
QUICK REFERENCE
PrepSEQ™ Residual DNA Sample Preparation Kit
For (Genomic DNA) CHO, E. coli, HEK293, Human, MDCK, NSO, Pichia, Sf9 and Baculovirus, and Vero
For (Plasmid DNA) Kanamycin resistance
Catalog Numbers 4402085, 4458435, A46014, A26366, 4464335, 4458441, 4464336, A46066, A41797, A50337
Pub. No. 4469839 Rev. G
Note: For safety and biohazard guidelines, see the “Safety” appendix in the PrepSEQ™ Sample Preparation Kits User Guide
(Pub. No. 4469838). Read the Safety Data Sheets (SDSs) and follow the handling instructions. Wear appropriate protective eyewear,
clothing, and gloves.
Prepare the reagents: before first use of the kit .................................................................. 1
■
Prepare reagents: before each use of the kit ..................................................................... 1
■
Manual protocol for DNA/RNA extraction ....................................................................... 2
■
Automated protocol for DNA/RNA extraction .................................................................... 4
■
Limited product warranty .................................................................................... 5
■
Prepare the reagents: before first use of the kit
Magnetic beads
1. Set a block heater to 37°C.
2. Incubate the Magnetic Particle suspension at 37°C for a minimum of 10 minutes with intermittent vortexing at setting #7, or until the
particles are completely suspended.
Binding Solution
1. Add 30 mL of 100% isopropanol to the Binding Solution bottle.
2. Label the bottle to indicate that it contains isopropanol, then store the bottle at ambient temperature.
Wash Buer Concentrate
1. Add 74 mL of 95% ethanol to one bottle of PrepSEQ™ Wash Buer Concentrate, then mix completely.
2. Label the bottle to indicate that it contains ethanol, then store the bottle at room temperature.
Prepare reagents: before each use of the kit
Proteinase K (PK) mix
• Use Proteinase K (PK) Buer II for new manual protocols and automated protocols.
Note: Proteinase K (PK) Buer is also provided in the kit for use with existing manual protocols that have been internally validated
with this buer.
• Prepare a fresh mix before each use of the kit.
• Include a 10% overage to account for pipetting losses.
For Research Use Only. Not for use in diagnostic procedures.
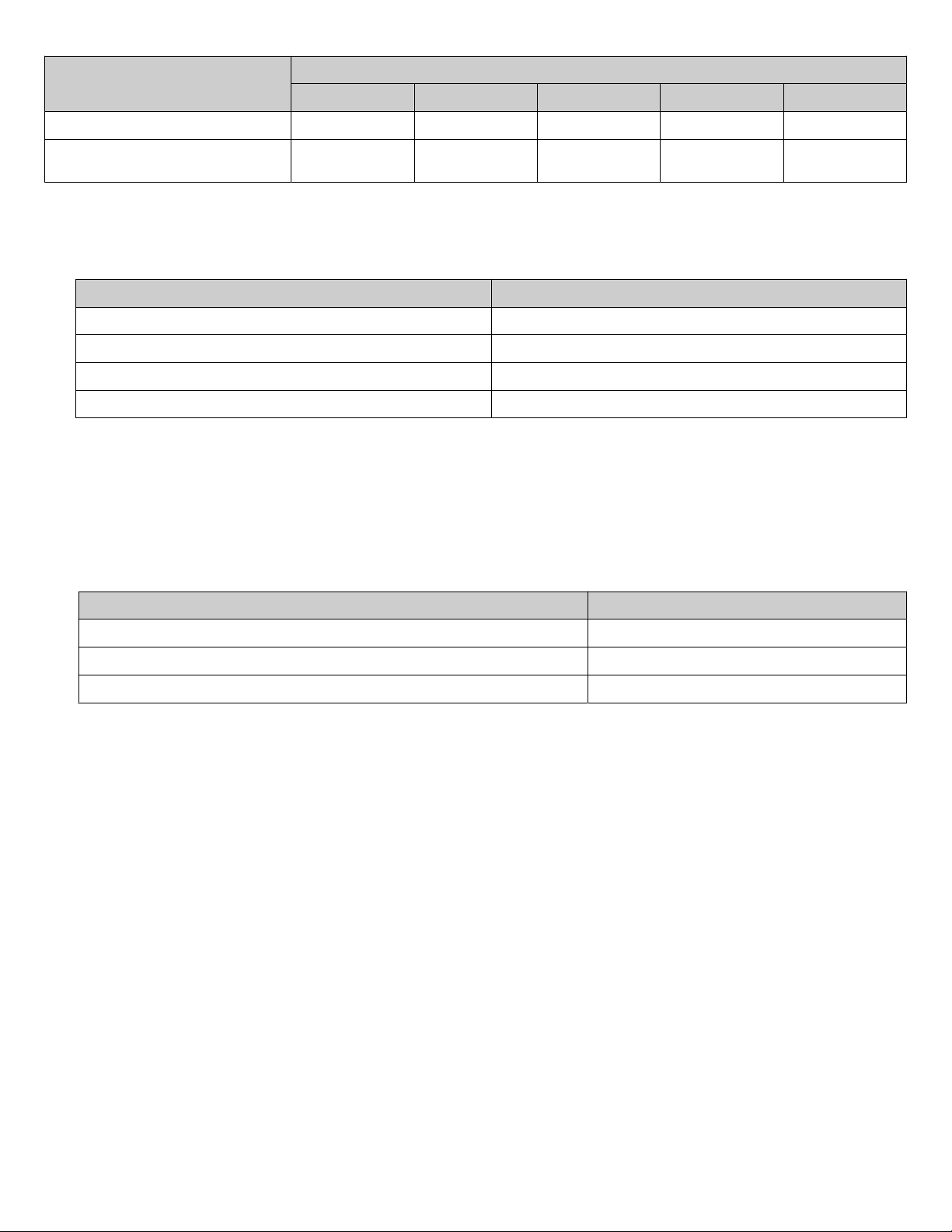
Component
1 7 10 13 25
Proteinase K, 20 mg/mL 10 μL 70 μL 100 μL 130 μL 250 μL
Number of extractions
Proteinase K (PK) Buer II or Proteinase K
(PK) Buer
60 μL 420 μL 600 μL 780 μL 1,500 μL
Lysis solution
• Prepare a fresh mixture immediately before use or during Proteinase K incubation.
• Prepare 360 µL (amount required) of lysis solution mix per sample.
Reagent Volume for ~20 extractions
Glycogen, 5 mg/mL 180 µL
Yeast tRNA, 10 mg/mL 4 µL
Lysis Buer 7,600 µL
Total 7,784 µL
Manual protocol for DNA/RNA extraction
(Plasmid samples only) Add Yeast tRNA
Plasmid samples require the use of Yeast tRNA as a carrier during the extraction process.
1. Dilute the Yeast tRNA.
Table 1 Diluted Yeast tRNA
Component
Yeast tRNA (10mg/mL) 5 μL
PBS (1X), pH 7.2 245 μL
Total 250 μL
Volume
2. Add 5 μL Diluted Yeast tRNA to 370 μL of each test sample before extraction. This is sucient for triplicate 100 μL extractions.
Digest the test samples and controls
1. Set a block heater to 56°C. If available, set a second block heater to 70°C.
2. Label 2‑mL Safe-Lock tubes:
• 3 for each sample
• 3 for each sample + ERC
• 3 for NEG
3. Add 100 µL of sample, sample + ERC, or 1X PBS to into each tube.
Note: Ensure that Diluted Yeast tRNA was added to each plasmid sample.
4. Add 10 µL of 5 M NaCl and 70 μLProteinase K/Proteinase K Buer II mix.
5. Briefly vortex and centrifuge.
6. Incubate at 56°C for 30 minutes.
If only one block heater is available, after this incubation step is complete, reset the block heater to 70°C for the elution step.
Note: For samples with high protein concentration, extending the incubation time to 60 minutes can increase recovery.
2 PrepSEQ
™
Residual DNA Sample Preparation Kit Quick Reference

7. Cool samples to room temperature.
8. Add 360 µL freshly made Lysis solution mix to each tube.
Bind the DNA/RNA
1. Vortex the Magnetic Particles to resuspend the particles.
Note: The appearance of the mixture should be homogeneous.
2. Add 30 µL of the Magnetic Particles to each tube.
3. Add 400 µL Binding Solution to each tube.
4. Mix and vortex the tubes:
a. Close the caps, immediately invert each tube twice to mix.
b. Vortex the tubes in the vortex adaptor for 5 minutes at setting #7.
5. Briefly centrifuge the tubes for 15 seconds at top speed (>15,000 × g) to collect the Magnetic Particles at the bottom of the tubes.
6. Place the tubes in the magnetic stand with the pellet against the magnet, then let the tubes stand for 5 minutes or until the solution
is clear.
7. Without disturbing the magnetic beads, remove the supernatant using a PIPETMAN™ pipette or by aspiration.
Wash the DNA/RNA
For aspiration of liquid supernatants and wash buers during sample preparation, we recommend attaching the waste-collection bottle to
the vacuum using tubing that can accommodate 200‑µL pipette tips.
1. Remove the tube rack (with tubes) from the magnetic stand, then add 300 µL of Wash Solution to the tubes. Vortex the tubes for
5 seconds at room temperature at setting #7.
2. Centrifuge the tubes in a microcentrifuge at top speed (>15,000 × g) for a maximum of 20 seconds. Do not centrifuge for >20
seconds.
3. Place the tubes in the magnetic stand, then let the tubes stand for 1 minute.
Note: The Magnetic Particles with the bound DNA/RNA are magnetically captured after approximately 1 minute.
4. Without disturbing the Magnetic Particles, remove the supernatant using a PIPETMAN™ pipette or by aspiration.
5. Remove the tube rack (with tubes) from the magnetic stand, then add 300 µL of Wash Solution to each tube for a second wash.
Vortex the tubes for 5 seconds at room temperature at setting #7.
6. Centrifuge the tubes in a microcentrifuge at top speed (>15,000 × g) for a maximum of 20 seconds. Do not centrifuge for >20
seconds.
7. Place the tubes in the magnetic stand, then let the tubes stand for 1 minute.
Note: The Magnetic Particles with the bound DNA/RNA are magnetically captured after approximately 1 minute.
8. Open all tubes, then start the 5‑minute timer.
9. Without disturbing the Magnetic Particles, remove the supernatant using a PIPETMAN™ pipette or by aspiration.
Use a P200 to remove the remaining solution from the bottom of the tube.
10. With the tube lid open, air-dry the Magnetic Particles pellet in the magnetic stand for no more than 5 minutes at room temperature.
IMPORTANT!
not over-dry; over-dried beads are not easily resuspended.
PrepSEQ™ Residual DNA Sample Preparation Kit Quick Reference 3
Air-dry to remove ethanol from the Wash Solution. After dried, the DNA/RNA stays bound to the magnetic beads. Do

Elute the DNA/RNA
1. Add 50 µL of Elution Buer to each tube.
2. Vortex the tubes for 20 seconds at high speed, then incubate the tubes at 70°C for 7 minutes. Vortex the tubes two to three times
during the incubation to help resuspension.
Note: (Optional) If vortexing does not result in full resuspension, then wash the beads o the tube by pipetting.
3. Centrifuge the tubes in a microcentrifuge at top speed (>15,000 × g) for a maximum of 20 seconds. Do not centrifuge for >20
seconds.
4. Place the tubes in the magnetic stand, then let the tubes stand for 1 minute.
Note: The Magnetic Particles with the bound DNA/RNA are magnetically captured after approximately 1 minute.
5. Without disturbing the Magnetic Particles, transfer the liquid phase containing the eluted DNA/RNA to a new nonstick 1.5‑mL
microcentrifuge tube.
6. Centrifuge the tube at top speed (>15,000 × g) for 3 minutes to collect the Magnetic Particles at the bottom, then place the tubes in
the magnetic stand for 1 minute.
7. Without disturbing the Magnetic Particles, transfer the liquid phase containing the eluted DNA/RNA to the nonstick 1.5‑mL
microcentrifuge tube.
Note: Magnetic Particles can inhibit PCR.
When you finish the sample extraction procedure, see the resDNASEQ™ Quantitative DNA Kits User Guide (Pub. No. 4469836) or the
ViralSEQ™ Real‑Time PCR Kits User Guide (Pub. No. 4445235) to set up PCR for DNA/RNA quantification.
Use 10 µL of the eluate in the real-time PCR.
Automated protocol for DNA/RNA extraction
You can use the KingFisher™ Flex or MagMAX™ Express 96-deep well automation platforms to automate the extraction of DNA/RNA. For
all chemicals, read the Safety Data Sheet (SDS) and follow the handling instructions. Wear appropriate protective eyewear, clothing, and
gloves.
(Plasmid samples only) Add Yeast tRNA
Plasmid samples require the use of Yeast tRNA as a carrier during the extraction process.
1. Dilute the Yeast tRNA.
Table 2 Diluted Yeast tRNA
Component
Yeast tRNA (10mg/mL) 5 μL
PBS (1X), pH 7.2 245 μL
Total 250 μL
2. Add 5 μL Diluted Yeast tRNA to 370 μL of each test sample before extraction. This is sucient for triplicate 100 μL extractions.
Prepare the plates
Prepare the Wash 1, Wash 2, and Elution plates:
Volume
Plate name
Wash 1 96 deep-well plate 300 µL of Wash buer
Wash 2 96 deep-well plate 300 µL of Wash buer
Elution 96 deep-well plate 200 µL of Elution buer
4 PrepSEQ
Plate type Volume of buer to add
™
Residual DNA Sample Preparation Kit Quick Reference

Prepare the lysis plate
In all steps that require pipetting, dispense liquid at bottom center of the wells.
1. Add 100 μL to the appropriate wells of the 96 deep-well Lysis plate:
• 3 wells for each sample
• 3 wells for each sample + ERC
• 3 wells for NEG
Note: Ensure that Diluted Yeast tRNA was added to each plasmid sample.
2. Add 10 μL of 5 M NaCl to each sample well.
3. Add 70 µL Proteinase K/Proteinase K (PK) Buer II mix to each sample well.
Process samples on the instrument
1. Select the script or program for the instrument you are using:
Instrument
KingFisher™ Flex PrepSEQ_resDNA_v1 script
MagMAX™ Express-96 PrepSEQ_resDNA_2011
PrepSEQ_1hr_resDNA (if installed)
2. Load the plates into the instrument in the order listed below. After loading each plate, press START to move the turntable.
a. Comb loading plate
b. Elution plate with 200 μL of Elution Buer
c. Wash 2 plate with 300 μL of wash buer
d. Wash 1 plate with 300 μL of wash buer
e. Lysis plate
Select
3. Press START to begin the PK digestion process.
The instrument mixes the samples for 10 seconds at fast speed, then incubates the samples at 56°C for 30 minutes, mixing at slow
speed. When digestion is complete, the instrument pauses and returns the Lysis plate to the loading position.
4. After the digestion step is complete, add additional components to the Lysis plate:
a. Remove the Lysis plate from the instrument.
b. Add 360 µL of Lysis Solution to each sample well.
c. Add 30 µL of Magnetic Particle suspension to each sample well.
d. Add 400 µL of Binding Solution to each sample well, then immediately pipet up-and-down three times to mix.
e. Place the plate back into the instrument loading position, then press START to begin binding.
5. When DNA/RNA extraction is finished, the instrument returns the Elution plate to the loading position.
When you finish the sample extraction procedure, see the resDNASEQ™ Quantitative DNA Kits User Guide (Pub. No. 4469836) or the
ViralSEQ™ Real‑Time PCR Kits User Guide (Pub. No. 4445235) to set up PCR for DNA/RNA quantitation.
Note: Use 10 µL of the eluate in the real-time PCR.
Limited product warranty
Life Technologies Corporation and/or its aliate(s) warrant their products as set forth in the Life Technologies' General Terms and
Conditions of Sale at www.thermofisher.com/us/en/home/global/terms-and-conditions.html. If you have any questions, please
contact Life Technologies at www.thermofisher.com/support.
PrepSEQ™ Residual DNA Sample Preparation Kit Quick Reference 5

Life Technologies Ltd | 7 Kingsland Grange | Woolston, Warrington WA1 4SR | United Kingdom
For descriptions of symbols on product labels or product documents, go to thermofisher.com/symbols-definition.
The information in this guide is subject to change without notice.
DISCLAIMER: TO THE EXTENT ALLOWED BY LAW, THERMO FISHER SCIENTIFIC INC. AND/OR ITS AFFILIATE(S) WILL NOT BE LIABLE FOR SPECIAL, INCIDENTAL, INDIRECT,
PUNITIVE, MULTIPLE, OR CONSEQUENTIAL DAMAGES IN CONNECTION WITH OR ARISING FROM THIS DOCUMENT, INCLUDING YOUR USE OF IT.
Revision history: Pub. No. 4469839
Revision Date Description
G 2 April 2021 Added the resDNASEQ™ Quantitative Plasmid DNA ‑ Kanamycin Resistance Gene Kit
F 5 August 2020 Added the resDNASEQ™ Quantitative Sf9 and Baculovirus DNA Kit (Cat. No. A46066).
Important Licensing Information: These products may be covered by one or more Limited Use Label Licenses. By use of these products, you accept the terms and conditions of all
applicable Limited Use Label Licenses.
©2021 Thermo Fisher Scientific Inc. All rights reserved. All trademarks are the property of Thermo Fisher Scientific and its subsidiaries unless otherwise specified.
(Cat. No. A50337).
thermofisher.com/support | thermofisher.com/askaquestion
thermofisher.com
2 April 2021
 Loading...
Loading...