinent 91 80 2837 1900, Ireland 353 1 448 1500, Italy 39 02 265 0983,
Japan 81 3 3471 7191, Korea 82 2 6300 4800, Mexico 52 55 52 00 1860, The Netherlands 31 76 508 7200, Norway 47 6 384 6050, Poland 48 22 833 4400, Puerto Rico 1 787 747 8445,
Russia/CIS 7 495 727 4490/ 290 9737, Singapore 65 6593 7100, Spain 34 93 600 9300, Sweden 46 8 555 115 00, Switzerland 41 56 676 7000, Taiwan 886 2 2501 9928,
United Kingdom 44 208 238 6100, All other countries: Waters Corporation U.S.A. 1 508 478 2000/1 800 252 4752
Waters Corporation
34 Maple Street
Milford, MA 01757 U.S.A.
T: 1 508 478 2000
F: 1 508 872 1990
www.waters.com
ACQUITY UPLC BEH SEC Columns
Waters, ACQUITY UP LC, Empower, and MassLynx are registered trademarks of
Waters Corporation. eCord and The Science of What’s Possible are trademarks of
Waters Corporation. Milli-Q is a registered trademark of Millipore Corporation.
All other trademarks are t he property of their respective owners.
October 2012 720003385EN Rev D IH-VW-P DF
9
 Loading...
Loading...