Page 1

OPERATION
&
MAINTENANCE
MANUAL
Tabletop Autoclaves
Models
1730, 2340, 2540, 3140, 3850, 3870 M & MK
1730MK Valueklave
Cat. No. MAN205-0007000EN Rev. Q
Tuttnauer U.S.A. Co, Ltd. 25 Power Drive Hauppauge, NY, 11788, USA. Tel (631) 737 4850, (800) 624 5836, Fax: (631) 737 0720
Page 2

Page 3

TABLE OF CONTENTS
PARAGRAPH PAGE NO.
1 GENERAL................................................................................................................3
1.1 Incoming Inspection .....................................................................................3
1.2 Warranty........................................................................................................3
1.3 Warranty Statement ......................................................................................3
2 TECHNICAL DATA ................................................................................................5
2.1 Introduction ..................................................................................................5
2.2 Storage Conditions ........................................................................................6
2.3 Operating Conditions ....................................................................................6
2.4 Standards.......................................................................................................6
2.5 Construction ..................................................................................................6
2.6 Utilities ..........................................................................................................7
2.7 Waste Water Disposal ...................................................................................7
2.8 Environment Emission Information .............................................................7
2.9 Dimensions, Models 1730 .............................................................................8
2.10 Dimensions, Models 2340, 2540 ...................................................................9
2.11 Dimensions, Models 3140 ...........................................................................10
2.12 Dimensions, Models 3850, 3870 .................................................................11
2.13 Technical Specifications .............................................................................12
2.14 Electrical Data ............................................................................................13
2.15 Maximum Solid Load Sizes.........................................................................13
2.16 Symbol Description .....................................................................................13
3 DESCRIPTION OF COMPONENTS....................................................................19
3.1 Control Panel ..............................................................................................19
3.2 Other Components ......................................................................................19
4 INSTALLATION INSTRUCTIONS ......................................................................20
4.1 Electrical .....................................................................................................20
4.2 Setup ............................................................................................................20
4.3 Lifting and Carrying ...................................................................................21
5 WATER QUALITY ................................................................................................22
6 PREPARATION BEFORE STERILIZATION .....................................................23
7 OPERATION .........................................................................................................27
7.1 Loading and Unloading the Device ............................................................27
7.2 Fill the Water Reservoir..............................................................................27
8 SERVICE AND MAINTENANCE INSTRUCTIONS...........................................32
8.1 Preventive and Scheduled Maintenance.....................................................32
8.2 Draining the Reservoir................................................................................33
8.3 Cleaning the Air Jet ....................................................................................34
8.4 Replacing the Door Gasket .........................................................................35
8.5 Checking the Safety Valve ..........................................................................36
8.6 Unclogging the Multi-Purpose Valve or Fill Piping. .................................37
9 CLEANING THE TABLETOP AUTOCLAVES WITH CHAMBER BRITE™ ...38
10 TROUBLESHOOTING .........................................................................................40
11 LIST OF ACCESSORIES......................................................................................50
1
Page 4

TABLE OF CONTENTS (Cont.)
DRAWINGS PAGE NO.
Front View Model 1730 M, MK-Valueklave.................................................................. 14
Front View Model 2340/2540 M, MK ............................................................................ 15
Front View Model 3140 M.............................................................................................. 16
Front View Model 3850/3870 M..................................................................................... 17
Rear View ........................................................................................................................18
Tray Handle CT530020 .................................................................................................. 48
Pouch Rack ..................................................................................................................... 48
Tray .................................................................................................................................. 48
Tray Holder ..................................................................................................................... 49
2
Page 5

1 GENERAL
Read the Operating Instructions carefully, before beginning any operation
on the autoclave!
1.1 Incoming Inspection
Upon receiving your Tuttnauer Autoclave carefully inspect the outside
of the shipping carton for signs of damage. If any damage to the carton
is found, note the location with respect to the autoclave and check that
area of the autoclave carefully once it is fully unpacked. Observe
packing method and retain packing materials until the unit has been
inspected. Mechanical inspection involves checking for signs of physical
damage such as: scratched panel surfaces, broken knobs, etc.
If any damage is found, contact your dealer as soon as possible so
that they can file a claim with the shipping carrier and also notify
Tuttnauer.
All Tuttnauer products are carefully inspected prior to shipment and all
reasonable precautions are taken in preparing them for shipment to
assure safe arrival at their destination.
Note: Lifting and carrying should always be done by two people.
1.2 Warranty
We certify that this instrument is guaranteed to be free from defects in
material and workmanship for one year against faulty components and
assembly.
This warranty does not include routine cleaning and preventive
maintenance to be performed according to instructions in
section 8.1 (Preventive and Scheduled Maintenance).
Tuttnauer warrantees all new Manual autoclaves for a period of one full
year, covering both parts and labor. This one year warranty covers
defects in materials and workmanship on every part in the autoclave
except door gaskets and HEPA filters (they are wear items).
Tuttnauer warrantees all chambers for a period of ten (10) years against
defects in materials and workmanship. This chamber warranty went
into effect January 1997.
This warranty does not apply to any instrument that has been subjected
to misuse, neglect, accident or improper installation or application, nor
shall it extend to autoclaves that have been repaired or altered outside
the factory without prior authorization from Tuttnauer.
Tuttnauer’s obligation is limited to the repair or replacement of parts
for the autoclave. This warranty will be void if the unit is not purchased
from an authorized Tuttnauer dealer. No other warranties or obligations
are expressed or implied.
The Autoclave should only be used in a manner described in this
manual!
1.3 Warranty Statement
To activate the warranty, the registration card must be completed and
returned to Tuttnauer within fourteen (14) days of purchase or you may
call our customer service department at the number listed below.
No product will be received or accepted for repair without prior return
authorization from Tuttnauer. All transportation charges to and from
Tuttnauer must be paid by the owner of the autoclave. During the first
3
Page 6

90 days after purchase of an autoclave, Tuttnauer will pay shipping
costs on an individually evaluated basis and ONLY with pre-approval.
Note:
If you have any questions or there are any difficulties with this
instrument and the solution is not covered in this manual, please
contact your dealer or our Technical Service Dept. at the address
below. Do not attempt to service this instrument yourself.
Tuttnauer USA Co., Ltd. 25 Power Drive Hauppauge, NY, 11788,
USA
: (800) 624 5836, (631) 737 4850, Fax: (631) 737 0720
e-mail:info@tuttnauerUSA.com
4
Page 7

2 TECHNICAL DATA
2.1 Introduction
This tabletop autoclave is designed for the sterilization of wrapped and
unwrapped instruments and related items found in dental, medical and
This autoclave is an electrically heated sterilizer using steam as the
The operator can select a sterilization temperature from within a range
All models feature an easy to use control panel. The machines are
This manual is intended for the user and gives the user a general
After reading this manual, operating the autoclave will be easy.
Only technical personnel having proper qualifications and holding
veterinary clinics, first aid rooms, laboratories, etc.
sterilizing agent. It is a manually operated device, with a control
system based upon steam pressure.
of 212ºF - 273ºF (100ºC - 134ºC). This allows for the sterilization of
heat sensitive material at a low sterilization temperature, as well as
providing for faster sterilization at higher temperatures for materials
able to withstand the higher sterilization temperatures.
ruggedly built using 316L stainless steel, copper, brass and aluminum.
To guard against rusting, no iron components are used. All models
include a drying system for wrapped items.
2.1.1 Safety features
The safety features include a double locking door
mechanism (door tightening bolt and locking bellows), a
mechanical pressure relief valve, over temperature
thermostats and a double pole circuit breaker.
Pressure Door Lock System (Door Bellow)
The Door Bellows is a safety device that prevents the door
from opening when the chamber is pressurized.
The system utilizes the buildup of pressure in the chamber
to expand a flexible Silicon-rubber bellows. The bellows
pushes a metal pin into a grove on the tightening bolt of the
Door Closing Device.
This prevents the operator from opening the door when
there is pressure in the chamber. When the steam is
released, this bellow returns to its original position, drawing
the pin with it and releasing the tightening bolt.
understanding of the instrument and the best ways to operate and take
care of it, in order to obtain optimum effective results.
However, since this instrument is built with high technology sensitive
components, no attempt should be made by the user or any other
unauthorized person to repair or recalibrate it.
technical documentation (including a technician manual) and
adequate information are authorized to service the apparatus.
5
Page 8

2.2 Storage Conditions
The packed or unpacked autoclave shall be stored in “indoor
conditions” (protected from rain and water).
2.3 Operating Conditions
This device is intended for indoor use.
This autoclave is intended for NORMAL environment conditions as
follows:
♦ Altitude up to 2000m.
♦ Minimum room temperature 41ºF (5ºC).
♦ Installation Category II.
♦ Pollution Degree 2.
♦ Maximum relative humidity 80% for temperature up to 31ºC
decreasing linearly to 50% relative humidity at 40ºC.
♦ Mains supply voltage fluctuations up to ±10% of nominal voltage.
♦ The sterilizer should be loaded only with autoclavable material.
2.4 Standards
2.4.1 Technical standards
1. A.S.M.E. Code, Section VIII div.1 for unfired pressure
vessels.
2. FDA Cleared.
3. UL61010-1 General Safety.
4. UL61010-2-041 Particular Safety for Autoclaves.
2.4.2 Quality standards
1. EN ISO 9001:2008– Quality System
2. ISO 13485:2003 – Quality systems – Medical devices.
2.5 Construction
The main parts of the autoclave are made of materials as indicated
below:
♦ Chamber is built of electro-polished stainless steel 316 L.
♦ Door is made of stainless steel CF8.
♦ Trays are made of stainless steel 304.
♦ Door handle is made of hard plastic material that is safe to touch
and thermo-insulated.
♦ Water reservoir is made of hard plastic material.
6
Page 9
2.6 Utilities
Utilities Unit
Value
V-A 1ph, 120V – 16A,50/60 Hz
Power supply (as appropriate)
V-A 1ph, 230V – 16A,50/60 Hz
Attention:
1. The electrical net must be protected with a current leakage safety
relay (GFI Receptical or Circuit Breaker).
2. The electrical network must comply with local rules or regulations.
3. The autoclave must be connected to a properly grounded outlet.
2.7 Waste Water Disposal
Caution !
Waste water must be brought into the public water piping in
accordance with the local rules or requirements, i.e. only nonhazardous liquids may be disposed in public sewage!
2.8 Environment Emission Information
A. The peak sound level generated by the sterilizer is < 70 / dBA with
background noise of 60 dB.
B. The total heat transmitted by the sterilizer is < 100 W/h for
1730/2340/2540 models and < 150 W/h for 3140/3850/3870
models.
7
Page 10
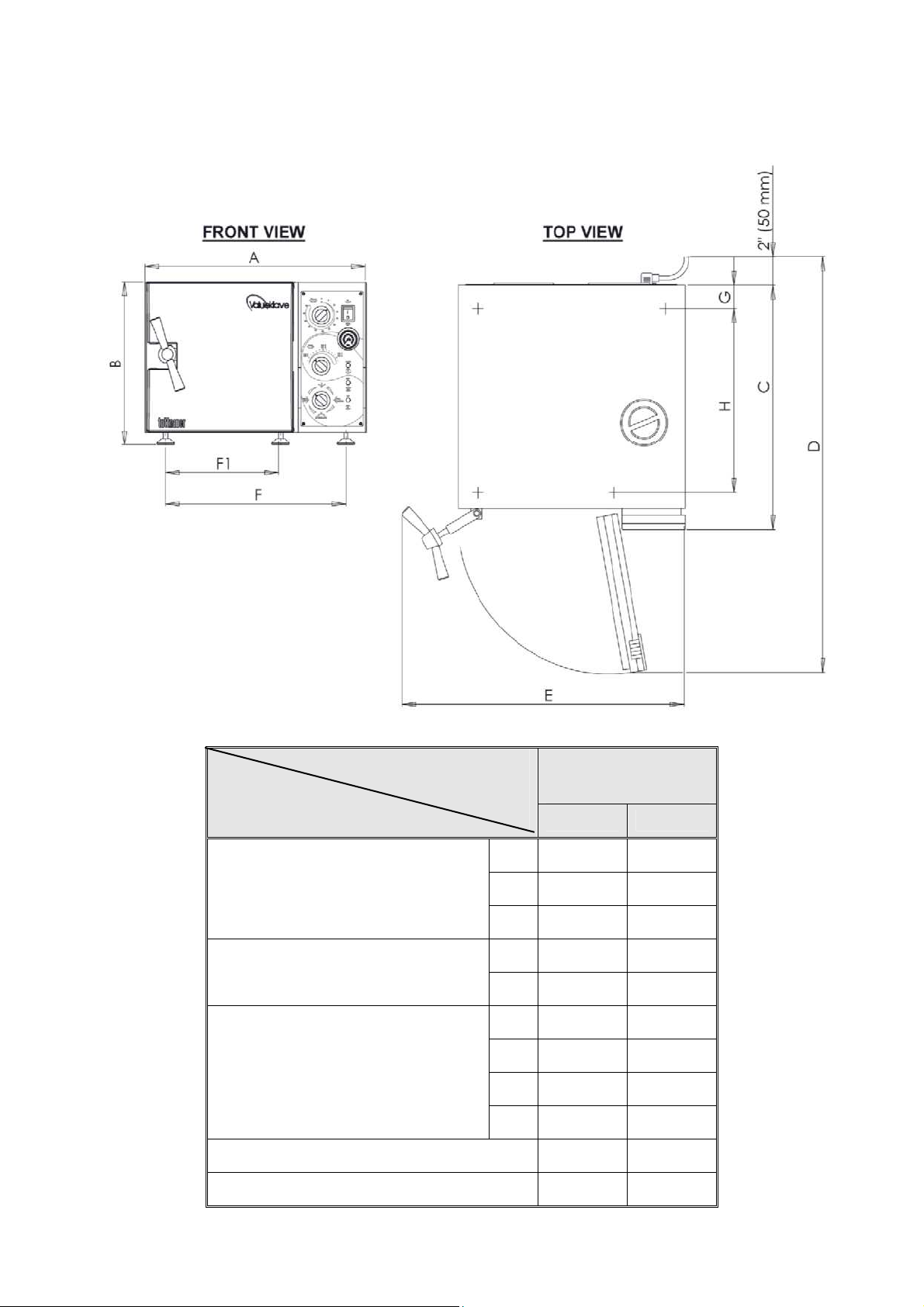
2.9 Dimensions, Models 1730
Dimensions
Model
M, MK-Valueklave
1730
in mm
A 17.4 440
Overall Dimensions
B 12.0 305
C 17.9 455
D 29.5 750
Maximum Dimensions (door open)
E 22.0 560
F1 13.7 347
Distance
F1-front legs
F -rear legs
Between Supporting
Legs
F 13.4 339
G 2.0 50
H 12.4 315
Chamber Diameter 6.7 170
Chamber Depth 13.4 340
8
Page 11
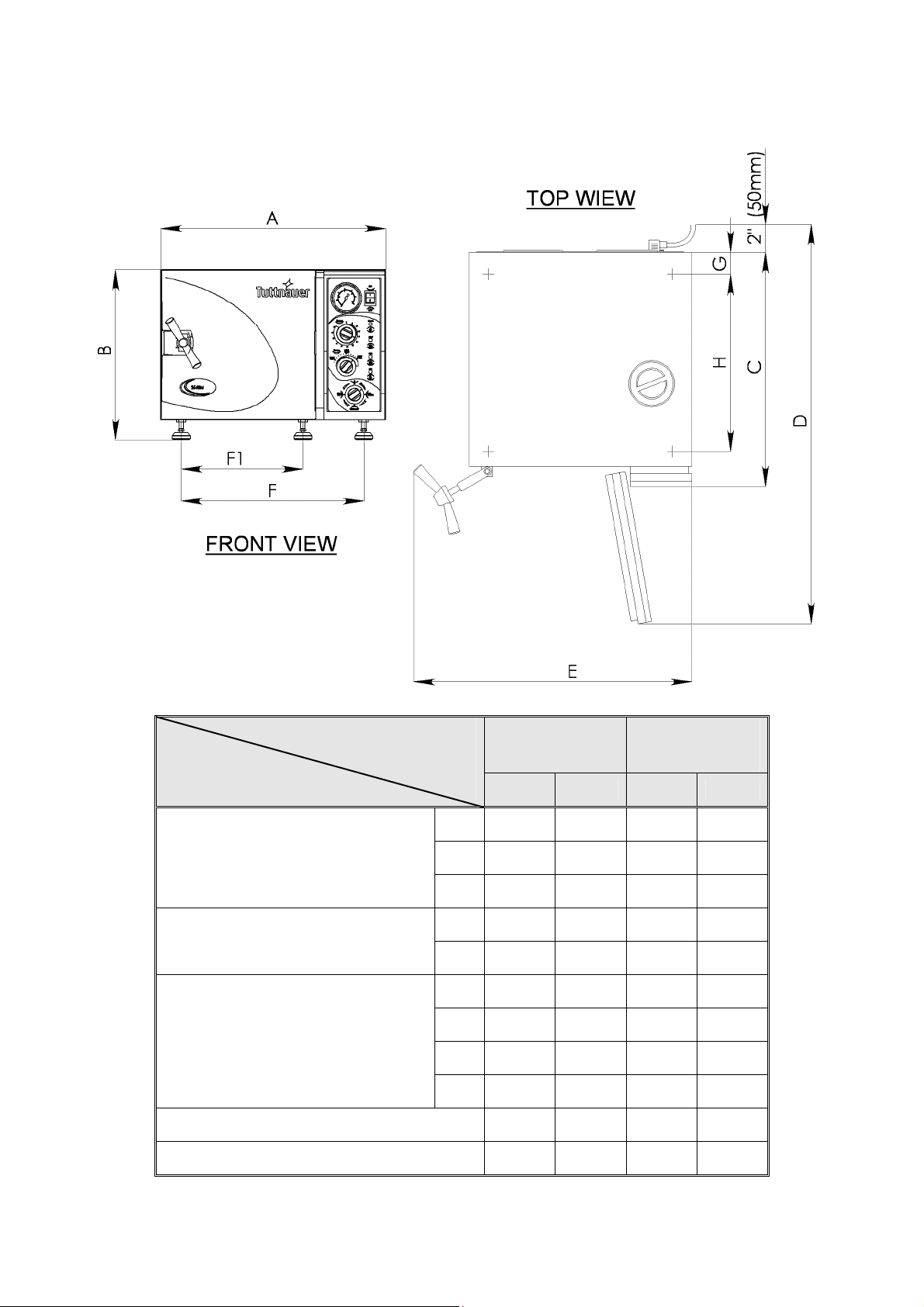
2.10 Dimensions, Models 2340, 2540
Dimensions
Model
2340
M, MK
2540
M, MK
in mm in mm
A 20.0 510 20.0 510
Overall Dimensions
B 14.4 365 14.4 365
C 21.5 545 21.5 545
D 35.8 910 35.8 910
Maximum Dimensions (door open)
E 25.8 655 25.8 655
F1 11.8 299 11.8 299
Distance
F1-front legs
F -rear legs
Between Supporting
Legs
F 16.6 422 16.6 422
G 2.0 50 2.0 50
H 15.8 400 15.8 400
Chamber Diameter 9.1 230 10 254
Chamber Depth 18.5 470 18.7 475
9
Page 12

2.11 Dimensions, Models 3140
Dimensions
Model
3140
M
in mm
A 23.2 590
Overall Dimensions
B 17.7 450
C 21.9 556
D 39.0 990
Maximum Dimensions (door open)
E 29.7 755
F1 19.2 488
Distance
F1-front legs
F -rear legs
Between Supporting
Legs
F 14.6 371
G 2.0 50
H 15.2 386
Chamber Diameter 12.3 312
Chamber Depth 15.4 391
10
Page 13
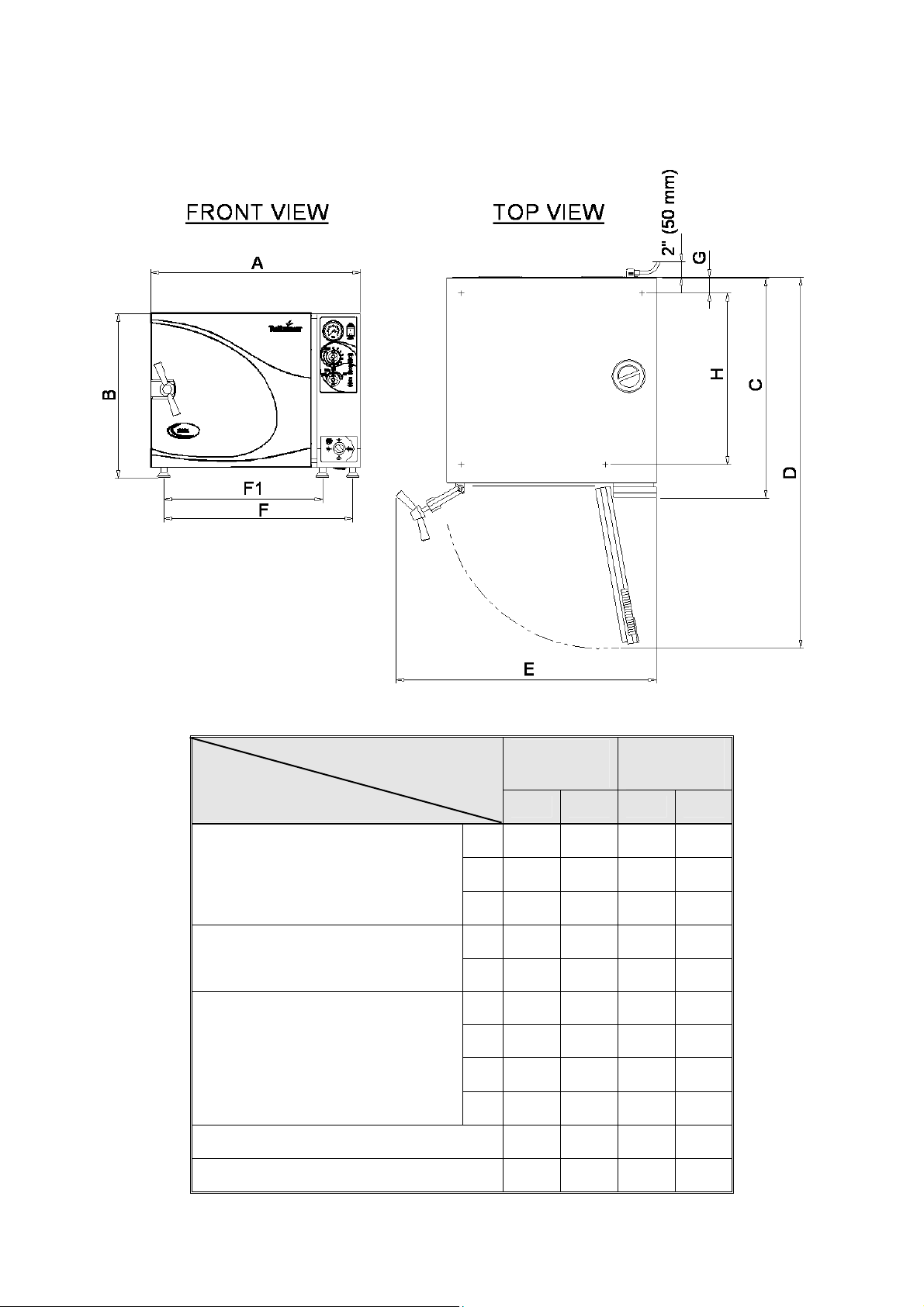
2.12 Dimensions, Models 3850, 3870
Dimensions
Model
3850
M
3870
M
in mm in mm
A 26.0 665 26.0 665
Overall Dimensions
B 20.7 525 20.7 525
C 27.5 695 34.5 875
D 45.5 1155 53.0 1335
Maximum Dimensions (door open)
E 32.0 815 32.0 815
F1 17.7 450 17.7 450
Distance
F1-front legs
F -rear legs
Between Supporting
Legs
F 22.2 564 22.2 564
G 2.0 50 2.0 50
H 21.8 555 30.5 725
Chamber Diameter 15.1 384 15.1 384
Chamber Depth 22.8 580 29.9 760
11
Page 14
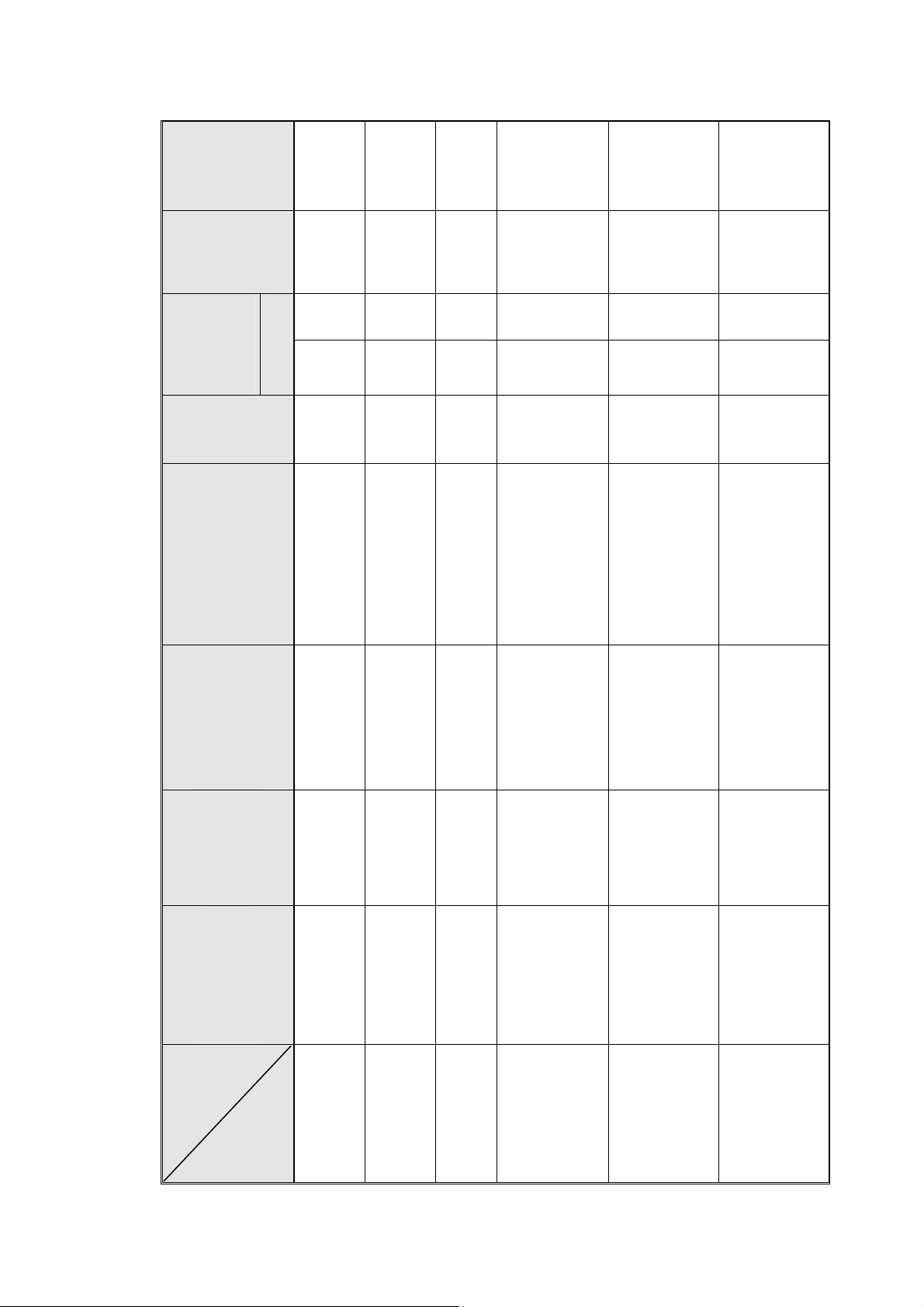
Full + Half
)
3
)
3
)
3
)
3
)
3
)
3
Volume
Shipping
Weight
Shipping
6.35 cu.f.
(0.18 m
54.7 lbs.
(24.8 kgs.)
2
No. of
Standard
(Optional)
Cassettes
of
No.
Trays
3
9.4 cu.f.
(0.27m
9.4 cu. f.
(0.27m
78.7 lbs.
2
2
3
83.3 lbs.
(35.7 kgs.)
(47.8 kgs.)
3
3
4
12.4 cu.f
(0.35 m
132 lbs.
(60 kgs)
4
4
2
22.2cu.f.
(0.63 m
196 lbs.
(89 kgs.)
10
2
26.8cu.f
(0.76m
225 lbs.
(102 kgs.)
15
2
D x W x H
Tray Dimensions
11.6" x 4.7" x 0.8"
(295 x 120 x 20 mm)
16.3 " x 6.7" x 0.8"
16.3 " x 6.7" x0.8"
(415 x 170 x 20 mm)
16.1" x10.1" x 1"
(415 x 170 x 20 mm)
16.1" x7.8" x 1"
(408 x 250.6x 25 mm)
20" x 11" x 1"
(408 x 198x 25 mm)
20" x 14" x 1"
(500 x 280 x 25 mm)
26" x 11" x 1"
(500 x 350 x mm)
26" x 14" x 1"
(670 x 280 x mm)
(670 x 350 x mm)
Volume of
Mineral Free
(3.0 liters)
0.66 US gal.
(3.0 liters)
0.66 US gal.
(3.0 liters)
0.66 US gal.
(3.0 liters)
0.66 US gal.
(7.5 liters)
2.0 US gal.
(7.5 liters)
2.0 US gal.
Water Reservoir
2 US gal.
Chamber
Volume of
(7.5 liters)
5 US gal.
6 US gal.
(19 liters)
(23 liters)
7.8 US gal.
(34.4 liters)
(65 liters)
17US gal.
(84 liters)
22 US gal.
ф x L
Chamber
Dimensions
2.13 Technical Specifications
6.7" x 13.4"
MK-
1730 M, MK
9" x 18.5"
(170 x 340 mm)
(230 x 470 mm)
10" x 18.7"
(254 x 475 mm)
12.3" x 15.4"
(312 x 391 mm)
3140 M
Valueklave
2340 M, MK
2540 M, MK
15" × 23"
(380 × 580 mm)
3850 M
15" x 30"
(380 x 760 mm)
3870 M
12
Page 15
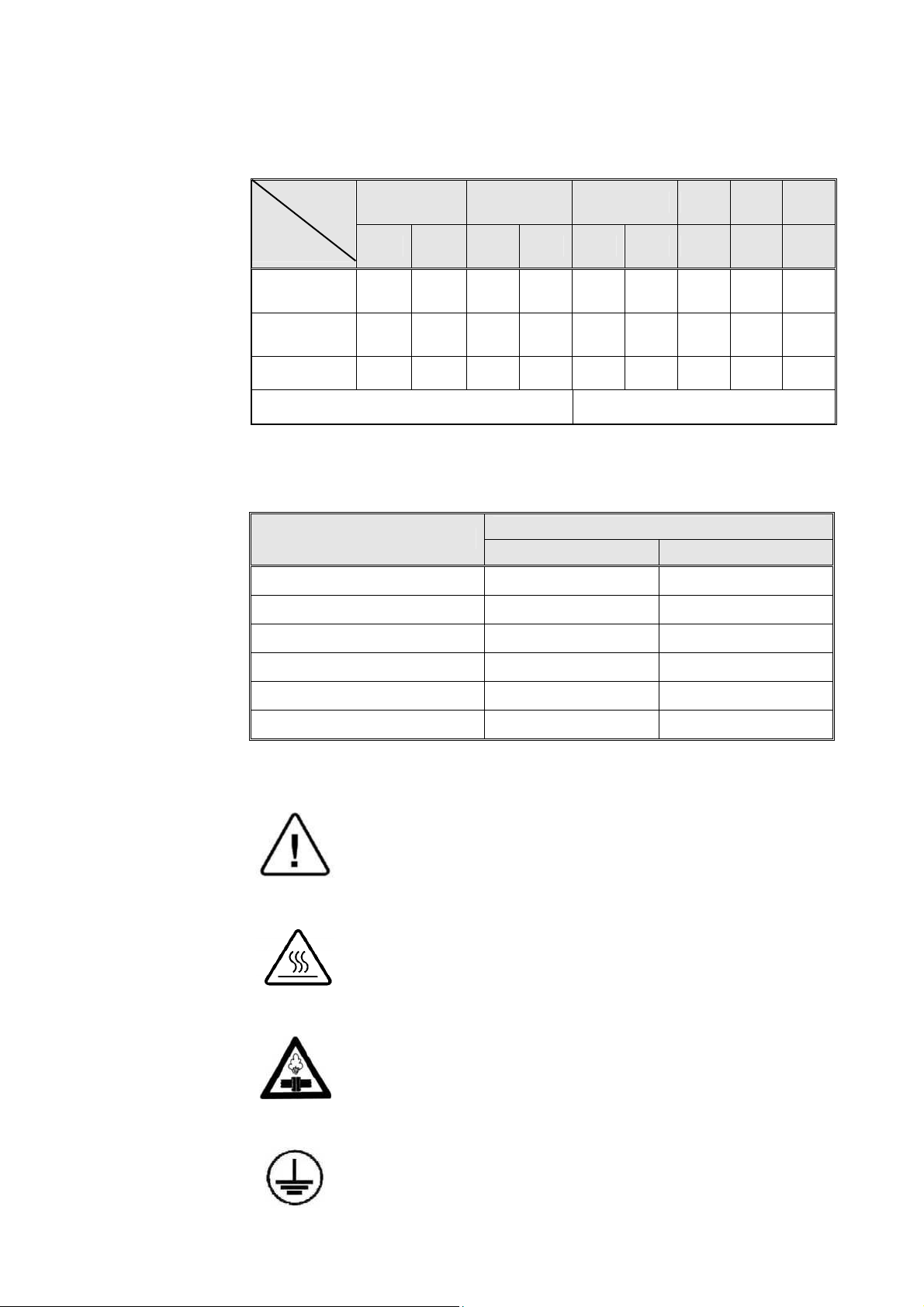
2.14 Electrical Data
Model
Specifications
Total power
model 120V
Total power
model 230V
1730 2340 2540 3140 3850 3870
M
MK
MK-V
M MK M MK M M M
8.8A 11.2A 11.7A - 11.7A - 20.0A - -
4.6A 5.9A 6.0A 9.6A 6.0A 9.6A 10.4A 10.4A 13A
Heaters W 1050 1350 1400 2200 1400 2200 2400 2400 3000
Degree of protection by enclosure IP31
2.15 Maximum Solid Load Sizes
(Textile load = 1/3 of solid load)
Models
Loads
lbs kg
1730 6.0 2.7
2340 7.0 3.2
2540 8.8 4.0
3140 11.0 5.0
3850 13.6 6.0
3870 14.0 6.4
2.16 Symbol Description
Caution! Consult accompanying documents
Caution! Hot Surface.
Caution! Hot steam.
Ground
13
Page 16
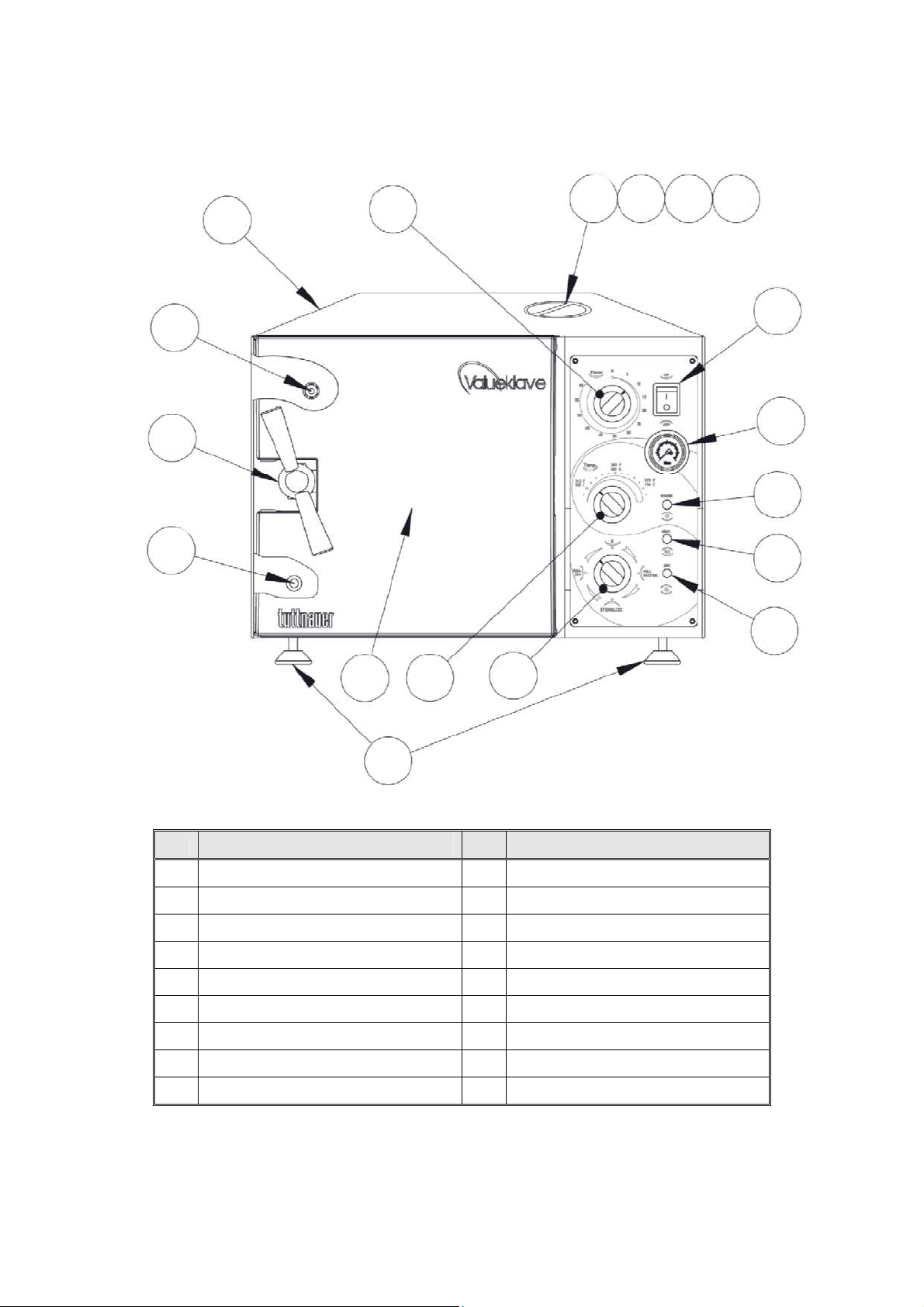
18
12
6
11
5
4
10
9
8 7
2
3
13
14
15
16
17
1
FRONT VIEW MODEL 1730 M, MK-Valueklave
No.
1. Front legs 10. Air trap jet
2. Reservoir water drain valve 11. Main power switch
3. Door closing device 12. Pressure gauge
4. Door microswitch 13. Power indicator light
5. Autoclave cover 14. Heat indicator light
6. Timer 15. Dry indicator light
7. Water reservoir cover 16. Multipurpose valve
8. Water reservoir 17. Thermostat (B10) knob
9. Safety valve 18. Door cover
Description No.
14
Description
Page 17

FRONT VIEW MODEL 2340/2540 M, MK
19
18
17
16
15
14
13
No.
1 2 3 4
Description No.
5
6
7
8
9
10
11
12
Description
1. Water reservoir cover 11. Thermostat (B10) knob
2. Water reservoir 12. Multipurpose valve
3. Safety valve 13. Front legs
4. Air trap jet 14. Rear legs
5. Pressure gauge 15. Reservoir water drain valve
6. Main power switch 16. Door closing device
7. Timer 17. Door microswitch
8. Power indicator light 18.
9. Heat indicator light 19. Autoclave cover
10. Dry indicator light
Door cover
15
Page 18

FRONT VIEW MODEL 3140 M
18
1 2 3 4
5
6
17
7
16
15
3140
8
9
10
14
11
13
12
No.
Description No.
Description
1. Water reservoir cover 10. Dry indicator light
2. Water reservoir 11. Thermostat (B10) knob
3. Safety valve 12. Multipurpose valve
4. Air trap jet 13. Front legs
5. Pressure gauge 14. Reservoir water drain valve
6. Main power switch 15. Door closing device
7. Timer 16. Door microswitch
8. Power indicator light 17. Door cover
9. Heat indicator light 18. Autoclave cover
16
Page 19

FRONT VIEW MODEL 3850/3870 M
18
17
16
15
14
13
No.
Description No.
1 2 3 4
Description
5
6
7
8
9
10
11
12
1. Water reservoir cover 10. Dry indicator light
2. Water reservoir 11. Thermostat (B10) knob
3. Safety valve 12. Multipurpose valve
4. Air trap jet 13. Front legs
5. Pressure gauge 14. Reservoir water drain valve
6. Main power switch 15. Door closing device
7. Timer 16. Door microswitch
8. Power indicator light 17. Door cover
9. Heat indicator light 18. Autoclave cover
17
Page 20

REAR VIEW
18
Page 21

water
This
air during
3 DESCRIPTION OF COMPONENTS
3.1 Control Panel
Description Operation
PRESSURE GAUGE
TIMER 0-60 min.
THERMOSTAT
Position
FILL WATER
MULTIPURPOSE
VALVE
STERILIZE
EXH.& DRY
“ 0 ”
HEAT INDICATOR
LIGHT
DRY INDICATOR LIGHT
POWER INDICATOR
LIGHT
MAIN SWITCH
0-60 psi, (0-4bar) indicates the chamber pressure
and includes maximum point indicator.
Sets the time for sterilization and drying cycles
(see section 7 for correct cycle time settings).
Note: The power to the heating elements is
switched off when the Timer reaches 0 minutes.
Sets the desired sterilization temperature for each
cycle within the range of 212ºF - 273ºF (100ºC 134ºC).
Water flows from the water reservoir into the
chamber.
Valve closed to all directions.
Exhausts the steam from the chamber into the
reservoir after the sterilization cycle is finished.
Heating elements are disconnected, no cycle is in
progress.
Lights to indicate that the heaters are activated.
It will cycle off and on when the temperature
reaches the preset valve.
Lights to indicate that drying cycle is in process.
Light to indicate that the main switch is on.
Main power switch, which supplies electric power
to the autoclave.
3.2 Other Components
Description Operation
WATER DRAIN VALVE
WATER RESERVOIR
SAFETY VALVE
AIR TRAP JET
SAFETY THERMOSTAT
CUT-OUT THERMOSTAT
Enables the drainage of water from the reservoir.
Holds water for sterilization and also serves as a
condenser for the hot steam during the exhaust
phase.
Located in the water reservoir. Protects the chamber
by releasing any pressure above 40psi (2.7bar).
Safety Valve is required and approved by ASME.
Located in the water reservoir. Eliminates
heat up phase to insure correct sterilization
temperature is reached. Also prevents air pockets
and pockets of cold steam from forming in the
chamber.
Prevents overheating during the sterilization and
drying stages, will automatically reset itself.
Cuts off the power in case of overheating if the
safety thermostat does not operate. This thermostat
does not reconnect automatically but must be reset.
19
Page 22

4 INSTALLATION INSTRUCTIONS
Caution:
The sterilizer must be placed on a rigid and leveled surface. The stand must
be able to hold the load of the device and loaded material.
Note:
Make sure, when placing the autoclave, to leave space around the machine,
to give the technician access to service the machine.
4.1 Electrical
The electrical connection should comply with the devices power
requirement. It must also comply with local installation and safety rules
and regulations. The voltage supplied to the device must comply with
the label ± 5%.
In order to avoid any injury by electrical hazard, it is mandatory for the
customer to have installed an earth leakage relay (GFI outlet or circuit
breaker) in the electrical circuit to which the autoclave is connected.
This relay disconnects all the poles of the electrical power line in case
of accidental contact by the power line, with the autoclave’s metal
Note: Keep the back and the right side of the autoclave approximately 1”
Connect the power cord to the socket on the rear side of the autoclave;
enclosure.
(25mm) away from the wall to allow for ventilation.
plug it into the supply outlet.
4.2 Setup
Proper adjustment of the chamber pitch is one of the most important
things you can do for the sterilizer. Proper chamber pitch insures that
among other things, the sterilizer will have the proper amount of water
in the chamber at the beginning of each cycle. Insufficient water in the
chamber, at the beginning of the cycle, will cause the unit to overheat
and activate one of the safety thermostats. This will occur at some
point during the cycle when the water level becomes too low. If, on the
other hand, there is too much water in the chamber, this will extend the
heating portion of the cycle. Extending the heat up time will shorten
the sterilization time, causing items to not be sterilized, indicator strips
to not change color and spore tests to fail.
For proper setup please follow these setup steps (see drawing below):
♦ The autoclave should be turned off and unplugged.
♦ Make sure the counter is level and sturdy (3).
♦ Make sure all the feet are on the autoclave and none have been lost.
♦ Make sure the feet are free to move in and out (2).
♦ Position the autoclave on the counter.
♦ Fill the reservoir with distilled water (see sec 7.2).
♦ The chamber should be empty of any instruments, trays or leftover
water.
20
Page 23

♦ The chamber pitch now needs to be adjusted.
350
15
450
480
23
690
27
810
♦ Measure to the proper amount of distilled water for the appropriate
model unit as listed below.
1730 2340/2540 3140 3850 3870
10-12
oz
300-
ml
12-
oz
350-
ml
14-16
oz
420-
ml
20-
oz
600-
ml
24-
oz
720-
ml
♦ Pour the proper amount of water into the chamber through the front
door of the unit (4).
♦ This water should cover the bottom of the chamber to within +/- ½
inch of the groove in the front (1).
♦ If necessary, adjust the front leveling feet, up or down, so that the
water lays in the chamber correctly (2).
♦ Once the chamber pitch adjustment is completed, the unit is ready to
operate.
Note: It is imperative to have the correct amount of water in the
chamber each cycle for proper operation of the autoclave.
4.3 Lifting and Carrying
Caution:
Before moving the autoclave, make sure that the electric cord is
disconnected from the power and there is no pressure in the
chamber.
1. Disconnect the power supply cord.
2. Drain the water from the reservoir and vessel.
To avoid injuries, lifting and carrying should be done by two people.
Do not drop this device!
21
Page 24

5 WATER QUALITY
The distilled or mineral free water supplied to the autoclave should have the
physical characteristics and maximum acceptable level of contaminants
indicated in the table below:
Physical Characteristics and Maximum acceptable contaminants levels
in steam for sterlizers
(According to EN 13060:2004).
Element Condensate – allowable content
Silicium oxide. SiO2 ≤0.1 mg/kg
Iron ≤0.1 mg/kg
Cadmium ≤0.005 mg/kg
Lead ≤ 0.05 mg/kg
Rest of metals except iron, cadmium,
lead
Chloride (Cl) ≤0.1 mg/kg
Phosphate (P2O5) ≤0.1 mg/kg
Conductivity (at 20°C) ≤3 µs/cm
≤0.1 mg/kg
pH value (degree of acidity) 5 to 7
Appearance Colourless clean without sediment
Hardness (Σ Ions of alkaline earth) ≤0.02 mmol/l
Compliance with the above data should be tested in accordance with
acknowledged analytical methods, by an authorized laboratory.
Attention:
We recommend testing the water quality once a month. The use of water for
autoclaves that does not comply with the table above may have severe impact
on the working life of the sterilizer and can invalidate the manufacturer’s
warranty.
22
Page 25

6 PREPARATION BEFORE STERILIZATION
The purpose of packaging and wrapping items for sterilization is to provide an
effective barrier against contamination during storage, once the items have
been sterilized.
VERY IMPORTANT!
When sterilizing cotton wool or pads, it is essential to wrap them in paper or
cotton bags in order to prevent the multi-purpose valve and the autoclave
openings from becoming clogged with remnants of the material.
Packaging and wrapping materials should be approved for use in a steam
sterilizer and permit the removal of air and penetration of the steam during the
sterilization process.
The basic principle of determining the size, mass and contents of instrument
and hollowware packs is that the contents are sterile and dry immediately upon
completion of the drying cycle.
Instruments to be sterilized must be clean and free from any residual matter,
such as debris, blood, pads or any other material. Such substances may cause
damage to the instruments themselves or the sterilizer.
1. Clean instruments immediately after use. It is recommended that
instruments be ultrasonically cleaned in a Tuttnauer Clean and Simple
Ultrasonic Cleaner, using Tuttnauer Clean and Simple enzymatic
cleaning solution.
2. After ultrasonic cleaning, rinse under tap water for 30 seconds and pat dry
to remove residual minerals. If your tap water has a high mineral content,
rinse a second time in a bath of distilled water and pat dry.
3. Launder textile wraps prior to reuse, but do not use bleach.
4. Follow the instrument manufacturer’s instructions for cleaning and
lubricating instruments.
5. Be sure that instruments of dissimilar metal (stainless steel, carbon steel,
etc.) are separated. Carbon steel instruments should be bagged or placed
on autoclavable towels and not directly on stainless steel trays. (Mixing
will result in the oxidation of these metals).
6. Load items within the boundaries of the tray so that they do not touch the
chamber walls, or fall off when the tray is inserted into the autoclave. The
chamber walls are very hot; items that come into contact with the wall can
be damaged
7. Check the manufacture’s instructions as to the proper procedure for
sterilizing each item. The instrument manufacturer can give specific
information on proper sterilization temperature and sterilization time for
any item.
8. Place a sterilization indicator in each tray or inside each wrapped pack.
9. When using a paper / plastic bag, the plastic side should always be down.
Use single-use wraps once only and discard after use.
10. Verify that the packaging method is in accordance with good practice
approach and the packaging materials are in accordance with the
applicable standards (e.g. EN868 series).
23
Page 26

Pouch Rack
11. At least once per week use a biological spore test (Bacillus
Stearothermophilus) in any load to insure proper sterilization. (Be aware
testing standards may vary).
12. All instruments must be sterilized in an open position. Place instruments
with ratchets opened and unlocked or clipped on the first ratchet position.
Surfaces that are hidden because the item is in a closed position will not
be exposed to the steam and will not be sterilized.
13. Disassemble or sufficiently loosen multiple-part instruments prior to
packaging to permit the sterilizing agent to come into contact with all
parts of the instrument.
14. Make sure that all instruments remain apart during the sterilization
process. Surfaces that are hidden because items are covering other items
will not be exposed to the steam and will not be sterilized.
15. Items prone to trapping air or moisture, e.g. hollowware, should be tilted
on edge. This will allow a minimal resistance to the removal of air or
condensate during sterilization and drying.
16. The operator may use racks to allow for adequate separation of packaged
instruments.
17. Do not overload the sterilizer trays. Overloading will cause inadequate
sterilization and poor drying. Load trays loosely to capacity. Instruments
should be loaded one level deep only. See table in section 2 for
recommended loading capacities.
18. Tubing should be rinsed after cleaning. When placed in the tray, make
sure that both ends of the tubing are open and there are no sharp bends or
twists.
19. Empty canisters should be placed upside-down, in order to prevent
accumulation of water.
20. Allow a distance of approximately 1” (2.5 cm) between trays or cassettes
to permit steam circulation.
21. Wrapped instruments should be packed in material which will allow steam
penetration and promote drying, such as autoclave bag, autoclave paper,
or muslin towels.
22. Do not stack pouches.
It is recommended that a Tuttnauer
Pouch Rack be used. This will allow
the operator to place pouches on their
side, which will increase capacity and
will allow for better exposure to
steam for sterilization and better
circulation of air for drying.
24
Page 27

23. If spotting is detected on the instruments, the first step would be to use an
ordinary eraser to remove the spot. If there is no pitting under the spot, the
spot was only dirt. Dirt spots on an instrument may be an indication that the
autoclave needs to be cleaned or that the instruments were not adequately
cleaned or dried. If removal of the spot reveals pitting, the spot was most
likely rust. Rust spots on an instrument are not uncommon on inexpensive
instruments. It may also be an indication that the instruments were rinsed in
tap water with a high content of minerals. These minerals when exposed to
high temperature and steam will accelerate the oxidation of the metal. One
suggestion would be to final rinse the instruments in distilled water.
24. If the instruments exhibit a discoloration, this can be due to the mixing of
carbon steel and stainless steel. When these two metals come into contact
with each other electrolysis occurs that breaks down the metal. The best
solution is to separately wrap the carbon steel to insulate it from other
instruments or the trays.
25. Packs
VERY IMPORTANT!
When sterilizing cotton wool or pads, it is essential to wrap them in paper
or cotton bags in order to prevent the multi-purpose valve and the
autoclave openings from becoming clogged with remnants of the
material.
1. Place packs upright on trays, side by side.
2. Packs should not touch the chamber walls.
3. Pack instrument sets in a manner that prevents damage to delicate items.
4. Pack hollowware sets so that all openings face the same direction and
so that the contents cannot move inside the pack.
5. Load packs of folded operating room drapes with layers vertical,
allowing air to be removed from the packs rapidly.
6. Do not place packs of hollowware and trays of instruments above
textile packs or soft goods in order to avoid wetting caused by
condensation from items above.
7. Load items packed in flexible packaging materials on edge with paper
to laminate, or flat with the plastic surface downwards.
Note: The instrument manufacturer’s recommendations shall be observed,
concerning the sterilization data for each type of material.
25
Page 28

26. Tubing
1. When placing in a tray, make sure that both ends are open, without
sharp bends or twists.
Wrong
Right
27. Cassettes
1. Instruments may be sterilized in cassettes. The advantage of the
cassettes is that the sterilized instruments may remain organized in
the cassettes ready for use, while stored in a sterile area.
2. If using models 2340 / 2540, remove the trays and slide the cassettes
into the chamber on the rack system.
3. If using models 3850 / 3870, place the cassettes directly on the tray,
either lying flat (but no stacking) or on edge.
26
Page 29

7 OPERATION
To avoid possible damage, do not leave the autoclave unattended while in
operation.
Make sure the power cord is plugged into the back of the unit and also
plugged into a power source.
7.1 Loading and Unloading the Device
7.1.1. Safety
Protective equipment, clothes and other safety instructions
should be implemented in accordance with local and national
regulations and/or rules!
For proper sterilization - Do not overload the chamber. Only
autoclavable products shall be used; please refer to the
material or instrument manufacturer's instructions for
sterilization of unknown materials or instruments.
7.1.2. Loading
Correct loading of the autoclave is essential to successful
sterilizing for several reasons. Efficient air removal from the
chamber and the load will permit steam penetration and
saturation, and allow proper drainage of condensate.
Additionally, correct loading will reduce damage to packs
and their contents and maximize efficient use of the
sterilizer.
For detailed loading instructions, see sec. 6 (Preparation
before sterilization).
7.1.3. Unloading
On completion of the cycle, the load shall be immediately
removed from the sterilizer and a visual inspection made to
ascertain that the load is dry, and that sterilizing indicators
have made the required color change.
7.2 Fill the Water Reservoir
7.2.1. Ensure that the drain valve is in a CLOSED position.
7.2.2. Remove the water reservoir cover.
7.2.3. Pour distilled water into the reservoir through the opening
on top of the autoclave, until it reaches the base of the safety
valve holder. Under no circumstances fill any higher than
the base of the safety valve holder.
7.2.4. For proper operation make sure the water level is above the
coils of the cooling coil.
Caution:
Under no circumstance should water be filled above the safety
valve holder.
27
Page 30

7.2.5. USE DISTILLED WATER ONLY. Use water-having
characteristics as per table in sec 5. The impurities in tap
water will create the need for more frequent cleaning and
maintenance, in addition, they will accumulate and block the
hole of the Air Jet. This will prevent the temperature in the
chamber from rising properly. This will cause spore tests to
fail and indicator strips will not change color. It is
essential from time to time, during heating and sterilization
phases, that a spray of steam should escape, from the Air Jet,
causing a hissing sound. If no escaping steam is evident or
no hissing sound heard, follow the instructions in sec 8.3 for
cleaning the Air Jet.
EXIT FOR STEAM SPRAY
Caution:
Daily before operation, check the water level in the reservoir and add
water when required. Once a week or after 20 cycles (the shorter
period) replace the water in the reservoir.
7.3. Move the ON / OFF rocker switch, located on the front panel, to the
ON position. The green Power Light will turn on, indicating that power
is ready to be supplied to the Heating Elements.
7.4. Turn the red tracking needle on the pressure gauge counterclockwise to
0 psi. The tracking needle will indicate the highest pressure reached
during the cycle.
7.5. Open the front door of the autoclave and set the Multi-purpose valve
knob to the FILL WATER position.
7.5.1. The water will now flow into the chamber.
7.5.2. The water should cover the bottom of the chamber up to the
groove in the front. This amount of water should be in
accordance with the table in sec 4.2.
7.5.3. When the water reaches the mark at the front of the autoclave,
set the multi-purpose valve knob to the STERILIZE
position.
Note:
When used for the first time, the multi-purpose valve requires slight
effort, but with use it will turn smoothly and easily.
Warning
The Multi-purpose valve knob should be turned in a clockwise direction only!
28
Page 31

7.6. Load the autoclave. See section “Preparation for Sterilization” for
C)
information on proper loading.
7.7. Shut the door, move the Door Closing Devise into position and tighten,
making sure that the Door Switch is activated.
NOTE:
Due to the inherent elasticity of the door gasket, it is important to
tighten the door bolt until “hand tight”. Do not overtighten the bolt as
this may result in damage to the gasket.
Should the autoclave fail to reach the sterilizing temperature/pressure,
always check first that the door is fully sealed. If not, tighten the door
bolt further, as described above, until completely sealed.
7.8. Turn the Thermostat knob to the desired sterilization temperature.
Note: This autoclave is designed according to all international
standards, which allows the temperature to raise 4ºF (2ºC)
over the working temperature.
Sterilization Time Table
TOTAL STERILIZATION TIME
(does not include drying)
Material
a. Unwrapped instruments,
open glass or metal
containers and any other
items where such
temperature is suitable.
Single Instruments 12min. 9min.
STE.
TEMP
273ºF
(134ºC)
MK M
Cold
Start
Hot
Start
Cold
Start
Hot
Start
16min. 11min. — — 1730
27min. 13min. 2340
30min. 14min. 2540
21min. 11min.
32min. 23min.
20min. 15min. — — 1730
b. Wrapped instruments,
standard cassettes,
rubber tubing and any
other items where such
temperature is suitable.
c. Packs and any other
items where such temp.
is suitable.
d. Any items where a
lower sterilization temp.
is required.
273ºF
(134ºC)
273ºF
(134ºC)
250ºF
(121
º
25min. 15min.
25min. 20min. 45min. 35min.
30min. 25min. 60min. 50min.
31min. 17min. 2340
34min. 18min. 2540
36min. 27min.
Note: The table shows different times for M (Standard Manual) & MK
(Kwiklave Manual) units as well as for hot and cold starts.
Make sure you are using the correct times for your model machine.
A hot start is any cycle that is begun within 1 hour of a previous cycle ending
(including drying time).
Note: These sterilization times are based on the unit being supplied the
correct voltage, as indicated on the Technical Label attached to the outer
Models
3140,
3850,
3870
3140,
3850,
3870
All
models
All
models
29
Page 32

cabinet. If the voltage supplied is substantially less than the indicated voltage,
additional time must be added to each cycle.
7.9. Set the Timer to the desired sterilization cycle time according to the
Sterilization Time Table.
7.9.1. The Heat Light will come on, indicating that power is being
supplied to the Heating Elements and remain on until the
correct sterilization pressure is achieved.
7.9.2. Once the correct pressure is reached the Heat Light will cycle
on and off, indicating that the Heating Elements are turning
on and off to maintain the correct sterilization pressure.
7.9.3. When the Timer reaches 0 min, the Heating Elements are
turned off and a buzzer will sound indicating that the
sterilization cycle is complete.
7.10. If unwrapped instruments were sterilized and no drying is required,
follow these steps.
7.10.1. Once the Timer has reached 0 min, turn the Multi-purpose
valve knob promptly to the Exhaust / Dry position. This will
allow the steam and leftover water to return to the reservoir.
7.10.2. When the white needle on the pressure gauge has reached
0 psi, the door can be opened.
7.10.3. Unscrew the Door Closing Device, move it to the side and
open the door to remove the instruments.
7.10.4. Now turn the Multi-purpose valve knob to the “0” or off
position.
Note: The sterility of instruments processed in unwrapped cycles
cannot be maintained if exposed to a non-sterile environment.
7.11. If wrapped instruments were sterilized and drying is required, follow
these steps.
7.11.1. Once the Timer has reached 0 min, turn the Multi-purpose
valve knob promptly to the Exhaust / Dry position. This will
allow the steam and leftover water to return to the reservoir.
Do not allow the pressure to drop below 10 psi before
beginning this procedure. This will cause water to remain in
the bottom of the chamber even after the Multi-purpose
valve has been turned to Exh / Dry. Resetting the Timer for
drying will only be heating up this water and not drying the
instruments.
If the pressure has dropped below 10 psi, leave the unit in
the STERILIZE position, leave the door closed and locked.
Now reset the Timer for 10 minutes.
When the Timer reaches 0 min, the pressure should be above
10 psi (if not, add 5 more minutes to the Timer). Now turn
the Muli-purpose valve to the Exh / Dry position. This will
insure that all the water has been returned to the reservoir.
Note: The sooner the Multi-purpose valve is turned to
Exh / Dry at the end of the sterilization cycle, the
more effective and efficient will be the drying.
7.11.2. When the white needle on the pressure gauge has reached
0 psi the door can be opened.
30
Page 33

7.11.3. Unscrew the Door Closing Devise as far as it will go, but do
not move it to the side, this will allow the door to open
about ¾ of an inch.
7.11.4. Leave the Multi-purpose valve knob in the Exhaust / Dry
position.
7.11.5. Reset the Timer for drying, 20 – 30 minutes, the Dry Light
will come on indicating that Drying is active and the
Heating Elements are back on.
7.11.6. When the Timer reaches 0 min., the drying is complete and
the Dry Light and Heating Elements will turn off.
7.11.7. Unscrew the Door Closing Device, move it to the side and
open the door to remove the instruments.
7.11.8. Now turn the Multi-purpose valve knob to the “0” or off
position.
Warning
Multi-purpose valve knob should be turned in a clockwise direction
only.
7.12. At the end of the day, turn the ON / OFF rocker switch to the OFF
position.
31
Page 34

8 SERVICE AND MAINTENANCE INSTRUCTIONS
8.1 Preventive and Scheduled Maintenance
The maintenance operations described in this chapter need to be
followed as indicated to keep the device in good working condition.
The instructions that follow can easily be carried out by the office
personnel and do not require a service technician.
Should the need arise, technical assistance or a service technician can be
requested by either calling your dealer or Tuttnauer USA.
8.1.1 Daily
Clean the door gasket with a mild detergent, water and a soft
cloth or sponge. The gasket should be clean and smooth.
8.1.2 Weekly
1. ONCE PER WEEK, clean the air jet. To ensure that the
temperature inside the chamber rises properly, it is
necessary to keep the air jet clean. A dirty air jet will
prevent indicator strips from changing color and cause
spore tests to fail. See sec. 8.3.
2. Once per week, clean and descale the chamber, copper
tubes and the reservoir using Chamber Brite (see sec. 9).
Caution
Do not use steel wool, steel brush or bleach as this can
damage the chamber and trays!
3. Take out the tray holder and trays. Clean the tray holder
and trays with detergent or a non-abrasive stainless steel
cleaner and water, using a cloth or sponge. Rinse the tray
holder and trays immediately with water to avoid staining
the metal.
4. Put a few drops of oil on the 2 door pins and door
tightening bolt screw shaft and bearing.
5. Clean the outer parts of the autoclave with a soft cloth.
8.1.3 Periodically
1. Once every month, clean and check the safety valve (see
sec. 8.5).
2. Replace the door gasket every 12 months, or as needed
(see sec. 8.4).
3. Once a year, inspect the locking device for excessive
wear.
32
Page 35

8.2 Draining the Reservoir
Caution
Before starting, ensure that the electric cord is disconnected and
there is no pressure in the autoclave.
The drain valve is located on the front left side of the autoclave after the
door is opened. The function of the drain valve is to drain the water
reservoir.
1. Connect the silicone hose, supplied with the autoclave, to drain
into a bucket.
2. Turn drain valve counterclockwise to the open position.
3. Fully drain the reservoir.
4. With a quart of tap water, flush out the reservoir.
5. Turn drain valve clockwise to the close position.
6. Connect the electric cord to power source.
7. Fill the reservoir with distilled water to just below the safety
valve (see sec 7.2).
8. Turn on the main power switch.
9. The autoclave is now ready for use.
33
Page 36

8.3 Cleaning the Air Jet
(Located in the water reservoir.)
A dirty air jet is the number one cause of failed spore tests
The elimination of air from the sterilization chamber during heat up is
critical to the proper operation of the autoclave. Failure of the air
removal system will be responsible for incomplete sterilization, indicator
strips that do not change color and failed spore tests.
The air jet consists of a small orifice with a clean out wire inserted in it
(wire is permanently installed and will not come out). It is required that
the air jet be cleaned once per week or more often if necessary, to
remove any accumulated dirt and debris.
It is preferred to clean the air jet when the unit is running a cycle and
under pressure. This is so that any loosened debris will be blown away,
however, it can be done while the unit is idle.
1. Remove the water reservoir cover.
2. Clean the hole of the jet by manipulating the air trap wire (A) back
and forth 10 times.
Note:
It is important to clean the hole of the air trap, as described in point 2
before starting operation of the autoclave, for the first time.
34
Page 37

8.4 Replacing the Door Gasket
Pull off the gasket from the door groove. Install the new gasket as
described in drawings 1, 2 and 3 below.
Caution!
This gasket is designed with a trapezoidal cross section. The gasket
should be placed with the widest side towards the door.
35
Page 38

8.5 Checking the Safety Valve
(Located in the water reservoir)
In order to prevent the safety valve (6) from becoming blocked, it is
necessary to allow the steam pressure to escape through the valve. This
procedure should be done every month as follows:
1. Run a sterilization cycle with a sterilization temperature of 273ºF
according to the manual.
2. Allow a pressure of approximately 30 psi (260 kpa ) to build up in
the chamber.
3. Turn the timer back to 0 minutes.
4. Remove the water reservoir cover.
Caution!
This next step will expose you to HOT STEAM
Caution!
To avoid being burned, by hot steam, do not place your face over the
safety valve.
5. Pull the ring of the safety valve using a tool, i.e. screwdriver, hook
etc. and open the safety valve for 2 seconds then release. Be careful
not to burn your hands.
6. Verify that the valve releases steam and closes immediately.
7. If the safety valve is stuck in the “open” position, let the pressure
decrease to zero (atmospheric pressure).
8. After the pressure in the chamber decreases to zero, pull the valve
ring to release the valve.
9. Repeat operations 1 to 6.
10. If the valve is stuck again in the open position, call for service.
11. After a successful check, turn the multi-purpose valve to the
Exh/Dry position.
12. Wait until the pressure decreases to zero, only then can the door be
opened.
36
Page 39

8.6 Unclogging the Multi-Purpose Valve or Fill Piping.
1. Pour distilled water into the chamber, according to quantities
mentioned in para. 4 (Installation Instructions).
2. Close the door.
3. Turn the multi-purpose valve to STERILIZE position.
4. Move the main switch to the ON position.
5. Turn the Thermostat knob to 273ºF (134ºC).
6. Turn the Timer knob to 20 minutes.
7. After the timer has reached “0” turn the multi-purpose valve
(clockwise) to the FILL WATER position, do not stop at any
other position.
In most cases, the pressure pushes the obstructing substance out
and the steam exhausts into the water reservoir.
8. When the pressure gauge reaches 0, turn the multi-purpose valve
to the "0" position, and the main switch to OFF.
9. Open the door.
10. Replace the water in the water reservoir.
The autoclave is ready for the next cycle.
11. If this procedure does not clear up the clogging, a technician will
be required to replace the multi-purpose valve or clear the piping.
VERY IMPORTANT!
When sterilizing cotton wool or pads, it is essential to wrap them in
paper or cotton bags in order to prevent the multi-purpose valve and
the autoclave openings from becoming clogged with remnants of the
material.
37
Page 40

9 CLEANING THE TABLETOP AUTOCLAVES WITH
CHAMBER BRITE™
CHAMBER BRITE ™ is a cleaning and descaling agent designed specifically
for the cleaning and removal of water deposits, oxides and other sediments
that are found in steam sterilizers. The material is a combination of acidic salts
and additional cleaning materials.
Chamber Brite™ autoclave cleaner has been formulated specifically to be a
fast, powerful and easy to use cleaner for steam sterilizers.”
If the autoclave is not cleaned regularly, dirt and debris will build up and clog
the tubing and valves. This dirt can also be transmitted to the instruments
during sterilization. In addition, a layer of dirt on the stainless steel chamber
traps moisture against the metal and will lead to the chamber becoming porous
and failing.”
“It is recommended that your autoclave be cleaned with
CHAMBER BRITE™ once per week”
Caution!
NEVER use bleach, steel wool, a steel brush
or anything abrasive to scrub or clean the
chamber.
Cleaning Procedure
1. Important – all steps in this procedure
must be completed without interruption.
2. When the autoclave chamber is cold,
remove instruments and trays from the
autoclave.
3. Open the door and spread the contents of a
packet in a straight even line along the
bottom of the chamber, from back to front.
4 Start a sterilization cycle* with water and
No Drying Cycle according to the
manufacturer's instructions. When the
cycle is finished, exhaust the unit.
5. At the end of the exhaust cycle, drain the
water from the reservoir.
6. Fill the water reservoir with distilled
water.
7. Repeat a sterilization cycle without
Chamber Brite™ powder, to remove any
excessive dirt in the pipes. Start a
sterilization cycle* with water and No
Drying Cycle according to the
manufacturer's instructions. When the
cycle is finished, exhaust the unit
8. At the end of the exhaust cycle, drain the
water from the reservoir.
9. Turn the autoclave off and allow chamber
to cool.
38
Page 41

10. Remove the tray holder; wipe the interior of the chamber with a damp
cloth.
11. Fill the reservoir with distilled water only.
12. Wipe the tray holder with a damp cloth and return it to the chamber.
13. Turn fill knob to fill position and allow a small amount of water (2-4
ounces) to fill chamber. Remove water from chamber.
14. The autoclave is ready to use.
IMPORTANT!
DO NOT sterilize instruments during the cleaning process!!!
CAUTION!
Keep out of reach of children. Contains mildly acidic ingredients. Avoid
contact with the skin, eyes or clothing. Wash hands well after touching the
powder, in the case of eye contact flush with continuous running water for
at least 15 minutes. If irritation persists get medical attention. If
accidentally swallowed, do not induce vomiting, drink large amounts of
water and obtain medical attention. MSDS available upon request.
For models 1730, 2340, 2540 use one packet of CHAMBER BRITE ™.
For models 3140, 3850, 3870 use two packets of CHAMBER BRITE ™.
Clean every 20 cycles or as needed.
* Total cycle time for cleaning Tuttnauer “M” series is 30 minutes at 273°F
(134°C). Total cycle time for cleaning Tuttnauer “MK” series is 15
minutes at 273°F (134°C). All cycles referenced are from a cold start.
39
Page 42

Corrections
the wall outlet and the back of the unit or
restore power to the wall outlet.
1.1 Make sure the power cord is plugged into
Possible Cause Checkup and Tests
has no power.
1.1 The unit is not plugged in or the wall outlet
1.2 Reset the circuit breaker.
1.3 Reset the cut out thermostat.
1.2 Circuit breaker is tripped.
light.
1.4 Have a technician replace the “Power”
1.3 Cut out thermostat is tripped.
15
to
the
timer
to
the
set
valve
and
purpose
-
position
”
multi
Dry
the
/
Exh
“
minutes.
1.4 Turn
burned out. When finished, turn the timer
If the “Dry” light is on, the “Power” light is
1.5 Have a technician checkout the unit.
back to 0 minutes.
1.5 If the “Dry” light does not come on, there is
2.1 Have a technician replace the “Heat” light.
an internal electrical problem.
2.1 The “Heat” light is burned out.
STERILIZE position.
3.1 Turn the multi-purpose valve to the
3.2 Close the door tightly.
STERILIZE position.
3.1 The multi-purpose valve is not in the
3.2 The door is not closed tightly.
activator. When adjusting – turn the
activator screw, in or out, by ¼ turn until
the door microswitch is pushed in when the
door is closed.
3.3 Replace or readjust the door switch
3.4 Turn the timer on.
3.5 Have a technician checkout the unit.
adjusted correctly.
3.3 The door switch activator is missing or not
3.4 The timer is off.
3.5 There is an internal problem.
Symptom
qualified technician needs to be called. Please call your dealer or Tuttnauer USA Co.
This troubleshooting section is for use by the Operator of the autoclave. If a problem develops that is not covered in this section, a
10 TROUBLESHOOTING
light up when the ON / OFF
switch is in the ON position.
1. Power indicator light does not
light up at the beginning of the
sterile cycle. The Power light is
on and the unit does heat up.
2. Heat indicator light does not
light up at the beginning of the
3. Heat indicator light does not
up.
sterile cycle. The Power light is
on and the unit does NOT heat
40
Page 43

to unclog
to unclog
. 6. A Tuttnauer Pouch Rack
Corrections
position.
4.1 Have a technician replace the “Dry” light.
Possible Cause Checkup and Tests
4.1 The “Dry” light is burned out.
5.1 Turn the multi-purpose valve to the DRY
5.2 Turn the timer on.
5.3 Have a technician check out the unit.
6.1 Fill the reservoir with distilled water.
position.
5.1 The multi-purpose valve is not in the DRY
5.2 The timer is off.
5.3 There is an internal problem.
6.1 There is no water in the reservoir.
the valve.
6.2 Follow the instructions in para. 8.6
6.2 The multi-purpose valve is clogged.
the piping.
6.3 Follow the instructions in para. 8.6
7.1 Clean the air jet, see para. 8.3
Load Sizes" table in sec. 2.
7.2 Adjust loading according to "Maximum
and not stacked. See preparations for
sterilization para
is recommended
7.3 Items to be sterilized should be separated
7.4 Check table para 7.
proper operating pressure”.
7.5 See para. 10.9 “unit does not reach the
pressure reading on the gauge.
7.1 Air jet is clogged.
7.2 Unit may be overloaded.
7.3 The load may be too densely packed.
7.4 Sterilization time may not be correct.
6.3 The fill piping is clogged.
7.5 Unit not reaching the correct sterilization
Symptom
Chamber when the multi-
light up at the beginning of the
dry cycle. The Power light is on
and the unit does heat up.
4. Dry indicator light does not
light up at the beginning of the
dry cycle. The Power light is on
and the unit does NOT heat up.
5. Dry indicator light does not
6. Water does not enter the
WATER position.
purpose valve is in the FILL
indicator strips are not changing
color according to instructions
of indicator's manufacturer.
7. Spore tests are failing or
41
Page 44

sterilization para. 6. A Tuttnauer Pouch Rack
operating
correct
for
Corrections
2.6
table
cycle, immediately turn the multi-purpose
valve to the “Exh / Dry” position. Once
venting is complete, start the drying cycle.
8.1 Once the timer rings at the end of the sterile
Possible Cause Checkup and Tests
after the sterile cycle.
8.1 The unit is not being vented immediately
Load Sizes" table in sec. 2.
8.2 Adjust loading according to "Maximum
8.3 Items to be sterilized should be separated
8.2 Units may be overloaded.
8.3 The load may be too densely packed.
is recommended.
and not stacked. See preparations for
8.4 Have a technician check the unit.
8.4 Units may not be heating properly.
voltages.
9.1 Check
9.1 Unit is not getting the correct voltage.
instructions.
procedures. See sec. 4 for installation
9.2 Check section 7 for correct operating
replace the door gasket.
9.3 Tighten the door more, if leaking persists,
amount of water.
9.2 Chamber was not filled with the correct
9.3 Door gasket is leaking steam.
replace the bellows.
9.4 Door bellows is leaking, have a technician
9.4 Steam is leaking at the closing device.
valve.
persists have technician replace the Safety
9.5 Follow procedure in section 8.5. If leaking
9.5 Safety Valve is leaking.
front of the unit higher to allow more water
into the chamber. See installation
instructions section 4.2/maximum load table
section 2.
9.6 Sterilize fewer towels or gowns or adjust the
available steam (cloth towels or gowns).
9.6 Items being sterilized are absorbing all
internal steam leak. Have a technician
check the unit.
9.7 Unit may be out of calibration or there is an
9.8 Have a technician check the unit.
is reached.
9.7 Heat light goes out before correct pressure
9.8 One or more of the heating elements is bad.
Symptom
not dry. See also section 10.32
and section 8.8.
8. Wrapped or bagged items do
operating pressure.
9. Unit does not reach the proper
42
Page 45

Corrections
instructions.
procedures. See sec. 4 for installation
10.1 Check section 7 for correct operating
Possible Cause Checkup and Tests
amount of water.
10.1 Chamber was not filled with the correct
of the chamber.
10.2 Make sure no items are touching the walls
10.2 Items are lying up against the chamber.
table in section 7.
10.3 Set the sterilization time according to the
10.3 The sterilization time is set too high.
the front of the unit higher to allow more
water into the chamber. See installation
instructions section 4.2/maximum load
table section 2.
10.4 Sterilize fewer towels or gowns or adjust
available steam (cloth towels or gowns).
.
10.4 Items being sterilized are absorbing all
replace the door gasket.
10.5 Tighten the door more. If leaking persists,
10.5 Door gasket is leaking steam.
replace the bellows.
10.6 Door bellows is leaking, have a technician
10.6 Steam is leaking at the closing device.
valve.
persists have technician replace the Safety
10.7 Follow procedure in section 8.5. If leaking
10.7 Safety Valve is leaking.
10.8 Have a technician check the machine.
10.8 There is an internal steam leak or electrical
11.1 Follow instruction in section 10.31.
problem.
11.1 Door bellow locking pin is trapped.
white lithium grease to the screw and
8.1.2.4.
bearing of the closing device, see sec
12.1 Apply 1 or 2 drops of 3 in 1 oil or some
12.1 Closing device needs oil.
burning or melting.
Symptom
10. Items in the chamber are
43
when pressure gauge reads 0
psi.
11. Closing device does not open
close.
12. Closing device is hard to
Page 46

Corrections
leaking persists, have technician
instructions.
procedures. See sec. 4 for installation
7.2.
sterilized, this is not a problem.
open the door right away.
reservoir. The open end of the cooling
coil should not be below the water line.
thermostat; if the problem persists have a
technician check the machine.
a) Check section 7 for correct operating
persists, replace the door gasket.
b) Tighten the door more; if leaking
replace the Safety valve.
c) Follow procedure in section 8.5. If
the front of the unit higher to allow more
water into the chamber.
See installation instructions section
4.2/maximum load table section 2.
d) Sterilize fewer towels or gowns or adjust
e) Have a technician check the machine.
13.1 Do not fill above the safety valve. See sec
Possible Cause Checkup and Tests
13.1 Water level too high in the reservoir.
13.2 If unwrapped instruments are being
13.2 Waiting too long to exhaust after
For wrapped instruments see sec 7.11.
14.1 Once the chamber is finished venting,
sterilization is complete.
14.1 Leaving the multi-purpose valve in the
Also, straighten the cooling coil in the
“Exh/Dry” position while leaving the door
closed and having a cooling coil that is
mispositioned in the reservoir.
15.1 Correct the cause and reset the Cut out
beginning of the cycle.
condition due to too little water in the
chamber caused by either:
a) Incorrectly filling the chamber at the
15.1 The unit has detected an overheating
b) A leaking door seal.
d) Absorbent material being sterilized.
c) A leaking safety valve.
e) An internal steam leak.
Symptom
the chamber at the end of
sterilization.
13. Water is left in the bottom of
the door is open.
14. Water spills on the floor when
15. Cut out thermostat trips.
44
Page 47

Corrections
to the up position. If the problem persists,
have a technician check the unit.
compressed air into the valve. This should
where it can be removed. Cover the
reservoir opening to avoid splatter.
blow the clog back into the reservoir
compressed air into the valve. This should
where it can be removed. Cover the
reservoir opening to avoid splatter.
blow the clog back into the reservoir
Chamber Brite, section 9.
Chamber Brite, section 9.
16.1 Reset the red lever of the circuit breaker
Possible Cause Checkup and Tests
16.1 A power surge or spike.
17.1 Open drain valve completely and blow
17.1 Drain valve is clogged.
18.1 Open drain valve completely and blow
properly.
18.1 Debris is stopping the valve from seating
18.2 Have a technician replace the o-rings.
18.2 Drain valve o-rings are worn.
19.1 Follow cleaning instructions using
19.1 Unit has not been cleaned recently.
20.1 Use 100% steam distilled water.
20.2 Follow cleaning instructions using
20.1 Not using distilled water.
20.2 Unit has not been cleaned recently.
21.1 Have a technician replace the door bellows.
21.1 The door bellows is leaking.
22.1 Tighten the door more.
22.2 Replace the door gasket (see sec. 8.4).
22.1 Door may not be tightened down enough.
22.2 Door gasket may be worn or cracked.
Symptom
looking.
16. Circuit breaker trips.
17. Reservoir does not drain.
18. Drain valve is leaking.
45
19. Chamber is black or dirty
along bottom.
20. Chamber has a water mark
device area.
21. Steam escaping from the closing
seal.
22. Steam leaking from the door
Page 48

Corrections
knob and the glass of the gauge. Turn the
knob back and forth to work the oil onto
purpose valve.
purpose valve.
the internal shaft.
small screw in the center of the silver
knob.
set hammer.
then pull on the ring of the safety valve.
This will relieve the vacuum in the
chamber.
23.1 Have a technician replace the multi-
Possible Cause Checkup and Tests
23.1 The internal spring in the multi-purpose
24.1 Have a technician replace the multi-
valve has broken.
24.1 Poor maintenance will result in the multi-
25.1 Place one drop of oil between the silver
purpose valve binding.
25.1 No lubrication on the needle shaft.
25.2 Use a small screwdriver to adjust the
26.1 Have a technician replace the timer.
27.1 Have a technician replace the timer.
27.2 Timer must be turned past 10 minutes to
off.
28.1 Follow the instructions in sec 8.5.
hammer
valve.
29.1 Follow instructions in sec 6.23.
dirt.
30.1 Make sure pressure gauge reads 0 psi, and
and a vacuum developed.
25.2 Internal spring is too tight.
26.1 Internal gearing has worn down.
27.1 The hammer on the timer bell has broken
27.2 Timer was not turned far enough to set
28.1 Debris has lodged in the seat of the safety
29.1 The first step is to determine if it is rust or
30.1 Unit was left to cool with the door closed
Symptom
backwards.
23. Multi-purpose valve turns
turn.
24. Multi-purpose valve does not
gauge is hard to set.
25. Red tracking needle on pressure
26. Timer does not time down.
27. Timer bell does not ring.
28. Safety valve is leaking.
29. Instruments are rusting.
device is open.
30. Door will not open, closing
46
Page 49

Corrections
recommended to provide proper spacing
of bagged instruments. This will allow
for better exposure to steam for
sterilization and better circulation
(closing direction). The pin will be
released, enabling the operator to
EXH. & DRY.
1. a. Turn the Multi-purpose valve to
Device ¼ of a turn in the clockwise
b. Turn the handle of the Door Closing
open the door.
Technician will need to be called
2. If this does not correct the problem, a
a. A Tuttnauer Pouch Rack is
of air for drying.
the instruments on the tray are only one
level deep and that the paper / plastic
bags are plastic side down.
b. If a Pouch Rack is not used, make sure
31.1
Possible Cause Checkup and Tests
31.1 Locking pin is trapped.
32.1
each other.
32.1 Bagged instruments are placed too close to
32.2 End cycle according to para. 7.
correctly.
32.2 Cycle ending has not been performed
Symptom
the
door to be opened
and there is no pressure in the
be turned counter-clockwise for
chamber.
31. Door handle cannot
are not drying properly (see also
sec 7.11).
32. Wrapped or bagged instruments
47
Page 50

TRAY HANDLE CT530020
For 1730, 2340, 2450 models only
POUCH RACK
TRAY
TYPE
CAT No.
2340
2540
AR910
AR920 3870
MODEL SIZE CAT. No.
1730 CU520010
2340 CT520010
2540 CT520010
Big TRY314-0001
3140
Small TRY314-0002
Big CF520010
3850
Small CF520020
Big CC520010
3870
Small CC520020
48
Page 51

TRAY HOLDER
MODEL TRAY HOLDER CAT. NO.
1730 CU510010
2340 CT510010
2540 CV510010
3140 TRH314-0000
3850 CF510010
3870 CC510010
49
Page 52

11 LIST OF ACCESSORIES
Description
1730 2340 2540 3140 3850 3870
Cat. No.
Tray Handle, TTA
1730, 2340, 2540
Pouch Rack
Big
Tray
Small
Tray Holder
Silicon drain tube
Chamber Brite 1 case
(12 boxes)
CT530020 CT530020 CT530020 — — —
— AR910 AR910 — — AR920
TRY314-0001 CF520010 CC520010
CU520010 CU520010 CU520010
TRY314-0002 CF520020 CC520020
CU510010 CT510010 CV510010 TRH314-0000 CF510010 CC510010
02620016 02620016 02620016 02620016 02620016 02620016
CB0010 CB0010 CB0010 CB0010 CB0010 CB0010
50
 Loading...
Loading...