ST Microelectronics MHBGW1 Product literature
Features
Single Lead AFE for ECG with self calibration
circuitry
Bio-impedance
STM32F M3 Cortex ARM cortex
BT radio class 2: STLC 2584
Digital 3 axis Accelerometer: LIS 331 DLH
External parallel NAND FLASH 2Gbit
Battery Level sensing
Rechargeable battery LiPo 380 mAh
3 LEDs
Mechanical ON/OFF button
Functions
• Heart rate detection
• One lead ECG recording and
transmission
• Physical activity estimation
• Breathing rate measurement
• Body position detection
SPMHBGW1
Body Gateway Electronic patch
Data brief 1.1
Applications
• Elderly people home monitoring
• Chronic cardiac disease monitoring
• Event monitoring
• Single lead holter
1/12

SPMHBGW1
Contents
1 Body Gateway device description ..................................................................................................................... 3
2 Body Gateway device architecture .................................................................................................................... 4
2.1 Hardware: ................................................................................................................................................... 4
2.2 Embedded SW Framework: ........................................................................................................................ 4
3 Embedded SW Framework ............................................................................................................................... 5
3.1 Connectivity: ............................................................................................................................................... 5
3.2 Embedded Data Aggregator........................................................................................................................ 5
3.2.1 ECG: extraction of .............................................................................................................................. 5
3.2.2 Bio-impedance: extraction of .............................................................................................................. 5
3.2.3 Accelerometer: extraction of ............................................................................................................... 5
4 Operating modes: ............................................................................................................................................. 6
5 Physiological parameters & personalization features ........................................................................................ 7
5.1 Parameters: ................................................................................................................................................ 7
5.2 Personalization: .......................................................................................................................................... 7
6 Body Gateway mechanical dimension .............................................................................................................. 8
6.1 Body Gateway device ................................................................................................................................. 8
7 Power consumption......................................................................................................................................... 10
7.1 Usage model example 1: streaming mode ................................................................................................ 10
7.2 Usage model example 2: monitoring mode ............................................................................................... 10
8 ECG EC38 signal snapshot ............................................................................................................................ 11
9 Regulatory Compliance ................................................................................................................................... 12
Safety and EMI Approval, ................................................................................................................................... 12
10 Revision history ......................................................................................................................................... 12
2/12

SPMHBGW1
RoHS compliance
ST modules are RoHS compliant and comply with ECOPACK® norms.
1 Body Gateway device description
The Body Gateway (BGW) Devices is a wearable electronic, battery operated device that is worn on the
chest for the acquisition, recording and transmission of physiological parameters to external devices which
can analyze or forward the data to additional storage elements or system.
The BGW device is also capable to record symptomatic and asymptomatic events and is indicated for
ambulatory monitoring of non lethal cardiac arrhythmias.
Additionally, resident in the Body Gateway device is a heart rate, respiration rate and activity level
calculation algorithm, which allows the system to manage information messages from/to the Server
according to specific settings defined by the physicians/operators.
The device is a part of a Multi-parameter Analysis System, Body Gateway System (BGW system) and
communicates via a BT radio link with the external device. Specification of BGW System is beyond the
scope of this document.
At its heart is a 32-bit ARM Cortex microcontroller with 768 kByte Flash, chosen for its flexible architecture
and low power processing capability. Bluetooth radio was selected for connectivity because its availability in
most commercial mobile phones ensures proper coverage and patient access.
3/12
SPMHBGW1
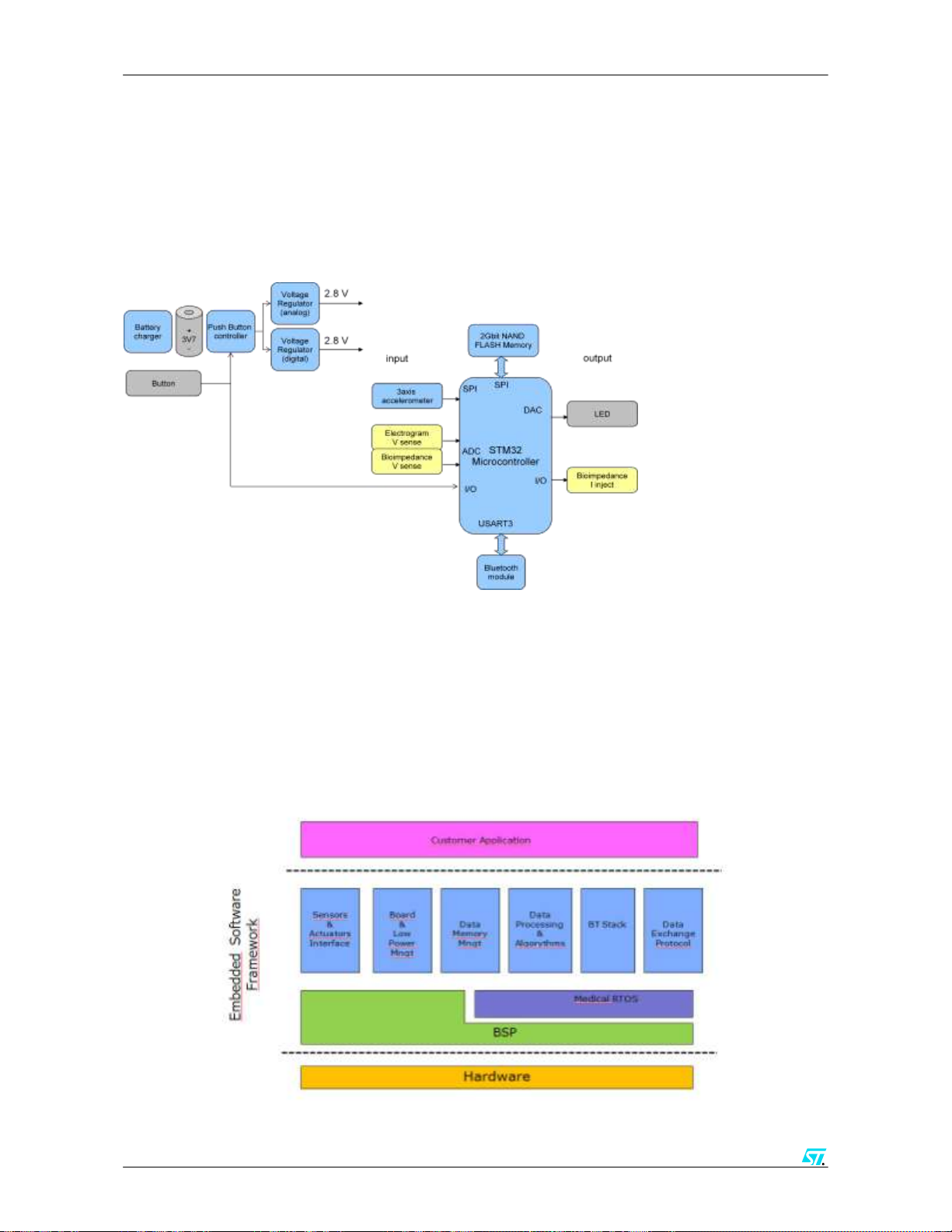
2 Body Gateway device architecture
2.1
Hardware:
The hardware architecture for the BGW device is shown here below.
2.2 Embedded SW Framework:
The embedded SW Frameworks includes a RT OS, board support package, proprietary data aggregation
algorithms, embedded memory management system, a BT stack and proprietary data exchange protocol.
This SW is modular and provides an application interface to allow integration in customer specific
environment including the possibility to plug in proprietary algorithms for physiological data elaboration.
4/12
 Loading...
Loading...