USER GUIDE
Dynabeads™ Intact Virus Enrichment (optimized for SARS-CoV-2)
Catalog Numbers 10700D, 10701D
Pub. No. MAN0019858 Rev. A.0
WARNING! Read the Safety Data Sheets (SDSs) and follow the handling instructions. Wear appropriate protective eyewear, clothing, and
gloves. Safety Data Sheets (SDSs) are available from thermofisher.com/support.
Product description
Biological study of viruses, including SARS-CoV-2, often requires isolation of intact virus particles from dilute samples. Current isolation methods
such as ultracentrifugation are tedious, dicult, and cannot be automated. The Dynabeads™ Intact Virus Enrichment (optimized for SARS-CoV-2)
provides a simple, fast and reliable method for concentration of intact viruses from various samples such as cell culture media and virus transport
media (VTM) for manual or automated handling. The procedure is simple, and performed in less than 30 minutes. The enriched virus particles can
be released from the magnetic beads for subsequent applications, if necessary.
Dynabeads™ Intact Virus Enrichment contains highly positively charged, monosized, superparamagnetic beads that strongly bind negatively
charged vesicles or molecules in the sample. The provided user protocols are tested for SARS-CoV-2 but can be further optimized for use with
other negatively charged enveloped viruses, virus-like particles (VLP’s), exosomes, or proteins. The enriched virus can be used for functional
studies, immunological studies, protein analysis (e.g., western blot) or nucleic acid (NA) extraction (e.g., for qRT-PCR).
Contents and storage
Amount
2 mL (100 reactions) 10700D
10 mL (500 reactions) 10701D
[1]
Contains 40 mg/mL of 1 µm sized strong anion exchange superparamagnetic
beads supplied in 30 mM sodium chloride (NaCl) and 0.05% sodium azide (NaN3).
[1]
Cat. No. Cat. No.
2–8°C
Required materials not supplied
• DynaMag™ Magnet (see thermofisher.com/magnets to find the
most suitable for your volumes).
• Sample mixer or roller allowing tilting and rotation of tubes (e.g.,
HulaMixer™ Sample Mixer).
• See “Automated enrichment protocol” for additional materials.
Buers and solutions
The following reagents are general recommendations. Alternative
buers may also be used.
• Binding & Washing Buer (B&W Buer): 10 mM NaCl in 20 mM
triethanolamine, pH 6
• Release Buer (optional): 0.25 M KI in 20 mM triethanolamine,
pH 6
• Phosphate buered saline (PBS)
General guidelines
• The protocols are optimized for SARS-CoV-2 virus and SARSCoV-2 VLPs in cell culture media and VTM. For other sample
types, further optimization may be required (e.g., bead amount
and incubation time).
• Isolated virus can be released in 10 minutes using Release Buer,
but further optimization may be required (e.g., release time and
salt concentration).
• Increasing the release volume can increase virus yield, whereas
reducing the release volume can be used to concentrate the virus
sample (e.g., for western blot).
• If necessary, use Exosome Spin Columns to exchange Release
Buer to a more appropriate buer after virus release.
• Use exosome-depleted fetal bovine serum to avoid co-enrichment
of exosomes.
• Perform procedures at room temperature, unless otherwise stated.
Manual enrichment protocol
This protocol provides a general procedure for enrichment of intact
infectious or inactivated SARS-CoV-2 and SAR-CoV-2 VLP’s from cell
culture media or VTM.
• One reaction is defined as 20 µL Dynabeads™ Intact Virus
Enrichment per 1 mL virus sample. Volumes can be scaled up
or down proportionally as required.
• Because virus concentration vary between samples, sample
volume must be optimized (e.g., 20 µL beads can be used with
a sample volume ranging from 200 µL to 1 mL).
• Remove the sodium azide by washing the beads prior to virus
binding (see “Wash magnetic beads”).
Table 1 Examples of total reagent volumes for virus enrichment
Virus starting volume
1 mL 20 µL ~1.5 mL
5 mL 100 µL ~7 mL
20 mL 400 µL ~30 mL
Wash magnetic beads
For multiple samples, the beads can be washed in one large bulk
volume sucient for all of the samples.
1. Resuspend the vial of Dynabeads™ magnetic beads (e.g., place
on a roller for ~5 minutes).
2. Immediately pipette 20 µL of resuspended beads from the vial to
a new tube.
3. Add 400 µL B&W Buer to the tube, then mix thoroughly.
4. Apply to a DynaMag™ magnet for 1 minute, then remove the
buer.
Enrich for virus particles
1. Add 1 mL of virus sample into the tube containing the pre-
washed beads (alternatively, add 20 µL pre-washed beads into
1 mL of sample).
2. Incubate on a roller for 10 minutes.
3. Apply to a DynaMag™ magnet for 1 minute, then remove the
supernatant.
4. Add 1 mL B&W Buer, then mix thoroughly.
Note: Alternatively, for downstream qRT-PCR, the bead-bound
virus can be directly resuspended in 1X PBS and Viral/Pathogen
Bead volume B&W Buer
For Research Use Only. Not for use in diagnostic procedures.
Kit Binding Buer (see “Extract RNA for qRT-PCR (Automated)”
on page 3).
5. Apply to a DynaMag™ magnet for 1 minute, then remove the
buer.
6. Go directly to “(Optional) Release virus particles” on page 2 or
resuspend the bead-bound virus in a suitable buer and volume
for your downstream assay.
(Optional) Release virus particles
Start with the bead-bound virus particles from Enrich for virus
particles (not resuspended). The protocol is scaled for 20 µL
beads/mL virus starting sample.
1. Add 50 µL Release Buer and mix well.
2. Incubate on a roller for 10 minutes.
3. Mix well by pipetting or vortexing.
4. Apply to a DynaMag™ magnet for 1 minute.
5. Transfer the supernatant containing the isolated virus to a new
tube.
The isolated and released virus is now ready for downstream
analysis.
Note: The sample is dissolved in high salt at the end of the
procedure. For buer exchange, use the Exosome Spin Columns.
(Optional) Prepare sample for electrophoresis
Volumes are adapted for 1 well (~40 μL/well). Further optimization
may be required for optimal results.
1. Resuspend the bead-bound virus from “Enrich for virus particles”
in 30 µL of distilled water.
Note: If your antibody requires reducing conditions reduce the
volume distilled water to 26 µL, and add 4 µL 10X Bolt™ Sample
Reducing Agent to the sample.
2. Add 10 μL 4X Bolt™ LDS Sample Buer.
3. Heat for 10 minutes at 70°C.
4. Apply to DynaMag™ magnet for 1 minute.
5. Load supernatant containing isolated virus into the wells of the
gel for electrophoresis.
Perform western blot
For convenience the iBlot™ 2 Gel Transfer Device can be used for
ecient blotting transfer within seven minutes after electrophoresis
without the need for liquid buers. For fast and automated
immunodetection, the iBind™ Western System can be used.
(Optional) Extract RNA for qRT-PCR
After virus enrichment, RNA extraction can be performed on the
sample using the MagMAX™ Viral/Pathogen Nucleic Acid Isolation Kit
for downstream qRT-PCR. See the MagMAX™ Viral/Pathogen Nucleic
Acid Isolation Kit (manual extraction) User Guide (MAN0018072) for
manual extraction instructions. For an automated procedure, see
“Extract RNA for qRT-PCR” on page 3.
Automated enrichment protocol
This section provides an automated protocol for the KingFisher™ Flex
instrument. Other KingFisher™ instruments can also be used. Go to
www.thermofisher.com/automation or contact Technical support
for more information regarding protocols for other KingFisher
instruments.
Required materials not supplied
• KingFisher™ Flex Magnetic Particle Processor with 96 Deep-Well
Head
• KingFisher™ Deep-Well 96 Plate, V-bottom, polypropylene
(50-1000 µL)
• KingFisher™ Flex 96 Tip Comb for Deep-Well Magnets
• BindIt™ 4.0 Software
See www.thermofisher.com/automation for alternative plates and
magnetic heads (e.g., for 24-wells).
Download BindIt™ software and protocol
1. Go to www.thermofisher.com/automation
2. In the left-hand panel, select Software and Protocols .
3. Open the BindIt Software tab, select Download BindIt
Software, and follow the instructions.
4. Open the Viruses and Vesicles Protocols, and select the
Dynabeads Intact Virus Enrichment-Flex for download.
General guidelines
• This protocol is for KingFisher Flex 96-deep well plates, but other
suitable KingFisher plastic for 96-well heads and 24-well heads
and corresponding plastic can be used as well.
• 1 reaction is defined as 10 µL beads from the original Dynabeads
vial in 200 µL virus cell culture or VTM media. Optimize the
volumes depending on the virus concentration (range from
~200-500 µL virus media per 10 µL beads). Note: Since these
volumes are half of the manual protocol volumes, the automated
#rxns are doubled (200 rxns per 2 mL beads, and 1000 rxns per
10 mL beads).
• It is important to resuspend the Dynabeads vial prior to starting
the procedure (e.g. leave on a roller for ~5 minutes).
• If you need the virus to be released from the beads, see protocol
guidelines in the manual section.
• Do not elute in a volume smaller than 30 µL/well.
Prepare plates
• Prepare 5 plates and 1 tip-comb plate for each run.
• A smaller plate than the deep-well plate may be more suitable to
use as the resuspension step (plate #5).
• Dilute the Dynabeads™ magnetic beads 1:10 in B&W buer prior
to loading the beads in the wells (e.g., 10 µL beads are diluted to
100 µL per reaction which is added to plate 1)
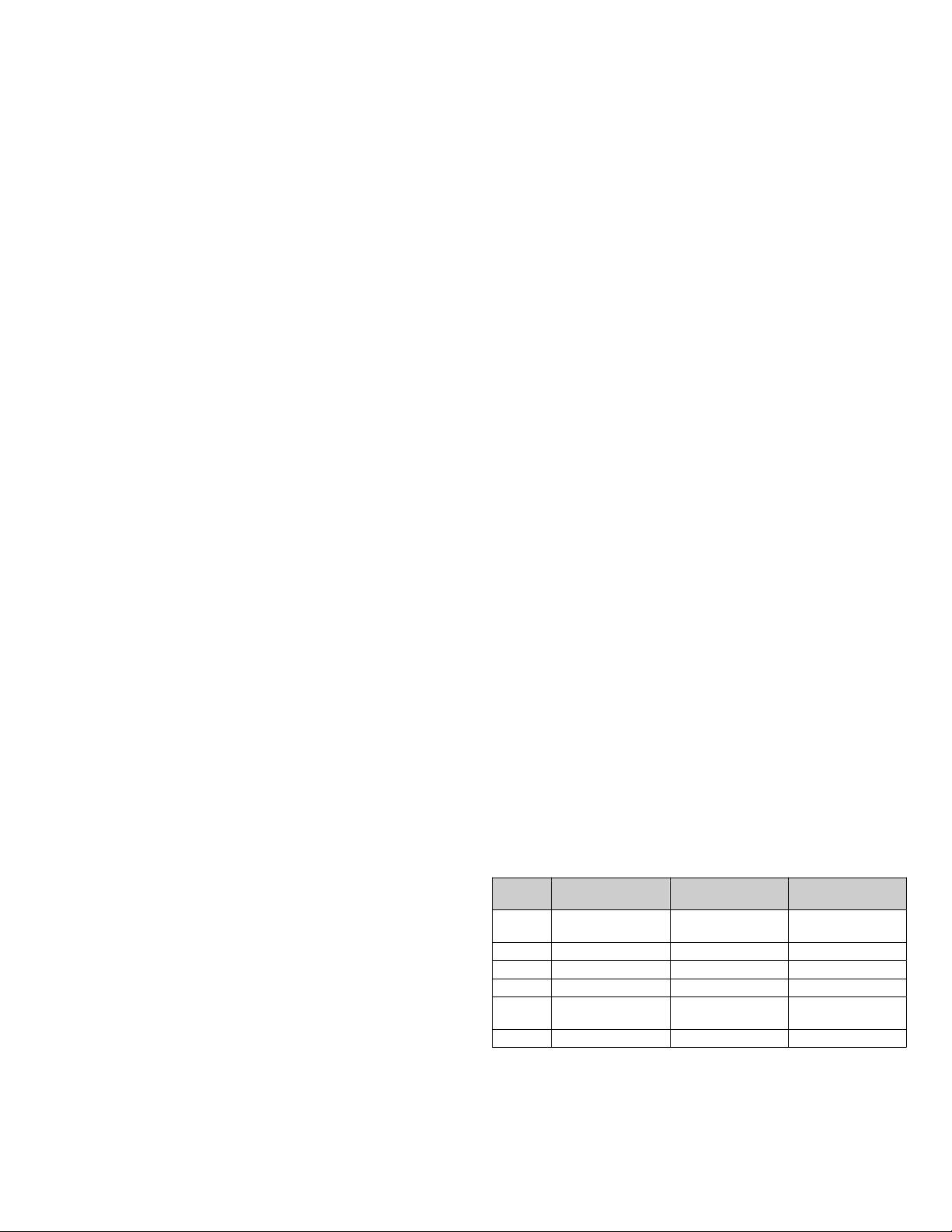
Table 2 Plate set-up and volume requirements per well
Plate
position
1 Dynabeads Diluted magnetic
2 Wash I B&W Buer 400 µL
3 Target Virus in medium 200 µL
4 Wash II B&W Buer 400 µL
5 Isolated virus with
6 Tip comb — —
Plate name Reagent Volume/well
beads
B&W Buer 200 µL
beads
™
100 µL
2 Dynabeads
™
Intact Virus Enrichment
 Loading...
Loading...