Spacelabs Healthcare Lifecard CF, Lifecard 12 User manual

Service Manual
Lifecard CF
and
Lifecard 12
Firmware revision 7
18-0583 Rev. C

This Page is intentionally Blank
2 |
18-0583 Rev.C |

Contents Page
1. |
PRODUCT OVERVIEW |
7 |
|
1.1 |
INTRODUCTION |
7 |
|
1.2 |
SpeciÞcation |
9 |
|
1.2.1 |
Standard Recording Mode – ECG Inputs |
9 |
|
1.2.2Standard Recording Mode - Pacemaker Pulse Detection 10
|
1.2.3 |
Standard Recording Mode - Data Storage |
10 |
|
|
1.2.4 |
User Interface |
10 |
|
|
1.2.5 |
Power Requirements |
11 |
|
|
1.2.6 |
Extended Recording Mode |
11 |
|
|
1.2.7 |
Additional SpeciÞcations in 12-Lead Mode |
12 |
|
|
1.2.8 |
Physical and Environmental |
12 |
|
|
1.2.9 |
Electro-Magnetic Compatibility |
13 |
|
2. |
SAFETY AND REGULATORY |
15 |
||
|
2.1 |
Intended Use of Equipment |
15 |
|
|
2.2 |
Safety ClassiÞcation |
15 |
|
|
2.5 |
Explanation of Markings |
17 |
|
3. |
LCF BUILT-IN TESTS |
21 |
||
|
3.1 |
Primary Built-in Tests |
21 |
|
|
3.2 |
Second Level Built-in Tests |
21 |
|
|
3.3 |
Third Level Built-in Tests |
21 |
|
|
3.4 |
User Level Tests |
22 |
|
|
3.5 |
Fault Protection While Recording |
22 |
|
|
3.6 |
Lifecard CF Menu Options |
23 |
|
|
3.6.1 |
Main Menu Options |
23 |
|
|
3.6.2 |
Setup Menu |
25 |
|
|
3.7 |
Lifecard CF Sounds |
28 |
|
|
3.8 |
Error and Warnings Displays |
29 |
|
|
3.9 |
Decontamination |
32 |
|
|
3.10 |
Patient Cable |
33 |
|
|
3.11 |
Battery |
34 |
|
|
3.12 |
Battery Check |
34 |
|
|
3.13 Checking the Hardware & Software Revision |
34 |
||
|
3.14 |
Compact Flashcard |
35 |
|
Lifecard CF Service Manual |
3 |

4. |
Test Procedure |
37 |
4.1 |
Checking the Patient Cable |
37 |
4.2 |
LC12 Varios Yoke Assembly |
37 |
4.3 |
Lifecard CF Check-out |
39 |
5. |
Spares & Part Numbers |
41 |
6. |
Assembly Views |
43 |
6.1 |
Front Assembly - Front View - Complete |
43 |
6.2 |
Front Assembly - Rear View |
44 |
6.3 |
Intermediate Moulding |
45 |
6.4 |
Circuit Board |
46 |
6.5 |
Patient Cable Mounting with Clip |
48 |
6.6 |
Short Patient Cable |
49 |
6.7 |
Varios with 46-1123 Cable 46-1152 |
50 |
6.8 |
Varios Lead Parts |
51 |
4 |
18-0583 Rev.C |

©Copyright 2010 Spacelabs Healthcare Ltd.
1 Harforde Court, John Tate Road, Hertford. SG13 7NW
Lifecard CF Service Manual |
5 |

This Page is intentionally Blank
6 |
18-0583 Rev.C |

1. PRODUCT OVERVIEW
1.1Introduction
The Lifecard CF is a compact Holter Ambulatory ECG Recorder utilising a digital storage technique to store the ECG recording onto a Compact Flash (CF) card. The Lifecard CF provides continuous recording of 2 or 3 leads of ECG for up to 48 hours in standard mode and up to 7 days in extended mode.
The Lifecard 12 option provides continuous recording of 12 leads of ECG for a period of 24 hours. The recorder has a built in display for you to monitor the ECG and pacing detection during hook-up. This enables you to verify the ECG quality before starting the recording. Menu options are selected using the 2 buttons on the front of the recorder unit.
The Lifecard CF requires one AAA battery. The patient cables for the Lifecard CF are designed to prevent accidental disconnection from the recorder by the patient.
The Patient Event button on the front of the recorder unit enables the patient to indicate symptomatic episodes in the recording for correlation with the patient diary. Pacemaker pulse detection may be enabled and disabled by the physician or cardiac technician. Recordings may be analysed using a Spacelabs Healthcare PathÞnder, Impresario, or Lifescreen Holter analysis system, if they have compatible hardware and software. (Lifescreen is incompatible with 12-lead recordings.)
The Lifecard CF comprises two sections, the ‘Recorder Unit’ and the ‘Patient Cable Unit’.
Lifecard CF Service Manual |
7 |

LIFECARD CF RECORDER UNIT
Recorder Unit
Lifecard CF
Display
Yellow Button |
Green Button |
Menu Navigation |
Menu |
and |
Selection |
Patient Event |
and |
|
Patient Event |
Microphone |
Loudspeaker |
Patient Cable Unit
Patient Cable
Electrode Color
Code label 
Belt Clip (long cables only)
Slot for Neck
Lanyard
Attachment
8 |
18-0583 Rev.C |
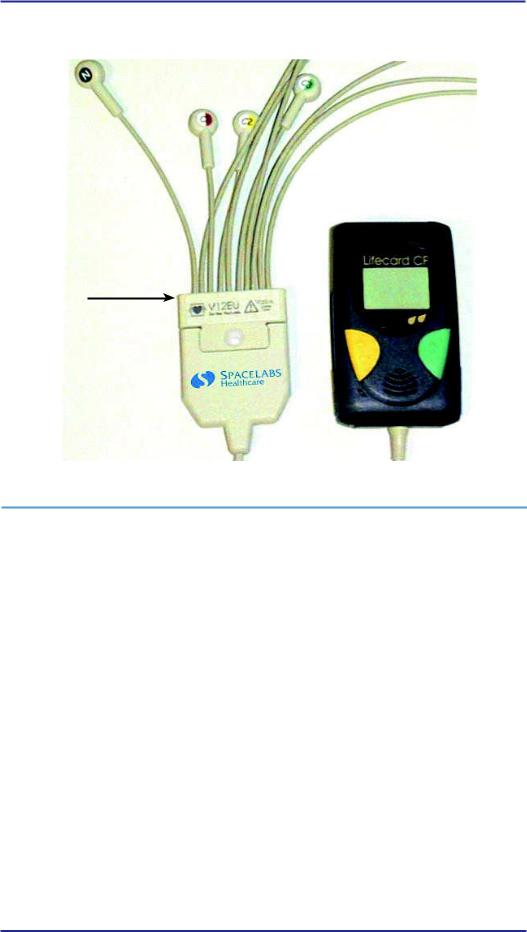
Patient Cable
Varios Active Yoke
1.2Specifi cation
1.2.1Standard Recording Mode – ECG Inputs
Channels |
3, type BF applied part patient isolation |
|
types 2 or 3 channel 3 electrode, 2 |
|
4 electrode, 3 channel 6 electrode with |
|
detachable leadwires. |
Input impedance |
> 5Mȍ Ohms |
Input |
DC offset ± 300 mV, with saturation |
|
recovery circuit (3 seconds max) |
CMRR |
> 60 dB at 10Hz, > 80 dB at 50 Hz and |
|
above, 2 Vpp signal |
Dynamic range |
10 mV |
Resolution |
2.5ȝV |
Lifecard CF Service Manual |
9 |

Calibration |
± 5% |
Bandwidth |
0.05 - 40 Hz (-3 dB) |
Sampling rate |
1024 samples per second per channel |
Noise fi lter |
Linear phase Þlter effective from 60Hz to |
|
> 1MHz, 128 samples per second out |
|
put rate |
1.2.2Standard Recording Mode - Pacemaker Pulse Detection
Sensitivity |
7 mV nominal, channels 1 and 2 only |
Noise rejection |
> 50 mVpp for sinusoids up to 200 Hz |
CMRR |
2 V common mode spikes are rejected |
Refractory period |
40 ms |
1.2.3Standard Recording Mode - Data Storage
Media type |
Removable card, CompactFlash Association |
|
standard (Type 1) |
Data types |
Full disclosure ECG, with pacing and patient |
|
event markers. Recording Time and Date. |
|
Patient name and record number |
|
(PathÞnder systems), Encrypted patient record |
|
Þle (CardioNavigator). 8 second voice recording. |
|
Recorder serial number |
Capacity req |
15 Mbytes per channel per 24 hours eg. |
|
a 48 hour three channel recording occupies |
|
90 Mbytes |
1.2.4 |
User Interface |
Type |
Text menus with audio cues and keys for up, down |
|
and select |
Languages |
English, German, French, Italian, Spanish, Danish |
|
and Polish languages also Hebrew patient ID support |
Clock |
Clock and calendar (to 2098), selectable 12/24 hour |
|
and US/European date formats. 13mm digit height for |
|
patient use |
|
|
|
10 |
18-0583 Rev.C |
|

Basic features |
Pacing detection on/off, hook-up display, |
|
voice recording for patient identiÞcation |
Ancillary features |
Identify and delete unread recordings, |
|
warning/error screens for battery and |
|
memory card conditions |
Hook-up display |
Real time display of each channel, with 60 |
|
ȝV/30 ms resolution and pacing annotation |
Set-up options |
Time and date, language, display contrast, |
|
recorder identiÞcation |
1.2.5Power Requirements
Disposable cell |
Single AAA alkaline (Duracell MN2400 or |
|
equivalent), two 24 hour recordings or one 48 |
|
hour recording |
Rechargable cell |
Single AAA nickel metal hydride (Ansmann |
|
600 mAh or equivalent), one 24 hour |
|
recording per charge |
Battery check |
User is warned of poor battery condition |
|
before recording |
Clock battery |
Internal rechargeable cell, charged during re |
|
cording. The clock is maintained |
|
for > 3 months between recordings |
1.2.6Extended Recording Mode
Channels |
2 channel recording, with pacing detection |
Cable Types |
2 channel 3 electrode or 2 channel 4 electrode |
Resolution |
10ȝV |
Sampling Rate |
256 samples per second per channel |
Compression |
10ȝV maximum compression error when |
|
tested with MIT-BIH Arrhythmia and |
|
Compression databases |
Capacity required |
90 Mbytes for dual channel 1 week |
Disposable Cell |
AAA alkaline (Duracell MN2400 or equivalent) |
|
for 1 week. |
User Interface |
Includes an audible alarm to alert the |
|
patient if an electrode becomes detached. |
|
Sense current is < 10 nA. |
Note: other specifi cations are the same as Standard Recording Mode.
Lifecard CF Service Manual |
11 |

1.2.7Additional Specifi cations in 12-Lead Mode
Channels |
Standard 12-lead, one neutral and nine active |
|
electrodes |
Cable types |
10 electrode, deÞbrillation protected, IEC |
|
or AHA code |
Isolation |
DEFIBRILLATION-PROOF TYPE |
|
CF APPLIED PART |
Input impedance |
10 Mohm |
Sampling rate |
4096 samples per second per channel |
CMRR |
> 80dB per IEC and ANSI/AAMI methods |
Suppression |
Active neutral system (‘right leg drive’) |
Resolution |
0.6ȝV |
Noise |
< 0.6ȝV RMS |
Pacing detection |
>2mV, 200ȝs to 5ms pulse in any electrode |
Capacity required |
256 MByte card for 24 hour recording |
Battery |
An alkaline AAA cell is required for |
|
24 hour recording |
Fault tolerance |
In the event of electrode detachment |
|
noise is suppressed, and the available leads |
|
are recorded (differential V leads only if R, L |
|
or F is detached) |
Note: other specifi cations are the same as Standard Recording Mode
1.2.8Physical and Environmental
Dimensions |
96 x 57 x 17.5mm with patient cable Þtted |
Weight |
Recorder body 55g: patient load 130g |
|
including battery, card and typical |
|
patient cable |
User labelling |
Area provided is 52 x 15 mm |
Temperature |
0 to 45°C operation, -20 to 65°C storage |
Humidity |
Operation or storage 5% to 95%, |
|
non-condensing |
Pressure |
Operation or storage air pressure |
|
700 - 1060 mbar |
Shock |
1 m drop |
|
|
|
12 |
18-0583 Rev.C |
|

1.2.9Electro-Magnetic Compatibility
General |
Complies with EN60601-1-2:1993 and ANSI/ |
|
AAMI EC38:1998 |
ESD (1) |
4 kV air and 2 kV contact discharges: no inter |
|
ruption in recorder function |
ESD (2) |
8 kV air and 6 kV contact discharges: no |
|
damage to the recorder, recording |
|
resumes automatically in < 10 s |
Radiated emissions |
CISPR 11:1997, EN55011:1998 Group 1 |
|
Class B |
Radiated immunity |
3 V/m 26 MHz - 1 GHz, 80% AM modulated |
|
at 5 Hz. Keyed carrier immunity to |
|
EN50082:1996 |
This equipment has been tested and found to comply with the limits for a class B computing device in accordance with the speciÞcations in Subpart J of Part 15 of FCC Rules, which are designed to provide reasonable protection against interference to radio and television reception.
This equipment generates and uses radio frequency energy and if not installed and used in accordance with the instructions it may cause interference. However, there is no guarantee that interference will not occur in a particular installation. If this equipment does cause interference to radio or television reception, which can be determined by turning the equipment off or on, the user is encouraged to try to correct the interference by one or more of the following measures:
•Reorient the receiving antenna
•Relocate the equipment with respect to the receiver
•Move the equipment away from the receiver
If necessary, the user should consult Spacelabs Healthcare or an experienced radio/television technician for additional suggestions. The user may Þnd the following booklet prepared by the Federal Communications Commission helpful:
“How to Identify and Resolve Radio-TV Interference Problems”
This booklet is available from the U.S. Government Printing OfÞce, Washington, DC 20402, Stock No. 004-000-00345-4.
Lifecard CF Service Manual |
13 |

This Page is intentionally Blank
|
|
|
14 |
18-0583 Rev.C |
|

2. SAFETY AND REGULATORY
2.1Intended Use of Equipment
The Lifecard CF Holter recorder is to be used for the non-invasive ambulatory recording of two or three channel electrocardiograms on a standard commercial compact ßash card.
The Lifecard 12 option is to be used for the non-invasive ambulatory recording of 12-lead electrocardiograms on a standard commercial compact ßash card.
The recorder allows data to be collected over a continuous period of up to 7 days whilst allowing the subject to perform most of their normal daily activities.
The recordings can be analysed on compatible analysis systems from Spacelabs Healthcare.
This device has been designed and supplied speciÞcally for the long term recording of electrocardiograms in ambulatory patients using standard Holter monitoring techniques. It shall not be used for any other purposes.
The device shall be operated only be suitably competent personnel trained in the use and procedures of Holter electrocardiography for diagnostic purposes.
The Lifecard CF comprises two sections; the ‘Recorder Unit’ and the ‘Patient Cable Unit’.
2.2Safety Classifi cation
This device has been designed in accordance with EN60601 - 1 , “Medical
electrical equipment, Part 1: General requirements for safety”, as follows:
1.EQUIPMENT with an INTERNAL ELECTRICAL POWER SOURCE. The equipment is designed to be battery operated only. Under NO circumstances shall a mains powered battery eliminator or any other external power source be used with the equipment.
Lifecard CF Service Manual |
15 |

2.EQUIPMENT having a TYPE BF APPLIED PART. or
3.EQUIPMENT having a TYPE CF APPLIED PART if so marked.
4.IPX4 EQUIPMENT protected against the ingress of splashing water, if so marked. Otherwise, ORDINARY EQUIPMENT, without protection against ingress of liquid.
5.Not suitable for use in the presence of a ßammable anesthetic mixture with air or with oxygen or nitrous oxide, or ßammable cleaning agents.
6.Rated for CONTINUOUS OPERATION.
7.EQUIPMENT with an APPLIED PART, speciÞcally designed for application where a CONDUCTIVE CONNECTION is made to the PATIENT, but not directly to the heart. According to ANSI/AAMI EC38:1998.Lifecard CF is Type 1 ambulatory ECG device.
2.3Adjustment, replacement of parts, maintenance and repair
The device requires no routine adjustments to maintain its operation.
The device contains no user serviceable parts. It shall be serviced only by Spacelabs Healthcare or by an agent accredited by them to service device of this type. Unauthorised repairs or dismantling of the device will invalidate the guarantee.
2.4Defects and abnormal stresses
For continued safety the device must not be maltreated, used outside its speciÞed operation conditions, or stored outside its speciÞed storage conditions.
Lifecard CF contains protection against electrostatic discharge, but there is no protection against deÞbrillators. To avoid damage the device should be removed before deÞbrillating. The Varios active yoke and 46-1123 / 46-1127 patient cables have deÞbrillator protection. (The protection is a combination of the cable and the yoke).
|
|
|
16 |
18-0583 Rev.C |
|
 Loading...
Loading...