PrimeQHelp@bibby
-
scientific.com
| www.bibby
-
scientific.com
+44(01785) 810433
1
Application Note A08-001A
Minimising Sample Volumes
Introduction
Intercalating dye chemistry is a very sensitive and flexible method for amplicon
detection in real-time nucleic acid detection systems. For a well-optimized assay it
may be possible to detect nucleic acid copies using as little as 10µl sample volumes.
It is apparent that the lower the sample volume, the greater the cost saving since
less master mix and primers are required. In this application note we demonstrate
that reducing the sample volume of an intercalating dye assay to 10µl has no effect
on reaction efficiency and minimal effect on sensitivity. This indicates that real cost
savings are achievable when using PrimeQ.
Methods
A region of the plasmid vector pBR322 was amplified in PrimeQ using GoTaq® qPCR Master Mix from Promega®,
with BRYT Green® as the intercalating fluorescent reporter dye and carboxy-X-rhodamine (CXR) as a passive
reference dye. 5, 10, 15 and 20µl reaction volumes were used in a black 96-well low profile plate, heat sealed with
Clear Seal Diamond Heat Sealing Film from Thermo Fisher Scientific Inc. BRYT Green® fluorescence was measured
at the end of the extension step of each thermal cycle using the FC02 filter to detect product amplification; CXR
was detected using filter FC04. The vector was diluted in a 10-fold series to give a range of concentrations from
approximately 1x105 copies per well to 1x109 copies per well in each reaction volume group.
Results
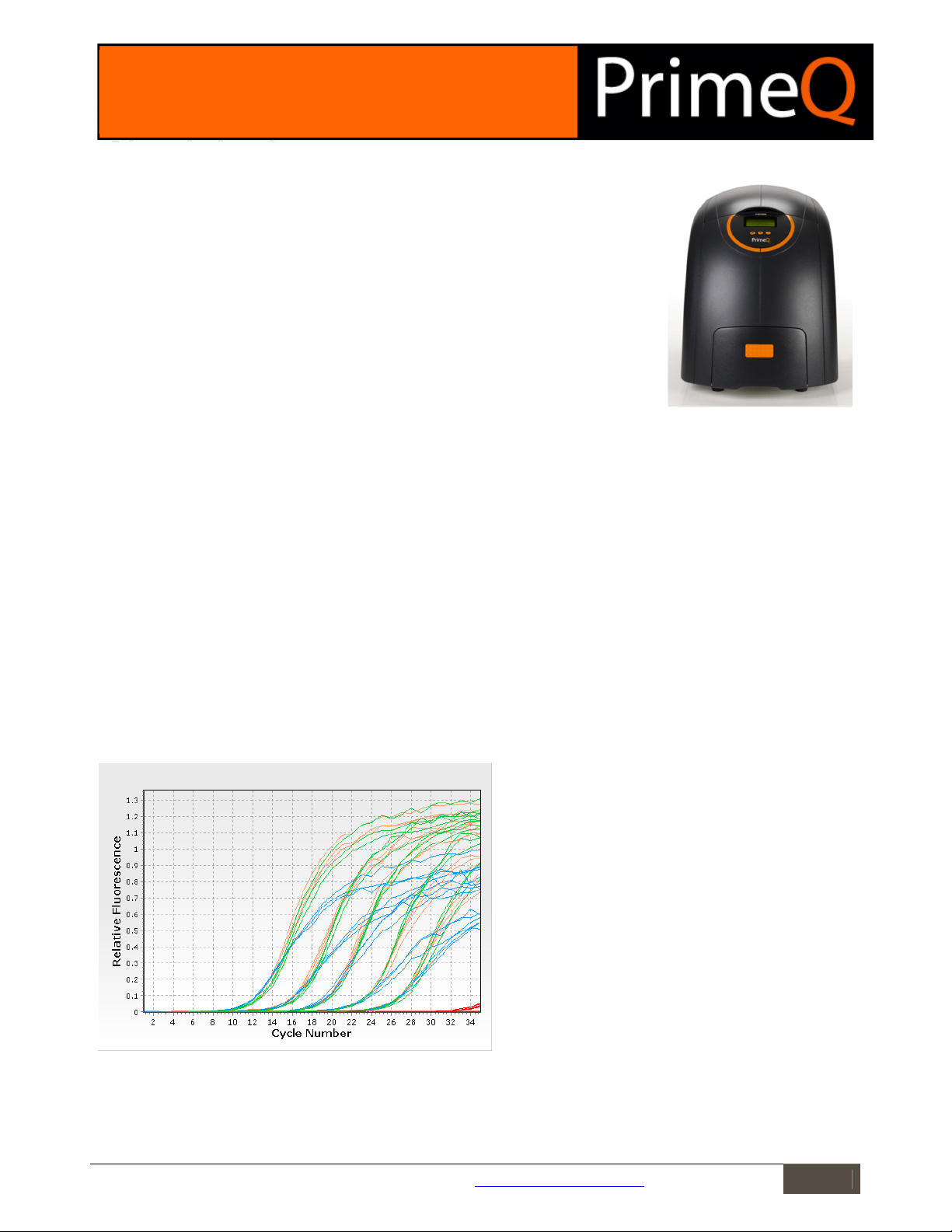
The amplification curves for 10, 15 and 20µl reaction volumes are shown in Figure 1. Results from the 5µl reaction
volume are not shown as this sample size was insufficient to yield a successful amplification curve. Although the
final fluorescence levels were reduced when using a smaller sample volume, the cycles at which amplification first
became apparent was very similar across sample groups. This is illustrated in Figure 2.
Using the fit points method of Quansoft™ Quantification analysis, the quantification cycle (Cq) for each standard
was calculated and plotted against the log of the copy number to give the standard curve.
Figure 1: Amplification curves. Triplicate standards are shown in
blue for the 10µl sample group, orange for the 15µl sample
group, and green for the 20µl sample group. The no template
control samples are shown in red.
PrimeQHelp@bibby
-
scientific.com
|www.bibby
-
scientific.com
+44(01785) 810433
2
A08-001A: Minimising Sample Volumes
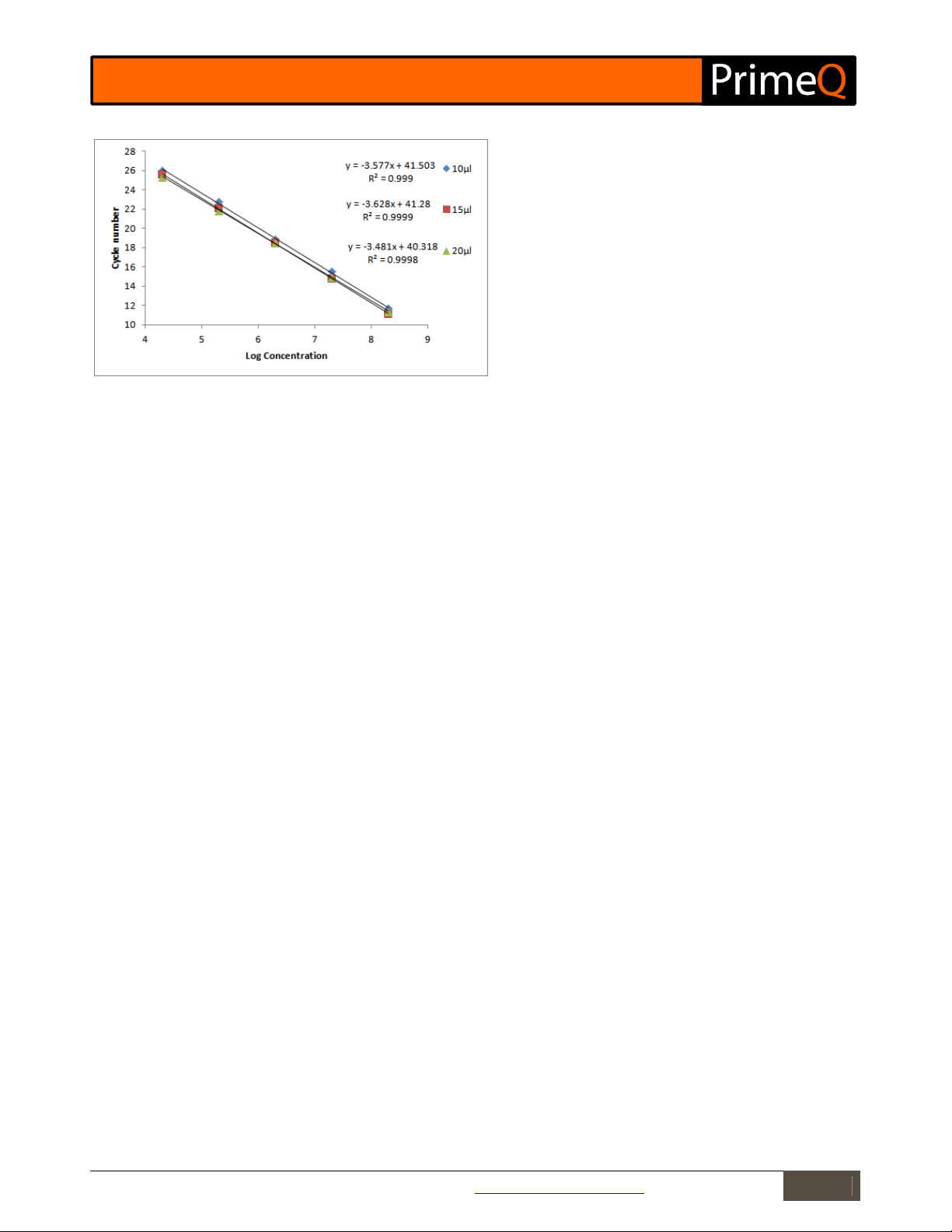
Figure 2
: Standard curve graphs for each sample volume group.
Parallel lines indicate equivalent efficiencies for each reaction.
The standard curves at each sample volume are parallel indicating that the reaction efficiencies in each sample
group are the same. Reducing the sample volume to 10µl does result in a slight reduction in sensitivity as shown by
the offset of the curve; however this is by less than 1 cycle.
Conclusions
The results presented above demonstrate that the reaction sample volume can be reduced by half, from 20 to 10µl
with no effect on reaction efficiency and only minimal effect in reaction sensitivity. Reaction efficiency (E) was
greater than 1.90 in each case and all groups showed excellent correlation of the standards to the curve (R2>0.99).
A 5µl sample volume was found to be insufficient for successful detection of PCR products. The use of low profile
plates in PrimeQ is also of benefit since there is reduced distance between the sample and detection mechanism.
In conclusion, real cost savings can be achieved by reducing reaction volumes as appropriate for the assay.
Trademarks
Quansoft™ is a trademark of Techne
Promega®, GoTaq® and BRYT Green® are registered trademarks of Promega Corporation.
 Loading...
Loading...