Venus Viva User Manual
User Manual
International
Venus VIVA User Manual |
Effective Date: 01.05.14 P/N: DCVIIRB2 Rev.02 |

Copyright 2009 Venus Concept. All rights reserved.
Print date: May 2014
Venus Concept Ltd. reserves the right to make changes to its products or specifications to improve performance, reliability, or manufacturability. Information furnished by Venus Concept Ltd. is believed to be accurate and reliable. However, Venus Concept Ltd. assumes no responsibility for its use. No license is granted by its implication or otherwise under any patent or patent rights of Venus Concept Ltd.
No part of this document may be produced or transmitted in any form or by any means, electronic or mechanical, for any purpose, without the express written permission of Venus Concept Ltd.
Data is subject to change without notification.
Venus Concept Ltd. has patents and pending patent applications, trademarks, copyrights, or other intellectual property rights covering subject matter in this document. The furnishing of this document does not give you any license to these patents, trademarks, copyrights, or other intellectual property rights except as expressly provided in any written agreement from Venus Concept Ltd.
International (M) |
Hachermesh Street Bld. 62, Karmiel 21652, Israel. |
Canada |
255 Consumers Rd. Suite 100, Toronto, Ontario. M2J 1R4. |
Europe (P) |
Obelis S.A. - Av.de Tervuren, 34 bte 44, B-1040 Brussels, Belgium. |
General Inquiries |
info@venus-concept.com |
Service Inquiries |
service@venus-concept.com |
Website |
www.venus-concept.com |
For Latin America and the Caribbean please call 1-888-907-0115.
All other inquires, please contact: Venus Concept is a subsidiary Of Venus Technologies
#3 HaBarzel St., Tel Aviv, Israel 6971005 Ph. +972 3 647 1882
Venus VIVA User Manual |
Effective Date: 01.05.14 P/N: DCVIIRB2 Rev.02 |
Table of Contents
|
Chapter 1 - Before You Start…………………………………………… ......................... |
…1-1 |
|
|
Glossary of Symbols Used in this Manual |
........................................................................ |
1-1 |
|
|
S/N Label ......................................................................................................................... |
|
1-2 |
|
|
WARNING........................................................................................................................ |
|
1-3 |
|
|
Chapter 2 - Introduction.................................................................................................. |
|
2-1 |
|
|
View of Venus Viva System ............................................................................................. |
|
2-1 |
|
|
System Description .......................................................................................................... |
|
2-2 |
|
|
Chapter 3 - Safety .......................................................................................................... |
|
3-1 |
|
|
Introduction ...................................................................................................................... |
|
3-1 |
|
|
The Operator.................................................................................................................... |
|
3-2 |
|
|
Electrical and Mechanical Safety ..................................................................................... |
|
3-4 |
|
|
Fire Hazards .................................................................................................................... |
|
3-4 |
|
|
Chapter 4 - System Installation ...................................................................................... |
|
4-1 |
|
|
Equipment List ................................................................................................................. |
|
4-1 |
|
|
Electrical Requirements ................................................................................................... |
|
4-2 |
|
|
Environmental Requirements........................................................................................... |
|
4-2 |
|
|
Connect the Applicators ................................................................................................... |
|
4-3 |
|
|
Connect/Disconnecting a Tip to Viva Fractional ................................................Applictor |
4-4 |
|
||
Chapter 5 - Operating the System.................................................................................. |
|
5-1 |
|
|
Turning the System On/Off .............................................................................................. |
|
5-1 |
|
|
Login Screen.................................................................................................................... |
|
5-1 |
|
|
Treatment Screen ............................................................................................................ |
|
5-2 |
|
|
Selecting Desired Applicator ............................................................................................ |
|
5-3 |
|
|
Fractional RF Treatment Mode ........................................................................................ |
|
5-3 |
|
|
Operating the Viva Fractional Applicator.......................................................................... |
|
5-7 |
|
|
(MP)2 Treatment Mode .................................................................................................... |
|
5-8 |
|
|
Tools Screen.................................................................................................................. |
|
5-10 |
|
|
Chapter 6 - Treatment Procedures................................................................................. |
|
6-1 |
|
|
Fractional RF Treatment Mode Using Viva ....................................Fractional Applicator |
6-1 |
|
||
Exclusion Criteria ............................................................................................................. |
|
6-1 |
|
|
Possible Side Effects ....................................................................................................... |
|
6-3 |
|
|
Pre-treatment ................................................................................................................... |
|
6-3 |
|
|
Test Treatment................................................................................................................. |
|
6-4 |
|
|
Treatment Procedure ....................................................................................................... |
|
6-5 |
|
|
Treatment Protocol........................................................................................................... |
|
6-6 |
|
|
Treatment Parameters ..................................................................................................... |
|
6-7 |
|
|
Post Treatment Care........................................................................................................ |
|
6-7 |
|
|
|
|
|
|
|
Venus VIVA User Manual |
Effective Date: 01.05.14 P/N: DCVIIRB2 Rev.02 |
|||

Follow-Up......................................................................................................................... |
6-8 |
Treatment Conclusion ...................................................................................................... |
6-8 |
(MP)2 Treatment Modality Using Diamondpolar Applicator .............................................. |
6-9 |
Exclusion Criteria ............................................................................................................. |
6-9 |
Pre-Treatment................................................................................................................ |
6-10 |
General Treatment Information ...................................................................................... |
6-11 |
Chapter 7 - Maintenance................................................................................................ |
7-1 |
Cleaning the System ........................................................................................................ |
7-1 |
Cleaning the applicators................................................................................................... |
7-1 |
Disinfection the applicators .............................................................................................. |
7-2 |
Cleaning the tip ................................................................................................................ |
7-2 |
Chapter 8 - Troubleshooting............................................................................................ |
8-1 |
Chapter 9 - Technical Specifications .............................................................................. |
9-1 |
Chapter 10 - Manufacturer Warranty ............................................................................. |
10-1 |
Venus VIVA User Manual |
Effective Date: 01.05.14 P/N: DCVIIRB2 Rev.02 |

Before You Start
Glossary of Symbols Used in this Manual
The following are symbols that you will find throughout this operating manual and their meanings:
WARNING sign: The information stated where you will see this symbol is extremely important and must be noted!
Provides general information that is important to keep in mind.
Waste of Electrical and Electronic Equipment (WEEE) Marking.
Fuse.
Type BF Equipment.
Manufacturer.
Authorized Representative in the European Community.
Manufacturer (accompanied by the name and address of the manufacturer).
Date of Manufacture.
Symbol used with a HF isolated patient circuit.
Venus VIVA User Manual |
1-1 |
Effective Date: 01.05.14 P/N: DCVIIRB2 Rev.02 |
|
|
Consult Instruction for Use.
System that includes RF transmitters or that applies RF electromagnetic energy for diagnosis or treatment.
Obelis S.A.
“Conformité Européene" Symbol (CE Marking).
S/N Label
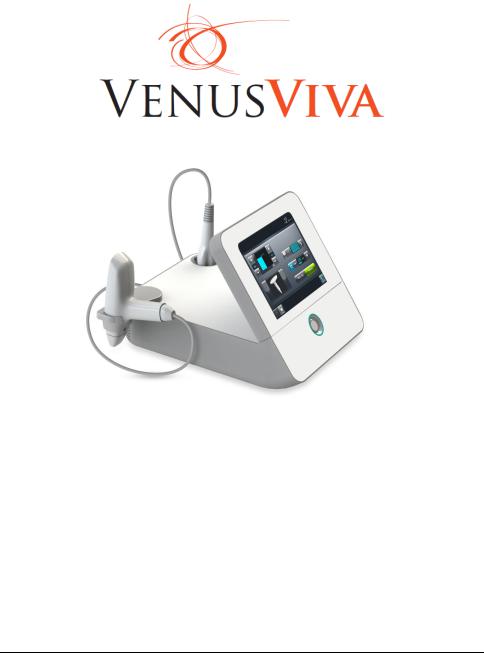
Figure 1-1, Venus Viva
Serial Number Label
Venus VIVA User Manual |
1-2 |
Effective Date: 01.05.14 P/N: DCVIIRB2 Rev.02 |
|
|

WARNING
The User Manual is for IRB only. Please read the User Manual instructions carefully before installing or using the System to
become familiar with all safety requirements and operating procedures thereby prevent accidents, injury and reduce the risk of damaging the machine.
The Venus VIVA is designed for professional use only. The manufacturer cannot be held responsible for damage or injury caused by improper use or for uses other than those for which this machine is intended.
Venus VIVA User Manual |
1-3 |
Effective Date: 01.05.14 P/N: DCVIIRB2 Rev.02 |
|
|

2. Introduction
View of Venus Viva System
a |
b |
c |
d |
|
|
|
e |
f |
|
|
|
g |
h |
i |
j |
|
Venus VIVA User Manual |
2-1 |
Effective Date: 01.05.14 P/N: DCVIIRB2 Rev.02 |
|
|

a.Diamondpolar Applicator. (detachable accessory)
b.Viva Fractional Applicator. (detachable accessory)
c.Diamonpolar Holder.
d.Viva Fractional Holder.
e.Diamondpolar Applicator Socket.
f.Viva Fractional Applicator Socket.
g.Touch Screen.
h.Serial Number Label.
i.On/Off Button.
j.Power Socket.
System Description
The Venus VIVA is a non invasive medical aesthetic device intended for use in dermatologic and general surgical procedures requiring:
Ablation and resurfacing of the skin, using Viva Fractional applicator
Treatment of moderate to severe facial wrinkles and rhytides in Fitzpatrick skin types I- IV, using Diamondpolar applicator
Skin Rejuvenation with Venus Viva is a unique procedure which uses radio frequency energy to deliver an effective but controlled ablative treatment.
The energies supplement each other to provide optimal treatment results with minimal risk of side effects.
Venus VIVA User Manual |
2-2 |
Effective Date: 01.05.14 P/N: DCVIIRB2 Rev.02 |
|
|

The system offers 2 main applicators for face area:
Diamondpolar Applicator. (detachable accessory)
Viva Fractional Applicator. (detachable accessory) Intended user and environment:
The device is intended to be used by professional practitioners in the medical aesthetic field.
The operator should stand near the client.
The operator is not allowed to leave a client during the entire treatment.
Venus VIVA User Manual |
2-3 |
Effective Date: 01.05.14 P/N: DCVIIRB2 Rev.02 |
|
|

3. Safety
This chapter describes safety issues regarding the use and maintenance of the System, with special emphasis on electrical safety.
Please carefully read this chapter and be familiar with all of its safety requirements and operating procedures prior to operating the System.
Caution
Federal (USA) law restricts this device to sale by or on the order of a physician or licensed practitioner.
Introduction
The System is designed for a safe and reliable treatment when used in accordance to proper operating and maintenance procedures as outlined in this operating manual. Only trained and qualified personnel, by an authorized trainer, can use the system and perform the treatments. The operator and all other personnel operating or maintaining the System should be familiar with all of the safety information provided in this manual.
The primary objective should always be in maximizing the safety of both the client and the treatment operator.
Venus VIVA User Manual |
3-1 |
Effective Date: 01.05.14 P/N: DCVIIRB2 Rev.02 |
|
|

The Operator
All operators MUST be familiar with the system controls and know how to shut down the system in case of trouble.
Always be aware of the possible dangers of using the System and take proper precautions as described in this manual.
Do not touch the inner parts of the System. The System services and repairs must be performed by qualified personnel only. Failure to do so will void all service agreements.
RF used in this (MP)² technology can cause injury if used improperly.
High voltage is present inside the System, do not attempt to open the casing.
Disconnect the System from the power supply before servicing (pull out the plug).
Do not use the System unless all enclosure panels are properly in place.
Do not tamper with the controls or attempt to open up the System.
Do not abuse, sit or lean on the system.
Venus Viva System should be kept out of the reach of Children.
Do not allow the Applicators to come in contact with metal elements this could damage the (MP)2 electrodes.
A patient history should be completed prior to treatment to ensure no complications could arise. It is important to verify client does not full under the exclusion criteria.
The PATIENT should not come into contact with metal parts which are earthed or which have an appreciable capacitance to earth (for example operating table supports, etc). The use of antistatic sheeting is recommended for this purpose.
The cables to the Applicators should be positioned in such a way that contact with the PATIENT or other leads is avoided.
The product should not be in contact with other equipment.
Failure of the System could result in an unintended increase of output power.
Interference produced by the operation of HF SURGICAL EQUIPEMENT may adversely influence the operation of other electronic EQUIPEMENT.
For PATIENT with cardiac pacemakers or other active implants, a
Venus VIVA User Manual |
3-2 |
Effective Date: 01.05.14 P/N: DCVIIRB2 Rev.02 |
|
|

possible hazard exists because interference with the action of the pacemakers may occur, or the pacemaker may be damaged. In case of doubt, approved qualification advice should be obtained.
This equipment/system may cause radio interference or may disrupt the operation of nearby equipment. It may be necessary to take mitigation measures. Such as reorienting or relocating.
Portable and mobile RF communications equipment can affect the System.
The patient should then be fully informed of the treatment protocol, expected results and should sign informed consent form prior to beginning treatments.
Only authorized person is allowed to stand near the system during the treatment.
Stop the treatment in case of unexpected changes in client’s condition.
Do not drop the Applicators. In case the Applicators were dropped, turn off the system immediately. Don’t use the broken applicator and call to local service support.
There are no user-serviceable parts inside the system. ONLY VenusAUTHORIZED PERSONNEL MAY SERVICE THE SYSTEM, ESPECIALLY INSIDE ITS CABINET.
Keep the bodies of the applicators clean; pay particular attention to the RF electrodes of the Viva Fractional applicator. Check the integrity of all components.
Tip of the Viva Fractional applicator should be used per patient only
Do not allow the applicators to come in contact with hard materials that could damage the tips.
Venus VIVA User Manual |
3-3 |
Effective Date: 01.05.14 P/N: DCVIIRB2 Rev.02 |
|
|

Electrical and Mechanical Safety
Keep all covers and panels of the System closed. Removing the covers creates a safety hazard.
Keep your hands away from the applicators during the System start-up.
Perform maintenance procedures when the System is shut down and disconnected from power.
Move the System slowly and carefully. The System weighs approximately 8kg (~17pounds) and may cause injury if proper care is not taken when moving it.
The System is grounded through the grounding conductor in the power cord. This protective grounding is essential for safe operation.
Fire Hazards
Do not use the System in the presence of explosive or flammable materials.
Do not use flammable substances when preparing the skin for treatment.
If germicide swipes is used for cleaning and disinfecting the system, it must be allowed to fully dry before the System can be used again.
Venus VIVA User Manual |
3-4 |
Effective Date: 01.05.14 P/N: DCVIIRB2 Rev.02 |
|
|

4. System Installation
The system is designed for operation in a clinical environment and it may be easily transported inside a protective carrying case. The Venus VIVA system is set up by doing the following:
1.Unpack the system.
2.Check the integrity of the system and all components.
3.Connect the Viva Fractional applicator to the system platform
4.Connect the Diamondpolar applicator to the system platform.
5.Connect the power cable to the system cable connection port.
6.Plug the system's power cable into an appropriate electrical outlet.
7.Turn the system on.
Equipment List
The System includes the following:
System platform (Console Venus VIVA).
Diamondpolar Applicator. (detachable accessory)
Viva Fractional Applicator. (detachable accessory)
Power cord.
User Manual.
Quick reference guide (only for non-English speakers’ countries).
Tips
Brush
Venus VIVA User Manual |
4-1 |
Effective Date: 01.05.14 P/N: DCVIIRB2 Rev.02 |
|
|

Electrical Requirements
The System can automatically accommodate the most of the local mains voltage. Specifically, the System can be energized of the mains voltage as follow:
Single phase 100/120/240V ~, 350W(MAX), 50/60Hz
For continued protection against fire, only fuses that are defined on the System label can be insulted in the System. Only power cord which is suitable to be connected to the System and approved for the local mains, must be used.
Environmental Requirements
Corrosive materials can damage electronic parts, ensure that the environment is free from corrosive material.
Metallic dust can damage electrical equipment.
For optimal operation, the System should be placed in a room with temperature between 10º35ºC (50º-95ºF) with a relative humidity of less than 80% and altitude up to 3000m.
For optimal storage, system should be stored in a place with temperature between -20 to 55°C (-13 to 131°F) with a relative humidity of 0-90 % @ 55°C noncondensing. For Optimal transportation system should be transport under the temperature range between -10º- 60ºC (14º-140ºF) with a relative humidity of less than 80% and altitude up to 15000m.
Venus VIVA User Manual |
4-2 |
Effective Date: 01.05.14 P/N: DCVIIRB2 Rev.02 |
|
|
 Loading...
Loading...