Page 1

ND-1000 Spectrophotometer
V3.3 User’s Manual
Page 2

NanoDrop Technologies, Inc.
3411 Silverside Road
Bancroft Building
Wilmington, DE 19810 USA
Voice: 302-479-7707
Fax: 302-792-7155
Email: info@nanodrop.com
www.nanodrop.com
NanoDrop is a registered trademark of NanoDrop Technologies, Inc. Other parties’
trademarks are the property of their respective owners and should be treated as such.
Copyright © 2007 NanoDrop Technologies, Inc.
rev 3/2007
Page 3

Table of Contents
1. Overview .................................................................................................1-1
Instrument Description.............................................................................. 1-1
Operation.................................................................................................. 1-1
Applications..............................................................................................1-1
Patents .....................................................................................................1-1
2. Initial Set Up............................................................................................ 2-1
Computer Requirements .......................................................................... 2-1
Software Installation.................................................................................2-1
Software Upgrades...................................................................................2-1
Registering Your Instrument..................................................................... 2-2
3. General Operation..................................................................................3-1
The Sample Retention System................................................................. 3-1
Cleaning the Sample Retention System...................................................3-1
Software Architecture and Features .........................................................3-2
User Preferences......................................................................................3-3
Utilities and Diagnostics ...........................................................................3-3
Account Management............................................................................... 3-3
Dye/Chromophore Editor..........................................................................3-5
4. Common Module Functions..................................................................4-6
Module Startup.........................................................................................4-6
Escape Key (ESC).................................................................................... 4-6
Measure (F1)............................................................................................ 4-6
Blank (F3).................................................................................................4-6
Blanking Cycle..........................................................................................4-6
Re-blank (F2)............................................................................................4-7
Print Screen (F4)......................................................................................4-7
Saving Current Screen as .JPG Image..................................................... 4-7
Start Report / Recording (F6) ...................................................................4-7
Print Report (F5)....................................................................................... 4-7
Show Report (F7).....................................................................................4-8
Sample ID................................................................................................. 4-8
Sample # ..................................................................................................4-8
Exit ........................................................................................................... 4-8
Show Context Help...................................................................................4-8
User’s Manual...........................................................................................4-8
5. Nucleic Acids..........................................................................................5-1
Sample Volume Requirements................................................................. 5-1
Measurement Concentration Range.........................................................5-1
Spectrum Normalization ...........................................................................5-2
Spectrum Overlay Control ........................................................................5-2
6. MicroArray...............................................................................................6-1
Fluorescent Dye Selection........................................................................ 6-1
Sample Volume Requirements................................................................. 6-1
Measurement Concentration Range.........................................................6-1
Baseline Calculation & Normalization.......................................................6-2
7. UV-VIS ..................................................................................................... 7-1
Sample Volume Requirements................................................................. 7-1
Measurement Concentration Range.........................................................7-1
Unique Screen Features........................................................................... 7-1
8. Protein A280............................................................................................8-1
Sample Volume Requirements................................................................. 8-1
Special Cleaning Requirements for Proteins ............................................8-1
Measurement Concentration Range.........................................................8-1
Unique Screen Features........................................................................... 8-1
Spectrum Normalization ...........................................................................8-2
Spectrum Overlay Control ........................................................................8-2
9. Proteins & Labels................................................................................... 9-1
Fluorescent Dye Selection........................................................................ 9-1
Sample Volume Requirements................................................................. 9-1
Special Cleaning Requirements for Proteins ............................................9-1
Page 4

Measurement Concentration Range.........................................................9-1
Unique Screen Features........................................................................... 9-1
Baseline Type...........................................................................................9-2
10. Protein BCA .......................................................................................... 10-1
Sample Volume Requirements............................................................... 10-1
Special Cleaning Requirements for Proteins ..........................................10-1
Measurement Concentration Range.......................................................10-1
BCA Kits, Protocols, and Sample Preparation........................................ 10-1
Unique Screen Features......................................................................... 10-1
Making BCA Measurements................................................................... 10-2
Standard Curve Features ....................................................................... 10-2
Exiting the BCA Module.......................................................................... 10-3
11. Protein Lowry........................................................................................11-1
Sample Volume Requirements............................................................... 11-1
Special Cleaning Requirements for Proteins ..........................................11-1
Measurement Concentration Range.......................................................11-1
Modified Lowry Kits, Protocols, and Sample Preparation.......................11-1
Unique Screen Features......................................................................... 11-1
Making Lowry Measurements.................................................................11-2
Standard Curve Features ....................................................................... 11-2
Exiting the Lowry Module .......................................................................11-3
12. Protein Bradford................................................................................... 12-1
Sample Volume Requirement.................................................................12-1
Special Cleaning Requirements for Proteins ..........................................12-1
Measurement Concentration Range.......................................................12-1
Bradford Kits, Protocols, and Sample Preparation ................................. 12-1
Unique Screen Features......................................................................... 12-1
Making Bradford Protein Measurements ................................................ 12-2
Standard Curve Features ....................................................................... 12-3
Exiting the Bradford Module ................................................................... 12-3
13. Cell Cultures ......................................................................................... 13-1
Sample Size Requirements....................................................................13-1
Cell Suspension Concentrations............................................................. 13-1
Sample Homogeneity .............................................................................13-1
Decontamination of Measurement Pedestals.........................................13-2
14. Archived Data and Data Viewer...........................................................14-1
Archive File Creation ..............................................................................14-1
Data Storage Hierarchy.......................................................................... 14-1
Data Viewer............................................................................................14-2
Archive File Converter............................................................................ 14-5
Opening Archived Data with Spreadsheet Programs .............................14-6
15. Troubleshooting................................................................................... 15-1
Error USB2000.......................................................................................15-1
Connection Error .................................................................................... 15-2
Signal Error.............................................................................................15-2
Saturated Detector ................................................................................. 15-3
Liquid Column Breakage ........................................................................ 15-3
Other Software Error Messages.............................................................15-4
Sample Accuracy and Reproducibility .................................................... 15-5
260/280 Ratio.........................................................................................15-6
Unusual Spectrum.................................................................................. 15-6
Technical Service...................................................................................15-7
16. Maintenance and Warranty..................................................................16-1
Cleaning .................................................................................................16-1
Calibration .............................................................................................. 16-1
Warranty.................................................................................................16-1
17. Appendices........................................................................................... 17-1
Instrument Specifications .......................................................................17-1
Blanking and Absorbance Calculations .................................................. 17-1
Concentration Calculation (Beer’s Law) .................................................17-1
Solvent Compatibility.............................................................................. 17-2
Decontamination of Measurement & Optical Surfaces ...........................17-2
Setting Up a Dymo 400 Label Writer Printer ........................................... 17-2
Page 5

Section 1- Overview
1. Overview
Instrument Description
The NanoDrop
reproducibility. It utilizes a patented sample retention technology that employs surface tension alone to hold the sample in place. This
eliminates the need for cumbersome cuvettes and other sample containment devices and allows for clean up in seconds. In addition,
the ND-1000 has the capability to measure highly conce ntrated samples without dilution (50X higher concentration than the samples
measured by a standard cuvette spectrophotometer).
Operation
A 1 ul sample is pipetted onto the end of a fiber optic cable (the receiving fiber). A second fiber optic cable (the source fiber) is then
brought into contact with the liquid sample causing the liquid to bridge the gap between the fiber optic ends. The gap is controlled to
both 1mm and 0.2 mm paths. A pulsed xenon flash lamp provides the light source and a spectrometer utilizing a linear CCD array is
used to analyze the light after passing through the sample. The instrument is controlled by special software run from a PC, and the
data is logged in an archive file on the PC.
Applications
UV/VIS spectrophotometry is simple for samples as small as 1 ul using the NanoDrop
requirement and ease of use make the NanoDrop
• Nucleic acid concentration and purity of nucleic acid samples up to 3700 ng/ul (dsDNA) wit ho ut dilution
• Fluorescent dye labeling density of nucleic acid microarray samples
• Purified protein analysis (A280) up to 100 mg/ml (BSA)
• Expanded spectrum measurement and quantitation of fluorescent dye labeled proteins, conjugates, and metalloproteins
• Bradford Assay analysis of protein
• BCA Assay analysis of protein
• Lowry Assay analysis of protein
• Cell density measurements
• General UV-Vis spectrophotometry
Patents
The sample retention technology used in the ND-1000 is covered under US patents 6,628,382 and 6,809,826. Other patents are
pending.
®
ND-1000 is a full-spectrum (220-750nm) spectrophotometer that measures 1 ul samples with high accuracy and
®
®
ND-1000 Spectrophotometer ideally suited for measuring:
ND-1000 Spectrophotometer. The small sample
1-1
Page 6
Section 2- Initial Set Up
2. Initial Set Up
Computer Requirements
The NanoDrop software will only run on an IBM compatible PC meeting the below criteria. No Mac versions of the software are
currently available.
• Microsoft Windows XP or 2000 operating system.
Windows Vista has also been tested successfully with NanoDrop software.
The operating software is not compatible with Windows NT, 95, 98 or ME.
• 233 MHz or higher processor
• CD ROM drive
• 32 MB or more of RAM
• 40 MB of free hard disk space
• Open USB port (the instrument can only be connected via the USB port)
• Microsoft Excel or other spreadsheet program to manipulate archived data (optional)
Software Installation
WARNING: The system software must be loaded onto the PC before the USB cable is connected. Administrator access on
the PC is required to install the software.
To properly install NanoDrop software:
1.
Close all programs and make sure that the USB cable is unplugged.
2. Insert the operating software CD in the CD drive of the PC. The software installation menu should appear automatically. If
software menu does not appear, choose ‘My Computer‘ to view the contents of the CD. Double click on the file named ‘nd1000…install.exe’.
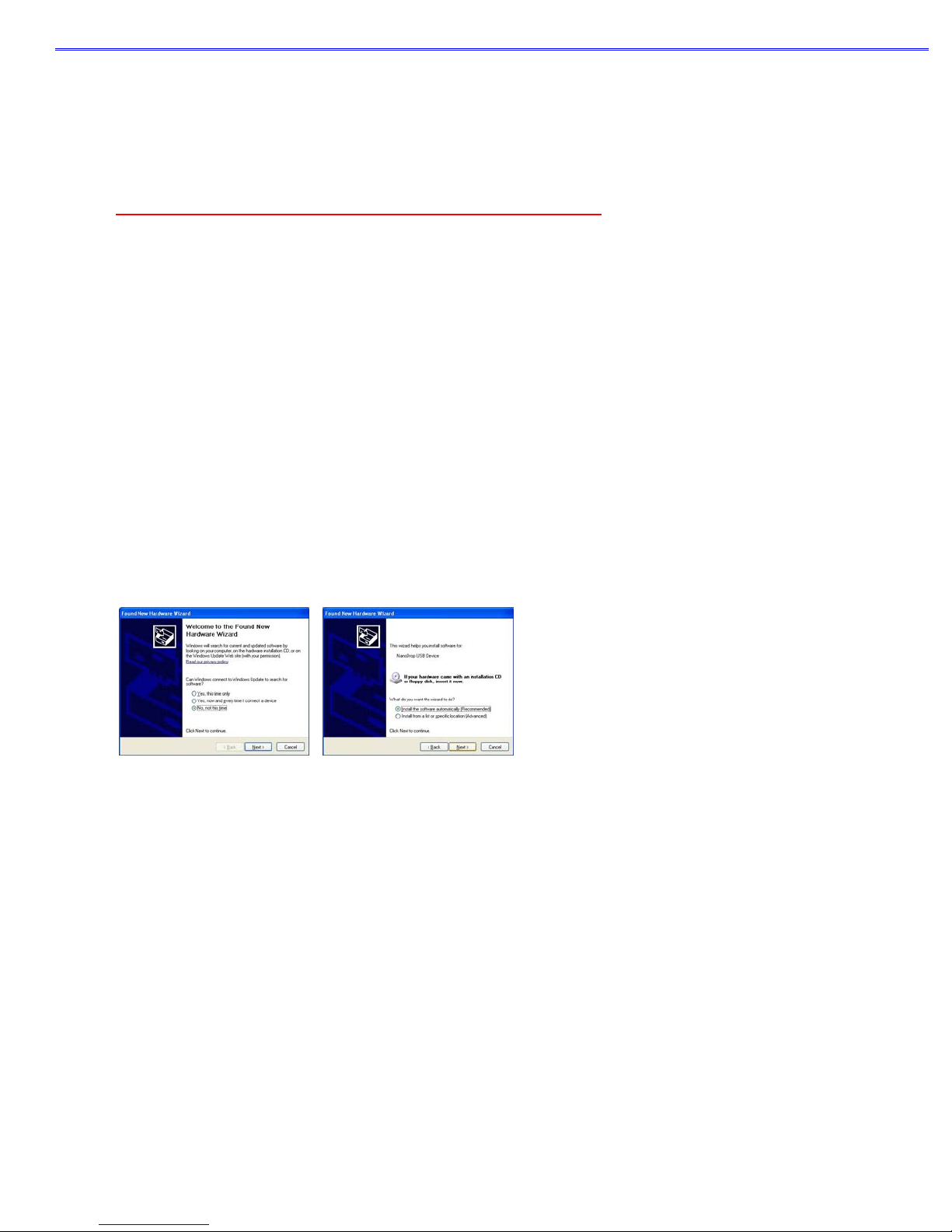
3. After software installation, connect the USB cable and the Found New Hardware Wizard should start as shown below.
(Windows XP SP2 operating system will ask to allow it to search the internet for the proper software as shown- Select ‘No, not
this time’). Follow the prompts for automatic installation of the software.
Intro Page: Windows XP- SP2
Your NanoDrop
All Windows Operating Systems
®
ND-1000 Spectrophotometer should now be ready for operation. If the software does not start properl y, refer to the
“Troubleshooting” section for possible solutions.
Configuring the System Font
The NanoDrop software is designed to look best with the MS Sans Serif font, 8 point. To check that the system font is set to the proper
selection:
1. Open the ‘Displays Properties’ b y right clicking on the desktop and select Properties
Æ
Appearance. (Additional step for
Windows XP: click on the ‘Advanced’ button).
2. From ‘item’ list select ‘icon’.
3. Select the ‘MS Sans Serif (western)’ font and select ‘8 point’ size.
4. Click OK.
Other selections can be used, but may either cause some text in the NanoDrop software window to not fit well or result in the function
selection tabs across the top to become inaccessible.
Software Upgrades
NanoDrop Technologies makes periodic upgrades to the NanoDrop software. These upgrades are available for download at
www.nanodrop.com.
2-1
Page 7

Section 2- Initial Set Up
Cable Connections
To make measurements with the instrument, connect the USB cable to instrument and the PC, plug in the 12V power supply and
connect to the power input at the back of the instrument.
Note: The power supply can remain plugged into the NanoDrop
®
ND-1000 Spectrophotometer while the instrument is not in use. When
the unit is in this “standby” mode, power consumption is ~1.5 W and the flashlamp is not energized. Also, the instrument does not
utilize a power switch or give a visual indication of the operability of the 12V power supply.
Registering Your Instrument
Please register your product! We periodically update our software and add new features free of charge. We would like to keep our user
list updated so that we may alert you to these updates. All information supplied to NanoDrop Technologies is completely confidential.
You can register at www.nanodrop.com.
2-2
Page 8

3. General Operation
The Sample Retention System
Basic Use
The main steps for using the sample retention system are listed below:
Section 4-Common Module Functions
1. With the sampling arm open, pipette the sample onto the lower
measurement pedestal.
2. Close the sampling arm and
initiate a spectral measurement
using the operating software on the
PC. The sample column is
automatically drawn between the
upper and lower measurement
pedestals and the spectral
measurement made.
3. When the measurement is
complete, open the sampling arm and
wipe the sample from both the upper
and lower pedestals using a soft
laboratory wipe. Simple wiping
prevents sample carryover in
successive measurements for
samples varying by more than 1000
fold in concentration. See
www.nanodrop.com for performance
data on sample carryover.
Cleaning the Sample Retention System
Wiping the sample from both the upper and lower pedestals (as shown above) upon completion of each sample measur ement is usually
sufficient to prevent sample carryover and avoid residue buildup. Although generally not necessary, 2 ul water aliquots can be used to
clean the measurement surfaces after particularly high concentration samples to ensure n o residual sample is retained on either
pedestal. After measuring a large number of samples, however, it is recommended that the areas around the upper and lower pedestals
be cleaned thoroughly. This will prevent the wiping after each measurement from carrying previous samples onto the measurement
pedestals and affecting low-level measurements. A final cleaning of all surfaces with de-ionized water is also recommended after the
user’s last measurement.
Decontamination of Measurement Pedestals
If decontamination is necessary, a sanitizing solution, such as a 5.25% solution of sodium hypochlorite (bleach – freshl y prepared), can
be used to ensure that no biologically active material is present on the measurement pede s tals. T he metal fiber optic fittings are made
from 303 stainless steel and are resistant to most common laboratory solvents (see “Solvent Compatibility” appendix).
Special Cleaning Requirements for Proteins
Proteins and solutions containing surfactants can “un-condition” the measurement pedestal surfaces so that the liquid column does not
form well with 1ul samples. If this occurs, “buff” the measurement pedestal surfaces by rubbing each with a dry laboratory wipe 30-40
times. This will “re-condition” the surface allowing the liquid sample column to form.
3-1
Page 9
Section 4-Common Module Functions
Sample Size Requirements
Although sample size is not critical, it is essential that the liquid column be formed so that the gap between the upper and lower
measurement pedestals is bridged with sample.
Field experience indicates that the following volumes are sufficient to ensure reproducibility:
• Aqueous solutions of nucleic acids: 1 ul
• Purified protein: 2 ul
• Bradford, BCA or Lowry assay: 2 ul
• Microbial cell suspensions: 1-2 ul
It is best to use a precision pipettor (0-2 ul) with precision tips to assure that sufficient sample (1-2 ul) is used. Lower precision pipettors
(0-10 ul and larger) are not as good at delivering 1 ul volumes to the measurement pedestal. If you are unsure about your sample
characteristics or pipettor accuracy, a 2 ul sample is recommended.
Sample Carryover
Prevention of sample being retained on the ND-1000 Spectrophotometer’s measurement pedestals is easily addressed. Simple wiping
of the upper and lower measurement pedestal with a dry laboratory wipe is highly effective in eliminating carryover for samples differing
in concentration by as much as three orders of magnitude (see data at www.nanodrop.com). This is possible since eac h measurement
pedestal is in actuality a highly polished end of a fiber optic cable. There ar e no cracks or crevices for residual sample to get trapped
within.
Sample Homogeneity
Sampling from non-homogeneous solutions – particularly when using small volumes – can cause significant deviations in the data
generated using all measurement technologies including spectrophotometr y. Genomic DNA, lambda DNA and viscous solutions of
other highly concentrated nucleic acids, such as resuspended nucleic acid preparations, are common examples known to the molecular
biologist. Proteins are subject to denaturation, precipitation, and aggregatio n and therefore may require special handling to ensure
sample homogeneity.
Effect of Evaporation and Solvents
Evaporation of the sample during the measurement cycle usually has just a minimal effect on abs orbance readings and may result in a
1-2% increase in sample concentration. This can be observed in the field by measuring the same sample successivel y over time.
Highly volatile solvents, such as hexane, will likely evaporate before the measurement can be completed. Less volatile solvents such as
DMSO can be used successfully.
Sample Recovery
One of the advantages of the sample retention system is that samples can be recovered from the upper and lower measurement
pedestals by extraction with a pipette.
Software Architecture and Features
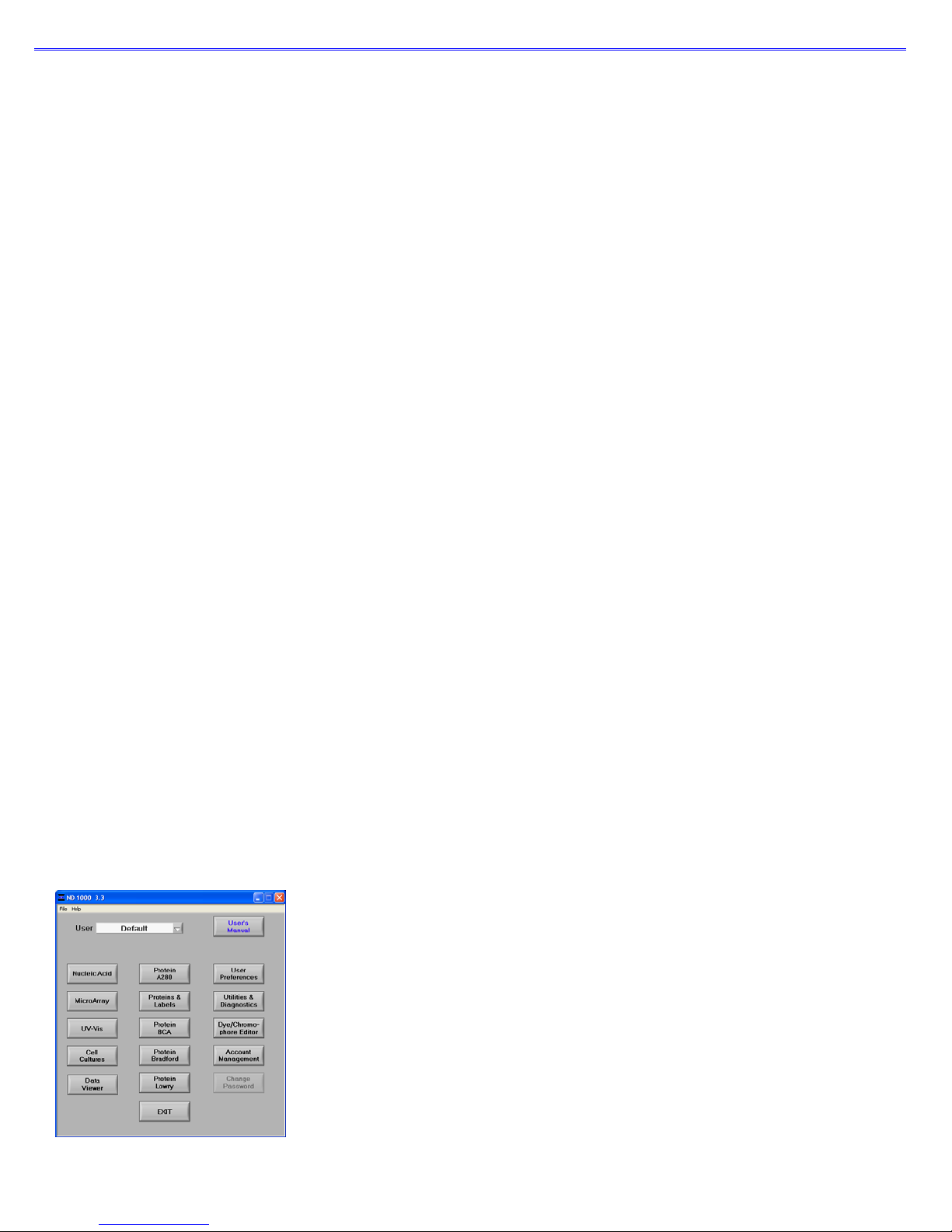
Main Menu
With the sampling arm in the down position, start the NanoDrop
Start
Æ
Programs Æ NanoDrop Æ ND-1000 (version)
software by selecting the following path:
3-2
Page 10
Section 4-Common Module Functions
Application Modules
The NanoDrop software has been tailored to meet the life scientist’s needs. It includes the following application modules:
• Nucleic Acid – concentration and purity of nucleic acid
• MicroArray – dye incorporation concentration and purity of nucleic acid
• UV-Vis – general UV-Vis measurements
• Cell Cultures – “absorbance” (light scattering) measurement of suspended microbial cells
• Protein A280 – concentration and purity of purified protein
• Proteins & Labels – concentration of dye-labeled proteins, conjugates, and metalloproteins
• Protein BCA – protein concentration using the BCA assay
• Protein Bradford – protein concentration using the Bradford assay
• Protein Lowry – protein concentration using the Modified Lowry assay
User Preferences
Each user has the option to configure a number of settings in the various application modules.
The user preferences options for each application module are self explanatory.
Some key features include:
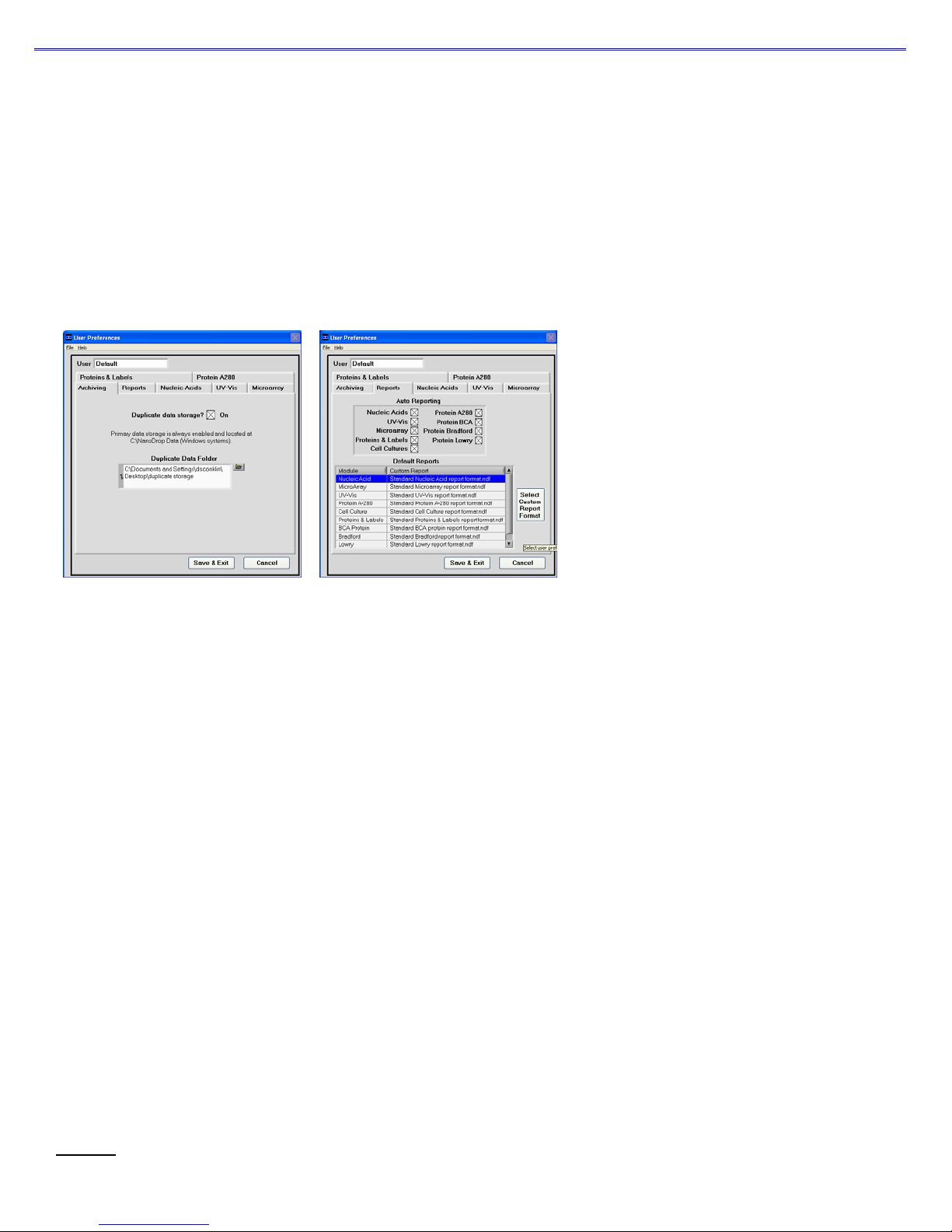
Duplicate data storage
In addition to the primary data storage of archive files at c:\nanodrop data, users may elect to save their data to an additional location.
This option can be chosen under the ‘Archiving.’ tab by selecting the ‘Duplicate data storage?’ box and then choosing the file path by
clicking on the file folder icon under ‘Duplicate Data Folder’. Save the alternative path by clicking on the ‘Save Preferences’ button
before exiting the User Preferences module.
Auto Reporting
Users may choose to select the ‘Auto Reporting’ option for any of the application modules. The auto reporting option allows data to
automatically be saved to the report for all samples. Users may choose this option under the Report tab by selecting the corresponding
box next to the modules listed under ‘Auto Reporting’. Save the auto reporting functions by clicking on the ‘Sav e Preferences’ button
before exiting the User Preferences window.
Note: User preferences are stored in a ‘.log’ file. When upgrading to a newer version of the software, this file shou ld be preserved. If
after upgrading to a new software version the user preferences do not appear correctly, the .log file should be manually copied to the
proper directory. See “Passwords. log’” for more detail.
Utilities and Diagnostics
This module is used to help troubleshoot operational problems with the instrument. For more information on using this module, refer to
the ”Troubleshooting” section of this manual.
Account Management
The Account Management module provides options for directing where specific data files are archived, allowing users to segregate their
data into personal folders. The Account Management module is accessible to the administrator onl y.
Account Types
There are three types of user accounts:
Level 10- this is the highest security setting and all level 10 users can add new users, modify a user, delete a user and set password
options. At the time of software installation, the only level 10 account is Administrator whose initial password is “nanodrop”. It is strongly
3-3
Page 11

Section 4-Common Module Functions
recommended that the password be changed after initial account set up. Any user can be set to a level 10 access, although this is not
recommended (see Level 5 below). Note: The administrator (or the last level 10 user) account may not be deleted.
Level 5- this is the security setting recommended for an ordinary user account. An account with this access will be password protected
and will be able to select specific user preferences. Also, all data generated will be automatically archived in to the user’s acco unt in
c:\nanodrop data (and the user specified location if that preference is selected).
Default (level 0 security) - this access level is reserved for the Default account only. This account enables any user without an account
to access all the active software measurement modules. Although it is not password protected, user preferences can be set for this
account. All data generated will be automatically archived into the Default folder within the c:\Nanodrop Data folder. Note: For
laboratories requiring that every user have a unique user-account, the administrator ma y disable the default user account.
Account Log-in/Log-out and Time Out
The user’s account will remain active until 1) a user logs out of his/her account by using the pull down menu to select either Default or
another user name or 2) the user closes the software.
A user account may also be logged out automatically if the software “System Idle Timeout” is exceeded. After 4 hours of inactivity the
software account will automatically revert back to the Default user. A screen will appear indicating that the time is about to expire, with a
30-second countdown. If the user elects ‘CANCEL’, the clock with reset and the user account and application module will remain active
for another 4 hours. If the time expires, the open application module will close, returning to the Main Menu an d the Default user.
Account Lockout
User-specific accounts can become locked out in several ways as noted below:
• Failure to change password within the allotted time
• Incorrectly entering the password 99 consecutive times
• The administrator locks a specific account
Only the administrator (level 10) can unlock a locked account. This is done by using the ‘Modify User’ entry in the Account
Management module. Note: All accounts (even the administrator) can be locked if the incorrect password entry occurs as described
above.
Change Password
This module enables each user having an authorized account ID to change their respective password.
Note: The administrator, using the ‘Options’ or the ‘Modify User’ entries in the ‘Account Management’ module, establishes whether
individual user passwords will expire and, if so, after how many days.
3-4
Page 12

Section 4-Common Module Functions
Passwords.log file
This file contains the User ID & password for all accounts and is readable only by the software. It can be found in the c:\nanodrop
data\log files folder.
It is strongly recommended that the administrator make a copy of that file and store it in the same log files folder as
above each time a new user account is added or a password is changed. If the administrator’s account becomes locked, the up-to-date
copy can be renamed and used as the password,log file.
Note: If upgrading from a previous version, the “passwords.log” and “user preferences.log” files should be automatically copied to the
c:\NanoDrop Data\Log Files directory. If for some reason these files are not copied automatically, they must be manual ly copied from
the c:\program files\NanoDrop {version} to the c:\program files\NanoDrop V(version) directory.
Dye/Chromophore Editor
The Dye/Chromophore Editor gives the user the ability to add their own dyes or chromophores in addition to the predefined fluorescent
dyes available for use with the MicroArray and Proteins and Labels modules. Note 1: Predefined dye methods are indicated by a
diamond and can’t be modified. Note 2: Absorbance contribution at 260nm from the respective dye can be corrected by entering the
appropriate decimal correction in the 260nm % field. Refer to the following Molecular probes Base:Dye Ratio Calculator to find the 260
nm % factor for dyes not pre-defined in the Dye/Chromophore List:
http://probes.invitrogen.com/resources/calc/basedyeratio.html
Note: If upgrading from a previous version, zero values (0) for 260 nm % and 280 nm % correction factors will be entered for all user
defined dyes. Please refer to the above link for a list of the correct factors to manually enter.
3-5
Page 13
4. Common Module Functions
Section 4-Common Module Functions
Module Startup
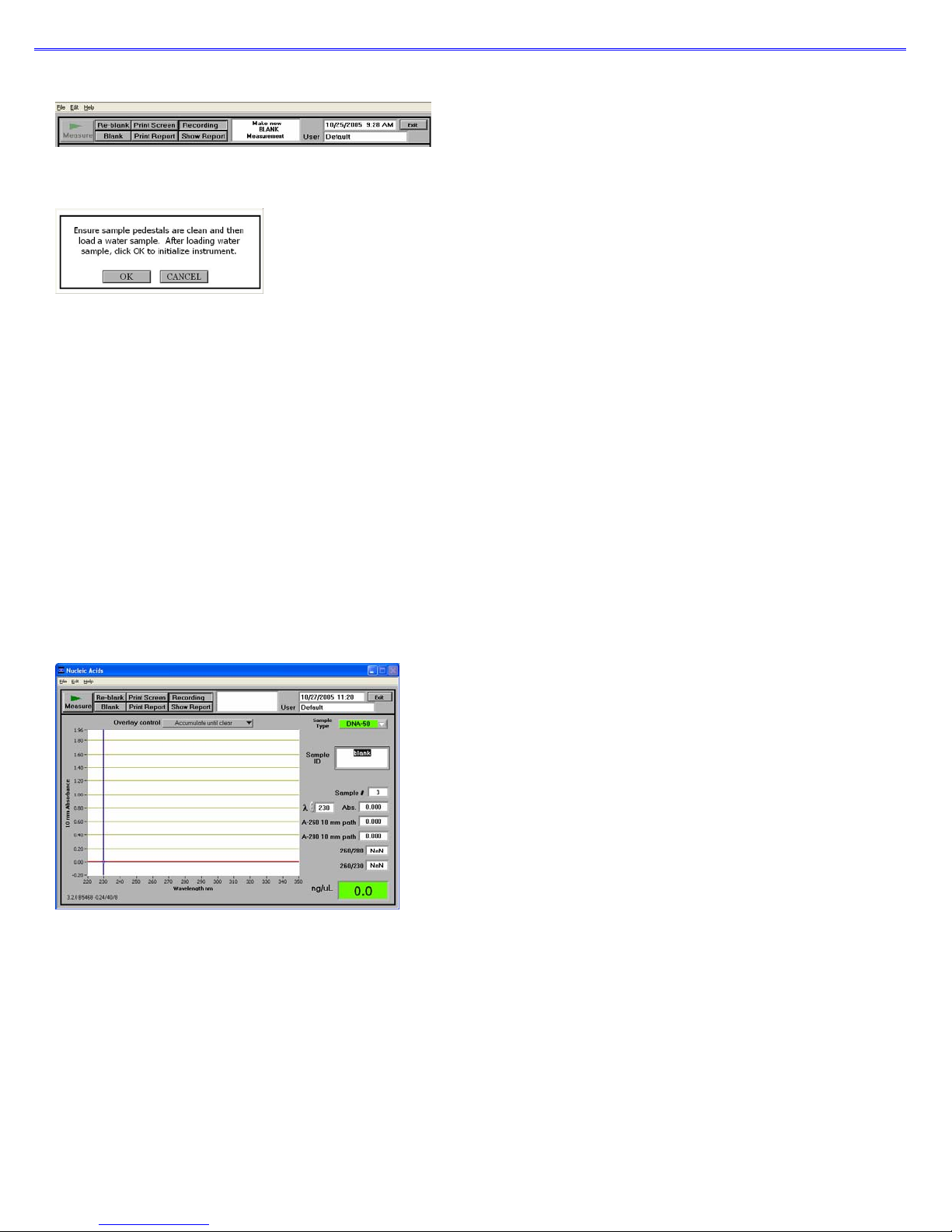
When the software starts, you should see this message:
For best results, ensure measurement pedestal surfaces are clean and load a water sample onto the lower measurement pedestal
and then click ‘OK’. After clicking OK, the message “Initializing Spectrometer- please wait” will appear. When this message
disappears, the instrument will be ready for use. All data taken will automatically be logged in the a ppropriate archive file.
Escape Key (ESC)
The escape key is set to exit out of all screens. Hitting the escape key twice will log the user out of an application module.
Measure (F1)
Each time a software module is opened (initiated), the Measure’ button is inactive as noted by its “grayed-out” appearance. A blank
must first be measured before the Measure button will become active.
The Measure button is used to initiate the measurement sequence for all samples (non-blanks). It is actuated by depressing the F1 key
or clicking the ‘Measure’ button. The entire measurement cycle takes approximately 1 0 se conds.
Blank (F3)
Before making a sample measurement, a blank must be measured and stored (see “Blanking and Absorbance Calculations” in the
appendix for more details on absorbance calculations). After making an initial blank measurement, a straight line will appear on the
screen; subsequent blanks will clear any sample spectrum and display a straight line as shown in the image below.
Blanking Cycle
For the most consistent results, it is best to begin any measurement session with a blanking cycle. This will assure the user that the
instrument is working well and that any non-specific fluorescence is not a concern. To perform a blanking cycle, perform the following:
1. Load a blank sample (the buffer, solvent, or carrier liquid used with your samples) onto the lower measurement pedestal and lower
the sampling arm into the ‘down’ position.
2. Click on the ‘Blank’ (F3) button.
3. Wipe the blanking buffer from both pedestals using a laboratory wipe.
4. Analyze an aliquot of the blanking solution as though it were a sample. This is done using the ‘Measure’ button (F1). The result
should be a spectrum with a relatively flat baseline. Wipe the blank from both measurement pedestal surfaces and repeat the
process until the spectrum is flat.
See “Blanking and Absorbance Calculations” in the appendix for more information on blanking and absorbance calculations.
4-6
Page 14
Section 4-Common Module Functions
Re-blank (F2)
The Re-blanking option (F2) establishes a new reference (blank) that is use d for the absorbance calculations of subsequent samples.
However, unlike the Blank (F3) function, the Re-blank feature recalculates the absorba nce spectrum for the most recent sample and
displays this on the screen. When the Re-blank function is used, the following message appears:
See the “Blanking and Absorbance Calculations” appendix for more information on absorbance calculations.
Print Screen (F4)
The ‘Print Screen’ button will print a copy of the current operating screen to the default printer attached to the operating PC.
Note: The system is configured to work with the Dymo Label Writer 400 printing on #30256 [2-5/16’ X 4’] shipping labels, but can print
on any printer connected to the PC.
Print Window
A Print dialogue can be initiated from the ‘File’ pull-down menu or by typing ‘Ctrl+P’. The user can specify any connected printer to print
to from the Print dialogue.
Saving Current Screen as .JPG Image
The current screen can be saved as a .jpg image file by selecting ‘Save Window’ from the ‘File’ pull down menu.
Start Report / Recording (F6)
The user can log measurement results in a report table and print them to the desired printer. To initiate this feature, select the ‘Start
Report’ button. The default setting has the Recording feature activated. Refer to section 14 (Data Viewer) for additional d etails. Note:
To override this feature, click on the ‘Recording’ button. Once de-selected, the button will read Start Report.
When the maximum number of entries for that specific report has been reached, there are 2 options:
Print Report & Clear or
Save Report & Clear.
All data is stored in the archive file at c:\NanoDrop Data (and in a duplicate location if selected in User Preferences).
Note: This feature can be set so that ‘Recording’ is the default mode. See “User Preferences” in section 3 for more information.
Print Report (F5)
Selecting the ‘Print Report’ (F5) button will print the existing sample report to the default printer. It can be configured to clear the sample
report contents. The user also has options as to how the buffer is handled. Refer to section 14 (Data Viewer) for additional details. All
data is stored in the archive file at c:\NanoDrop Data.
Note: The system is configured to work with the Dymo Label Writer 400 printing on #30256 [2-5/16’ X 4’] shipping labels, but can print to
any printer connected to the PC.
4-7
Page 15

Section 4-Common Module Functions
Show Report (F7)
The user can display the entries comprising the current Sample Report at any time by selecting the ‘Show Report’ button. This function
will enable the Data Viewer software described in section 14. Parameters specific for the individual application modules are populated
for each individual Sample ID. Descriptive parameters specific for the individual application modules are pop ulated for each individual
sample ID.
Sample ID
The ‘Sample ID’ is highlighted for overtyping or barcode scanning. The user may input a sample ID that will be used to identif y the
measurement in a report print and in the archived data file. The sample ID entry is “key focused”, meaning it is the default selection on
the screen and should have a flashing text cursor when the instrument is waiting to make a new measurement.
Sample #
The ‘Sample #’ indicator is activated when a sample report is being recorded. It indicates the sample number of the last sample
processed in the current report and increments with each successive measurement until the sample report is fully populated. The
sample buffer limit can be modified on the report page.
Exit
This command closes all application modules and supporting options. After clicking the ‘Exit’ button, the user has 10 seconds to cancel
the exit command. If no action is taken within 10 seconds, the exit command is carried out. Note: All measurement data is
automatically saved to an archive file and requires no user action.
Show Context Help
Context Help is enabled in the Main Menu, all function modules, and the application modules. The help feature is enabled by choosing
‘Show Context Help’ from the ‘Help’ menu pull down or by selecting ‘Ctrl+H’. Once enabled, placing the cursor on elements of the
screen will automatically generate an explanation of that element. Context Help remains active until deselected.
User’s Manual
A .PDF version of this User’s Manual is accessible from the Main Menu and from the Help menu in all of the application modules. It can
Æ
also be accessed by selecting from the Help pull down menu in any application mo dule or from Start
Programs Æ NanoDrop ÆND-
1000 (version).
4-8
Page 16
5. Nucleic Acids
Nucleic acid samples can be readily checked for concentration and quality using the NanoDrop
measure nucleic acid samples select the ‘Nucleic Acid’ application module.
Section 5- Nucleic Acids
®
ND-1000 Spectrophotometer. To
Sample Volume Requirements
Field experience has indicated that 1ul samples are sufficient to ensure accurate and reproducible results when measuring aqueous
nucleic acid samples. However, if you are unsure about your sample or your pipettor accuracy, a 1.5-2ul sample is recommended to
ensure that the liquid sample column is formed and the light path is completely covered by sample.
Measurement Concentration Range
The NanoDrop
®
ND-1000 Spectrophotometer will accurately measure dsDNA samples up to 3700 ng/ul without dilution. To do this, the
instrument automatically detects the high concentration and utilizes the 0.2mm pathlength to calculate the absorb ance.
Detection
Limit
(ng/ul)
2
Approx.
Upper Limit
(ng/ul)
3700 ng/ul (dsDNA)
3000 (RNA)
2400 (ssDNA)
Typical Reproducibility
(minimum 5 replicates)
(SD= ng/ul; CV= %)
sample range 2-100 ng/ul: ± 2 ng/ul
sample range >100 ng/ul: ± 2%
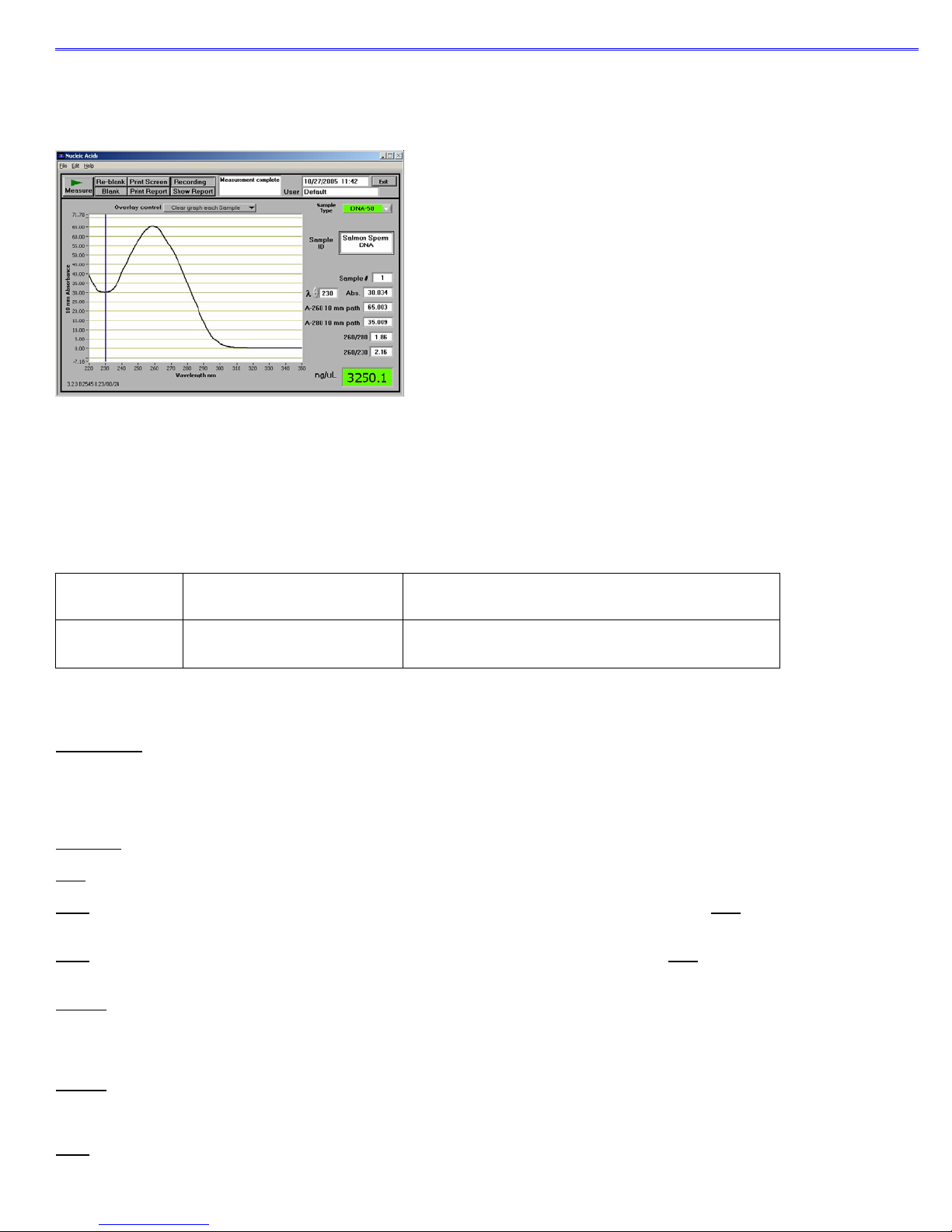
Unique Screen Features
Sample Type: used to select the (color-keyed) type of nucleic acid being measured. The user can select ‘DNA-50’ for dsDNA, ‘RNA-
40’ for RNA, ‘ssDNA-33’ for single-stranded DNA, or ‘Other’ for other nucleic acids. The default is DNA-50. If ‘Other’ is selected, the
user can select an analysis constant between15-150. When navigating amongst the three gener al sample types within the Nucleic
Acids module, the last constant value entered within the ‘Constant’ sample type will be retained. See the “Concentration Calculation
(Beer’s Law)” Appendix for more details on this calculation.
λ and Abs: the user selected wavelength and corresponding absorbance. The wavelength can be selected by moving the cursor or
using the up/down arrows to the left of the wavelength box.
Note: The user-selected wavelength and absorbance are not utilized in any calculations.
A260: absorbance of the sample at 260 nm represented as if measured with a conventional 10 mm path. Note: This is 10X the
absorbance actually measured using the 1 mm path length and 50X the absorbanc e actually measured using the 0.2 mm path length.
A280: sample absorbance at 280 nm represented as if measured with a conventional 10 mm path. Note: This is 10X the absorbance
actually measured using the 1 mm path length and 50X the absorbance actually measured using the 0.2 mm path length.
260/280: ratio of sample absorbance at 260 and 280 nm. The ratio of absorbance at 260 and 280 nm is used to asses s the purity of
DNA and RNA. A ratio of ~1.8 is generally accepted as “pure” for DNA; a ratio of ~2.0 is generally accepted as “pure” for RNA. If the
ratio is appreciably lower in either case, it may indicate the presence of protein, phenol or other contaminants that absorb strongly at or
near 280 nm. See “260/280 Ratio” section of the Troubleshooting section for more details on factors that can affect this ratio.
260/230: ratio of sample absorbance at 260 and 230 nm. This is a secondary measure of nucleic acid purity. The 260/230 values for
“pure” nucleic acid are often higher than the respective 260/280 values. They are commonly in the range of 1.8-2.2. If the ratio is
appreciably lower, this may indicate the presence of co-purified contaminants.
ng/ul: sample concentration in ng/ul based on absorbance at 260 nm and the selected analysis constant. See the “Concentration
Calculation (Beer’s Law)” in the appendix for more details on this calculation.
5-1
Page 17

Section 5- Nucleic Acids
Show Report: formatted for 200 samples although the buffer size can be increased to accommodate thousands of samples.
Spectrum Normalization
The baseline is automatically set to the absorbance value of the sample at 340 nm, which should be very nearly zero absorbance. All
spectra are referenced off of this zero.
Spectrum Overlay Control
The user can display more than one spectrum in the same display using this feature. T he current samp le plot will be displayed in bold
and previous plots will be distinguished by different colors. See example below:
The default option is set to clear the display for the next reading. The user may set the overlay control to clear after each sample plot,
(default setting), after each new report, or accumulate plots until prompted to clear. The Clear Now setting will clear all current and
previous plots. When the overlay function is active, the software will auto scale the y-axis based on the sample with the highest
absorbance at 260 nm. Note: When the overlay function is active, the ‘Blank’ function does not clear the existing overlaid sample
spectra.
5-2
Page 18

Section 6- MicroArray
6. MicroArray
The capability to pre-select viable fluorescent-tagged hybridization pr obes for gene expression in microarrays can eliminate potentially
flawed samples and improve research effectiveness. The NanoDrop
fluorescent dye, allowing detection at dye concentrations as low as 0.2 picomole per microliter.
Fluorescent Dye Selection
There are currently nine fluorescent dyes that are hard-coded for use with the MicroArray module (see table below). Users can also
enter & save fluorescent dyes not coded within the ND-1000 software using the ‘Dye/Chromophore Edit or’ button found in the main
menu.
Dyes can be selected using the scroll arrows or by highlighting the Dye 1 or Dye 2 box. The respective absorb ance wavelength,
extinction coefficient, and 260nm and 280nm % corrections will be automatically utilized for measurement and concentration calculation.
The default settings from NanoDrop remain Dye 1 set to Cy3 and Dye 2 set to Cy5. In addition to the fluorescent dyes available from
the drop-down menu, an option entitled ‘None’ is also available. Selecting ‘None’ disables the respective calculations & numeric
displays corresponding to that dye.
Note: Please refer to the dye manufacturer for the appropriate correction factors for user entered dyes.
®
ND-1000 Spectrophotometer measures the absorbance of the
Sample Volume Requirements
Field experience has indicated that 1 ul samples are sufficient to ensure accurate and repr oducible results when measuring aqueous
nucleic acid samples containing incorporated fluorescent dyes. However, if you are unsure ab out the surface tension properties of your
sample or your pipettor accuracy, a 1.5-2 ul sample is recommended to ensure that the liquid sample column is formed and the light
path is completely covered by sample.
Measurement Concentration Range
The NanoDrop
®
ND-1000 Spectrophotometer will accurately measure fluorescent-dye and nucleic acid concentrations up to 100
pmols/ul (Cy3) and 750 ng/ul (DNA) respectively without dilution. A table of sample concentration ranges is listed below.
Sample
Type
Cy3, Cy3.5, Alexa Fluor
555 and Alexa Fluor 660
Cy5, Cy5.5 and Alexa
Fluor 647
Alexa Fluor 488 and
Alexa Fluor 594
Alexa Fluor 546 0.30 145
Detection
Limit
(pmol/ul)
0.20 100
0.12 60
0.40 215
Approx. Upper
Limit (pmol/ul)
sample range 0.20-4.0 pmol/ul: ± 0.20 pmol/ul
sample range 0.12-2.4 pmol/ul: ± 0.12 pmol/ul
sample range 0.40-8.0 pmol/ul: ± 0.40 pmol/ul
sample range 0.30-6.0 pmol/ul: ± 0.30 pmol/ul
Typical Reproducibility
(minimum 5 replicates)
(SD= pmol/ul; CV= %)
sample range >4.0 pmol/ul: ± 2%
sample range >2.4 pmol/ul: ± 2%
sample range >8.0 pmol/ul: ± 2%
sample range >6.0 pmol/ul: ± 2%
Unique Screen Features
6-1
Page 19

Section 6- MicroArray
Max Absorbance:
used to rescale the upper limit of the vertical axis.
Sample Type
: used to select the (color-keyed) type of nucleic acid being measured. The user can select ‘DNA-50’ for dsDNA, ‘RNA-
40’ for RNA, ssDNA-33’ for single-stranded DNA, or ‘Other’ for other nucleic acids. The default is ssDNA-33’. If ‘other’ is selected, the
user can select an analysis constant between 15-150. When navigating amongst the three (3) general sample types within the Micro
Array module, the last value of the constant entered within the ‘Constant’ Sample Type will be retained. See “Concentrati on Calculation
(Beer’s Law)” in the appendix for more details on this calculation.
λ and Abs
: the user selected wavelength (black cursor) and corresponding absorbance at the 1mm pathlength. The wavelength can be
selected by dragging the black cursor or using the up/down arrows to the left of the wavelength box. Note: The user-selected
wavelength and absorbance at the 1 mm pathlength are not utilized in any calculations.
Dye 1 (or 2)
Abs. Norm
pmol/ul
: user selected dye
: normalized absorbance of selected Dye at the 1 mm pathlength.
: concentration based upon selected Dye’s extinction coefficient. See “Concentration Calculation (Beer’s Law)” in the
appendix for more details on this calculation.
: concentration of nucleic acids in the sample calculated using the absorbance at 260 nm minus the absorbance at 340 nm (i.e.
ng/ul
normalized at 340 nm) and the nucleic acid analysis constant. See “Concentration Calculation (Beer’s Law)” in the appendix for more
details on this calculation.
Show Report
: formatted for 200 samples although the buffer size can be increased to accommodate thousands of samples.
260/280
: ratio of sample absorbance at 260 and 280 nm. The ratio of absorbance at 260 and 280 nm is used to assess the purity of
DNA and RNA. A ratio of ~1.8 is generally accepted as “pure” for DNA; a ratio of ~2.0 is generally accepted as “pure” for RNA. If the
ratio is appreciably lower in either case, it may indicate the presence of protein, phenol or other contaminants that absorb strongly at or
near 280 nm. See “260/280 Ratio’” section of the Troubleshooting section for more details on factors that can affect this ratio.
Baseline Calculation & Normalization
The software normalizes the visual spectrum display for all readings at 750nm and will automatically calculate a baseline between 400
and 750 nm for dye concentration calculations. The green vertical line on the scree n represents the peak wavelength position for Dye 1,
and the red vertical line represents the peak wavelength position for Dye 2.
6-2
Page 20

Section 7- UV-VIS
7. UV-VIS
The ‘UV/VIS Absorbance’ module allows the NanoDrop
Sample absorbance is displayed on the screen from 220 nm to 750 nm and cursors permit the measurement of individual peaks.
Sample Volume Requirements
Field experience has indicated that 1 ul samples are sufficient to ensure accurate and reproducible results when measuring most
aqueous samples. If you are unsure about your sample composition or your pipettor accuracy, a 1.5-2 ul sample is recommended to
ensure that the liquid sample column is formed and the light path completely is covered by sample.
Measurement Concentration Range
All ND-1000 spectrophotometers will measure absorbance up to the 10mm pathlength equivalent of 15 A. ND-1000s with serial
numbers >500 or those that have been field retrofitted, can utilize the short path length (0.2mm) which enables the 10mm pathlength
equivalent of 75 A.
Unique Screen Features
®
ND-1000 Spectrophotometer to function as a conventional spectrophotometer.
λ1/Abs1 and λ2/Abs2
: current values of the user-selectable wavelength cursors and correspon ding absorbencies for a 1 mm
pathlength. The wavelengths can be set by dragging the cursor, using the up/down arrows or typing in the desired wavelength.
Baseline:
the absorbance of the user selectable-baseline (horizontal) cursor. The user ma y drag this cursor to a new vertical position
to create a new baseline. The absorbance value of the baseline is subtracted from the absorbance of the spectrum.
Max Absorbance:
used to rescale the upper limit of the vertical axis.
: samples with high absorbance (up to 75 A equivalent @ 10 mm path) can be measured dir ectly (applicable for instruments with
Hi Abs
serial numbers >500 only or that have been field-retrofitted). This capability is selected by choosing the ‘Hi Abs’ button on the header
bar. When this is selected, the absorbance is measured using the short path (0.2mm) and plotted as a red line normalized to a 0.1mm
path for easier visual comparison as shown above. Sample ID label will be stored with sample data in the file fold er “C:\NanoDrop
Data\User name\ HiAbs”. This data cannot be imported into the Data Viewer but can be opened with Excel type spreadsheets.
Normalize: This is a user-selectable feature in this module. If selected, the software will automatically normalize the spectrum
based on the lowest absorbance value in the range 400-700 nm.
Show Report
: formatted for 200 samples although the buffer size can be increased to accommodate thousands of samples.
7-1
Page 21

Section 8- Protein A280
8. Protein A280
Proteins, unlike nucleic acids, can exhibit considerable diversity. The A280 method is ap plicable to purified proteins exhibiting
absorbance at 280nm. It does not require generation of a standard curve and is ready for quantitation of protein samples at startup. This
module displays the UV spectrum, measures the protein’s absorbance at 280 nm (A280) and calculates the concentration (mg/ml). Like
the Nucleic Acid module, it automatically switches to the 0.2 mm pathlength at very high concentrations of protein. Also analogous to
the Nucleic Acid module, the Protein A280 module displays and records 10 mm (1 cm) equivalent data on the screen and in the
archived data file.
Sample Volume Requirements
Some proteins are hydrophobic and others hydrophilic giving rise to variable surface tension in the sample to be measured. Additionally
the presence of surfactants or detergents in reagents, such as the Bradford reagent, can significantly alter surface tension. That
occurrence can be overcome without affecting the sample’s absorbance by using a larger sample volume. A 2 ul sample size is
recommended for protein measurements.
Special Cleaning Requirements for Proteins
Proteins and solutions containing surfactants are known to “un-condition” the measurement pedestal surfaces so that the liquid column
does not form. If this occurs, “buff” the measurement pedestal surfaces by rubbing each with a dry laboratory wipe 15-20 times. This
will “re-condition” the surface allowing for the liquid sample column to form.
Measurement Concentration Range
The NanoDrop
this, the instrument automatically detects the high concentration and utilizes the 0.2mm pathlength to calculate the absorbance. A table
of concentration range and typical reproducibility is listed belo w.
Sample Type
Purified BSA 0.10 mg/ml 100 mg/ml
Unique Screen Features
®
ND-1000 Spectrophotometer will accurately measure protein samples up to 100 mg/ml (BSA) without dilution. To do
Detection
Limit
Approx. Upper
Limit
sample range 0.05-10 mg/ml: ± 0.10 mg/ml
Typical Reproducibility
(minimum 5 replicates)
(SD= mg/ml; CV= %)
sample range >10mg/ml: ± 2%
Sample Type
: There are six sample types (options) available for purified protei n analysis and concentration measurement. All of the
options can be viewed by clicking the mouse while it is positioned within the ‘Sample Type’ box. The sample type (color-keyed) can be
selected by clicking on the preferred option or by scrolling through the selections using the up or down arrow keys located to the left of
the sample type box. A description of each sample type is given below.
A general reference setting based on a 0.1% (1 mg/ml) protein solution producing an Absorbance at 280 nm of
1.0 A (where the pathlength is 10 mm or 1 cm).
Bovine Serum Albumin reference. Unknown (sample) protein concentrations are calculated using the mass
extinction coefficient of 6.7 at 280 nm for a 1% (10 mg/ml) BSA solution.
8-1
Page 22

Section 8- Protein A280
IgG reference. Unknown (sample) protein concentrations are calculated using the mass extinction coefficient of
13.7 at 280 nm for a 1% (10 mg/ml) IgG solution.
Lysozyme reference. Unknown (sample) protein concentrations are calculated using the mass extinction
coefficient of 26.4 at 280 nm for a 1% (10 mg/ml) Lysozyme solution.
User-entered values for molar extinction coefficient (M-1 cm-1) and molecular weight (MW) in kilo Daltons for their
respective protein reference. Maximum value for e is 999 X 1000 and maximum value for M.W. is 9999 X 1000.
User-entered mass extinction coefficient (L gm-1cm-1) for a 10 mg/ml (1%) solution of the respective reference
protein.
λ and A:
current value of the user-selectable wavelength cursor and corresponding absorbance. The wavelength can be set by
dragging the cursor, using the up/down arrows or typing in the desired wavelength. Note: The user-selected wavelength and
absorbance are not utilized in any calculations.
A280 10-mm Path
: 10 mm-equivalent absorbance at 280 nm for the protein sample measured
A260/280
: ratio of sample absorbance at 260 and 280 nm
Show Report
: formatted for 200 samples although the buffer size can be increased to accommodate thousands of samples.
Spectrum Normalization
The baseline is automatically set to the absorbance value of the sample at 340 nm, which should be very nearly zero absorbance. All
spectra are referenced off of this zero. All data are now normalized and archived in the same format.
Spectrum Overlay Control
The user can display more than one spectrum in the same display using this feature. T he current samp le plot will be displayed in bold
and previous plots will be distinguished by different colors. See example below:
The default option is set to clear the display for the next reading. The user may set the overlay control to clear after each sample plot
(default setting), after each new report, or accumulate plots until prompted to clear. The clear now setting will clear all current and
previous plots. When the overlay function is active, the software module will auto scale the y-axis based on the sample with the highest
absorbance at 280nm. Note: When the overlay function is active, the ‘Blank’ function doesn’t clear the existing ov erlaid sample spectra.
8-2
Page 23

Section 9- Proteins & Labels
9. Proteins & Labels
This software module can be used to determine protein concentration (A280nm) as well as fluorescent dye concentration (protein array
conjugates), or to measure the purity of metalloproteins (such as hemoglobin) using wavele ngth ratios.
Fluorescent Dye Selection
There are currently nine fluorescent dyes that are hard-coded for use with the Proteins and Labels module (see table below). Users can
also enter & save fluorescent dyes not coded within the ND-1000 software using the ‘Dye/Chromophore Editor’ button found in the main
menu.
Dyes can be selected using the scroll arrows or by highlighting the Dye 1 or Dye 2 box. The respective absorb ance wavelength,
extinction coefficient, and 280nm % corrections will be automatically utilized for measurement and concentration calculation. The
default settings from NanoDrop remain Dye 1 set to Cy3 and Dye 2 set to Cy5. In addition to the fluorescent dyes available from the
drop-down menu, an option entitled ‘None’ is also available. Selecting ‘None’ disables the respective calculations & numeric displays
corresponding to that dye.
Note: Please refer to the dye manufacturer for the appropriate correction factors for user entered dyes.
Sample Volume Requirements
Some proteins are hydrophobic and others hydrophilic giving rise to variable surface tension in the samples to be measured.
Additionally the presence of surfactants or detergents in reagents, such as the Bradford reagent, can signific antly alter surface tension.
This occurrence can be overcome without affecting the sample’s absorbance by using a larger sample volume. A 2 ul sample size is
recommended for protein measurements.
Special Cleaning Requirements for Proteins
Proteins and solutions containing surfactants are known to “un-condition” the measurement pedestal surfaces so that the liquid column
does not form. If this occurs, “buff” the measurement pedestal surfaces by rubbing each with a dry laboratory wipe 15-20 times. This
will “re-condition” the surface allowing for the liquid sample column to form.
Measurement Concentration Range
The NanoDrop
®
ND-1000 Spectrophotometer will accurately measure protein samples up to 20 mg/ml (BSA) without dilution. A table of
concentration range and typical reproducibility is liste d below.
Sample
Type
Purified BSA 0.10 mg/ml 20 mg/ml
Cy3
Detection
Limit
0.2uM
Approx.
Upper
Limit
100uM
sample range 0.05-10 mg/ml: ± 0.10 mg/ml
sample range 0.20-4.0 pmol/ul: ± 0.20 pmol/ul
Typical Reproducibility
(minimum 5 replicates)
(SD= mg/ml; CV= %)
sample range >10mg/ml: ± 2%
sample range >4.0 pmol/ul: ± 2%
Unique Screen Features
9-1
Page 24

Section 9- Proteins & Labels
Max Absorbance:
used to rescale the upper limit of the vertical axis.
Sample Type
: The same six sample types (options) listed under Protein A280 (Section 8) are availabl e for purified Proteins & Labels
analysis and concentration measurement. All of the options can be viewed by clicking the mouse while it is positioned within the
sample type box. The sample type (color-keyed) can be selected by clicking on the preferred option or b y scrol ling through the
selections using the up or down arrow keys located to the left of the sample type box. See Protein A280 (Section 8) for a detailed
description of each sample type.
λ 3
: user-selected wavelength
Norm Abs @ λ 3
: 10mm equivalent normalized absorbance at the respective wavelength.
Dye 1 (or 2)
Norm Abs @ λ1
uM
: user-selected dye
: normalized 10 mm equivalent absorbance of selected Dye
: concentration based upon selected Dye’s extinction coefficient. See the “Concentration Calculation (Beer’s Law)” Appendix for
more details on this calculation.
Abs ratio λ1/λ3
: ratio of the absorbance of Dye 1 to the absorbance at the user-selected wavelength (λ3).
concentration of proteins in the sample calculated using the absorbance at 280 nm minus the absorbance at 340 nm (i.e.
mg/ml:
normalized at 340 nm). See the “Concentration Calculation (Beer’s Law)” Appendi x for more details on this calculation.
Show Report
: formatted for 200 samples although the buffer size can be increased to accommodate thousands of samples.
Baseline Type
This application module has two user-selectable “Baseline Type” options. The default setting is set to normalize the display spectrum at
750nm. Alternatively, the 400-750 Slope Baseline Type will normalize the display at 750nm and accommodate any linear baseline offset
across the 400 to 750nm range.
9-2
Page 25

Section 10- Protein BCA
10. Protein BCA
The BCA (Bicinchoninic Acid) Protein Assay is an alternative method for determining protein concentration. It is often used for more
dilute protein solutions and/or in the presence of components that also have significant UV (280 nm) absorbance. Unlik e the Protein
A280 method, the BCA Assay requires a standard curve to be generated each time it is run, before unknown proteins ca n be measured.
The resulting Cu-BCA chelate formed in the presence of protein is measured at its wavelength maxim um of 562 nm and normalized at
750 nm. Pre-formulated reagents of BCA and CuSO
Follow their recommendations when mixing the respective reagents at the time the assay is to be performed.
Sample Volume Requirements
Some proteins are hydrophobic and others hydrophilic giving rise to variable surface tension in the sample to be measured. Additionally
the presence of surfactants or detergents in reagents, such as the Bradford reagent, can significantly alter surface tension. This
occurrence can be overcome without affecting the sample’s absorbance by using a larger sample volume. A 2 ul sample size is
recommended for protein measurements.
Special Cleaning Requirements for Proteins
Proteins and solutions containing surfactants are known to “un-condition” the measurement pedestal surfaces so that the liquid column
does not form. If this occurs, “buff” the measurement pedestal surfaces by rubbing each with a dry laboratory wipe 15-20 times. This
will “re-condition” the surface allowing for the liquid sample column to form.
Measurement Concentration Range
On the NanoDrop
®
ND-1000 Spectrophotometer, the regular BCA assay can measure samples from ~0.20 mg/ml up to 8.0 mg/ml. A
mini-BCA assay covers an approximate range of 0.01 – 0.20 mg/ml.
Assay
Type
Regular BCA 0.2 mg/ml 8.0 mg/ml
Mini BCA 0.01 mg/ml 0.20 mg/ml
Approx.
Lower
Limit
BCA Kits, Protocols, and Sample Preparation
Commercial BCA Protein kit manufacturers typically outline procedures for two different protein concentration ranges:
• A regular assay – using a 20:1 reagent / sample volume ratio
sample volume of 4 ul in 80 ul of BCA reagent (larger sample volume is preferable).
• A mini assay – using a 1:1 reagent / sample volume ratio
using a minimum of 10 ul of sample and 10 ul of BCA reagent in a PCR tube. Using the same pipettor for both volumes will
eliminate any pipette-to-pipette accuracy differences.
Note: If you run the assay at 60°C, doubling the volumes may afford greater insurance against skewed results from
evaporation / condensation within the sealed reaction tube.
In addition to the kit reagents, protein standards (BSA) for generating a standard curve are provided for the BCA method by the
manufacturer. Follow the manufacturer’s protocol for the assay including recommended incubation times and temperature. Additionally,
use the respective standard (e.g., BSA) and dilutions that cover the analytical range (mg/ml) of interest. Note: Since the ND-1000 can
measure higher protein concentrations, you may need to supply your own protein standards at higher concentrations than provided by
the manufacturer.
Unique Screen Features
View Standard Curve (F8):
selecting this button allows the user to view the standard curve at any time.
Sample Type
: choices are Reference, Standards 1-7, and Sample. The software will guide you to measure your reference, then at
least one standard before allowing measurement of samples.
Replicate #:
counter for tracking replicate number during reference and standard measurement.
Reset Window (F11)
: clears all replicates of all standards.
Reset This Std (F12):
clears all replicates of the selected standard.
Absorbance at 562nm
: the Cu-BCA complex’s absorbance at 562 nm for the 1mm pathlength.
Cursor λ and Absorbance
: this feature allows the user to adjust the cursor wavelength and view the corresponding absorbance. The
cursor wavelength can be set by the user. Note: The user-selected wavelength and absorbance are not utilized in any calculations.
, utilized in the assay, are available in kit form from numerous manufacturers.
4
Approx.
Upper
Limit
Typical Reproducibility
(minimum 5 replicates)
(SD= mg/ml; CV= %)
± 2% (over entire range)
± 0.01 mg/ml (over entire range)
. To accurately prepare standards, we suggest using a minimum
. To prepare sufficient volume of these 1:1 mixtures, we suggest
10-1
Page 26

Section 10- Protein BCA
mg/ml
: the concentration of the sample (unknown).
Show Report
: formatted for 200 samples although the buffer size can be increased to accommodate thousands of samples.
Making BCA Measurements
A standard curve is required every time the BCA assay is run. Although curves can be saved and re loaded in the NanoDrop
®
ND-1000
Spectrophotometer software, it is recommended that the user follow manufacturers’ guidelines and gener ate fresh standard curves for
each assay. Both single and multi-point standard curve generation is incorporated into the software. A standard curve can be developed
using a reference (BCA reagent only – no protein) and a single replicate of one standard. The multi-point standard curve generator
allows a maximum of five replicates for each of seven different standards. There is no set order in which standards must be run.
There are only three general procedural steps to unknown protein concentration measurements. The requisite order, including
generating the standard curve, is as follows:
Step 1: Measure the ‘Reference’
(BCA reagent – a ‘zero’ Standard)
The software will not allow
measurement of samples until at
least 1 standard and references –
or 2 standards – are measured.
Software will guide the user
Note:
with instructions in the large text
box on the right side of the screen.
The red light indicates the
standard curve is incomplete and
not yet ready for sample
measurements.
Step 2: Measure Standards
Up to 5 replicates of each
standard can be measured. The
software will not allow
measurement of samples until a
minimum of 1 standard and
references – or 2 standards – are
measured. Polynomial curve fitting
requires more standard points
depending on the polynomial
degree selected.
Step 3: Measure Samples
Once a minimum standard curve
has been established, the red
indicator light will turn green,
allowing the user to start
measuring samples. Sample
concentrations are calculated by
using linear interpolation (point-topoint) between the two standards
flanking the unknown sample or
by using polynomial fitting. [Note:
In order to obtain a concentration
value (mg/ml) the sample
(unknown) must fall within the
limits of the standard curve].
Standard Curve Features
Standard curves can be saved and reloaded for reference use by using the ‘Standard Curve’ pull down menu and choosing save or
reload functions. Selecting the ‘View Standard Curve’ button allows the user to review the standard curve at an y time.
The Delete Point button allows the user to delete points by first selecting the data point to be deleted in the table and then choosing the
‘Delete Point’ button. If erroneous or non-representative readings are encountered for a specific standard, all replicates of that standard
are cleared by selecting ‘Reset this std’. Additionally, all standards can be deleted at once using the ‘R eset This Window’ (F11) button.
10-2
Page 27

Section 10- Protein BCA
Regular BCA Standard Curve:
0.2 – 8.0 mg/ml
mini-BCA Standard Curve:
0.01 – 0.20 mg/ml
Exiting the BCA Module
It is recommended that you process all of the unknowns to be assayed before exciting the BCA software module.
10-3
Page 28

Section 11-Protein Lowry
11. Protein Lowry
The Modified Lowry Protein Assay is an alternative method for determining protein concentration based on the widely used and cited
Lowry procedure for protein quantitation. Like the BCA and Bradford Assays, the Modified Lowry Assay requires standard curve
generation each time it is run, before unknown proteins can be measured. The Modified Lowry procedure involves reaction of protein
with cupric sulfate in alkaline solution, resulting in formation of tetradentate copper-protein complexes. The Folin-Ciocalteu Reagent is
effectively reduced in proportion to the chelated copper-complexes resulting in a water-soluble blue product that is measured at 650 nm
and normalized at 405 nm. Pre-formulated reagents, utilized in the assay, are available in kit form from numerous manufacturers.
Follow their recommendations when mixing the respective reagents at the time the assay is to be performed.
Sample Volume Requirements
Some proteins are hydrophobic and others hydrophilic giving rise to variable surface tension in the sample to be measured. Additionally
the presence of surfactants or detergents in reagents, such as the Bradford reagent, can significantly alter surface tension. This
occurrence can be overcome without affecting the sample’s absorbance by using a larger sample volume. A 2 ul sample size is
recommended for protein measurements.
Special Cleaning Requirements for Proteins
Proteins and solutions containing surfactants are known to “un-condition” the measurement pedestal surfaces so that the liquid column
does not form. If this occurs, “buff” the measurement pedestal surfaces by rubbing each with a dry laboratory wipe 15-20 times. This
will “re-condition” the surface allowing the liquid sample column to form.
Measurement Concentration Range
On the NanoDrop
Modified Lowry Kits, Protocols, and Sample Preparation
Commercial Modified Lowry Protein kit manufacturers typically outline procedures for their assa ys.
Modified Lowry assay – using a 5:1 reagent / sample volume ratio
• To accurately prepare Standards, we suggest using a minimum sample volume of 20 ul and 100ul of Modified Lowry reagent.
Using the same pipettor for both volumes will eliminate any pipette-to-pipette accuracy diff erences.
• After the initial 10 minute room temperature (RT) incubation period, add 10 ul of the Folin –Ciocalteu reage nt and incubate for
30 minutes at RT.
In addition to the kit reagents, protein standards (BSA) for generating a standard curve are also provided by the manufacturer for the
Modified Lowry method. Follow the manufacturer’s protocol for the assay including recommended incubation times and temperature.
Additionally, use the respective standard (e.g., BSA) and dilutions that cover the analytical range (mg/ml) of interest. Note: Since the
ND-1000 can measure higher protein concentrations, you may need to supply your own protein stan dards at higher concentrations than
provided by the manufacturer.
Unique Screen Features
View Standard Curve (F8):
Sample Type
least one standard before allowing measurement of samples.
Replicate #:
Reset Window (F11)
Reset This Std (F12):
Absorbance at 650nm
Cursor λ and Absorbance
cursor wavelength can be set by the user. Note: the user-selected wavelength and absorbance are not utilized in any calculations.
: the concentration of the sample (unknown).
mg/ml
®
ND-1000 Spectrophotometer, the Modified Lowry assay can run from ~0.20 mg/ml up to 8.0 mg/ml.
Assay
Type
Modified Lowry 0.2 mg/ml 4.0 mg/ml
Approx.
Lower
Limit
Approx.
Upper
Limit
Typical Reproducibility
(minimum 5 replicates)
(CV= %)
± 2% (over entire range)
.
selecting this button allows the user to view the Standard Curve at any time.
: choices are Reference, Standards 1-5, and Sample. The software will guide you to measure your reference, then at
counter for tracking replicate number during reference and standard measurement.
: clears all replicates of all standards.
clears all replicates of the selected standard.
: the Cu-complex’s absorbance at 650 nm for the 1 mm pathlength.
: this feature allows the user to adjust the cursor wavelength and view the corresponding absorbance. The
11-1
Page 29

Section 11-Protein Lowry
Show Report
: formatted for 200 samples although the buffer size can be increased to accommodate thousands of samples.
Making Lowry Measurements
A standard curve is required every time the Modified Lowry assay is run. Although curves can be sav ed and reloaded in the NanoDrop
®
ND-1000 Spectrophotometer software, it is recommended that the user follow manufacturers’ guidelines and generate fresh standard
curves for each assay. Both single and multi-point standard curve generation is inc orporated into the software. A standard curve can be
developed using a reference (Modified Lowry reagent only – no protein) and a single replicate of one standard. The multi-point standard
curve generator allows a maximum of five replicates for each of seven different standards. There is no set order in which standards
must be run.
There are only three general procedural steps to unknown protein concentration measurement. The requisite order, including
generating the standard curve, is as follows:
Step 1: Measure the ‘Reference’
(Lowry reagent – a ‘zero’
Standard)
The software will not allow
measurement of samples until a
minimum of 1 standard and
Reference – or 2 Standards – are
measured.
Software will guide the user
Note:
with instructions in the large text
box on the right side of the screen.
The red light indicates the
standard curve is incomplete and
not yet ready for sample
measurements.
Step 2: Measure Standards
Up to 5 replicates of each
standard can be measured. The
software will not allow
measurement of samples until a
minimum of 1 standard and
references – or 2 standards – are
measured. Polynomial curve fitting
requires more standard points
depending on the polynomial
degree selected.
Step 3: Measure Samples
Once a minimum standard curve
has been established, the red
indicator light will turn green,
allowing the user to start
measuring samples. Sample
concentrations are calculated by
using linear interpolation (point-topoint) between the two standards
flanking the unknown sample or by
using polynomial fitting. [Note: In
order to obtain a concentration
value (mg/ml), the sample
(unknown) must fall within the
limits of the standard curve.]
Standard Curve Features
Standard curves can be saved and reloaded for reference use by using the ‘Standard Curve’ pull down menu and choosing save or
reload functions. Selecting the ‘View Standard Curve’ button allows the user to review the standard curve at an y time.
The Delete Point button allows the user to delete points by first selecting the data point to be deleted in the table and then choosing the
‘Delete Point’ button. If erroneous or non-representative readings are encountered for a specific standard, all replicates of that standard
are cleared by selecting ‘Reset this std’. Additionally, all standards can be deleted at once using the ‘R eset This Window’ (F11) button.
11-2
Page 30

Modified Lowry Standard
Curve: 0.2 – 4.0 mg/ml
Exiting the Lowry Module
It is recommended that you process all of the unknowns before exciting the Lowry software module.
Section 11-Protein Lowry
11-3
Page 31

12. Protein Bradford
The Bradford Assay is a second alternative method commonly utilized for determining protein concentration. It is often used for more
dilute protein solutions where lower detection sensitivity is needed and/or in the presence of components that also have significant UV
(280 nm) absorbance. Like the BCA method, the Bradford method requires a standard curve to be generated each time the assay is
run, before unknown proteins can be measured.
The Bradford uses the protein-induced Absorbance shift of Coomassie Bl ue dye to 595 nm as a measure of protein concentration. The
bound protein-dye complex is measured at 595 nm and normalized at 750 nm. A single stabilized reagent mixture containing
Coomassie Blue dye, alcohol, and surfactant in kit form is available from numerous manufacturers. Follow the respective
manufacturer’s recommendations for all standards and samples (unknowns), ensuring they are subjected to the ide ntical conditions and
timing throughout the assay.
Sample Volume Requirement
Some proteins are hydrophobic and others hydrophilic giving rise to variable surface tension in the sample to be measured. Additionally
the presence of surfactants or detergents in reagents, such as the Bradford reagent, can significantly alter surface tension. This
occurrence can be overcome without affecting the sample’s absorbance by using a larger sample volume. A 2 ul sample size is
recommended for protein measurements.
Special Cleaning Requirements for Proteins
Proteins and solutions containing surfactants are known to “un-condition” the measurement pedestal surfaces so that the liquid column
does not form. If this occurs, “buff” the measurement pedestal surfaces by rubbing each with a dry laboratory wipe 15-20 times. This
will “re-condition” the surface allowing for the liquid sample column to form.
Measurement Concentration Range
On the NanoDrop
to several thousand micrograms/ml (ug/ml) can be determined. The best linearity is in the 100-1000 ug/ml range. A mini-Bradford assay
covers the approximate range of 15-125 ug/ml.
Coomassie dye-dye and Coomassie dye-protein aggregates are frequently encountered in Coomassie dye-based protein assays. With
time, particulate can be observed, which can cause significant fluctuations in Absorbance readings. It is also important to note the total
analyte (protein-dye) signal at 595nm is limited to ~ 0.150 A as a result of the 1.0mm pathlength of the instrument, the Bradford
(Coomassie dye) reagent concentration, and the acidic pH. Making measurements in triplicate of standards and samples (unknowns)
is good practice, particularly with the limited assay signal obtained with the Bradford Assay.
Regular Bradford 100 ug/ml 8000 ug/ml
Mini Bradford 15 ug/ml 100 ug/ml
Bradford Kits, Protocols, and Sample Preparation
Commercial Bradford Protein kit manufacturers typically outline procedures for two different concentration ran ges:
• A regular assay – using a 50:1 reagent / sample volume ratio
sample volume of 4 ul in 200 ul of Bradford reagent (larger sample volume is preferable).
• A mini assay– using a 1:1 reagent / sample volume ratio
a minimum of 10 ul of sample and 10 ul of Bradford reagent in a PCR tube. Using the same pipettor for both volumes will
eliminate any pipette-to-pipette accuracy differences.
In addition to the kit reagents, protein standards (e.g., BSA) for generating a standard curve are provided by the manufacturer. Follow
the manufacturer’s recommendation using standard (BSA) dilutions that cover the analytical (ug/ml) range of interest. Note: Since the
ND-1000 Spectrophotometer can measure higher protein concentrations, you may need to supply your own protein standards at higher
concentrations than provided by the manufacturer.
Unique Screen Features
View Standard Curve (F8):
Sample Type
least one standard before allowing measurement of samples.
Replicate #:
®
ND-1000 Spectrophotometer using the regular Bradford assay, unknown protein concentrations from ~100ug/ml up
Assay
Type
Approx.
Lower
Limit
selecting this button allows the user to view the standard curve at any time.
: choices are Reference, Standards 1-5, and Sample. The software will guide you to measure your reference, then at
counter for tracking replicate number during reference and standard measurement.
Approx.
Upper
Limit
Section 12- Protein Bradford
Typical Reproducibility
(minimum 5 replicates)
(SD= ug/ml; CV= %)
sample range 100-500 ug/ml: ± 25 ug/ml
sample range 500-8000 ug/ml: ± 5%
sample range 15-50 ug/ml: ± 4 ug/ml
sample range 50-125 ug/ml: ± 5%
. To accurately prepare standards, we suggest using a minimum
. To prepare sufficient volume of these 1:1 mixtures, we suggest using
12-1
Page 32

Section 12- Protein Bradford
Reset Window (F11)
: clears all replicates of all standards.
Reset This Std (F12):
clears all replicates of the selected standard.
Absorbance at 595nm
: protein-dye complex’s absorbance at 595 nm at the 1mm pathlen gth.
Cursor λ and Absorbance
: this feature allows the user to adjust the cursor wavelength and view the corresponding absorbance. The
cursor wavelength can be set by the user. Note: The user-selected wavelength and absorbance are not utilized in any calculations.
: concentration of the sample (unknown).
ug/ml
Show Report
: formatted for 200 samples although the buffer size can be increased to accommodate thousands of samples.
Making Bradford Protein Measurements
A standard curve is required every time the Bradford assay is run. Although curves can be saved and reloaded in the Nano Drop
®
ND1000 Spectrophotometer software, it is recommended that the user follow manufacturers’ guidelines and generate fresh standard
curves for each assay. Both single and multi-point standard curve generation is inc orporated into the software. A standard curve can be
developed using a reference (Modified Lowry reagent only – no protein) and a single replicate of one standard. The multi-point standard
curve generator allows a maximum of five replicates for each of seven different standards. There is no set order in which standards
must be run.
There are only three general procedural steps to unknown protein concentration measurement. The requisite order, including
generating the standard curve, is as follows:
Step 1: Measure the ‘Reference’
(Bradford reagent – a ‘zero’
Standard)
The software will not allow
measurement of samples until at
least 1 standard and reference – or
2 standards – are measured.
software will guide the user
Note:
with instructions in the large text
box on the right side of the screen.
The red light indicates the standard
curve is incomplete and not yet
ready for sample measurements.
Step 2: Measure Standards
Up to 5 replicates of each standard
can be measured. The software will
not allow measurement of samples
until a minimum of 1 standard and
references – or 2 standards – are
measured. Polynomial curve fitting
requires more standard points
depending on the polynomial
degree selected.
Step 3: Measure Sample
Once a minimum standard curve
has been established, the red
indicator light will turn green,
allowing the user to start measuring
samples. Sample concentrations
are calculated by using linear
interpolation (point-to-point)
between the two standards flanking
the unknown sample or by using
polynomial fitting of the data. [Note:
In order to obtain a concentration
value (ug/ml) the sample (unknown)
must fall within the limits of the
standard curve.
12-2
Page 33

Section 12- Protein Bradford
Standard Curve Features
Standard curves can be saved and reloaded for reference use by using the ‘Standard Curve’ pull down menu and choosing save or
reload functions. Selecting the ‘View Standard Curve’ button allows the user to review the standard curve at an y time.
The Delete Point button allows the user to delete points by first selecting the data point to be deleted in the table and then choosing the
‘Delete Point’ button. If erroneous or non-representative readings are encountered for a specific standard, all replicates of that standard
are cleared by selecting ‘Reset this std’. Additionally, all standards can be deleted at once using the ‘R eset This Window’ (F11) button.
Regular Bradford curve covers 2008000 ug/ml. Note the linear range
is 100-1000 ug/ml
A mini-Bradford assay covers an
approximate range of 15-100 ug/ml.
Exiting the Bradford Module
It is recommended that you process all of the unknowns to be assayed before exciting the Bradford software module.
12-3
Page 34

Section 13- Cell Cultures
13. Cell Cultures
Using an absorbance spectrophotometer to monitor light scattered by non-absor bing suspended cells is common practice in life science
laboratories. Such applications, more than any other, accentuate the differences amongst the optical systems of the numerous
spectrophotometer designs.
Note: The most distinct difference between the NanoDrop
and those observed using classical cuvette based systems will be attributable to the shorter pathlength (1 mm vs. 1 cm.) Values may
not be exactly 10 fold different as readings are dependent on both the optics of a specific spectrophotometer as well as the cell type in
suspension.
The ‘Cell Cultures’ module displays the sample spectrum from 250 nm to 700 nm. One cursor is fixed at the frequently used wavelength
for monitoring cell suspensions (600nm) while the second cursor can be set to the wavelength of interest.
Sample Size Requirements
Field experience has indicated that 1ul samples are sufficient to ensure accurate and reproducible results when measuring aqueous
samples. However, if you are unsure about your sample composition or your pipettor accuracy, a 2 ul sample is recommend ed to
ensure that the liquid sample column is formed and the light path completely covered by sample.
Cell Suspension Concentrations
Due to its shorter pathlength, the ND-1000 can measure absorbencies that are 10-fold h igher than those measurable on a standard
cuvette spectrophotometer. This makes it possible to directly monitor concentrated cell suspensions. Since the entire spectrum is
displayed, diluted samples exhibiting very low ‘Absorbance’ at 600 nm can be monitored at lower wavelengths, for example 280 nm.
Unique Screen Features
®
ND-1000 spectrophotometer “absorbance” values for cell microbial cultures
600nm Absorbance
: current value of the absorbance at the λ1 cursor with the Baseline absorbance subtracted. Note: the actual 1
mm absorbance is displayed.
λ and Absorb.: current value of the user-selectable wavelength cursor and corresponding absorbance. The wavelength can be set by
dragging the cursor, using the up/down arrows or typing in the desired wavelength. Note: The user-selected wavelength and
absorbance are not utilized in any calculations.
Baseline:
to create a new baseline. The absorbance value of the baseline is subtracted from the absorbance of the spectrum.
Max Absorbance:
Show Report
Sample Homogeneity
The user must be sure to homogeneously suspend the cells when sampling for “absorbanc e” measurements and read the sample
immediately to avoid significant cell settling. Vigorous mixing may be required particularly when measuring concentrated samples
the absorbance of the user selectable-baseline (horizontal) cursor. The user ma y drag this cursor to a new vertical position
used to rescale the upper limit of the vertical axis.
: formatted for 200 samples although the buffer size can be increased to accommodate thousands of samples.
13-1
Page 35

Section 13- Cell Cultures
Decontamination of Measurement Pedestals
If decontamination is necessary, a 5.25% solution of sodium hypochlorite (bleach – freshly prepared) or other decontaminating solutions
can be used to ensure that no biologically active material is present on the measurement pedestals. The metal fiber optic fittings are
made from 303 stainless steel and are very resistant to most common laboratory solvents (see “Solvent Compatibility” appendix.)
13-2
Page 36

Section 14-Archived Data and Data Viewer
14. Archived Data and Data Viewer
Sample data from all application modules is automatically stored in archive files and can be opened by either the integrated Data
Viewer software program or spreadsheet programs such as MS Excel.
Archive File Creation
Every time an application module is started, an application-specific archive file is created for the user that is logged in. All
measurements made by the user (in that application module) for a given calendar day are stored in a single archive file. These files
bear the name of the respective application module with the date appended. For example, an archived file entitled “Nucleic Acid 2005
11 07.ndj” corresponds to Nucleic Acid data from the software session that began on November 7, 2005. A unique file extension (.ndj)
has been given to these files to enable automatic startup with the Data Viewer (see section on Data Viewer below).
The data may be edited and/or reformatted and stored under names of the user’s choice. The spectrum can be re-plotted from the
wavelength data if needed for further analysis.
Note1: Absorbance data shown in archive files are represented as they are displayed on the screen. F or Nucleic Aci ds, Protein A280,
and Protein and Labels application modules, data are stored based on a 1.0 cm (10.0 mm) path. For MicroArray, UV-Vis, BCA Protein,
Bradford, Lowry and Cell Culture application modules the data is normalized to a 1.0 mm (0.1 cm) path. For high absorbance UV-Vis
samples, data are stored based on a 0.1 mm path.
Note 2: For data from all modules, a column entitled ‘Measurement Type’ is included. For each measurement, this column will contain
‘Measure’, ‘Blank’, or ‘Reblank’. If the value is ‘Measure’, then the values in that row are from a normal measurement that has utilized
the stored blank value. If the value is ‘Blank’, it indicates that the measurement is the initial blank recorded. If the value is ‘Reblank’, it is
the re-analysis of the previous measurement with a new blank.
Data Storage Hierarchy
The hierarchy for archive files is as follows:
Æ
C:\NanoDrop Data
User name Æ Application Module (BCA Protein, Lowry, Bradford, Cell Culture, MicroArray, Nucleic Acid, Protein
A-280, Proteins & Labels, UV-Vis or UV-Vis HiAbs)
All archived data files are stored within an application module folder that is within th e User folder as shown below:
User-Defined Archive File Location
In addition to the primary data storage, users may elect to save their data to an additional location. This option can be chosen under
the Archiving Tab in User Preferences on the Main Menu by setting the ‘Duplicate data storage box ’ to ‘On’ and then choosing the file
path by clicking on the file folder icon under Duplicate Data Folder. Save the alternative path by clicking on the ‘Save Preferences’
button before exiting the User Preferences window.
All data are written to the archive file immediately upon completion of the measurement. Inadvertent software or PC shutdowns should
not affect the archive file.
14-1
Page 37

Section 14-Archived Data and Data Viewer
Data Viewer
Data Viewer is a versatile data reporting software program incorporated into the NanoDrop software that offers the user the ability to
customize report structures, import stored data and re-plot data generated from NanoDrop instruments. Using the Data Viewer is the
most expedient method to review data. This feature may be accessed during measurement sessions from the Show Report function
found within each method module. It may also be accessed from the Main Menu page. An ND-1000 Spectrophotometer does not need
to be connected to the PC to use the Data Viewer module.
Data Viewer Features
The Data Viewer is composed of two or three pages in a tabular form consisting of Plots, Reports and Standard Curves (where utilized).
The user may access any page by clicking on the tabs.
The software opens to the Report page (see Plots section below) whether accessed through the Main Menu or Show Report. Note:
Recording rather than Start Report must be selected in order to access the Data Viewer via Show Report.
Tool Bar Features common to all three pages include:
Configuration: Options controlled by this tool bar function include ‘Auto Scale’, ‘Include graph in printout’ and ‘Include
standards in printout’.
Data: Includes options to import data, rename samples and delete sample data. Note: After deleting all samples, it is important
to exit out of the Data Viewer module and re-enter if importing data for a different application type.
Report: This tool bar function allows the user to select columns of interest to be included in a report. See section on Reports
Page for more detail.
File Page Set-up: Allows the user to define the page set up for printing out the spectra, the report and the standard curve.
Print Window: The current Plot, Report, or Standards screen may be printed by selecting ‘Print Window’ or ‘CTL +P’.
Save Window: Saves files as .JPGs.
Help/Context Help: This feature is enabled in the Main Menu, all function modules, and the application modules. The help
feature is enabled by choosing ‘Show Context Help’ from the Help menu pull down or by selecting ‘Ctrl H’. Once enabled,
placing the cursor on elements of the screen will automatically generate an explanation of that element. Context Help remains
active until deselected.
Import
To import samples for viewing, select “Import Samples” from the “Data” menu of either the Plots or Report pages within the Data Viewer
software. This will bring up a new window with an Import Folder box and a Directory Tree as shown belo w.
Features include:
Import folder: Used to select folder where data is to be imported from. Folder selection must be at the level of user or higher--
may not select an application or method folder within a user in this activity box.
Directory tree: Used to select specific data to be imported. Clicking on the square to the left of each file name will provide
further detail to each level. Users may choose to select either individual samples within a file or the entire file. All impor t
selections must be of the same application or method type. Note: Hi Abs data cannot be imported into the Data Viewer but can
be opened with Excel type spreadsheets.
Archive File Converter:
data will need to be copied and converted to 3.2 version compatible .ndj files. Refer to the Archive File Converter secti on
below for more details. Note: The original archive files will not be altered during this process.
ND-1000 data generated with earlier versions of NanoDrop software and not stored in c:\nanodrop
14-2
Page 38

Section 14-Archived Data and Data Viewer
>>> or <<<: Used to move the highlighted sample choices to or from the Selected Samples box. Note: The software defaults
to a buffer size of 200 samples.
Search: Function allows the user to locate specific data by searching through sample ID names.
Sample Information and Spectrum: Are populated with the information associated with the most recently highlighted sample.
Import and Return: Uses selected sample data to populate Plots and Reports windows. Note: Holding down the shift or control
PC function keys will allow the user to select multiple samples and/or files for importing. The keys can also be used to deselect
multiple samples. See example below:
Plots
The Plots page is where selected sample spectra are displayed.
Features include:
Method: Auto fills in module name.
Date:
Selected Plot:
Plots/Sets:
Auto fills in date and time of report.
There are two methods of selecting or highlighting individual sample data. The user ma y simpl y move the
cursor over the plot of interest and click or use the ‘Selected Plot’ drop down box (which will also display the legend). The
selected sample will show up as a bold plot line.
Users may select the maximum number of individual plots (up to 20) graphed per page. Since a report can hol d
data for many samples and a graph page is limited to 20 plots, additional sample spectra are displayed on new pages. Each
page is then referred to as a set.
Legend: Positioning the cursor over the legend box will bring up a visual display matching the sample name to a plot color.
The user is not able to select or highlight a sample from the legend.
Sample information: Automatically populates with data associated with selected sample. Data displayed is appropriate for
data type chosen. Note: Information is based on data collected at the time the sample was measured and is not mod ified by a
change in cursor position on the Data Viewer real time display.
Movable x and y axis
one of the outer limit numbers. The cursor absorbance information displayed at the bottom of the page is determined by the
position of the movable cursors. The movable X determines the baseline from which the peak of the Y position is calculated.
Reset Baseline will reposition the x axis back to zero.
: Available for all data types. If the cursor is out of view in either direction- rescale the axis by typing over
14-3
Page 39

Section 14-Archived Data and Data Viewer
Reports
The Reports page displays the data for selected samples in a table format. The user may modify colum n config urations for each
method type and save multiple customized formats.
Some key options useful for the Reports page are accessible through the Report tool bar drop down.
Choosing the ‘Configure Report’ option brings up the following box:
Selecting ‘OK’ will return the user to the reports page displaying only the columns of interest.
Features include:
Sort: Allows users to sort data by column (example-by date or sample name) and by either ascending or descendi ng order.
Save Report Format:
format as the default format, exit to the Main Menu, choose ‘Users Preferences’ and click ’Reports’. Use the “Select Default
Report Format’ to see the list of saved formats available for the specific method type.
Load Report Format
Print report:
pages by selecting these options under the Configuration drop down on the tool bar.
Will print out only the Report page by default. Users may choose whether or not to print out the standards or plots
Saves the current report format as an .ndf file for retrieval and future use. To designate a saved report
: Allows saved report formats to be loaded either before or after data is imported.
Save Report and Load Report: There are several options for this feature as seen in the following window:
14-4
Page 40

Section 14-Archived Data and Data Viewer
Using the Full Report option will allow the user to use the Data Viewer to reload the report at a later date. The saved report may be
recalled using the pull down Load Report. If using the Load Report feature- the report will be displayed with the default column
configuration. Note: Access the User Preferences module on the main menu to modify and save preferred default configurations.
Reports are saved in an .ndv format.
The other two options are meant for reports that are expected to be opened in Excel type spreadsheets. To open these reports, go to
the C:\NanoDrop Data\Reports folder and right click on the file of interest.
Additional features of the Report page:
Method: Automatically populated with data method type.
Date and time: Automatically populated when report is generated.
Report Name: User defined designation for the current report.
Buffer mode: Drop down box defining options for managing reports.
Max Buffer size:
Default number is set at 200.
When the selected buffer size is reached, the Report will either be ‘Saved & Cleared’ or ‘Printed and Cl eared’.
Standards Page
The Standards page will display the actual reference standards applied to each particular sample at the time of measurement. Note:
This page is available for software modules utilizing a Standard Curve.
Archive File Converter
All archive files located in the c:\Nanodrop Data folder generated with earlier versions of NanoDrop software will automatically be
copied and converted to 3.3 version-compatible .ndj files upon first use of the software. Archive files generated with earlier versions of
software and not stored in the folder c:\NanoDrop Data will need to be converted manually before reviewing with the Data Viewer. The
File Converter can be accessed from the Import Data page and the conversion can be done one file at a time or by converting an entire
file folder. Note: The original archive files are not altered during this process.
14-5
Page 41

Section 14-Archived Data and Data Viewer
Opening Archived Data with Spreadsheet Programs
The archived files are in tab-delimited format and can be opened in Microsoft Excel or an equivalent spreadsheet program.
14-6
Page 42

15. Troubleshooting
Error USB2000
Section 15- Troubleshooting
This error might appear upon software startup and usually indicates that the USB cable is not properly connected or th e software is not
loaded properly. To troubleshoot, do the following:
1. Confirm that the USB cable is connected to both the PC and the instrument.
Æ
2. If the cable is connected properly but the error persists, run the ‘USB Reset’ application located in Start
Æ
Utilities Æ USB Reset. (If USB Reset is not installed on your PC, you can download it from the ‘Downloads’ section of
Programs Æ NanoDrop
www.nanodrop.com).
3. Follow the onscreen instructions from USB Reset. After unplugging and then reconnecting the USB cable, the Found New
Hardware Wizard should start as shown below. (Windows XP SP2 operating system will ask to allow it to search the internet for the
proper software as shown- Select ‘No, not this time’). Follow the prompts for automatic installation of the software.
Intro Page: Windows XP- SP2
Other Windows Operating Systems
4. Try the NanoDrop software, if it works properly you are finished. If it does not operate properly, go to step 5.
5. Shutdown the NanoDrop software and open the USBView utility to confirm proper USB communication: Start
Æ
NanoDrop
Utilities Æ USBView. If USBView is not installed on your PC, you can download it from the ‘Downloads’ section of
Æ
Programs Æ
www.nanodrop.com.
6. Click on the ‘Device Connected’ as shown below. If more than one USB device is connected, view each of them. One of the
connected devices should give an “idVendor’”and “idProduct” as 0x2457 and 0x1002 respectively as shown below. If these are
present, the USB function of the instrument should be OK. If the “idVendor” and “idProduct” are different than below, or if no USB
device is present in the list, go to step 7.
7. Install the instrument on another PC to rule out a faulty USB hub/port on the original PC. Run USBVIEW on the 2
behavior is exhibited, then the instrument may need service. Contact NanoDrop Technologies or your distributor if this occurs.
nd
PC. If the same
15-1
Page 43

Connection Error
Section 15- Troubleshooting
This error occurs whenever the USB connection is disrupted while operating a software module. In most cases, selecting ‘Retry’ will
reconnect properly. Some possible causes and solutions are listed below:
Power management scheme on the PC:
If your PC is automatically going into standby or hibernate mode, the USB communication will be lost whenever it occurs and ‘Retry’ will
NOT reconnect the instrument. If this occurs, the USB cable will need to be disconnected/reconnected before selecting ‘Retry’.
Æ
You can confirm that the power management settings are correct by opening the Power Options Properties pag e by choosing Start
Control Panel
Æ
Power Options. The ‘System Standby’ and ‘System Hibernate’ should be set to ‘never’ for the ‘Plugged In’ column as
shown below.
Static Electricity Discharge
Discharge from the user to the instrument can be a problem in very dry environments. It may be necessary for the user to wear a
grounding strap to prevent the discharges from occurring.
Defective USB Port on PC
If your instrument operates properly most of the time, but the Connection Error appears intermittently, it could be caused by the USB
port on the PC. If this occurs, install the software and operate on another PC. If the error does not occur on the second PC, it may be
necessary to replace the USB card on the original PC.
Signal Error
This error occurs because no light or not enough light is reaching the detector. If the troubleshooting steps outlined in the message do
not fix the problem, perform an Intensity Check:
• Open the Utilities and Diagnostics module from the main menu.
15-2
Page 44

Section 15- Troubleshooting
• With the sampling arm down, select OK to initialize the spectrometer and then select ‘Intensity Check’. You should see a red
and black spectrum and a bias value greater than 65 as shown below. This indicates that the USB communication is normal,
the power supply is operational and the flashlamp is functioning.
If no spectra appear in the image, confirm that the power supply is firmly connected to the instrument and the plug is
connected to a working outlet. Next, confirm that the power supply is operating properly. To do this, connect the leads of a
volt-ohmmeter to the outlet of the supply. The voltage should be 12-20 Vdc, center positive.
If none of the troubleshooting steps above solve the problem, refer to the Technical Service section for getting help from your distributor
or NanoDrop Technologies.
Saturated Detector
This error message can occur when the software calculates too high integration times during initializati on. This is most likely due to a
dried sample left on the measurement surface after the last use of the instrument. Cleaning both the top and bottom pedestals with deionized water and exiting out of the software module to the main menu should alleviate the problem. It is not necessary to close the
software completely as each module is re-initialized when it is opened. If the error persists, contact NanoDrop Technologies or your
local distributor.
Liquid Column Breakage
This warning occurs when a possible problem with the column is detected. The software compares the long path and short path
absorbances and issues a warning to the user if the short path is not 20% of the long path absorba nce, wit hin a tolerance. The most
common explanation is that the column is not forming properly due to the pedestal being “unconditi oned”.
15-3
Page 45

Section 15- Troubleshooting
Proteins and solutions containing surfactants are known to “un-condition” the measurement pedestal surfaces so that the liquid column
does not form. “Re-condition the pedestal as follows:
• Apply 5-10 ul of ~0.5M of HCl to the bottom pedestal, lower the upper pedestal into the solution and let it sit for approximately 2
to 3 minutes. Wipe both pedestals with a clean lab wipe and repeat this using 5-10 ul of deionized water to wash away any
residue.
• Fold a cle an dry lab wipe over several times to increase its thickness. Press the lab wipe firmly down on the lower pedestal and
"buff/rub" very aggressively at least 50 times - the lab wipe will rip during this procedure and will have to be refolded several
times throughout the procedure. The upper pedestal may also be buffed but care should be taken not to put too much force on
the upper arm.
• Test the effectiveness of the re-conditioning by pipetting a 1-2ul sample of water onto the lower measurement pedestal and
making sure the water "beads up" on the pedestal surface
.
If the warning persists and the user visually confirms that the liquid column is forming, contact NanoDrop Technologies or your local
distributor.
Error: Code 8
This error code is most likely to occur if the Windows account that is currently logged into Windows does not have read and write
access to the folder c:\nanodrop data or one of its subfolders. See your PC administrator to make sure that all users of the NanoDrop
software operate with a Windows account that has the appropriate access.
Can’t Find LabView RunTime Engine…
This error message likely means that one or more of the software components have been removed or corrupted. If this occurs, reinstall
Labview Runtime engine by selecting Start Æ ProgramsÆ NanoDropÆUtilitiesÆ Runtime Installer.
the
Report Format Loading Error
This error occurs when the user does not have “Write” access to one of the report format files located at c:\nanodrop data\custom report
formats or the file has been moved from this folder. This error is similar to Error Code 8 (see above). Contact your PC administrator to
give all users Read and Write access to this folder. Replace the files if they have been removed. If the file cannot be located, reinstall
the software.
Other Software Error Messages
Can’t find file OOIDRV.INI…
This error occurs when trying to install the software without administrator privileges. Contact your system administrator to install the
software.
Source Error…
15-4
Page 46

Section 15- Troubleshooting
This error indicates that there is insufficient light getting through to make good absorbance measurements. Check that the sampling
arm is in the down position and the power is connected.
Error 9003
This error indicates that the monitor resolution is below the 1024x768 required. Check the computer settings. Be sure that the start
menu tool bar is set to the bottom and not along the side.
Low Detector Bias…
This occurs when the software has detected a problem with the detector. Contact NanoDrop Technologies or your dis tributor if you
encounter this error.
OOIDRV\ Timeout
This error occurs when a necessary file is deleted as a previous version of the software is uninstalled. Reinstall the newest software to
correct the problem.
EZUSB.SYS Cannot Be Found…
If this error message appears, do the following:
• Windows 2000: Type C:\WINNT\INF in the file path text box.
• Windows XP: Type C:\WINDOWS\INF in the file path text box.
This should allow the installation to complete successfully.
Driver X Configuration Failed- You Must Manually Edit the Registry
This error message (or others with similar wording) occurs when attempting to install the operating software on a comput er running
Windows 2000 or XP. It occurs because the user does not have the necessary authorization to install the software. Contact your
system administrator if this occurs.
Insufficient Memory…
This error message (or others with similar wording) occurs when attempting to install the operating software on a comput er that does
not have at least 40MB of free hard disk space.
No Printer Connected…
This error appears when attempting to print when a printer is not attached to the PC. It is non-fatal and will not cause the software to
shut down.
Sample Accuracy and Reproducibility
If you are obtaining results that seem inaccurate or not reproducible, it could be the result of sample or aliquot non-homogeneity or
liquid column breakage. It may be helpful to try the following to ensure representative results:
Make sure the sample surfaces are clean before starting the software module
A dirty sample pedestal on startup can cause erroneous absorbance readings (even neg ative val ues) and signal saturation. It is always
a good practice to clean the sample surfaces with de-ionized water to remove any dried sample that might be present.
Use a 1.5-2 ul sample size
Very strange results can occur when the liquid sample column is not completely formed during a measurement. While making a
measurement, visually confirm that the liquid column is formed. If necessary, try 1.5-2 ul samples to ensure the column is formed.
Also, proteins and solutions containing surfactants are known to “un-condition” the measurement pedestal surfaces so that the liquid
column does not form. If this occurs, “buff” the measurement pedestal surfaces by rubbing each with a dry laboratory wipe 15-20 times.
This will “re-condition” the surface allowing for the liquid sample column to form.
Heat DNA samples to 55 °C and vortex before measurement
Due to the small volumes required by the ND-1000, it is extremely important to ensure that the sample being measured is
homogeneous. Field experience has shown that samples containing large molecu les such as genomic or lambda DNA are particularly
susceptible to this phenomenon. Note: Larger volumes used by cuvette-based spectrophotometers will minimize or mask the effect of
sample non-homogeneity.
Perform a Blanking Cycle
This will confirm that the instrument is working well and that any sample carryover from previous measurements is not a concern. To
run a blanking cycle, perform the following:
Open the application software module.
Load an aliquot of the blank (the buffer, solvent, or carrier liquid used with your samples) onto the lower measurement pedestal
and lower the sampling arm into the ‘down’ position.
Click on the ‘Blank’ (F3) button to store the blank reference.
15-5
Page 47

Section 15- Troubleshooting
Analyze a fresh replicate of the blanking solution as though it were a sample by selectin g ‘Measure’ (F1). The result should be
a spectrum that varies no more than 0.050 A (10mm absorbance equivalent).
Wipe the blank from both measurement pedestal surfaces with a laboratory wipe and repeat the process until the spectrum is
within 0.005 A (1mm path).
Confirm that reference (blank) solution and solvent are the same material
Buffers often absorb in the UV range and therefore it is critical to blank the instrument with exactly the same material that the sample is
suspended in.
Confirm that your sample is not too dilute
Measuring samples at or near the detection limit will result in measurements that can vary a significant amount. Refer to the
‘Measurement Concentration Range’ of the application module that you are using for the applicable measurement range.
Confirm instrument accuracy and reproducibility with CF-1
This is a potassium dichromate calibration standard available from NanoDr op Technologies and its distributors. It is a good practice to
check the instrument’s performance every six months with a fresh vial of CF-1.
260/280 Ratio
Many researchers encounter a consistent 260/280 ratio change when switching from a standard cuvette spectrophotometer to the
NanoDrop
®
ND-1000 spectrophotometer. The three main causes for this are listed below:
Change in sample acidity
Small changes in solution pH will cause the 260:280 to vary
basic solution will over-represent the ratio by 0.2-0.3. If comparing the NanoDrop
**
. Acidic solutions will under-represent the 260:280 ratio by 0.2-0.3, while a
®
ND-1000 Spectrophotometer to other
spectrophotometers, it is important to ensure that the pH of an undiluted sample measured on the ND-1000 is at the same pH as the
diluted sample measured on the second spectrophotometer.
**
William W. Wilfinger, Karol Mackey, and Piotr Chomczynski, Effect of pH and Ionic Strength on the Spectrophotometric Assessment of Nucleic Acid
Purity: BioTechniques 22:474-481 (March 1997)
Wavelength accuracy of the spectrophotometers
Although the absorbance of a nucleic acid at 260nm is generally on a plateau, the absorbance curve at 280nm is quite steeply sloped.
A slight shift in wavelength accuracy will have a large effect on 260:280 ratios. For example, a +/- 1 nm shift in wavelength accuracy
will result in a +/- 0.2 change in the 260:280 ratio. Since many spectrophotometers claim a 1 nm accuracy specification, it is possible to
see as much as a 0.4 difference in the 260:280 ratio when measuring the same nucleic acid sample on two spectrophotometers that are
both within wavelength accuracy specification.
Nucleotide mix in your sample
The five nucleotides that comprise DNA and RNA exhibit widely varying 260:280 ratios
**
. The following represent the 260:280 ratios
estimated for each nucleotide if measured independently:
Guanine: 1.15
Adenine: 4.50
Cytosine: 1.51
Uracil: 4.00
Thymine: 1.47
The resultant 260:280 ratio for the nucleic acid being studied will be approximately equal to the weighted average of the 260:280 ratios
for the four nucleotides present. It is important to note that the generally accepted ratios of 1.8 and 2.0 for DNA and RNA are “rules of
thumb”. The actual ratio will depend on the composition of the nucleic acid. Note: RNA will typically have a higher 260:280 ratio due
to the higher ratio of Uracil compared to that of Thymine.
***
Leninger, A. L. Biochemistry, 2nd ed., Worth Publishers, New York, 1975
Unusual Spectrum
A sample that exhibits jagged “cuts” out of the spectrum, but an otherwise normal shape, may be the result of detector saturation. This
can be caused by the software selecting too high of an integration time due to a dirty sample pedestal upon startup. Try cleaning both
sample pedestals thoroughly and restarting the software. For reference, examples of spectra generated wit h a saturated detector are
shown below.
15-6
Page 48

Section 15- Troubleshooting
Detector saturation- nucleic acid
measurement
Detector saturation- Bradford
measurement
A spectrum that is very “un-smooth” or “ragged” can be caused by insufficient light intensity reaching the spectrometer. If you suspect
that this is occurring, refer to the “Technical Service” section for instructions on how to contact NanoDrop Technologies and what
information must be sent to allow for diagnosing the problem.
Technical Service
If after referring to the above troubleshooting tips you are unable to resolve your problem, please contact your local distributor or
NanoDrop Technologies at
info@nanodrop.com for help. The following information will be very helpful:
JPG image of Utilities and Diagnostics module
To get this, open this module and select ‘OK’ to initialize the module. Select ‘Intensity Check’. Once the spectrum has been created,
choose File Æ Save Window as shown below. Save to your hard drive and email as an attachment to your distri butor or to
info@nanodrop.com.
Application Module Screen Captures
Screen captures of the actual spectrum as seen on your PC are of great use in diagnosing problems. Making a screen capture is quite
easy. When in an application module, press ‘Alt+Print Screen’. This copies the highlighted screen window to the PC’s clipboard. Next,
paste this screen capture into MS Word, MS Paint (this program usually comes standard with the PC and can usually be found in the
Start Æ Accessories menu), or other graphics programs. Save this as a .jpg or .doc file and send as email attachment to your
distributor or to
info@nanodrop.com.
Data Archive Files
If you have questions about your data, please send the archive file containing the suspect data as an email attachment to your
distributor or to
Protein, Bradford, Cell Culture, Protein Lowry, Proteins and Labels, MicroArray, Nucleic Acid, Protein A-280, UV-Vis or UV-Vis HiAbs).
info@nanodrop.com. The archived file can be found at C:\NanoDrop Data Æ User name Æ Application Module (BCA
15-7
Page 49

Section 16- Maintenance and Warranty
16. Maintenance and Warranty
Cleaning
The primary maintenance requirement of the NanoDrop
clean. Upon completion of each sample measurement, wipe the sample from the upper and lower pedestals to prevent sample
carryover and avoid residue buildup. It is also recommended that both measurement pedestals be cleaned with de-ionized water upon
completion of a testing series.
Calibration
Wavelength
The wavelength is auto-calibrated based on known peaks in the xenon lamp spectra each time the software is started. No calibr ation is
required by the user.
Pathlength (Accuracy)
Generally, this is not required as field experience has shown that the path does not change appreciably ev en after several years of
heavy use. However, it is good practice to check the calibration every six months using CF -1 calibration fluid. A calibration check
procedure is available from the Downloads section on the Support tab at
Parts That Require Replacement
In general, the only part that should periodically require replacement is the flash lamp. The flash lamp should last a minimum of 30K
measurements. There is no way to test the flash lamp to confirm its remaining life. When the flash lamp fails, the light output will
become very erratic or stop altogether.
Warranty
All spectrophotometers and accessories manufactured by NanoDrop Technologies are warranted against manufacturing defects in
parts and labor for a period of one year. Preventive Maintenance as well as addition al one, two, and three year warranty extensions are
available. Plan descriptions can be found at the following link:
www.nanodrop.com.
®
ND-1000 Spectrophotometer is keeping the measurement pedestal surfaces
www.nanodrop.com.
16-1
Page 50

Section 17- Appendices
17. Appendices
Instrument Specifications
• Sample Size: 1microliter
• Path Length: 1 mm (with auto-ranging to 0.2 mm)
• Light Source: Xenon flash lamp
• Detector Type: 2048-element linear silicon CCD array
• Wavelength Range: 220-750 nm
• Wavelength Accuracy: 1 nm
• Wavelength Resolution: 3 nm (FWHM at Hg 546 nm)
• Absorbance Precision: 0.003 absorbance ( 1mm path)
• Absorbance Accuracy: 2% (at 0.76 absorbance at 257 nm)
• Absorbance Range: 0.02-75 (10 mm equivalent absorbance)
• Detection Limit: 2 ng/microliter (dsDNA)
• Maximum Concentration: 3700 ng/microliter (dsDNA)
• Measurement Cycle Time: 10 seconds
• Dimensions: 20 cm X 15 cm x 12 cm
• Weight: 3 kg
• Sample Pedestal Material of Construction: 303 stainless steel and quartz fiber
• Operating Voltage: 12 Vdc
• Operating Power Consumption: 6 W
• Standby Power Consumption: 1.5 W
• CE Approval: Units sold in Europe, Australia and New Zealand
• UL/CSA Approval: Units sold in N. America, S. America, Asia and Africa
• Included in system: software, compatible with Windows 2000 or XP
Blanking and Absorbance Calculations
When the NanoDrop
memory as an array of light intensities by wavelength. When a measurement of a sample is taken, the intensity of light that has
transmitted through the sample is recorded. The sample intensities along with the blank intensities are used to calculate the sample
absorbance according to the following equation:
®
ND-1000 Spectrophotometer is “blanked”, a spectrum is taken of a reference material (blank) and stored in
Absorbance = -log (Intensity
sample
/Intensity
blank
)
Thus, the measured light intensity of both the sample and of the blank are required to calculate the absorbance at a given wavelength.
Concentration Calculation (Beer’s Law)
General
The Beer-Lambert equation is used to correlate the calculated absorbance with concentration:
A = E * b * c
Where A is the absorbance represented in absorbance units (A), E is the wavelength-depende nt molar absorptivity coefficient (or
extinction coefficient) with units of liter/mol-cm, b is the path length in cm, and c is the analyte concentration in moles/liter or molarity
(M).
Fluorescent Dyes (Microarray Measurement)
The NanoDrop software uses the general form of the Beer-Lambert equation to calculate fluorescent dye concentrations in the
Microarray module. The table of extinction coefficients for each dye is below:
Dye Type
Cy3 150000 550
Cy5 250000 650
Alexa Fluor 488 71000 495
Alexa Fluor 546 104000 556
Alexa Fluor 555 150000 555
Alexa Fluor 594 73000 590
Alexa Fluor 647 239000 650
Alexa Fluor 660 132000 663
Cy3.5 150000 581
Cy5.5 250000 675
Nucleic Acids
Extinction
Coefficient
(liter/mol-cm)
Measurement
Wavelength
(nm)
17-1
Page 51

Section 17- Appendices
For nucleic acid quantification, the Beer-Lambert equation is modified to use an extinction coefficient with units of ng-cm/ml. Using this
extinction coefficient gives a manipulated equation:
c = (A * e)/b
Where c is the nucleic acid concentration in ng/microliter, A is the absorbance in AU, e is the wavelength-dependent extinction
coefficient in ng-cm/microliter and b is the path length in cm.
The generally accepted extinction coefficients for nucleic acids are:
• Double-stranded DNA: 50 ng-cm/ul
• Single-stranded DNA: 33 ng-cm/ul
• RNA: 40 ng-cm/ul
For the NanoDrop
spectrophotometer using a 10.0 mm path. Thus, the NanoDrop
are 50 times more concentrated than can be measured in a standard spectrophotometer.
Note: Absorbance data shown in archive files are represented as displayed on the software screen. For Nucleic Acid, Protein A280 and
Proteins and Labels modules, data are normalized to a 1.0 cm (10.0 mm) path. For MicroArray, UV-Vis, Protein BCA, Protein Bradford,
Protein Lowry and Cell Culture modules the data are normalized to a 0.1 cm (1.0 mm) path. For high absorbance UV-Vis samples, data
are normalized to a 0.1mm path.
Solvent Compatibility
The NanoDrop
methanol, ethanol, n-propanol, isopropanol, butanol, acetone, ether, chloroform, carbon tetrachlorid e, DMSO, DMF, Acetonitrile, THF,
toluene, hexane, benzene, sodium hydroxide, sodium hypochlorite (bleach), dilute HCl, dilute HNO3, dilute acetic acid.
All forms of Hydrofluoric Acid (HF) are incompatible, as the fluoride ion will
dissolve the quartz fiber optic cable.
Decontamination of Measurement & Optical Surfaces
If decontamination is necessary, a sanitizing solution, such as a 5.25% solution of sodium hypochlorite (bleach – freshl y prepared), can
be used to ensure that no biologically active material is present on the measurement pede s tals. T he metal fiber optic fittings are made
from 303 stainless steel and are resistant to most common laboratory solvents (see “Solvent Compatibility” appendix).
Setting Up a Dymo 400 Label Writer Printer
To set DYMO default label to print with ND-1000:
1. Open Control Panel, then Printers and Faxes
2. Right click on Dymo printer then select Properties
3. Under General tab, choose ‘Printing Prefere nces’ then ‘Advanced’. Then set the following:
• Paper Size: 30256
• Halftoning: Super Cell
• Print Quality: Barcodes and Graphics
4. Click ‘OK’ to close this window then click ‘Apply’
5. Next, click on the ‘Advanced’ tab. Ensure that ‘Enable advanced printing features’ box is checked. Then click on ‘Printing
Defaults’ then ‘Advanced’. Then set the following:
• Paper Size: 30256
• Halftoning: Super Cell
• Print Quality: Barcodes and Graphics
6. Click ‘OK’ to close this window then click ‘Apply’
7. Last, click on the ‘Device Settings’ tab an d ensure 30256 Shipping Label is selected as the ‘Default’ label.
®
ND-1000 Spectrophotometer, path lengths of 1.0 mm and 0.2 mm are used compared to a standard
®
ND-1000 Spectrophotometer is compatible with most solvents typically used in life science laboratories. These include:
®
ND-1000 Spectrophotometer is capable of measuring samples that
17-2
 Loading...
Loading...