Thermo Fisher Scientific SureTect User Manual

SureTect™ Campylobacter jejuni, C. coli and
C. lari PCR Assay
USER GUIDE
Lysis and real-time PCR detection of Campylobacter jejuni,
C. coli, and C. lari in food and environmental samples
for use with:
Applied Biosystems™ QuantStudio™ 5 Food Safety Real-Time PCR Instrument
with Thermo Scientific™ RapidFinder™ Analysis Software v1.1 or later
Applied Biosystems™ 7500 Fast Food Safety Real-Time PCR Instrument with
Applied Biosystems™ RapidFinder™ Express Software v2.0 or later
Catalog Number A44251
Publication Number MAN0018692
Revision B.0
For testing of Food and Environmental samples only.
The information in this guide is subject to change without notice.
DISCLAIMER: TO THE EXTENT ALLOWED BY LAW, THERMO FISHER SCIENTIFIC INC. AND/OR ITS AFFILIATE(S) WILL NOT BE
LIABLE FOR SPECIAL, INCIDENTAL, INDIRECT, PUNITIVE, MULTIPLE, OR CONSEQUENTIAL DAMAGES IN CONNECTION WITH OR
ARISING FROM THIS DOCUMENT, INCLUDING YOUR USE OF IT.
Revision history: Pub. No. MAN0018692
Revision Date Description
B.0 5 February 2021 Update of the instrument software versioning.
A.0 29 January 2020 New document
Trademarks: All trademarks are the property of Thermo Fisher Scientific and its subsidiaries unless otherwise specified. AOAC is a
trademark and Performance Tested Methods is a service mark of AOAC INTERNATIONAL. Windows is a trademark of Microsoft.
TaqMan is a registered trademark of Roche Molecular Systems, Inc., used under permission and license.
©2021 Thermo Fisher Scientific Inc. All rights reserved.

Contents
■
CHAPTER 1 Product information .................................................. 5
Product description ............................................................. 5
Name and intended use ..................................................... 5
Principle of the test ......................................................... 5
Procedure overview ......................................................... 6
Limitations ................................................................. 6
Contents and storage ............................................................ 6
Required materials .............................................................. 7
Materials for enrichment ..................................................... 7
Materials for lysis ........................................................... 8
Materials for PCR .......................................................... 9
Materials for confirmation testing ............................................ 10
Workflows .................................................................... 10
■
CHAPTER 2 Before you begin .................................................... 11
Procedural guidelines ........................................................... 11
Guidelines for sample enrichment ............................................ 11
Guidelines for sample lysis .................................................. 11
Guidelines for PCR ........................................................ 11
■
CHAPTER 3 Enrich food samples ................................................ 13
Enrich food samples ............................................................ 13
■
CHAPTER 4 Prepare the lysate ................................................... 15
Prepare the lysate using the thermal cycler method ................................ 15
SureTect
™
Campylobacter jejuni, C. coli and C. lari PCR Assay User Guide—AOAC
3

Contents
■
■
CHAPTER 5 Perform PCR ........................................................ 17
PCR with the QuantStudio™ 5 Instrument and RapidFinder™ Analysis Software
v1.1 or later ................................................................. 17
Set up the plate layout in RapidFinder™ Analysis Software ...................... 17
Set up the PCR reactions ................................................... 17
Load and run the reactions .................................................. 18
View results and data analysis ............................................... 18
PCR with the 7500 Fast Instrument and RapidFinder™ Express Software v2.0 or later ... 20
Set up the plate layout ..................................................... 20
Set up the PCR reactions ................................................... 20
Load and run the reactions .................................................. 21
View results and data analysis ............................................... 22
CHAPTER 6 Confirm positive results ............................................ 23
Recommended confirmation methods ........................................... 23
Isolate presumptive positives .................................................... 23
Confirmation in case of co-infection .............................................. 23
■
APPENDIX A Troubleshooting .................................................... 24
Test control organisms .......................................................... 26
RapidFinder™ Express Software results warnings .................................. 26
■
APPENDIX B Supplemental information ........................................ 27
AOAC Performance Tested MethodsSM Certification ................................. 27
Good laboratory practices for PCR ............................................... 28
Symbol definitions ............................................................. 30
■
APPENDIX C Safety ............................................................... 31
Chemical safety ................................................................ 32
Biological hazard safety ......................................................... 33
■
Documentation and support ....................................................... 34
Food Safety support ............................................................ 34
Related documentation ......................................................... 34
References ................................................................................ 35
4
SureTect™ Campylobacter jejuni, C. coli and C. lari PCR Assay User Guide—AOAC
1
IMPORTANT! Before using this product, read and understand the information in the “Safety” appendix
in this document.
Product description
Name and intended use
The Thermo Scientific™ SureTect™ Campylobacter jejuni, C. coli and C. lari PCR Assay enables real-time
PCR detection of Campylobacter jejuni, C. coli, and C. lari from food and environmental samples. This
kit is for use in laboratories undertaking microbiological analysis, and they are compatible with the
following instruments and software:
Product information
PCR instrument
Applied Biosystems
QuantStudio™ 5 Food Safety RealTime PCR Instrument
Applied Biosystems™ 7500 Fast
Food Safety Real-Time PCR
Instrument
[1]
or equivalents manufactured by Thermo Fisher Scientific and/or subsidiaries.
[2]
Assay files and instructions are available at thermofisher.com/molecular-microbiology-software.
[1]
™
Thermo Scientific™ RapidFinder
Analysis Software v1.1 or later
Applied Biosystems™ RapidFinder
Express Software v2.0 or later
Software Pathogen Assay File
™
™
Principle of the test
This assay is based on TaqMan™ PCR technology. Dye-labeled probes target unique DNA sequences
specific to Campylobacter jejuni, C. coli, and C. lari, and an internal positive control (IPC). Target DNA,
if present, is detected by real-time PCR. Analysis software provides interpretation of results. For more
information about real-time PCR, go to thermofisher.com/qpcreducation.
The IPC template, primers, and probe provide an internal control with each reaction to show that the
PCR process has occurred. It is unnecessary to incorporate positive control organisms with routine
testing of samples.
Campylobacter Multiplex SureTect QS5
version 1.0 or later
C.jejuni Multiplex SureTect 1.0 or later
[2]
[2]
SureTect™ Campylobacter jejuni, C. coli and C. lari PCR Assay User Guide—AOAC
5
Chapter 1 Product information
1
Contents and storage
Procedure overview
Enriched food or environmental samples are combined directly with ready-to-use Lysis Reagent 1 and
Proteinase K, to lyse bacterial cells present in the sample and release their DNA into solution.
Lysates are transferred to the SureTect™ Campylobacter jejuni, C. coli and C. lari PCR Tubes to
rehydrate the lyophilized PCR pellets. The pellets contain lyophilized target-specific primers, dyelabelled probes, and PCR master mix components. The PCR tubes are sealed, loaded into the real-time
PCR instrument, then the run is started using the RapidFinder™ software. After the run is complete,
the software displays the interpreted results as simple positive or negative symbols. The results can be
reported, stored, printed, and downloaded as required.
Results are achieved approximately 80 minutes after loading the prepared sample into the instrument.
Limitations
•
The test is designed to detect DNA from target organisms that have been present at a minimum
level of 1 CFU/sample, and have grown to detectable levels during the enrichment.
•
The customer is responsible for validation of sample matrices or culture media not described in this
document.
•
When testing a sample type or culture medium that has not been validated, we recommend testing
a selection of known negative and positive samples, to ensure that expected results are achieved.
See “Test control organisms” on page 26 and EN ISO 22174:2005.
•
See Appendix A, “Troubleshooting” for additional information.
Contents and storage
Store the kit protected from light, at 2–8°C. Bring to room temperature before opening.
Table 1 SureTect™ Campylobacter jejuni, C. coli and C. lari PCR Assay, 96 tests (Cat. No. A44251)
Contents
Lysis Reagent 1 Tubes (clear, pale blue liquid containing fine white
particles)
Lysis Tube Caps, domed 12 strips of 8 caps
Proteinase K (clear colorless liquid) 1 tube
SureTect™ Campylobacter jejuni, C. coli and C. lari PCR Tubes 12 strips of 8 tubes
PCR Caps 12 strips of 8 caps
Amount
12 strips of 8 tubes
1 pellet each
6
SureTect™ Campylobacter jejuni, C. coli and C. lari PCR Assay User Guide—AOAC
Required materials
Unless otherwise indicated, all materials are available through the Thermo Fisher Microbiology ordering
process or thermofisher.com. MLS: Fisher Scientific (fisherscientific.com) or other major laboratory
supplier.
Note: Parts may ship separately depending on configuration and storage conditions.
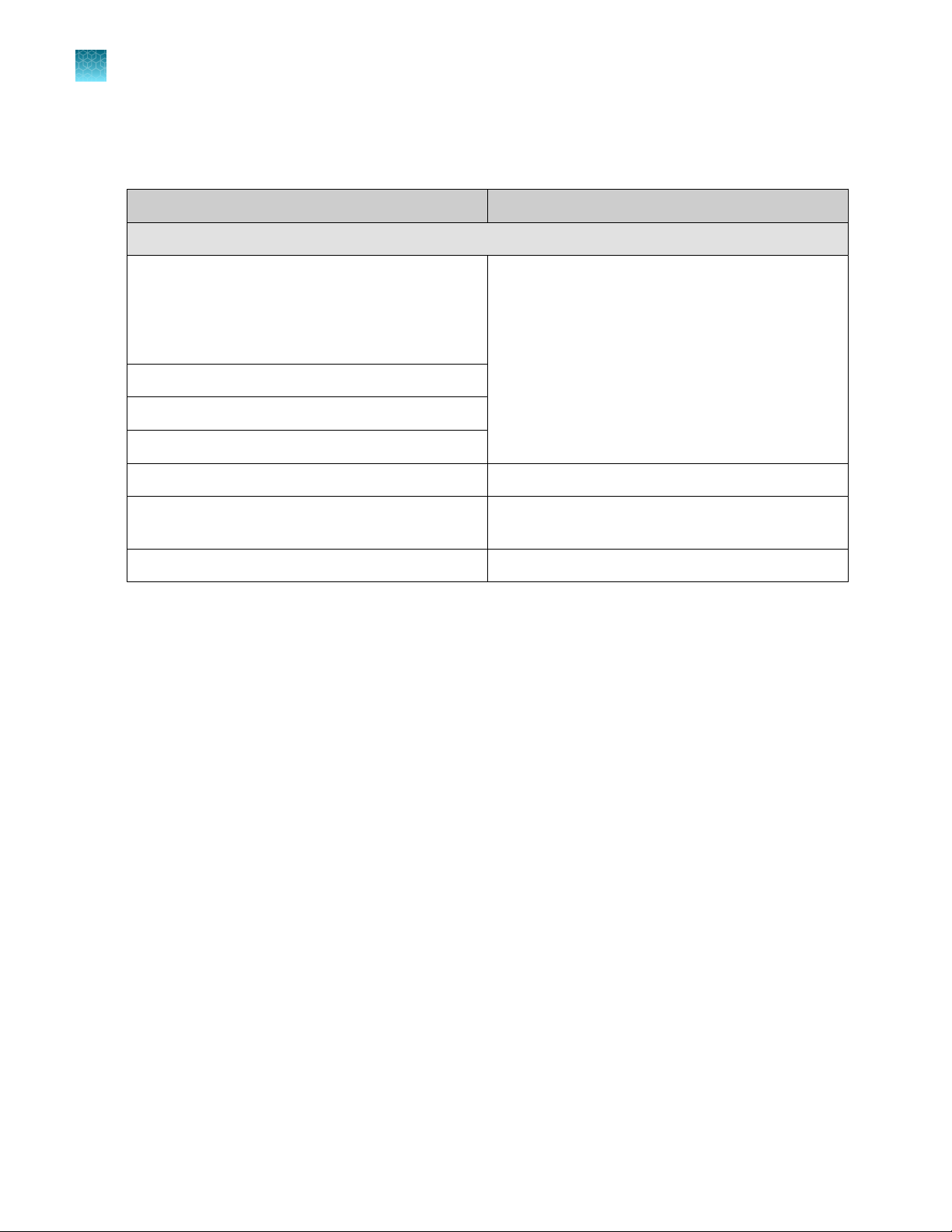
Materials for enrichment
Table 2 Equipment, accessories, and consumables
Item Source
Chapter 1 Product information
Required materials
1
Homogenizer Laboratory Blender or Dilutor, one of
the following, or equivalent
Homogenizer bags appropriate for the sample type
and size
Incubator fitted with racks for homogenizer bags, set
to 42±1°C
Disposable gloves MLS
Variable volume single-channel pipette, 1‑ to 10‑mL
96-well rack
Filtered pipette tips, 1‑ to 10‑mL
Sample tubes, 1.5‑mL
Available through the Thermo Fisher Microbiology
For DB4100A or DB4150A:
DB5000A
DB4100A
DB4150A
DB4011A
DB4012A
DB4013A
DB4014A
thermofisher.com
ordering process.
Table 3 Media
Item
Bolton Broth, 500 g of base CM0983B
Oxoid™ Modied Bolton Broth Selective Supplement SR0208E
Buered Peptone Water (BPW) MLS
SureTect™ Campylobacter jejuni, C. coli and C. lari PCR Assay User Guide—AOAC
Source
7
Chapter 1 Product information
1
Required materials
Materials for lysis
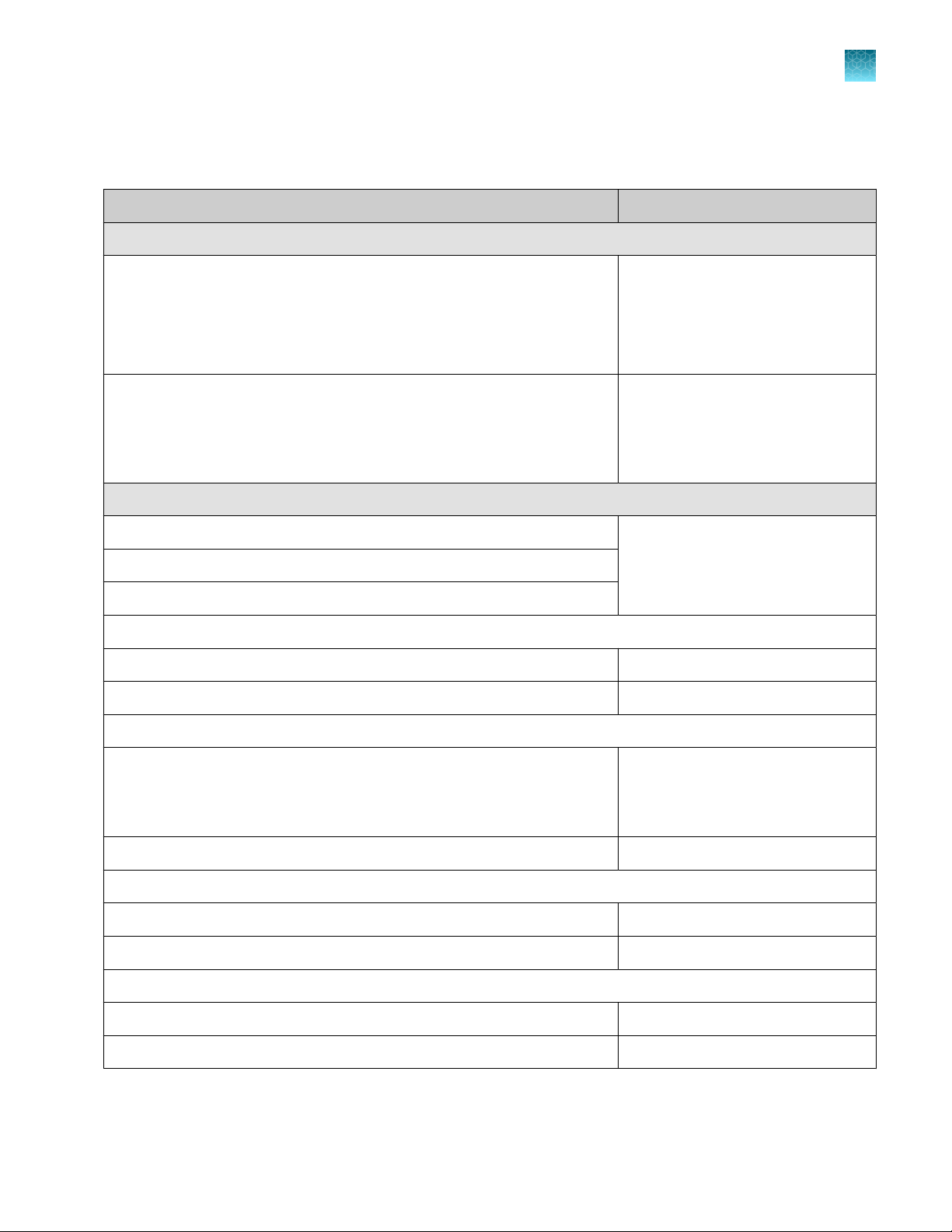
Table 4 Materials for lysis of enriched cultures
Plastics, consumables, and reagents
Single-channel pipette, 10‑ to 100‑µL
or
Electronic adjustable spacing, multichannel pipette,
10‑ to 100‑µL
Single-channel stepper pipette, 10‑ to 100‑µL
Filtered pipette tips, 10‑ to 100‑µL
Tool for capping and decapping
Applied Biosystems™ SimpliAmp™ Thermal Cycler A24811
MicroAmp™ 96-Well Tray/Retainer Set for Veriti
Systems
Item Source
Available through the Thermo Fisher Microbiology
ordering process.
™
4381850
MicroAmp™ Splash-Free 96-Well Base 4312063
8
SureTect™ Campylobacter jejuni, C. coli and C. lari PCR Assay User Guide—AOAC
Materials for PCR
Table 5 Materials for PCR
Chapter 1
Product information
Required materials
Item Source
1
Real-time PCR instrument and accessories, one of the following instrument packages
QuantStudio™ 5 Food Safety Real-Time PCR Instrument, 0.1-mL block, with
RapidFinder™ Analysis Software v1.1 or later
For use with SureTect™ Campylobacter jejuni, C. coli and C. lari PCR
Assay and Pathogen Assay File: Campylobacter Multiplex SureTect QS5
Contact your local microbiology
version 1.0 or later
7500 Fast Food Safety Real-Time PCR Instrument with RapidFinder
™
Express Software v2.0 or later
For use with SureTect™ Campylobacter jejuni, C. coli and C. lari PCR Assay
and Pathogen Assay File: C.jejuni Multiplex SureTect 1.0 or later
Contact your local microbiology
[1]
A36320 (desktop)
A36328 (laptop)
sales representative
A30304 (desktop)
A30299 (laptop)
sales representative
Additional materials for PCR
Vortex mixer
8-channel pipette, 10‑ to 100‑µL
Filtered pipette tips, 10‑ to 100‑µL
Available through the Thermo Fisher
Microbiology ordering process. See
thermofisher.com/plastics for more
information.
For the QuantStudio™ 5 Food Safety Real-Time PCR Instrument
MicroAmp™ 96-Well Tray for VeriFlex™ Block 4379983
MicroAmp™ Splash-Free 96-Well Base 4312063
For the 7500 Fast Food Safety Real-Time PCR Instrument
Precision Plate holder for SureTect™ assays
or
7500 Fast Precision Plate Holder, for 0.1 mL tube strips
PT0690
or
A29252
PCR Carry plate for SureTect™ assays PT0695
If using Precision Plate holder for SureTect™ assays (Cat. No. PT0690):
VersiPlate PCR Strip Tube Plate, 96-well, low profile
Ultra Clear qPCR Caps, strips of 8
[2]
[2]
AB1800
AB0866
If using 7500 Fast Precision Plate Holder, for 0.1 mL tube strips (Cat. No. A29252):
MicroAmp™ Fast 8-Tube Strip, 0.1 mL
MicroAmp™ Optical 8-Cap Strips
[1]
or equivalents manufactured by Thermo Fisher Scientific and/or subsidaries.
[2]
Used for balancing.
[3]
Required to balance the lid pressure if less than 2 full strips are processed.
[3]
[3]
4358293
4323032
SureTect™ Campylobacter jejuni, C. coli and C. lari PCR Assay User Guide—AOAC
9
Chapter 1 Product information
1
Workflows
Materials for confirmation testing
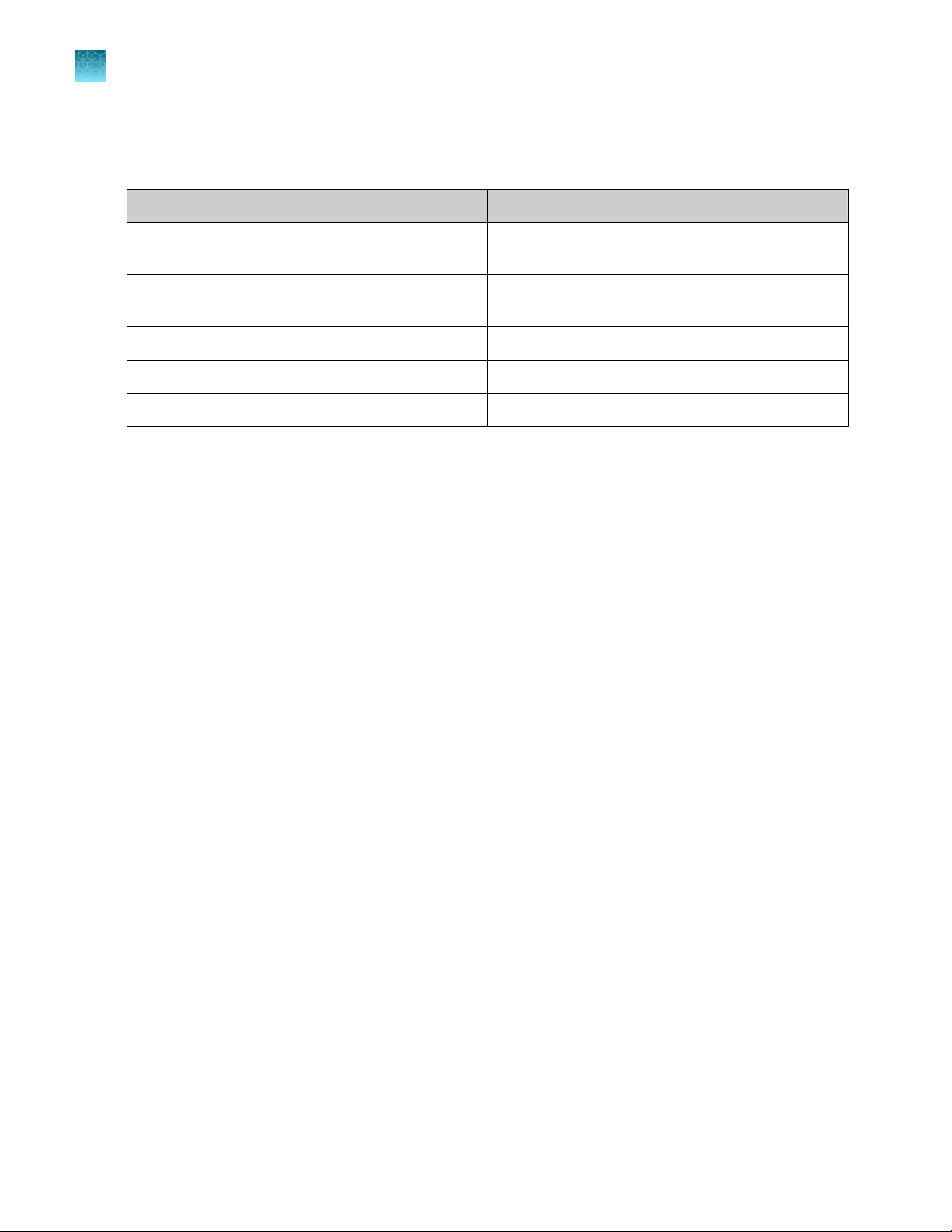
Table 6 Materials for confirmation of positive results
Item Source
Oxoid™ Campylobacter Blood-Free Selective Agar
Base (Dehydrated), 500 g
Oxoid™ CCDA Selective Supplement, 10 freeze-dried
vials
Remel™ Campy Cefex Agar R110138 (USA)
Brilliance™ CampyCount Agar R110168, PO1185A (Europe)
Oxoid™ O.B.I.S. campy Kit ID0800M
Workflows
“PCR with the QuantStudio™ 5 Instrument
and RapidFinder™ Analysis Software v1.1 or
Enrich food samples (page 13)
▼
Prepare the lysate using the thermal cycler method (page 15)
▼
PCR with the 7500 Fast Instrument and
RapidFinder™ Express Software v2.0 or later
later” on page 17
CM0739B
SR0155E
(page 20)
10
▼
Confirm positive results (page 23)
SureTect™ Campylobacter jejuni, C. coli and C. lari PCR Assay User Guide—AOAC

2
Procedural guidelines
Guidelines for sample enrichment
•
For preparation of master suspensions, follow the instructions of EN ISO 6887 and EN ISO 16654
standards. Comply with Good Laboratory Practices (refer to EN ISO 7218 standard).
•
Follow the manufacturer's instructions for preparation of culture media.
•
Use non-filtered homogenizer bags to help with fat and particle separation.
•
For consistent PCR results, use a ventilated incubator.
•
Follow the specified temperature allowances.
•
Dispose of all inoculated culture media as hazardous microbiological waste, even if shown to be
negative for the target organism, according to local guidelines.
Before you begin
Guidelines for sample lysis
•
For downstream PCR on the 7500 Fast instrument or the QuantStudio™ 5 Instrument —Prepare
a mock-purified sample using sterile enrichment media as a negative extraction control. (The
negative extraction control is required for RapidFinder™ Express Software; it is optional but
recommended for RapidFinder™ Analysis Software.)
Add the enriched sample or negative extraction control to the bottom of the lysis tube.
•
For the thermal cycler method — To prevent crushing tubes, use the tray only from the
MicroAmp™ 96-Well Tray/Retainer Set provided with the SimpliAmp™ Thermal Cycler. See the
SimpliAmp™ Thermal Cycler User Guide (Pub. No. MAN0009889). Alternatively, use at least 4
complete tube strips in the heat block. We recommend spacing the strips evenly across the heat
block. If needed, add empty SureTect™ tubes to make 4 complete strips.
Guidelines for PCR
•
IMPORTANT! After the lysate has been added to the pellets, ensure that the pellet rehydrates
immediately by tapping the tubes on the lab bench. Start the PCR run within 30 minutes.
•
Tube and cap strips can be cut when less than a full strip is required.
Do not cut the strips of caps or tubes too close to the wall of the tube or the cap lid, otherwise the
lid might not seal adequately during PCR.
•
After the PCR tubes have been opened, add lysate within 10 minutes.
SureTect™ Campylobacter jejuni, C. coli and C. lari PCR Assay User Guide—AOAC
11
 Loading...
Loading...