Thermo Fisher Scientific Polyethylenimine-Transferrinfection User Manual
Polyethylenimine-Transferrinfection (Tf-PEI) Kit
Catalog Number BMS1003/a and BMS1003
Pub. No. MAN0024927 Rev. A.0 (30)
WARNING! Read the Safety Data Sheets (SDSs) and
follow the handling instructions. Wear appropriate protective
eyewear, clothing, and gloves. Safety Data Sheets (SDSs) are
available from thermofisher.com/support.
Product description
Transfection of DNA into eukaryotic cells is a common method to
study biological mechanisms. A major goal is the ecient and specific
delivery of genes into the desired target cells. Although a wide panel
of techniques and vectors (viral and nonviral) have been developed
that work with variable eciency, most vectors lack a target cell
specificity. Nonviral vectors are attractive because of their ease of
manipulation, safety, and high flexibility in the size of the delivered
transgene. The Transferrinfection is a high-eciency nucleic acid
delivery system based on transferrin receptor-mediated endocytosis
to carry DNA into cells. Furthermore it was shown that the cationic
polymer polyethylenimine (PEI) mediates ecient gene transfer into a
variety of cells. In the Polyethylenimine-Transferrinfection system, the
gene transfer eciency of PEI/DNA complexes are combined with the
specific mechanism of receptor-mediated endocytosis via Transferrin
receptor.
The method results in a 30–1,000-fold enhanced transfection
eciency depending on the cell line. It is an extremely gentle DNA
transfection method that employs physiological uptake mechanisms
of the cell. Transfection ecacy depends on the cell type, the level
of surface transferrin receptor expression. Very high transfection rates
have been shown for the tested tumor cell lines B16F10 melanoma,
Neuro 2A neuroblastoma, and a variety of primary human melanoma
cell lines. In other established cell lines, such as HeLa, CHO, Jurkat,
K562, HepG2, and COS, the PEI-Transferrinfection works with high
eciences, excellent reproducibility and with the advantage of being
an extremely gentle procedure.
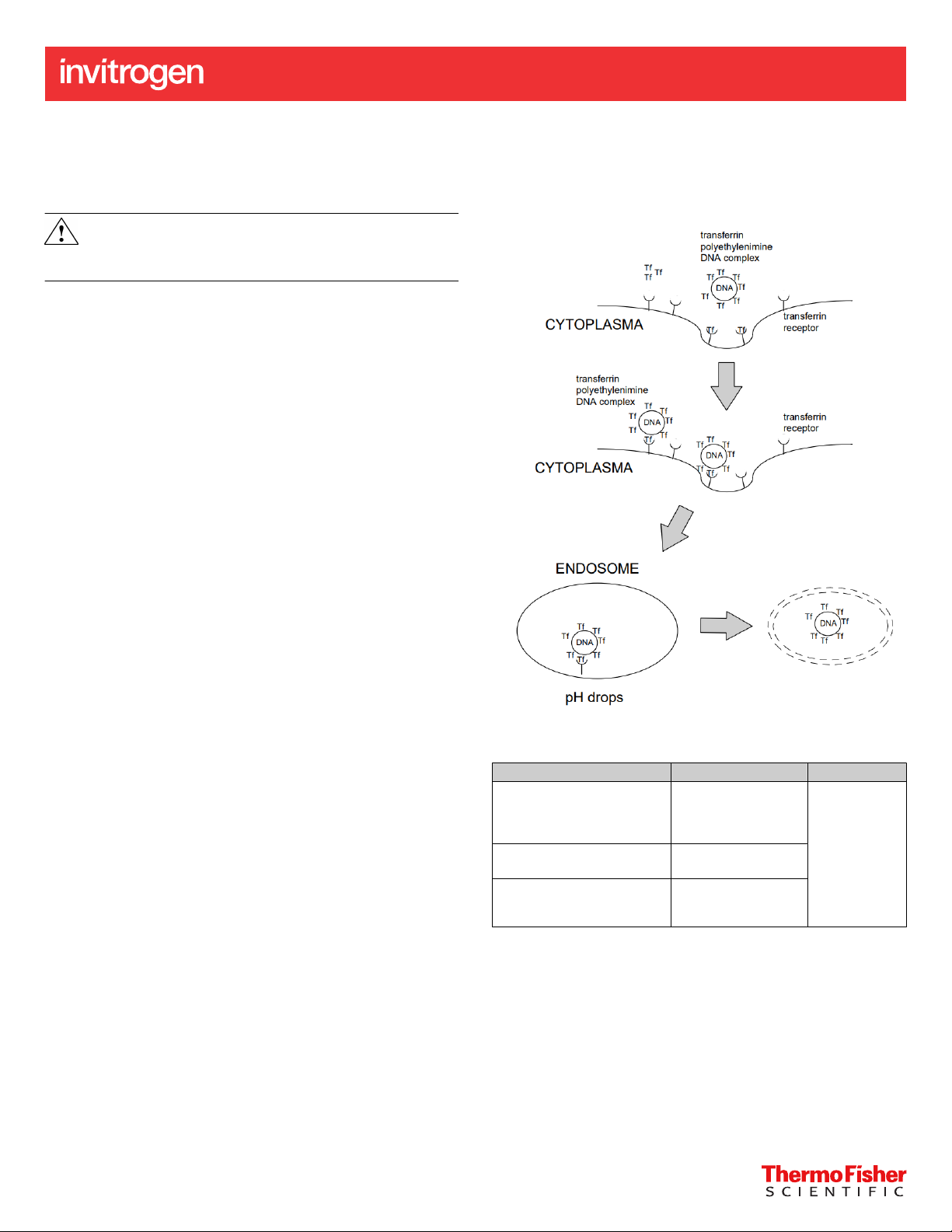
Human transferrin is covalently linked to a polycationic carrier
(Polyethylenimine/PEI) with intrinsic/ inherent endosomal activity.
PEI has the capacity to condense DNA and deliver it into cells,
presumably by adsorptive endocytosis. Furthermore, PEI is only
partially protonated at physiological pH. Upon acidification within
the endosome/ lysosome, PEI presumably acts as proton sponge
with the protonation triggering chloride influx, osmotic swelling, and
destabilization of the endosomal/ lysosomal vesicle enabling the
release of the DNA into the cytosol. As a result, ecient gene transfer
is obtained without the need for additional endosome destabilizing
agents. The modified, PEI-conjugated, transferrin molecules maintain
their ability to bind their cognate receptor and to mediate ecient
iron transport into the cell. Transferrin receptor-mediated endocytosis
occurs followed by the expression of the imported DNA (see Fig.
1). Deferrioxamine, a cell permeable iron chelator, increases the
transferrin receptor density on the cell surface thus further enhancing
gene delivery to the cell.
Contents and storage
Component
Transferrin-Polyethylenimine
Conjugate, lyophilized
(1 mg/mL upon reconstitution;
iron incorporated)
Deferrioxamine, lyophilized
(50 mM upon reconstitution)
HBS Buer Concentrate (20X)
(400 mM Hepes, pH 7.3;
3 M NaCl)
USER GUIDE
Quantity Storage
1 vial (BMS1003/a)
2 vials (BMS1003)
1 vial
1 vial (1 mL)
Store at 2–8°C.
For Research Use Only. Not for use in diagnostic procedures.

Prepare reagents
TransferrinPolyethylenimine
Deferrioxamine
HBS-Buer
Add 120 µL of distilled, sterile water to the vial
containing iron-corporated Transferrin-Polyethylenimine.
Ensure complete solubilization. Aliquot and freeze at –
20°C.
Add 500 µL of distilled, sterile water to the vial
containing deferrioxamine. Ensure complete solubilization.
The solution obtained is a 1,000X stock solution (50 mM).
Aliquot and freeze at –20°C.
Add contents of HBS-Buer concentrate (1.0 mL) to 19
mL of distilled, sterile water and mix gently. Store at 2° to
8°C. If crystals have formed, warm gently until they have
completely dissolved.
Protocol
The following protocol is calculated for the Transferrinfection of 2.5–
5 × 105 cells plated in a 24-well cluster plate in 1.5 mL of medium.
It can accordingly be adjusted to any other cell number, medium
volume, and tissue culture plate.
• Prepare cells
Method
Suspension
cells
Adherent cells The cells are plated one day before Transferrinfection
12 to 20 hrs before Transferrinfection, exponentially
growing suspension cultures are collected by
centrifugation and suspended in fresh medium
(with serum) containing 50 µM deferrioxamine
(from 1,000X stock solution). As an option before
Transferrinfection, the cells may be collected and
resuspended in fresh medium (with or without) serum
containing 50 µM deferrioxamine.
with medium containing 50 µM deferrioxamine (from
1,000X stock solution) and serum. As an option
directly before transfection, the medium may be
exchanged with fresh medium (with or without serum)
containing 50 µM deferrioxamine. At this step, cells
should be 50–70% confluent.
• Formation of transferrin-polyethylenimine conjugate/DNA
complex
a. Dilute purified DNA to a final concentration of 40 µg/mL in 1X
HBS buer.
b. Prepare transfection cocktail (500 µL) according to the table:
N/P Ratio
[1]
PEI Nitrogen to DNA Phosphate groups
[1]
3.6 4.5 µL 250 µL 245.5 µL
4.0 5.0 µL 250 µL 245.0 µL
4.8 6.0 µL 250 µL 244.0 µL
5.2 6.5 µL 250 µL 243.5 µL
6.0 7.5 µL 250 µL 242.5 µL
TfPEI
(1 mg/mL)
Description
DNA
(40 µg/mL)
1X HBS
c. Mix cocktail thoroughly by repeated aspiration and ejection for
~30 times. The ratios (N/P) may be crucial for the transfection
eciency. N/P ratios of at least 3.6 were shown to be
necessary for successful gene transfer. For a number of cell
lines, an N/P ratio of 4.8 to 6 were found to be optimal
for gene transfer. However, to achieve optimal results, the
individual optimum has to be titrated for each cell line/ DNA
combination.
d. Incubate transfection cocktail for 20 mins at room temperature
before adding to the cells.
• Transferrinfection of transferrin-polyethylenimine/DNA
complex
a. Add 1.5 mL of fresh medium containing 50 µM deferrioxamine
to the cells as indicated above.
b. Add 500 µL of transfection cocktail to the cells plus medium.
c. Incubate at 37°C for 4 hours.
d. Remove the transfection medium, optionally wash cells once
with prewarmed medium, and incubate in medium with the
usual amount of serum.
Additional information
• Addition of serum
The Transferrinfection method can be done in the presence
or absence of heat-inactivated fetal calf serum (FCS). Dierent
batches of FCS might influence the transfection eciency.
Decreasing (or omitting) FCS during transfection will increase
transfection ecacy, but may also result in increased toxicity.
• Addition of Deferrioxamine
This Transferrinfection method can be done in the presence
or absence of Deferrioxamine, with Deferrioxamine increasing
transfection ecacy in some cell lines. In most cases, treatment
with Deferrioxamine is not necessary for ecient transfection.
• Quality of DNA preparation
The DNA quality is a critical parameter for Transferrinfection.
The DNA transfected should be purified by cesium chloride
gradients or commercial purification columns and should not be
contaminated with RNA because RNA competes for complex
formation of transferrin-polyethylenimine conjugate. Importantly,
DNA has to be free of endotoxin because endotoxin was shown to
increase toxicity and decrease ecacy of transfection procedure.
Limited product warranty
Life Technologies Corporation and/or its aliate(s) warrant their
products as set forth in the Life Technologies' General Terms and
Conditions of Sale at www.thermofisher.com/us/en/home/global/
terms-and-conditions.html. If you have any questions, please
contact Life Technologies at www.thermofisher.com/support.
Bender MedSystems GmbH | Campus Vienna Biocenter 2 | 1030 Vienna, Austria
For descriptions of symbols on product labels or product documents, go to thermofisher.com/symbols-definition.
The information in this guide is subject to change without notice.
DISCLAIMER: TO THE EXTENT ALLOWED BY LAW, THERMO FISHER SCIENTIFIC INC. AND/OR ITS AFFILIATE(S) WILL NOT BE LIABLE FOR SPECIAL, INCIDENTAL, INDIRECT,
PUNITIVE, MULTIPLE, OR CONSEQUENTIAL DAMAGES IN CONNECTION WITH OR ARISING FROM THIS DOCUMENT, INCLUDING YOUR USE OF IT.
Revision history: Pub. No. MAN0024927
Revision Date Description
A.0 (30) 10 March 2021 New manual.
Important Licensing Information: These products may be covered by one or more Limited Use Label Licenses. By use of these products, you accept the terms and conditions of all
applicable Limited Use Label Licenses.
©2021 Thermo Fisher Scientific Inc. All rights reserved. All trademarks are the property of Thermo Fisher Scientific and its subsidiaries unless otherwise specified.
thermofisher.com/support | thermofisher.com/askaquestion
thermofisher.com
10 March 2021
 Loading...
Loading...