Thermo Fisher Scientic ViiA Reference Guide
ViiA™ 7 Real-Time PCR System
QUICK REFERENCE
QuantStudio™ Real‑T
Pub. No. MAN0018831 Rev. C.0
Note: For safety and biohazard guidelines, see the “Safety” appendix in the Applied Biosystems™ ViiA™ 7 Real-Time PCR System User
Guide: Calibration, Maintenance, Networking, and Security (Pub. No. MAN0018830). Read the Safety Data Sheets (SDSs) and follow the
handling instructions. Wear appropriate protective eyewear, clothing, and gloves.
ime PCR Software v1.6.1
About the ViiA™ 7 system
The Applied Biosystems™ ViiA™ 7 Real-Time PCR System uses uorescent-based polymerase chain reaction (PCR) reagents to provide:
• Quantitative research detection of target nucleic acid sequences (targets) using real-time analysis.
• Qualitative research detection of targets using post-PCR (endpoint) analysis.
• Qualitative analysis of the PCR product (achieved by melt curve analysis that occurs post-PCR).
Calibrate and maintain the ViiA™ 7 Real-Time PCR System
The ViiA™ 7 Real-Time PCR System r
Calibration with QuantStudio™ Real‑Time PCR Software v1.6.1
IMPORTANT! The spectral calibration plates for the QuantStudio
QuantStudio™ Real‑Time PCR Software v1.6.1. For more information, see the Applied Biosystems™ ViiA™ 7 Real-Time PCR System User
Guide: Calibration, Maintenance, Networking, and Security (Pub. No. MAN0018830).
QuantStudio™ Real‑Time PCR Softwar
plate block. For this version of the software, normalization calibration is only required for the TaqMan™ Array Card block.
After the software is upgraded, the instrument must be calibrated before starting a run with a 96-well plate block or a 384-well plate
block.
After the software is upgraded, the instrument does not need to be calibrated before starting a run with a TaqMan™ Array Card block.
The feature to override a calibration can only be used for a data le with compatible calibration data.
• A data le generated with a previous version of the software cannot have the calibration overridden after an upgrade to
• A data le generated with QuantStudio™ Real‑Time PCR Software v1.6.1 cannot have the calibration overridden if it is opened using
QuantStudio™ Real‑Time PCR Software v1.6.1.
a previous version of the software.
equires regular calibration and maintenance to ensure optimal instrument performance.
™
3 and 5 Instruments ar
e v1.6.1 does not require a normalization calibration for the 96-well plate blocks or the 384-well
e used for the calibration procedure with
For Research Use Only. Not for use in diagnostic procedures.

Recommended calibration and maintenance schedule
Note: After the software is upgraded to QuantStudio™ Real‑Time PCR Software v1.6.1, the instrument must be calibrated before starting a
run with a 96-well plate block or a 384-well plate block. The instrument does not need to be calibrated before starting a run with a
TaqMan™ Array Card block.
IMPORTANT! Calibrate the ViiA
™
7 System at the same ambient temperatur
e at which you will run experiments. Extreme variations in
ambient temperature can affect the heating and cooling of the ViiA™ 7 System and, in extreme cases, inuence experimental results.
IMPORTANT! Do not use or
ganic solvents to clean the ViiA™ 7 System.
Frequency User-performed maintenance task
Check the computer disk space. If necessary, archive or back up your experiment files and instrument settings.
Power off the computer that controls the ViiA™ 7 System, then after 30 seconds, power on the computer
Weekly
Clean the surface of the ViiA™ 7 System with a lint-free cloth.
Perform a ViiA™ 7 Instrument self test.
Check the lamp status. If necessary, replace the lamp.
Monthly
Perform a background calibration.
[1]
Run disk cleanup and disk defragmentation.
Perform a regions of interest (ROI) calibration.
Perform a background calibration.
Perform a uniformity calibration.
Semi-annually (6 months)
Perform a dye calibration.
(TaqMan™ Array Card block only) Perform a normalization calibration.
.
Perform an instrument verification run.
Decontaminate the ViiA™ 7 System.
Replace the ViiA™ 7 System fuses.
As needed
Update the Windows™ operating system.
Update the QuantStudio™ Real‑Time PCR Software and firmware.
[1]
Y
ou can perform a background calibration to check for contamination. If any parts of the optics are replaced or moved, you must perform all calibrations, including an RNase P
instrument verification run.
Calibrate the ViiA™ 7 Real-Time PCR System
The ViiA™ 7 Real-Time PCR System uses the following calibration types:
• Regions of interest (ROI)
• Background
• Uniformity
• Dye
•
Instrument verication
• (TaqMan™ Array Card block only) Normalization
2 Applied Biosystems
™
ViiA™ 7 Real-T
ime PCR System Quick Reference Guide
Calibration and verification consumables
Note: For reagent or consumable shelf-life expiration date, see the package label.
96-well 0.2-mL consumables
Note: Spectral calibration plates for the QuantStudio™ 3 and 5 Instruments are used for the calibration procedure with QuantStudio
Real‑Time PCR Software v1.6.1.
™
Consumable Cat. No. Storage
QuantStudio™ 3/5 10‑Dye Spectral Calibration Kit, 96‑W
ell 0.2‑mL
(Contains all 3 spectral calibration plates listed below)
QuantStudio™ 3/5 Spectral Calibration Plate 1
(FAM™, VIC™, ROX™, and SYBR™ dyes), 96‑Well 0.2‑mL
QuantStudio™ 3/5 Spectral Calibration Plate 2, 96‑Well 0.2‑mL
(ABY™, JUN™, and MUSTANG PURPLE™ dyes)
QuantStudio™ 3/5 Spectral Calibration Plate 3, 96‑Well 0.2‑mL
(TAMRA™, NED™, and Cy®5 dyes)
A26343
A26331
A26332
A26333
–25°C to –15°C
Shelf life at storage
temperatur
Use the consumable
by the expiration date
on the packaging.
Region of Interest (ROI) and Background Plates, 96‑Well 0.2‑mL (2 plates) 4432364
TaqMan™ RNase P Instrument Verification Plate, 96‑Well 0.2‑mL 4432382
96-well 0.1-mL consumables
Note: Spectral calibration plates for the QuantStudio™ 3 and 5 Instruments are used for the calibration procedure with QuantStudio
Real‑Time PCR Software v1.6.1.
Consumable Cat. No. Storage
QuantStudio™ 3/5 10‑Dye Spectral Calibration Kit, 96‑well, 0.1‑mL
(Contains all 3 spectral calibration plates listed below)
A26342
Shelf life at storage
temperatur
e
™
e
QuantStudio™ 3/5 Spectral Calibration Plate 1,
AM™, VIC™, ROX™, and SYBR™ dyes), 96‑well, 0.1 mL
(F
QuantStudio™ 3/5 Spectral Calibration Plate 2 (ABY™, JUN™, MUSTANG
PURPLE™ dyes), 96‑well Fast (0.1‑mL) Plate
QuantStudio™ 3/5 Spectral Calibration Plate 3 (TAMRA™, NED™ and Cy®5
dyes) 96‑well Fast (0.1‑mL) Plate
Region of Interest (ROI) and Background Plates, Fast 96‑Well 0.1‑mL
(2 plates)
TaqMan™ RNase P Instrument V
erification Plate, Fast 96-Well 4351979
A26336
A26337
A26340
4432426
–25°C to –15°C
Use the consumable
by the expiration date
on the packaging
Applied Biosystems™ ViiA™ 7 Real-Time PCR System Quick Reference Guide 3
384-well consumables
Note: Spectral calibration plates for the QuantStudio™ 3 and 5 Instruments are used for the calibration procedure with the QuantStudio
Real‑Time PCR Software v1.6.1.
™
Consumable Cat. No. Storage
QuantStudio™ 5 10-Dye Spectral Calibration Kit, 384-well
(Contains the 2 spectral calibration plates listed below)
QuantStudio™ 5 Spectral Calibration Plate 1, (F
TAMRA™, and SYBR™ dyes), 384-well
QuantStudio™ 5 Spectral Calibration Plate 2 (ABY™, JUN™, MUSTANG
PURPLE™, NED™, and Cy®5 dyes), 384-well
Region of Interest (ROI) and Background Plates, 384-well 4432320
TaqMan™ RNase P Instrument Verification Plate, 384-well 4455280
AM™, VIC™, ROX
™
A26341
A26334
A26335
Array card sample block consumables
Consumable Cat. No. Storage
ViiA™ 7 Array Car
Includes FAM™ Dye, VIC™ Dye, ROX™ Dye, ROI Dye, FAM™/ROX™ Dye,
VIC™/ROX™ Dye, and Background Buffer
ViiA™ 7 Array Card RNaseP Verification Kit 4432265
d Spectral Calibration Kit
4432314
–25°C to –15°C
–25°C to –15°C
Shelf life at storage
temperatur
Use the consumable
by the expiration date
on the packaging.
Shelf life at storage
temperatur
Use the consumable
by the expiration date
on the packaging.
e
e
Prepare the calibration plate or array card
IMPORTANT! Wear powder-free gloves and safety glasses when you prepare plates or array cards.
1.
Remove the calibration plate or the calibration solution from the freezer, then allow it to thaw to room temperature in the dark
(approximately 5 minutes).
IMPORTANT! Pr
otect the calibration plate and the calibration solution from light. The uorescent dyes in the calibration plate wells
and calibration solutions are photosensitive. Prolonged exposure to light can diminish the uorescence of the dye.
Do not remove the calibration plate from its packaging until you are ready to run it.
2.
epare the calibration plate or array card.
Pr
• To prepare an array card, ll it with calibration solution. For a normalization calibration, ll two cards with separate normalization
solutions.
• To prepare a plate:
a. Remove the plate from its packaging.
IMPORTANT! Do not discar
d the packaging. The plate can be used up to three times if it is stored in its original packaging
sleeve.
b. Vortex the plate for 5 seconds, then centrifuge it for 2 minutes.
Verify that the liquid is at the bottom of each well of
c.
the plate. If not, centrifuge the plate again at a
higher rpm and for a longer period of time.
IMPORTANT! Do not allow the bottom of the plate
to become dirty
. Fluids and other contaminants that
adhere to the bottom of the plate can contaminate
the sample block(s) and cause an abnormally high
background signal.
4 Applied Biosystems
™
ViiA™ 7 Real-Time PCR System Quick Reference Guide
Perform the calibration
A1
1.
In the Home screen of the QuantStudio™ Real‑Time PCR Software, click Instrument Console.
2.
In the Instrument Console, select your ViiA™ 7 Instrument from the list of instruments on the network, then click Add to My
Instruments.
Note: You must add an instrument to your list before you can manage it.
3.
After the instrument is added to your list of instruments, select it, then click Manage Instrument.
4.
In the Instrument Manager, select the calibration, then click Start Calibration.
5.
Click Next, then perform the calibration as instructed. When
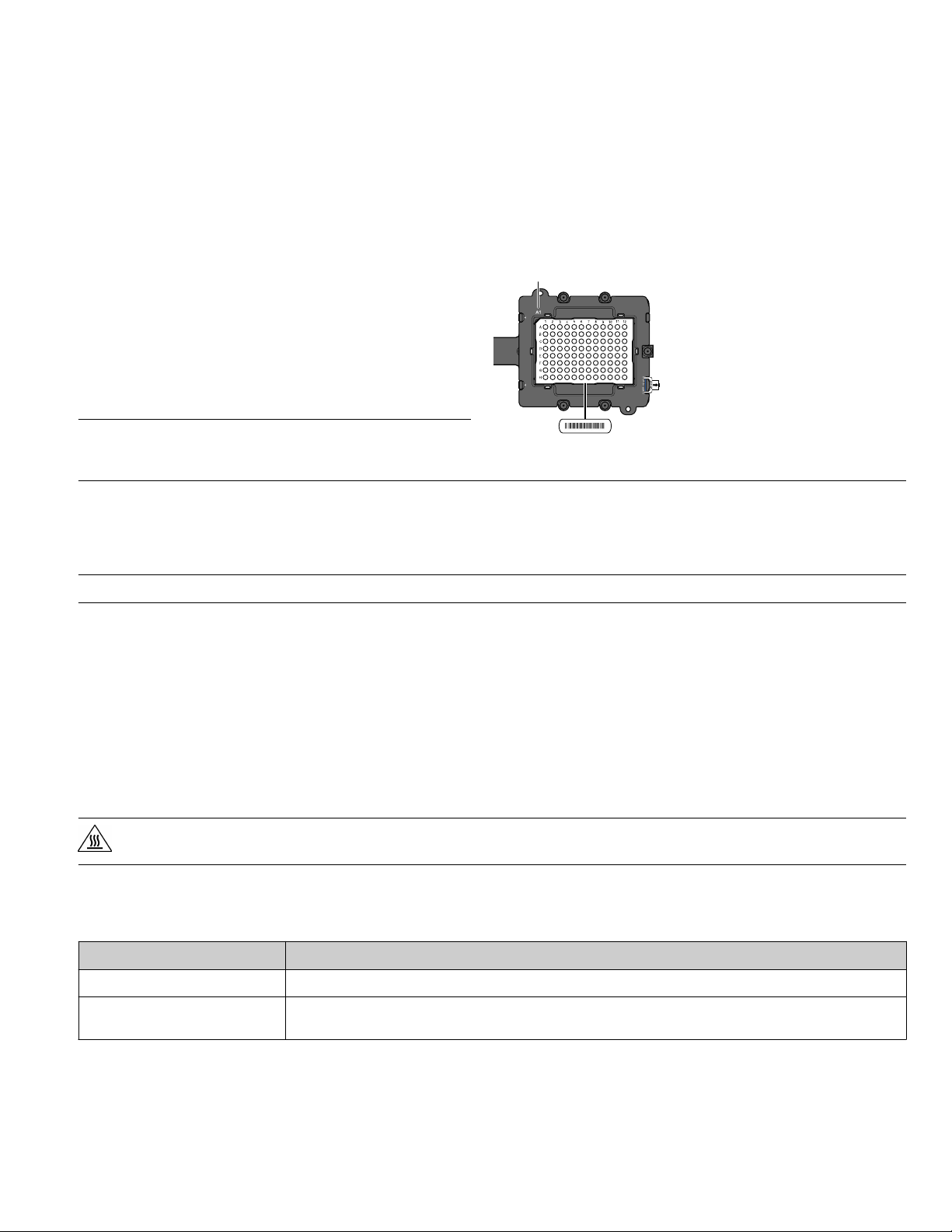
the side door opens, load the calibration plate or array card.
Ensure that the plate or array card is properly aligned in the
holder.
• Load 96/384-well plates with the A1 position at the topleft corner of the plate adapter.
• Load both plates and array cards with the bar code
facing the front of the instrument.
IMPORTANT! Plates should be loaded and unloaded by
operators who have been war
and have been adequately trained.
6.
After loading the plate or array car
a.
In the Setup tab, select Check the box when the calibration plate has been loaded, then click Next.
b.
In the Run screen, click START RUN
ned of the moving parts hazard
d, start the calibration:
IMPORTANT! Do not attempt to open the access door during the run. The door is locked while the instrument is in operation.
Review the results and complete the calibration
1.
When the run is complete and the softwar
the QC tab and review the quality check summary. If the run failed, troubleshoot the failed calibration as described in the Applied
Biosystems™ ViiA™ 7 Real-Time PCR System User Guide: Calibration, Maintenance, Networking, and Security (MAN0018830).
Note: You can accept a calibration that passes the Analysis Status check, but fails the QC Status check. We recommend using
calibrations that yield passing results for both status reports.
2.
After you inspect results, click Next, then remove the plate or array card when the instrument ejects the plate adapter.
WARNING! PHYSICAL INJUR
removing the plate, wait until it reaches room temperature.
3.
Click Finish to complete the calibration.
4.
d or store the plate or array card.
Discar
Consumable Action
Array card Discard the array card.
Plate
Discard the plate, unless it can be reused. If you can store the plate for reuse, return the calibration plate to
its packaging sleeve, then r
e displays the Analysis screen, conrm the analysis status of the calibration, then select
Y HAZARD. During instrument operation, the plate can be heated to 100°C. Before
eturn the packaged plate to the freezer.
Applied Biosystems™ ViiA™ 7 Real-Time PCR System Quick Reference Guide 5
Maintain the ViiA™ 7 system
Decontaminate the sample block
Perform this procedure to eliminate uorescent contaminants from the ViiA™ 7 System sample block. Contamination is generally evident in
failed background calibrations where one or more wells consistently exhibit abnormally high signals.
CAUTION! PHYSICAL INJUR
instrument that you can safely service yourself. If you suspect a problem, contact a Technical Support.
CAUTION! PHYSICAL INJUR
performing the following procedure, be sure to wait until the sample block reaches room temperature.
CAUTION! Befor
Fisher Scientic that the proposed method will not damage the equipment.
e using a cleaning or decontamination method other than those recommended in this guide, verify with Thermo
Y HAZARD. Do not remove the ViiA™ 7 Instrument cover. There are no components inside the
Y HAZARD. During instrument operation, the sample block can be heated to 100°C. Before
Materials required
•
Bleach, 10% solution
• Tissue, lint-free
• Cotton or nylon swabs and lint-free cloths
• Ethanol, 95% solution
• Safety glasses
• Pipette (100‑µL) with pipette tips
• Powder-free gloves
• Screwdriver
• Deionized water
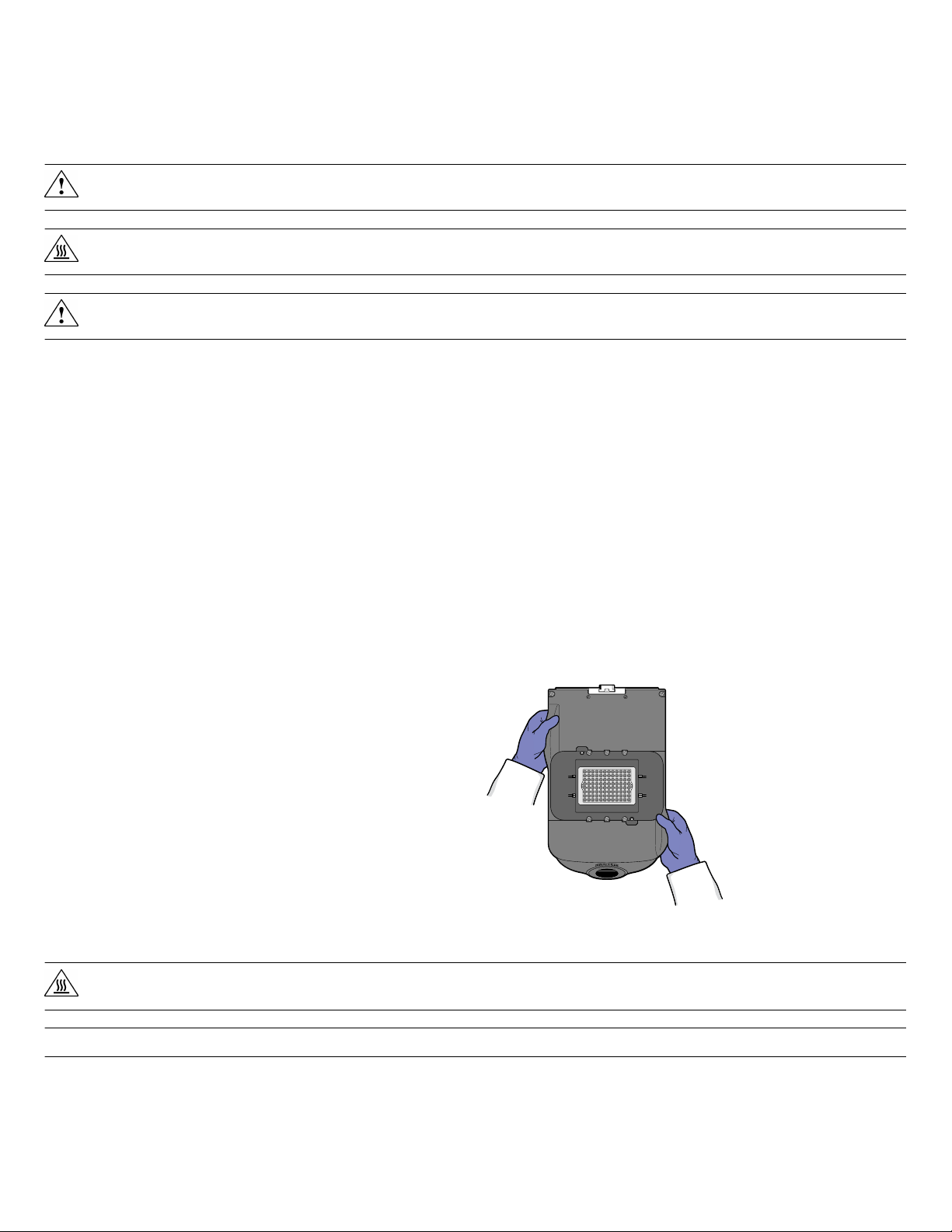
How to handle the sample block
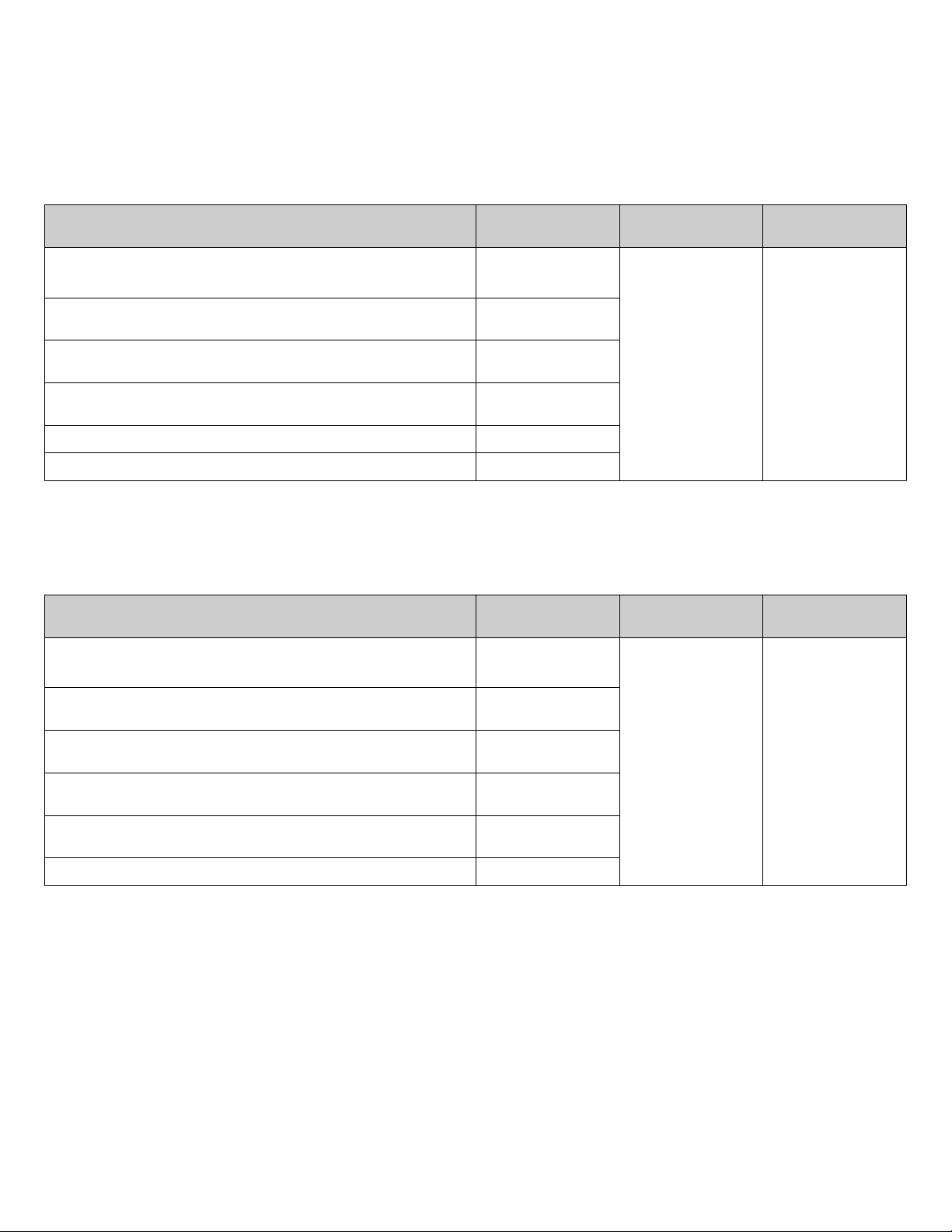
To prevent damaging or contaminating the sample block, handle the
assembly as shown below. After the assembly has been removed
from the ViiA™ 7 Instrument, place the sample block on a clean, dry
surface or in its shipping container.
Clean the sample block
WARNING! PHYSICAL INJUR
100°C. Before removing the sample block, be sure to wait until it reaches room temperature.
IMPORTANT! W
1.
Identify the contaminated wells of the sample block.
ear powder-free gloves when you perform this procedure.
Y HAZARD. During instrument operation, the sample block and heated cover can be heated to
2.
Power of
3.
Open the access door.
6 Applied Biosystems
f and unplug the instrument, then allow it to cool for 15 minutes.
™
ViiA™ 7 Real-Time PCR System Quick Reference Guide
 Loading...
Loading...