Page 1
[ Care and Use ManUal ]
Waters AccellPlus QMA and CM Bulk Media
I. calculatIng bulk medIa needs
II. packed column use
a. Slurry Column Packing
b. Dry Column Packing
c. Column Equilibration
d. Packed Column Testing
III. bulk medIa use
IV. addItIonal InformatIon
a. Chemical Compatibility
b. Sanitization
c. Regeneration
d. Short-Term Storage (less than 72 hours)
e. Long-Term Storage (more than 72 hours)
f. Troubleshooting
Waters AccellPlus™ QMA anion exchange and Waters AccellPlus CM
cation exchange packing materials are polymer-coated, silica-based
media of 37-55 µm particles with a 300Å pore size. AccellPlus
media is prepared by a patented copolymerization process that
encapsulates the rigid silica base with a hydrophilic bonding layer
and a highly stable cross-linked functional layer. AccellPlus media
provides excellent recovery and high resolution of bio-molecules. The
rigid and non-compressible structure of AccellPlus media makes it
well suited for the purification and isolation of proteins, enzymes and
immunoglobulins, particularly when preparative or process scale-up is
intended.
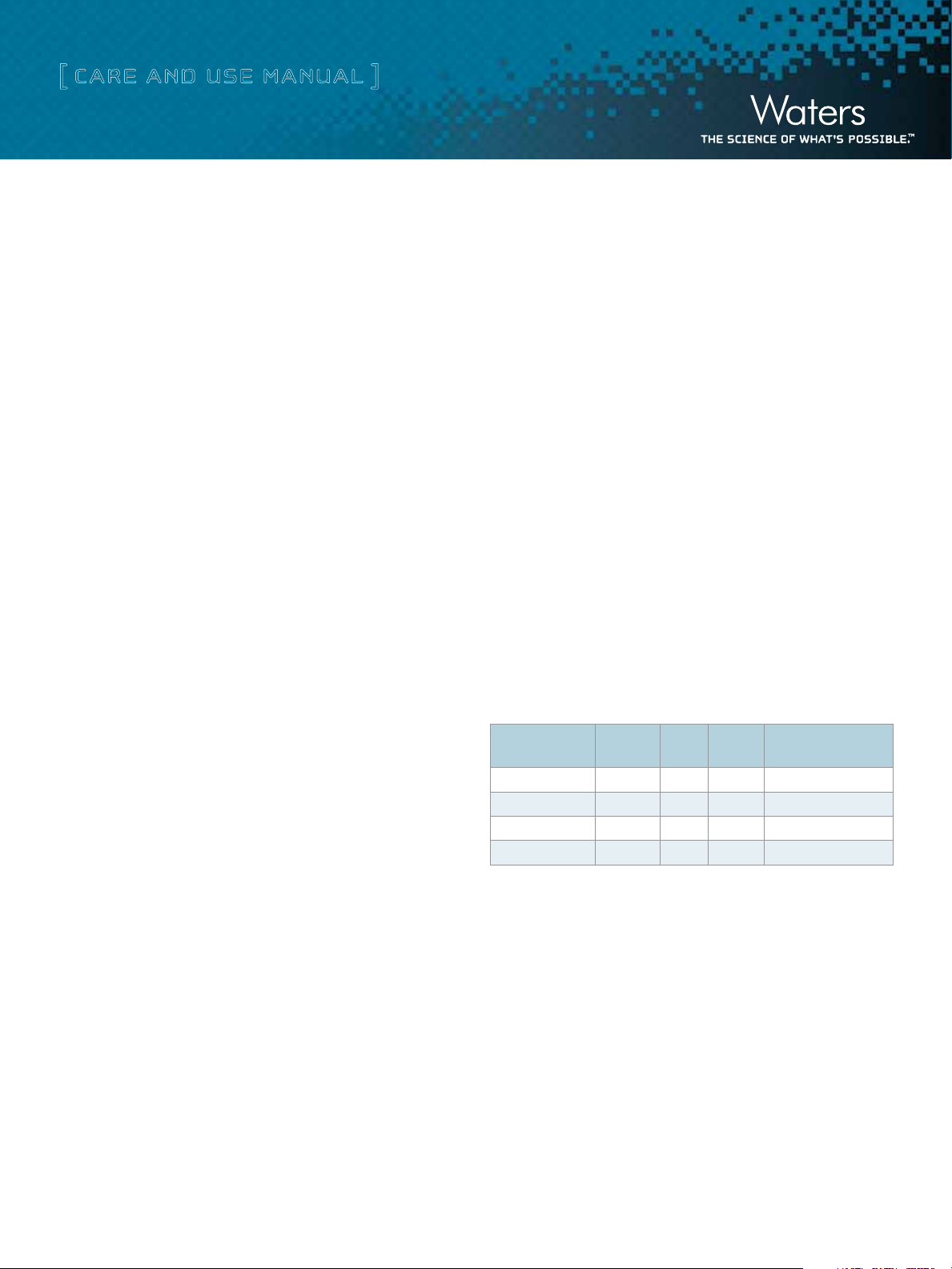
AccellPlus ion exchange media is available in 100 gm and 500 gm
bottles (Table 1). Larger quantities are available on request. The
media is shipped as a dry white finely divided powder and can be
stored dry.
Table 1. AccellPlus Ion-Exchange Bulk Packings
Particle
Description
AccellPlus QMA
Anion Exchanger
AccellPlus CM
Cation Exchanger
Particle
Size
40
µm 300Å
40
µm 300Å
40
µm 300Å
40
µm 300Å
Pore
Size
Qty. Part No.
100 g WAT010742
500 g WAT010741
100 g WAT010740
500 g WAT010739
Waters AccellPlus QMA and CM Bulk Media 1
Page 2
[ Care and Use ManUal ]
Required column volume (mL)
I. calculatIng bulk medIa needs
AccellPlus ion exchange media can be used for batch processes or can
be packed into columns. Columns may be dry- or slurry-packed using
AccellPlus media.
The amount of ion exchange medium and the column size to be used
depends on the amount of sample to be purified. Typically, optimum
performance is achieved when the total sample amount represents
less than 20% of the protein capacity of the AccellPlus medium used
(Tables 2 and 3). Samples exceeding 20% of the column’s maximum
protein binding capacity can be applied with somewhat lower than
optimal resolution of the components contained in the mixture. The
demands of the separation will determine the sample load for amount
of AccellPlus medium used.
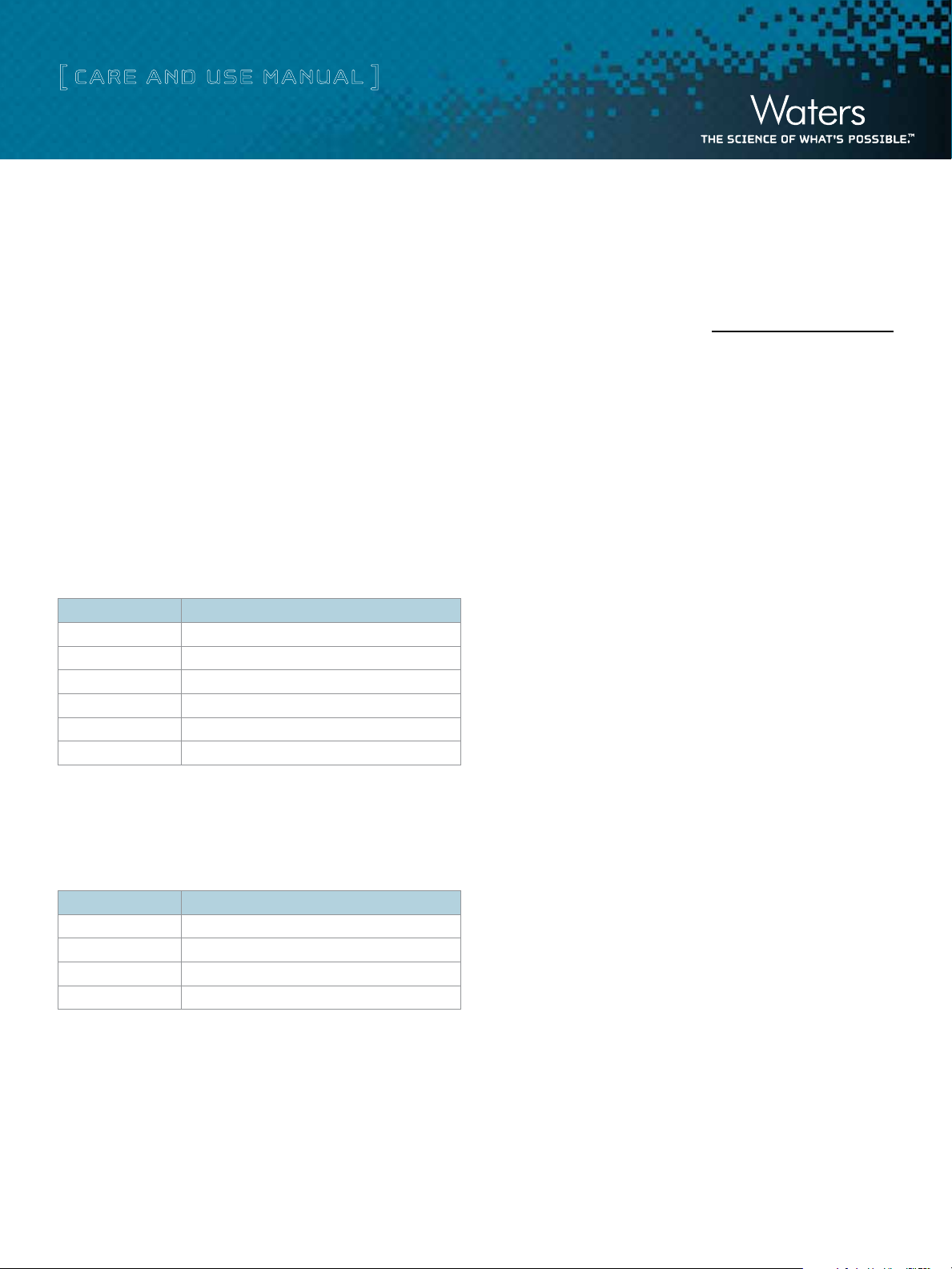
Table 2. AccellPlus QMA - Protein Binding Capacity
Measured using Bovine Serum Albumin in 0.02 M Tris/HCl buffer.
pH Mg BSA/gm Accell® (± 5%)
6.0 122
6.5 180
7.0 180
7.5 171
8.0 105
8.5 62
To pack a column with AccellPlus media:
The bulk density of AccellPlus is 0.5 g/mL. Thus, 1 gm of media will
produce 2 mLs of packed column bed.
AccellPlus media required (grams) =
2
a. Slurry Column Packing
Slurry packing using methanol solvent provides an efficient means
to column packing. We strongly recommend that columns greater
than 5 cm in diameter be slurry packed. Methanol slurry packing
produces more efficient columns providing better peak shapes than
slurry packing using dilute buffer or water eluents. Typically a
3-5:1 v/v methanol:AccellPlus slurry should be used. Pour slurry
directly into the column with the outlet attached to a water aspirator.
Alternatively, the columns can be packed using conventional HPLC
slurry packing techniques. AccellPlus media can withstand pressure of
up to 2000 psi without any problems.
b. Dry Column Packing
To dry pack AccellPlus media into any column follow these steps:
1. Pour the media slowly into the column while either tapping the
side or the base of the column. This will help the media settle
into a densely packed bed.
Protein binding capacity varies considerably based on selected
protein, buffer type, and pH.
Table 3. AccellPlus CM - Protein Binding Capacity
Measured using Cytochrome C in 0.02 M sodium phosphate.
pH Mg Cytochrome C/gm Accell (± 5%)
5.0 157
6.0 171
6.5 172
7.0 155
Note: For best results do no exceed 20% of protein binding capacity.
II. packed column use
Rigid AccellPlus particles greatly simplify column packing compared
to use of many traditional soft gel media. Columns can be easily
packed using either slurry- or dry-column packing techniques.
Waters AccellPlus QMA and CM Bulk Media 2
2. Once the column has been packed, flow mobile phase through
the column for 2-3 minutes at as high a flow rate as the pump
or column pressure limit will allow. This will settle the column
bed slightly. Voids should be filled by stirring the packing at the
column inlet and topping off with a thick slurry media.
3. Adjust the plunger or tap up with media to adjust voids. Repeat
this procedure until a stable column bed is obtained.
c. Column Equilibration
1. Wash the column with 5 column volumes of dilute buffer.
Note: If the column has been packed in methanol, flush with 5
column volumes HPLC-grade water prior to the dilute buffer wash.
2. After the dilute buffer wash, wash the column with 5 column
volumes of concentrated buffer.
3. Wash the column with starting buffer until the pH and ionic
strength of the column eluate match those of the solvent being
pumped onto the column.
Page 3

[ Care and Use ManUal ]
2
The AccellPlus column should now be tested (see Packed Column
Testing). Use methanol as the mobile phase to run the efficiency and
asymmetry test.
d. Packed Column Testing
Waters recommends that columns be tested prior to use. There are
many different ways of doing this, but one of the simplest is to inject
a small volume of a 500:1 buffer:acetone solution. This will absorb
effectively at 280 nm. The peak should be symmetrical. A typical
calculated plate count an AccellPlus IEX slurry packed, 1 x 10 cm
column should be greater than 500 plates. Lower observed values
would be normal for columns that are packed using the less efficient,
dry packing process.
N = 16
Rt
W
Rt = retention time in minutes
W = peak width at baseline in minutes
III. bulk medIa use
Determine the amount of bulk ion exchange media required for
application needs (see Section II, Table 2 or Table 3).
• 100% methanol
• 100% acetone
• 100% ethanol
• 0.1% sodium azide
• 70% ethanol
• 2 M sodium chloride
• 0.25 M hydrochloric acid (limited)
• 0.1 M sodium hydroxide (limited)
b. Sanitization
70% ethanol, 0.10% sodium azide or 0.5% Hibitane are effective
sanitizing agents.
c. Regeneration
Clean protein solutions, carefully filtering of buffers, and occasional
column regeneration will yield 100 or more runs. Occasionally, a
reduction in capacity is observed. When this happens, the column
should be regenerated by pumping 5 column volumes of 2 M sodium
chloride at pH 7.5 for the AccellPlus QMA media and pH 4 for the
AccellPlus CM media. More aggressive cleaning solutions may be
found in Table 4.
Slurry the media with 5 times the media’s volume of dilute buffer
for 2-3 minutes. The media should be allowed to settle and then the
excess buffer drained off. A similar volume of concentrated buffer
(0.1 M) should be added, slurried up 2-3 minutes, and then drained
off. The same procedure should be repeated with dilute buffer until pH
and salt concentration remain at the desired values.
IV. addItIonal InformatIon
a. Chemical Compatibility
AccellPlus is fully stable in aqueous buffers between pH 2-9. The
following reagents are compatible with AccellPlus media for use in
cleaning and as anti-microbials.
• 10% acetic acid (pH 2.5)
• 2.5 M sodium acetate (pH 8) 20% aqueous propanol,
0.1% trifluoroacetic acid 5 M urea
• 0.5% Hibitane™ (chlorohexidine)
• 5 M urea
• 1% Triton™ X-100
• 0.1% sodium azide
Table 4. Suggested Cleaning Solutions
Agent Concentration Exposure
Sodium Hypochlorite 1000 ppm 2 hours
Hydrochloric Acid 0.25 M less than 30 minutes
Sodium Hydroxide 0.1 M
d. Short-Term Storage (less than 72 hours)
AccellPlus media does not require special storage or treatment for
periods less than 72 hours. We recommend, however, that the
columns be stored at 4 °C.
e. Long-Term Storage (more than 72 hours)
Although AccellPlus media is not susceptible to microbial attack, an
anti-microbial such as Hibitane, sodium azide or 70% ethanol can be
added (see Chemical Compatibility). If the medium is to be stored dry,
pump the medium out of the column, wash with 10 column volumes
of 1 M sodium chloride, 10 column volumes of HPLC-grade water, and
10 column volumes of methanol. Dry the media under vacuum at 80 °C.
3 column volumes pump through
in less than 20 minutes
Waters AccellPlus QMA and CM Bulk Media 3
Page 4

[ Care and Use ManUal ]
f. Trouble Shooting
Problem Cause Remedy
Poor Resolution Sample overload Reduce sample amount (see Bulk Media Packing).
Flow rate too high Reduce flow rate.
Media fouling Reverse flow direction. Flush with suitable cleaning agent (see Chemical Compatibility).
Column voiding Check efficiency, and if low, repack or fill column void.
Low Flow Rate Media fouling Reverse flow direction. Flush with suitable cleaning agent (see Chemical Compatibility).
Frits plugged
Low Capacity Salt concentration too high
pH too high (QMA)
pH too low (CM)
Inappropriate buffer salt
Reverse flow. Replace connector frits.
Reduce salt concentration.
Lower pH if protein stability is not affected.
Raise pH if protein stability is not affected.
Change buffer salt.
Waters AccellPlus QMA and CM Bulk Media 4
Page 5

[ Care and Use ManUal ]
Sales Offices
Austria and European Export
(Central South Eastern Europe, CIS
and Middle East) 43 1 877 18 07
Australia 61 2 9933 1777
Belgium 32 2 726 1000
Brazil 55 11 5094 3788
Canada 1 800 252 4752
China 86 10 5293 6688
Czech Republic 420 2 617 11384
Denmark 45 46 59 8080
Finland 358 9 5659 6288
France 33 1 30 48 72 00
Germany 49 6196 400 600
Hong Kong 852 2964 1800
Hungary 36 1 350 5086
Norway 47 6 384 6050
Poland 48 22 833 4400
Puerto Rico 1 787 747 8445
Russia/CIS 7 495 727 4490/ 290 9737
Singapore 65 6593 7100
Spain 34 93 600 9300
Sweden 46 8 555 115 00
Switzerland 41 56 676 7000
Taiwan 886 2 2501 9928
United Kingdom 44 208 238 6100
All other countries:
Waters Corporation U.S.A.
1 508 478 2000
1 800 252 4752
www.waters.com
India 91 80 2837 1900
Ireland 353 1 448 1500
Italy 39 02 265 0983
Japan 81 3 3471 7191
Korea 82 2 6300 4800
Mexico 52 55 52 00 1860
The Netherlands 31 76 508 7200
©2012 Waters Corporation. Waters, AccellPlus, Accell, and
The Science of What's Possible are trademarks of Waters
Corporation. Hibitane is a trademark of Ayerst Labs and
Imperial Chemical Industries, Ltd. Triton is a trademark of Rohm
and Haas Company.
December 2012 WAT010737 Rev D LS--KK-PDF
Waters Corporation
34 Maple Street
Milford, MA 01757 U.S.A.
T: 1 508 478 2000
F: 1 508 872 1990
www.waters.com
Waters AccellPlus QMA and CM Bulk Media 5
 Loading...
Loading...