Page 1
1288 HD Urology
Camera Head
User Guide
1288310130
Page 2

Page 3

Contents
User Guide ......................................................................................... 1
Guide de l’utilisateur................................................................. 23
Benutzerhandbuch ..................................................................... 45
Manuale d’uso ...............................................................................67
Manual do Utilizador ................................................................. 89
Guía del usuario ......................................................................... 113
Gebruikershandleiding ........................................................... 135
Brugervejledning ....................................................................... 157
Käyttöohje .....................................................................................179
Brukerhåndbok ........................................................................... 201
Användarhandbok ..................................................................... 223
Podręcznik użytkownika ......................................................... 245
Οδηγός χρήσης ........................................................................... 267
用户指南 .............................................................................................. 289
ユーザーガイド .................................................................................. 311
사용 설명서 ........................................................................................ 333
Page 4

Page 5

Contents
Warnings and Cautions ........................................................3
Symbol Definitions .................................................................5
Product Description and Intended Use ......................6
Indications/Contraindications ......................................................6
Product Features .........................................................................6
Setup ...............................................................................................8
Operation ...................................................................................10
Using the Urology Camera Head Buttons .................................10
Adjusting the Focus ...................................................................13
Cleaning, Reprocessing, and Maintenance ...........14
Reprocessing the Urology Camera Head ..................................14
Using Sterile Drapes ..................................................................20
Technical Specifications ...................................................21
Page 6

Page 7

Warnings and Cautions
Please read this manual and follow its instructions carefully.
IMPORTANT SAFETY NOTICE: Before operating this device, please read
this operating manual thoroughly and carefully. When using this device
with a light source, re and/or severe injury may result to the patient, user,
or inanimate objects if the instructions in this manual are not followed.
All light sources can generate signicant amounts of heat at the scope
tip, the scope light post, the light cable tip, and/or near the light cable
adapter. Higher levels of brightness from the light source result in higher
levels of heat. Always adjust the brightness level of the camera and the
monitor before adjusting the brightness level of the light source. Adjust the
brightness level of the light source to the minimum brightness necessary
to adequately illuminate the surgical site. In addition, adjust the internal
shutter of the camera higher in order to run the light source at a lower
intensity. Avoid touching the scope tip or the light cable tip to the patient,
and never place them on top of the patient, as doing so may result in burns
to the patient or user. In addition, never place the scope tip, the scope light
post, the light cable adapter, or the light cable tip on the surgical drapes or
other ammable material, as doing so may result in re. Always place the
light source in standby mode whenever the scope is removed from the light
cable or the device is unattended. e scope tip, scope light post, light cable
adapter, and light cable tip will take several minutes to cool o aer being
placed in standby mode, and therefore may still result in re or burns to the
patient, user, or inanimate objects.
Warnings and Cautions
To avoid potential serious injury to the user and the patient and/or damage
tothis device, please note the following warnings:
1. Be a qualied physician, having complete knowledge of the use of this device.
2. Carefully unpack this device and check if any damage occurred during
shipment. If damage is detected, refer to the standard warranty.
3. Read this operating manual thoroughly, especially the warnings, and be
familiar with its contents before connecting and using this device.
4. Read the entire instruction section of the manual before assembling
orconnecting the camera.
5. Pay close attention to the care, cleaning, disinfection, and sterilization
instructions in this manual. Any deviation may cause damage.
6. Test this device prior to a surgical procedure. is device was fully tested
at the factory before shipment. Never use this device in the presence of
ammable or explosive gases.
3
Page 8

7. Avoid disassembling any part of the camera head, as doing so may break the
seals, causing leakage and/or electric shock. is device has been factory
sealed to prevent moisture from entering electronic components. If the
camera head or cable seal is intentionally broken, the warranty will be void.
8. Before each use, check the outer surface of the camera and endoscope to
ensure that there are no rough surfaces, sharp edges, or protrusions.
9. Using the camera head with broken connector tabs may damage the CCU. If
any tab is missing or damaged, please refer to the Stryker Standard Warranty.
10. Ensure that readjustments, modications, and/or repairs are carried out by
persons authorized by Stryker Endoscopy. Attempt no internal repairs or
adjustments not specically detailed in this operating manual. Doing so may
result in unintended performance or product damage.
11. Always treat the urology camera with care. e camera system contains
sensitive parts that are precisely aligned and may suer damage if dropped
ormistreated.
12. Repeated sterilization via Ethylene Oxide may result in degradation in image
quality.
e warranty is void if any of these warnings are disregarded.
4
Page 9
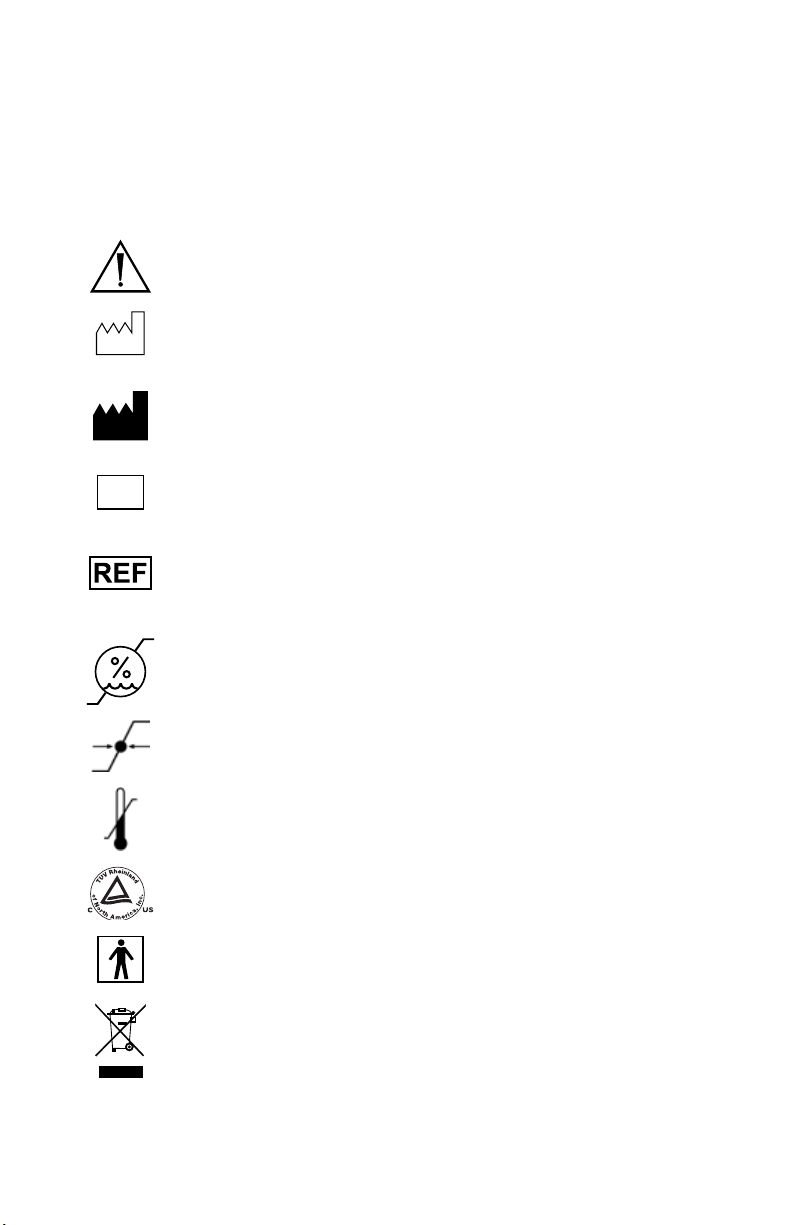
Symbol Definitions
In addition to the cautionary symbols already listed, other symbols found on the
1288 HD Urology Camera Head and in this manual have specic meanings that
clarify the proper use and storage of the 1288 HD Urology Camera Head. e
following list denes the symbols associated with this product:
Warns of presence of important operating and maintenance
instructions in the manual
Date of manufacture
Legal manufacturer
SN
Serial number
Catalogue number
Operating humidity ratings
Operating pressure ratings
Operating temperature ratings
Denotes compliance to CAN/CSA C22.2 No601.1-M90
UL60601-1.
Type BF applied part
is symbol indicates that the waste of electrical and electronic
equipment must not be disposed as unsorted municipal waste
and must be collected separately. Please contact the manufacturer
or other authorized disposal company to decommission your
equipment.
5
Page 10

Product Description and Intended Use
e 1288 HD Urology Camera Head is a high-denition camera used to capture
still and video images of endoscopic urology procedures. It is designed with a 90°
angle between the camera head and the scope to allow for easier access during
urology procedures.
e urology camera head is used in conjunction with the 1288 HD Camera
Console (REF 1288010000; REF 1288010001).
Indications/Contraindications
e 1288 HD Urology Camera is indicated for use in general laparoscopy,
nasopharyngoscopy, ear endoscopy, sinuscopy, and plastic surgery wherever
alaparoscope/endoscope/arthroscope is indicated for use. A few examples
ofthe more common endoscopic surgeries are laparoscopic cholecystectomy,
laparoscopic hernia repair, laparoscopic appendectomy, laparoscopic pelvic
lymph node dissection, laparoscopically assisted hysterectomy, laparoscopic
andthorascopic anterior spinal fusion, anterior cruciate ligament reconstruction,
knee arthroscopy, shoulder arthroscopy, small joint arthroscopy, decompression
xation, wedge resection, lung biopsy, pleural biopsy, dorsal sympathectomy,
pleurodesis, internal mammary artery dissection for coronary artery bypass,
coronary artery bypass graing where endoscopic visualization is indicated
and examination of the evacuated cardiac chamber during performance of
valve replacement. e users of the camera are general surgeons, gynecologists,
cardiac surgeons, thoracic surgeons, plastic surgeons, orthopedic surgeons, ENT
surgeons and urologists.
ere are no known contraindications.
Product Features
e urology camera head connects to the camera console and captures video
and photographic images, which it relays to the camera console. It features several
controls that are accessible through a button keypad located on the top of the
urology camera head (see the “Operation Instructions” section of this manual).
6
Page 11
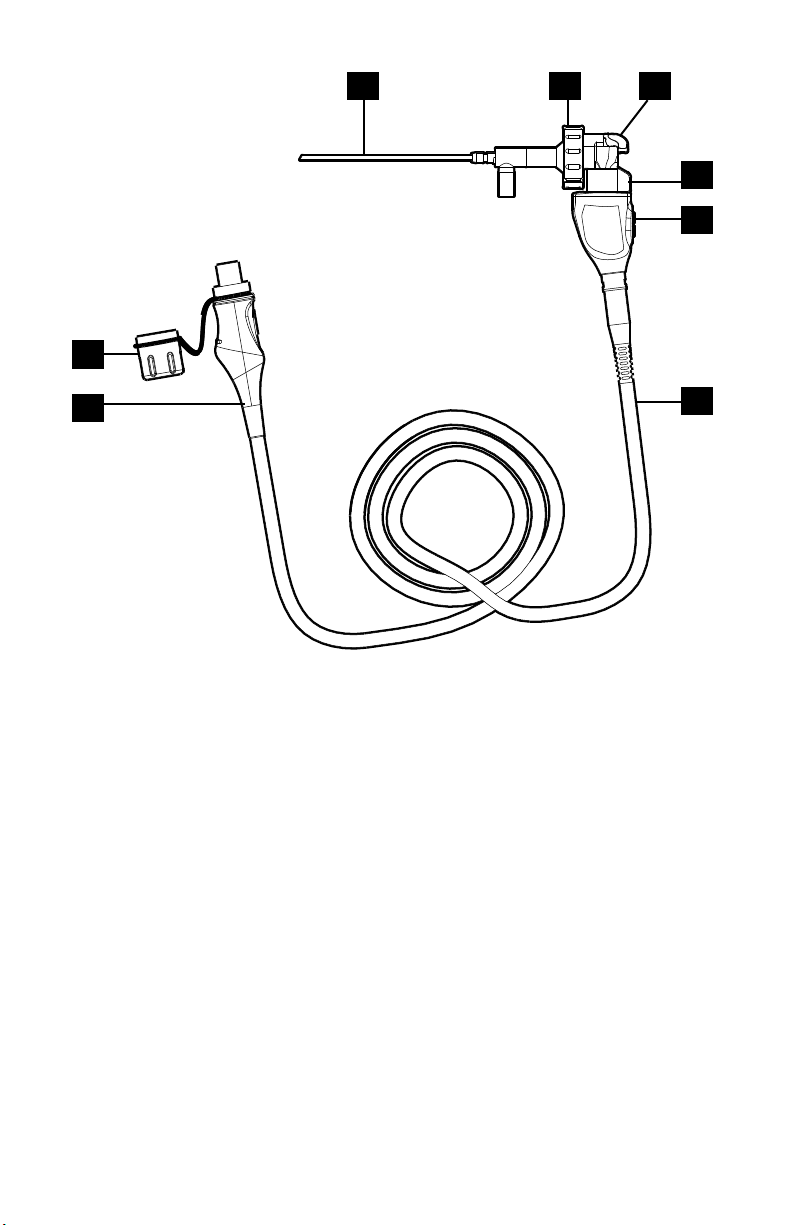
1
2 3
4
5
8
7
1. Endoscope —
2. Endobody clamp Secures the scope to the camera head
3. Endobody brake Prevents rotation of scope
4. Focusing knob Adjusts the focus of the camera head
5. 1288 HD Urology
camera head
6. Urology camera
cable
Captures photographic and video images and provides
camera controls
—
6
7. Cable connector Connects the camera head to the camera console
8. Soaking cap Protects the cable connector during cleaning and
sterilization
7
Page 12
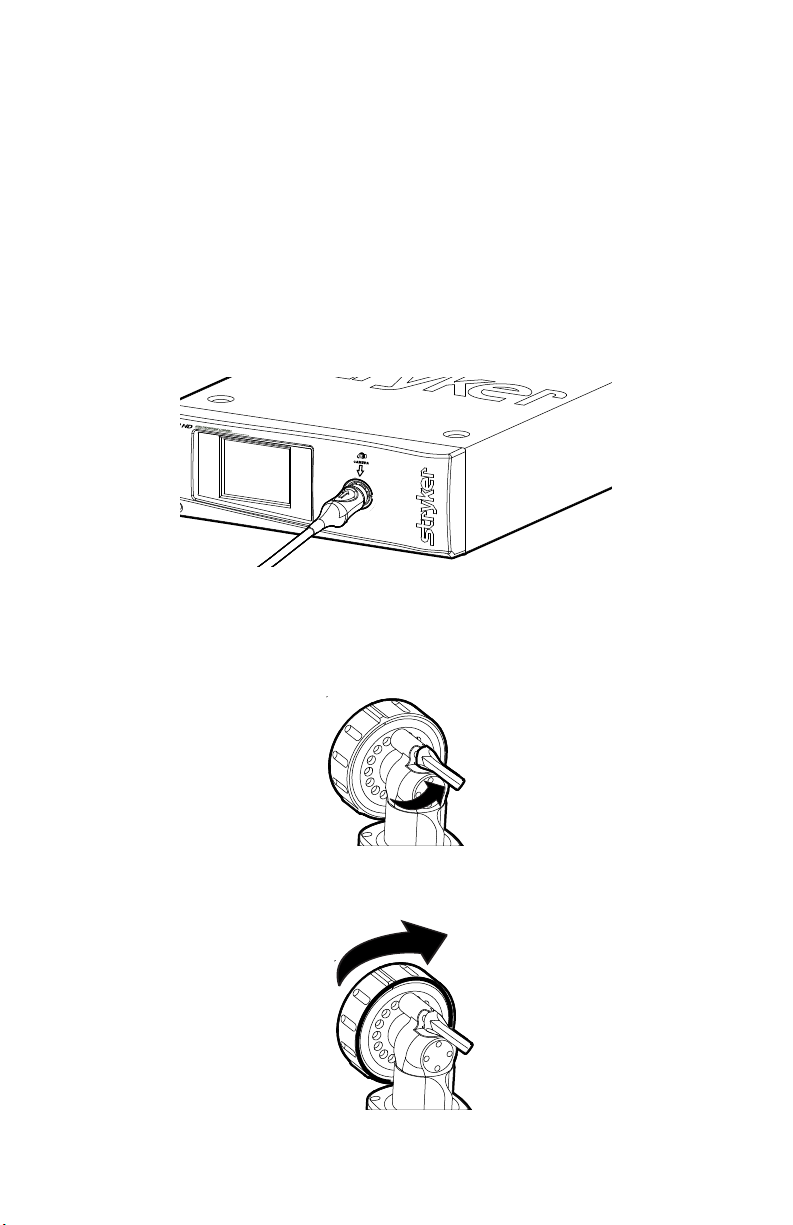
Setup
1. Set up the 1288 HD Camera Console according to the instructions provided
in its user manual.
2. Connect the urology camera head to the console.
• Unscrew the soaking cap from the cable connector if necessary.
• Align the blue arrow on the cable connector with the blue arrow
onthe urology camera-connector port on the front console panel.
• Push in the connector until it locks in place.
• (To unplug the urology camera from the control unit, grasp the
knobbed portion of the connector and pull straight out.)
3. Attach an endoscope to the urology camera head.
• Remove the red dust cap if it is present.
• Lock the endobody brake by pushing it to the right.
• Twist the endobody clamp and hold it open.
8
Page 13
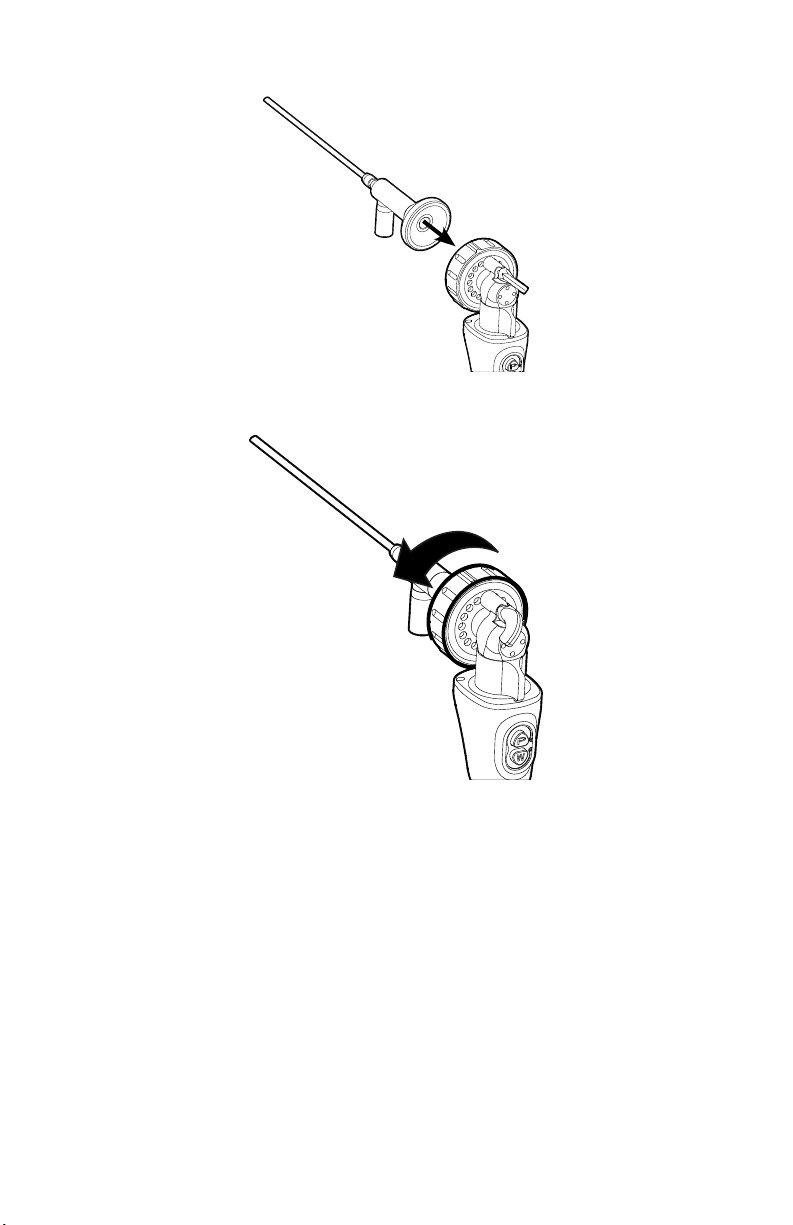
• Insert the endoscope into the endobody clamp.
• Twist the endobody brake in the reverse direction to secure the
endoscope.
4. Attach a light cable from the light source to the light post on the endoscope.
9
Page 14
Operation
Warning: Before using the 1288 HD Urology Camera in a
surgical procedure, test all components to ensure proper
function. Ensure that a video image appears on all video
monitors before beginning any procedure.
e 1288 HD Urology Camera can be controlled using buttons on the camera
head or the touchscreen interface on the console.
Using the Urology Camera Head Buttons
e urology camera head features an oval, two-button keypad for controlling the
1288 urology camera. ese buttons are labeled P and W.
P (Picture) Button
e P button controls up to two remote video accessories.
• Press the P button for less than two seconds to select Remote 1. One beep
will sound.
• Press the P button for more than two seconds to select Remote 2. Two beeps
will sound.
W (White Balance) Button
e W button activates the white-balance function or the light/zoom function.
e white balance function is used to correct slight color dierences that exist
between dierent light sources or endoscopes.
• Press the W button for more than two seconds to activate the white balance
function.
• Press the W button for less than two seconds to increase the zoom one of
eight levels. (e zoom will cycle to the lowest level aer completing the
cycle.)
Perform the white balance procedure before every surgical procedure.
10
Page 15
Note: Ensure that a scope and light source are attached to the camera, and that
the camera, light source and monitor are powered on before adjusting the white
balance.
1. Point the scope at several stacked 4 × 4 white gauze pads, a white laparoscopic
sponge, or any clean white surface.
2. Look at the monitor and make sure that no glare is visible o of the white
surface.
3. Press and hold the W button until “WHITE BALANCE IN PROGRESS”
begins ashing on the video monitor.
4. Continue pointing the scope at the white surface until the video monitor
indicates that white balance is “WHITE BALANCE COMPLETE.” e video
picture may change color. If you cannot achieve an acceptable white balance,
refer to the 1288 HD Camera Console user guide.
Using the Touchscreen Interface
e touchscreen interface on the console provides controls for operating the
camera and selecting system settings. From the touchscreen, you can:
• choose camera settings for urology procedures
• capture photos
• capture video
• activate white balance
11
Page 16
Scroll through preset camera settings designed for
surgical specialties. Choose from:
• Arthroscopy
• Cystoscopy
• ENT
• Flexi-Scope
• Hysteroscopy
Capture photo.
Press and hold button for two seconds before
itactivates.
Capture video.
Press and hold button for two seconds before
itbegins recording. Press again to stop.
Activate white balance.
Press and hold button for two seconds before
itactivates.
• Press once to proceed to the Menu screen.
• Laparoscopy
• Laser
• Microscope
• Standard
12
Page 17

Adjusting the Focus
Slide the focusing knob to the le or right to adjust the focus.
13
Page 18

Cleaning, Reprocessing, and
Maintenance
Reprocessing the Urology Camera Head
ese reprocessing instructions are provided in accordance with ISO 17664,
AAMI TIR12, AAMI ST79, and AAMI ST81. While they have been validated
by Stryker as being capable of preparing the device for re-use, it remains the
responsibility of the processor to ensure that the reprocessing as actually
performed, using equipment, materials, and personnel in the reprocessing
facility, achieves the desired result. is normally requires validation and
routine monitoring of the process. Stryker recommends users observe these
standards when reprocessing medical devices.
Warnings
• is device must be cleaned and sterilized prior to the rst use and aer
every subsequent use.
• Use only the sterilization cycles outlined in this document. Using
unspecied sterilization cycles may damage the device or result in
incomplete sterilization.
• Separate the urology camera head and scope prior to cleaning,
disinfection, or sterilization.
• Wear appropriate protective equipment: gloves, eye protection, etc.
Cautions
• Always install the soaking cap prior to processing the urology camera.
Failure to properly tighten the soaking cap will corrode the connector
pins and void the warranty.
• Inspect the camera cable for cuts and breaks before soaking in any uid.
Return any damaged camera to Stryker for service.
• Do not use brushes or pads with metal or abrasive tips during manual
cleaning, as permanent scoring or damage could result.
• To minimize galvanic corrosion, avoid soaking dissimilar metals in close
proximity.
• Never soak the camera in the same tray with sharp instruments.
• Repeated sterilization via Ethylene Oxide may result in degradation
inimage quality.
14
Page 19
Limitations on Reprocessing
• Do not cross-sterilize the device. Using multiple sterilization methods
may signicantly reduce the performance of the device.
• Do not leave the device in solutions longer than necessary. is may
accelerate normal product aging.
• Proper processing has a minimal eect on this device. End of life is
normally determined by wear and damage due to use.
• Damage incurred by improper processing will not be covered by the
warranty.
Instructions
Point of Use
• Wipe excess soil from the device using disposable paper towels.
• If an automated reprocessing method will be used, rinse any channels
inthe device with 50mL of sterile distilled water immediately aer use.
Containment and Transportation
• Reprocess the device as soon as reasonably practical following use1.
• Transport the device in a tray to avoid damage.
1
A 30 minute wait time was used during cleaning validation.
Preparation for Cleaning
1. Disassemble the coupler from the scope and camera head.
2. Prepare an enzymatic detergent according to the manufacturer’s
recommendations (one ounce per gallon of tap water at 35 - 40°C)2.
3. Wipe the entire device with the detergent, using a clean cloth.
4. Immerse the device in the detergent. Using a syringe, inject any inside
regions of the device with 50mL of the detergent to ensure all parts of the
device are reached.
5. Soak the device in the detergent for a minimum of 15 minutes.
15
Page 20

Cleaning: Manual
1. Brush
• Prepare a fresh solution of enzymatic detergent according to the
manufacturer’s recommendations (one ounce per gallon of tap water
at35 - 40°C)
• oroughly brush the exterior of the device with a so-bristled brush,
focusing on any mated or rough surfaces.
• Using a syringe, inject any lumen or mated surface a minimum of
5times with 50mL of the detergent.
• Brush any lumens a minimum of 5 times from each end, using an
appropriate bottle brush.
• Brush any movable parts in all extreme positions.
2. Rinse
• Rinse the device with reverse osmosis/de-ionized (RO/DI) water at
ambient temperature until all detergent residue is removed. Flush any
lumens or mated surfaces a minimum of 5 times. Once all detergent
residue is removed, continue to rinse for a minimum of 30 seconds.
• Drain excess water from the device and dry it using a clean cloth or
pressurized air.
• Visually inspect the device for cleanliness, paying close attention to
hard-to-reach areas. If visible soil remains, repeat steps 1 and 2.
3. Soak
• Prepare a non-enzymatic detergent according to the manufacturer’s
recommendations (0.25 ounces per gallon of tap water at 35 - 40°C)
• Fully immerse the device and use a syringe to inject any lumens and
mated surfaces with 50mL of the detergent.
• Soak the device for a minimum of 15 minutes.
4. Brush
• oroughly brush the exterior of the device using a so-bristled brush.
• Using a syringe, inject 50mL of the detergent into any cannulae, lumens,
or mated surfaces a minimum of 5 times.
• Brush any lumens a minimum of 5 times from each end, using an
appropriate bottle brush.
• Actuate the device, brushing around any movable parts in all extreme
positions.
5. Rinse
• oroughly rinse the device with RO/DI water until all detergent
residue is removed. Flush any lumens or crevices a minimum of 5 times.
Once all detergent residue is removed, continue to rinse for a minimum
of 30 seconds.
• Drain the excess water from the device and dry it using a clean cloth or
pressurized air.
2
.
3
.
16
Page 21

Cleaning: Automated
1. Brush
• Using a syringe, inject 50mL of the enzymatic detergent (from the
“Preparation for Cleaning” section) into any lumen and mated surface
aminimum of one time.
• Brush from both ends of any lumens a minimum of 1 time, using an
appropriate bottle brush.
2. Rinse
• Rinse the device with RO/DI water at ambient temperature until there
is no visible detergent residue. Continue to rinse for a minimum of
30seconds aer all detergent residue has been removed.
• Place the device in the washer on an incline to facilitate drainage.
3. Automated wash
• Program the washer using the following parameters:
Phase Recirculation
Time
Pre Wash 2 minutes Cold tap
Enzyme
2 minutes Hot tap water Enzymatic Detergent
Wash
Wash 1 2 minutes Set point
Rinse 1 2 minutes Hot tap water N/A
Dry Phase 7 minutes 115°C N/A
• Upon completion of the draining phase aer Rinse 1, stop the cycle and
open the washer door.
• Remove the device from the washer during the thermal phase and place
the device back into the washer for the dry phase.
• If necessary, use pressurized air to aid in drying. Visually inspect each
device for cleanliness.
Water
Temperature
water
(66°C)
Detergent Type and
Concentration
(if applicable)
N/A
Non-enzymatic Detergent
2
3
2
ENZOL® Enzymatic Detergent is validated for cleaning ecacy.
3
Renu-Klenz® is validated for cleaning ecacy.
17
Page 22

Low Level Disinfection (optional)
1. Disinfect the device in a disinfecting solution that has one of the following
active Ingredients:
• ≥ 2.4% glutaraldehyde4 with a minimum soaking time
of45minutesat25°C
or
• ≥ 3.4% glutaraldehyde5 with a minimum soaking time
of20minutesat25°C
or
• ≥ 0.55% ortho-phthalaldehyde6 with a minimum soaking time
of12minutes at 25 °C.
2. Prepare the disinfecting solution according to the manufacturer’s
instructions.
3. Per manufacturer’s recommendations, immerse the device, lling all
lumens, in the disinfecting solution for the required time at the appropriate
temperature.
4. oroughly rinse and ush all parts and lumens with running,
demineralized water to remove the disinfectant.
5. Dry all parts with a lint-free towel immediately aer rinsing.
4
CIDEX Activated® is validated for disinfection ecacy.
5
CIDEX Plus® is validated for disinfection ecacy.
6
CIDEX® OPA is validated for disinfection ecacy.
Drying
• For automated drying, use the drying cycle provided with the washer/
disinfector.
• For manual drying, use a lint-free cloth.
• Dry any lumens with compressed air.
Maintenance, Inspection, and Testing
• Inspect the device on a continual basis. If a problem is observed
orsuspected, the device should be returned for repair.
• Inspect all components for cleanliness. If uid or tissue buildup
ispresent, repeat the above cleaning and disinfection procedures.
• Inspect the camera cable for cuts and breaks. Return any damaged
urology camera to Stryker for service.
Packaging
N/A
18
Page 23

Sterilization
Aer performing the cleaning instructions specied above, perform one of the
following sterilization cycles.
Ethylene Oxide (EtO)
• Double wrap camera head and cable prior to sterilization.
Preconditioning parameters
Temperature 55°C (131°F)
Chamber Humidity 70% RH
Vacuum Set Points 1.3 psia
Time 30 minutes
Exposure
Concentration (100% EtO) 725 mg/L
Temperature 55 ± 2°C (131 ± 5°F)
Time 1 hour
Chamber Humidity 70 ± 5% RH
Aeration parameters
Aeration Time 12 hours
Temperature 35 – 54°C (95 – 129°F)
Steris® System 1
1. Clean and prepare the urology camera head and cable as recommended in
the Cleaning section.
2. Sterilize the urology camera head and cable using Steris® System 1 with
Steris® Sterilant 20.
3. Allow the urology camera head, cable, and scope to completely dry before
reassembly. Any moisture may cause the camera window to fog during use.
Sterrad®
1. Clean and prepare the urology camera head and cable as recommended
inthe Cleaning section.
2. Sterilize the urology camera head and cable using the Sterrad™ NX or
100SSterilization System.
Storage
Never store the device in a non-ventilated, humid environment such
asacarrying case. is may present an infection control risk.
19
Page 24
Using Sterile Drapes
Using sterile drapes will ensure maximum longevity of your 1288 HD
Camera Head. For best results, follow the instructions provided by the drape
manufacturer.
Disposal
is product contains electrical waste or electronic equipment.
It must not be disposed of as unsorted municipal waste and
must be collected separately in accordance with applicable
national or institutional related policies relating to obsolete
electronic equipment.
e 1288 HD must be disposed of according to local laws and
hospital practices.
20
Page 25

Technical Specifications
60Hz settings are displayed rst. (50Hz settings follow in parentheses.)
Imaging System 1/3” Progressive Scan CCDs
High Denition
Operating Conditions Temperature: 5 – 40°C
Relative Humidity: 30 – 95%
Transport and Storage
Conditions
Total Shipping Weight 1.5 lbs. (0.680 kg) Urology Camera head
Dimensions Camera Head Cable to Camera Console:
Enhancement 8 levels (switchable)
Classication Type BF Applied Part
Complies with Medical
Safety Standards
Temperature: -20 – 60°C
Relative Humidity: 10 – 95%
Atmospheric Pressure: 700 – 1060 hPa
10 (3.0 m) sealed cable
20 (6.1 m) cable extension available
Water Ingress Protection, IPX7—protected
against the eects of temporary immersion
inwater
IEC 60601-1:1988 + A1:1991 + A2:1995
IEC 60601-2-18:1996 + A1:2000
CAN/CSA C22.2 No 601.1-M90
UL 60601-1:2003
AS/NZS 3200.1.0:1998
CSA 22.2.601.1.1:2002
CAN/CSA C22.2 No. 601.2.18:1990
Please contact your local Stryker Endoscopy sales representative for information
on changes and new products.
21
Page 26

Page 27

Table des matières
Avertissements et précautions d’emploi ................25
Définition des symboles ....................................................27
Description et utilisation du produit .........................28
Indications/Contre-indications ...................................................28
Caractéristiques du produit .......................................................28
Montage ......................................................................................30
Utilisation ...................................................................................32
Utilisation des boutons de la tête de caméra urologique ..........32
Mise au point .............................................................................35
Nettoyage, retraitement etentretien ........................36
Retraitement de la tête de caméra urologique ..........................36
Utilisation de housses stériles ...................................................42
Caractéristiques techniques ..........................................43
Page 28

Page 29

Avertissements et précautions
d’emploi
Il convient de lire intégralement ce manuel et d’en suivre attentivement
lesinstructions.
AVIS DE SÉCURITÉ IMPORTANT: Lire attentivement ce manuel
d’utilisation avant d’actionner ce matériel. Lors de l’utilisation de ce matériel
avec une source de lumière, le non-respect des instructions contenues dans
cemanuel peut entraîner un incendie, des dommages auxobjets inanimés
et/ou des blessures graves au patient ou à l’utilisateur. Toutes lessources
delumière peuvent générer des volumes importants dechaleur àl’extrémité
de l’endoscope, au port d’éclairage de l’endoscope, à l’extrémité du câble
d’éclairage et/ou à proximité de l’adaptateur de câble d’éclairage. Desniveaux
plus élevés de luminosité de la source de lumière génèrent desniveaux
supérieurs de chaleur. Toujours régler le niveau de luminosité de la caméra
etdu moniteur avant de régler le niveau de luminosité de la source de
lumière. Régler le niveau de luminosité dela source de lumière surla
luminosité minimum nécessaire à l’éclairage adéquat du site chirurgical.
Enoutre, régler l’obturateur interne de la caméra à un niveau plus élevé
ande faire fonctionner la source de lumière à une intensité inférieure.
Éviterque l’extrémité de l’endoscope ou du câble d’éclairage touche le patient
et ne jamais les placer sur le patient, sous risque d’entraîner des brûlures pour
le patient ou l’utilisateur. Deplus, ne jamais placer l’extrémité de l’endoscope,
le port d’éclairage de l’endoscope, l’adaptateur du câble d’éclairage ou
l’extrémité du câble d’éclairage sur les champs opératoires ou tout autre
matériau inammable. En eet, cela pourrait entraîner un incendie.
Toujoursplacer la source de lumière en mode de veille si l’endoscope
estretirédu câble d’éclairage ou si l’équipement est laissé sans surveillance.
Une fois que la source est placée en mode de veille, le refroidissement de
l’extrémité de l’endoscope, du port d’éclairage de l’endoscope, de l’adaptateur
de câble d’éclairage etde l’extrémité du câble d’éclairage prend plusieurs
minutes. Ces parties pourraient dès lors toujours entraîner un incendie
oudes brûlures pour lepatient, l’utilisateur ou des objets inanimés.
Avertissements et précautions d’emploi
Pour éviter des blessures graves à l’utilisateur et au patient et/ou des dommages
àcet équipement, il convient de tenir compte des avertissements suivants:
1. L’utilisateur doit être un praticien qualié, possédant une parfaite connaissance
du fonctionnement de l’équipement.
2. Déballer l’équipement avec précaution et vérier qu’il n’a pas été endommagé
pendant le transport. Le cas échéant, consulter les termes de la garantie
standard.
25
Page 30

3. Lire intégralement le présent manuel d’utilisation, en particulier les
avertissements, et se familiariser avec son contenu avant de connecter
etd’utiliser l’équipement.
4. Lire intégralement les instructions du manuel avant d’assembler
etdeconnecter la caméra.
5. Il convient de prêter une grande attention aux recommandations d’entretien,
denettoyage, de désinfection et de stérilisation présentées dans ce manuel.
Tout manquement dans ce domaine peut entraîner des dommages.
6. Tester l’équipement préalablement à toute procédure chirurgicale.
Cetéquipement a été intégralement contrôlé en usine avant son transport.
Nejamais utiliser cet équipement en présence de gaz inammables
ouexplosifs.
7. Éviter de démonter les composants de la tête de caméra an de ne pas rompre
les joints et de provoquer des fuites ou d’éviter tout risque d’électrocution.
Cetéquipement a été scellé en usine an d’éviter toute inltration d’humidité
au niveau des composants électroniques. Si les scellés de la tête de caméra
oudu câble viennent à être rompus intentionnellement, la garantie est annulée.
8. Avant chaque utilisation, vérier que la surface externe de la caméra et
del’endoscope ne présente aucune rugosité, arête aiguë ou protubérance.
9. L’utilisation de la tête de caméra avec des broches cassées peut endommager
la console. Si la moindre broche est manquante ou endommagée,
consulterlestermes de la garantie standard de Stryker.
10. S’assurer que les réajustements, modications et/ou réparations sont
eectués par des personnes autorisées par la société Stryker Endoscopy.
N’eectuer aucun réglage ou réparation interne qui ne soit pas spéciquement
détaillé dans le manuel d’utilisation, sous peine d’altérer les performances
oud’endommager l’instrument.
11. Toujours manipuler délicatement la caméra urologique. Le système de caméra
comporte des pièces sensibles qui sont alignées avec précision et peuvent
êtreendommagées en cas de chute ou de mauvaise manipulation.
12. L’emploi répété de la méthode de stérilisation à l’oxyde d’éthylène peutentraîner
une dégradation de la qualité d’image.
La garantie sera nulle et non avenue si ces avertissements ne sont pas respectés.
26
Page 31

Définition des symboles
Outre les symboles de sécurité déjà répertoriés, d’autres symboles rencontrés
surla tête de caméra urologique 1288 HD et dans le présent manuel possèdent
unesignication particulière qui explique l’usage et le stockage appropriés de
latête de caméra urologique 1288 HD. La liste ci-dessous présente la dénition
dessymboles associés à ce produit:
Avertit de la présence d’instructions d’utilisation et d’entretien
importantes dans le manuel
Date de fabrication
Fabricant légal
SN
Numéro de série
Référence catalogue
Taux d’humidité opérationnels
Niveaux de pression opérationnels
Plages de températures opérationnelles
Conformité aux normes CAN/CSA C22.2 N°601.1-M90
UL60601-1.
Pièce appliquée de type BF
Ce symbole indique que les équipements électriques et
électroniquesusagés ne doivent pas être mis au rebut comme
lesdéchets ménagers ordinaires et doivent être collectés séparément.
Contacter le fabricant ou une société agréée d’élimination desdéchets
pour organiser la mise au rebut du matériel.
27
Page 32

Description et utilisation
duproduit
La tête de caméra urologique 1288 HD est une caméra de haute dénition
utilisée pour l’acquisition d’images xes et d’images vidéo au cours de procédures
urologiques endoscopiques. Elle est conçue avec un angle de 90° entre la tête de
caméra et l’endoscope pour faciliter l’accès pendant les interventions urologiques.
La tête de caméra urologique s’utilise en association avec la console de caméra1288HD
(réf. 1288010000; réf. 1288010001).
Indications/Contre-indications
La caméra urologique 1288 HD peut être utilisée pendant les interventions
générales de laparoscopie, de nasopharyngoscopie, d’endoscopie de l’oreille,
de sinuscopie et de chirurgie plastique lorsque l’utilisation d’un laparoscope,
d’un endoscope ou d’un arthroscope est indiquée. Voici quelques exemples
des interventions chirurgicales endoscopiques les plus courantes utilisant cette
caméra: cholécystectomie laparoscopique, réparation laparoscopique des
hernies, appendectomie laparoscopique, dissection laparoscopique des ganglions
lymphatiques pelviens, hystérectomie assistée par laparoscopie, fusion spinale
antérieure par laparoscopie et thorascopie, reconstruction des ligaments croisés
antérieurs, arthroscopie des genoux, des épaules et des petites articulations,
xation des décompressions, résection en coin, biopsie pulmonaire et pleurale,
sympathectomie dorsale, pleurodèse, dissection de l’artère mammaire interne
pour pontage de l’artère coronarienne, grees de pontage de l’artère coronarienne
lorsqu’une visualisation endoscopique est indiquée, et examen de la chambre
cardiaque évacuée pendant un remplacement de valve. L’utilisation de la caméra
est destinée aux chirurgiens généralistes, aux gynécologues, aux chirurgiens
cardiaques, thoraciques, plasticiens, orthopédiques et oto-rhino-laryngologistes,
ainsi qu’aux urologues.
Il n’existe pas de contre-indications connues.
Caractéristiques du produit
La tête de caméra urologique est connectée à la console. Elle lui transmet les
images photographiques et vidéo capturées. Elle est dotée de plusieurs commandes
accessibles depuis le clavier à boutons situé sur le dessus de la tête de caméra
urologique (consulter le paragraphe «Instructions d’utilisation» du présent manuel).
28
Page 33

1
2 3
4
5
8
7
1. Endoscope —
2. Pince du porte-endoscope Fixe l’endoscope sur la tête de caméra
3. Frein du porte-endoscope Empêche la rotation de l’endoscope
4. Bouton de mise au point Ajuste la mise au point de la tête de caméra
5. Tête de caméra
urologique1288 HD
6. Câble de la caméra
urologique
Capture les images photographiques et vidéo
etpermetde contrôler la caméra
—
6
7. Connecteur de câble Connecte la tête de caméra à la console
8. Capuchon étanche Protège le connecteur de câble pendant
lenettoyageetla stérilisation
29
Page 34

Montage
1. Monter la console de caméra 1288 HD conformément aux instructions fournies
dans le manuel d’utilisation.
2. Connecter la tête de caméra urologique à la console.
• Si nécessaire, dévisser le capuchon étanche du connecteur de câble.
• Sur le panneau avant de la console, aligner la èche bleue du
connecteur de câble avec celle du port de connexion de la caméra
urologique.
• Pousser le connecteur à fond jusqu’au verrouillage.
• (Pour déconnecter la caméra urologique de la console de commande,
saisir la partie enrobée du connecteur et tirer vers soi.)
3. Raccorder un endoscope à la tête de caméra urologique.
• Enlever le capuchon de protection rouge s’il est en place.
• Verrouiller le frein du porte-endoscope en le poussant vers la droite.
30
• Tourner la pince du porte-endoscope et la maintenir ouverte.
Page 35

• Insérer l’endoscope dans la pince du porte-endoscope.
• Tourner le frein du porte-endoscope dans le sens inverse pour
xerl’endoscope.
4. Relier par un câble la source de lumière et le port d’éclairage de l’endoscope.
31
Page 36

Utilisation
Avertissement: Avant d’utiliser la caméra urologique 1288HD
au cours d’une procédure chirurgicale, vérier le bon fonctionnement de tous ses composants. Vérier qu’une image apparaît
sur tous les moniteurs vidéo avant de commencer uneprocédure.
La caméra urologique 1288 HD peut être contrôlée à l’aide des boutons présents
surla tête de caméra ou de l’interface tactile de la console.
Utilisation des boutons de la tête de caméra
urologique
La tête de caméra urologique comporte un clavier ovale, doté de deux boutons,
qui permettent de contrôler la caméra urologique 1288. Ces boutons portent
lamention P et W.
Bouton P (image)
Le bouton P permet de contrôler jusqu’à deux accessoires vidéo distants.
• Appuyer sur le bouton P pendant moins de deux secondes pour sélectionner
lasortie 1. Un bip sonore retentit.
• Appuyer sur le bouton P pendant plus de deux secondes pour sélectionner
lasortie 2. Deux bips sonores retentissent.
Bouton W (équilibrage des niveaux de blanc)
Le bouton W active la fonction d’équilibrage des niveaux de blanc ou la fonction
de luminosité/zoom. La fonction d’équilibrage des niveaux de blanc permet de
corriger les diérences de couleurs légères qui existent entre les sources de lumière
ou les endoscopes.
• Appuyer sur le bouton W pendant plus de deux secondes pour activer
lafonction d’équilibrage des niveaux de blanc.
• Appuyer sur le bouton W pendant moins de deux secondes pour augmenter
leniveau de zoom d’un niveau (sur huit possibles). Après le huitième niveau,
lezoom est ramené au réglage le plus bas.
32
Page 37

Eectuer l’équilibrage des niveaux de blanc avant chaque procédure chirurgicale.
Remarque: Avant d’ajuster l’équilibrage des niveaux de blanc, vérier qu’un
endoscope et une source de lumière sont xés à la caméra et que la caméra,
lasource de lumière et le moniteur sont sous tension.
1. Pointer l’endoscope sur une pile de compresses de gaze blanches de 10 x 10 cm,
uneéponge laparoscopique blanche ou une surface blanche quelconque.
2. Regarder le moniteur et s’assurer qu’il n’existe pas d’éclat visible en dehors
delasurface blanche.
3. Maintenir le bouton W enfoncé jusqu’à ce que «ÉQUILIBRAGE DES NIVEAUX
DE BLANC EN COURS» commence à clignoter sur le moniteur vidéo.
4. Maintenir l’endoscope pointé sur la surface blanche jusqu’à ce que le moniteur
vidéo indique «ÉQUILIBRAGE DES NIVEAUX DE BLANC TERMINÉ».
L’image vidéo peut changer de couleur. Se référer au Guide de l’utilisateur de la
console de caméra 1288 HD s’il est impossible d’obtenir un équilibrage correct
des niveaux de blanc.
Utilisation de l’interface tactile
L’interface tactile de la console fournit les commandes pour contrôler la caméra
etsélectionner les réglages du système. Sur l’écran tactile, l’utilisateur peut:
• choisir les réglages de la caméra pour les interventions urologiques
• capturer des photos
• capturer des vidéos
• activer l’équilibrage des niveaux de blancs
33
Page 38

Faire déler les réglages prédénis de la caméra en
fonction de la spécialité chirurgicale. Choix possibles:
• Arthroscopie
• Cystoscopie
• ORL
• Flexi-Scope
• Hystéroscopie
Capturer une photo.
Maintenir le bouton enfoncé pendant deux secondes
pour activer cette fonction.
Capturer une vidéo.
Maintenir le bouton enfoncé pendant deux secondes
pour activer l’enregistrement. Appuyer de nouveau
surle bouton pour arrêter cette fonction.
Activer l’équilibrage des niveaux de blancs.
Maintenir le bouton enfoncé pendant deux secondes
pour activer cette fonction.
• Appuyer une fois pour passer à l’écran demenu.
• Laparoscopie
• Laser
• Microscope
• Standard
34
Page 39

Mise au point
Déplacer le bouton de mise au point vers la gauche ou la droite pour régler l’image.
35
Page 40

Nettoyage, retraitement
etentretien
Retraitement de la tête de caméra urologique
Ces instructions de retraitement sont fournies conformément aux normes
ISO 17664, AAMI TIR12, AAMI ST79 et AAMI ST81. Bien qu’elles aient été
validées par Stryker comme étant capables de préparer l’équipement en vue de sa
réutilisation, il incombe à l’opérateur de s’assurer que le retraitement, tel que réalisé,
permet d’obtenir le résultat souhaité en utilisant du matériel, des matériaux et du
personnel sur le site de retraitement. Cela requiert normalement une validation
et un suivi de routine du traitement. Stryker recommande aux utilisateurs de
respecter ces normes lors du retraitement d’équipements médicaux.
Avertissements
• Cet équipement doit être nettoyé et stérilisé avant la première utilisation
et après chaque utilisation ultérieure.
• Utiliser uniquement les cycles de stérilisation décrits dans ce document.
L’utilisation de cycles de stérilisation non spéciés risque d’endommager
l’équipement ou d’entraîner une stérilisation incomplète.
• Séparer la tête de caméra urologique et l’endoscope avant le nettoyage,
ladésinfection ou la stérilisation.
• Porter des équipements de protection appropriés: gants, protections
oculaires, etc.
Précautions d’emploi
• Toujours mettre en place le capuchon étanche avant de traiter la caméra
urologique. Un serrage incorrect du capuchon étanche risque de provoquer
une corrosion des broches du connecteur et d’annuler la garantie.
• Rechercher toutes coupures ou cassures éventuelles sur le câble de
lacaméra avant de l’immerger dans un liquide. Retourner une caméra
endommagée à Stryker pour réparation.
• Pendant le nettoyage manuel, ne pas employer de brosses ou de tampons
comportant des embouts métalliques ou abrasifs sous peine d’iniger
auxinstruments des éraures ou des dommages permanents.
• Afin de minimiser les risques de corrosion galvanique, éviter de faire
tremper des métaux différents à proximité les uns des autres.
• Ne jamais faire tremper la caméra dans le même plateau que des
instruments tranchants.
• L’emploi répété de la méthode de stérilisation à l’oxyde d’éthylène
peutentraîner une dégradation de la qualité d’image.
36
Page 41

Limitations du retraitement
• Ne pas stériliser l’équipement en recourant à diérentes méthodes
destérilisation. L’utilisation de plusieurs méthodes de stérilisation
peutréduire considérablement les performances de l’équipement.
• Ne pas laisser l’équipement dans des solutions plus longtemps que
nécessaire. Cela pourrait accélérer le vieillissement normal du produit.
• Le traitement normal n’a que peu d’incidence sur cet équipement.
Lande vie est normalement déterminée par l’usure et les dommages
liésà l’utilisation.
• Les dommages dus à un traitement inapproprié ne sont pas couverts
parlagarantie.
Instructions
Point d’application
• Nettoyer toute salissure sur l’équipement à l’aide de serviettes enpapierjetables.
• Si une méthode de retraitement automatisée est utilisée, rincertous les
canaux dans l’équipement avec 50mL d’eau distillée stérile immédiatement
après utilisation.
Connement et transport
• Retraiter l’équipement dès que cela est possible après utilisation.
• Transporter l’équipement sur un plateau pour éviter tout dommage.
1
Un temps d’attente de 30 minutes a été utilisé lors de la validation du nettoyage.
1
Préparation au nettoyage
1. Dissocier le coupleur de l’endoscope et de la tête de caméra.
2. Préparer un détergent enzymatique conformément aux recommandations
dufabricant (7,49 grammes pour 1 litre d’eau du robinet à 35 – 40 °C).
3. Essuyer tout l’équipement avec le détergent à l’aide d’un chion propre.
4. Immerger l’équipement dans le détergent. À l’aide d’une seringue, injecter
50mL de détergent dans les zones intérieures de l’équipement de manière
àatteindre toutes les parties de l’équipement.
5. Laisser tremper l’équipement dans le détergent pendant 15minutes au moins.
2
37
Page 42

Nettoyage : Manual (Manuel)
1. Brosse
• Préparer une solution fraîche de détergent enzymatique conformément
aux recommandations du fabricant (7,49 grammes pour 1 litre d’eau
durobinet entre 35 et 40 °C).
• Brosser soigneusement l’extérieur de l’équipement avec une brosse à poils
doux en se concentrant sur les surfaces rugueuses ou connexes.
• À l’aide d’une seringue, injecter 50mL de détergent au minimum cinq fois
dans chaque lumière et surface connexe.
• Brosser chaque lumière au minimum 5 fois en partant de chacune des
extrémités à l’aide d’un goupillon adapté.
• Brosser les pièces mobiles dans toutes les positions extrêmes.
2. Rincer
• Rincer l’équipement avec de l’eau désionisée/à osmose inverse (DI/OI)
àtempérature ambiante jusqu’à ce que tous les résidus de détergents soient
éliminés. Rincer chaque lumière ou surface connexe au minimum cinq
fois. Une fois tous les résidus de détergent éliminés, poursuivre le rinçage
pendant 30secondes au moins.
• Purger l’eau restante dans l’équipement, puis le sécher à l’aide d’un chion
propre ou d’air sous pression.
• Inspecter visuellement l’équipement pour s’assurer qu’il est propre, en prêtant
une attention toute particulière aux endroits d’accès dicile. Si des salissures
sont toujours visibles, répéter les étapes1 et2.
3. Trempage
• Préparer un détergent non enzymatique conformément aux recommandations
du fabricant (1,87 gramme pour 1 litre d’eau du robinet entre 35 et 40 °C).
• Immerger entièrement l’équipement et injecter 50mL de détergent dans
chaque lumière et surface connexe à l’aide d’une seringue.
• Laisser tremper l’équipement pendant 15minutes au moins.
4. Brosser
• Brosser soigneusement l’extérieur de l’équipement à l’aide d’une brosse
àpoils doux.
• A l’aide d’une seringue, injecter au minimum 5 fois 50mL de détergent
dans chaque canule, lumière ou surface connexe.
• Brosser chaque lumière au minimum 5 fois en partant de chacune des
extrémités à l’aide d’un goupillon adapté.
• Faire fonctionner l’équipement et brosser autour de chaque pièce mobile
dans toutes les positions extrêmes.
5. Rincer
• Rincer soigneusement l’équipement avec de l’eau DI/OI jusqu’à ce qu’il
ne reste plus de résidu de détergent. Rincer chaque lumière ou ssure
au minimum cinq fois. Une fois tous les résidus de détergent éliminés,
poursuivre le rinçage pendant 30secondes au moins.
• Purger l’eau restante hors de l’équipement, puis le sécher à l’aide d’un
chion propre ou d’air sous pression.
38
2
3
Page 43

Nettoyage: Automatique
1. Brosse
• A l’aide d’une seringue, injecter 50mL de détergent enzymatique
(consulter la section «Préparation au nettoyage») dans chaque lumière
etsurface connexe au moins une fois.
• Brosser les deux extrémités de toutes les lumières au moins 1 fois, à l’aide
d’un goupillon adapté.
2. Rincer
• Rincer l’équipement avec de l’eau DI/OI à température ambiante jusqu’à
disparition de tout résidu de détergent. Continuer à rincer pendant au
moins 30secondes après élimination de tout résidu de détergent.
• Placer l’équipement dans l’appareil de lavage sur un plan incliné pour
faciliter l’égouttage.
3. Lavage automatisé
• Programmer l’appareil de lavage en respectant les paramètres suivants:
Phase Délai de
recirculation
Température
de l’eau
Type et concentration
dedétergent
(le cas échéant)
Prélavage 2minutes Eau du robinet
S/O
froide
Lavage
enzymatique
Lavage1 2minutes Point de
2minutes Eau du robinet
chaude
réglage
Détergent enzymatique
Détergent non
enzymatique
3
(66°C)
Rinçage1 2minutes Eau du robinet
S/O
chaude
Phase de
7 minutes 115°C S/O
séchage
• Une fois la phase de purge qui suit le premier rinçage terminée, arrêter
lecycle et ouvrir la porte de l’appareil de lavage.
• Sortir l’équipement de l’appareil de lavage pendant la phase thermique
etle replacer à l’intérieur pour la phase de séchage.
• Si nécessaire, utiliser de l’air sous pression pour faciliter le séchage.
Contrôler visuellement la propreté de chaque équipement.
2
L’ecacité du détergent enzymatique ENZOL® pour le nettoyage a été validée.
3
L’ecacité du Renu-Klenz® pour le nettoyage a été validée.
2
39
Page 44

Désinfection de bas niveau (en option)
1. Désinfecter l’équipement dans une solution désinfectante contenant l’un des
ingrédients actifs suivants:
4
• ≥ 2,4% de glutaraldéhyde
avec un temps de trempage minimum
de45minutes à 25°C
ou
5
• ≥ 3,4 % de glutaraldéhyde
avec un temps de trempage minimum
de20minutes à 25°C
ou
6
• ≥ 0,55% d’ortho-phthalaldéhyde
avec un temps de trempage minimum
de 12 minutes à 25 °C.
2. Préparer la solution désinfectante conformément aux instructions du fabricant.
3. Conformément aux recommandations du fabricant, immerger l’équipement,
en remplissant toutes les lumières, dans la solution désinfectante pendant
letemps nécessaire et à la température appropriée.
4. Rincer soigneusement toutes les pièces et lumières à l’eau courante
déminéralisée pour éliminer le désinfectant.
5. À l’aide d’une serviette non pelucheuse, essuyer toutes les pièces
immédiatement après rinçage.
4
L’ecacité du CIDEX Activated® pour la désinfection a été validée.
5
L’ecacité du CIDEX Plus® pour la désinfection a été validée.
6
L’ecacité du CIDEX® OPA pour la désinfection a été validée.
Séchage
• Pour le séchage automatisé, utiliser le cycle de séchage de l’appareil
delavage/désinfection.
• Pour le séchage manuel, utiliser un chion non pelucheux.
• Sécher chaque lumière à l’air comprimé.
Entretien, inspection et tests
• Inspecter systématiquement l’équipement. Si un problème est observé
oususpecté, l’équipement doit être renvoyé pour réparation.
• Vérier la propreté de tous les éléments. En présence d’une accumulation
de tissus ou de uides, répéter les procédures de nettoyage et de désinfection décrites ci-dessus.
• Vérier que le câble de la caméra ne présente pas de coupure ni decassure.
Retourner une caméra urologique endommagée à Stryker pourréparation.
Conditionnement
S/O
40
Page 45

Stérilisation
Après avoir eectué les opérations de nettoyage décrites ci-dessus,
procéderàl’un des cycles de stérilisation suivants.
Oxyde d’éthylène (EtO)
• Doubler l’emballage de la tête de caméra et du câble avant la stérilisation.
Paramètres de préconditionnement
Température 55 °C
Humidité de la chambre 70 % HR
Points de réglage du vide 1,3psia
Temps 30minutes
Exposition
Concentration (100 % EtO) 725mg/L
Température 55 ± 2 °C
Temps 1heure
Humidité de la chambre 75 ± 5% HR
Paramètres d’aération
Temps d
Température 35 à 54 °C
Système Steris® 1
1. Nettoyer et préparer la tête de caméra urologique et le câble conformément
2. Stériliser la tête de caméra urologique et le câble au moyen du système
3. Laisser la tête de caméra urologique, le câble et l’endoscope sécher
Sterrad®
1. Nettoyer et préparer la tête de caméra urologique et le câble conformément
2. Stériliser la tête de caméra urologique et le câble à l’aide du système
’aération 12heures
aux recommandations du paragraphe «Nettoyage».
Steris®1 avec la solution stérilisante Steris® 20.
complètement avant de les remonter. La présence d’humidité provoque
l’apparition de buée sur la lentille de la caméra lors de son utilisation.
aux recommandations du paragraphe «Nettoyage».
destérilisation Sterrad™ NX ou 100S.
41
Page 46

Stockage
Ne jamais stocker l’équipement dans un environnement humide et non ventilé,
comme par exemple dans une boîte de transport. Cela peut comporter un risque
de contrôle infectieux.
Utilisation de housses stériles
L’emploi de housses stériles garantit une longévité maximale de la tête de caméra
1288 HD. Pour obtenir les meilleurs résultats, observer les instructions fournies par
le fabricant.
Mise au rebut
Ce produit comprend des composants électriques ou
électroniques. Il ne doit pas être mis au rebut avec les déchets
ménagers ordinaires mais doit être collecté séparément
conformément aux réglementations nationales ou aux politiques
institutionnelles relatives aux équipements électroniques
obsolètes.
La caméra 1288 HD doit être recyclée conformément
auxréglementations locales et hospitalières en vigueur.
42
Page 47

Caractéristiques techniques
Les paramètres pour 60 Hz sont indiqués en premier (les paramètres pour 50Hz
suivent entre parenthèses).
Système d’imagerie
Conditions d’utilisation Température: 5 – 40°C
Conditions de transport
etde stockage
Poids total à l’expédition 0,680kg (tête de caméra urologique)
Dimensions Câble reliant la tête de caméra à la console:
Amélioration 8niveaux (commutables)
Classication Pièce appliquée de type BF
Conforme aux normes
desécurité médicales
Couplage CCD progressif de 1/3 pouce
Haute dénition
Humidité relative : 30 – 95 %
Température: -20 – 60°C
Humidité relative : 10 – 95 %
Pression atmosphérique: 700 – 1060hPa
câble scellé de 3,0m
rallonge de 6,1m disponible
Protection contre la pénétration de liquide,
IPX7 — Protégé contre les eets de l’immersion
temporaire dans l’eau
CEI 60601-1:1988 + A1:1991 + A2:1995
CEI60601-2-18:1996 + A1:2000
CAN/CSA C22.2 N° 601.1-M90
UL 60601-1:2003
AS/NZS 3200.1.0:1998
CSA 22.2.601.1.1:2002
CAN/CSA C22.2 No. 601.2.18:1990
Pour plus d’informations sur les modications et les nouveaux produits,
contacterle représentant local de Stryker Endoscopy.
43
Page 48

Page 49

Inhalt
Warnhinweise und Vorsichtsmaßnahmen ...............47
Erläuterung der Symbole ..................................................49
Produktbeschreibung und Verwendungszweck ..50
Indikationen/Kontraindikationen ................................................50
Produkteigenschaften ................................................................50
Vorbereitung .............................................................................52
Bedienung ..................................................................................54
Verwenden der Tasten des Urologie-Kamerakopfs ...................54
Einstellen des Fokus ..................................................................57
Reinigung, Aufbereitung und Wartung .....................58
Aufbereitung des Urologie-Kamerakopfes.................................58
Verwenden von sterilen Abdecktüchern ....................................65
Technische Daten .................................................................66
Page 50

Page 51

Warnhinweise und
Vorsichtsmaßnahmen
Lesen Sie dieses Handbuch sorgfältig durch, und halten Sie sich genau an die
darin enthaltenen Anweisungen.
WICHTIGER SICHERHEITSHINWEIS: Lesen Sie vor Inbetriebnahme dieses
Produkts aufmerksam dieses Benutzerhandbuch durch. Bei Verwendung
dieses Produkts mit einer Lichtquelle können sich Patienten oder Benutzer
Brandverletzungen und/oder andere ernsthae Verletzungen zuziehen,
oder Objekte können Feuer fangen, wenn die Anweisungen in diesem
Handbuch nicht befolgt werden. Alle Lichtquellen können an der Spitze,
am Lichtanschluss, am Ende des Lichtleiterkabels und/oder nahe des
Lichtleiterkabeladapters des Endoskops sehr heiß werden. Eine stärkere
Helligkeit der Lichtquelle bewirkt eine stärkere Hitzeentwicklung. Stellen
Sie immer zuerst die Helligkeit der Kamera und des Monitors ein, bevor
Sie die Helligkeit der Lichtquelle anpassen. Stellen Sie die Helligkeit
der Lichtquelle so ein, dass sie für eine adäquate Ausleuchtung des
Eingrisbereichs gerade ausreicht. Stellen Sie darüber hinaus die interne
Blende der Kamera so ein, dass Sie die Lichtquelle mit geringerer Intensität
verwenden können. Achten Sie darauf, dass der Patient nicht mit der Spitze
des Endoskops bzw. des Lichtleiterkabels in Berührung kommt, und legen
Sie diese Teile nie auf dem Patienten ab, da dies zu Verbrennungen des
Patienten bzw. des Benutzers führen kann. Legen Sie außerdem die Spitze
des Endoskops, den Lichtanschluss, den Lichtleiterkabeladapter oder das
Ende des Lichtleiterkabels nie auf den OP-Abdeckungen oder sonstigem
entammbarem Material ab, da dies zu einem Brand führen kann. Schalten
Sie die Lichtquelle immer in den Standby-Modus, wenn das Endoskop vom
Lichtleiterkabel getrennt wird oder das Gerät unbeaufsichtigt ist. Die Spitze
und der Lichtanschluss des Endoskops sowie der Lichtleiterkabeladapter
und das Ende des Lichtleiterkabels brauchen einige Minuten, um
abzukühlen, nachdem das Gerät in den Standby-Modus geschaltet wurde.
Sie können daher immer noch einen Brand oder Brandverletzungen am
Patienten oder Benutzer verursachen.
Warnhinweise und Vorsichtsmaßnahmen
Um ernsthae Verletzungen des Benutzers und des Patienten und/oder
Beschädigungen am Produkt zu vermeiden, sind folgende Warnhinweise
zu beachten:
1. Der Benutzer muss ein qualizierter Arzt sein und über vollständige
Kenntnisse hinsichtlich des Gebrauchs dieses Produkts verfügen.
47
Page 52

2. Das Produkt vorsichtig auspacken und auf eventuelle Versandschäden
überprüfen. Bei eventuellen Beschädigungen bitte die Angaben zur
Standardgarantie lesen.
3. Vor dem Anschluss und der Verwendung des Produkts dieses Handbuch
und insbesondere die Warnhinweise gründlich durchlesen und sich mit dem
Inhalt vertraut machen.
4. Vor dem Zusammenbau oder Anschluss der Kamera das gesamte Handbuch
vollständig durchlesen.
5. Die in diesem Handbuch enthaltenen Anweisungen bezüglich Pege,
Reinigung, Desinfektion und Sterilisation genau beachten. Andernfalls kann
das Gerät beschädigt werden.
6. Das Produkt vor einem chirurgischen Eingri testen. Dieses Produkt wurde
vor dem Versand im Werk gründlich getestet. Das Produkt niemals in der
Nähe entzündlicher oder explosiver Gase verwenden.
7. Die Teile des Kamerakopfs nicht auseinandernehmen, da ansonsten
möglicherweise Versiegelungen beschädigt werden oder es zu Kriechverlust
und/oder Stromschlägen kommen kann. Dieses Produkt wurde im Werk
versiegelt, damit keine Feuchtigkeit in die elektronischen Komponenten
eindringen kann. Wenn der Kamerakopf oder das Kabel absichtlich
beschädigt werden, erlischt die Garantie.
8. Die Außenächen der Kamera und des Endoskops vor der Verwendung
auf raue, scharantige und vorstehende Stellen überprüfen.
9. Die Verwendung des Kamerakopfs mit gebrochenen Anschlussstien kann
zu einer Beschädigung der Kamerasteuerungseinheit führen. Sollte ein
Anschlusssti beschädigt sein oder fehlen, bitte die Standardgarantie von
Stryker zurate ziehen.
10. Sicherstellen, dass Nachjustierungen, Modikationen und/oder Reparaturen
ausschließlich von Fachpersonal durchgeführt werden, das von Stryker
Endoscopy dazu autorisiert wurde. Keine Reparaturen oder Einstellungen
an internen Komponenten versuchen, die nicht ausdrücklich in diesem
Handbuch beschrieben sind. Andernfalls kann dies zu unvorhergesehenen
Betriebseigenschaen oder zur Beschädigung des Produkts führen.
11. Die Urologie-Kamera stets mit großer Vorsicht behandeln. Das
Kamerasystem enthält empndliche exakt ausgerichtete Teile, die beim
Herunterfallen oder einer falschen Behandlung beschädigt werden können.
12. Wiederholte Sterilisation mit Ethylenoxid kann zu einer geringeren
Bildqualität führen.
Jede Nichtbeachtung dieser Warnhinweise führt zum Erlöschen der Garantie.
48
Page 53

Erläuterung der Symbole
Zusätzlich zu den bereits beschriebenen Warnsymbolen benden sich noch
weitere Symbole am Urologie-Kamerakopf 1288HD und in diesem Handbuch,
die eine besondere Bedeutung hinsichtlich der korrekten Verwendung und
Lagerung dieses Kamerakopfs haben. Die folgende Liste enthält Erklärungen der
im Zusammenhang mit diesem Produkt verwendeten Symbole:
Weist auf wichtige Bedienungs- und Wartungsanweisungen im
Handbuch hin
Herstellungsdatum
Rechtmäßiger Hersteller
SN
Seriennummer
Katalognummer
Angaben zur Lufeuchtigkeit im Betrieb
Angaben zu den Druckverhältnissen im Betrieb
Betriebstemperaturbereich
Einhaltung von CAN/CSA C22.2 Nr. 601.1-M90 und UL60601-1.
Eingesetztes Bauteil: Typ BF
Dieses Symbol bedeutet, dass von elektrischen und elektronischen
Geräten stammender Abfall nicht dem Hausmüll zugeführt werden
darf, sondern gesondert entsorgt werden muss. Wenden Sie sich
bezüglich der Entsorgung des Produkts an den Hersteller oder an
ein entsprechendes Entsorgungsunternehmen.
49
Page 54

Produktbeschreibung und
Verwendungszweck
Der Urologie-Kamerakopf 1288HD ist eine hochauösende Kamera für
die Erfassung von Stand- und Videobildern bei endoskopisch-urologischen
Eingrien. Der Winkel von 90° zwischen Kamerakopf und Endoskop sorgt für
einen vereinfachten Zugang bei urologischen Verfahren.
Der Urologie-Kamerakopf wird zusammen mit der Kamerakonsole 1288 HD
(REF 1288010000; REF 1288010001) verwendet.
Indikationen/Kontraindikationen
Die Urologie-Kamera 1288HD kann in den Bereichen der allgemeinen
Laparoskopie, Nasopharyngoskopie, Ohrendoskopie, Sinuskopie und plastischen
Chirurgie verwendet werden, in denen der Einsatz von Laparoskopen,
Endoskopen oder Arthroskopen indiziert ist. Einige Beispiele für geläugere
endoskopische Eingrie sind laparoskopische Cholezystektomie, laparoskopische
Hernienreparatur, laparoskopische Appendektomie, laparoskopische
Beckenlymphknotendissektion, laparoskopisch assistierte Hysterektomie,
laparoskopische und thoraskopische anteriore Spondylodese, Rekonstruktion
des vorderen Kreuzbands, Kniearthroskopie, Schulterarthroskopie, KleingelenkArthroskopie, Dekompressionsxierung, Keilresektion, Lungenbiopsie,
Pleuralbiopsie, dorsale Sympathektomie, Pleurodese, Dissektion der inneren
Brustwandarterie für eine koronare Bypass-Operation, koronare BypassTransplantation mit indizierter endoskopischer Visualisierung sowie
Untersuchung der evakuierten Herzkammer während eines Klappenersatzes.
Anwender der Kamera sind Chirurgen aus dem Bereich der Allgemeinmedizin,
Kardiologie, oraxchirurgie, plastischen Chirurgie, Orthopädie, HNO-Medizin
sowie Gynäkologen und Urologen.
Kontraindikationen sind nicht bekannt.
Produkteigenschaften
Der Urologie-Kamerakopf wird an die Kamerakonsole angeschlossen und erfasst
Videobilder und Fotos, die er an die Kamerakonsole überträgt. Er verfügt über
mehrere Bedienelemente in Form eines Tastenfelds an der Oberseite (siehe
Abschnitt „Bedienungsanleitung“ in diesem Handbuch).
50
Page 55

1
2 3
4
5
8
7
1. Endoskop —
2. Endoskopklammer Zur Befestigung des Endoskops am Kamerakopf
3. Endoskopbremse Verhindert die Drehung des Endoskops
4. Fokussierknopf Zum Anpassen des Fokus des Kamerakopfs
5. Urologie-
Kamerakopf 1288
HD
6. Urologie-
Kamerakabel
Erfasst Fotos und Videobilder und verfügt über
Kamerabedienelemente
—
6
7. Kabelstecker Verbindet den Kamerakopf mit der Kamerakonsole
8. Schutzkappe Schützt den Kabelstecker während der Reinigung
und Sterilisation
51
Page 56

Vorbereitung
1. Die Kamerakonsole 1288HD wie im zugehörigen Benutzerhandbuch
beschrieben einrichten.
2. Den Urologie-Kamerakopf an die Konsole anschließen.
• Falls erforderlich, die Schutzkappe vom Kabelstecker abschrauben.
• Den blauen Pfeil am Kabelstecker am blauen Pfeil des UrologieKameraanschlusses an der Vorderseite der Konsole ausrichten.
• Den Stecker in den Anschluss einschieben, bis er einrastet.
• (Um die Kamera wieder von der Steuereinheit zu trennen, den
Stecker am gerielten Teil greifen und gerade herausziehen.)
3. Ein Endoskop am Urologie-Kamerakopf anbringen.
• Rote Staubschutzkappe, falls vorhanden, entfernen.
• Die Endoskopbremse nach rechts drücken, um sie zu schließen.
52
• Die Endoskopklammer drehen und oen halten.
Page 57

• Das Endoskop in die Endoskopklammer einführen.
• Die Endoskopbremse in entgegengesetzter Richtung drehen,
um das Endoskop zu sichern.
4. Ein Lichtkabel zwischen Lichtquelle und Lichtanschluss des Endoskops
anschließen.
53
Page 58

Bedienung
Warnung: Bevor Sie die Urologie-Kamera 1288HD bei einem
chirurgischen Eingri einsetzen, überprüfen Sie alle
Komponenten auf einwandfreie Funktion. Stellen Sie sicher,
dass auf allen Videobildschirmen ein Videobild angezeigt wird,
bevor Sie mit einem Eingri beginnen.
Der Urologie-Kamerakopf 1288HD wird über die Tasten am Kamerakopf oder
die Touchscreen-Oberäche auf der Konsole bedient.
Verwenden der Tasten des Urologie-Kamerakopfs
Der Urologie-Kamerakopf verfügt über ein ovales Zwei-Tasten-Bedienfeld zur
Steuerung der Urologie-Kamera 1288. Diese Tasten sind mit den Buchstaben
P und W beschriet.
P-Taste (Bildtaste)
Mit der P-Taste können Sie bis zu zwei Videokomponenten fernsteuern.
• Die P-Taste weniger als zwei Sekunden drücken, um den Fernsteuerungsausgang 1 auszuwählen. Es ertönt ein Signalton.
• Die P-Taste mehr als zwei Sekunden drücken, um den Fernsteuerungsausgang 2 auszuwählen. Es ertönen zwei Signaltöne.
W-Taste (Weißabgleich)
Mit der W-Taste kann die Weißabgleich-Funktion oder die Funktion für
Belichtung/Zoom aktiviert werden. Mit der Weißabgleich-Funktion korrigieren
Sie leichte Farbunterschiede, die zwischen verschiedenen Lichtquellen und
Endoskopen vorhanden sein können.
• Die W-Taste mehr als zwei Sekunden drücken, um die WeißabgleichFunktion zu aktivieren.
• Die W-Taste weniger als zwei Sekunden drücken, um den Zoom um jeweils
eine der acht Stufen zu erhöhen. (Wenn die höchste Zoomstufe erreicht ist,
wird durch erneutes Drücken der W-Taste wieder die niedrigste Zoomstufe
eingestellt.)
54
Page 59

Führen Sie den Weißabgleich vor jedem chirurgischen Verfahren durch.
Hinweis: Stellen Sie vor dem Einstellen des Weißabgleichs sicher, dass die
Kamera mit einem Endoskop und einer Lichtquelle verbunden ist und dass
die Kamera, die Lichtquelle und der Monitor eingeschaltet sind.
1. Das Endoskop auf mehrere Stapel von weißen 10 x 10 cm Tupfern, einen
weißen laparoskopischen Tupfer oder eine andere saubere weiße Oberäche
ausrichten.
2. Auf den Monitor schauen und sicherstellen, dass die weiße Oberäche nicht
blendet.
3. Die W-Taste gedrückt halten, bis auf dem Videomonitor „WEIßABGLEICH
WIRD DURCHGEFÜHRT“ zu blinken beginnt.
4. Das Endoskop weiter auf die weiße Oberäche ausrichten, bis auf dem
Videomonitor „WEIßABGLEICH ABGESCHLOSSEN“ angezeigt wird.
Möglicherweise verändert sich die Farbe des Videobilds. Falls es Ihnen nicht
gelingt, einen akzeptablen Weißabgleich einzustellen, nden Sie weitere
Informationen im Benutzerhandbuch zur Kamerakonsole 1288 HD.
Verwenden des Touchscreens
Über den Touchscreen auf der Konsole können die Kamera gesteuert und
Systemeinstellungen ausgewählt werden. Sie haben folgende Möglichkeiten:
• Kameraeinstellungen für urologische Verfahren auswählen
• Foto aufnehmen
• Video aufzeichnen
• Weißabgleich aktivieren
55
Page 60

Blättern Sie die voreingestellten Kameraeinstellungen
für chirurgische Sparten durch. Wählen Sie aus den
folgenden Optionen aus:
• Arthroskopie
• Zytoskopie
• HNO
• Flexscope
• Hysteroskopie
Foto aufnehmen.
Die Taste zwei Sekunden gedrückt halten, um die
Funktion zu aktivieren.
Video aufzeichnen.
Die Taste zwei Sekunden gedrückt halten, um mit
der Aufzeichnung zu beginnen. Die Taste erneut
drücken, um die Aufzeichnung zu beenden.
Weißabgleich aktivieren.
Die Taste zwei Sekunden gedrückt halten, um die
Funktion zu aktivieren.
• Die Taste einmal drücken, um den Bildschirm
„Menü“ anzuzeigen.
• Laparoskopie
• Laser
• Mikroskop
• Standard
56
Page 61

Einstellen des Fokus
Den Fokussierknopf nach links oder rechts schieben, um den Fokus anzupassen.
57
Page 62

Reinigung, Aufbereitung und
Wartung
Aufbereitung des Urologie-Kamerakopfes
Diese Anweisungen zur Auereitung entsprechen den Normen ISO 17664,
AAMI TIR12, AAMI ST79 und AAMI ST81. Sie wurden von Strykerals
geeignet für die Auereitung des Produkts zur Wiederverwendung validiert.
Allerdings muss das auereitende Unternehmen sicherstellen, dass mit der
Auereitung (so wie sie derzeit unter Verwendung von Geräten, Materialien
und Personal der Auereitungseinrichtung durchgeführt wird) das gewünschte
Ergebnis erzielt wird. Dazu ist in der Regel die Validierung und routinemäßige
Überwachung des Prozesses erforderlich. Stryker empehlt Benutzern die
Einhaltung dieser Standards bei der Auereitung von Medizinprodukten.
Warnhinweise
• Das Produkt muss vor dem ersten und nach jedem weiteren Einsatz
gereinigt und sterilisiert werden.
• Nur die in diesem Dokument beschriebenen Sterilisationszyklen anwenden.
Andere Sterilisationszyklen können das Produkt beschädigen oder zu
einer unzureichenden Sterilisation führen.
• Urologie-Kamerakopf und Endoskop vor der Reinigung, Desinfektion
oder Sterilisation voneinander trennen.
• Geeignete Schutzausrüstung tragen: Handschuhe, Augenschutz etc.
Achtung
• Vor der Auereitung der Urologie-Kamera stets die Schutzkappe anbringen.
Wenn die Schutzkappe nicht ordnungsgemäß aufgesetzt und fest
aufgeschraubt wird, kommt es zur Korrosion der Steckverbinderstie
und zum Erlöschen der Garantie.
• Das Kamerakabel vor dem Einweichen in eine Flüssigkeit auf Einschnitte
und Bruchstellen überprüfen. Eine beschädigte Kamera an den Kundendienst
von Stryker zurücksenden.
• Für die manuelle Reinigung keine Bürsten oder Reinigungspads mit
metallenen oder scheuernden Spitzen verwenden, da dies zu bleibenden
Einkerbungen oder Schäden führen könnte.
• Um die galvanische Korrosion möglichst gering zu halten,
ungleichartige Metalle nicht nebeneinander einweichen.
• Die Kamera keinesfalls zusammen mit scharantigen Instrumenten
einweichen.
• Wiederholte Sterilisation mit Ethylenoxid kann zu einer geringeren
Bildqualität führen.
58
Page 63

Einschränkungen bei der Aufbereitung
• Keine wechselnden Sterilisationsverfahren für das Produkt verwenden.
Bei Verwendung mehrerer Sterilisationsmethoden kann die Leistung
des Produkts erheblich beeinträchtigt werden.
• Das Produkt nicht länger als nötig in Lösungen eintauchen. Ansonsten
wird der normale Produktalterungsprozess beschleunigt.
• Eine einwandfreie Auereitung hat geringfügige Auswirkungen auf
das Produkt. Das Ende der Betriebsdauer ist in der Regel von der
Abnutzung und den Schäden, die auf die Verwendung des Produkts
zurückzuführen sind, abhängig.
• Schäden durch unsachgemäße Auereitung sind nicht durch die
Garantie abgedeckt.
Anweisungen
Einsatzort
• Grobe Verunreinigungen mit Einweg-Papiertüchern vom Produkt
abwischen.
• Wenn eine automatisierte Auereitungsmethode verwendet wird,
die Kanäle unmittelbar nach der Verwendung mit 50 ml sterilem,
destillierten Wasser spülen.
Sicherheitsbehälter und Transport
• Das Produkt sollte schnellstmöglich nach Gebrauch auereitet werden1.
• Das Produkt in einer Kassette transportieren, um Beschädigungen
zuvermeiden.
1
Während der Reinigungsvalidierung wurde eine Wartezeit von 30 Minuten
angewendet.
Reinigungsvorbereitung
1. Koppler von Endoskop und Kamerakopf trennen.
2. Eine enzymatische Reinigungslösung gemäß den Herstellerempfehlungen
herstellen. (Dazu 7,49 g auf l Leitungswasser mit einer Temperatur von
35–40°C verwenden.)
3. Ein sauberes Tuch in die Reinigungslösung tauchen und das gesamte
Produkt abwischen.
4. Das Produkt in die Reinigungslösung tauchen. Mit einer Spritze 50ml
Reinigungslösung in das Produktinnere injizieren, um sicherzustellen,
dassalle Teile des Produkts erreicht werden.
5. Das Produkt mindestens 15 Minuten in der Reinigungslösung einweichen.
2
59
Page 64

Reinigung: Manuelle
1. Bürsten
• Eine frische enzymatische Reinigungslösung gemäß den
Herstellerempfehlungen herstellen (7,49 g auf l Leitungswasser
miteinerTemperatur von 35 – 40°C)
• Das Äußere des Produkts gründlich mit einer weichen Bürste abbürsten.
Insbesondere Verbindungsstellen oder raue Oberächen abbürsten.
• Mindestens fünfmal mit einer Spritze 50 ml Reinigungslösung in jedes
Lumen und jede Verbindungsstelle injizieren.
• Die beiden Enden aller Lumina mindestens fünfmal mit einer
geeigneten Flaschenbürste reinigen.
• Alle beweglichen Teile in allen extremen Positionen abbürsten.
2. Spülen
• Das Produkt mit Umkehrosmosewasser/entionisiertem Wasser
bei Raumtemperatur spülen, bis alle Reste der Reinigungslösung
entfernt wurden. Alle Lumina oder Verbindungsstellen mindestens
fünfmal spülen. Das Produkt nach dem vollständigen Entfernen der
Reinigungslösung noch mindestens weitere 30 Sekunden lang spülen.
• Überschüssiges Wasser vom Produkt entfernen und mit einem sauberen
Tuch oder Drucklu trocknen.
• Das Produkt einer Sichtprüfung auf Sauberkeit unterziehen, dabei
besonders auf schlecht erreichbare Bereiche achten. Falls noch
Verunreinigungen sichtbar sind, die Schritte 1 und 2 wiederholen.
3. Einweichen
• Eine nicht enzymatische Reinigungslösung gemäß den
Herstellerempfehlungen herstellen. (Dazu 1,87 g auf l Leitungswasser
miteiner Temperatur von 35 – 40°C verwenden.)
• Das Produkt vollständig eintauchen und mit einer Spritze in alle
Lumina und Verbindungsstellen 50ml Reinigungslösung injizieren.
• Das Produkt mindestens 15 Minuten einweichen.
2
.
3
60
Page 65

4. Bürsten
• Das Äußere des Produkts gründlich mit einer weichen Bürste abbürsten.
• 50ml der Reinigungslösung mit einer Spritze mindestens fünfmal in alle
Kanülen, Lumina oder Verbindungsstellen injizieren.
• Die beiden Enden aller Lumina mindestens fünfmal mit einer
geeigneten Flaschenbürste reinigen.
• Alle beweglichen Teile des Produkts in allen extremen Positionen
abbürsten.
5. Spülen
• Das Produkt mit Umkehrosmosewasser/entionisiertem Wasser
gründlich spülen, bis alle Rückstände der Reinigungslösung entfernt
sind. Alle Lumina und Zwischenräume mindestens fünfmal spülen.
DasProdukt nach dem vollständigen Entfernen der Reinigungslösung
noch mindestens weitere 30 Sekunden lang spülen.
• Überschüssiges Wasser vom Produkt entfernen und mit einem sauberen
Tuch oder Drucklu trocknen.
61
Page 66

Reinigung: Automatisiert
1. Bürsten
• 50ml der enzymatischen Reinigungslösung (aus dem Abschnitt
„Reinigungsvorbereitung“) mit einer Spritze in alle Lumina oder
Verbindungsstellen mindestens einmal injizieren.
• Von beiden Enden aller Lumina mindestens einmal mit einer geeigneten
Flaschenbürste reinigen.
2. Spülen
• Den Handgri mit Umkehrosmose-/entionisiertem Wasser
bei Umgebungstemperatur spülen, bis keine Rückstände der
Reinigungslösung mehr sichtbar sind. Das Produkt nach dem
vollständigen Entfernen der Reinigungslösung noch mindestens weitere
30 Sekunden lang spülen.
• Das Produkt schräg in den Waschautomaten stellen, um das Ablaufen
von Wasser zu erleichtern.
3. Waschautomat
• Den Waschautomaten mit folgenden Parametern programmieren:
Phase Rückfüh-
rungszeit
Wasser-
temperatur
Reinigungsmitteltyp
und -konzentration
(sofern zutreend)
Vorwäsche 2Minuten Kaltes
–
Leitungswasser
Enzymwäsche 2Minuten Heißes
Leitungswasser
Waschen 1 2Minuten Einstellpunkt
(66 °C)
Spülen 1 2Minuten Heißes
Enzymatische
Reinigungslösung
Nichtenzymatisches
Reinigungsmittel
–
Leitungswasser
Trocknen 7Minuten 115 °C –
• Bei Abschluss der Trockenlegung nach Spülen 1 den Zyklus anhalten
und die Tür des Waschautomaten önen.
• Das Gerät während der Heizphase aus dem Waschautomaten entfernen
und das Gerät für die Trockenphase wieder in den Waschautomaten
platzieren.
• Ggf. kann Drucklu zur Unterstützung des Trockenvorgangs eingesetzt
werden. Jedes Produkt einer Sichtprüfung auf Sauberkeit unterziehen.
2
Die Reinigungskra des enzymatischen Reinigungsmittels ENZOL® wurde
validiert.
3
Die Reinigungskra von Renu-Klenz® wurde ebenfalls validiert.
62
2
3
Page 67

Desinfektion auf niedriger Stufe (optional)
1. Das Produkt in einer Desinfektionslösung mit einem der folgenden
wirksamen Bestandteile desinzieren:
• ≥ 2,4% Glutaraldehyd4 mit einer Mindesteinweichzeit von 45 Minuten
bei25°C
oder
• ≥ 3,4% Glutaraldehyd5 mit einer Mindesteinweichzeit von 20 Minuten bei
25°C
oder
• ≥ 0,55% Orthophthalaldehyd6 mit einer Mindesteinweichzeit von
12Minuten bei 25°C.
2. Desinfektionslösung gemäß den Anweisungen des Herstellers zubereiten.
3. Das Produkt gemäß den Empfehlungen des Herstellers für die erforderliche
Zeit und bei der richtigen Temperatur in die Desinfektionslösung
eintauchen, dabei alle Lumina füllen.
4. Alle Teile und Lumina gründlich mit ießendem, entmineralisiertem
Wasser spülen, um das Desinfektionsmittel zu entfernen.
5. Sofort nach dem Spülen alle Teile mit einem fusselfreien Tuch abtrocknen.
4
Die Wirksamkeit von CIDEX Activated® zur Desinfektion wurde validiert.
5
Die Wirksamkeit von CIDEX Plus® zur Desinfektion wurde validiert.
6
Die Wirksamkeit von CIDEX® OPA zur Desinfektion wurde validiert.
Trocknen
• Zur automatischen Trocknung den Trocknungszyklus des
Wasch-/Desinfektionsautomaten verwenden.
• Zur manuellen Trocknung ein fusselfreies Tuch verwenden.
• Alle Lumina mit Drucklu trocknen.
Wartung, Inspektion und Testing
• Das Produkt regelmäßig kontrollieren. Wird ein Problem erkannt
oder vermutet, sollte das Produkt zur Reparatur eingesendet werden.
• Alle Komponenten auf Sauberkeit überprüfen. Falls Flüssigkeits- oder
Geweberückstände vorliegen, die vorstehend aufgeführten Reinigungsund Desinfektionsverfahren wiederholen.
• Das Kamerakabel auf Einschnitte und Bruchstellen überprüfen. Eine
beschädigte Urologie-Kamera an den Kundendienst von Stryker
zurücksenden.
Verpackung
–
63
Page 68

Sterilisation
Nach der Reinigung gemäß der oben genannten Vorgehensweise einen der
folgenden Sterilisationszyklen anwenden.
Ethylenoxid (EO)
• Wickeln Sie Kamerakopf und Kabel vor der Sterilisation doppelt ein.
Vorbehandlungsparameter
Temperatur 55°C
Feuchtigkeit in Kammer 70% RF
Vakuum-Einstellpunkte 1,3 psia
Zeit 30Minuten
Exposition
Konzentration (100% EO) 725 mg/l
Temperatur 55 ± 2°C
Zeit 1 Stunde
Feuchtigkeit in Kammer 70 ± 5% relative Lufeuchtigkeit
Belüungsparameter
Belüungsdauer 12 Stunden
Temperatur 35 – 54°C
Steris®-System 1
1. Den Urologie-Kamerakopf und das Kabel wie im Abschnitt „Reinigung“
beschrieben reinigen und vorbereiten.
2. Den Urologie- Kamerakopf und das Kabel mit dem Steris®-System 1 und
dem Sterilisationsmittel Steris® 20 sterilisieren.
3. Den Urologie-Kamerakopf, das Kabel und das Endoskop vor dem erneuten
Zusammensetzen vollständig trocknen lassen. Durch Feuchtigkeit
beschlägt das Fenster der Kamera beim Gebrauch.
64
Page 69

Sterrad®
1. Den Urologie-Kamerakopf und das Kabel wie im Abschnitt „Reinigung“
beschrieben reinigen und vorbereiten.
2. Den Urologie-Kamerakopf und das Kabel mit dem Sterilisationssystem
Sterrad™ NX oder 100S sterilisieren.
Auewahrung
Das Gerät niemals in einer nicht belüeten, feuchten Umgebung wie einem
Tragekasten auewahren. Dies kann ein Infektionsrisiko darstellen.
Verwenden von sterilen Abdecktüchern
Durch die Verwendung steriler Abdecktücher wird die Langlebigkeit des
Kamerakopfes 1288HD maximiert. Um bestmögliche Ergebnisse zu erzielen,
beachten Sie die Anweisungen des Herstellers der Abdecktücher.
Entsorgung
Bei diesem Produkt fällt Elektromüll an. Er muss
inÜberein-stimmung mit den geltenden örtlichen
Gesetzenund Klinik-richtlinien fachgerecht gesondert
entsorgt werden. Die Entsorgung über den Hausmüll
istnichtzulässig.
Die Kamera 1288HD muss in Übereinstimmung mit
den geltenden örtlichen Gesetzen und Klinikrichtlinien
entsorgt werden.
65
Page 70

Technische Daten
60-Hz-Einstellungen werden als Erstes angezeigt. (50-Hz-Einstellungen folgen
in Klammern.)
Bildgebungssystem 1/3” Progressive Scan CCDs
Hochauösend
Betriebsbedingungen Temperatur: 5 – 40°C
Relative Lufeuchtigkeit: 30 – 95 %
Transport- und
Lagerbedingungen
Gesamtversandgewicht 0,680 kg Urologie-Kamerakopf
Abmessungen Verbindungskabel zwischen Kamerakopf
Optimierung 8 Stufen (umschaltbar)
Klassikation Eingesetztes Bauteil: Typ BF
Einhaltung medizinischer
Sicherheitsnormen:
Temperatur: -20 – 60°C
Relative Lufeuchtigkeit: 10 – 95 %
Ludruck: 700 – 1060hPa
und Kamerakonsole:
3,0 m abgedichtetes Kabel
6,1 m Kabelverlängerung erhältlich
Schutz gegen Eindringen von Wasser,
IPX7 – Schutz gegen die Wirkungen beim
zeitweiligen Untertauchen in Wasser
IEC 60601-1:1988 + A1:1991 + A2:1995
IEC 60601-2-18:1996 + A1:2000
CAN/CSA C22.2 Nr. 601.1-M90
UL 60601-1:2003
AS/NZS 3200.1.0:1998
CSA 22.2.601.1.1:2002
CAN/CSA C22.2 Nr. 601.2.18:1990
Wenden Sie sich bezüglich Angaben zu Änderungen und neuen Produkten
an den zuständigen StrykerEndoscopy-Vertreter.
66
Page 71

Indice
Messaggi di avvertenza ediattenzione ..................69
Definizioni dei simboli ........................................................71
Descrizione del prodotto eusoprevisto .................72
Indicazioni/Controindicazioni .....................................................72
Caratteristiche del prodotto .......................................................72
Configurazione ........................................................................75
Funzionamento .......................................................................77
Uso dei pulsanti della testa della videocamera urologica..........77
Regolazione della messa a fuoco ..............................................80
Pulizia, rigenerazione emanutenzione ....................81
Rigenerazione della testa della videocameraurologica .............81
Uso di telini sterili .......................................................................87
Specifiche tecniche .............................................................88
Page 72

Page 73

Messaggi di avvertenza
ediattenzione
Leggere il manuale e attenersi scrupolosamente alle istruzioni.
AVVISO IMPORTANTE SULLA SICUREZZA: prima di utilizzarequesto
dispositivo, leggere attentamente e accuratamente il presente manuale
d’uso. Se non si rispettano le istruzioni riportate in questo manuale,
l’usodi questo dispositivo con una sorgente luminosa può causare ustioni
e/o gravi lesioni al paziente, all’utente oppure danni a oggetti inanimati.
Tutte le sorgenti luminose possono generare una notevole quantità
dicalore sulla punta dell’endoscopio, sul supporto luce dell’endoscopio,
sulla punta del cavo luce e/o in prossimità dell’adattatore del cavo luce.
Livelli elevati di luminosità prodotti dalla sorgente luminosa generano
una maggiore quantità dicalore. Regolare sempre il livello di luminosità
della videocamera e del monitor prima di regolare quello della sorgente
luminosa. Regolare la luminosità della videocamera sul livello minimo
necessario per illuminare in modo adeguato il sito chirurgico. Inoltre,
regolare l’otturatore interno della videocamera su un valore maggiore per
poter utilizzare la sorgente luminosa con un livello d’intensità minore.
Evitare il contatto tra lapunta dell’endoscopio o del cavo luce e il paziente,
in quanto si possono causare gravi ustioni alpaziente o all’utente.
Inoltre, non appoggiare mai la punta dell’endoscopio, il supporto luce
dell’endoscopio, l’adattatore del cavo luce o la punta del cavo luce sui teli
chirurgici o altro materiale inammabile, inquanto si possono causare
incendi. Mettere sempre la sorgente luminosa in modalità di standby tutte
le volte che l’endoscopio viene rimosso dalcavo luce o il dispositivo è privo
di sorveglianza. Dal momento che la punta dell’endoscopio, il supportoluce
dell’endoscopio, l’adattatore del cavo luce e la punta del cavo lucerichiedono
diversi minuti per rareddarsi una volta attivata la modalità di standby,
sussiste il pericolo di incendi o ustioni alpaziente o all’utente o di danni
agli oggetti inanimati.
Messaggi di avvertenza e di attenzione
Per evitare gravi lesioni all’utente e al paziente e/o danni al dispositivo,
attenersialle seguenti avvertenze:
1. Il dispositivo deve essere utilizzato esclusivamente da medici qualicati
einpossesso delle conoscenze e delle nozioni necessarie al suo impiego.
2. Disimballare con cautela il dispositivo e vericare che non si siano vericati
danni durante il trasporto. Se vengono rilevati danni, consultare la garanzia
standard.
3. Prima di collegare e usare il dispositivo, leggere attentamente il presente
manuale, con particolare attenzione ai messaggi di avvertenza, e acquisire
familiarità con il suo contenuto.
69
Page 74

4. Prima di assemblare o collegare la videocamera, leggere attentamente l’intera
sezione di istruzioni del manuale.
5. Leggere attentamente le istruzioni di trattamento, pulizia, disinfezione
esterilizzazione riportate nel presente manuale. In caso contrario ildispositivo
potrebbe venire danneggiato.
6. Testare questo dispositivo prima di una procedura chirurgica.
Questodispositivo è stato completamente vericato in fabbrica prima
della spedizione. Nonutilizzare mai questo dispositivo in presenza di gas
inammabili oesplosivi.
7. Evitare di rimuovere qualsiasi componente della testa della videocamera,
inquanto ciò potrebbe rompere le guarnizioni e causare dispersioni e/o
shock elettrico. Questo dispositivo è stato sigillato in fabbrica per impedire
la penetrazione dell’umidità nei componenti elettronici. Se la testa
della videocamera o la tenuta del cavo vengono rotti intenzionalmente,
lagaranziasarà invalidata.
8. Prima di ogni uso, controllare la supercie esterna della videocamera
edell’endoscopio per accertarsi che non vi siano superci ruvide,
borditaglienti o sporgenze.
9. L’uso della testa della videocamera con i piedini del connettore spezzati può
danneggiare la CCU. In caso di piedini mancanti o danneggiati, consultare
lagaranzia standard Stryker.
10. Accertarsi che le regolazioni e/o le modiche e/o le riparazioni vengono
eseguite da persone autorizzate da Stryker Endoscopy. Non eseguire
riparazioni o regolazioni non espressamente descritte nel presente manuale.
In caso contrario, potrebbero vericarsi prestazioni impreviste o danni
alprodotto.
11. Maneggiare sempre con cura la testa della videocamera urologica.
Lavideocamera contiene parti sensibili allineate con precisione
epotrebbedanneggiarsi se fatta cadere o maltrattata.
12. La sterilizzazione ripetuta con ossido di etilene potrebbe comportare
ladegradazione della qualità delle immagini.
Il mancato rispetto di queste avvertenze annulla la garanzia.
70
Page 75

Definizioni dei simboli
Oltre ai simboli di avvertenza già elencati, altri simboli trovati sulla testa della
videocamera urologica 1288 HD e in questo manuale hanno signicati specici
che chiariscono l’uso e la conservazione corretti della testa della videocamera
urologica 1288 HD. L’elenco seguente riporta i simboli associati a questo prodotto:
Avverte della presenza di importanti istruzioni di funzionamento
emanutenzione nel manuale
Data di produzione
Produttore legale
SN
Numero di serie
Numero catalogo
Umidità d’esercizio nominale
Pressione d’esercizio nominale
Temperatura d’esercizio nominale
Denota conformità alla norma CAN/CSA C22.2 N. 601.1-M90
UL60601-1.
Parte applicata di tipo BF
Questo simbolo indica che le apparecchiature elettriche
edelettroniche non devono essere smaltite come riuti
cittadini indierenziati maseparatamente. Per lo smaltimento
dell’apparecchiatura rivolgersi al produttore o ad altre aziende
dismaltimento autorizzate.
71
Page 76

Descrizione del prodotto
eusoprevisto
La testa della videocamera urologica 1288 HD è una videocamera adalta
denizione usata per acquisire immagini sse e video di applicazioni urologiche
endoscopiche. È stata disegnata con un angolo di 90° tra la testa dellavideocamera
e l'endoscopio per consentire un accesso più facile durante le procedure
diurologia.
La testa della videocamera urologica è usata insieme alla Console dellavideocamera
1288 HD (REF 1288010000; REF 1288010001).
Indicazioni/Controindicazioni
Questa videocamera urologica 1288 HD è indicata per l’uso in laparoscopie,
nasofaringoscopie, endoscopie delle orecchie, sinuscopie e chirurgia plastica,
quando risulta indicato l’uso di laparoscopi/endoscopi/artroscopi. Alcuni esempi
delle chirurgie endoscopiche più comuni sono la colecistectomia laparoscopica,
correzione laparoscopica dell’ernia, appendicectomia laparoscopica, dissezione
laparoscopica del linfonodo pelvico, isterectomia con laparoscopia, fusione spinale
anteriore laparoscopica e toracoscopica, ricostruzione del legamento crociato
anteriore, artroscopia del ginocchio, artroscopia della spalla, artroscopia per
piccole articolazioni, ssaggio a decompressione, resezione a cuneo, biopsia
polmonare, biopsia pleurica, simpatectomia dorsale, pleurodesi, dissezione
dell’arteria mammaria interna per bypass coronarico, innesto di bypass coronarico
quando è consigliata la visualizzazione endoscopica ed esame della cavità
cardiaca evacuata durante le procedure di sostituzione della valvola. La videocamera
viene utilizzata da chirurghi generici, ginecologi, cardiochirurghi, chirurghi
toracici, chirurghi plastici, chirurghi ortopedici, chirurghi otorinolaringoiatri
eurologi.
Non esistono controindicazioni note.
Caratteristiche del prodotto
La testa della videocamera urologica collega alla console della videocamera
eacquisisce immagini video e fotograche, che invia alla console della videocamera.
È caratterizzata da diversi comandi accessibili mediante un tastierino di pulsanti
situato sulla parte alta della testa della videocamera urologica (vedere la sezione
"Istruzioni di funzionamento" di questo manuale).
72
Page 77

1
2 3
4
5
8
7
1. Endoscopio —
2. Morsetto interno Fissa l’endoscopio alla testa della videocamera
3. Freno interno Evita la rotazione dell’endoscopio
4. Manopola per
lamessa a fuoco
5. Testa della
videocamera
urologica 1288HD
Regola il livello della testa della videocamera
Acquisisce immagini fotograche e video e fornisce
i controlli della videocamera
6
73
Page 78

6. Cavo della
videocamera
urologica
7. Connettore del cavo Collega la testa della videocamera alla console della
—
videocamera
8. Cappuccio
diprotezione
Protegge il connettore del cavo durante la pulizia
ela sterilizzazione
74
Page 79

Configurazione
1. Impostare la videocamera 1288 HD secondo le istruzioni fornite
nelmanualeutente.
2. Collegare la testa della videocamera urologica alla console
• Svitare il cappuccio d’immersione dal connettore del cavo
senecessario.
• Allineare la freccia blu sul connettore del cavo con la freccia
blusulla porta videocamera urologica-connettore sul pannello
anteriore della console.
• Spingere il connettore nché non scatta in posizione.
• (Per scollegare la videocamera urologica dall’unità di controllo,
prendere la parte in rilievo del connettore e tirare).
3. Collegare un endoscopio alla testa della videocamera urologica.
• Rimuovere il cappuccio antipolvere rosso, se presente.
• Bloccare il freno interno spostandolo verso destra.
• Ruotare il morsetto interno e tenerlo aperto.
75
Page 80

• Inserire l’endoscopio base nel morsetto interno.
• Ruotare il freno interno nella direzione opposta per ssare
l’endoscopio.
4. Collegare un cavo luce dalla sorgente luminosa al supporto luce
sull’endoscopio.
76
Page 81

Funzionamento
Avvertenza: prima di usare la videocamera urologica 1288HD
in una procedura chirurgica, testare tutti i componentiper
accertarsi che funzionino correttamente. Accertarsi chel’immagine
video appaia su tutti i monitor prima di iniziare una procedura.
La videocamera urologica 1288 HD può essere controllata usando i pulsanti sulla
testa della videocamera o sull’interfaccia touchscreen sulla console.
Uso dei pulsanti della testa della videocamera
urologica
Le funzioni della testa della videocamera urologica dispongono di un tastierino
ovale a due pulsanti per il controllo manuale della videocamera urologica 1288.
Idue pulsanti sono P e W.
Pulsante P (immagine)
Il pulsante P controlla no a due accessori video remoti.
• Premere il pulsante P per meno di due secondi per selezionare Controllo
adistanza 1. Viene emesso un segnale sonoro.
• Premere il pulsante P per più di due secondi per selezionare Controllo
adistanza 2. Vengono emessi due segnali sonori.
Pulsante W (bilanciamento del bianco)
Il pulsante W attiva la funzione di bilanciamento del bianco o la funzione
luminosità/zoom. La funzione di bilanciamento del bianco si usa per correggere
lievi dierenze di colore che esistono tra diverse sorgenti luminose o endoscopi.
• Premere il pulsante W per più di due secondi per attivare la funzione
dibilanciamento del bianco.
• Premere il pulsante W per meno di due secondi per aumentare il livello
dizoom di uno degli otto livelli (lo zoom tornerà al livello minimo dopo
ilcompletamento del ciclo).
Eseguire il bilanciamento del bianco prima di ogni procedura chirurgica.
77
Page 82

Nota: accertarsi che alla videocamera siano collegati un endoscopio e una
sorgente luminosa e che la videocamera, la sorgente luminosa e il monitor siano
accesi prima di regolare il bilanciamento del bianco.
1. Puntare l’endoscopio su una pila di tamponi di garza 10 x 10 cm, una spugna
laparoscopica bianca o una supercie bianca pulita.
2. Osservare il monitor e accertarsi che non siano visibili bagliori fuori della
supercie bianca.
3. Mantenere premuto il pulsante W nché “BILANCIAMENTO DEL
BIANCO IN CORSO” non inizia a lampeggiare sulmonitor.
4. Continuare a puntare l’endoscopio sulla supercie bianca nché il monitor
non indica “BILANCIAMENTO DEL BIANCO COMPLETATO”.
L’immagine video potrebbe cambiare colore. Se non si riesce ad ottenere un
bilanciamento del bianco accettabile, fare riferimento alla guida utente della
Console della videocamera 1288 HD.
Uso dell’interfaccia Touchscreen
L’interfaccia Touchscreen sulla console presenta i comandi per far funzionare
la videocamera e per selezionare le impostazioni di sistema. Dal touchscreen,
èpossibile:
• scegliere le impostazioni della videocamera per le procedure
diurologia
• acquisire foto
• acquisire video
• azionare il bilanciamento del bianco
78
Page 83

Scorrere le impostazioni predenite della videocamera
concepite per le specialità chirurgiche. Scegliere tra:
• Artroscopia
• Cistoscopia
• ORL
• Flexi-Scope
(Endoscopio
essibile)
• Isteroscopia
Acquisizione foto.
Per l’attivazione premere il pulsante per più di due
secondi.
Acquisizione video.
Per iniziare la registrazione premere il pulsante per
due secondi. Premere Stop per interrompere.
Azionamento bilanciamento del bianco.
Per l’attivazione premere il pulsante per più di due
secondi.
• Premere una sola volta per procedere alla
schermata Menu.
• Laparoscopia
• Laser
• Microscopio
• Standard
79
Page 84

Regolazione della messa a fuoco
Per regolare la messa a fuoco, spostare la manopola per la messa a fuoco
asinistra o a destra.
80
Page 85

Pulizia, rigenerazione
emanutenzione
Rigenerazione della testa della
videocameraurologica
Queste istruzioni per la rigenerazione vengono fornite ai sensi di ISO 17664,
AAMI TIR12, AAMI ST79 e AAMI ST81. Nonostante siano state validate da
Stryker e considerate idonee alla preparazione del dispositivo per il riutilizzo,
resta responsabilità dell’operatore garantire che la rigenerazione così come
realmente eseguita utilizzando attrezzatura, materiali e personale nella sede
deputata alla rigenerazione, ottenga il risultato desiderato. Ciò di norma
richiede la validazione e il monitoraggio di routine del processo. Stryker
consiglia agli utenti di osservare questi standard durante il reprocessing
diquesti dispositivi medicali.
Avvertenze
• Pulire e sterilizzare questo dispositivo quando lo si utilizza per la prima
volta e dopo ogni impiego successivo.
• Utilizzare esclusivamente i cicli di sterilizzazione descritti in questo
documento. Cicli di sterilizzazione diversi da quelli prescritti possono
danneggiare il dispositivo o risultare in una sterilizzazione insuciente.
• Separare la testa della videocamera urologica e l’endoscopio prima della
pulizia, disinfezione o sterilizzazione.
• Indossare l’apposita attrezzatura protettiva: guanti, protezione per gli
occhi e così via.
Attenzione
• Installare sempre il cappuccio per immersione prima della rigenerazione
della videocamera urologica. Un errato serraggio del cappuccio
diprotezione corroderebbe i piedini del connettore e invaliderebbe
lagaranzia.
• Prima dell’immersione in un qualsiasi liquido, ispezionare il cavo della
videocamera per rilevarne eventuali tagli e crepe. Restituire eventuali
videocamere danneggiate al reparto riparazioni Stryker.
• Non utilizzare spazzole o tamponi con punte metalliche o abrasive
durante la pulizia manuale per evitare gra o danni permanenti.
• Per ridurre la corrosione galvanica, evitare l’immersione di metalli
diversi in stretta vicinanza.
• Mai immergere la videocamera nello stesso vassoio in cui si ripongono
strumenti alati.
• La sterilizzazione ripetuta con ossido di etilene potrebbe comportare
ladegradazione della qualità delle immagini.
81
Page 86

Limiti della rigenerazione
• Non sterilizzare il dispositivo adottando metodi diversi. L’impiego
di diversi metodi di sterilizzazione può ridurre signicativamente
leprestazioni del dispositivo.
• Non lasciare il dispositivo immerso in soluzioni più a lungo del necessario.
Ciò potrebbe accelerare l’invecchiamento del prodotto.
• Una procedura corretta esercita un eetto minimo su questo dispositivo.
La durata utile viene normalmente determinata dall’usura e dai danni
dovuti all’uso.
• I danni dovuti a procedure di sterilizzazione non corrette non sono
coperti dalla garanzia.
Istruzioni
Punto d’uso
• Eliminare l’eccesso di sporco dal dispositivo utilizzando salviette
monouso.
• Qualora venga utilizzato un metodo di rigenerazione automatico,
risciacquare i canali nel dispositivo con 50 ml di acqua distillata sterile
immediatamente dopo l’uso.
Contenimento e trasporto
• Rigenerare il dispositivo non appena risulti ragionevolmente pratico
dopo l’uso1.
• Trasportare il dispositivo su un vassoio per evitare di danneggiarlo.
1
Durante la validazione della pulizia è stato utilizzato un tempo di attesa
di30minuti.
Preparazione alla pulizia
1. Smontare l’innesto dall’endoscopio e dalla testa della videocamera.
2. Preparare un detergente enzimatico, seguendo le istruzioni della ditta
produttrice (7,49 g per litro di acqua a 35 – 40 °C)2.
3. Stronare l’intero dispositivo con un panno pulito e il detergente.
4. Immergere il dispositivo nel detergente. Utilizzando una siringa, iniettare
50 ml di soluzione detergente facendo in modo da raggiungere tutte le parti
del dispositivo.
5. Immergere il dispositivo nel detergente per un minimo di 15 minuti.
82
Page 87

Pulizia: manuale
1. Spazzola
• Preparare una soluzione nuova di detergente enzimatico, seguendo le
istruzioni della ditta produttrice (7,49 g per litro di acqua a35–40 °C)2.
• Spazzolare a fondo la parte esterna del dispositivo con una spazzola
a setole morbide concentrandosi principalmente su tutte le superci
accoppiate e su quelle ruvide.
• Utilizzando una siringa, iniettare i lumi e le superci accoppiate per
unminimo di cinque volte con 50 ml del detergente.
• Spazzolare i lumi per un minimo di 5 volte da ogni estremità,
utilizzando uno scovolino idoneo.
• Spazzolare tutte le parti mobili in tutte le posizioni estreme.
2. Risciacquo
• Risciacquare il dispositivo con acqua di osmosi/deionizzata
(RO/DI) atemperatura ambiente no a rimuovere qualsiasi residuo
di detergente. Risciacquare i lumi o le superci accoppiate per un
minimo di 5 volte. Una volta rimosso ogni residuo di detergente,
continuare a risciacquare per un minimo di 30 secondi.
• Eliminare tutta l’acqua in eccesso dal dispositivo e asciugarlo con
unpanno pulito o aria compressa.
• Ispezionare visivamente il dispositivo per controllarne la pulizia,
prestando attenzione alle aree dicili da raggiungere. Se sono presenti
residui di sporco, ripetere le fasi 1 e 2.
3. Immersione
• Preparare un detergente non enzimatico, seguendo le istruzioni della
ditta produttrice (1,87 g per litro di acqua a 35 – 40 °C)3.
• Immergere completamente il dispositivo e utilizzare una siringa per
iniettare i lumi e le superci accoppiate con 50 ml di detergente.
• Immergere il dispositivo per un minimo di 15 minuti.
4. Spazzolamento
• Spazzolare a fondo la parte esterna del dispositivo utilizzando una
spazzola a setole morbide.
• Utilizzando una siringa, iniettare 50 ml di detergente in ogni cannula,
lume o supercie accoppiata per un minimo di 5 volte.
• Spazzolare i lumi per un minimo di 5 volte da ogni estremità,
utilizzando uno scovolino idoneo.
• Azionare il dispositivo e spazzolare attorno alle parti mobili in ogni
posizione estrema.
5. Risciacquo
• Risciacquare completamente il dispositivo utilizzando acqua O/DI no
alla rimozione di ogni residuo di detergente. Risciacquare i lumi ole
fenditure per un minimo di 5 volte. Una volta rimosso ogni residuo
didetergente, continuare a risciacquare per un minimo di 30 secondi.
• Eliminare tutta l’acqua in eccesso dal dispositivo e asciugarlo con un
panno pulito o aria compressa.
83
Page 88

Pulizia: automatizzata
1. Spazzola
• Con una siringa, iniettare 50 ml di detergente enzimatico (dalla sezione
“Preparazione alla pulizia”) almeno una volta in tutti i lumi e in tutte
lesuperci accoppiate.
• Spazzolare almeno 1 volta da entrambe le estremità di tutti i lumi,
utilizzando uno scovolino idoneo.
2. Risciacquo
• Risciacquare il dispositivo con acqua RO/DI a temperatura ambiente
no a quando non è più visibile alcun residuo di detergente. Continuare
a sciacquare il dispositivo per almeno 30 secondi dopo aver rimosso
tutti i residui di detergente.
• Tenere inclinato dispositivo nella macchina per il lavaggio per favorire
lo spurgo.
3. Lavaggio automatizzato
• Programmare il dispositivo per il lavaggio attenendosi ai seguenti
parametri:
Fase Tempo
diricircolo
Temperatura
dell’acqua
Tipo di detergente
e concentrazione
(seapplicabile)
Pre-Lavaggio 2 minuti Acqua di rubinetto
N/A
calda
Lavaggio
enzimatico
Lavaggio 1 2 minuti Set point
Risciacquo 1 2 minuti Acqua di rubinetto
2 minuti Acqua di rubinetto
fredda
(66 °C)
Detergente
enzimatico
Detergente non
enzimatico
N/A
fredda
Fase di
7 minuti 115 °C N/A
asciugatura
• Dopo il completamento della fase di asciugatura dopo Risciacquo 1,
interrompere il ciclo e aprire lo sportello della macchina per il lavaggio.
• Rimuovere il dispositivo dalla macchina per il lavaggio durante la fase
termica e riposizionare il dispositivo nella macchina per il lavaggio per
la fase di asciugatura.
• Per agevolare l’asciugatura, se necessario, utilizzare aria compressa.
Controllare visivamente che ogni dispositivo sia pulito.
2
ENZOL® Enzymatic Detergent è validato per ecacia di pulizia.
3
Renu-Klenz® è validato per ecacia di pulizia.
84
2
3
Page 89

Disinfezione a basso livello (opzionale)
1. Disinfettare il dispositivo in una soluzione disinfettante che possieda uno
dei seguenti ingredienti attivi:
• ≥ 2,4% di glutaraldeide4 con un tempo di immersione minimo
di45minuti a 25 °C
oppure
• ≥ 3,4% di glutaraldeide5 con un tempo di immersione minimo
di20minuti a 25 °C
oppure
• ≥ 0,55% di orto-alaldeide6 con un tempo di immersione minimo
di12minuti a 25 °C.
2. Preparare una soluzione disinfettante in base alle istruzioni del produttore.
3. Come da raccomandazioni del produttore, immergere il dispositivo,
riempiendo tutti i lumi nella soluzione disinfettante per il tempo necessario
alla temperatura appropriata.
4. Sciacquare e irrigare tutti i componenti e i lumi con abbondante acqua
corrente demineralizzata per rimuovere il disinfettante.
5. Immediatamente dopo il risciacquo asciugare tutte le parti con un panno
privo di lanugine.
4
CIDEX Activated® è validato per ecacia disinfettante.
5
CIDEX Plus® è validato per ecacia disinfettante.
6
CIDEX® OPA è validato per ecacia disinfettante.
Asciugatura
• Per l’asciugatura automatica, utilizzare il ciclo di asciugatura
indotazione con il dispositivo per il lavaggio/disinfezione.
• Per l’asciugatura manuale, utilizzare un panno privo di lanugine.
• Asciugare ogni lume con aria compressa.
Manutenzione, ispezione e collaudo
• Controllare il dispositivo sistematicamente. Se si osserva o si sospetta
unproblema, il dispositivo va restituito per essere riparato.
• Controllare che tutti i componenti siano puliti. Se sono presenti residui
di tessuto o uidi, ripetere le procedure di pulizia e disinfezione
sopraesposte.
• Ispezionare il cavo della videocamera per rilevarne eventuali tagli
e danneggiamenti. Restituire eventuali videocamere urologiche
danneggiate al reparto riparazioni Stryker.
Confezionamento
N/A
85
Page 90

Sterilizzazione
Dopo aver eseguito la pulizia come da istruzioni sopraindicate, eseguire uno
dei cicli di sterilizzazioni seguenti.
Ossido di etilene (EtO)
• Avvolgere in un doppio involucro la testa della videocamera prima
diprocedere alla sterilizzazione.
Parametri di precondizionamento
Temperatura 55 °C
Umidità della camera 70% UR
Set point vuoto 1,3 psia
Tempo 30 minuti
Esposizione
Concentrazione (100% EtO) 725 mg/l
Temperatura 55 ± 2 °C
Tempo 1 ora
Umidità della camera 70% ± 5% UR
Parametri di aerazione
Tempo d’aerazione 12 ore
Temperatura 35 – 54 °C
Steris® System 1
1. Pulire e preparare la testa della videocamera urologica e il cavo così come
indicato nella sezione Pulizia.
2. Sterilizzare la testa e il cavo della videocamera urologica impiegando
Steris®System 1 con sterilizzante Steris® Sterilant 20.
3. Lasciare asciugare completamente la testa della videocamera urologica,
il cavo e l’endoscopio prima di rimontarli. L’umidità potrebbe causare
l’annebbiamento del vetro della videocamera durante l’uso.
Sterrad®
1. Pulire e preparare la testa della videocamera urologica e il cavo così come
indicato nella sezione Pulizia.
2. Sterilizzare la testa e il cavo della videocamera urologica impiegando il sistema
di sterilizzazione Sterrad™ NX o 100S.
Conservazione
Non conservare mai il dispositivo in un ambiente umido e non areato come
lacustodia di trasporto. Ciò può costituire rischio d’infezione.
86
Page 91

Uso di telini sterili
L’uso di telini sterili assicurerà la massima durata della testa della videocamera
HD 1288. Per ottenere i risultati migliori seguire le istruzioni fornite dal produttore
dei telini.
Smaltimento
Questo prodotto contiene apparecchiature elettriche
edelettroniche da smaltire. Non deve essere smaltito nel
sistema di raccolta comunale collettivo ma separatamente nel
rispetto delle normative nazionali o istituzionali in materia
diapparecchiature elettroniche obsolete.
La 1288 HD deve essere smaltita sulla base delle leggi e delle
pratiche ospedaliere locali.
87
Page 92

Specifiche tecniche
Le impostazioni a 60 Hz sono visualizzate per prime (quelle a 50 Hz gurano
fraparentesi).
Sistema di imaging 1/3” CCD a scansione progressiva
Alta denizione
Condizioni
difunzionamento
Condizioni per il trasporto
e la conservazione
Peso totale di spedizione 0,680 kg videocamera sopratesta urologica
Dimensioni Dal cavo della testa videocamera alla console
Ottimizzazione 8 livelli (con interruttore)
Classicazione Componente applicato tipo BF
Conforme agli standard
di sicurezza in ambito
medicale
Temperatura: 5 – 40 °C
Umidità relativa: 30 – 95%
Temperatura: -20 – 60 °C
Umidità relativa: 10 – 95%
Pressione barometrica: da 700 a 1060 hPa
della videocamera:
3,0 tenuta cavo
6,1 estensione cavo
Protezione dall’ingresso dell’acqua,
IPX7– protezione dagli eetti
dell’immersione temporanea nell’acqua
IEC 60601-1:1988 + A1:1991 +A2:1995
IEC 60601-2-18:1996 + A1:2000
CAN/CSA C22.2 No 601.1-M90
UL 60601-1:2003
AS/NZS 3200.1.0:1998
CSA 22.2.601.1.1:2002
CAN/CSA C22.2 N. 601.2.18:1990
Per informazioni su modiche e prodotti nuovi, contattare il rappresentante
locale Stryker Endoscopy.
88
Page 93

Índice
Advertências e Precauções ............................................91
Explicação dos Símbolos ..................................................93
Descrição e Finalidade do Produto ............................94
Indicações/Contra-indicações ...................................................94
Funções do Produto ..................................................................94
Instalação ..................................................................................97
Funcionamento .......................................................................99
Utilização dos Botões da Cabeça da Câmara para Urologia ....99
Ajuste da Focagem ..................................................................102
Limpeza, Reprocessamento eManutenção ........103
Reprocessamento da Cabeça da Câmara para Urologia ........103
Utilização de Capas Esterilizadas ............................................110
Especificações Técnicas.................................................111
Page 94

Page 95

Advertências e Precauções
Leia atentamente este manual e siga as suas instruções rigorosamente.
AVISO DE SEGURANÇA IMPORTANTE: Antes de utilizar este dispositivo,
ler atentamente o presente manual de utilização. Ao utilizar este dispositivo
com uma fonte luminosa, poderá provocar um incêndio e/ou lesões graves
no paciente, no utilizador ou em objectos inanimados, se as instruções neste
manual não forem seguidas. Todas as fontes de luz podem gerar quantidades
de calor signicativas na ponta do endoscópio, no suporte deluz, na ponta
do cabo de bra óptica e/ou próximo do adaptador do cabo de bra óptica.
Uma maior luminosidade da fonte de luz resulta numa maior quantidade
de calor. Ajustar sempre o nível de luminosidade da câmara e do monitor
antes de ajustar o nível de luminosidade da fonte de luz. Ajustar o nível
de luminosidade da fonte de luz para o mínimo necessário para iluminar
adequadamente o local da cirurgia. Ajustar também o obturador interno
dacâmara para uma velocidade mais elevada, para utilizar a fonte de luz
com uma intensidade mais baixa. Evitar tocar na ponta do endoscópio
ouna ponta do cabo de bra óptica do lado do paciente, e nunca colocá-las
sobre o paciente, porque tal poderá resultar em queimaduras no paciente
ou no utilizador. Por outro lado, nunca colocar a ponta do endoscópio,
osuporte de luz, o adaptador do cabo de bra óptica ou a ponta do cabo
debra óptica sobre capas cirúrgicas ou outros materiais inamáveis,
porque tal poderá provocar um incêndio. Colocar a fonte luminosa no modo
em espera sempre que o endoscópio for removido do cabo de bra óptica ouo
dispositivo for deixado sem vigilância. A ponta do endoscópio, o suporte
de luz do endoscópio, o adaptador do cabo de bra óptica e a ponta do cabo
de bra óptica demorarão vários minutos a arrefecer depois de terem sido
colocados no modo em espera, pelo que poderão ainda provocar incêndios
ou queimaduras no paciente, no utilizador ou em objectos inanimados.
Advertências e Precauções
Para evitar ferimentos graves no utilizador e no paciente e/ou danos neste
equipamento, é necessário ter em conta as seguintes advertências:
1. Ser um médico qualicado, com um conhecimento aprofundado
dautilização deste dispositivo.
2. Retirar cuidadosamente este dispositivo da embalagem e vericar se sofreu
algum dano durante o transporte. Se forem detectados quaisquer danos,
consulte a garantia padrão.
3. Ler atentamente o presente manual de utilização, especialmente as advertências,
e estar familiarizado com o seu conteúdo antes de ligar e utilizar este dispositivo.
4. Ler integralmente a secção de instruções do manual de instruções antes
deproceder à montagem ou ligação da câmara.
91
Page 96

5. Prestar especial atenção às instruções existentes neste manual relativamente
aos cuidados a ter com o equipamento, à limpeza, à desinfecção e à esterilização
do mesmo. O seu não cumprimento poderá provocar danos.
6. Testar este dispositivo antes de realizar qualquer procedimento cirúrgico.
Este dispositivo foi completamente testado na fábrica antes do respectivo
envio. Nunca utilizar este dispositivo na presença de gases inamáveis
ouexplosivos.
7. Evitar desmontar qualquer parte da cabeça da câmara, porque fazê-lo poderá
quebrar os vedantes, causando fugas e/ou choque eléctrico. Este dispositivo
é fornecido selado de fábrica para impedir que a humidade penetre nos
componentes electrónicos. Se a vedação da cabeça da câmara ou do cabo
forintencionalmente quebrada, a garantia perderá a sua validade.
8. Antes de cada utilização, vericar a superfície exterior da câmara
edoendoscópio para se certicar de que não apresenta superfícies
ásperas,arestas aadas ou saliências.
9. A utilização da cabeça da câmara com patilhas partidas no conector poderá
causar danos na Unidade de Controlo da Câmara. Se existir alguma patilha
danicada ou em falta, consulte a Garantia Padrão da Stryker.
10. Certicar-se de que todos e quaisquer reajustes, modicações e/ou reparações
serão levados a cabo por pessoas autorizadas pela Stryker Endoscopy.
Nãotentar realizar quaisquer reparações ou ajustes internos que não estejam
especicamente explicados neste manual de operação. Tal acção poderá
resultar num desempenho não intencional ou em danos no produto.
11. Manusear sempre a câmara para urologia com todo o cuidado. O sistema
de câmara contém componentes sensíveis que se encontram alinhados
com precisão e que podem sofrer danos se forem deixados cair ou se forem
manuseados incorrectamente.
12. A esterilização repetida por óxido de etileno pode resultar na degradação
daqualidade da imagem.
A garantia é anulada caso qualquer um destes avisos não seja respeitado.
92
Page 97

Explicação dos Símbolos
A par dos símbolos de advertência já enumerados, existem outros símbolos
naCabeça da Câmara para Urologia 1288 HD e neste manual que têm signicados
especícos que esclarecem o uso e o armazenamento correctos da Cabeça
da Câmara para Urologia 1288 HD. A lista que se segue explica os símbolos
associados a este produto:
Alerta para a presença de instruções importantes de operação
emanutenção no manual.
Data de fabrico
Fabricante legal
SN
Número de série
Número de catálogo
Valores de humidade de operação
Valores de pressão de operação
Valores de temperatura de operação
Indica o cumprimento do estipulado nas normas
CAN/CSAC22.2N.º 601.1-M90 e UL60601-1.
Peça aplicada de tipo BF
Este símbolo indica que os resíduos de equipamentos eléctricos
e electrónicos não devem ser eliminados juntamente com o lixo
municipal indiferenciado, e devem ser recolhidos em separado.
Contactar o fabricante ou outra empresa de eliminação autorizada
para inutilizar o equipamento.
93
Page 98

Descrição e Finalidade do Produto
A Cabeça da Câmara para Urologia 1288 HD é uma câmara de alta denição
utilizada para captar imagens paradas e de vídeo de procedimentos endoscópicos
de urologia. Está concebida com um ângulo de 90° entre a cabeça da câmara
e o endoscópio para permitir um acesso mais fácil durante procedimentos
deurologia.
A cabeça da câmara para urologia é utilizada em conjunto com a Consola
daCâmara 1288 HD (REF 1288010000; REF 1288010001).
Indicações/Contra-indicações
A Câmara para Urologia 1288 HD está indicada para ser utilizada em laparoscopia
geral, nasofaringoscopia, endoscopia do ouvido, sinuscopia e cirurgia plástica
sempre que for indicada a utilização de um laparoscópio/endoscópio/artroscópio.
Alguns exemplos das cirurgias endoscópicas mais comuns são a colecistectomia
laparoscópica, reparação de hérnia por laparoscopia, apendicectomia laparoscópica,
dissecção dos nódulos linfáticos pélvicos por laparoscopia, histerectomia assistida
por laparoscopia, fusão espinal anterior por laparoscopia e toracoscopia, reconstrução
do ligamento cruzado anterior, artroscopia do joelho, artroscopia do ombro,
artroscopia de articulações pequenas, descompressão-xação, ressecção emcunha,
biópsia pulmonar, biópsia pleural, simpatectomia dorsal, pleurodese, dissecção
da artéria mamária interna para cirurgia de bypass da artéria coronária, cirurgia
de enxerto de bypass da artéria coronária onde a visualização endoscópica
é indicada, e exame da câmara cardíaca evacuada durante a realização
dasubstituição da válvula. Os utilizadores da câmara são cirurgiões gerais,
ginecologistas, cirurgiões cardíacos, cirurgiões torácicos, cirurgiões plásticos,
cirurgiões ortopédicos, cirurgiões otorrinolaringologistas e urologistas.
Não existem contra-indicações conhecidas.
Funções do Produto
A cabeça da câmara para urologia liga-se à consola da câmara e capta imagens
fotográcas e de vídeo que transmite à consola da câmara. Dispõe de várias funções
que estão acessíveis através de um conjunto de botões localizado naparte superior
da cabeça da câmara para urologia (ver a secção “Instruções de Operação”
destemanual.)
94
Page 99

1
2 3
4
5
8
7
1. Endoscópio —
2. Dispositivo
de xação do
suporte do
endoscópio
3. Dispositivo
de bloqueio
do suporte do
endoscópio
Prende o endoscópio à cabeça da câmara
Evita a rotação do endoscópio
6
4. Botão de focagem Ajusta a focagem da cabeça da câmara
5. Cabeça da câmara
para Urologia
1288 HD
Capta imagens fotográcas e de vídeo e permite
ocomando da câmara
95
Page 100

6. Cabo da câmara
para urologia
7. Conector do cabo Liga a cabeça da câmara à consola da câmara
—
8. Tampa
deimersão
Protege o conector do cabo durante a limpeza
eaesterilização
96
 Loading...
Loading...