Steelco VS Sterilizer User and maintenance manual

0398
0051
Steelco VS series
Steelco S.p.A.
Via Balegante, 27
31039 - Riese Pio X (TV)
ITALY
Tel. - +39 0423 7561
Fax. - +39 0423 755528
www.steelcospa.com
Use and Maintenance Manual
1

2
NOTIZIE DI COPYRIGHT
The Copyright 2014 of this document is property of Steelco S.p.A. No part of this document may be copied,
transmitted or divulged to third parties without the written authorization of the president of Steelco S.p.A.
AUTHORS APPROVED
Stefano Beni
Version Request
Modifications
1.0 Emission 08/01/2014 All Martina De Lorenzi
1.1
2.0 Modification 06/03/2014 All Lucia Marletta
2.1 Modification 16/05/2014 All Lucia Marletta
2.2 Modification 24/06/2014 3, 38 Lucia Marletta
Table
modification
10/02/2014 19-21 Martina De Lorenzi
_________________
_________________
PUBLICATION TABLE
Date Pages
affected
Issued
by
2.3 Modification 07/08/2014 3, 18-28 Lucia Marletta
2.4 Modification 10/11/2014
3.0 Modification 10/11/2014
3.1 Modification 04/12/2014
3.2 Modification 12/12/2014 All Lucia Marletta
3.3 Modification 28/01/2015 All Lucia Marletta
All publications must be promptly inserted in this document.
After 5 corrections – with relative publication – this document must be drafted with a higher publication
number.
3, 11, 12,
18-104
3, 11, 12,
18-104
3, 11-29,
27-29, 37,
78, 82-93
Lucia Marletta
Lucia Marletta
Riccardo Carpin
3

CONTENTS
1. INTRODUCTION ...........................................................................................................11
1.1 Dear Customer .....................................................................................................11
1.2 Product Presentation ...........................................................................................11
1.3 Identification of the various model .....................................................................12
1.4 Purpose of the manual .........................................................................................12
1.5 General notices .....................................................................................................13
1.5.1 Clauses of supply ...........................................................................................13
1.6 Conditions of guarantee ......................................................................................14
1.7 Fields of use ..........................................................................................................14
1.8 Applicable Standards ...........................................................................................15
2. CONSTRUCTIONAL CHARACTERISTICS .............................................................16
2.1.1 Autoclaves accessories ..................................................................................42
2.1.2 Steelco VS series performances ...................................................................44
2.1.3 Unit classification (for all versions) .............................................................44
2.1.4 Noise/Vibrations ............................................................................................44
2.1.5 Heat loss of the unit ......................................................................................44
2.1.6 Electromagnetic compatibility .....................................................................45
2.1.7 Warning tags .................................................................................................48
2.2 Table Of Symbols .................................................................................................49
2.3 Characteristics of storage and conservation ......................................................50
2.4 Residual risks .......................................................................................................50
2.5 Options ..................................................................................................................50
2.5.1 Steam generator ............................................................................................50
2.5.2 Number of doors ...........................................................................................51
2.5.3 Technical Compartment ...............................................................................51
2.5.4 Reference pressure transmitter ...................................................................51
2.5.5 Other options .................................................................................................51
3. OPERATOR GUIDE .......................................................................................................52
3.1 Operator position .................................................................................................52
3.2 Control system ......................................................................................................54
3.3 Main menù page ...................................................................................................56
3.3.1 State of the sterilizer .....................................................................................56
3.3.2 Keyboards ......................................................................................................57
3.3.3 “STERILIZATION” Menu ..........................................................................58
3.3.4 “LAST CYCLE” Menu ................................................................................65
3.3.5 “UTILITY” Menu .........................................................................................66
3.3.6 “MAINTENANCE” menu ...........................................................................70
3.3.7 “CONFIGURATION” menu .......................................................................74
3.3.8 Characteristics of the phases of the sterilization programs ......................78
3.4 Graphs ...................................................................................................................83
3.5 PC INTERFACE ..................................................................................................87
4. ALARMS ..........................................................................................................................88
5. MAINTENANCE ...........................................................................................................101
5.1 Periodic Cleaning ...............................................................................................103
5.1.1 External paneling cleaning .........................................................................103
5.1.2 Sterilization chamber cleaning ..................................................................104
5.2 Safety Devices .....................................................................................................105
5.3 Procedure to extract the load in Emergency ...................................................106
6. TREATMENT AND CONSERVATION OF STERILIZED MATERIAL ..............107
6.1 Treatment of Materials Before Sterilization ...................................................107
6.2 Arrangement of the load ...................................................................................108
4

6.3 Conservation of sterilized material ..................................................................109
7. PRINTOUTS ..................................................................................................................110
7.1 Printout management ........................................................................................110
7.2 Complete cycle report ........................................................................................112
7.3 Cycle Report Data ..............................................................................................114
7.4 Cycle Result ........................................................................................................114
7.5 Report Trend ......................................................................................................114
7.6 Digital Inputs/Outputs Status Report ..............................................................114
7.7 Analogical Values Status Report ......................................................................114
7.8 Set Point Report .................................................................................................114
7.9 Machine History Report ....................................................................................114
7.10 Machine Settings Report ...................................................................................114
8. GLOSSARY ...................................................................................................................115
9. ATTACHMENT 1 P&I DRAWING ...........................................................................116
10. ATTACHMENT 2 ELECTRICAL DIAGRAM ........................................................117
5

INDEX OF FIGURES
Figure 1 –Main components ______________________________________________________________________________ 17
Figure 2 – Labelling design ______________________________________________________________________________ 48
Figure 3 – Operator position VS1 _________________________________________________________________________ 53
Figure 4 – Operator position VS12 ________________________________________________________________________ 53
Figure 5 – Operator position VS 18 ________________________________________________________________________ 53
Figure 6 – Load side ____________________________________________________________________________________ 54
Figure 7 – Sterile side ___________________________________________________________________________________ 55
Figure 8 – Main menu __________________________________________________________________________________ 56
Figure 9 – State of the sterilizer ___________________________________________________________________________ 56
Figure 10 – Keyboards __________________________________________________________________________________ 57
Figure 11 – Menu ______________________________________________________________________________________ 58
Figure 12 – Sterilization _________________________________________________________________________________ 58
Figure 13 – Basic program _______________________________________________________________________________ 59
Figure 14 – User program _______________________________________________________________________________ 59
Figure 15 – Work state __________________________________________________________________________________ 60
Figure 16 – Sterilization describe _________________________________________________________________________ 61
Figure 17 – Input and output diagram ______________________________________________________________________ 61
Figure 18 – Parameters diagram __________________________________________________________________________ 62
Figure 19 – Finished cycle _______________________________________________________________________________ 62
Figure 20 – Cycle suspend _______________________________________________________________________________ 63
Figure 21 – Alarm _____________________________________________________________________________________ 63
Figure 22 – Alarm describe ______________________________________________________________________________ 64
Figure 23 – Cycle suspend _______________________________________________________________________________ 64
Figure 24 – Active alerts ________________________________________________________________________________ 64
Figure 25 – Last cycle __________________________________________________________________________________ 65
Figure 26 – Cycle diagram _______________________________________________________________________________ 65
Figure 27 – Utility menu _________________________________________________________________________________ 66
Figure 28 – Machine data _______________________________________________________________________________ 66
Figure 29 – Maintenance state ____________________________________________________________________________ 67
Figure 30 – State I/O ___________________________________________________________________________________ 68
Figure 31 – Inlet _______________________________________________________________________________________ 68
Figure 32 – Outlet _____________________________________________________________________________________ 68
Figure 33 – Graph. state ________________________________________________________________________________ 69
Figure 34 – Printer utility ________________________________________________________________________________ 69
Figure 35 – Maintenance menu ___________________________________________________________________________ 70
Figure 36 – Update clock ________________________________________________________________________________ 70
Figure 37 – Manual control ______________________________________________________________________________ 71
Figure 38 – Disabled manuals ____________________________________________________________________________ 71
Figure 39 – Outlet control _______________________________________________________________________________ 72
Figure 40 – Inlet state ___________________________________________________________________________________ 72
Figure 41 – Graph. state ________________________________________________________________________________ 73
Figure 42 – Constructor data restore _______________________________________________________________________ 73
Figure 43 – Configuration _______________________________________________________________________________ 74
Figure 44 – System data _________________________________________________________________________________ 74
Figure 45 -Temperature _________________________________________________________________________________ 75
Figure 46 – Operators authorized list ______________________________________________________________________ 76
Figure 47 – Insert a new operator _________________________________________________________________________ 76
Figure 48 - Program ____________________________________________________________________________________ 77
Figure 49 – Program management _________________________________________________________________________ 77
Figure 50 – Programs set ________________________________________________________________________________ 78
Figure 51 – Properties phase set __________________________________________________________________________ 78
Figure 52 – Seal control _________________________________________________________________________________ 78
Figure 53 - Homogenization ______________________________________________________________________________ 79
Figure 54 – Sterilization _________________________________________________________________________________ 80
Figure 55 - Aeration ____________________________________________________________________________________ 80
Figure 56 – Vacuum test _________________________________________________________________________________ 81
Figure 57 – Heating ____________________________________________________________________________________ 81
Figure 58 – Cooling ____________________________________________________________________________________ 82
Figure 59 – Electric Control Board _______________________________________________________________________ 106
Figure 60 – Printout of the PLC checking __________________________________________________________________ 110
Figure 61 – Complete Cycle Report Example _______________________________________________________________ 112
6

INDEX OF TABLES
Table 1 – Classification as standard EN 61010-1 _____________________________________________________________ 44
Table 2 – Heat loss _____________________________________________________________________________________ 44
Table 3 – Environmental characteristics ____________________________________________________________________ 50
Table 4 – Alarms ______________________________________________________________________________________ 100
Table 5 – Routine maintenance __________________________________________________________________________ 103
Table 6 – Safety devices ________________________________________________________________________________ 105
Table 7 – Available reports content _______________________________________________________________________ 111
Table 8 – Analogical Data for report ______________________________________________________________________ 113
Table 9 – Values and meaning of Result ____________________________________________________________________ 113
7

WARNINGS
Please note that the use of this manual and attached documents is for the exclusive use of the personnel
specified for each skill and in the environment where the autoclave is installed.
These restrictions for use are underlined and
UNAUTHORIZED COPYING IS STRICTLY FORBIDDEN
And their distribution particularly to other companies.
Steelco S.p.A. Reserves the right to act under the law should these warnings not be complied with.
Steelco S.p.A. warns that all operations concerning software and hardware must be carried out with the
utmost care. Such operations can lead to the machine not behaving as required, to be damaged or to treat the
load in a different way.
Any exception must have the authorization of
Steelco S.p.A.
8
SALE AND SUPPORT CENTERS
Steelco S.p.A.
Via Balegante, 27
31039 Riese Pio X (TV)
www.steelcospa.com
Tel. 0039 0423 7561
Fax 0039 0423 755528
9
REFERENCE NOTES
The warning conditions are highlighted in the following way:
Operators must pay due care to these types of messages.
In the manual, the term operator describes the person in charge of usually operating the machine, and who
has the following tasks:
For other technical terms, please refer to the GLOSSARY on page 115.
NOTES
This machine requires trained personnel even for normal loading and pick up operations.
Description of warning
1. Loading and picking up the product;
2. Cycle selection;
3. Cycle start and cycle reset;
4. Alarms reset;
5. Stop the cycle by using the emergency push buttons, when required.
10

1. INTRODUCTION
1.1 Dear Customer
Thank you for placing your trust in Steelco S.p.A. We hope that the performance of this product will meet
your complete satisfaction.
Here you will find a description of all procedures for proper safe use and how to take full advantage of the
features of this unit.
Important notes:
1.2 Product Presentation
The new range of Steelco VS Series autoclaves includes models with 1, 1.5, 2, 3, 4, 6, 8, 10, 12, 12H, 15H,
18H sterilization units.
Steelco VS Series is characterized by an innovative line and a careful and flexible design. The models are
available both with a single and double door, and left- or right- technical compartment.
Ergonomic load height and integrated railways allow the easy positioning of the load.
Into the front panel is mounted a monitor LCD “Touch-screen” and a push-button membrane with six button
for setting. The LCD monitor depending on the position of state: program or management, displays some
buttons that selected by press in the display area what they check specific control functions. It indicates the
various phases, the parameters of the cycle both in numeric form and graphic form, any anomalies or
failures, audit-trails etc. Each sterilization cycle is automatically recorded into the physics memory of the
computer . At the end of the cycle a report containing the identification data and the graphical trend of the
pressure and temperature is printed in compliance with the requirements of the technical standard UNI EN
285 on a 112 mm thermal printer also installed on the front panel of the machine (access to the autoclave is
managed using passwords with different access levels).
Maximum integration with the client’s IT systems.
Information found in this manual is subject to modifications without prior notice
Steelco S.p.A. is not liable for direct, indirect, accidental or other damages regarding the issue and use
of this information
Reproduction, adaptation or translation of this document or any part of it without prior written
consent by Steelco S.p.A.. is strictly forbidden.
The figures contained in this manual are purely for indicative purposes.
This manual must always be available to the sterilization operator
The key to turn on the sterilizer, where applicable, must be available to the sterilization
operator, and must be removed if the unit is not in operation.
External panels must always be closed, the keys must be removed and kept by the person
responsible for the sterilizer
11

1.3 Identification of the various model
The machine model described in this manual is identified by the symbol "VS" followed by:
The first number indicates the capacity of the sterilization chamber:
1 sterilization units
1,5 sterilization units
2 sterilization units
3 sterilization units
4 sterilization units
6 sterilization units
8 sterilization units
10 sterilization units
12 sterilization units
15 sterilization units
18 sterilization units
The letter “H” in case of high machine only for size 12, 15, 18
The second number indicates the number of doors:
1 = single front door, “Single Door Models”
2 = two doors, one opposite the other, “Double Door Models”
The letter, if applicable, indicate the type of steam generator:
E = internal generator with Electric heating
V = direct centralized steam ("Vapour") without internal generator
I = internal generator with Indirect steam heating
EV = internal generator with Electric heating + direct steam ("Vapour")
EI = internal generator with Electric heating + Indirect steam ("Vapour")
Example:
Autoclave Model VS 4/1 E
VS Series
4 Indicates the range's smallest model (4 units)
1 Single Door Model
E Generator equipped with electric heating
1.4 Purpose of the manual
The purpose of this manual is to provide the operator with instructions for:
- General product knowledge;
- Safe and efficient use;
- Treatment of the material before and after sterilization;
- Cleaning and maintenance;
- Analysis of problems and alarms, and how to deal with them;
- Operating diagrams.
12
1.5 General notices
The product and/or its accessories must always be used in compliance with the procedures set forth in this
manual. They must never be used for other than their intended purposes.
1.5.1 Clauses of supply
Concerning installation and assembly carried out by third parties, the manufacturer shall in no way be held
liable for:
a) work carried out improperly, not in accordance with good construction practices, concerning:
- pipes and accessories for the adding and discharge of various fluids,
- electrical connections,
b) work or modifications of any sort carried out on the unit and its accessories without advance written
approval from the manufacturer,
c) associated stresses (seismic, wind, etc.) or dynamic loads originating outside the unit,
d) ladders, metallic structures, foundations, masonry work in general and protective structures, realized by
third parties specifically for the unit,
e) removal of panels and electrical protective parts,
f) insertion of metallic objects in the unit,
g) for special installation (e.g. unit installed in pre-existing service compartment, recessed, placed along side
other units), all internal parts of the machine must be protected in such a way that they cannot be directly
accessed by unqualified personnel (inspection doors with keys or other locking methods that require a
tool for opening). Check the installation diagram and if necessary contact the manufacturer for further
clarification.
The legal representatives of the health and private structures, and public and private health
providers, on the basis of what they detect in carrying out their activities, must immediately notify the
Ministry of Health, directly or via the relevant health structure, any alteration to the characteristics
In order to prevent hazardous situations, with possible damage to individuals and/or property, please
observe the following precautions:
and performance of a device, or the unsuitableness of the instructions for use that may cause the death
of a patient or an operator or their health condition to worsen.
The Ministry of Health notifies the manufacturer.
(excerpt from Legislative Decree dated 24/02/97 n° 46, art. 9 and 10; 93/42 EEC)
The user is responsible for legal obligations connected with installation and use of the product. If the
product is not installed, used, and maintained properly by qualified personnel authorized by the
manufacturer, the manufacturer shall no longer be bound by any sort of responsibility. The unit's
guarantee is to be considered void and the manufacturer shall not be held liable for any breakdowns,
malfunctions, or direct or indirect damage to individuals or property.
Do NOT pour water or other liquids on the unit.
Do NOT pour flammable liquids onto the machine.
Do not apply alcohol or any substance containing it on the Plexiglas panels.
Do NOT use the machine if there are explosive or flammable gases or vapours present.
Before carrying out any maintenance or cleaning, always disconnect the electrical power supply.
Make sure the electrical system is provided with an earth connection in compliance with current laws
and/or standards
Do NOT remove any label or tag from the machine. If this is necessary, ask for new ones
13

1.6 Conditions of guarantee
The following guarantee conditions are offered:
- 12 months from the date of testing and commissioning of the machine;
- 18 months from the date of shipment.
1.7 Fields of use
The Steelco VS series autoclaves are designed for hospital sterilization, i.e. they are suitable to operate
on porous and rubber materials, on surgical instruments, on materials pre-packaged into bags, provided they
can withstand working temperatures of up to 140°C and maximum pressure of 2.5 bar.
It is preferable to avoid sterilizing materials that include additives and/or chemical solvents.
Use only original spare parts
Failure to comply with the measures outlined above relieves the manufacturer of any liability
The guarantee is not applicable to expendable materials and routine maintenance.
The use of sterilizing autoclaves must be entrusted to responsible personnel
It is advisable to carry out the Bowie & Dick test to check the effectiveness of the sterilization process.
It is possible to sometimes carry out the test to check for vacuum seal, to see if the machine has any
seal problems.
If the Bowie & Dick test is successful, the machine can be used (each cycle should in any case be
validated by a manager, and the result of the Bowie & Dick test does not exempt the user from the
obligation of validating the entire sterilization process as required by the technical standard UNI EN
ISO 17665-1).
Warning: test documentation must be conserved in compliance with current legislation.
Improper use of the autoclave relieves the manufacturer of any liability
14

1.8 Applicable Standards
The sterilizers dealt with by this document comply with directives 97/23/EC and standards:
- ASME VIII div 1 & div 2 - Rules for Construction of Pressure Vessels
- EN13445-3 annex B & C – EN13445-3 Cl.18 - Pressure Vessel Code
- UNI EN 288-3 / EN15614-1 - Manufacturing and control procedure for welded joints
- UNI EN 473 - Qualification of personnel responsible for non-destructive controls
- UNI EN ISO 9712:2012 - non-destructive testing — qualification and certification of ndt personnel
- UNI EN ISO 5817:2008 - Welding - Fusion-welded Joints In Steel, Nickel, Titanium And Their Alloys
(beam Welding Excluded) - Quality Levels For Imperfections
- EN10028-7 :2008 - Flat products made of steels for pressure purposes. Part 7: Stainless steels
- EN571-1 - Non-destructive tests - examination with penetrating liquids
- EN1435 A1 - - non-destructive control of welds – radiographic testing of welded joints
- UNI EN ISO 17637 - Non-destructive testing of welds-visual testing of fusion-welded joints
- EN 287.1:2012 – Qualification test of welders - fusion welding - part 1: steels
- UNI EN ISO 15609-1:2006 – Specification And Qualification Of Welding Procedures For Metallic
Materials - Welding Procedure Specification - Part 1: Arc Welding
The sterilizers dealt with by this document comply with directives 93/42/CEE+2007/47/CE and standards:
- EN 285:2009 – Steam sterilizers – Large sterilizers
- CEI EN 61010-1:2011 – Safety requirements for electrical equipment for measurement, control and
laboratory use – General requirements
- CEI EN 61010-2-040:2005 – Safety requirements for electrical equipment for measurement, control and
laboratory use – Parte 2-040: Particular requirements for sterilizers and washer-disinfectors used to treat
medical materials.
- UNI EN ISO 14971:2012 Medical devices – Application of risk management to medical devices.
- CEI EN 60601-1-6:2010 Medical electrical equipment Part 1: General requirements for basic safety and
essential performance – Collateral standard: Usability.
- UNI EN ISO 17665 – 1:2007 Sterilization of health care products - Moist heat - Part 1: Requirements for
the development, validation and routine control of a sterilization process for medical devices.
- CEI IEC 60601-1-2:2007 Medical electrical equipment .Part 1-2:General requirements for basic safety
and essential performance .Collateral standard: Electromagnetic compatibility .Requirements and tests
- EN 62366:2008-01 “Medical devices – Application of usability engineering to medical devices”
- GAMP5 (Good Automated Manufacturing Practice - Version 5)
- EN 61326-1 Electrical equipment for measurement, control and laboratory use - EMC requirements -
Part 1: General requirements
- CEI EN 60204-1/2006 - Safety of machinery. Electrical equipment of machines. General requirements
15

2. CONSTRUCTIONAL
CHARACTERISTICS
The new Steelco VS series autoclaves are characterized by a body completely in stainless steel AISI
316L/316Ti with parallelepiped chamber and tilting towards the drain, with cylindrical chamber for models
1-1,5-2; thermal insulation is guaranteed by an innovative and practical textile cover.
The vertical sliding doors are molded and do not have any welding. The doors' vertical movement is carried
out by a toothed belt motorized system or chain (models 1-1,5). This system is more precise and quieter
during opening and closing. The doors' anti-crushing plate, compliant with safety standards CEI EN 61010-1
and CEI EN 61010-2-040, is characterized by a new design that is easy to clean. In addition, it is sufficient
to apply minimum pressure to stop the door, maximizing the responsiveness of the safety system.
The new Steelco VS series autoclaves guarantee excellent performance in sterilization cycles whilst
optimizing water and steam consumption.
Tri-clamp hydraulic connections make it easy to maintain the systems which have been arranged on the
front part of the autoclave. The vacuum pump has two-stages; electrical versions are equipped with a new
steam generator with power up to 64 kW. The autoclave's front part houses a tray for the vacuum pump,
which allows the latter to be easily extracted for maintenance (when necessary).
The base is equipped with guides for pallet hand trucks (when necessary), in order to be able to lift the
autoclave and transport it safely and easily. The load-bearing structure rests on 4 height-adjustable feet to
ensure that the unit is balanced.
The autoclave, except for models 1-1,5-2, is designed to rapidly reduce the total width to just 950 mm, to
allow easy access to the technical panels, which can be placed on both the right and left side.
Temperatures of accessible parts and internal parts are within the limits set forth by safety standards CEI EN
61010-1 and CEI EN 61010-2-040 and by product standard UNI EN 285;
Manifolds compliant with technical standard UNI EN 285 for insertion of additional probes (in the
sterilization chamber or in the well) and for connection of instruments required for physical qualification
required by technical standard UNI EN ISO 17665-1;
The seal of the autoclave is obtained via a silicon gasket, housed in a cavity obtained by precision working
of a ring welded to the body, pushed against the door by steam injection. Before the door is opened, a
vacuum is created in the cavity that houses the gasket so that the gasket is detached from the door before it
is possible to move the door (reducing wear).
Control of fluids with pneumatic valves installed with fittings with mechanical seal;
Equipped with sterile filters with degree of filtering of 99.999% according to D.O.P. test;
Equipped with the following indicator instruments: gauge to indicate pressure in the chamber (scale 0÷6 bar
relative); gauge to indicate pressure in jacket (scale -1÷5 bar relative) and gauge to indicate the pressure of
the steam (scale 0÷6 bar relative);
Instruments for measuring temperature and pressure of both control system and recording system compliant
with the requirements of technical standard UNI EN 285;
Equipped with a pressure safety switch for chamber pressure, a safety pressure switch for pressure in the
generator (if installed), a service pressure switch for gasket pressure; a safeguard pressure switch for water
supply and a safeguard pressure switch for compressed air supply;
Equipped with internal guides for any mobile load structures (optional).
The design pressure of the pressurized containers goes from vacuum to 3 bar relative.
16

Sterilization chamber
Electrical panel
Technical compartment
containing the hydraulic system
Control panel
Steam generator
(only versions “E”, “I”, “EV” and “EI” )
Figure 1 –Main components
Upon the client's request at the moment of the order, the electrical panel, the technical compartment
containing the hydraulic system and the control panel can be installed on the left side of the sterilization
chamber instead of the right side.
For the smallest models, where the technical compartment is much smaller, the electrical panel may be
placed other than as illustrated.
17
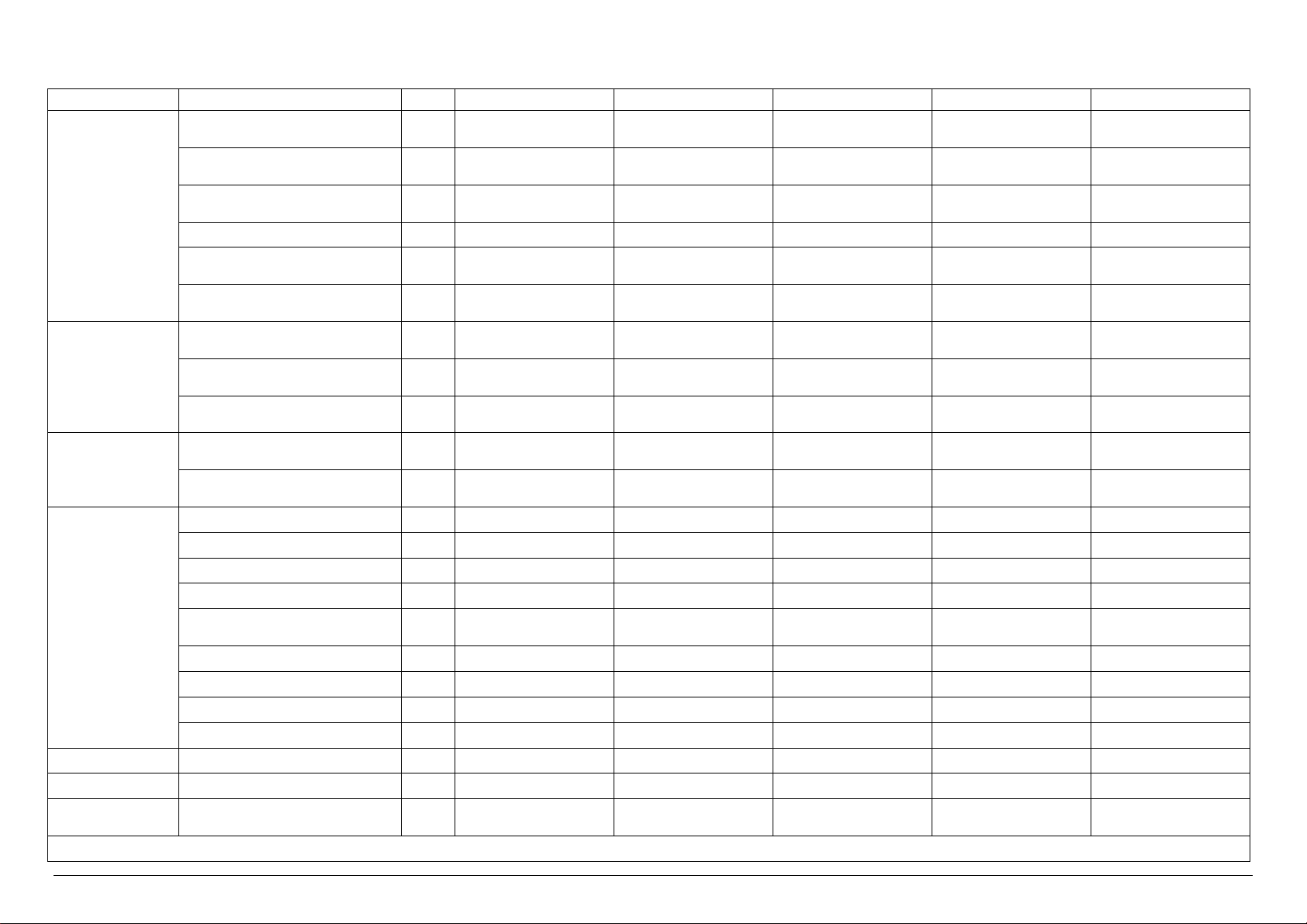
Technical characteristics of VS 1 VERSIONS
Chamber
dimensions
Footprint
dimensions
Weights
Connections
Electrical Power kW 14.0 2.0 2.0 14.0 14.0
Electrical Current A 20.0 5.0 5.0 20.0 20.0
Steam Consumption
* Upon the client’s request, it is possible to have the same models with power supply at 480V 3~ +T 60 Hz
MODEL
Width
Height
Depth
Sterilization Unit N° 1 1 1 1 1
Usable Volume
Total Volume
Width
Height
Depth
Net Weight
Hydraulic Test Weight
A-General Drain / 1 ¼” Female DN32 1 ¼” Female DN32 1 ¼” Female DN32 1 ¼” Female DN32 1 ¼” Female DN32
B-Safety Valve Drain / n°3 Ø 18 n°3 Ø 18 n°3 Ø 18 n°3 Ø 18 n°3 Ø 18
C-Condensation Discharge / - - ½” male DN 15 - ½” male DN15
D-Steam Input / - ½” male DN 15 - ½” male DN15 -
E-Softened Water Input
Demi Water Input
F-Compressed Air Input / ¼” male ¼” male ¼” male ¼” male ¼” male
G-Power Supply / 400V 3~ + T50Hz* 400V 3~ + T50Hz* 400V 3~ + T50Hz* 400V 3~ + T50Hz* 400V 3~ + T50Hz*
H-City Water Input / ¾” male DN 20 ¾” male DN 20 ¾” male DN 20 ¾” male DN 20 ¾” male DN 20
I-Steam Input / - - ½” male DN 15 - ½” male DN15
U.M. VS1/1E VS1/1V VS1/1I VS1/1EV VS1/1EI
mm
inch
mm
inch
mm
inch
litres
Cft
litres
Cft
mm
inch
mm
inch
mm
inch
kg
lb
kg
lb
/ ¾” male DN20 - ¾” male DN 20 ¾” male DN 20 ¾” male DN 20
kg/h
lb/h
-
Ø 420.0
Ø 16.5
Ø 420.0
Ø 16.5
782.0
30.8
68.0
2.4
108.0
3.8
800.0
31.5
1700.0
66.9
1000.0
39.4
400.0
881.8
515.0
1135.4
Ø 420.0
Ø 16.5
Ø 420.0
Ø 16.5
782.0
30.8
68.0
2.4
108.0
3.8
800.0
31.5
1700.0
66.9
1000.0
39.4
357.0
787.0
473.0
1042.8
25.0
55.1
Ø 420.0
Ø 16.5
Ø 420.0
Ø 16.5
782.0
30.8
68.0
2.4
108.0
3.8
800.0
31.5
1700.0
66.9
1000.0
39.4
400.0
881.8
515.0
1135.4
30.0
60.1
Ø 420.0
Ø 16.5
Ø 420.0
Ø 16.5
782.0
30.8
68.0
2.4
108.0
3.8
800.0
31.5
1700.0
66.9
1000.0
39.4
400.0
881.8
515.0
1135.4
25.0
55.1
Ø 420.0
Ø 16.5
Ø 420.0
Ø 16.5
782.0
30.8
68.0
2.4
108.0
3.8
800.0
31.50
1700.0
66.9
1000.0
39.4
400.0
881.8
515.0
1135.4
30.0
60.1
18

MODEL
Width
Height
Chamber
dimensions
Footprint
dimensions
Weights
Connections
Electrical Power kW 14.0 2.0 2.0 14.0 14.0
Electrical Current A 20.0 5.0 5.0 20.0 20.0
Steam Consumption
* Upon the client’s request, it is possible to have the same models with power supply at 480V 3~ +T 60 Hz
Depth
Sterilization Unit N° 1 1 1 1 1
Usable Volume
Total Volume
Width
Height
Depth
Net Weight
Hydraulic Test Weight
A-General Drain / 1 ¼” Female DN32 1 ¼” Female DN32 1 ¼” Female DN32 1 ¼” Female DN32 1 ¼” Female DN32
B-Safety Valve Drain / n°3 Ø 18 n°3 Ø 18 n°3 Ø 18 n°3 Ø 18 n°3 Ø 18
C-Condensation Discharge / - - ½” male DN 15 - ½” male DN15
D-Steam Input / - ½” male DN 15 - ½” male DN15 -
E-Softened Water Input
Demi Water Input
F-Compressed Air Input / ¼” male ¼” male ¼” male ¼” male ¼” male
G-Power Supply / 400V 3~ + T50Hz* 400V 3~ + T50Hz* 400V 3~ + T50Hz* 400V 3~ + T50Hz* 400V 3~ + T50Hz*
H-City Water Input / ¾” male DN 20 ¾” male DN 20 ¾” male DN 20 ¾” male DN 20 ¾” male DN 20
I-Steam Input / - - ½” male DN 15 - ½” male DN15
U.M. VS1/2E VS1/2V VS1/2I VS1/2EV VS1/2EI
mm
inch
mm
inch
mm
inch
litres
Cft
litres
Cft
mm
inch
mm
inch
mm
inch
kg
lb
kg
lb
/ ¾” male DN20 - ¾” male DN 20 ¾” male DN 20 ¾” male DN 20
kg/h
lb/h
-
Ø 420.0
Ø 16.5
Ø 420.0
Ø 16.5
718.0
28.3
68.0
2.4
100.0
3.5
800.0
31.5
1700.0
67.0
1000.0
39.4
447.0
985.5
554.0
1221.4
Ø 420.0
Ø 16.5
Ø 420.0
Ø 16.5
718.0
28.3
68.0
2.4
100.0
3.5
800.0
31.5
1700.0
67.0
1000.0
39.4
404.0
890.7
512.0
1128.8
25.0
55.1
Ø 420.0
Ø 16.5
Ø 420.0
Ø 16.5
718.0
28.3
68.0
2.4
100.0
3.5
800.0
31.5
1700.0
67.0
1000.0
39.4
447.0
985.5
554.0
1221.4
30.0
60.1
Ø 420.0
Ø 16.5
Ø 420.0
Ø 16.5
718.0
28.3
68.0
2.4
100.0
3.5
800.0
31.5
1700.0
67.0
1000.0
39.4
447.0
985.5
554.0
1221.4
25.0
55.1
Ø 420.0
Ø 16.5
Ø 420.0
Ø 16.5
718.0
28.3
68.0
2.4
100.0
3.5
800.0
31.50
1700.0
67.0
1000.0
39.4
447.0
985.5
554.0
1221.4
30.0
60.1
19
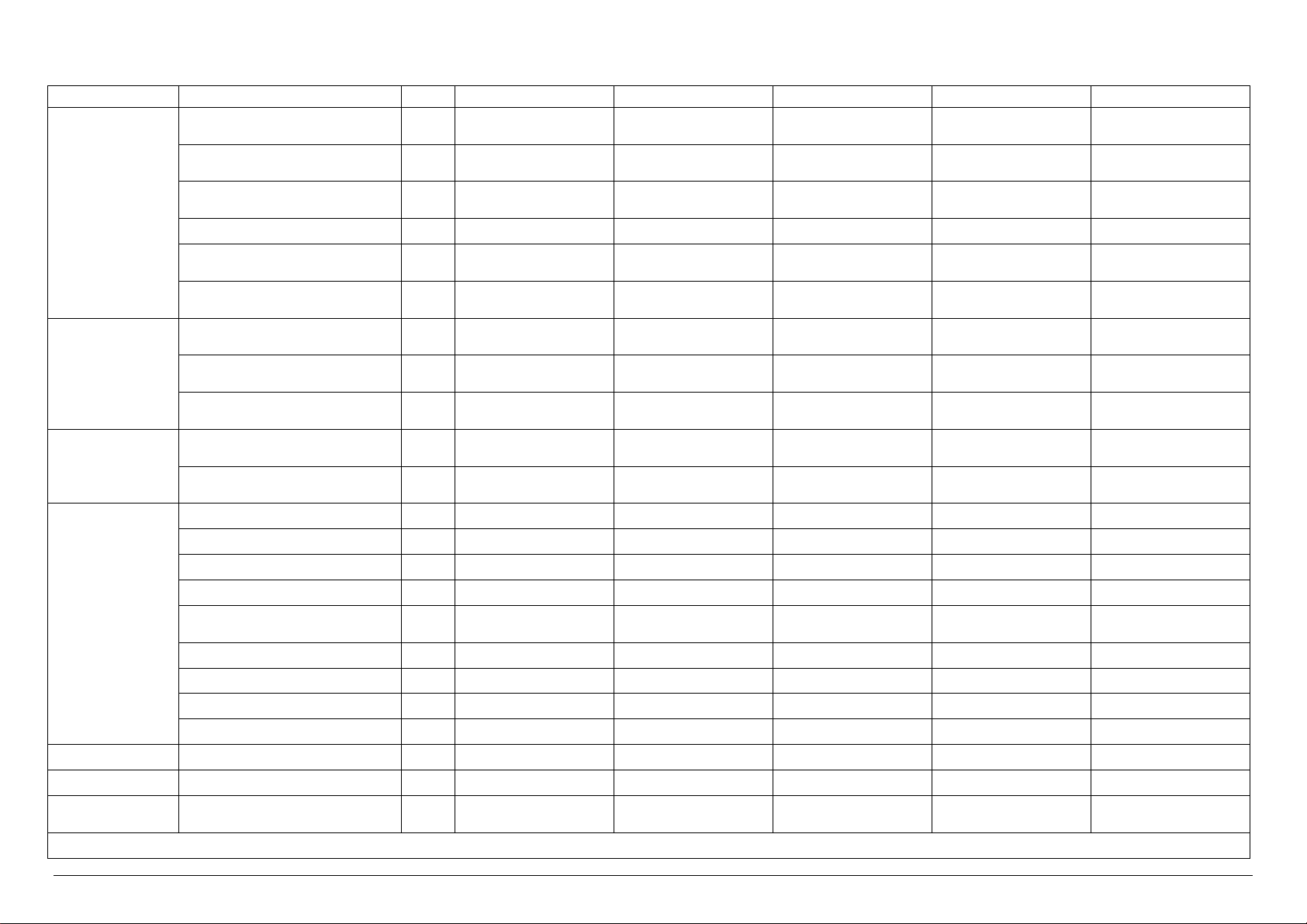
Technical characteristics of VS 1,5 VERSIONS
Chamber
dimensions
Footprint
dimensions
Weights
Connections
Electrical Power kW 14.0 2.0 2.0 14.0 14.0
Electrical Current A 20.0 5.0 5.0 20.0 20.0
Steam Consumption
* Upon the client’s request, it is possible to have the same models with power supply at 480V 3~ +T 60 Hz
MODEL
Width
Height
Depth
Sterilization Unit N° 1.5 1.5 1.5 1.5 1.5
Usable Volume
Total Volume
Width
Height
Depth
Net Weight
Hydraulic Test Weight
A-General Drain / 1 ¼” Female DN32 1 ¼” Female DN32 1 ¼” Female DN32 1 ¼” Female DN32 1 ¼” Female DN32
B-Safety Valve Drain / n°3 Ø 18 n°3 Ø 18 n°3 Ø 18 n°3 Ø 18 n°3 Ø 18
C-Condensation Discharge / - - ½” male DN 15 - ½” male DN15
D-Steam Input / - ½” male DN 15 - ½” male DN15 -
E-Softened Water Input
Demi Water Input
F-Compressed Air Input / ¼” male ¼” male ¼” male ¼” male ¼” male
G-Power Supply / 400V 3~ + T50Hz* 400V 3~ + T50Hz* 400V 3~ + T50Hz* 400V 3~ + T50Hz* 400V 3~ + T50Hz*
H-City Water Input / ¾” male DN 20 ¾” male DN 20 ¾” male DN 20 ¾” male DN 20 ¾” male DN 20
I-Steam Input / - - ½” male DN 15 - ½” male DN15
U.M. VS1.5/1E VS1.5/1V VS1.5/1I VS1.5/1EV VS1.5/1EI
mm
inch
mm
inch
mm
inch
litres
Cft
litres
Cft
mm
inch
mm
inch
mm
inch
kg
lb
kg
lb
/ ¾” male DN20 - ¾” male DN 20 ¾” male DN 20 ¾” male DN 20
kg/h
lb/h
-
Ø 420.0
Ø 16.5
Ø 420.0
Ø 16.5
1082.0
42.6
96.0
3.4
150.0
5.3
800.0
31.5
1700.0
66.9
1300.0
51.2
450.0
992.1
612.0
1349.2
Ø 420.0
Ø 16.5
Ø 420.0
Ø 16.5
1082.0
42.6
96.0
3.4
150.0
5.3
800.0
31.5
1700.0
66.9
1300.0
51.2
407.0
897.3
569.0
1254.4
35.0
77.2
Ø 420.0
Ø 16.5
Ø 420.0
Ø 16.5
1082.0
42.6
96.0
3.4
150.0
5.3
800.0
31.5
1700.0
66.9
1300.0
51.2
450.0
992.1
612.0
1349.2
40.0
88.2
Ø 420.0
Ø 16.5
Ø 420.0
Ø 16.5
1082.0
42.6
96.0
3.4
150.0
5.3
800.0
31.5
1700.0
66.9
1300.0
51.2
450.0
992.1
612.0
1349.2
35.0
77.2
Ø 420.0
Ø 16.5
Ø 420.0
Ø 16.5
1082.0
42.6
96.0
3.4
150.0
5.3
800.0
31.50
1700.0
66.9
1300.0
51.2
450.0
992.1
612.0
1349.2
40.0
88.2
20
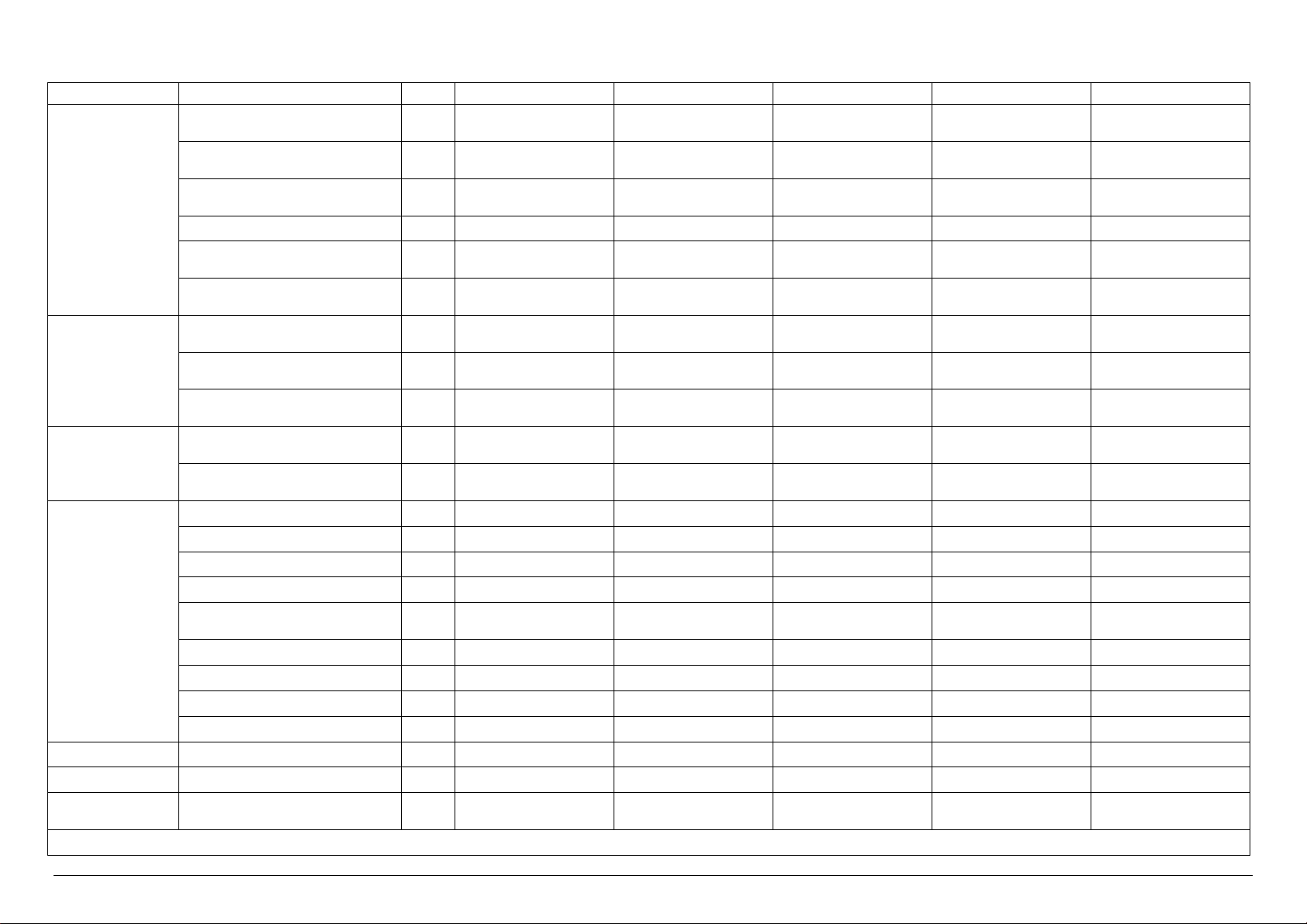
MODEL
Width
Height
Chamber
dimensions
Footprint
dimensions
Weights
Connections
Electrical Power kW 14.0 2.0 2.0 14.0 14.0
Electrical Current A 20.0 5.0 5.0 20.0 20.0
Steam Consumption
* Upon the client’s request, it is possible to have the same models with power supply at 480V 3~ +T 60 Hz
Depth
Sterilization Unit N° 1.5 1.5 1.5 1.5 1.5
Usable Volume
Total Volume
Width
Height
Depth
Net Weight
Hydraulic Test Weight
A-General Drain / 1 ¼” Female DN32 1 ¼” Female DN32 1 ¼” Female DN32 1 ¼” Female DN32 1 ¼” Female DN32
B-Safety Valve Drain / n°3 Ø 18 n°3 Ø 18 n°3 Ø 18 n°3 Ø 18 n°3 Ø 18
C-Condensation Discharge / - - ½” male DN 15 - ½” male DN15
D-Steam Input / - ½” male DN 15 - ½” male DN15 -
E-Softened Water Input
Demi Water Input
F-Compressed Air Input / ¼” male ¼” male ¼” male ¼” male ¼” male
G-Power Supply / 400V 3~ + T50Hz* 400V 3~ + T50Hz* 400V 3~ + T50Hz* 400V 3~ + T50Hz* 400V 3~ + T50Hz*
H-City Water Input / ¾” male DN 20 ¾” male DN 20 ¾” male DN 20 ¾” male DN 20 ¾” male DN 20
I-Steam Input / - - ½” male DN 15 - ½” male DN15
U.M. VS1.5/2E VS1.5/2V VS1.5/2I VS1.5/2EV VS1.5/2EI
mm
inch
mm
inch
mm
inch
litres
Cft
litres
Cft
mm
inch
mm
inch
mm
inch
kg
lb
kg
lb
/ ¾” male DN20 - ¾” male DN 20 ¾” male DN 20 ¾” male DN 20
kg/h
lb/h
-
Ø 420.0
Ø 16.5
Ø 420.0
Ø 16.5
1018.0
40.1
96.0
3.4
141.0
5.0
800.0
31.5
1700.0
66.9
1300.0
51.2
497.0
1095.7
650.0
1433.0
Ø 420.0
Ø 16.5
Ø 420.0
Ø 16.5
1018.0
40.1
96.0
3.4
141.0
5.0
800.0
31.5
1700.0
66.9
1300.0
51.2
454.0
1000.9
607.0
1338.2
35.0
77.2
Ø 420.0
Ø 16.5
Ø 420.0
Ø 16.5
1018.0
40.1
96.0
3.4
141.0
5.0
800.0
31.5
1700.0
66.9
1300.0
51.2
497.0
1095.7
650.0
1433.0
40.0
88.2
Ø 420.0
Ø 16.5
Ø 420.0
Ø 16.5
1018.0
40.1
96.0
3.4
141.0
5.0
800.0
31.5
1700.0
66.9
1300.0
51.2
497.0
1095.7
650.0
1433.0
35.0
77.2
Ø 420.0
Ø 16.5
Ø 420.0
Ø 16.5
1018.0
40.1
96.0
3.4
141.0
5.0
800.0
31.50
1700.0
66.9
1300.0
51.2
497.0
1095.7
650.0
1433.0
40.0
88.2
21
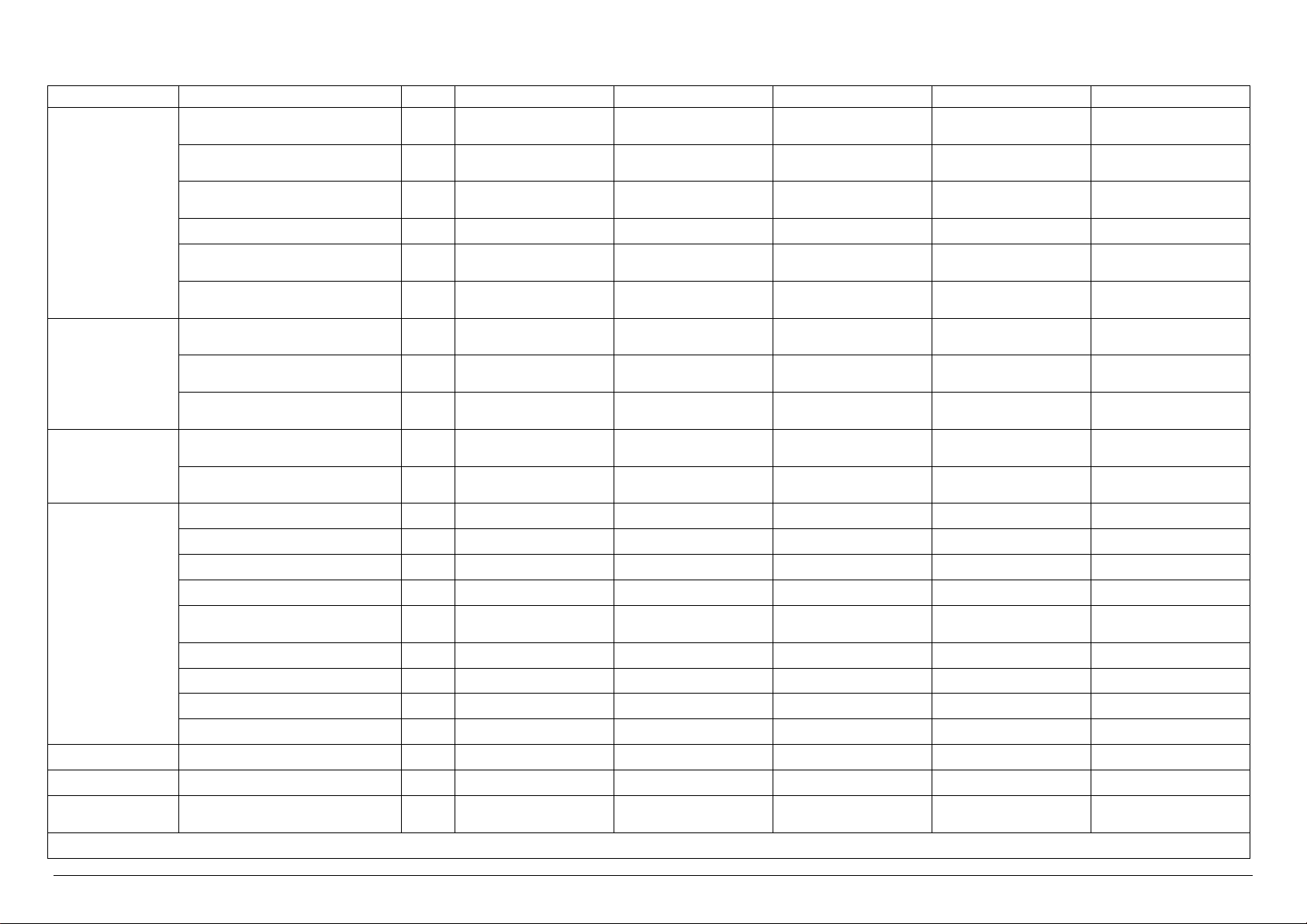
Technical characteristics of VS 2 VERSIONS
Chamber
dimensions
Footprint
dimensions
Weights
Connections
Electrical Power kW 26.0 2.0 26.0 26.0 26.0
Electrical Current A 41.5 5 5 41.5 41.5
Steam Consumption
* Upon the client’s request, it is possible to have the same models with power supply at 480V 3~ +T 60 Hz
MODEL
Width
Height
Depth
Sterilization Unit N° 2 2 2 2 2
Usable Volume
Total Volume
Width
Height
Depth
Net Weight
Hydraulic Test Weight
A-General Drain / 1 ¼” Female DN32 1 ¼” Female DN32 1 ¼” Female DN32 1 ¼” Female DN32 1 ¼” Female DN32
B-Safety Valve Drain / n°3 Ø 18 n°3 Ø 18 n°3 Ø 18 n°3 Ø 18 n°3 Ø 18
C-Condensation Discharge / - - ½” male DN 15 - ½” male DN15
D-Steam Input / - ½” male DN 15 - ½” male DN15 -
E-Softened Water Input
Demi Water Input
F-Compressed Air Input / ¼” male ¼” male ¼” male ¼” male ¼” male
G-Power Supply / 400V 3~ + T50Hz* 400V 3~ + T50Hz* 400V 3~ + T50Hz* 400V 3~ + T50Hz* 400V 3~ + T50Hz*
H-City Water Input / ¾” male DN 20 ¾” male DN 20 ¾” male DN 20 ¾” male DN 20 ¾” male DN 20
I-Steam Input / - - ½” male DN 15 - ½” male DN15
U.M. VS2/1E VS2/1V VS2/1I VS2/1EV VS2/1EI
mm
inch
mm
inch
mm
inch
litres
Cft
litres
Cft
mm
inch
mm
inch
mm
inch
kg
lb
kg
lb
/ ¾” male DN20 - ¾” male DN 20 ¾” male DN 20 ¾” male DN 20
kg/h
lb/h
-
440.0
17.3
700.0
27.6
645.0
25.4
196.0
6.9
200.0
7.1
1000.0
39.4
1900.0
74.8
992.0
39.1
600.0
1322.8
800.0
1763.7
440.0
17.3
700.0
27.6
645.0
25.4
196.0
6.9
200.0
7.1
1000.0
39.4
1900.0
74.8
992.0
39.1
557.0
1228.0
757.0
1668.9
40.0
88.2
440.0
17.3
700.0
27.6
645.0
25.4
196.0
6.9
200.0
7.1
1000.0
39.4
1900.0
74.8
992.0
39.1
600.0
1322.8
800.0
1763.7
45.0
99.2
440.0
17.3
700.0
27.6
645.0
25.4
196.0
6.9
200.0
7.1
1000.0
39.4
1900.0
74.8
992.0
39.1
600.0
1322.8
800.0
1763.7
40.0
88.2
440.0
17.3
700.0
27.6
645.0
25.4
196.0
6.9
200.0
7.1
1000.0
39.4
1900.0
74.8
992.0
39.1
600.0
1322.8
800.0
1763.7
45.0
99.2
22
MODEL
Width
Height
Chamber
dimensions
Footprint
dimensions
Weights
Connections
Electrical Power kW 26.0 2.0 2.0 26.0 26.0
Electrical Current A 41.5 5.0 5.0 41.5 41.5
Steam Consumption
* Upon the client’s request, it is possible to have the same models with power supply at 480V 3~ +T 60 Hz
Depth
Sterilization Unit N° 2 2 2 2 2
Usable Volume
Total Volume
Width
Height
Depth
Net Weight
Hydraulic Test Weight
A-General Drain / 1 ¼” Female DN32 1 ¼” Female DN32 1 ¼” Female DN32 1 ¼” Female DN32 1 ¼” Female DN32
B-Safety Valve Drain / n°3 Ø 18 n°3 Ø 18 n°3 Ø 18 n°3 Ø 18 n°3 Ø 18
C-Condensation Discharge / - - ½” male DN 15 - ½” male DN15
D-Steam Input / - ½” male DN 15 - ½” male DN15 -
E-Softened Water Input
Demi Water Input
F-Compressed Air Input / ¼” male ¼” male ¼” male ¼” male ¼” male
G-Power Supply / 400V 3~ + T50Hz* 400V 3~ + T50Hz* 400V 3~ + T50Hz* 400V 3~ + T50Hz* 400V 3~ + T50Hz*
H-City Water Input / ¾” male DN 20 ¾” male DN 20 ¾” male DN 20 ¾” male DN 20 ¾” male DN 20
I-Steam Input / - - ½” male DN 15 - ½” male DN15
U.M. VS2/2E VS2/2V VS2/2I VS2/2EV VS2/2EI
mm
inch
mm
inch
mm
inch
litres
Cft
litres
Cft
mm
inch
mm
inch
mm
inch
kg
lb
kg
lb
/ ¾” male DN20 - ¾” male DN 20 ¾” male DN 20 ¾” male DN 20
kg/h
lb/h
-
440.0
17.3
700.0
27.6
670.0
26.4
204.0
7.2
205.0
7.2
1000.0
39.4
1900.0
74.8
992.0
39.1
665.0
1466.1
870.0
1918.0
440.0
17.3
700.0
27.6
670.0
26.4
204.0
7.2
205.0
7.2
1000.0
39.4
1900.0
74.8
992.0
39.1
622.0
1371.3
827.0
1823.2
40.0
88.2
440.0
17.3
700.0
27.6
670.0
26.4
204.0
7.2
205.0
7.2
1000.0
39.4
1900.0
74.8
992.0
39.1
665.0
1466.1
870.0
1918.0
45.0
99.2
440.0
17.3
700.0
27.6
670.0
26.4
204.0
7.2
205.0
7.2
1000.0
39.4
1900.0
74.8
992.0
39.1
665.0
1466.1
870.0
1918.0
40.0
88.2
440.0
17.3
700.0
27.6
670.0
26.4
204.0
7.2
205.0
7.2
1000.0
39.4
1900.0
74.8
992.0
39.1
665.0
1466.1
870.0
1918.0
45.0
99.2
23
Technical characteristics of VS 3 VERSIONS
Chamber
dimensions
Footprint
dimensions
Weights
Connections
Electrical Power kW 34.0 2.0 34.0 34.0 34.0
Electrical Current A 50.0 5.0 5.0 50.0 50.0
Steam Consumption
* Upon the client’s request, it is possible to have the same models with power supply at 480V 3~ +T 60 Hz
MODEL
Width
Height
Depth
Sterilization Unit N° 3 3 3 3 3
Usable Volume
Total Volume
Width
Height
Depth
Net Weight
Hydraulic Test Weight
A-General Drain / 1 ¼” Female DN32 1 ¼” Female DN32 1 ¼” Female DN32 1 ¼” Female DN32 1 ¼” Female DN32
B-Safety Valve Drain / n°3 Ø 18 n°3 Ø 18 n°3 Ø 18 n°3 Ø 18 n°3 Ø 18
C-Condensation Discharge / - - ½” male DN 15 - ½” male DN15
D-Steam Input / - ½” male DN 15 - ½” male DN15 -
E-Softened Water Input
Demi Water Input
F-Compressed Air Input / ¼” male ¼” male ¼” male ¼” male ¼” male
G-Power Supply / 400V 3~ + T50Hz* 400V 3~ + T50Hz* 400V 3~ + T50Hz* 400V 3~ + T50Hz* 400V 3~ + T50Hz*
H-City Water Input / ¾” male DN 20 ¾” male DN 20 ¾” male DN 20 ¾” male DN 20 ¾” male DN 20
I-Steam Input / - - ½” male DN 15 - ½” male DN15
U.M. VS3/1E VS3/1V VS3/1I VS3/1EV VS3/1EI
mm
inch
mm
inch
mm
inch
litres
Cft
litres
Cft
mm
inch
mm
inch
mm
inch
kg
lb
kg
lb
/ ¾” male DN20 - ¾” male DN 20 ¾” male DN 20 ¾” male DN 20
kg/h
lb/h
-
440.0
17.3
700.0
27.6
945.0
37.2
287.0
10.1
290.0
10.2
1000.0
39.4
1900.0
74.8
1292.0
50.9
750.0
1653.5
1040.0
2292.8
440.0
17.3
700.0
27.6
945.0
37.2
287.0
10.1
290.0
10.2
1000.0
39.4
1900.0
74.8
1292.0
50.9
707.0
1558.7
997.0
2198.0
45.0
99.2
440.0
17.3
700.0
27.6
945.0
37.2
287.0
10.1
290.0
10.2
1000.0
39.4
1900.0
74.8
1292.0
50.9
750.0
1653.5
1040.0
2292.8
60.0
132.3
440.0
17.3
700.0
27.6
945.0
37.2
287.0
10.1
290.0
10.2
1000.0
39.4
1900.0
74.8
1292.0
50.9
750.0
1653.5
1040.0
2292.8
45.0
99.2
440.0
17.3
700.0
27.6
945.0
37.2
287.0
10.1
290.0
10.2
1000.0
39.4
1900.0
74.8
1292.0
50.9
750.0
1653.5
1040.0
2292.8
60.0
132.3
24
MODEL
Width
Height
Chamber
dimensions
Footprint
dimensions
Weights
Connections
Electrical Power kW 34.0 2.0 2.0 34.0 34.0
Electrical Current A 50.0 5.0 5.0 50.0 50.0
Steam Consumption
* Upon the client’s request, it is possible to have the same models with power supply at 480V 3~ +T 60 Hz
Depth
Sterilization Unit N° 3 3 3 3 3
Usable Volume
Total Volume
Width
Height
Depth
Net Weight
Hydraulic Test Weight
A-General Drain / 1 ¼” Female DN32 1 ¼” Female DN32 1 ¼” Female DN32 1 ¼” Female DN32 1 ¼” Female DN32
B-Safety Valve Drain / n°3 Ø 18 n°3 Ø 18 n°3 Ø 18 n°3 Ø 18 n°3 Ø 18
C-Condensation Discharge / - - ½” male DN 15 - ½” male DN15
D-Steam Input / - ½” male DN 15 - ½” male DN15 -
E-Softened Water Input
Demi Water Input
F-Compressed Air Input / ¼” male ¼” male ¼” male ¼” male ¼” male
G-Power Supply / 400V 3~ + T50Hz* 400V 3~ + T50Hz* 400V 3~ + T50Hz* 400V 3~ + T50Hz* 400V 3~ + T50Hz*
H-City Water Input / ¾” male DN 20 ¾” male DN 20 ¾” male DN 20 ¾” male DN 20 ¾” male DN 20
I-Steam Input / - - ½” male DN 15 - ½” male DN15
U.M. VS3/2E VS3/2V VS3/2I VS3/2EV VS3/2EI
mm
inch
mm
inch
mm
inch
litres
Cft
litres
Cft
mm
inch
mm
inch
mm
inch
kg
lb
kg
lb
/ ¾” male DN20 - ¾” male DN 20 ¾” male DN 20 ¾” male DN 20
kg/h
lb/h
-
440.0
17.3
700.0
27.6
970.0
38.2
295.0
10.4
300.0
10.6
1000.0
39.4
1900.0
74.8
1292.0
50.9
815.0
1796.8
1115.0
2458.2
440.0
17.3
700.0
27.6
970.0
38.2
295.0
10.4
300.0
10.6
1000.0
39.4
1900.0
74.8
1292.0
50.9
772.0
1702.0
1073.0
2365.6
45.0
99.2
440.0
17.3
700.0
27.6
970.0
38.2
295.0
10.4
300.0
10.6
1000.0
39.4
1900.0
74.8
1292.0
50.9
815.0
1796.8
1115.0
2458.2
60.0
132.3
440.0
17.3
700.0
27.6
970.0
38.2
295.0
10.4
300.0
10.6
1000.0
39.4
1900.0
74.8
1292.0
50.9
815.0
1796.8
1115.0
2458.2
45.0
99.2
440.0
17.3
700.0
27.6
970.0
38.2
295.0
10.4
300.0
10.6
1000.0
39.4
1900.0
74.8
1292.0
50.9
815.0
1796.8
1115.0
2458.2
60.0
132.3
25
Technical characteristics of VS 4 VERSIONS
Chamber
dimensions
Footprint
dimensions
Weights
Connections
Electrical Power kW 34.0 2.0 2.0 34.0 34.0
Electrical Current A 50.0 5.0 5.0 50.0 50.0
Steam Consumption
* Upon the client’s request, it is possible to have the same models with power supply at 480V 3~ +T 60 Hz
MODEL
Width
Height
Depth
Sterilization Unit N° 4 4 4 4 4
Usable Volume
Total Volume
Width
Height
Depth
Net Weight
Hydraulic Test Weight
A-General Drain / 1 ¼” Female DN32 1 ¼” Female DN32 1 ¼” Female DN32 1 ¼” Female DN32 1 ¼” Female DN32
B-Safety Valve Drain / n°3 Ø 18 n°3 Ø 18 n°3 Ø 18 n°3 Ø 18 n°3 Ø 18
C-Condensation Discharge / - - ½” male DN 15 - ½” male DN15
D-Steam Input / - ½” male DN 15 - ½” male DN15 -
E-Softened Water Input
Demi Water Input
F-Compressed Air Input / ¼” male ¼” male ¼” male ¼” male ¼” male
G-Power Supply / 400V 3~ + T50Hz* 400V 3~ + T50Hz* 400V 3~ + T50Hz* 400V 3~ + T50Hz* 400V 3~ + T50Hz*
H-City Water Input / ¾” male DN 20 ¾” male DN 20 ¾” male DN 20 ¾” male DN 20 ¾” male DN 20
I-Steam Input / - - ½” male DN 15 - ½” male DN15
U.M. VS4/1E VS4/1V VS4/1I VS4/1EV VS4/1EI
mm
inch
mm
inch
mm
inch
litres
Cft
litres
Cft
mm
inch
mm
inch
mm
inch
kg
lb
kg
lb
/ ¾” male DN20 - ¾” male DN 20 ¾” male DN 20 ¾” male DN 20
kg/h
lb/h
-
670.0
26.4
700.0
27.6
686.0
27.0
319.0
11.3
322.0
11.4
1250.0
49.2
1900.0
74.8
992.0
39.1
675.0
1488.1
995.0
2193.6
670.0
26.4
700.0
27.6
686.0
27.0
319.0
11.3
322.0
11.4
1250.0
49.2
1900.0
74.8
992.0
39.1
630.0
1388.9
955.0
2105.4
45.0
99.2
670.0
26.4
700.0
27.6
686.0
27.0
319.0
11.3
322.0
11.4
1250.0
49.2
1900.0
74.8
992.0
39.1
675.0
1488.1
995.0
2193.6
60.0
132.3
670.0
26.4
700.0
27.6
686.0
27.0
319.0
11.3
322.0
11.4
1250.0
49.2
1900.0
74.8
992.0
39.1
675.0
1488.1
995.0
2193.6
45.0
99.2
670.0
26.4
700.0
27.6
686.0
27.0
319.0
11.3
322.0
11.4
1250.0
49.2
1900.0
74.8
992.0
39.1
675.0
1488.1
995.0
2193.6
60.0
132.3
26
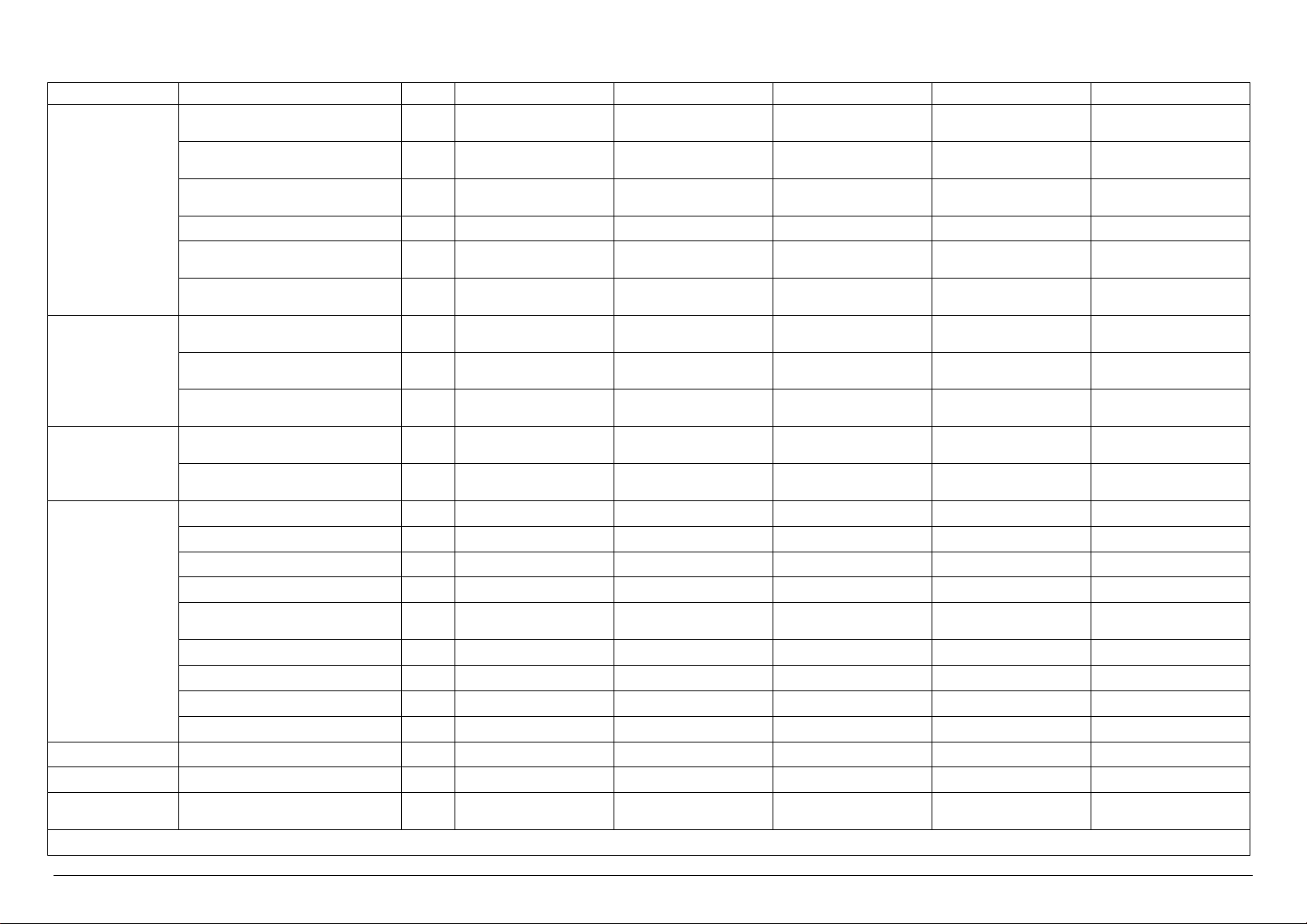
MODEL
Width
Height
Chamber
dimensions
Footprint
dimensions
Weights
Connections
Electrical Power kW 34.0 2.0 2.0 34.0 34.0
Electrical Current A 50.0 5.0 5.0 50.0 50.0
Steam Consumption
* Upon the client’s request, it is possible to have the same models with power supply at 480V 3~ +T 60 Hz
Depth
Sterilization Unit N° 4 4 4 4 4
Usable Volume
Total Volume
Width
Height
Depth
Net Weight
Hydraulic Test Weight
A-General Drain / 1 ¼” Female DN32 1 ¼” Female DN32 1 ¼” Female DN32 1 ¼” Female DN32 1 ¼” Female DN32
B-Safety Valve Drain / n°3 Ø 18 n°3 Ø 18 n°3 Ø 18 n°3 Ø 18 n°3 Ø 18
C-Condensation Discharge / - - ½” male DN 15 - ½” male DN15
D-Steam Input / - ½” male DN 15 - ½” male DN15 -
E-Softened Water Input
Demi Water Input
F-Compressed Air Input / ¼” male ¼” male ¼” male ¼” male ¼” male
G-Power Supply / 400V 3~ + T50Hz* 400V 3~ + T50Hz* 400V 3~ + T50Hz* 400V 3~ + T50Hz* 400V 3~ + T50Hz*
H-City Water Input / ¾” male DN 20 ¾” male DN 20 ¾” male DN 20 ¾” male DN 20 ¾” male DN 20
I-Steam Input / - - ½” male DN 15 - ½” male DN15
U.M. VS4/2E VS4/2V VS4/2I VS4/2EV VS4/2EI
mm
inch
mm
inch
mm
inch
litres
Cft
litres
Cft
mm
inch
mm
inch
mm
inch
kg
lb
kg
lb
/ ¾” male DN20 - ¾” male DN 20 ¾” male DN 20 ¾” male DN 20
kg/h
lb/h
-
670.0
26.4
700.0
27.6
710.0
28.0
330.0
11.7
333.0
11.8
1250.0
49.2
1900.0
74.8
992.0
39.1
750.0
1653.5
1085.0
2392.0
670.0
26.4
700.0
27.6
710.0
28.0
330.0
11.7
333.0
11.8
1250.0
49.2
1900.0
74.8
992.0
39.1
705.0
1554.3
1040.0
2292.8
45.0
99.2
670.0
26.4
700.0
27.6
710.0
28.0
330.0
11.7
333.0
11.8
1250.0
49.2
1900.0
74.8
992.0
39.1
750.0
1653.5
1085.0
2392.0
60.0
132.3
670.0
26.4
700.0
27.6
710.0
28.0
330.0
11.7
333.0
11.8
1250.0
49.2
1900.0
74.8
992.0
39.1
750.0
1653.5
1085.0
2392.0
45.0
99.2
670.0
26.4
700.0
27.6
710.0
28.0
330.0
11.7
333.0
11.8
1250.0
49.2
1900.0
74.8
992.0
39.1
750.0
1653.5
1085.0
2392.0
60.0
132.3
27
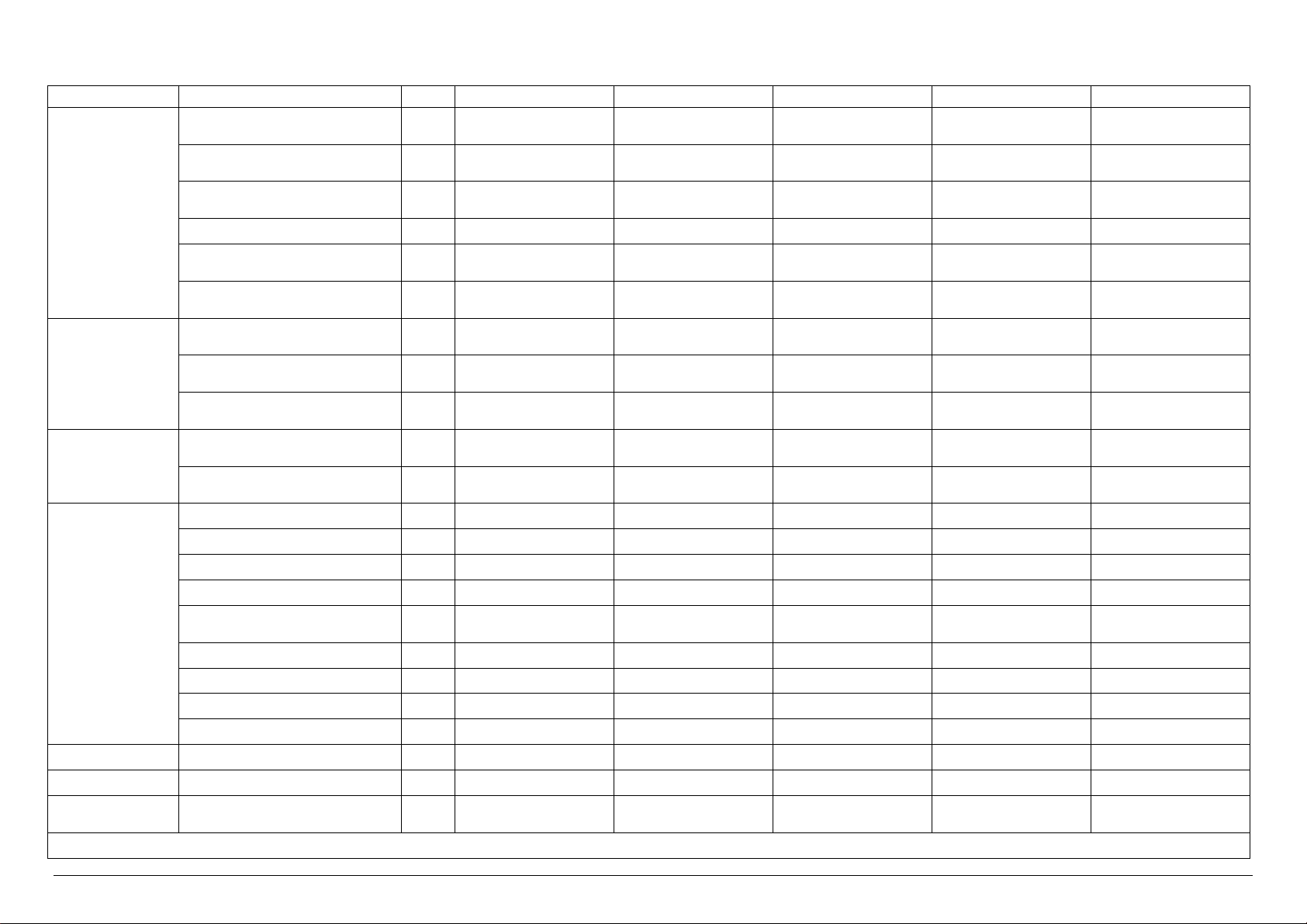
Technical characteristics of VS 6 VERSIONS
Chamber
dimensions
Footprint
dimensions
Weights
Connections
Electrical Power kW 50.5 2.5 2.5 50.5 50.5
Electrical Current A 75.0 5.0 5.0 75.0 75.0
Steam Consumption
* Upon the client’s request, it is possible to have the same models with power supply at 480V 3~ +T 60 Hz
MODEL
Width
Height
Depth
Sterilization Unit N° 6 6 6 6 6
Usable Volume
Total Volume
Width
Height
Depth
Net Weight
Hydraulic Test Weight
A-General Drain / 1 ¼” Female DN32 1 ¼” Female DN32 1 ¼” Female DN32 1 ¼” Female DN32 1 ¼” Female DN32
B-Safety Valve Drain / n°3 Ø 18 n°3 Ø 18 n°3 Ø 18 n°3 Ø 18 n°3 Ø 18
C-Condensation Discharge / - - ½” male DN 15 - ½” male DN15
D-Steam Input / - ½” male DN 15 - ½” male DN15 -
E-Softened Water Input
Demi Water Input
F-Compressed Air Input / ¼” male ¼” male ¼” male ¼” male ¼” male
G-Power Supply / 400V 3~ + T50Hz* 400V 3~ + T50Hz* 400V 3~ + T50Hz* 400V 3~ + T50Hz* 400V 3~ + T50Hz*
H-City Water Input / ¾” male DN 20 ¾” male DN 20 ¾” male DN 20 ¾” male DN 20 ¾” male DN 20
I-Steam Input / - - ½” male DN 15 - ½” male DN15
U.M. VS6/1E VS6/1V VS6/1I VS6/1EV VS6/1EI
mm
inch
mm
inch
mm
inch
litres
Cft
litres
Cft
mm
inch
mm
inch
mm
inch
kg
lb
kg
lb
/ ¾” male DN20 - ¾” male DN 20 ¾” male DN 20 ¾” male DN 20
kg/h
lb/h
-
670.0
26.4
700.0
27.6
986.0
38.8
458.0
16.2
462.0
16.3
1100.0
43.3
1900.0
74.8
1292.0
50.9
835.0
1840.9
1300.0
2866.0
670.0
26.4
700.0
27.6
986.0
38.8
458.0
16.2
462.0
16.3
1100.0
43.3
1900.0
74.8
1292.0
50.9
780.0
1719.6
1180.0
2601.5
60.0
132.3
670.0
26.4
700.0
27.6
986.0
38.8
458.0
16.2
462.0
16.3
1100.0
43.3
1900.0
74.8
1292.0
50.9
835.0
1840.9
1300.0
2866.0
75.0
165.4
670.0
26.4
700.0
27.6
986.0
38.8
458.0
16.2
462.0
16.3
1100.0
43.3
1900.0
74.8
1292.0
50.9
835.0
1840.9
1300.0
2866.0
60.0
132.3
670.0
26.4
700.0
27.6
986.0
38.8
458.0
16.2
462.0
16.3
1100.0
43.3
1900.0
74.8
1292.0
50.9
835.0
1840.9
1300.0
2866.0
75.0
165.4
28
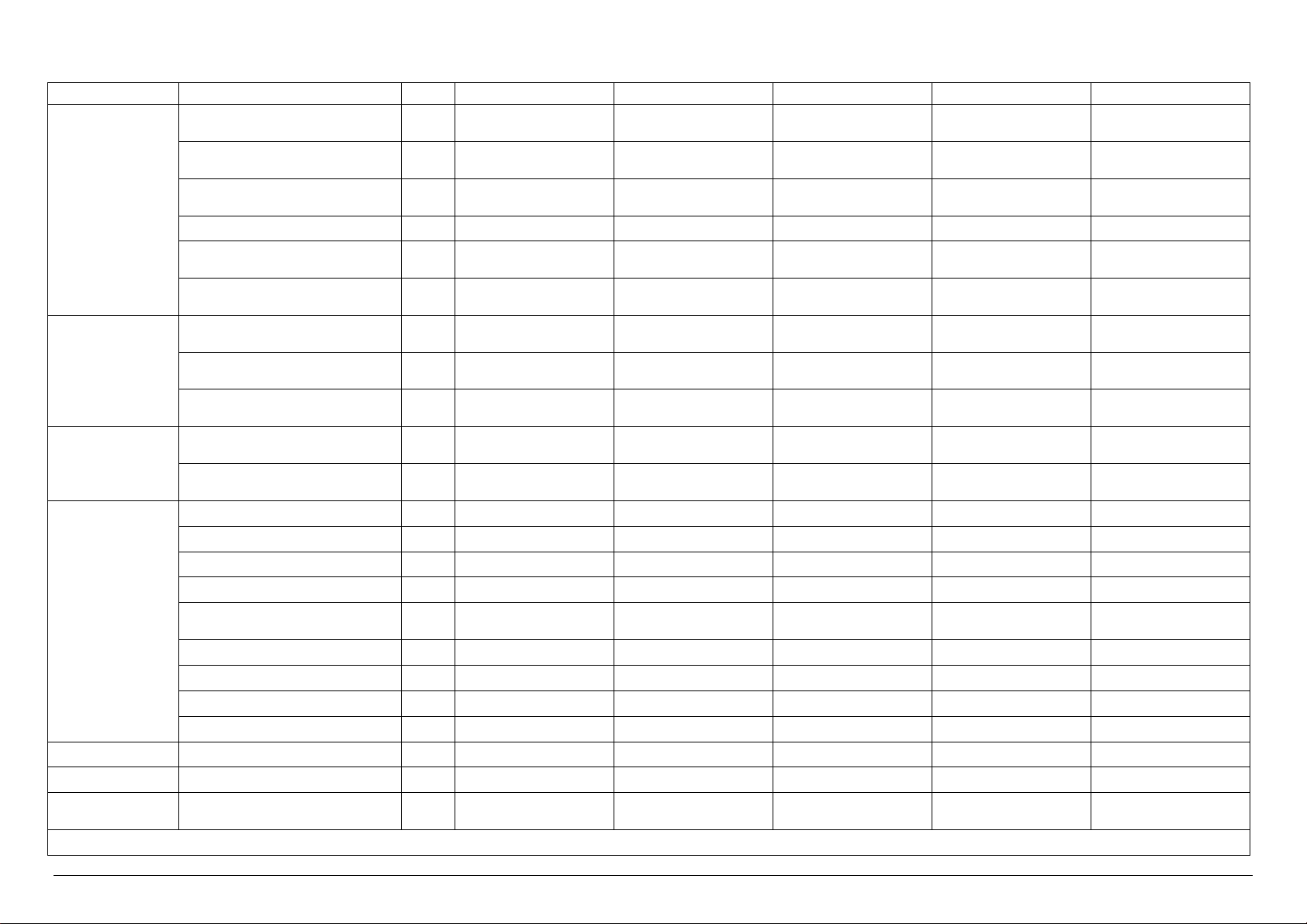
MODEL
Width
Height
Chamber
dimensions
Footprint
dimensions
Weights
Connections
Electrical Power kW 50.5 2.5 2.5 50.5 50.5
Electrical Current A 75.0 5.0 5.0 75.0 75.0
Steam Consumption
* Upon the client’s request, it is possible to have the same models with power supply at 480V 3~ +T 60 Hz
Depth
Sterilization Unit N° 6 6 6 6 6
Usable Volume
Total Volume
Width
Height
Depth
Net Weight
Hydraulic Test Weight
A-General Drain / 1 ¼” Female DN32 1 ¼” Female DN32 1 ¼” Female DN32 1 ¼” Female DN32 1 ¼” Female DN32
B-Safety Valve Drain / n°3 Ø 18 n°3 Ø 18 n°3 Ø 18 n°3 Ø 18 n°3 Ø 18
C-Condensation Discharge / - - ½” male DN 15 - ½” male DN15
D-Steam Input / - ½” male DN 15 - ½” male DN15 -
E-Softened Water Input
Demi Water Input
F-Compressed Air Input / ¼” male ¼” male ¼” male ¼” male ¼” male
G-Power Supply / 400V 3~ + T50Hz* 400V 3~ + T50Hz* 400V 3~ + T50Hz* 400V 3~ + T50Hz* 400V 3~ + T50Hz*
H-City Water Input / ¾” male DN 20 ¾” male DN 20 ¾” male DN 20 ¾” male DN 20 ¾” male DN 20
I-Steam Input / - - ½” male DN 15 - ½” male DN15
U.M. VS6/2E VS6/2V VS6/2I VS6/2EV VS6/2EI
mm
inch
mm
inch
mm
inch
litres
Cft
litres
Cft
mm
inch
mm
inch
mm
inch
kg
lb
kg
lb
/ ¾” male DN20 - ¾” male DN 20 ¾” male DN 20 ¾” male DN 20
kg/h
lb/h
-
670.0
26.4
700.0
27.6
1010.0
39.8
470.0
16.6
474.0
16.7
1100.0
43.3
1900.0
74.8
1292.0
50.9
910.0
2006.2
1385.0
3053.4
670.0
26.4
700.0
27.6
1010.0
39.8
470.0
16.6
474.0
16.7
1100.0
43.3
1900.0
74.8
1292.0
50.9
855.0
1885.0
1330.0
2932.1
60.0
132.3
670.0
26.4
700.0
27.6
1010.0
39.8
470.0
16.6
474.0
16.7
1100.0
43.3
1900.0
74.8
1292.0
50.9
910.0
2006.2
1385.0
3053.4
75.0
165.4
670.0
26.4
700.0
27.6
1010.0
39.8
470.0
16.6
474.0
16.7
1100.0
43.3
1900.0
74.8
1292.0
50.9
910.0
2006.2
1385.0
3053.4
60.0
132.3
670.0
26.4
700.0
27.6
1010.0
39.8
470.0
16.6
474.0
16.7
1100.0
43.3
1900.0
74.8
1292.0
50.9
910.0
2006.2
1385.0
3053.4
75.0
165.4
29
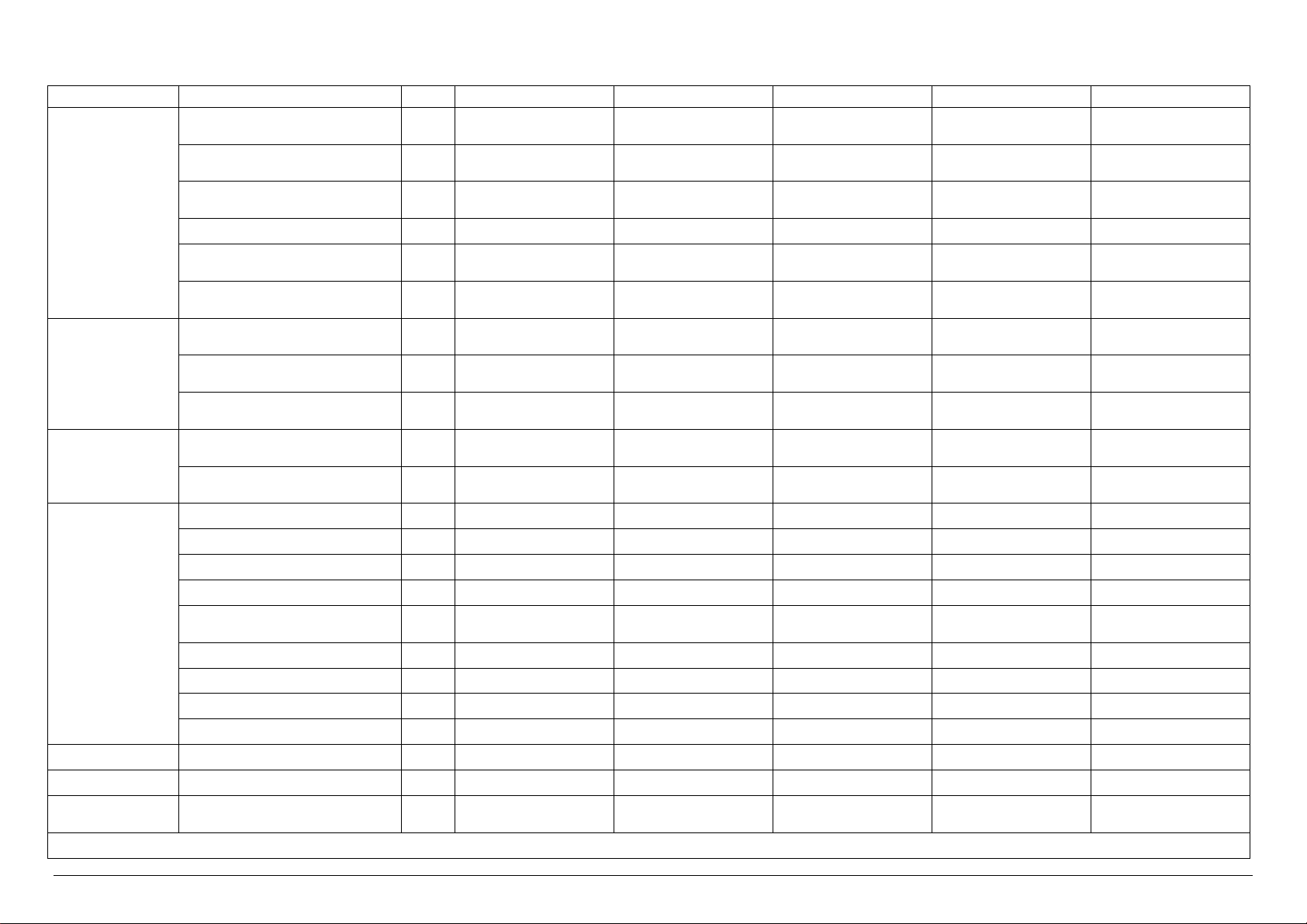
Technical characteristics of VS 8 VERSIONS
Chamber
dimensions
Footprint
dimensions
Weights
Connections
Electrical Power kW 50.5 2.5 2.5 50.5 50.5
Electrical Current A 75.0 5.0 5.0 75.0 75.0
Steam Consumption
* Upon the client’s request, it is possible to have the same models with power supply at 480V 3~ +T 60 Hz
MODEL
Width
Height
Depth
Sterilization Unit N° 8 8 8 8 8
Usable Volume
Total Volume
Width
Height
Depth
Net Weight
Hydraulic Test Weight
A-General Drain / 1 ¼” Female DN32 1 ¼” Female DN32 1 ¼” Female DN32 1 ¼” Female DN32 1 ¼” Female DN32
B-Safety Valve Drain / n°3 Ø 18 n°3 Ø 18 n°3 Ø 18 n°3 Ø 18 n°3 Ø 18
C-Condensation Discharge / - - ½” male DN 15 - ½” male DN15
D-Steam Input / - ½” male DN 15 - ½” male DN15 -
E-Softened Water Input
Demi Water Input
F-Compressed Air Input / ¼” male ¼” male ¼” male ¼” male ¼” male
G-Power Supply / 400V 3~ + T50Hz* 400V 3~ + T50Hz* 400V 3~ + T50Hz* 400V 3~ + T50Hz* 400V 3~ + T50Hz*
H-City Water Input / ¾” male DN 20 ¾” male DN 20 ¾” male DN 20 ¾” male DN 20 ¾” male DN 20
I-Steam Input / - - ½” male DN 15 - ½” male DN15
U.M. VS8/1E VS8/1V VS8/1I VS8/1EV VS8/1EI
mm
inch
mm
inch
mm
inch
litres
Cft
litres
Cft
mm
inch
mm
inch
mm
inch
kg
lb
kg
lb
/ ¾” male DN20 - ¾” male DN 20 ¾” male DN 20 ¾” male DN 20
kg/h
lb/h
-
670.0
26.4
700.0
27.6
1286.0
50.6
598.0
21.1
603.0
21.3
1100.0
43.3
1900.0
74.8
1592.0
62.7
1045.0
2303.8
1650.0
3637.6
670.0
26.4
700.0
27.6
1286.0
50.6
598.0
21.1
603.0
21.3
1100.0
43.3
1900.0
74.8
1592.0
62.7
990.0
2182.6
1590.0
3505.3
70.0
154.3
670.0
26.4
700.0
27.6
1286.0
50.6
598.0
21.1
603.0
21.3
1100.0
43.3
1900.0
74.8
1592.0
62.7
1045.0
2303.8
1650.0
3637.6
90.0
198.4
670.0
26.4
700.0
27.6
1286.0
50.6
598.0
21.1
603.0
21.3
1100.0
43.3
1900.0
74.8
1592.0
62.7
1045.0
2303.8
1650.0
3637.6
70.0
154.3
670.0
26.4
700.0
27.6
1286.0
50.6
598.0
21.1
603.0
21.3
1100.0
43.3
1900.0
74.8
1592.0
62.7
1045.0
2303.8
1650.0
3637.6
90.0
198.4
30
 Loading...
Loading...