trackit Mk3 User Manual

Digitally signed by Michael
Hulin
Date: 2018.03.02 10:39:47 Z
Digitally signed by David Hulin
DN: cn=David Hulin, o=Lifelines Ltd,
ou=Development, email=david.hulin@llines.com,
c=GB
Date: 2018.03.02 11:05:11 Z
Digitally signed by Michael
Hulin
Date: 2018.03.02 11:58:46 Z
Trackit Mk3
User Manual
Part no. 1114
Issue 2.4
28 February 2018
Created Checked Approved

Trackit Mk3 User Manual
Version History
V2.4 (February 2018)
• Added disinfec tion information in sec tion 2.7.
• Changed N.B. to 0086 (BSI).
V2.3 (May 2014)
• Latest 4
• Bluetooth recording times a nd range details added
• Latest version low-power XPOD Pulse Oximeter details added in Appendix 2
V2.2 (September 2011)
• Trackit Mk3 in tr oduced: USB Interface detail added to Sections 1.2, 1.3, 3.1, 3.2 and 4.2.
• Large memory cards (FAT32) a dded in Section 5.6.
• Aux Box 2 added in Appendix 2.
• More Setup parameters added to Appendix 5.
• Quick Setup Guide adjuste d in Appendix 6.
• Troubleshooting adjusted i n Appendix 8.
V2.1 (June 2010)
• Added Bluetooth indicator to Figure 6
• Added ‘Internal Bluetooth’ pa r a gr a ph to Appendix 5
• Minor amendments to section 5.7 ‘Reading an ambulatory recording’
• Minor amendmen ts to Appendix 4 ‘Network Connection’
V2.0 (April 2010)
• Reformatted to A4 page s ize
• Added full documentation to Appendix 4:
• Added full documentation to Appendix 5: Bluetooth Wireless
• Appendix 7: Troubleshooting guide moved to Appendix 8
• Appendix 8: Manufa c turer’s Declaration m oved to Appendix 9
• Added Appendix 7: Trackit Setup Wizard
V1.8 (28 January 2010)
• Virus protection recommendations added, Page 5.
• Lifelines logo adjusted, P a ge 3.
• References to Windows 98 a nd ME removed, Sections 1.4 and 4.
• Check with distributor for later software version added, Section 4.
• Picture of latest main Tool ba r updated, Figure 16, and table below u pda ted.
• Picture of main ongoing dis pla y window updated, Figure 23.
• Appendix 3 added, Photic and Hyperventilation. Photic refers to separate documentation. Old
• Appendix 4 added, Trackit Plus and Plus with Video Software. B oth r efer to separate docu-
• Appendix 5 added, Bluetoo th Wireless. Refer to separate docu m entation. Old Appendix 5 now
th
Generation internal Bluetooth d etails added in A ppendix 5
o Section 1 Record to PC
o Section 2 Network Connection
o Section 3 Video
Appendix 3 now Appendix 4.
mentation. Old Appendix 4 now Appendix 5.
Appendix 6.
2

Trackit manufactured by:
Lifelines Ltd, 7 C larendon Court,
Over Wallop, near Stockbridge,
Hampshire SO20 8HU, UK
Telephone +44 (0)1264 782226
www.LLines.com
sales@LLines.com
Trackit Mk3 User Manual
0086
3

Trackit Mk3 User Manual
Disclaimers & Warranties
The information in this section is sub je c t to change without n otice.
Except as stated below, Lifelines Ltd m a kes no warranty of an y kind with regard to this material,
including, but not limited to, the implied warranties of merchantability and fitness for a p a r ticular
purpose. Lifelines shall not be liable for errors contained herein or for inc id ental or consequentia l
damages in conn e c tion with the furn ishing, performanc e or use of this material.
Lifelines shall w a r rant its products a g a inst all defects in m a terial and workman sh ip for one year
from the date of delivery.
Misuse, accident, modification, unsuitable physica l or operating envir onment, improper main tenance or damage caused by a product for which Lifelines is not responsible will void the warranty.
Lifelines do not w a rrant uninterrupted or error-free oper ation of its products .
Lifelines or its authorised agents will repair or replace any products that pr ove to be defective dur-
ing the warranty period, provided that these products are used as prescribed in the operating instructions in the user’s and service manuals.
No other party is authorised to make any warranty to assu m e lia bility for Lifelines products. Lifelines will not recog nise any other wa r r a nty, either implied or in writing. In ad dition, services performed by someone other than Lifelin e s or its authorised agents or any technic al mod ification or
changes of products without Lifelin es prior, written consent may be cause for voiding this warr anty.
Defective products or par ts must be return ed to Lifelines or its auth orised agents, alon g with an
explanation of th e failure. Shipping c os ts must be prepaid.
Lifelines Ltd. manuf a c tures hardware and software to be used on or w ith standard PC-compatible
computers and operating s oftware. Lifelines, however, assumes no responsibilit y for the use or reliability of its software or hardware with equipment that is not furnished by th ir d-party manufacturers accepted by Lifelines at th e da te of purchase.
All warranties for third-party products used within the T rackit system are the responsibility of the
relevant manufacturer. Please refer to the relevant d ocumentation on each product for further details.
This document c ontains proprietary information that is protected by copyright. All rights are reserved. No part of this document may be p hotocopied, reproduced in any other form or translated
into another language without the prior written consent of Lifelines.
Trademarks
Microsoft, Windows and Windows N T are registered tradema r ks of the Microsoft Corporation. All
other trademarks and prod uct names are the property of their relevant owners.
Responsibility of manufacturer
The manufacturer and distributor con sider themselves responsible for the equipment’s sa fety, reliability and performanc e only if:
any peripheral equipment to be used with Trackit is supplied by third-party providers recom-
mended by the manufacturer;
assembly operations, extension s , readjustments, m odifications, or repa irs are carried out by
persons authorised by the manufacturer;
the electrical ins tallation of the rele vant room complies with the appropriate requirements;
the equipment is used by a hea lth-care professional and in accordance with the instruc tions for
use.
Note: the manufacturer has a policy of continual product imp r ovement; hence the equipment
specifications a re subject to change without notice.
Check with Lifelines or your distributor if a software upda te is available.
4

Trackit Mk3 User Manual
Note: Medical electrical equipment needs s pec ia l precautions regarding E M C and needs to be installed and put into service according to the EMC informa tion provided in the Ap pe ndix.
Software and V irus Protection
Lifelines takes all reasonable steps to ensure that it’s software is virus-free. In line with modern
computing practice, it is advisable that con tinual protection a g a inst viruses, trojans, malware, adware etc. is provid ed on the PC used for installation and the surrounding systems. Please note the
following recommendations which should be su pported by your internal IT/Computing department
procedures and practices:
1. Virus protection softw a r e s hould be installed on e very computer at risk of infection. This soft-
ware should have a resident ( on line) shield and provide email sca nning if appropriate.
2. Virus scanning should be s et to m anual mode or auto m a tic if desired but at a time when the
system is not being used.
3. All programs offering au to-update features, inclu ding Windows, should be set to manual or
automatic if desired but at a time when the system is not bein g used.
4. Adopt f ormal departmental or organisational procedures to en sure the integrity a nd safe op-
eration of the me dic al equipment and supporting systems.
5

Trackit Mk3 User Manual
Contents
Version History 2
Disclaimers & Warranties 4
Trademarks 4
Responsibility of manufacturer 4
Software and V irus Protection 5
Contents 6
Illustrations 8
1 System Overview 10
1.1 General descrip tion 10
1.2 Cautions and Warnings 10
1.3 Explanation of s ymbols 11
1.4 The system and its parts 12
1.5 Specifications and safety 12
Description of th e c om p onents 13
2 Installation an d Maintenance 15
2.1 Checks for completeness and integrity 15
2.2 Environmental parameters for ope r a tion 15
2.3 Use in the home environment 15
2.4 Power supply connections 15
2.5 Use with other equipment 16
2.6 Interference 16
2.7 Maintenance and cleaning 16
2.8 Disposal of equipment 16
3 Connections f or Trackit Mk3 setup 17
3.1 Overview 17
3.2 Connecting the Trackit Mk3 18
3.3 Switching on 18
3.4 Warning symb ols on the display 19
4 The setup software 20
4.1 Setting up a recording protoc ol 20
4.2 Configuring the recorder 25
4.3 Montage Editor 31
5 The ambulatory recording 33
5.1 Changing batteries and ca r ds 33
5.2 Fitting the Click on PCU 37
5.3 Event marking 38
5.4 Ending a recording 38
5.5 Identifying a recording 39
5.6 Advanced Settings 39
5.7 Reading an ambulatory rec ording 43
Appendix 1: Trackit Mk3 Specifications 44
EEG inputs 44
Polygraphy in puts 44
Aux. high-level DC Inputs 45
Modes of operation 45
Connections, p or ts and controls 45
Back-light display 45
Recording format 45
Physical characteristics 45
Safety and EMC standards 46
6

Trackit Mk3 User Manual
Appendix 2: Trackit Mk3 options 47
Nonin XPOD pulse oximeter 47
Appendix 3: Photic Stimulator and Hyperventilation 50
Photic Stimulation 50
Hyperventilation 51
Appendix 4: Record to PC and S yn c hronised Video 53
1 Record to PC 53
2 Network Conne c tion 53
3 Video 57
Appendix 5: Bluetooth Wireles s 61
Introduction 61
System overview 62
Connection and use 63
Application PC Setup 66
Trackit and Bluetooth Module Setu p 69
Trackit Bluetooth Module Specifications 78
Parameter Data 78
Regulatory 79
FCC Statement 80
IC Compliance 80
Guidelines for E fficient and Saf e Use 81
Trackit Bluetooth Battery Power Con sumption 83
Bluetooth Ran g e 83
Bluetooth Knowledge Base 85
Appendix 6: Trackit quick setup and operation guide 86
Appendix 7: Trackit Setup W iz a r d 87
Appendix 8: Troubleshooting guide 88
COM port problems with Bluetooth com m unication to Trac kit Mk3 88
Problems startin g the recording 88
File review problems 89
Appendix 9: Manufacturer’s Declaration 90
EMC Compatibility 90
7

Trackit Mk3 User Manual
Illustrations
Figure 1 Connecting the Trackit Mk3 for recorder set-up 17
Figure 2 Connec ting to the Trackit M k3 recorder 18
Figure 3 Trackit Mk3 recorder : front panel 19
Figure 4 The Trackit Mk3 display 19
Figure 5 New Patient dialog 20
Figure 6 New Patient databa s e 21
Figure 7 Signal list 21
Figure 8 Signal editing tool 22
Figure 9 EEG setup 22
Figure 10 Setup Recording dialog 23
Figure 11 View Hookup 23
Figure 12 Channel setu p 24
Figure 13 Recording Cha nnel editing 25
Figure 14 Track it s oftware toolbar 25
Figure 15 Trackit Control Panel 26
Figure 16 Trackit status 'B' 27
Figure 17 Recording Control 27
Figure 18 Ongoing trace display 29
Figure 19 Adjust display pa r a meter s 30
Figure 20 Impedance chec k 30
Figure 21 The online event viewer 31
Figure 22 Montage Editor 32
Figure 23 Opening the rear battery box/door 33
Figure 24 Removing and replac ing PP3 batteries 34
Figure 25 Removing and replacing Lithium rechargeable batter y 34
Figure 26 Closing the rear Battery box/door 35
Figure 27 Removing and replacing the flash card 36
Figure 28 Removing the flash card 36
Figure 29 Clickon PCU Retaining scre w location 37
Figure 30 Detach the PCU 37
Figure 31 Lift the PCU away from the Trackit Mk3 38
Figure 32 Locating the PCU 38
Figure 33 Trackit Defaults Tab 1 40
Figure 34 Trackit Defaults | Tab 2 41
Figure 35 Pulse oximeter an d oximeter probe 47
Figure 36 Connec ting the oximeter to Trackit Mk3 48
Figure 37 Connecting the oximeter to the oximeter probe 48
Figure 38 Attaching the oximeter probe to the finger 48
Figure 39 Display of SaO2 49
Figure 40 Photic Stim ulation 50
Figure 41 Photic Stim ulation control window 50
Figure 42 Photic trigger signal definition 51
Figure 43 Hyperventilation 51
Figure 44 Hyperven tila tion control win dow 52
Figure 45 Trackit video prev iew 59
Figure 46 Track it M k3 with Internal Blu etooth Module 62
Figure 47 The Trackit Control Panel 63
Figure 48 A typical Bluetooth Place s view 64
Figure 49 Trackit Main Sc reen 65
Figure 50 Options Tab 1 66
Figure 51 Options Tab 2 67
Figure 52 Trackit Defaults Tab1 69
Figure 53 Trackit Defaults Tab2 70
Figure 54 Trackit Defaults Tab 3 70
Figure 55 Trackit Defaults Tab 4 72
8

Trackit Mk3 User Manual
Figure 56 Trackit Defaults | Bluetooth Setup Tab 1 (General) 73
Figure 57 Trackit Def a ults | Bluetooth Setu p Tab 2 (Profile) 74
Figure 58 Track it De faults | Bluetooth S e tup Tab 3 (Serial) 75
Figure 59 Track it De faults | Bluetooth S e tup Tab 4 (Version) 76
Figure 60 Track it De faults | Bluetooth S e tup Tab 5 (Misc) 76
Figure 61 Trackit Def a ults | Bluetooth Setu p Tab 6 (Pairing) 77
9

Trackit Mk3 User Manual
1 System Overview
1.1 General description
Intended use
The Trackit Mk3 is intended to measure and record EEG signals.
Indications for use
The Trackit Mk3 is used as an aid in the diagnosis of neurophysiological disorders such a s epilepsy.
General description
The Trackit Mk3 is a multi-channel, ambulator y, electroencepha lograph recorder. It is a compact
body-worn device that is battery powered and the data is stored on a Compact Flash card.
The device is suita ble for use in a clinica l environment and in a n outpatient setting. The EEG
electrodes are fitted to the patient by a trained clinician prior to the patient being sent home. No
subsequent intervention is required by the patient.
Upon completion of the rec or d ing, the data which is stored on a Compact Flash card is reviewed b y
a clinician using review and analysis software on a PC.
This device is intended only as an adjunct device in patient assess m ent; it must be used in
conjunction w ith other methods of patient diagnosis.
The device does not s ustain or suppor t life.
Intended User
The intended user of the device is a healthcare professional who h a s the training and knowledge to
undertake EE G examinations and is familiar with EEG equipm ent and practice.
1.2 Cautions and Warnings
CONTRAINDICATIONS: Do not use the Trackit Mk3 in an MRI envir onment, in an exp los ive
atmosphere or during defibrillation.
WARNING: This devic e is intended to be used by a healthcar e professional and in accordance with
these instructions for u s e w hich must be read in their entirety before the device is used.
WARNING: This devic e in intended only as an adjunct dev ic e in pa tient assessment; it must be
used in conjunc tion with other methods of patient diagnos is.
WARNING: Lifelines does not supply EEG electrodes. The unit accepts standard 1.5 mm
touchproof electrodes u s in g D IN 42802-style connect ors. To ensure patient safety, the electr odes
used must be approved to the Medic a l Device Directive 93/42/EEC in Europe or to the relevant
local standards outside Europe.
CAUTION: The conduc tive part of electrodes and their connectors , including the Neutral electrode,
should not contact other conductive parts including earth.
WARNING: Lifel ines does not supply the Nonin sensor. Only use the ‘PureLight’ sensor s s pec if ied
by Nonin to be used with their Oximeters.
WARNING: Strangulation hazard due to long cables. A s with all medical equipme nt, carefully
route patient cabling to reduce the possib ility of patient entanglement or strangulation.
CAUTION: When in close proxim ity to the recorder, do not use mobile phones, transmitters, p ower
transformers, motors, or other equipment that gener a tes magnetic fields. Refer to the Appendix for
more information. Medical electrical equipment needs special precaution s r ega r ding EMC and needs
to be installed and put into service according to the EMC in formation provided in the Appendix.
WARNING: The f unction or safety of the equipment could be impaired if it has been subjected to
unfavourable c onditions in storage or in transit. If at any tim e function or safety is thought to be
impaired, the instrumen t s hould be taken out of operation and secured against unintended us e.
WARNING: Do not open the equ ipment.
WARNING: Do not modify this equipmen t without the authorization of the manufacturer.
10
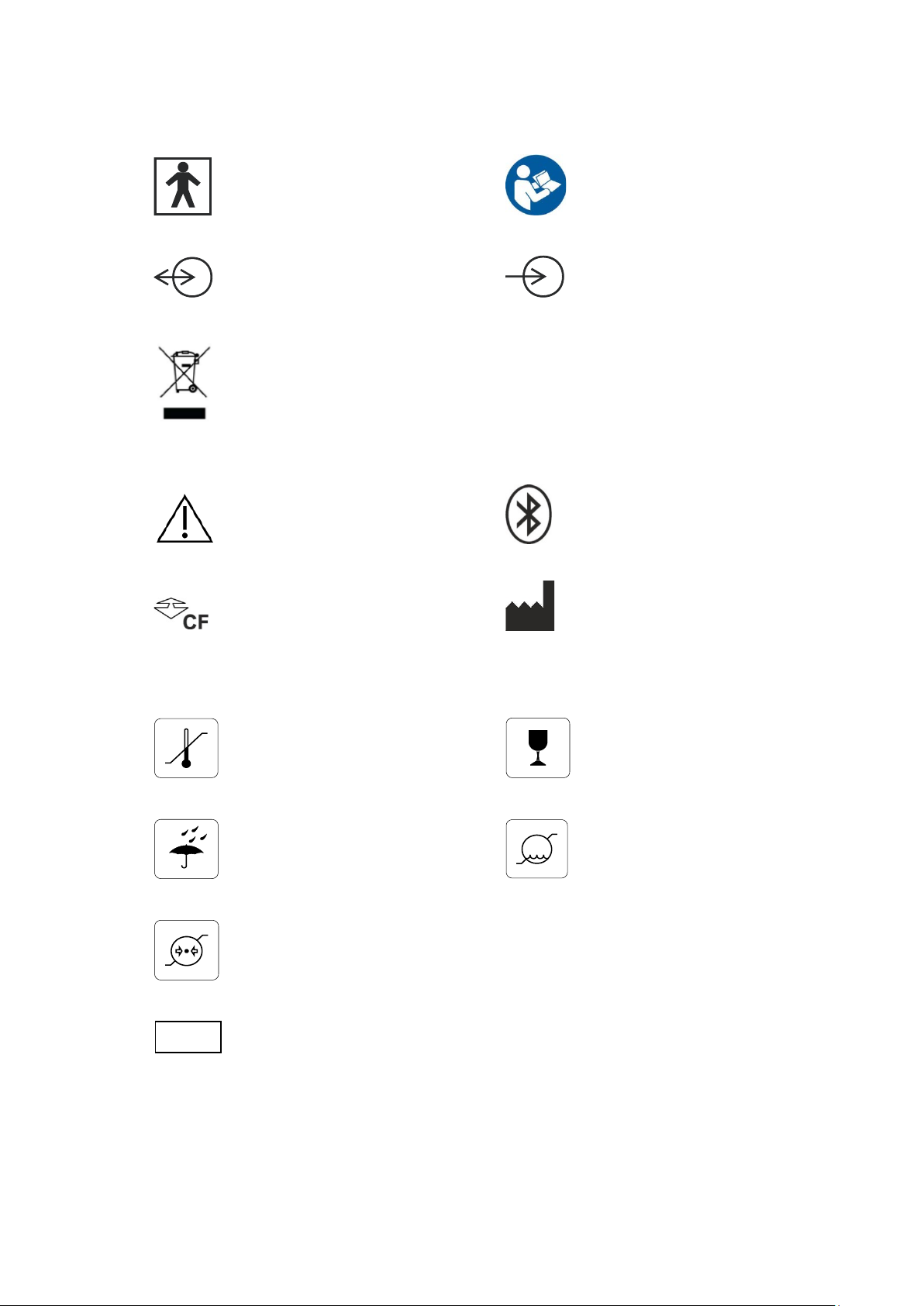
1.3 Explanation of symbols
-10
+50
°C
10
95
%RH
500
1060
hPa
IP22
Type BF equipment Follow operatin g instructions
Input/output connection Input connection
Special recycling required, do not dispose of in la ndfill. When this eq uipment has
reached the end of its useful life, it mu s t b e disposed of in an en vironmentallyfriendly way. Waste electrical and electronic equipment (WEEE) requires special
procedures for recycling or disposal. This includes batteries, printed circuit boards,
electronic comp onents, wiring and other elements of electronic devices. Follow all
of your respective local laws and r eg ulations for the proper d is p os al of such
equipment. Contact your local distributor for information concerning this.
Trackit Mk3 User Manual
Consult warnings in User Manual Bluetooth
Push to eject Compact Flash card Manufacturer
Storage and transport symbols
Temperature limits Fragile
Keep dry Relative humidity limi ts
Barometric pressure limits
International p r otection code
Protected against ingress of solid object 12.5 mm diameter.
Protected against access to hazardous parts with finger.
Protected against ingress of water dripping (15° tilted).
11

Trackit Mk3 User Manual
EN60601-2-26
quirements and EEG systems.
ments.
quirements.
ments, calling:
EN55011
Conducted Emissions, Group 1, Class B
EN55011
Radiated Emissions, Group 1, Class B
EN61000-4-2
Electrostatic D ischarges
1.4 The system and its parts
The Trackit Mk3 recorder is a multi-channel1 ambulatory ele c troencephalograph designed for use in
a variety of mon itor ing applications, including those concerned with neurological and sleep disorders.
1
Note
: Trackit Mk3 is available in a number of versions, including a 32-channel (Trackit-32/0 or
Trackit-24/8), a 24-channel (Trackit 24/0) and a 12-channel (Trackit-24/0). The version is displayed on the Trackit Mk3 LCD at switch-on. This manual applies to all versions, the only difference
being the number of channels.
The Trackit Mk3 recorder comprises the follow ing components:
Recorder
Trackit-32/0 (32 EEG) part number 1186
Trackit-24/0 (24 EEG) part number 1187
Trackit-12/0 (12 EEG) part number 1189
Trackit-20/4 (20 EEG, 4 POLYGRAPHIC) part number 1184
Trackit-18/8 (18 EEG, 8 POLYGRAPHIC) part number 1185
Trackit-24/8 (24 EEG, 8 POLYGRAPHIC) part number 1188
Recorder with inter nally fitted wirel e s s Bluetooth option
Trackit-32/0 (32 EEG) part number 1171
Trackit-24/0 (24 EEG) part number 1172
Trackit-12/0 (12 EEG) part number 1174
Trackit-20/4 (20 EEG, 4 POLYGRAPHIC) part number 1169
Trackit-18/8 (18 EEG, 8 POLYGRAPHIC) part number 1170
Trackit-24/8 (24 EEG, 8 POLYGRAPHIC) part number 1173
Patient connection unit (PCU) Clic kon
PCU-clickon Short part number 1181
Patient connection unit (PCU) Cabled
PCU-cabled 24/0 part number 1104
PCU-cabled-Extended 32/0 (univer s a l) part number 1136
Cable, PCU 32ch 1m part number 1106
Cable, PCU 32ch 0.5m part nu m ber 1105
Cable, USB 3m part number 1277
Trackit Mk3 strap, adult part number 1117
Trackit Mk3 strap, child part number 1118
Trackit Mk3 bag with PCU-clickon part number 1259
Trackit Mk3 set up software part number 1009
Trackit Mk3 User Manual part number 1114
Battery Box PP3 part number 1111
Battery Box PP3-Small part number 1140
Battery Box Li part number 1112
1.5 Specifications and safety
Refer to Appendix 1 for specifica tions.
The system has been certified and complies with the following s ta ndards:
EN60601-1 and
European standard for medical electrical equipment, general re-
UL60601-1:2003 USA standard for medical electrical equipment, gener a l require-
CAN/CSA 22.2 No 601.1 M90 Canadian standard for medical electrical equipment, general re-
EN60601-1-2:2001 European standard for medical electrical equipment, EMC require-
12

EN61000-4-3
Immunity - Radiated RF Field
EN61000-4-4
Immunity - Transients Bursts
EN61000-4-5
Immunity – Surges
EN61000-4-6
Immunity – Conducted
EN61000-4-8
Immunity – Power frequency fields
EN61000-4-11
Immunity – Voltage dips, interruptions
EN61000-3-2
Harmonic Emissions
EN61000-3-3
Voltage Fluctuations/flicker
or USB powered (Mk3)
Degree of protection against harmful ingress
Ordinary (no protection)
Mode of operation
Continuous
Degree of safety of application in the presence of a
or nitrous oxide
Not suitable
Degree of protec tion against electr ic al shock (when connected to host system )
Trackit Mk3 User Manual
Type BF
Type of protection against electrical s hock (when connected to host system )
of water
flammable anaes thetic mixture with air or with oxygen
Internally powered
or Mains powered Class 1 or 2 (Mk2)
Description of the components
The Trackit Mk3 recorder
The Trackit Mk3 is a multi-channel recording device designed for use in recording a patient’s E EG
signals. It comprises a 24-channel EEG (monopolar) amplifier acquisition board, an 8-channel polygraphic acquisition board and contr ol b oa r d with all the I/O inter face for serial an d patient communication. When connected to a host PC , the device has built-in is olation for patie nt safety. The device may be powered either by its ow n batteries or a PC USB port. EEG data is stored on an internal CF card. The data format is native European Data Format (EDF) , allowing the EEG files to be
reviewed by any EDF-compatible EEG reader.
Patient Connection Unit
The Patient Con nection Unit (PCU) connects the stan dard 1.5mm touchproof EEG recording electrodes attached to the patient to the Trackit Mk3 unit. It is available either as a ‘Cabled’ type which
is connected via a screened cable or a ‘C lickon’ type which fits on the Trackit Mk3 directly without
needing a cable.
WARNING: Lifelines does not supply EEG electrodes. T he PCU accepts st a nda rd 1.5 mm
touchproof elec t r odes using DIN 42802-style connectors. To ensure pati ent safety, the
electrodes used must be approved to the Medical Device Directive 93/42/EEC in Europe
or to the relevant l oca l standards outside Europe.
CAUTION: The conductive part of el ectrodes and their connectors, incl uding the Neutral
electrode, shoul d not contact other c onductive parts including earth.
PC Connection Cable
The Trackit Mk3 plugs directly into a USB port on the PC.
Batteries
3 PP3 disposable alkaline ba tteries are optionally supplied with the Trackit Mk3 recorder. Alternatively, a recha r g ea b le L ithium battery option is available and a small (single) PP3 opt ion.
CF flash card
A Compact Flash (‘CF’) card is used to store the EEG data recorded by Trackit Mk3. Storage cards
of varying capacity are available in the CF format.
13

Trackit Mk3 User Manual
Trackit setup software
The Trackit setup software runs under Microsoft Windows 2000 (with SP2), Windows XP, Windows
Vista or Windows 7 on the host PC and is used to setup and review the Trackit Mk3 recorder for an
ambulatory recording session.
The Trackit Mk3 recorder is connected to the P C via the connection c a ble or wirelessly with Bluetooth. The recording setup/montage, and patient info r m ation/ID is downloade d to th e device, and a
short review is made to verify that all the electrodes have been attached correctly.
The recorder is then disconnected from the PC and the ambu la tory recording is started.
Functions of the setup software:
Download the recording template. This includes:
which electrodes are turned on or off;
the recording montage;
any timed recording modes.
Download the un ique patient identifier onto the card so tha t no confusion can arise as to whom
the recording belongs to.
Perform a calibra tion of the Track it Mk3 device
Synchronise the Trackit Mk3 time and date to that of the host.
Perform a routin e EEG recording prior to the patient’s am bulatory EEG recordin g.
What does a recording consist of?
2–36 channels of recorded E EG/polygraphic signals
Signals recorded over a period usually not less tha n 24 hours
Patient event mar kers correlated in tim e with the real time cloc k displayed on Tra ckit Mk3 LCD
display
Data and results stored to card for future evaluation
Data review post-recording using any compatible EDF review program
14

Trackit Mk3 User Manual
2 Installation and Maintenance
WARNING: The following section m ust be read and under st ood before the equipment is
switched ON.
Note: Medical electrical equipment needs s pec ia l precautions regarding E M C and needs to be in-
stalled and put into service according to the EMC information provided in the Appendix.
The function or safety of the equ ipm ent could be impaired if it has been su bjec ted to unfavourable
conditions in storage or in transit. If at any time function or s afety is thought to be im pa ired, the
instrument should be taken out of operation and secured again s t unintended use.
The manufacturer should be contacted (details on page 3) for a s s ista nce, if needed, in setting up,
using or maintaining the equipment; or to report unex pec ted operation or events.
2.1 Checks for completeness and integrity
1 Remove the equipment from the packaging case(s).
2 Use the parts list to check that all ordered items have been received.
3 Assembly instructions for third -party products will b e found in their packing cases. It is recom-
mended that these instructions be file d with Trackit Mk3 technical reference materials.
4 Check for signs of damage that may have occurred during transit or storage. If any damage is
found, do not u s e the instrument; conta c t your distributor.
2.2 Environmental p arameters for operation
Operation
The instrument is designed to operate w ithin the following ranges:
Temperature +10°C to +40°C
Relative humidi ty 25% to 95% non-condensing
Atmospheric pressure 700mB to 1060mB
Do not obstruct any c ooling slots.
Position the instrument so that ai r flows freely.
Storage and transport
When the instr ument is in store or being tra nsported, the following ranges are tolerated:
Temperature -10°C to +50°C
Relative humidi ty 10% to 95% non-condensing
Atmospheric pressure 500mB to 1060mB
2.3 Use in the home environment
The equipment is intended to be operated in its bag where it is protected against ingress of solid
objects and water to a degree of I P 22.
Keep the equipment away from sources of heat.
Do not use mobile phones.
Do not allow pets or children to interfer e with the sensor cable s .
2.4 Power supply connections
Power requi rem ents
Standard PC USB port or 9V PP 3 ba tteries when operating independently .
No protective earth requir ed.
Power consumption
Maximum power from USB port: 2. 5W
Leakage curre nt
This instrument is designed to comply with IEC 601-1, the international standar d for medical electronic equipmen t, which specifies th e p ermissible levels of leakage current fr om individual products. A potential hazard exists in the summation of leakage currents caused by connecting several
pieces of equipment together. Because this instrumen t c a n be used in conjunction with standa r d
electronic devices, the total leakage current should be tes ted at regular intervals.
15

Trackit Mk3 User Manual
2.5 Use with other equipment
Defibrillators and HF surgical equipment
The equipment is not defibrillator proof and should not be u sed in situations where a defibrillator is
likely to be used.
The equipment should not be u s ed w ith high frequency surgica l equ ipm ent.
Other patient-connected equipment
When used simulta neously with other patient-connected equipment, for example a cardiac pa c emaker or other electrical stimulat or , it is unlikely that a safety hazard will a r ise. However always
consult the docu m entation supplied with the other patient-c on nected equipment to ensure that all
hazards, warnings an d c autions a r e c on s ider ed before the equipment is used to gether.
2.6 Interference
Trackit Mk3 will continue to operate in the presence of r a d io frequency magnetic fields (RF) and the
effects of electrostatic dis charges (ESD) and other in terference, in accordance with the requirements of EN60601-1-2. However, Trackit Mk3 records signals of very low amplitude, and these s ignals themselves a r e not immune to the effects of RF, ESD and low-frequency magnetic field interference. Such interference may cause signal artefacts.
Caution: when in c lose proximity to t he recorder, do not use mobile phones, tra n smitters, power transf ormers, motor s, or other equipment t hat generates magnetic fields.
Refer to the Appendix for more informa tion.
Note: Medical electrical equipment needs s pec ia l precautions regarding E M C and needs to be in-
stalled and put into service according to the EMC informa tion provided in the Ap pe ndix.
2.7 Maintenance and cleaning
The Trackit Mk3 contains no user-serviceable parts (apart from replaceable batteries). The system
uses solid-state components a nd requires no routine testing or mainten ance procedures apa rt from
occasional cleaning and checking for wear and damage to all parts including the accessories.
All the outer surfaces of the individua l p ieces of equipment of the Trackit sy stem may be cleaned
using a soft cloth moistened with wate r a nd a mild detergent. Each item may also be cleaned using
a low-pressure air-line or a vacuum cleaner.
Disinfection of the equipment can be carried out by the use of QAC-based disinfectants. Wipes are
recommended in order to prev ent the ingress of any liquid into the equipment. Suitable produc ts
include Mikrozid Sensitive Wipes (Schülke & Mayr GmbH), Microbac forte (Paul Hartmann AG), Distel Wipes (Tristel Ltd.).
Caution: Do not al low any liquid to e nt er the case of any inst r ument or connector.
Do not use acetone on any of the instru ments.
2.8 Disposal of equipment
The expected service life of the equipment is five years. When it has reached the end of its operating life, it should b e disposed of in accordance with local w aste regulation au thority that is typically
within the local gov e r nment office.
Caution: do not dis pose of batteries by incineration.
16
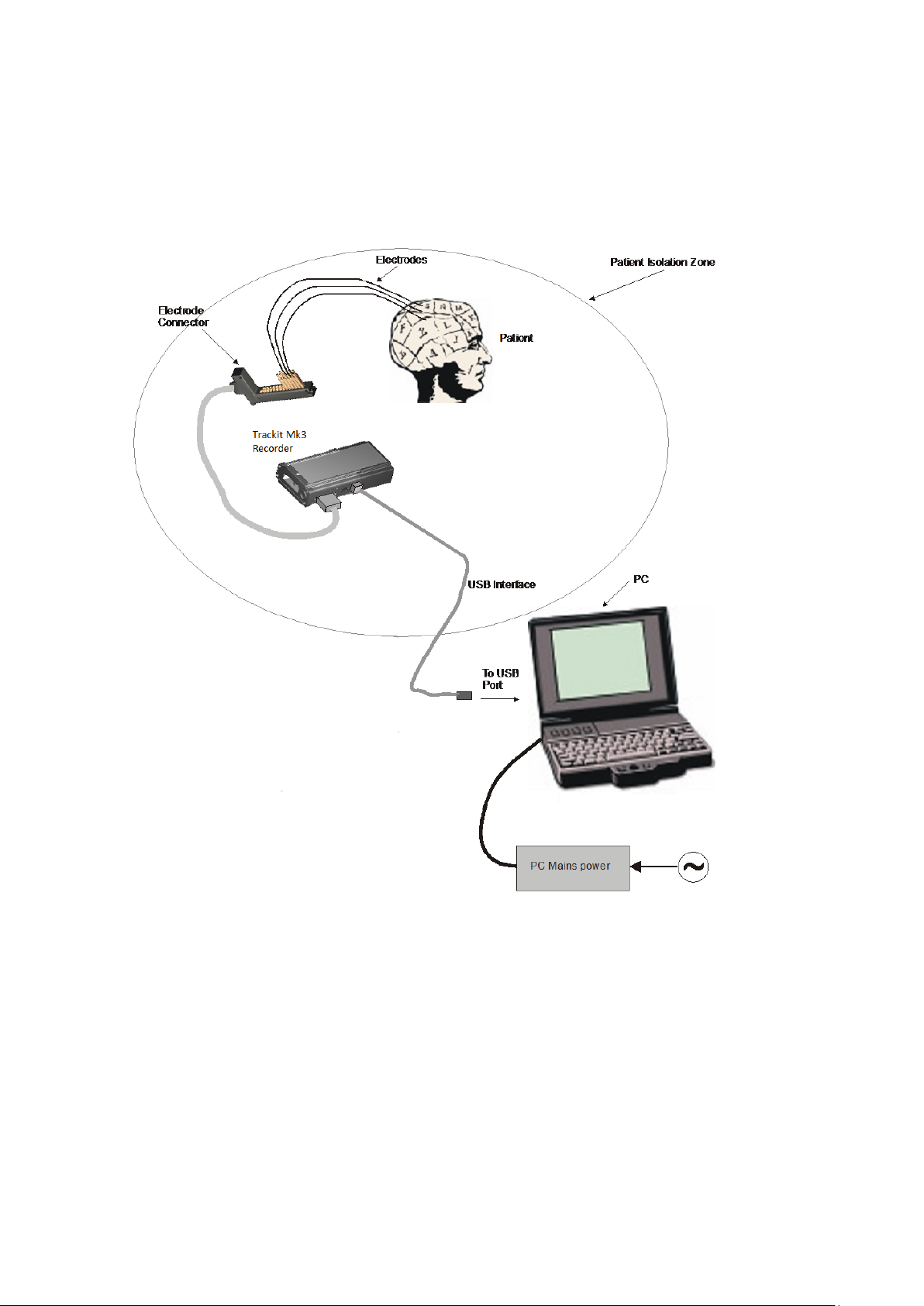
Trackit Mk3 User Manual
3 Connections for Tr ackit Mk3 setup
3.1 Overview
Below is an overview diagram showing the principal compon ents when conn ected to a PC during
system set up.
Figure 1 Connecting the Trackit Mk3 for re c order set-up
List of parts supplied by Lifelines:
Trackit Mk3 recorder plus bag
Patient Connec tion Unit (PCU), for e lectrode connection
1 x CF flash card (optional)
3 x PP3 batteries (optional)
PC USB cables
List of optional parts supplied by third parties:
Host PC/laptop with p ower cable
CF cards
Batteries
Battery charger
17
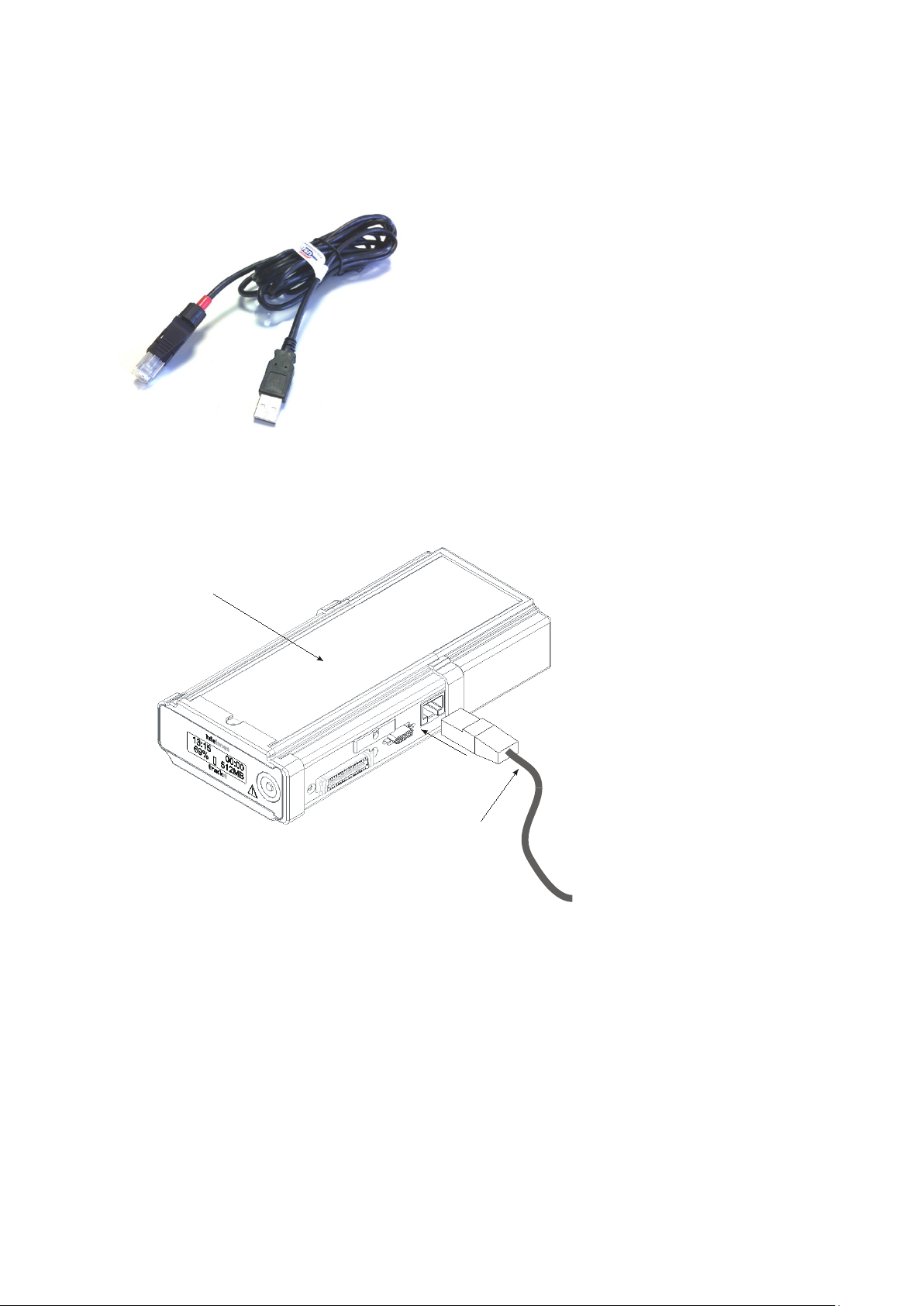
Trackit Mk3 User Manual
Trackit
RJ45 Connection
3.2 Connecting the Trackit Mk3
The Trackit Mk3 is simply plugged into the PC USB port usin g th e c a ble s upplied. Note: only u s e
the USB cable part number 1277 (with the red tip) for the Trackit Mk3 as shown below:
Figure 2 Connecting to the Trackit Mk3 recorder
The necessary U SB drivers will be found on the installation CD. Upon first connection of the Trackit
Mk3 to the PC USB por t, at the Windows promp t, browse to the folder CD Drive:\USB Drivers. From
there, Windows will find the correct dr ivers for the version of Windows being used.
3.3 Switching on
Turn on the unit by pressing th e button on the right of the LCD display on the front panel of the recorder.
18
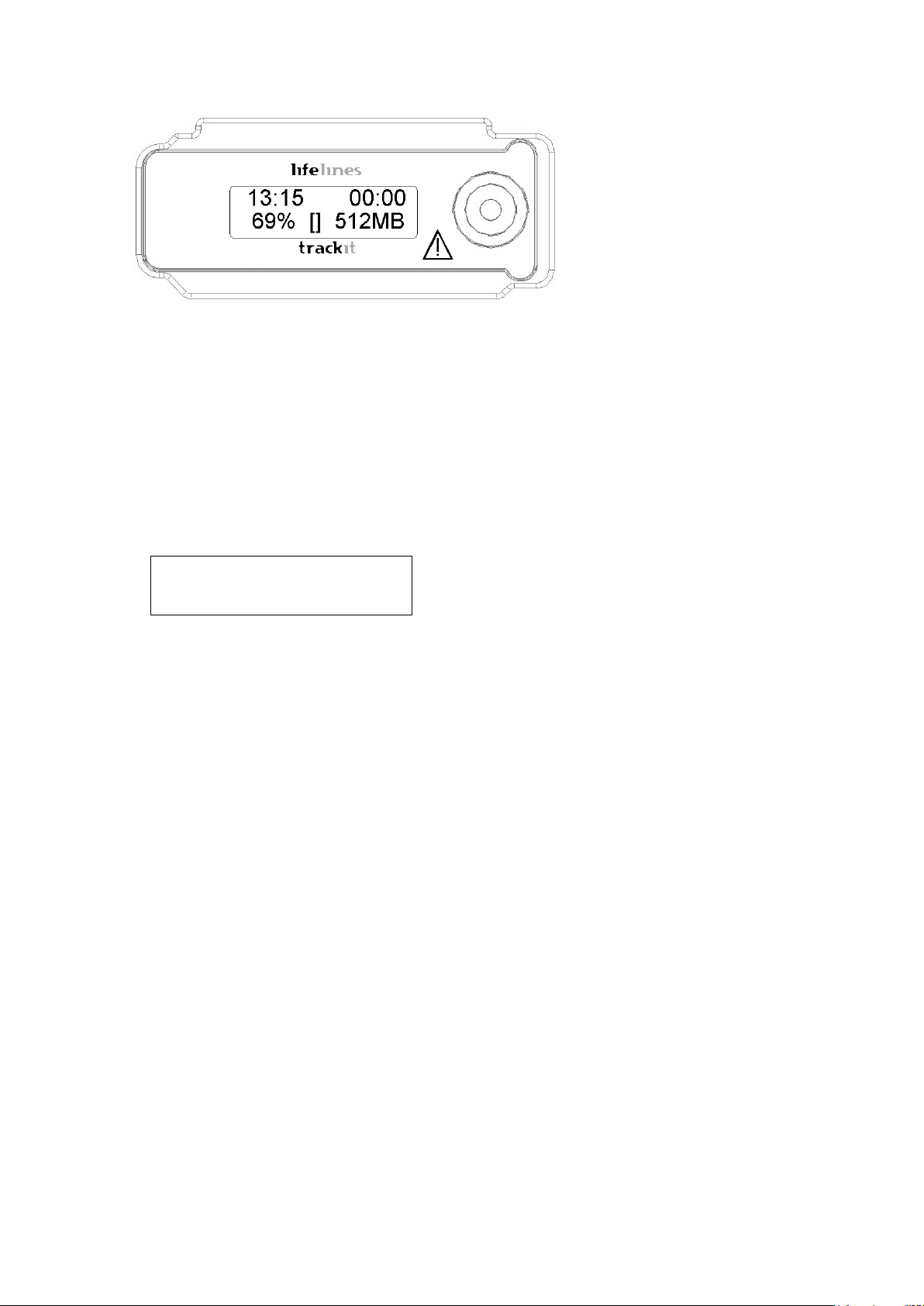
Trackit Mk3 User Manual
to the host during s etup.
Recording time of this recording
If not recording, ‘00:00’ is dis played.
sent, ‘0’ is displayed.
tery capacity).
Rear-door open warning symbol
Flashes when the rear battery door is open .
Internal beeper
Warning of low batte r y or rear-door open.
Special recording mode
Timed or sampled recordings a re indicated by the symbol
S.
Recording to card
The symbol R next to card capacity.
Refer to Appendix 5 for further inform ation on Bluetooth.
2 1 4 5 3 6
Figure 3 Trackit Mk3 recorder: front panel
After several seconds the LCD display indicates that Trackit Mk3 is performing a self-test for system integrity. On completion of th e s elf-test the LCD indicates the status of the Tr a ckit Mk3 recorder.
Indicators
The following ind ic a tors are available depending on Trackit Mk3 status:
Û~11:49 Û~00:00
Û~71 %âÛ Û~2049M
Figure 4 The Trackit Mk3 display
Key:
1 Time of day
2 Recording time
3 Battery capacity remaining
4 Internal Bluetooth on/off
5 Rear door open warning
6 CF Card capacity remaining
Time of day
Disk capacity Remaining storage capacity in MB. If there is no card pre-
Battery capacity Approximate battery capacity remaining (as % of full bat-
The battery-backed, real tim e clock which is syn chronized
3.4 Warning symbols on the display
Low card capacity Symbol L accompanied by beep in g every 30 seconds.
Internal Blu e tooth on/off When the Internal Bluetooth option is fitted to the Trackit
Mk3, the unit will display whether it is enabled or disabled
with a large ‘B’ (enabled) or small ‘β’ (disabled) next to
the ‘%’ indication. To toggle the current selection the
pushbutton is pressed 5 times w ithin 3 seconds. The display will change to indic a te the new state.
19

Trackit Mk3 User Manual
4 The setup software
The setup softw a r e is available on CD. A r eadme file describes installation. Check with your distributor or Lifelines if a newer version of software is available.
Trackit setup software is supported on Microsoft Window s 2000 ( w ith SP2), Windows XP, Windows
Vista and Windows 7.
When Trackit Mk3 is connected to the h ost PC, the software a llows the user to define p a rameters
for the recording, such as recording montage, start time and stop time, m ode of recording etc .
The setup softw a r e has the following func tions:
Define signal type s : create labels to atta c h to inputs
Attach the desired signal ty pe ( la bel) to the recording input. For ex a m ple , input 1 with a signal
type FP1
Create a recording montage and down load it to the recorder
Perform a calibra tion of the inputs
View signals online and adjust display parameters such as chart speed and display sensitivity
Start and stop a Trackit recording session
4.1 Setting up a recording protocol
Summary
Step 1 Define the patient ID
Step 2 Define the signals
Step 3 Define the inputs
Step 4 Define the recording channels
Step 5 Activate the r ec ording control
Step 6 Connect the Trackit Mk3 for setup
Step 7 Check Trackit Mk3 status
Step 8 Start a recording
Step 9 View the ongoing EEG tra c es
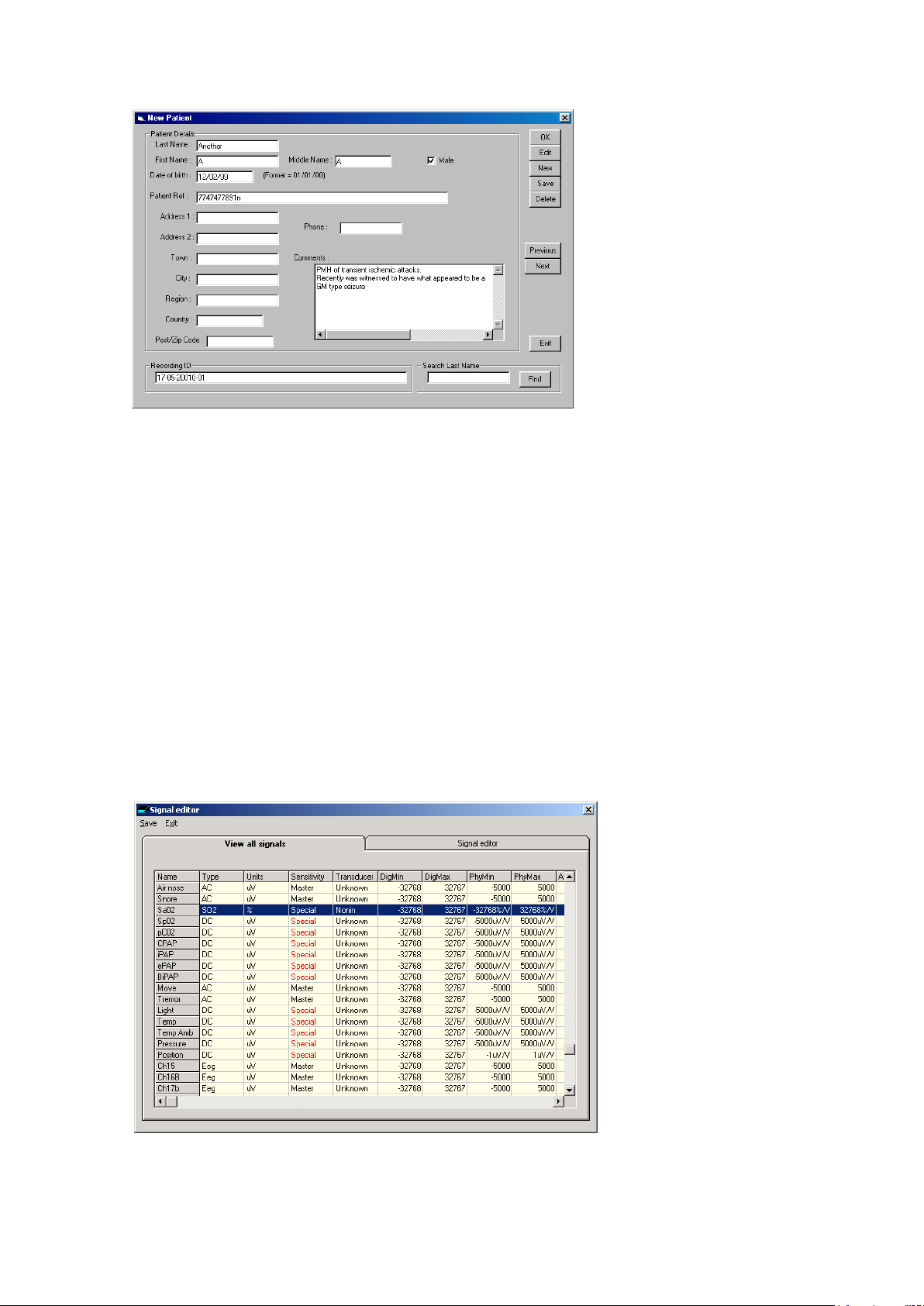
Step 1 Define the patient ID
1 Select the New patient icon on th e toolbar.
New Patient icon
2 Enter the patent name and Recording ID into the New Patient di-
alog.
This information is saved with the r ecording setup for d ownload
to the recorder in a future recording.
Figure 5 New Patient dialog
It is possible to con figure the system to use a patient database (Figure 6) instead of the simple dialog shown above .
20
Trackit Mk3 User Manual
Figure 6 New Patient database
The database allow s you to enter more exten s ive information about the patient and recording, and
save it for future refer ence. See the section entitled ‘Adv a nced options’.
Step 2 Define the signals
Defining signals is usually done once only – before using Track it Mk3 for th e first time. The Trackit
Mk3 recorder arrives w ith a default set of signals that should suffice for most applications in ambulatory EEG, hen ce it may only necessa r y to a dd s ig nal types for polygraphic recordings (airflow,
respiration etc).
If for any reason the signals have not been created, it is necessary to define all the signals (labels)
that are to be used f or m ontage creation in Step 3. The signal editor allows the creation of up to 64
distinct signals ranging from the standard 10/20 EEG signals such as FP1 O2, to Respiration, Pulse
and other polygraphy inpu ts .
Step 3 explains how to calib r ate an AUX input.
To define a signal:
1 Click the View all signals tab in the Signal editor dialog box . See Figure 7.
Figure 7 Signal list
21
Trackit Mk3 User Manual
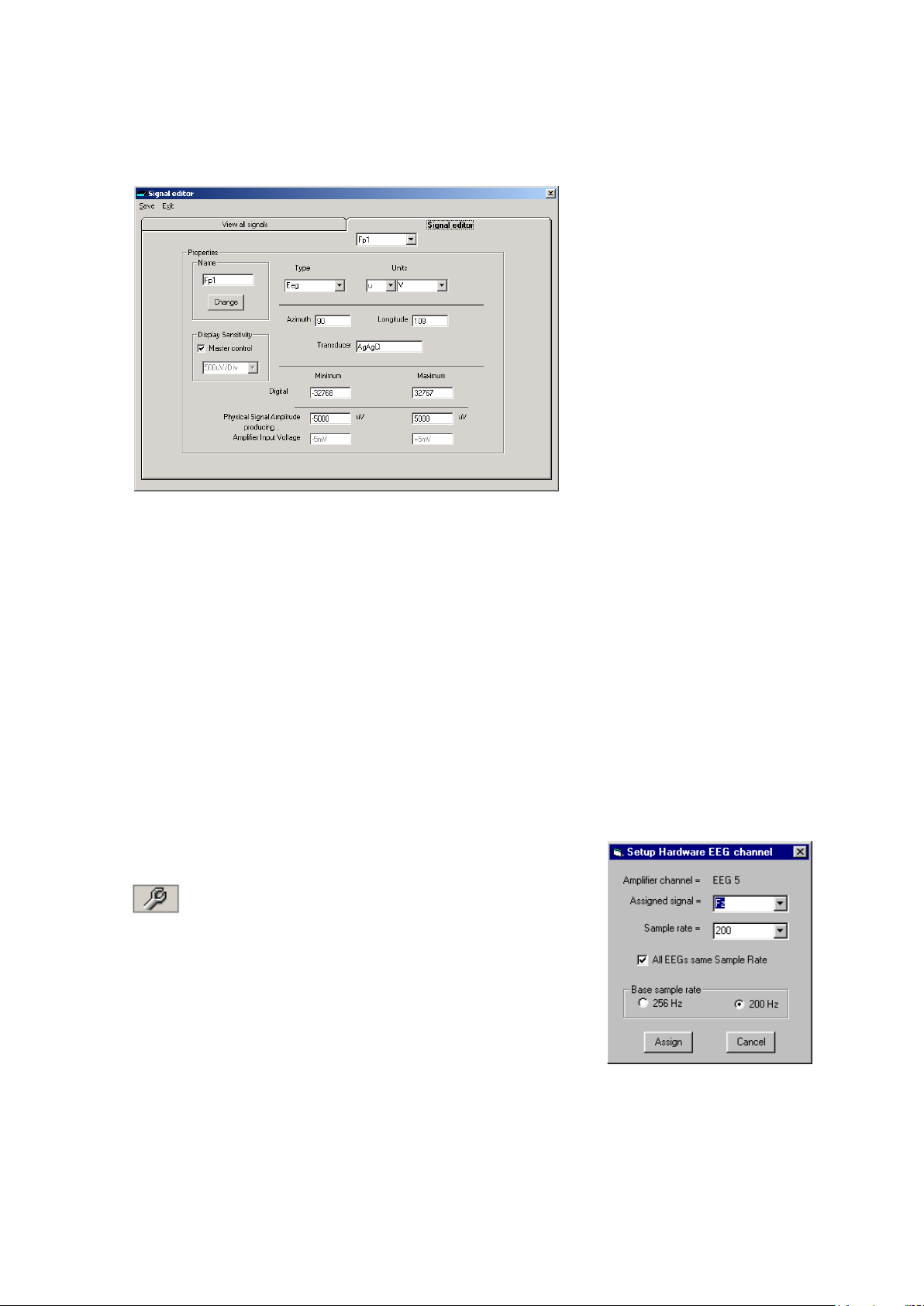
2 Double click on the signal you want to edit. This brings up the Signal editor tab), allowing you to
create a relevant signal or label to be en te red into the signa l lis t.
Figure 8 Signal e diting tool
3 Type in the Signal name (e.g. Fp1). N ote that for EEG signals this must be case-sensitive.
4 Select a signal type (in this case EEG).
5 Click on the Change button. The signal is now entered into the list under the View a ll signals
tab.
6 If the signal is not an EEG signal, it ma y be necessary to insert a display sensitivity value by un -
checking the M aster control check box.
Signals that ha ve been defined with their own independent sensitivities appear in red in the
trace display. F urther editing an d ch a nges to these sensitivity values in th e trace display will be
saved back into the signal library.
Caution: editing signals that are already used in an a c tive montage may r ender that
montage invali d. Y ou will need to re-enter the original m ontage in the Set-up rec ord-
ing tabbed dialog box – see steps 3, 4 an d 5.
Step 3 Define the inputs
1 Select the Spanner icon on the toolb a r . This opens the tabbed
Setup Recording dialog.
Spanner icon
2 Under the Amplifier Channels tab select the signals (la bels ) to be
attached to the phy sical inputs.
For example, EEG input 1 may require the label Fp1 and so on
according to the standard 10/ 20 nomenclature.
Double click the ch ann el name and s elect the r elevant sig nal label
from the Setup Hardwar e EE G cha nnel dialog.
Figure 9 EEG setup
22
Trackit Mk3 User Manual
Figure 10 Setup Re c ording dialog
The order of the sig nal labels in the pull dow n list is the same as the order of the signals in the signal list defined us ing the signal-editin g tool.
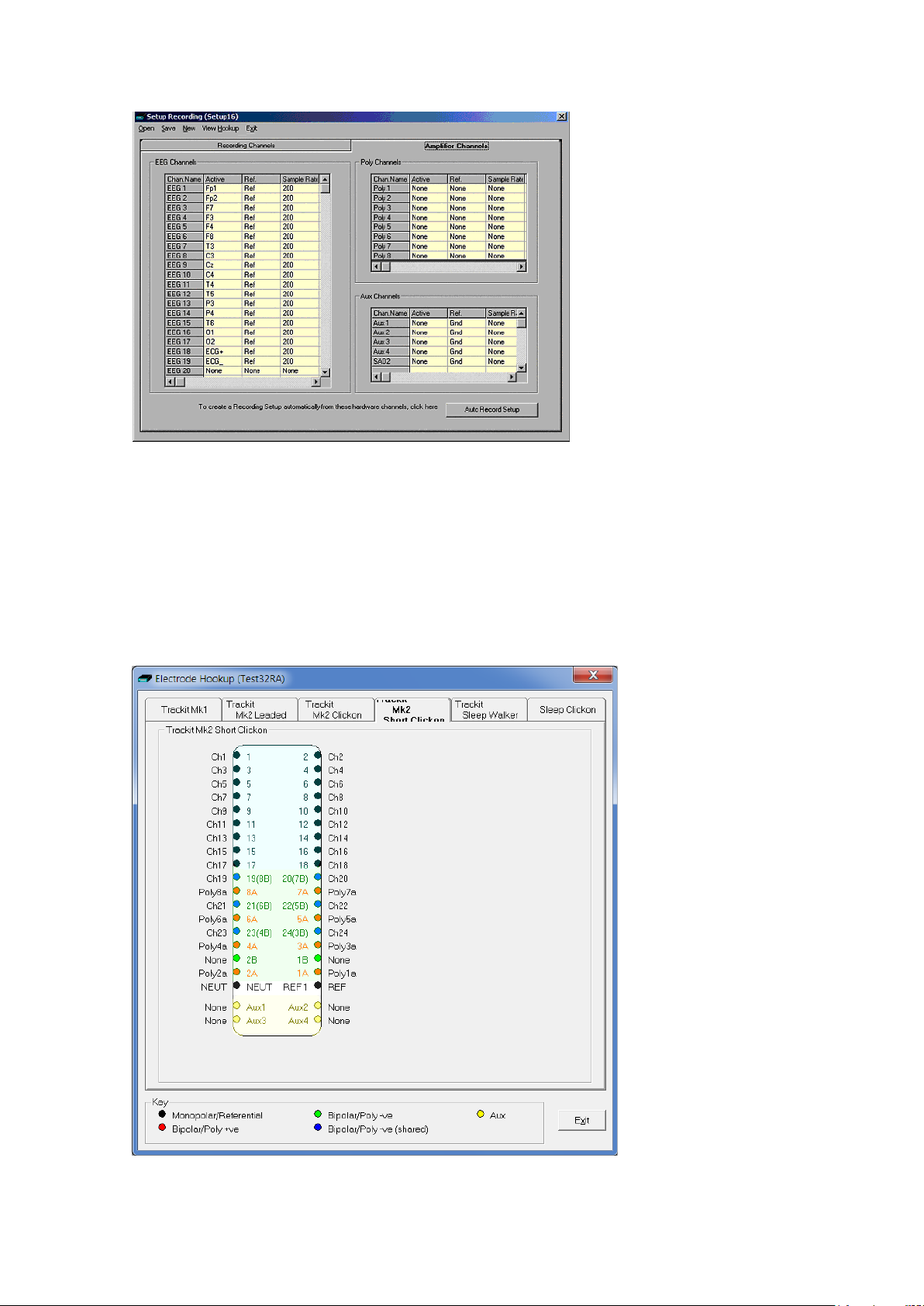
To see the user-defined la b els (signals) on the inputs, click on V iew Hook-up in the Menu Bar. See
Figure 11.
The View Hookup display shows all the currently av a ilable PCU options. C lick on the appropriate tab
to access the specific type.
Figure 11 View Hookup
23

Trackit Mk3 User Manual
Amplifier setup: amplifier setup ac tivates the recordin g inputs in preparation for a recording. For
most applications you n eed per form amplifier setup on ly once – when the sy stem is first installed –
since the amplifier setup is s a ved with the recording montage for f uture recall and usage. See Step
4 below.
If you want to use the recording ch a nnel order defined in amplifier setup, click on Auto Record
Setup in the Setu p R e c or d ing dialog box. You can then skip Step 4 (Define the recording channels).
Overall sampling rate: you can sele c t the overall sampling ra te for EEG channels from the Setup
Hardware EEG dialog (Figure 9). Once a sampling rate has been selec ted f or on e EEG input, you
can apply it to all EE G inputs by putting a checkmark against ‘All EEGs same Sample Rate’. If there
is no checkmark, you can select different sample rates on each EEG input – useful if you want to
apply an EOG signal to an EEG input.
Independent sample rates: if the independent sample rates feature is enabled in the Trackit
Mk3 firmware, it may be activated when required from a checkbox in th e Options dialog box
(choose View > O ther Options).
Notes:
1 Not all review software supports independent sample rates . Check with the vendor that your re-
view software does suppor t th em .
2 The Trackit Mk3 firmware must b revision 2.1.X or la te r .
3 It may be necessary to enter an unloc k code into Trackit Mk3 to enable this feature. If the fea-
ture is not enabled, a warning m es s a ge a ppea r s w hen a setup is sent to the recorder.
To enable multiple independent sample rates in the recorder:
1 Click on Trackit Control Panel in th e Trackit toolbar.
2 Click on Advanced Operations, then choose Configu r a tion from the Setting s Menu.
3 Copy the key code and email it to your Lifelines re pr esentative stating that you wish to have the
multiple independent sample r a te f ea ture enabled. You will then r ec eiv e ba c k an activation code
that you shou ld c op y and paste into the empty Trackit configur ation string field .
4 Press the Set Trackit button to enable the feature.
5 Choose Other Options from the Vie w Menu, and put a checkmark in the Allow Multi-sample rates
check box.
Poly and AUX inputs: poly inputs are low-le vel inputs, just lik e the EEG inputs, but dif fer in that
they can be set to either referential (EEG mode), bipolar AC or bipolar DC. They are ideal for p olygraphic signals such as respiration, airflow, EKG, body position (DC mode) etc. See the table in appendix 2 describing the inpu ts a nd their application.
The AUX inputs are high-level DC , and should not be us ed for low-level signals obtained from
transducers such as passive respiration sensors (piezo electric), airflow thermocouples etc. The
AUX inputs are designed to receiv e a high-level isolated DC signal (calibra ted output from a CPAP
machine, oxygen saturation meter etc).
To calibrate an AUX input to reflect a required unit of scale for a given voltage input use the signal
editor - see Figure 8). Select the appropriate u nits, e.g. %, or mm Hg, and enter the Physical Signal Amplitude required to generate the Amplifier Input Voltage.
Step 4 Define the recording channels
Step 4 can usually be skipped, s in c e the Auto Record Setup button w ill copy what you have defined
under the inputs in Step 3 into the list of recording chan nels.
However, you can defin e an d s a ve recording montages for specific recording needs, and recall them for f uture usage. You can
define up to 40 recording cha nnels in a montage.
Creating a montag e follows the same principle as the signal
creation and input definition tool: c lick on the channel number
to define the active and reference label of choice. See Figure
12.
Figure 12 Channel setup
24
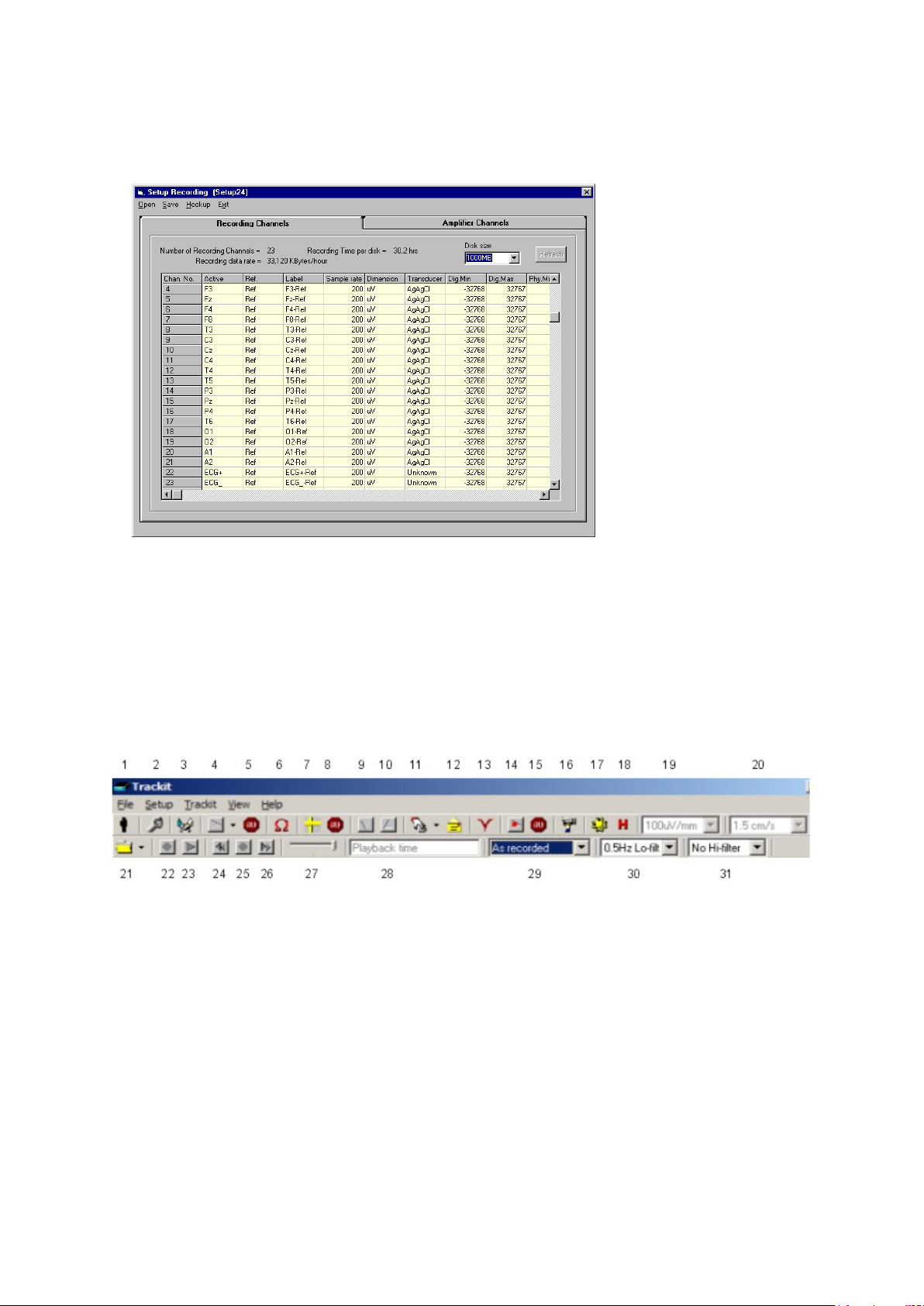
Key:
1 New Patient
2 Setup Recording
3 Trackit Control Panel
4 Ongoings On
5 Ongoings Off
6 Impedance Check On
7 Calibration On
8 Calibration Off
9 Page Down
10 Page Up
11 Get Trackit Events
12 Email Events L is t
13 Notch Filter On/Off
14 PC Record On
15 PC Record Off
16 Videometry (optional)
17 Photic Stimulation
18 Hyperventilation
19 Vertical sensi tivity
20 Chart speed
21 Open files for playback
22 Stop playback
23 Start playback
24 Page back
25 Stop paging
26 Page forward
27 Paging speed
28 Playback time
29 Montage selection
30 Lo-filter select ion
31 Hi-filter selection
An example of a r e c or d ing montage is shown in Figure 13 below.
Trackit Mk3 User Manual
Figure 13 Recordi ng Channel edit ing
4.2 Configuring the recorder
When you have f inished setting up the rec or d ing protocol, connect the Trackit Mk3 recorder to the
host computer. Steps 5 to 9 describe c on figuration and set-up of an ambulatory EEG.
Step 5 Activate the recording control
From the Track it toolbar (Figure 14) select the Trackit Contr ol Panel (‘handshake’) icon.
Figure 14 Trackit software toolbar
25
Trackit Mk3 User Manual
not.
Ext Supply
Shows whether the Trackit Mk3 is being powered by the medical grade DC
power supply.
Battery
Shows if a battery is pr e s ent or not.
CF card
Shows whether a CF card is present and its capacity.
corder.
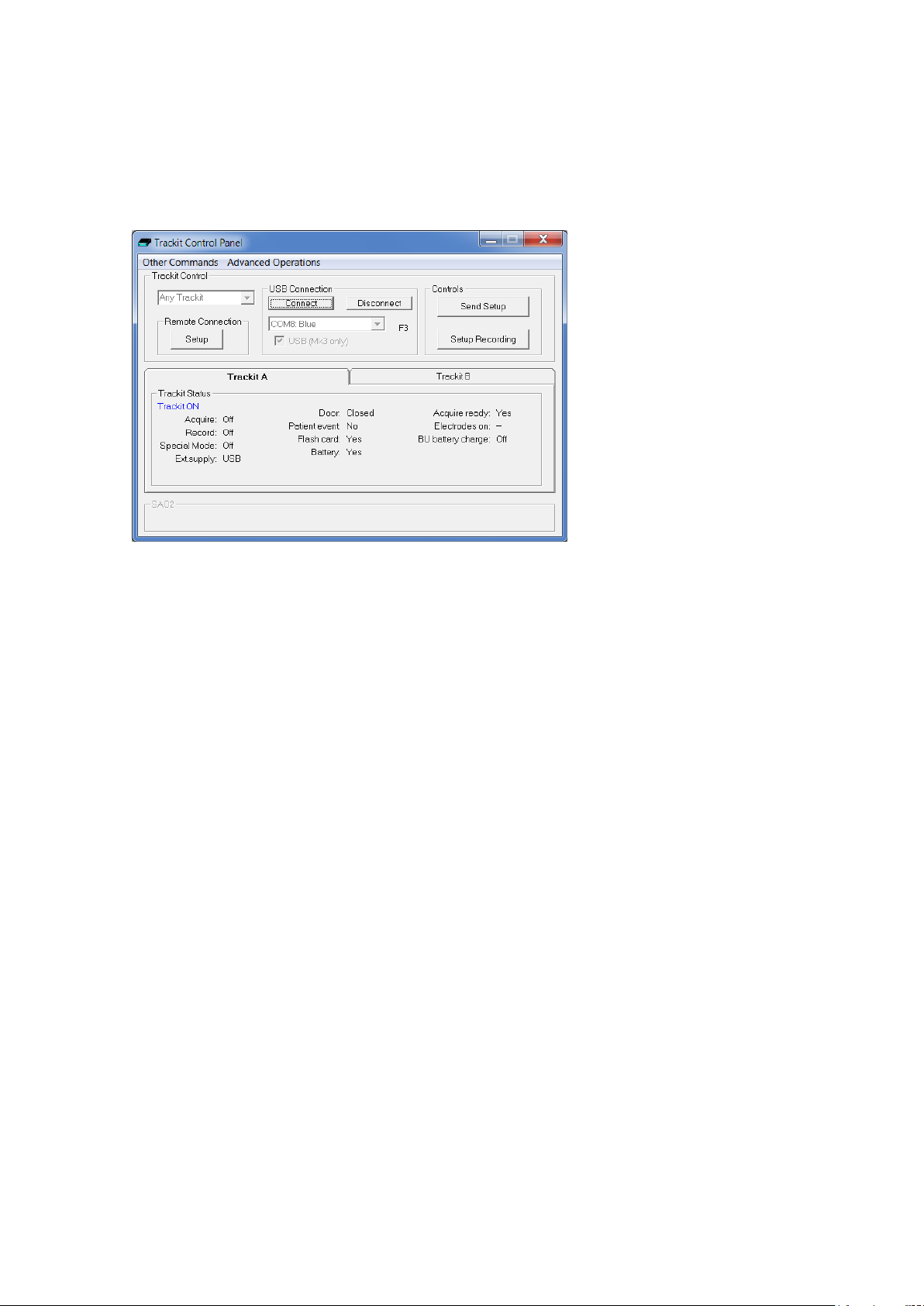
Clicking on the ‘ handshake’ icon brings up the Trackit Control Panel dialog box (Figure 15).
Step 6 Connect Trackit Mk3 for setup
Check that the Trackit Mk3 recorder is switched on and that the cables are all con nected properly.
In the Trackit C ontrol Panel either select the USB option for the Mk 3 Tr a c kit or check that the cor rect COM port is selected an d click on the Connect button.
Figure 15 Trackit Control Panel
After a couple of s e c onds Trackit Statu s shows ‘Trackit Online’ and the software displays the recording paramete r s loaded into Trackit Mk3 in the status bar at the bottom of the screen.
Trackit Status a lso shows whether th e b a tte r y and PC card are present, whether the door is open
or closed, whether the electrode conn ec tor is attached, a nd the recording status of the device.
The ‘F’ or ‘F1’ next to the port selection box indicates that the fast connection speed, which is available with the Trac kit Mk2, is being used. This will be automatica lly enabled as long as the PC serial
port hardware is capable of operating at 230 kBaud. ‘F2’ a nd ‘F3’ are displayed to indicate the substantially fa s te r c onnection speeds av a ila b le for the Mk3 USB interface.
Step 7 Check Trackit status
Use the Trackit C ontrol Panel to check that the Trackit Mk3 recorder is correctly online. The Trackit
Status part of th e Control Panel gives you the following information:
Acquire – on or off
Record – on or off Shows whether or not the Trackit Mk3 is recording data to flash card.
Shows whether an online view of the data on the host PC is takin g plac e or
Status Shows how much ba tte r y life is left.
Patient event Shows that the external patient event marker is connected.
Door Shows whether the door to the CF card and battery is open or closed.
Electrodes connected Shows that the P a tie nt Connection Unit is connected to the recorder (Mk1).
Acquire ready
26
Shows that a valid recording setup has been loaded to the Trackit Mk3 re-
Trackit Mk3 User Manual
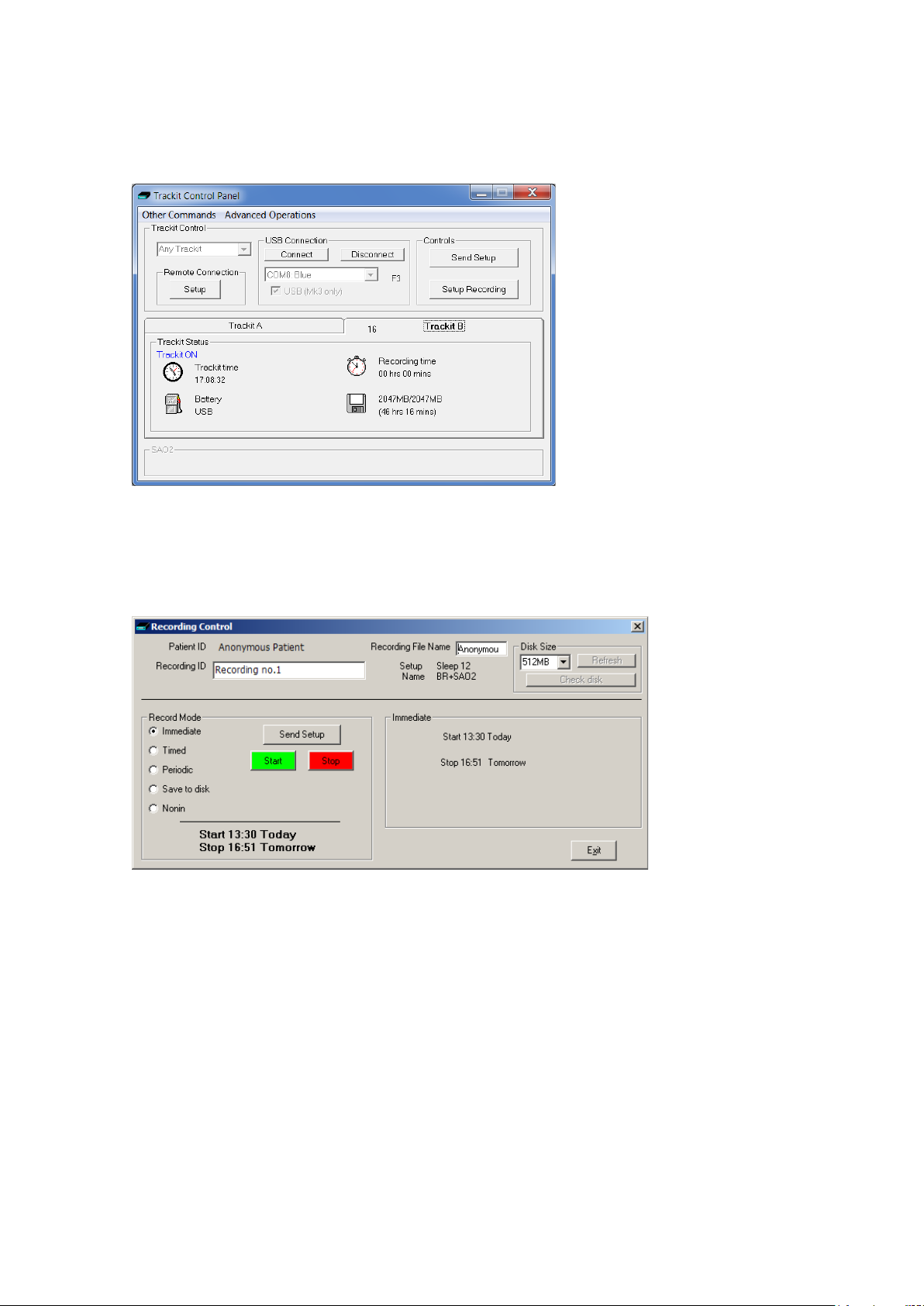
Note that further status information is available on th e 2nd tab shown below. This includes Trackit
Mk3 time, Battery capacity, Recording time a nd CF card MB total an d MB remaining.
Figure 16 Trackit status ' B'
Step 8 Start a recording
Click on the Setup Recording button in the Trackit Control Panel to open the Recording Control dialog box (Figure 17). The patient’s name and the file name for the recording are displayed.
Figure 17 Recordi ng Control
You can accept the default filename, or you can insert a filename (up to 8 characters long) of your
own choosing.
Default file names
To make the recording file name the same as the patient name:
1 Choose Other Options from the Vie w Menu.
2 Put a checkmark by Default to Patient Name.
3 Click on Exit.
Ways of starting a recording
You now have four ways to start a recording:
Immediate
Timed
27
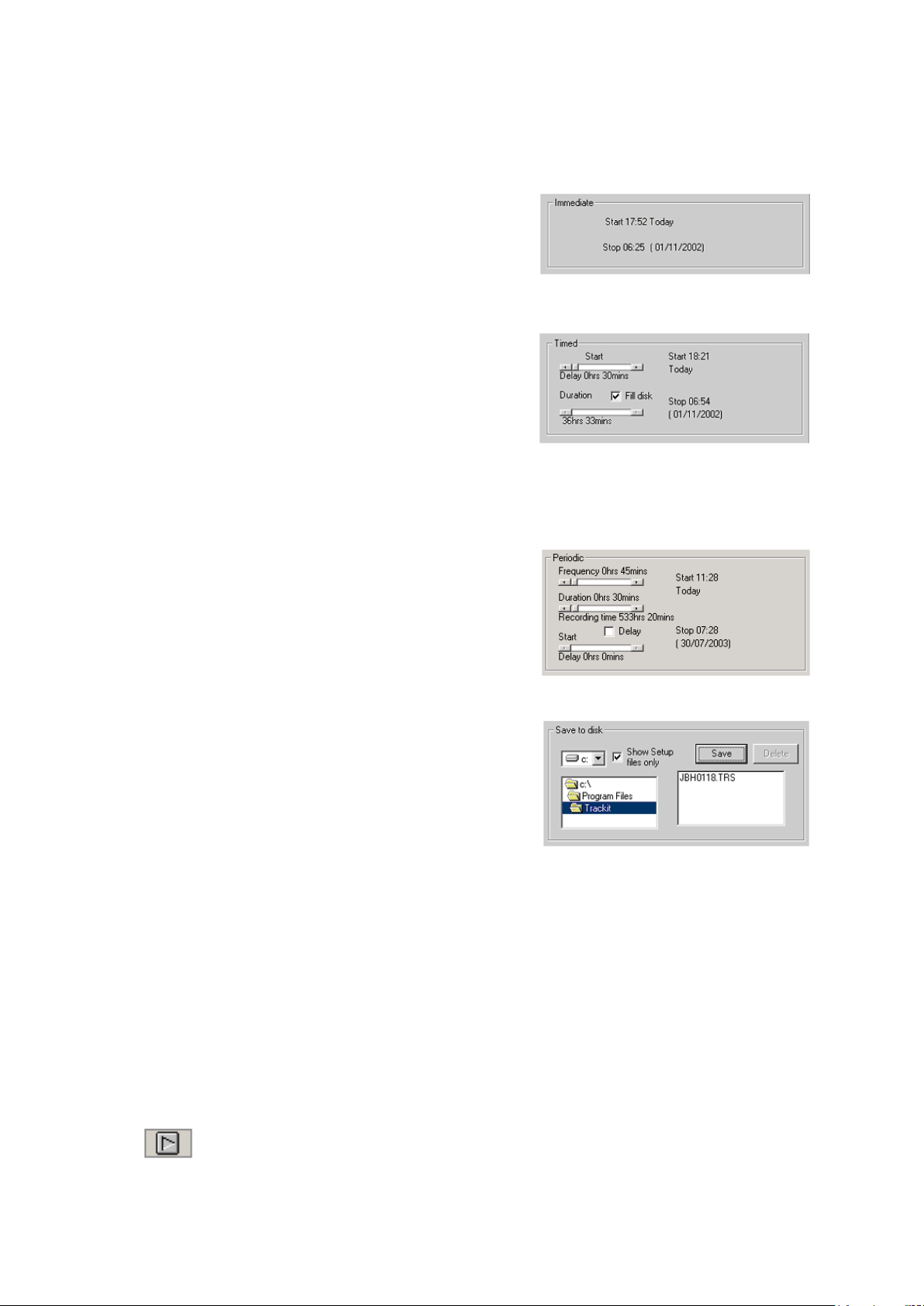
Trackit Mk3 User Manual
Periodic
Save to Disk
Nonin
Immediate: the recording starts as soon as you click
Send Setup, then Start (see Figure 17). Recording finishes
when the Trackit Mk3 is turned off.
1 Under Record Mode, choose Immediate.
2 Click the Send Setup button, then the Start button.
Timed: Trackit Mk3 records for a specified period of time.
1 Under Record Mode, choose Timed.
2 In the Recording Control dialog box, s e t a start time
for the recording using the Start slider.
3 Either put a checkmark by ‘Fill Disk’, or use the Dura-
tion slider to tell Trackit Mk3 how long to record for.
4 Click the Send Setup button.
Periodic: Trackit Mk3 records for specified periods of time at a defined interval (eg for pe r iod s of
30 minutes, with a 45-minute interval):
1 Under Record Mode, choose Periodic.
2 Use the frequency and duration sliders to define the
length of the recording period, a nd the interval be-
tween periods.
3 For a delayed start (eg in an MSLT study), put a
checkmark by the Delay box, and use the Start slider
to set a start time for the recording.
4 Click the Send Setup button, then the Start button.
Save to disk: a setup is created, then saved on the flash
card for use in a later recording. This is particularly useful
for home sleep recordings where the patient can switch on
the recorder (and so start the recording) just before going
to bed.
1 Create a setup in the normal way, with patient name an d recording chann els.
2 Place the flash card in the flash card reader in the host PC.
3 Under Record Mode, choose Save to disk. The setup is sav ed on t h e flash card as a *.trs (trackit
recording setup ) file. The name of the s e tup file is the patient’s last name.
4 The Trackit Mk3 recorder starts recording automatically, us ing this setup file, when it is
switched on.
Nonin: Recording starts as soon as the Nonin SaO2 probe is connected an d a ttached.
To initiate an imm e diate online recording
1 Click on the Send Setup button. Wa it for the setup to upload t o the Trackit Mk3 recorder. This
should take a few seconds.
2
In the Trackit toolba r , click the Ongoings O n icon to view the traces.
3 Make sure what is seen is what should be recorded.
28
 Loading...
Loading...