Page 1

COAGULATION
ANALYZER
OPERATION MANUAL
Instrument manufactured by
Sigma Amelung,
Lemgo, Germany
REVISION DATE 10/23/01
Page 2

Page 3

SIGMA DIAGNOSTICS INSTRUMENT WARRANTY
Sigma-Aldrich Co., Inc. ("Sigma"), warrants that instruments it sells to be free from
defects in workmanship and materials during normal use by the original purchaser.
This Warranty shall continue for a period of one year from the date of invoice to the
original purchaser, or until title is transferred from the original purchaser, whichever
occurs first (the "Warranty Period").
If any defects occur during the Warranty Period, contact the Sigma Service Center
immediately, and be prepared to furnish pertinent details concerning the defect, the
model number, and the serial number.
Warranty service is provided 8:30 a.m. through 5:00 p.m., Monday through Friday,
except on Sigma observed holidays. Any service performed at other times, and all
service required to correct defects or malfunctions not covered by this Warranty, will be
billed on a time-and-material basis at Sigma's labor rates then in effect.
This Warranty does not cover defects or malfunctions which: (1) are not reported to
Sigma during the Warranty Period and within one week of occurrence; (2) result from
chemical decomposition or corrosion; (3) are described in the applicable Sigma
Operation Guide; (4) result from maintenance, repair, or modification performed
without Sigma's prior written authorization; or (5) result from misuse, abuse or
accident.
Sigma's liability for all matters arising from the supply, installation, use, repair, and
maintenance of the instrument, whether arising under this Warranty or otherwise, shall
be limited solely to the repair or (at Sigma's sole discretion) replacement of the
instrument or of components thereof. In no event shall Sigma be liable for injuries
sustained by third parties, incidental or consequential damages, or lost profits.
Replaced parts shall become the property of Sigma.
THE FOREGOING IS THE SOLE WARRANTY MADE BY SIGMA REGARDING THE
INSTRUMENT, AND SIGMA SPECIFICALLY DISCLAIMS ALL OTHER WARRANTIES,
EXPRESSED OR IMPLIED, INCLUDING THE WARRANTIES OF MERCHANTABILITY
AND OF FITNESS FOR A PARTICULAR PURPOSE.
KC4
(Software Version 1.1B)
© 2001 Sigma-Aldrich Co.
October 2001 EN
™ User Manual, October 2001
∆
Page 4

Page 5

KC4 ∆∆∆∆™
0
Table of Contents
1 INTRODUCTION.................................................................................................... 1-1
1.1 INTENDED USE ................................................................................................. 1-1
1.2 PRINCIPLES OF OPERATION.............................................................................. 1-1
1.3 PHYSICAL SPECIFICATIONS .............................................................................. 1-2
1.4 PERFORMANCE SPECIFICATIONS ..................................................................... 1-3
1.5 PHYSICAL DESCRIPTION ................................................................................... 1-6
1.6 FRONT VIEW (FIGURE 1) ................................................................................... 1-7
1.7 KEYPAD (FIGURE 2)........................................................................................... 1-8
1.8 BACK VIEW (FIGURE 3) ..................................................................................... 1-9
1.9 MULTIPETTE (FIGURE 4 A & B) ....................................................................... 1-10
1.10 BALL DISPENSER (FIGURE 5) .......................................................................... 1-11
1.11 THERMAL PRINTER (FIGURE 6) ....................................................................... 1-11
1.12 PRINTER OPTIONS........................................................................................... 1-12
2 INSTALLATION ..................................................................................................... 2-1
2.1 UNPACKING ....................................................................................................... 2-1
2.2 KC4 ∆™ COAGULATION ANALYZER START-UP KIT............................................ 2-1
2.3 LOCATION REQUIREMENTS .............................................................................. 2-2
2.4 ELECTRICAL REQUIREMENTS .......................................................................... 2-2
2.5 PRELIMINARY CHECK OF THE INSTRUMENT OPERATION ................................ 2-3
3 GENERAL OPERATION ......................................................................................... 3-1
3.1 INSTRUMENT PREPARATION ............................................................................. 3-1
3.2 TEMPERATURE INDICATOR SCREEN ................................................................ 3-1
3.3 MAIN MENU FUNCTIONS ................................................................................... 3-2
3.4 PASSWORD MODIFICATION .............................................................................. 3-2
3.5 PROGRAM MODIFICATION ................................................................................ 3-3
3.6 REAGENT HANDLING ........................................................................................ 3-4
3.7 CUVETTE PREPARATION ................................................................................... 3-4
3.8 SAMPLE PREPARATION ..................................................................................... 3-5
3.9 PIPETTING ......................................................................................................... 3-6
3.10 TO DISPENSE SAMPLE ...................................................................................... 3-7
3.11 TO DISPENSE FIRST REAGENT ......................................................................... 3-8
3.12 TO DISPENSE START REAGENT ........................................................................ 3-9
3.13 SELECTING A TEST TO BEGIN A RUN ............................................................. 3-10
3.14 PATIENT IDENTIFICATION ............................................................................... 3-11
3.15 TESTING .......................................................................................................... 3-11
3.16 MANUAL START PROCEDURE ......................................................................... 3-12
3.17 AUTOMATIC START PROCEDURE.................................................................... 3-12
3.18 PRINTING RESULTS ......................................................................................... 3-13
4 MODE PROGRAMMING ......................................................................................... 4-1
4.1 ROUTINE PROGRAMMING ................................................................................. 4-1
4.2 EMERGENCY PROGRAMMING ........................................................................... 4-2
4.3 INDIVIDUAL PROGRAMMING............................................................................. 4-3
October 2001 TOC-1 EN
Page 6

KC4 ∆∆∆∆™
0
Table of Contents
5 TEST PROGRAMMING........................................................................................... 5-1
5.1 INR .................................................................................................................... 5-1
5.2 INR FLOW CHART.............................................................................................. 5-3
5.3 PROTHROMBIN TIME (PERCENT ACTIVITY CURVE) .......................................... 5-4
5.4 PT (PERCENT ACTIVITY) FLOW CHART.............................................................. 5-7
5.5 RATIO FLOW CHART ......................................................................................... 5-8
5.6 ACTIVATED PARTIAL THROMBOPLASTIN TIME................................................. 5-9
5.7 APTT FLOW CHART ......................................................................................... 5-11
5.8 FIBRINOGEN ................................................................................................... 5-12
5.9 FIBRINOGEN FLOW CHART............................................................................. 5-15
5.10 FACTORS......................................................................................................... 5-16
5.11 FACTORS FLOW CHART .................................................................................. 5-19
5.12 STATS.............................................................................................................. 5-20
6 QUALITY CONTROL .............................................................................................. 6-1
7 MAINTENANCE ..................................................................................................... 7-1
8 TROUBLESHOOTING............................................................................................. 8-1
8.1 TROUBLESHOOTING FLOW DIAGRAM .............................................................. 8-1
8.2 TROUBLESHOOTING PROCEDURES TABLE...................................................... 8-1
A APPENDIX ............................................................................................................ A-1
A.1 INR FAST TRACK ............................................................................................... A-1
A.2 APTT FAST TRACK............................................................................................. A-2
A.3 FIBRINOGEN FAST TRACK ................................................................................ A-3
A.4 FIBRINOGEN CALIBRATION CURVE DILUTION ................................................. A-4
A.5 EXTRINSIC FACTORS II, V, VII AND X FAST TRACK .......................................... A-5
A.6 EXTRINSIC FACTOR STANDARD CURVE DILUTIONS ........................................ A-6
A.7 INTRINSIC FACTORS VIII, IX, XI AND XII FAST TRACK...................................... A-7
A.8 INTRINSIC FACTOR STANDARD CURVE DILUTIONS ......................................... A-8
TOC-2 EN October 2001
Page 7

KC4 ∆∆∆∆™
1
Introduction
1Introduction
Contents
1.1 INTENDED USE...................................................................................... 1-1
1.2 PRINCIPLES OF OPERATION.................................................................. 1-1
1.3 PHYSICAL SPECIFICATIONS .................................................................. 1-2
1.4 PERFORMANCE SPECIFICATIONS ......................................................... 1-3
1.5 PHYSICAL DESCRIPTION ....................................................................... 1-6
1.6 FRONT VIEW (FIGURE 1) ....................................................................... 1-7
1.7 KEYPAD (FIGURE 2)............................................................................... 1-8
1.8 BACK VIEW (FIGURE 3).......................................................................... 1-9
1.9 MULTIPETTE (FIGURE 4 A & B).............................................................1-10
1.10 BALL DISPENSER (FIGURE 5)............................................................... 1-11
1.11 THERMAL PRINTER (FIGURE 6)........................................................... 1-11
1.12 PRINTER OPTIONS ...............................................................................1-12
October 2001 1-0 EN
Page 8

Page 9
KC4 ∆∆∆∆™
1
Introduction
1.1 Intended Use
The KC4∆™ Coagulation Analyzer is a semi-automated mechanical clot detection
system designed for the determination of prothrombin times (PT), activated partial
thromboplastin times (APTT), fibrinogen concentrations determined by Clauss
methodology, and other clotting assays. Any clotting based assay, which has fibrin
formation as its endpoint may be performed on the KC4
Measurement can be qualitative or quantitative. When used in conjunction with
appropriate reagents, the sample can be plasma, or whole blood.
The additions of both sample and reagents are manual. Time measurement of the
clotting endpoint is automated.
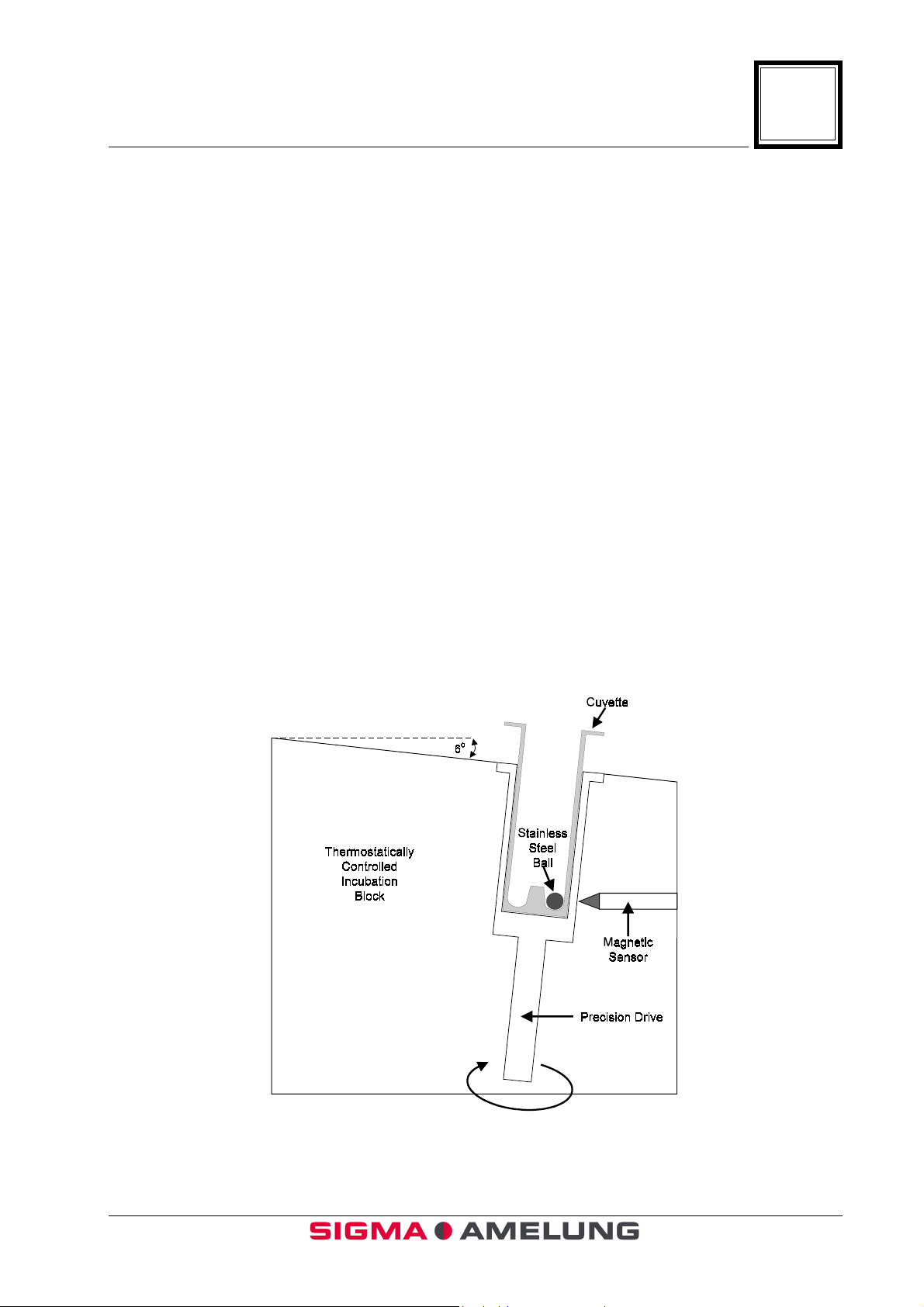
1.2 Principles of Operation
The KC4∆™ is an electromechanical clot detection system. The system utilizes a
special cuvette in which there is a stainless steel ball. Sample is added to the cuvette.
After an appropriate incubation period, the cuvette is placed into the measuring well of
∆
the KC4
along its longitudinal axis. Because the cuvette is positioned at a slight angle, gravity
and inertia always position the ball at the lowest point of the cuvette. Exactly opposite
the ball-position is a magnetic sensor. With the addition of appropriate reagent, a timer
is started. As coagulation takes place, fibrin strands form in the reaction mixture. The
fibrin strands pull the ball away from its position and triggers an impulse in the
magnetic sensor. This impulse electronically stops the timer (see diagrams).
™. The measuring well rotates slowly (50 rpm) causing the cuvette to rotate
∆
™ Coagulation Analyzer.
October 2001 1-1 EN
Page 10

1
Introduction
1.3 Physical Specifications
Type: Coagulation Analyzer, Bench Top
Online: Unidirectional
Principle: Ball Method
Measuring Channels: 4
Display: LCD
Incubation Wells: 8
Reagent Wells: 5
Dimensions
Height: 12.0 cm
Width: 35.4 cm
KC4 ∆∆∆∆™
Depth: 45.0 cm
Weight 6.3 kg
Power Supply
Voltage 110–220V/50–60 HZ
Power Consumption 1.5A at 100V; 0.4A at 220V
Temperature Control
Reagent Warming Wells: 37.0°C ± 0.5°C
Reaction Incubation Wells: 37.0°C ± 0.5°C
Measurement Wells: 37.0°C ± 0.5°C
Measurement Time
Minimum: 4.5 seconds
Maximum: 999.9 seconds
1-2 EN October 2001
Page 11
KC4 ∆∆∆∆™
1
Introduction
1.4 Performance Specifications
The overall performance of any testing performed on the KC4∆™ Coagulation Analyzer
is dependent not only on the instrument performance, but is also a function of
specimen integrity (collection and handling) as well as accuracy and precision of the
sample and reagent dispensing system being used.
Correlation:
The following linear regression data was obtained during evaluation to show
equivalence with a commercially available mechanical coagulation analyzer.
Activated Partial
Prothrombin Time
Number of Samples 121 110
Correlation Coefficient (r) 0.998 0.896
Slope 1.051 1.235
Intercept –0.241 0.873
Thromboplastin Time
The following linear regression data was obtained during evaluation to show
equivalence with a commercially available photo-optical coagulation analyzer.
Fibrinogen Factor X Factor IX
Number of Samples 109 112 101
Correlation Coefficient (r) 0.930 0.974 0.897
Slope 1.067 1.010 0.958
Intercept 30.749 –0.166 3.403
The following linear regression data was obtained in three physician’s office laboratories
(POL) during evaluation to show equivalence with manufacturer derived results on the
∆
KC4
™ Coagulation Analyzer.
Activated Partial
POL #1 Prothrombin Time
Number of Samples 47 44
Correlation Coefficient (r) 0.991 0.960
Slope 0.981 1.066
Intercept 0.492 0.379
Thromboplastin Time
Activated Partial
POL #2 Prothrombin Time
Number of Samples 45 46
Correlation Coefficient (r) 0.989 0.965
Slope 1.019 1.029
Intercept –0.248 1.021
October 2001 1-3 EN
Thromboplastin Time
Page 12

KC4 ∆∆∆∆™
1
Introduction
Activated Partial
POL #3 Prothrombin Time
Number of Samples 52 47
Correlation Coefficient (r) 0.974 0.927
Slope 1.012 0.786
Intercept 0.326 9.470
Precision: Prothrombin Time (PT)
Imprecision was evaluated at three levels according to the NCCLS EP5-T2 protocol.
Low Mid High
Mean (seconds) 13.20 33.53 39.66
Total Imprecision (CV%) 2.03 2.50 4.18
Within-Run Imprecision (CV%) 1.02 1.28 1.53
PT total imprecision was evaluated in three physician’s office laboratories (POL) at three
levels according to NCCLS EP10-T protocol. Within-Run imprecision was evaluated in
three physician’s office laboratories at two levels.
Thromboplastin Time
POL #1 Low Mid High
Mean (seconds) 13.1 26.8 42.9
Total Imprecision (CV%) 1.97 1.63 2.47
Mean (seconds) 12.7 44.1
Within-Run Imprecision (CV%) 1.3 1.1
POL #2 Low Mid High
Mean (seconds) 12.1 23.3 40.7
Total Imprecision (CV%) 2.59 7.12 3.0
Mean (seconds) 12.1 41.7
Within-Run Imprecision (CV%) 2.6 1.8
POL #3 Low Mid High
Mean (seconds) 11.3 22.8 34.2
Total Imprecision (CV%) 1.57 7.41 0.50
Mean (seconds) 11.4 34.7
Within-Run Imprecision (CV%) 2.0 1.3
1-4 EN October 2001
Page 13
KC4 ∆∆∆∆™
1
Introduction
Precision: Activated Partial Thromboplastin Time (APTT)
Imprecision was evaluated at three levels according to the EP5-T2 protocol.
Low Mid High
Mean (seconds) 28.55 51.01 75.78
Total Imprecision (CV%) 3.12 3.41 3.21
Within-Run Imprecision (CV%) 1.47 1.60 1.37
APTT total imprecision was evaluated in three physician’s office laboratories (POL) at
three levels according to NCCLS EP10-T protocol. Within-Run imprecision was
evaluated in three physician’s office laboratories at two levels.
POL #1 Low Mid High
Mean (seconds) 29.0 43.3 57.6
Total Imprecision (CV%) 2.83 3.15 1.87
Mean (seconds) 30.8 57.5
Within-Run Imprecision (CV%) 2.7 1.6
POL #2 Low Mid High
Mean (seconds) 29.2 42.7 57.0
Total Imprecision (CV%) 4.38 2.29 2.84
Mean (seconds) 28.2 57.1
Within-Run Imprecision (CV%) 2.1 1.7
POL #3 Low Mid High
Mean (seconds) 30.0 54.7 68.6
Total Imprecision (CV%) 1.87 1.80 2.13
Mean (seconds) 26.9 64.4
Within-Run Imprecision (CV%) 1.4 2.5
Precision: Fibrinogen
Imprecision was evaluated at three levels according to the NCCLS EP5-T2 protocol.
Low Mid High
Mean (mg/dl) 104.09 154.10 323.53
Total Imprecision (CV%) 3.53 6.21 4.36
Within-Run Imprecision (CV%) 2.05 2.86 2.12
October 2001 1-5 EN
Page 14

KC4 ∆∆∆∆™
1
Introduction
Precision: Factor X
Imprecision was evaluated at three levels according to the NCCLS EP5-T2 protocol.
Low Mid High
Mean (%) 31 57 102
Total Imprecision (CV%) 8.28 5.71 5.22
Within-Run Imprecision (CV%) 2.63 2.22 2.20
Precision: Factor IX
Imprecision was evaluated at three levels according to the NCCLS EP5-T2 protocol.
Low Mid High
Mean (%) 24 49 98
Total Imprecision (CV%) 5.88 6.89 4.06
Within-Run Imprecision (CV%) 3.96 4.04 2.54
1.5 Physical Description
The external features of the KC4∆™ Coagulation Analyzer are shown in Figure 1
(Front), Figure 2 (Keypad), Figure 3 (Rear), Figure 4 (Automatic Multipette with starter
cable), Figure 5 (Ball Dispenser) and Figure 6 (Thermal Printer).
1-6 EN October 2001
Page 15
KC4 ∆∆∆∆™
Introduction
1
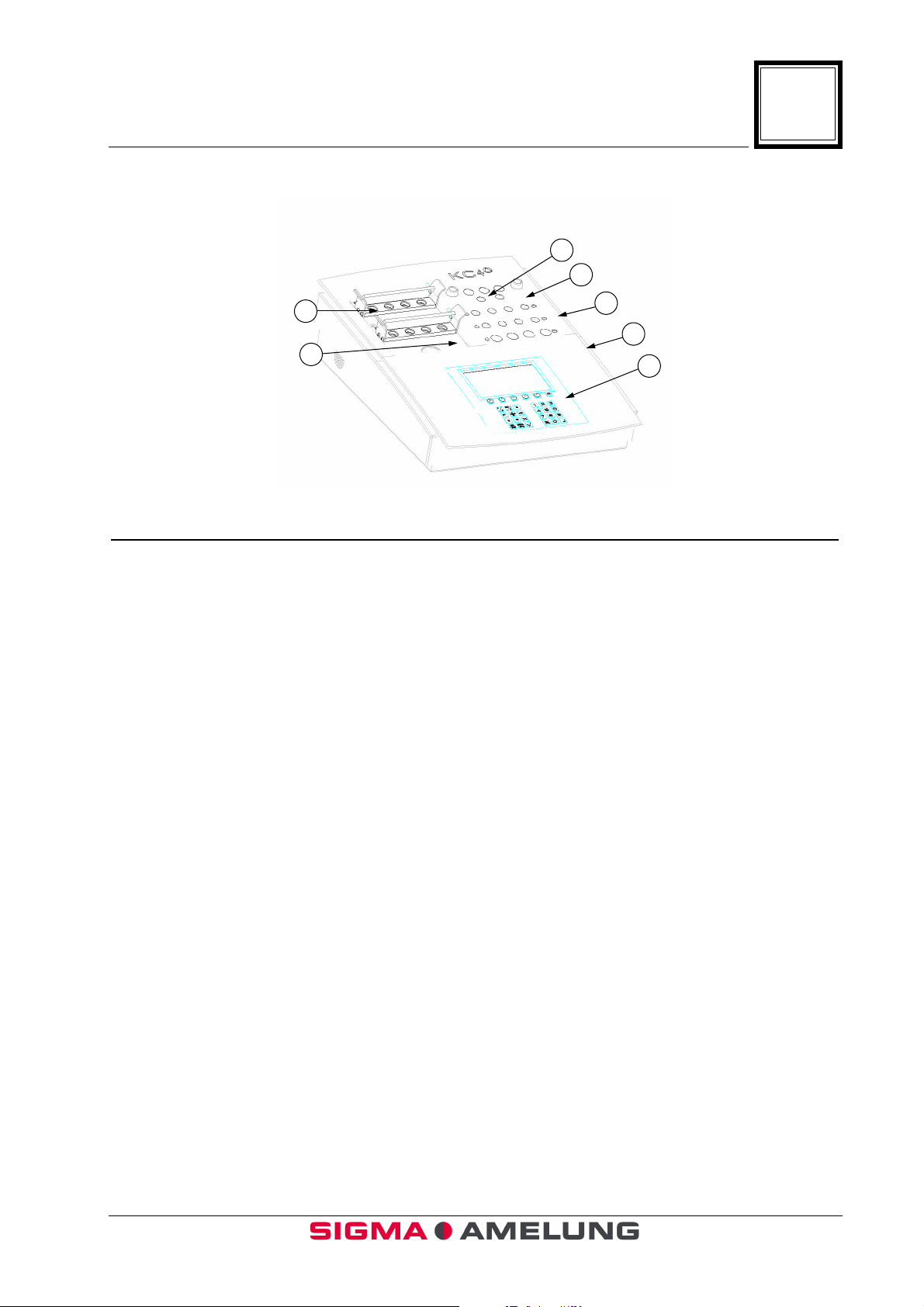
1.6 Front View (Figure 1)
1
3
4
1
2
Item Function or Description
1. Rack Used for transferring cuvettes from
preparation area to reaction incubation
wells and rotating test positions.
5
6
7
2. Preparation Area Room temperature wells used for sample
preparation prior to incubation.
3. Pipette Tubes Used to store the pipettes when not in use.
4. Reagent Warming Wells (5) Three 15 mm, and two 11 mm heated wells
used to warm reagents.
5. Reaction Incubation Wells (8) Heated wells used for incubation of sample
and first reagent.
6. Rotating Test Positions (4) Positions where start reagent is added and
the clotting time is measured.
7. Display Screen Displays elapsed time in seconds during
incubation for each of 4 channels. Displays
elapsed time in seconds and tenths of
seconds during clot time measurement.
Displays incubation times, clotting times,
programming selections and other menus.
October 2001 1-7 EN
Page 16
1
KC4 ∆∆∆∆™
Introduction
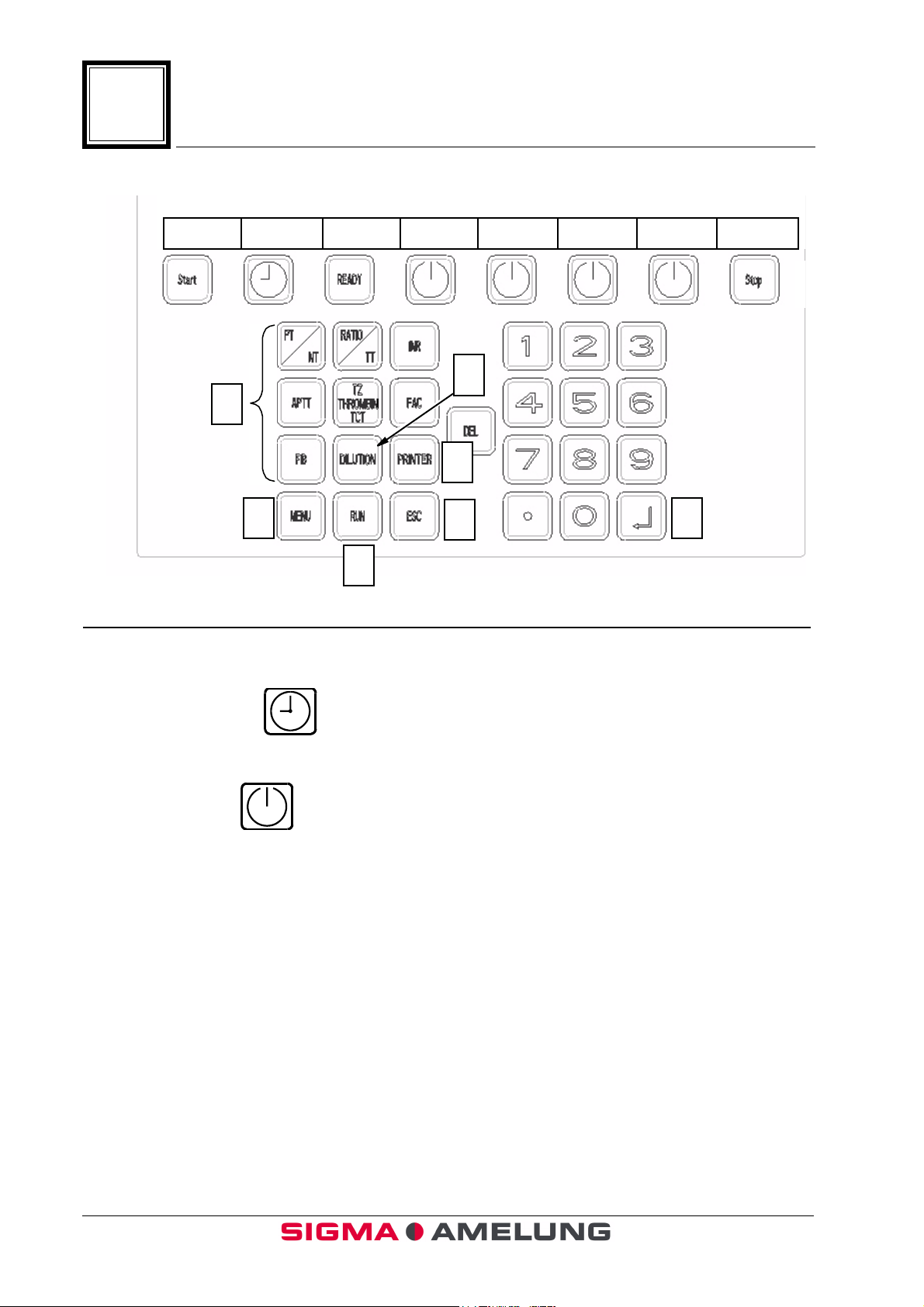
1.7 Keypad (Figure 2)
2
12 3 4 4 4 45
12
6
11
10
8
Item Function or Description
97
1. Start Key Activates automatic measurement timer.
2. Incubation Key Starts Incubation timers.
3. Ready Key Not functional at this time.
4. Channel Key Starts manual timing and incubation.
5. Stop Key Terminates measurements, and aborts
testing.
6. Function Keys Used in programming tests.
7. Menu Key Returns to Main Menu from Operating
Screen.
8. Run Key Returns to Test Program Selection Screen
from Operating Screen.
↵↵↵↵
9.
10. ESC Key Escapes back to previous function
11. Printer Key Used to turn printer on or off
ENTER
12. Dilution Key Used to change the patient dilution.
13. DEL key
1-8 EN October 2001
Page 17
KC4 ∆∆∆∆™
Introduction
1
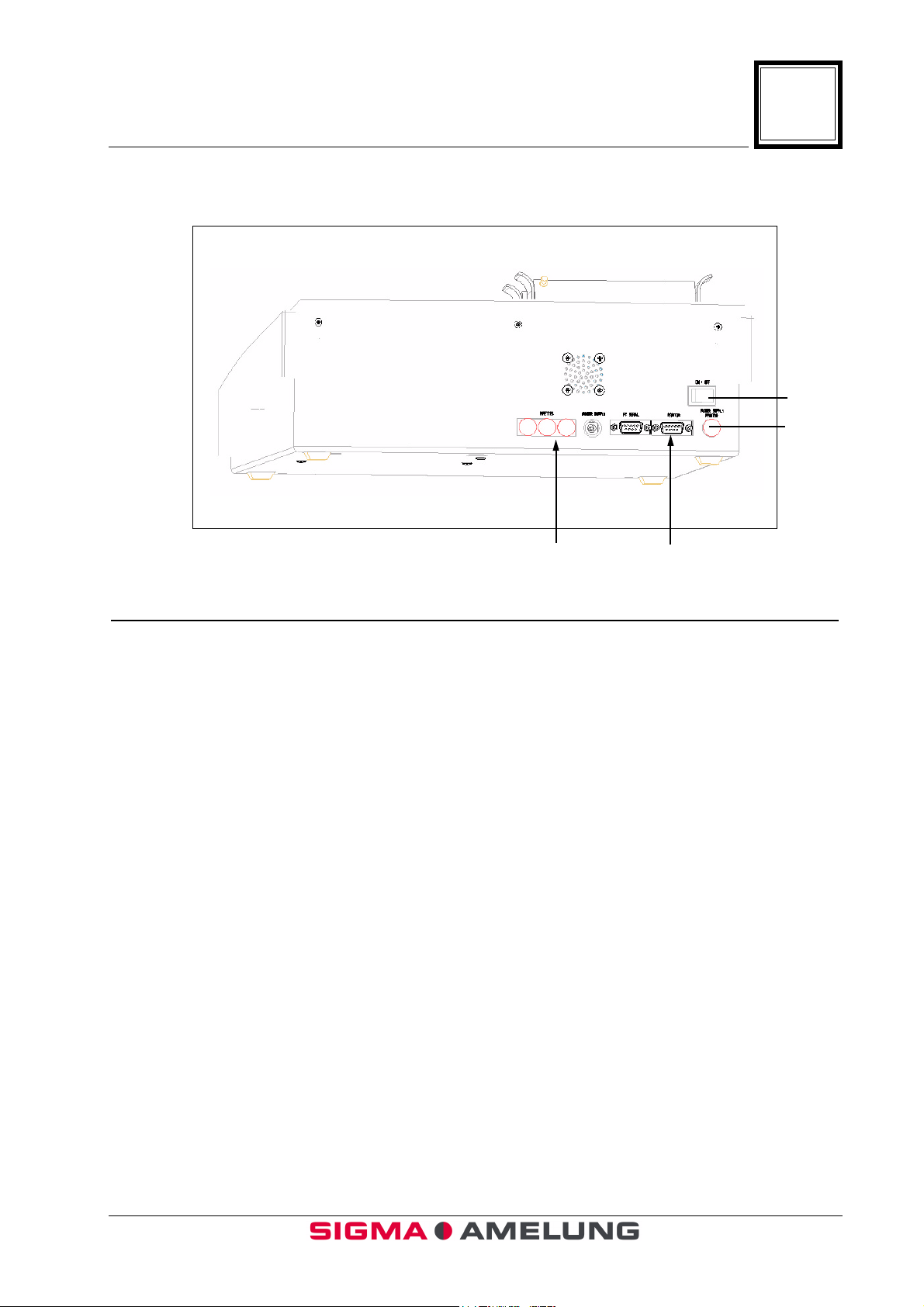
1.8 Back View (Figure 3)
3
2. 1.
Item Function or Description
3.
4.
1. Thermal Printer Port Thermal Printer connection.
2. Automatic Multipette Socket(s) Used to connect pipettes (Remove the capplug in the Multipette).
3. Power Switch Powers instrument off/on.
4. Power Supply Socket Connects instrument to power cord.
October 2001 1-9 EN
Page 18
1
KC4 ∆∆∆∆™
Introduction
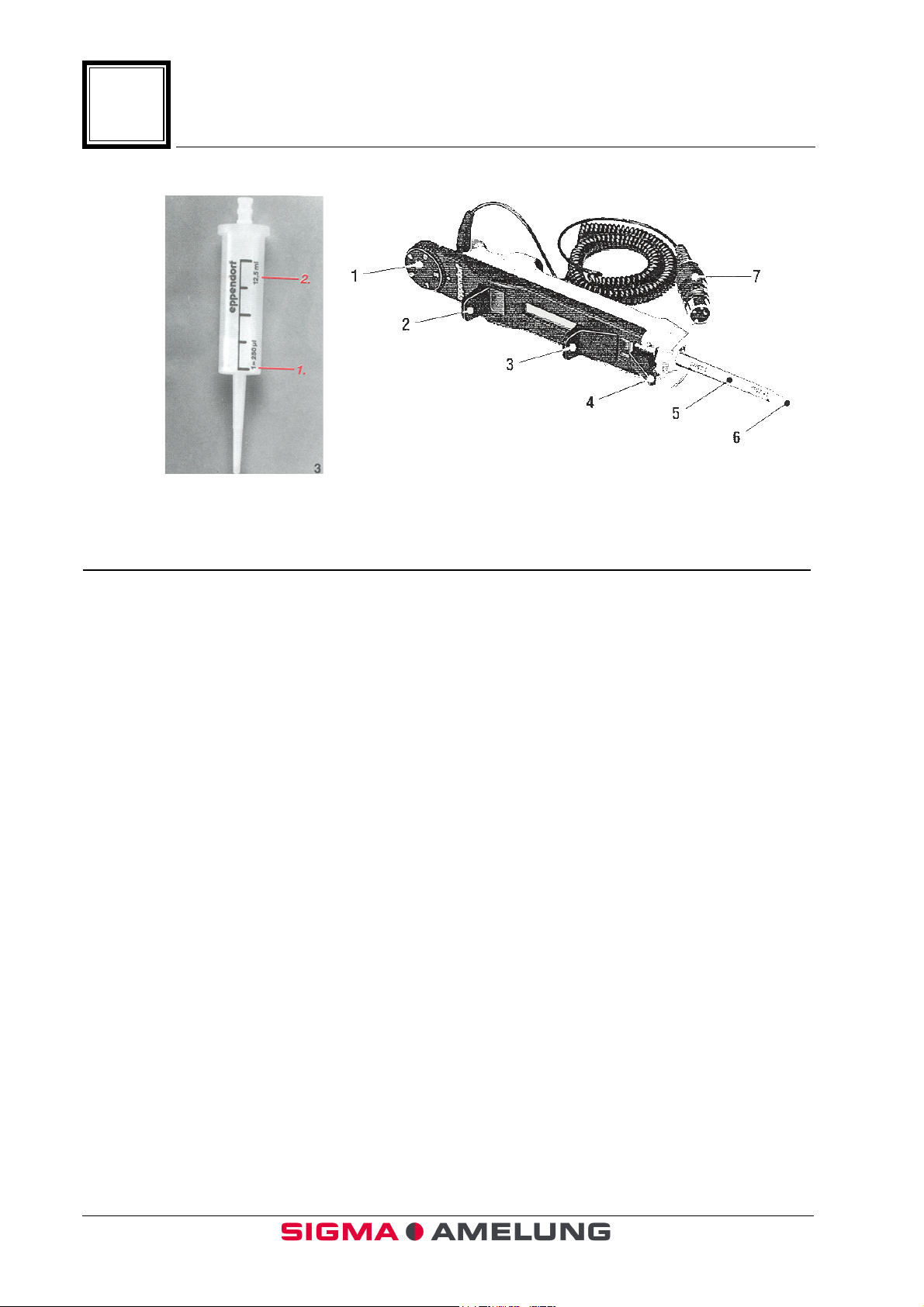
1.9 Multipette (Figure 4 A & B)
4
Combitip
4-A
Item 4-B Function or Description
1. Volume selection dial Determines pipetting volume: setting (1–5)
multiplied by the minimum pipetting
volume of the Combitip (1.25 ml or 2.50 ml
pipette tips).
Multipette
4-B
2. Pipetting lever The volume is pipetted by pressing the
pipetting lever down until it stops.
3. Filling lever The Combitip is filled by sliding this lever
upward.
4. Locking clamp The locking clamp serves to firmly clamp
the Combitip.
5. Combitip The pipette tip used with the automatic
multipette.
6. Combitip Cone Portion of the pipette tip that aspirates
reagent.
7. Starter Cable Connects pipette to instrument.
1-10 EN October 2001
Page 19
KC4 ∆∆∆∆™
Introduction
1
1.10 Ball Dispenser (Figure 5)
5
Item Description or Function
1. Dispenser For loading microballs into cuvettes
1.11 Thermal Printer (Figure 6)
6
October 2001 1-11 EN
Page 20
1
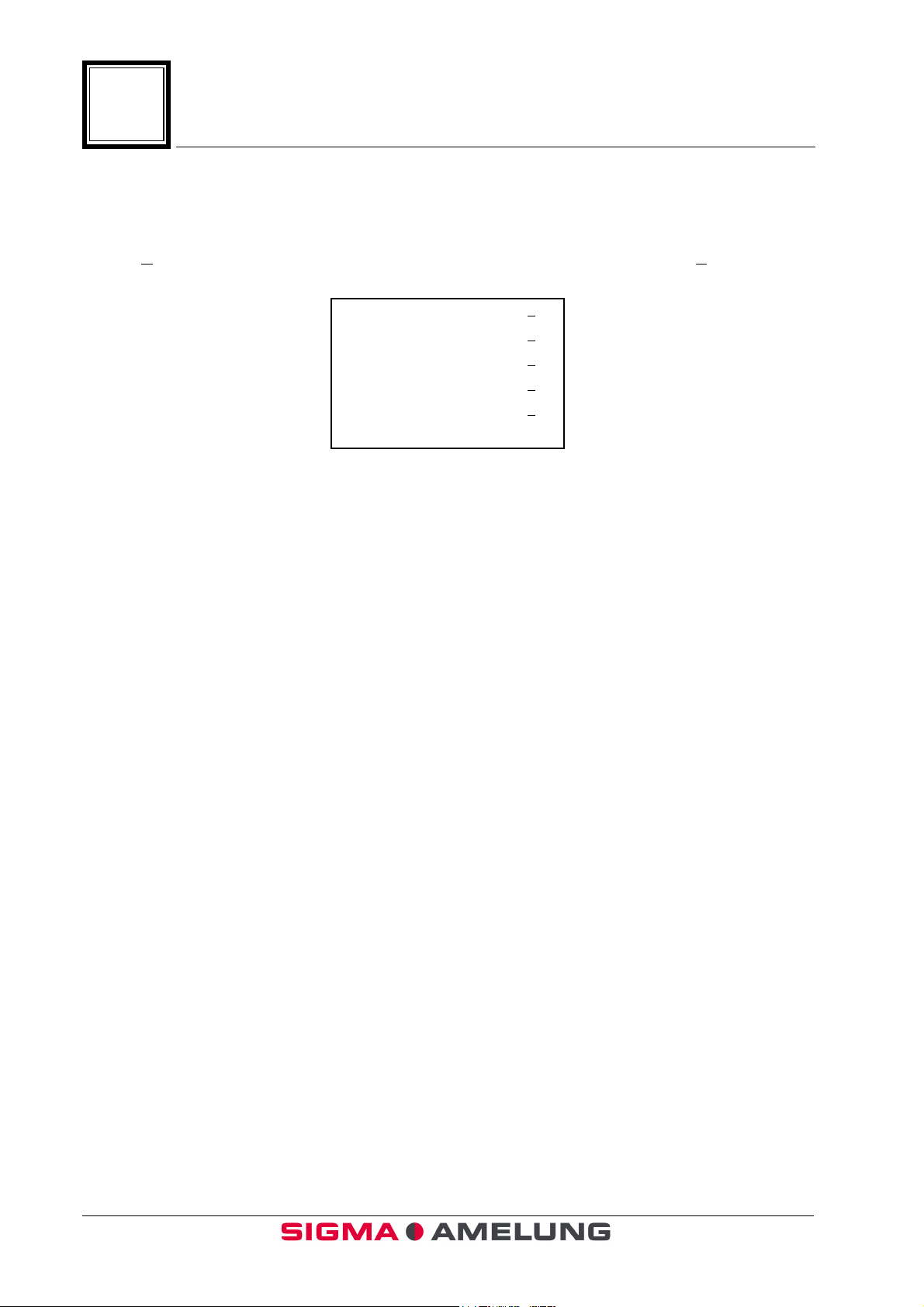
1.12 Printer Options
KC4 ∆∆∆∆™
Introduction
From the Main Menu, press <
options have been selected. If the analyzer is connected to a host LIS, ensure that
option 4
is selected. If the analyzer is not connected to a host LIS, select 5.
↵↵↵↵
>. Press the <PRINTER> key. The (*) shows which
Print Test Page 1
Print Switch On 2*
Print Switch Off 3
Line - out incl. Error Val. 4*
Line - out excl. Error Val. 5
Press ENTER <
> to continue
↵↵↵↵
1-12 EN October 2001
Page 21

KC4 ∆∆∆∆™
2
Installation
2Installation
Contents
2.1 UNPACKING........................................................................................... 2-1
2.2 KC4
2.3 LOCATION REQUIREMENTS................................................................... 2-2
2.4 ELECTRICAL REQUIREMENTS............................................................... 2-2
2.5 PRELIMINARY CHECK OF THE INSTRUMENT OPERATION..................... 2-3
∆∆∆∆
™ COAGULATION ANALYZER START-UP KIT ................................ 2-1
October 2001 2-0 EN
Page 22

Page 23

KC4 ∆∆∆∆™
Installation
2.1 Unpacking
2
The KC4
instrument from damage during shipment. If damage is apparent, immediately notify
the shipping company. Note the damage on the shipping bill of lading and notify your
Sigma Diagnostics Sales Representative.
2.2 KC4
Carefully remove the instrument and accessories from the transport box. Check that
the following items have been included:
KC4
∆∆∆∆
™ Coagulation Analyzer is shipped in a transport box designed to protect the
∆∆∆∆
™ Coagulation Analyzer Start-Up Kit
∆∆∆∆
™ Coagulation Analyzer
1. Power Cable
2. KC Micro Tetravettes
3. KC Delta Multipette with Starter Cable and Adapter Cord
4. Combitips (1.25 ml); 5 each
µ
5. KC Pipette Tips, Yellow 200
6. Tubes, Plastic (14.5 x 85 mm); 100 each
7. Tubes, Glass (15 x 85 mm), 50 each
8. Power Supply 12V
l; 1 tray
9. Lead for Power Supply
10. Protective Dust Cover; 1 each
Optional Items
Catalog Number Item
1. P2864 KC Series Printer with Power Adapter
2. K1638 * KC Series Thermal Printer Paper
3. K4882 KC4
4. K0508 * KC4
5. K1510 * KC4
6. K4257 * KC Pipette Tips, Yellow, 200
7. T9304 * Tubes, Glass (15 x 85 mm), 50 each
8. K4887 * KC4
9. T2242* Tubes, plastic (14.5 x 85 mm)
10. K1635* KC Micro Cuvettes with Ball Dispencer
11. A6083 Coated Stir Bar
12. K6208 APTT Stir Bar
∆
™ Multipette with Starter Cable
∆
™ Combitips 1.25 ml
∆
™ Combitips 2.50 ml
µ
l, 10 trays
∆
™ Tetravettes
13. K0633 KC Pittette Tube Sleeves
October 2001 2-1 EN
Page 24

KC4 ∆∆∆∆™
2
Installation
*These are consumable items and should be ordered as needed.
Pipettes are required for the test performance. Although the use of a Multipette will
ensure the start of the timing measurement is simultaneous with the addition of the
reagent, it is not mandatory.
Read the Operation Manual carefully prior to using the KC4
The Operation Manual has been written to provide the most comprehensive
∆
understanding of the operation of the KC4
you to fully utilize the features of the instrument.
™ Coagulation Analyzer and to enable
∆
™ Coagulation Analyzer.
2.3 Location Requirements
1. Place the KC4∆™ Coagulation Analyzer on a stable, vibration and dust free work
surface. It should not be positioned next to a centrifuge or other equipment, which
may cause vibration. The KC4
from moisture.
2. To avoid exceeding the control range of the instrument, place the KC4
Coagulation Analyzer in an area with a maximum room temperature of 30°C.
It should not be positioned in an area directly below ventilating ducts which
produce strong air currents. Do not expose the KC4
to direct sunlight. Sunlight influences the temperature control.
3. It is preferable to place the KC4
further than (6 ft.) 1.8 m from an electrical outlet. The instrument should not be
operated from an extension cord which does not employ protective grounding. The
electrical outlet used should not be shared with any devices, which consume large
amounts of power on a cyclic basis (e.g., centrifuges, air conditioners, and
refrigerators). When these type of devices cycle on and off, there may be a voltage
drop in the line which could interfere with the proper functioning of the instrument.
∆
™ Coagulation Analyzer should also be protected
∆
™
∆
™ Coagulation Analyzer
∆
™ Coagulation Analyzer in an area which is no
2.4 Electrical Requirements
The KC4∆™ Coagulation Analyzer is designed with a factory equipped three-pronged
grounding plug designed to be connected to the Power Supply, which is then plugged
into the analyzer. Under no circumstances should it be connected to an ungrounded
two-pronged receptacle. This procedure is in accordance with the National Electrical
Code and other applicable ordinances for this type of installation.
1. Do not use an extension cord which cannot to provide protective grounding.
2. It is recommended that any repair work other than routine maintenance
be performed by a trained specialist familiar with the hazards involved.
3. If safe operation of the KC4
the instrument must be taken out of service.
2-2 EN October 2001
∆
™ Coagulation Analyzer is no longer possible,
Page 25

KC4 ∆∆∆∆™
2
Installation
2.5 Preliminary Check of the Instrument Operation
The preliminary function checks of instrument operation should be performed prior to
using the instrument. This preliminary function check is to ensure that the instrument
is functioning properly prior to reporting patient results.
1. Connect the power cable to the power cable socket on the back of the
instrument (DC6.5V 2A).
2. Connect the Data Cable from the Serial Port on the Printer to the Printer port
on the back of the Analyzer if utilizing the KC4
∆
3. Activate the KC4
located on the left hand side of the back of the instrument.
4. Observe that the display screen lights up; a screen appears giving the operator
the option to select the operating language. After the language selection, a
screen showing a thermometer appears and will remain displayed while the
instrument warms up to 37°C.
5. Observe that four of the measurement wells are rotating. The wells will rotate
continuously whenever the instrument is on.
™ Coagulation Analyzer by pressing the off/on switch
∆
™ printer.
∆
6. Place a KC4
rack. Place the cuvette rack on the rotating test positions such that the cuvettes
are sitting flush in the holes. If using a KC4
ball into each cuvette using the ball dispenser. Observe that the ball falls to the
front of the cuvette and stays there.
7. Verification of temperature can be performed by placing approximately 3 ml of
water into a 15-mm reagent tube. Place a thermometer into the tube and allow
to equilibrate until the temperature has stabilized. Approximately 15 minutes
will be required for temperature stabilization. The temperature should be
37° ±0.5°C.
Note: The use of smaller diameter tubes is not recommended due to inadequate
heat transfer.
8. To verify the operation of the timers, use the pipette with the start cable, and
the measuring wells, a program modification is needed. From the Main Menu,
select 3
9. Enter the password; default password is 1 2 3 4. Press <
10. Select 4
Press <
11. To access the program settings, press <
at the top of the screen. Press 1
Program Selection.
Individual Program Modify; press TZ (Thrombin Clotting Time) key.
↵↵↵↵
™ Micro cuvette or Tetravette into each position of the cuvette
∆
™ Micro cuvette, dispense one
↵↵↵↵
>.
>.
↵↵↵↵
>. Press 2
(Yes) to modify TZ.
(No) until “Test TZ” appears
12. Enter 1
13. Enter incubation time of 10 seconds, press <
modifications are done.
14. Press <
(Thrombin Clotting Time) key; press <
October 2001 2-3 EN
(duplicate testing), and 10% for allowed CV; press <
↵↵↵↵
>. Press 2
↵↵↵↵
> to continue. Press Run; select 3
Start Individual Program. Press TZ
↵↵↵↵
>.
↵↵↵↵
> to continue.
(No) when
Page 26

2
KC4 ∆∆∆∆™
Installation
15. Enter 2 samples per rack; press <
Screen up.
16. If the automatic Multipette with the starter cable is being used, plug the pipette
cable connection into the pipette cable socket on the rear of the KC4
the locking mechanism on the Multipette into place.
17. Start timers by pressing the <START> key , followed by the individual well
timers . When all timers are showing 0.0, press and hold the <START>
key , while at the same time, depress the trigger switch on the Multipette 4
times. All wells should begin timing.
18. After at least 10 seconds, remove the cuvette rack from the rotating test
positions. Observe that the timers stop and are indicating the elapsed time in
seconds and tenths of seconds.
19. The first result will print automatically (if the optional printer has been
installed), or will appear on the screen. Press <
and clear the memory.
With the completion of the Preliminary Checks of Instrument Operation, installation is
complete and the instrument is ready for operation. If the instrument fails to perform
any of the tests with the specifications listed, call Sigma Diagnostics Technical Service
for assistance.
↵↵↵↵
>. Press <
↵↵↵↵
> again to bring the Operating
∆
™. Snap
↵↵↵↵
> to print the second result,
2-4 EN October 2001
Page 27

KC4 ∆∆∆∆™
3
General Operation
3General Operation
Contents
3.1 INSTRUMENT PREPARATION................................................................. 3-1
3.2 TEMPERATURE INDICATOR SCREEN..................................................... 3-1
3.3 MAIN MENU FUNCTIONS........................................................................ 3-2
3.4 PASSWORD MODIFICATION ................................................................... 3-2
3.5 PROGRAM MODIFICATION..................................................................... 3-3
3.6 REAGENT HANDLING............................................................................. 3-4
3.7 CUVETTE PREPARATION ....................................................................... 3-4
3.8 SAMPLE PREPARATION ......................................................................... 3-5
3.9 PIPETTING ............................................................................................ 3-6
3.10 TO DISPENSE SAMPLE........................................................................... 3-7
3.11 TO DISPENSE FIRST REAGENT.............................................................. 3-8
3.12 TO DISPENSE START REAGENT............................................................. 3-9
3.13 SELECTING A TEST TO BEGIN A RUN ...................................................3-10
3.14 PATIENT IDENTIFICATION ...................................................................3-11
3.15 TESTING...............................................................................................3-11
3.16 MANUAL START PROCEDURE ...............................................................3-12
3.17 AUTOMATIC START PROCEDURE .........................................................3-12
3.18 PRINTING RESULTS..............................................................................3-13
October 2001 3-0 EN
Page 28

Page 29

KC4 ∆∆∆∆™
3
General Operation
3.1 Instrument Preparation
The KC4∆™ is activated by the on/off button located on the back of the instrument.
Turn instrument on and select appropriate language. Press <
Note: This screen will only appear the first time the analyzer is turned on. After
a language has been selected, the analyzer will automatically display the
temperature indicator screen (shown below) on each subsequent power on.
Sprachwahl.
Deutsch 1
Englisch *2
Franzoesisch 3
Italienisch 4
Niederlaendisch 5
Spanisch 6
Weiter mit Taste ENTER
↵↵↵↵
>.
3.2 Temperature Indicator Screen
In this figure, the thermometer indicates operating temperature has not been reached.
In approximately 20 minutes the analyzer will reach operating temperature
(37° ±0.5°C). The display will change to the MAIN MENU:
October 2001 3-1 EN
Page 30

3
3.3 Main Menu Functions
S I G M A - A M E L U N G
KC 4 DELTA
DATE: 12 Sep 2001 1
TIME: 10 : 08 2
PROGRAM SELECTION 3
PRINT PARAMETERS 4
KC4 ∆∆∆∆™
General Operation
Press
to continue
↵↵↵↵
1. To enter DATE, select 1. The date is in European format: dd,mm,yy.
12-09-2001 is September 12, 2001. The entry is confirmed with <
terminated with <ESC>. If terminated, the previous values remain valid.
2. To enter TIME, select 2
3. To modify Program Selection, select 3
. The format for time is hh:mm; example: 10:08
.
4. To PRINT a copy of all programmed tests and parameters, select 4
5. To proceed to the Operating Screen, press <
↵↵↵↵
>.
3.4 Password Modification
From the Main Menu screen, press 3 Program Selection.
Password modify 1
Routine Program modify 2
Emergency Program modify 3
Individual Program modify 4
Delete all programs 5
Language modify 6
Press
Parameter Check with Enter
to continue
↵↵↵↵
↵↵↵↵
> or
.
Select 1
, Password modify. Enter a new password, Press <
↵↵↵↵
>. The default password
for a new analyzer is 1 2 3 4.
Enter Password:
Press
to continue.
↵↵↵↵
3-2 EN October 2001
Page 31

KC4 ∆∆∆∆™
3
General Operation
3.5 Program Modification
Selecting the tests to be available for each program
The programs ( Routine, Emergency, Individual) are modified by selecting tests from the
keypad to form a desired group.
From the Main Menu screen, select 3
Selection screen, select:
Modify the Routine Program: This function allows the operator to select the tests
2
available to be run in the Routine Program. The tests are selected by pressing keys
(INR, APTT, FIB, etc.) and confirming with <
<ESC>. Upon termination, the previously set values will remain valid. In this
program, the analyzer will allow a sequential operation of tests (Batch – mode).
Modify the Emergency Program: This function allows the operator to select the
3
tests available to be run in the Emergency Program. The tests are selected by
pressing keys (INR, APTT, FIB, etc.) and confirming with <
with <ESC>. Upon termination, the previously set values will remain valid. The
analyzer will do a selected profile of tests on a single patient (Patient – Selective).
Modify the Individual program: This function allows the operator to individualize
4
the test menu by sample if desired. The tests in the Individual Program are chosen
directly from the keyboard by pressing keys (INR, APTT, FIB, etc) and confirming
with <
values will remain valid.
5
6
↵↵↵↵
>. Terminating is possible with <ESC>. Upon termination, the previously set
To delete all programs.
Language Modify
Parameter Check with Enter
Program Selection. From the Program
↵↵↵↵
>. Terminating is possible with
↵↵↵↵
>. Terminating is possible
WARNING: All tests
one keystroke. A complete new installation of programs is necessary if all
programs are deleted.
See Section 4 for a more detailed description of Program Modification.
in the programs and all test parameters
can be deleted with
October 2001 3-3 EN
Page 32

3
3.6 Reagent Handling
KC4 ∆∆∆∆™
General Operation
Reagents for the appropriate test are prepared according to the manufacturer’s
instructions. Refer to the manufacturer’s reagent application for specific instructions
on preparation and handling of reagents. Any reagent requiring pre-heating should be
placed into a 15-mm tube and inserted into the reagent incubation well. The fluid level
in the tube should not be above the top edge of the incubation well. A minimum of
15 minutes will be required to warm reagent to 37°C±0.5°C. (Note: Reagents direct
from the refrigerator may take slightly longer to warm up.) All reagents should be
used before the expiration date listed by the manufacturer for each reagent. Do not
place any opened reagent bottles on the instrument.
3.7 Cuvette Preparation
Tetravettes or micro cuvettes are placed in racks in the unheated preparation area.
Up to twelve (12) cuvettes can be placed on the instrument. The exact size and surface
quality of the cuvettes is critical to the proper performance of testing. Absolute
cleanliness of cuvettes is mandatory to ensure correct performance. The cuvettes are
intended as one-time use items. Rewashing cuvettes is not recommended. The
performance of substitute cuvettes cannot be guaranteed; therefore, substitute
cuvettes should not be used.
Tetravettes are cuvettes in pre-packaged groups of 4. They contain a stainless steel ball
and can go directly into a loading rack and onto the instrument.
The stainless steel balls are manufactured from a special stainless steel. Purity, weight,
size, surface quality and magnetic characteristics of the balls are all critical to the
proper performance of testing. The balls, which are manufactured by Sigma/Amelung
have been tested to ensure compatibility with the instrument measurement process
and that they are inert when used with plasma and coagulation reagents. Rust, slight
impurities or oil residue can have an adverse effect on coagulation testing results.
The balls are intended as one-time use items. Recycling balls is not recommended.
The performance of substitute balls cannot be guaranteed; therefore, substitute balls
should not be used. The ball dispenser used in conjunction with the micro cuvettes,
has been designed to facilitate transfer of balls into the cuvettes.
3-4 EN October 2001
Page 33

KC4 ∆∆∆∆™
3
General Operation
3.8 Sample Preparation
Plasma samples and reagents are added with appropriate microliter pipettes. Refer to
the manufacturer’s reagent application to determine the sample and reagent(s) volume
required for each test. Pipetting technique is critical to the performance of testing. Refer
to the Pipetting section for guidelines in proper pipetting technique. Although the use of
a Multipette will facilitate initiation of measurement timing, special pipettes are not
required. If a Multipette is not available, measurement timing can be started using the
start button and channel buttons.
Sample is dispensed into the cuvette. Twelve o’clock is the recommended dispense
position. After sample has been dispensed, close the Plexiglas flap, pick the cuvette
rack up and swirl gently to evenly disperse the sample over the bottom of the cuvettes.
Place the rack with cuvettes in the rotating test positions ensuring that the cuvettes are
pressed firmly down to the bottom. Two additional racks of cuvettes (4 each) may also
have samples pipetted and placed in the reaction incubation wells.
Care must be taken not to over incubate the samples. It is advisable to stagger the time
interval between the cuvette racks in the heated incubation wells and rotating test
positions to prevent over incubation. Allow the samples to pre-incubate for the
recommended time. It will take slightly longer (a minimum of 120 seconds) for a sample
that has been stored at refrigerator temperature (2–8°C) to reach 37°C than for samples
stored at room temperature (18–26°C). Several of the coagulation factors, (Factors V,
VIII, XIII, and fibrinogen) are labile at 37°C. To avoid loss of these factors, samples
should not be pre-incubated longer than 5 minutes.
Timing is critical in coagulation testing and the reagent manufacturer’s guidelines for
incubation times should be followed. Any particulate reagent must be well mixed prior
to use. For those tests, having more than one reagent, all incubations prior to
measurement start can be accomplished in the reaction incubation wells. Nine o’clock
is the recommended first-reagent dispense position.
Care must be taken to avoid contact of the reagent pipette tip with previously dispensed
sample. After addition of reagent, close the Plexiglas lid, pick up the cuvette rack, and
swirl gently 5–6 times to mix the reagent and previously pipetted sample. The reaction
mixture should be evenly dispersed around the channel at the bottom of the cuvette. No
more cuvettes should be prepared for testing than can be completed within the
specified guidelines.
In most instances, addition of a start reagent begins the coagulation process. It is
important that the Measurement Timer or Multipette be started simultaneously to
the addition of the start reagent in order to ensure accuracy and precision of the assay.
Test measurement timing can be started either manually using the Channel Key or
automatically by using the Multipette fitted with a starter cable. Use of a Multipette will
ensure that the reagent addition starts measurement timing. Directions for both
manual start and automatic start are described below.
The location of the start reagent dispense is important. To ensure that mixing of start
reagent with the previously pipetted sample or sample/reagent mixture begins
immediately, the start reagent should be dispensed just to the right side of the ball.
This is best accomplished by holding the pipette angled obliquely from the right rear
side of the cuvette towards the ball position and dispensing the reagent just to the right
October 2001 3-5 EN
Page 34

KC4 ∆∆∆∆™
3
General Operation
of the ball. Care must be taken to avoid splashing the reagent out of the cuvette. The
dispense rate should be of moderate speed and forcefulness.
3.9 Pipetting
The overall performance of the KC4∆™ Coagulation Analyzer is dependent on the
accuracy and precision of pipetting both sample and reagent(s).
Testing can be performed with either standard microliter pipette(s) or with the
Multipette fitted with a starting cable. When the Multipette is used to dispense the final
start reagent, the timer is automatically started simultaneously with reagent dispense.
When a standard microliter pipette is used for addition of the final start reagent, the
timer is started manually using the Specific Channel Key.
Regardless of what kind of pipette is used, the care taken with pipetting is directly
proportional to the overall accuracy and precision of testing.
To avoid contamination of reagents, if the same pipette is being used for both sample
and reagent, a new tip must be used when transitioning between sample and reagent.
To avoid cross-contamination between samples, a new tip should be used for each
sample, whether running plasma or whole blood samples.
Pipetting Technique for Non-Repeating Pipettes
To fill the pipette tip: Depress the button to the first stop. With the button depressed,
insert the tip into the sample or reagent to a depth of approximately 2–3 mm. If
pipetting plasma directly from a centrifuged tube of blood, the tip should be kept well
away from the blood/plasma interface. This will assure that no red cells or platelets will
be aspirated into the tip. If pipetting a particulate reagent, the reagent should be well
mixed prior to aspiration.
Release the button slowly in such a manner that the sample or reagent flows smoothly
into the pipette tip. Slow aspiration will assure that the volume aspirated into the tip is
accurate. If the button is allowed to snap back, an incorrect volume may be aspirated.
In addition, sample or reagent can be aspirated into the barrel of the pipette. This can
result in contamination of subsequent samples or reagents. Unless the pipette is
dismantled and cleaned, inadvertent aspiration into the pipette barrel will result in
eventual obstruction and incorrect operation of the pipette.
Once the tip is filled, no dripping should be observed. If dripping is observed, either the
tip is not seated correctly on the pipette or the pipette requires maintenance. In such a
circumstance, replace the tip. If this does not correct the problem, the pipette should
not be used until maintenance can be performed.
Pipetting Technique for Multipettes
Only pipette tips recommended for use with the pipette should be used. Any pipette tip
whose insertion opening is out-of-round should be discarded. Any pipette tips that are
bent or otherwise damaged should be discarded. The tip opening must not be occluded.
Slide the filling lever down until it stops, then raise the locking clamp upward.
Insert the combitip until it clicks into position. Be sure the combitip plunger is fully
inserted into the barrel before attaching it to the Multipette. Be sure the filling lever is
completely down and then lower the locking clamp to secure the combitip in place.
3-6 EN October 2001
Page 35

KC4 ∆∆∆∆™
3
General Operation
Verify that the volume selection dial is set to dispense the correct volume. Immerse the
combitip cone into the liquid. Fill by slowly sliding the filling lever upward. Wipe the
combitip cone with a lint-free tissue. Follow the guidelines for correct dispense
positions described in the following sections.
3.10 To Dispense Sample
Sample should be dispensed to the 12 o'clock
position of the cuvette (see diagram). Aim the
pipette to the 12 o'clock position. Position the
tip approximately 3–4 mm above the bottom
of the cuvette. Depress the pipette button to
the first stop and hold for 1–2 seconds to
allow the residual contents of the tip to
collect at the bottom of the tip. Press the
button to the second stop. This will deliver
any residual sample into the cuvette. To
avoid bubbling and splattering, the tip
should not be placed so close to the bottom
that at the completion of pipetting the tip is
submersed in the dispensed sample. An
alternative method is to touch the tip to the
cuvette sidewall approximately 3–4 mm
above the bottom of the cuvette and then
depress the button slowly to the first stop.
Wait 1–2 seconds and press the button to the
second stop position. Sample should not be
dispensed by touching the tip to the upper
sidewalls of the cuvette. Any sample that is
left on the upper walls will not participate in
the coagulation reaction.
October 2001 3-7 EN
Page 36

KC4 ∆∆∆∆™
3
General Operation
3.11 To Dispense First Reagent
For those tests utilizing more than one reagent, the first reagent should be dispensed to
the 9 o'clock position of the cuvette (see diagram). Aim the pipette to the 9 o'clock
position. Position the tip approximately 3–4 mm above the bottom of the cuvette.
Depress the pipette button to the first stop and hold for 1–2 seconds to allow the
residual contents of the tip to collect at the bottom of the tip. Press the button to the
second stop. This will deliver any residual reagent into the cuvette. To avoid bubbling
and splattering, the tip should not be placed so close to the bottom that at the
completion of pipetting the tip is submersed in the dispensed reagent. An alternative
method is to touch the tip to the cuvette sidewall approximately 3–4 mm above the
bottom of the cuvette and then depress the button slowly to the first stop. Wait 1–2
seconds and press the button to the second stop position. To avoid contamination of
reagent during subsequent reagent pipetting, care must be taken to avoid contact of the
tip with the previously dispensed sample.
3-8 EN October 2001
Page 37

KC4 ∆∆∆∆™
3
General Operation
3.12 To Dispense Start Reagent
The Start Reagent is the reagent that, when added, begins the coagulation reaction.
Start Reagent should be dispensed just to the right side of the ball. This positioning
assures that mixing of reagent with the other reaction constituents begins immediately.
Holding the pipette obliquely from the right side, aim the pipette tip to the right side of
the ball. Position the tip approximately 5–6 mm above the ball. Depress the pipette
button to the last stop position. The dispense rate should not be so rapid or forceful
that reagent is splashed out of the cuvette. To avoid contamination of reagent during
subsequent reagent pipetting, care must be taken to avoid contact of the tip with the
previously dispensed sample and/or reagent (see diagram).
October 2001 3-9 EN
Page 38

3
3.13 Selecting a Test to Begin a Run
KC4 ∆∆∆∆™
General Operation
From the Main Menu, Press <
Pat. ID: 333
Position 1234
Inc. Time 0000
Meas. Time 0000
Start Inc.
Ready
The upper left shows the current patient ID and the upper right shows the temperature
of the measuring block in °C.
<MENU> Accesses up Main Menu functions.
<RUN> accesses the Program Menu.
↵↵↵↵
>. The Operating Screen will be displayed.
Operating Screen
37°C
1234
Select 1
Enter the Patient ID, press <
into the Routine Program (see Section 4). For example, if INR, APTT and FIB are in
the Routine Program, then the first test to be performed will be INR. INR will
appear in the upper right corner of the Operating Screen (replacing the
temperature). To scroll to the next test in the program (ie. APTT), press <
the number of samples per rack, press <
will appear in the upper right corner of the Operating Screen. To end the program,
press <RUN>.
Select 2
programmed in the Emergency Program will appear in the upper right corner of the
Operating Screen. Only one sample at a time can be run in the Emergency
Program. At the end of the first test, the result will be displayed. Record the result,
Press <
<RUN>.
Select 3
Individual Program will be displayed (see section 4). Select a test by pressing the
test Function Key. For example, INR, APTT and FIB are displayed on the grid. To
begin INR testing, press the INR key. Press <
press <
corner, and the Patient ID will be displayed in the upper left corner of the Operating
Screen.
Routine Program. Select number of samples per rack, press <
↵
↵
>. The analyzer will display the first test programmed
↵ ↵
↵
↵
>. The next test in the program (APTT),
↵ ↵
↵
Emergency Program. Enter Patient ID, press <
↵
↵
> to scroll to the next test in the program. To end the program, press
↵ ↵
Individual Program. A grid of available tests programmed into the
↵
↵
>. Enter number of samples per rack,
↵ ↵
↵
↵
>; enter Patient ID, press <
↵ ↵
↵
↵
>. INR will be displayed in the upper right
↵ ↵
↵
>. The first test
↵ ↵
↵↵↵↵
>.
↵
↵
>. Enter
↵ ↵
3-10 EN October 2001
Page 39

KC4 ∆∆∆∆™
3
General Operation
Note: When entering the number of samples per rack: if testing in duplicate, Max
would be 2 and the choice of 1 pair or 2 pair per rack would be made. It is
recommended that samples be analyzed in duplicate.
Samples per rack
Count: 2
Max = 2
3.14 Patient Identification
After the number of samples per rack has been entered, the analyzer will ask for a
Patient ID number. Press 1
increment up from this number. Press 2
be analyzed.
Note: This screen must be re-accessed to enter subsequent Patient ID
numbers. To do this, press the RUN key, select 3
Press the appropriate test key. Theer the number of samples per rack
(2 when analyzing in duplicate); select 2
to enter a Patient ID. The instrument will automatically
to enter a specific Patient ID for the samples to
Individual Program.
for manual Patient ID entry.
ROUTINE PROGRAM
Patient ID:
Start Patient ID Modify 1
Manual Patient ID 2
↵↵↵↵
Press
to Continue
3.15 Testing
1. With the transfer of the first rack to the rotating wells, Press Incubation timer:
Operating Screen
Patient ID: 333 INR
Position 1234
Inc. Time 0000
Meas. Time 0.0 0.0 0.0 0.0
Start Inc. *** ***
READY
2. Press Channel timer for each sample to be tested.
October 2001 3-11 EN
Page 40

KC4 ∆∆∆∆™
3
General Operation
3. Each channel will begin the incubation countdown. An audible beep will occur
when 5 seconds remain.
4. Press and hold down the <START> key and begin dispensing the start reagent.
3.16 Manual Start Procedure
Install a pipette tip onto an appropriately sized pipette.
Mix particulate reagent by covering the tube with Parafilm® and inverting gently. Do
not shake. Many coagulation reagents are mixtures of lipids, which will undergo spatial
rearrangement when shaken.
Aspirate reagent into the pipette tip. Dispense one aliquot of reagent into reagent
container to prime the pipette tip.
Press the <START> key.
Dispense reagent just to the right side of the ball and simultaneously press the Specific
Channel Key .
Note: Measurement can be started automatically if the reagent is dispensed with
enough force to move the ball from its steady position. This is best
accomplished by dispensing the reagent directly onto the right side of the
ball. This dispense should not be so forceful that any of the reaction
mixture is splashed out of the cuvette. This is not as reliable a way to start
timing. It is particularly unreliable when dispensing a volume less than
100 µl.
3.17 Automatic Start Procedure
The KC4∆ Multipette is recommended for use in the Automatic Start Procedure.
Assure that the Multipette cable is plugged into the Automatic Pipette socket located on
the rear of the KC4
Install appropriately sized pipette tip.
Mix particulate reagent by covering the tube with Parafilm® and inverting it gently. Do
not shake. Many coagulation reagents are mixtures of lipids, which will undergo spatial
rearrangement when shaken. The reactivity of such reagents is dependent on the
specific lipid arrangement.
Aspirate start reagent into the pipette tip. Dispense one aliquot of reagent into reagent
container to prime the pipette tip.
∆
Coagulation Analyzer.
Press the <START> key.
Fully depress the pipetting lever to dispense reagent. This is best accomplished by
holding the pipette angled obliquely from the right rear side and dispensing the reagent
just to the right side of the ball. Measurement timing is started automatically.
Return the pipette to the heated pipette tube holder. Note: Replace the pipette tube
holder sleeves daily and for each new reagent. Pipette tube holder sleeves should be
treated as biohazardous waste.
3-12 EN October 2001
Page 41

KC4 ∆∆∆∆™
3
General Operation
Note: To increase speed when using an automatic multipette with starting cable,
simple keep the Start Key of the first channel depressed while pipetting.
Thus, when channel 1 is pipetted and the timer for channel 1 is started, the
timer for channel 2 is activated. When channel 2 is pipetted, its timer
begins and channel 3’s timer is activated, etc.
With the addition of start-reagent, initiation of the coagulation process begins. As
coagulation takes place, fibrinogen polymerizes to form strands of fibrin. The formed
fibrin sweeps the ball out of its steady position, which triggers an impulse in the
magnetic sensor. This impulse electronically stops the timer signifying the end of
measurement time. The elapsed time will be displayed in seconds and tenths of
seconds.
3.18 Printing Results
1. The printer will immediately print out the results of the first test. The second test
results will be printed after <
Individual Program: INR
Date : 25 Nov 2001
Time : 01:11
Patient ID : 25
Measured Values:
Value 1 : 12,4 sec
Value 2 : 12,1 sec
Average Value : 12,25 sec
Difference : 2,4 %
↵↵↵↵
> is pressed.
Result : 1,02
Individual Program: INR
Date: 25 Nov 2001
Time: 01:12
Patient ID: 26
Measured Values:
Value 1 : 12,0 sec
Value 2 : 12,3 sec
Average Value : 12,15 sec
Difference : 2,4 %
Result : 1,01
Individual Program: INR
Date: 25 Nov 2001
Time: 01:14
Patient ID: 27
Measured Values:
Val ue 1 : 11 , 5 s ec
Val ue 2 : 11 , 2 s ec
Average Value : 11,35 sec
Difference : 2,6 %
Result : 0,93
October 2001 3-13 EN
Page 42

Page 43

KC4 ∆∆∆∆™
4
Mode Programming
4Mode Programming
Contents
4.1 ROUTINE PROGRAMMING...................................................................... 4-1
4.2 EMERGENCY PROGRAMMING................................................................ 4-2
4.3 INDIVIDUAL PROGRAMMING ................................................................. 4-3
October 2001 4-0 EN
Page 44

Page 45

KC4 ∆∆∆∆™
Mode Programming
4.1 Routine Programming
From the MAIN MENU:
Select 3
Enter Password, press
ENTER <
, Program Selection.
↵↵↵↵
>.
Main Menu
S I G M A - A M E L U N G
KC 4 DELTA
DATE: 12 Sep 2001 1
TIME: 10 : 08 2
PROGRAM SELECTION 3
PRINT PARAMETERS 4
Password Screen
Enter Password:
Press
to continue.
↵↵↵↵
4
Press 2
, Routine Program Modify.
Select from the keypad all tests
desired for this group. When
complete, press ENTER <
↵↵↵↵
>.
Tests programmed will cycle,
allowing parameter changes to be
made.
See Section 5 regarding
programming tests.
Modify Screen
Password Modify 1
Routine Program Modify 2
Emergency Program Modify 3
Individual Program Modify 4
Delete all programs 5
Language Modify 6
Parameter Check to Enter
Routine Program
Tests
In Routine Program
INR APTT FIB
New entry press ENTER to store
Press ESC to cancel
Programming Screen
Password Modify 1
Routine Program Modify 2
Emergency Program Modify 3
Individual Program Modify 4
Delete all programs 5
Press ENTER to continue
October 2001 4-1 EN
Page 46

4
4.2 Emergency Programming
S I G M A - A M E L U N G
From the MAIN MENU:
Press 3
Enter Password, press ENTER <
to continue.
, Program Selection.
DATE: 12 Sep 2001 1
TIME: 10 : 08 2
PROGRAM SELECTION 3
PRINT PARAMETERS 4
↵↵↵↵
>
Enter Password:
KC4 ∆∆∆∆™
Mode Programming
Main menu
KC 4 DELTA
Password Screen
Press 3
, Emergency Program
Modify.
Choose tests from the keypad for
this group. When complete, press
ENTER <
↵↵↵↵
>.
Tests will cycle allowing for
Parameter changes to be made.
See Section 5 for programming
changes.
Press
to continue.
↵↵↵↵
Modify Screen
Password Modify 1
Routine Program Modify 2
Emergency Program Modify 3
Individual Program Modify 4
Delete all programs 5
Press ENTER to continue
Emergency Screen
Tests
In Emergency program
INR APTT FIB
New entry press ENTER to store
Press ESC to cancel
Programming Screen
S I G M A - A M E L U N G
KC 4 DELTA
DATE: 12 Sep 2001 1
TIME: 10 : 08 2
PROGRAM SELECTION 3
PRINT PARAMETERS 4
4-2 EN October 2001
Page 47

KC4 ∆∆∆∆™
Mode Programming
4.3 Individual Programming
S I G M A - A M E L U N G
From the MAIN MENU:
Press 3
Enter Password, press ENTER<
, Program Selection.
↵↵↵↵
>.
DATE: 12 Sep 2001 1
TIME: 10 : 08 2
PROGRAM SELECTION 3
PRINT PARAMETERS 4
Enter Password:
Press
Main Menu
KC 4 DELTA
Password Screen
to continue.
↵↵↵↵
4
Press 4
, Individual Program Modify
Choose tests from the keypad for this
group. When complete, press
ENTER <
When complete, press ENTER <
↵↵↵↵
>.
↵↵↵↵
>.
Tests will cycle for parameter
changes.
See Section 5 for programming
information.
Modify Screen
Password Modify 1
Routine Program Modify 2
Emergency Program Modify 3
Individual Program Modify 4
Delete all programs 5
Press ENTER to continue
Individual Screen
Tests
In Individual Programming
INR APTT FIB
New entry press ENTER to store
Press ESC to cancel
Programming Screen
Password Modify 1
Routine Program Modify 2
Emergency Program Modify 3
Individual Program Modify 4
Delete all programs 5
Press ENTER to continue
October 2001 4-3 EN
Page 48

Page 49

KC4 ∆∆∆∆™
5
Test Programming
5Test Programming
Contents
5.1 INR........................................................................................................ 5-1
5.2 INR FLOW CHART.................................................................................. 5-3
5.3 PROTHROMBIN TIME (PERCENT ACTIVITY CURVE)............................... 5-4
5.4 PT (PERCENT ACTIVITY) FLOW CHART.................................................. 5-7
5.5 RATIO FLOW CHART ............................................................................. 5-8
5.6 ACTIVATED PARTIAL THROMBOPLASTIN TIME..................................... 5-9
5.7 APTT FLOW CHART ..............................................................................5-11
5.8 FIBRINOGEN ........................................................................................5-12
5.9 FIBRINOGEN FLOW CHART ..................................................................5-15
5.10 FACTORS..............................................................................................5-16
5.11 FACTORS FLOW CHART .......................................................................5-19
5.12 STATS ..................................................................................................5-20
October 2001 5-0 EN
Page 50

Page 51

KC4 ∆∆∆∆™
5
Test Programming
5.1 INR
The INR (International Normalized Ratio) is the preferred method for reporting
Prothrombin Times in the United States. The P T percent activity curve and ratio are
preferred in Europe.
Allow the instrument to reach 37°C prior to testing.
S I G M A - A M E L U N G
KC 4 DELTA
DATE: 12 Sep 2001 1
TIME: 10 : 08 2
PROGRAM SELECTION 3
PRINT PARAMETERS 4
Prior to testing, a Test Program or profile needs to be established. The three programs
available are discussed in Section 4. The Individual Program will be used here.
At the MAIN MENU,
1. Press 3
2. Enter 4
3. Press <
, Program Selection.
digit Password:_ _ _ _;
↵↵↵↵
>.
4. Select Individual Program Modify 4
5. Choose tests from the keypad.
6. Press <
↵↵↵↵
> to store.
Enter Password:
Press
:
Password Modify 1
Routine Program Modify 2
Emergency Program Modify 3
Individual Program Modify 4
Delete all programs 5
Tests
In individual program
New entry press ENTER
Press ESC to cancel
to continue.
↵↵↵↵
Press ENTER to continue
INR
to store
↵↵↵↵
October 2001 5-1 EN
Page 52

5
KC4 ∆∆∆∆™
Test Programming
7. Select Yes (1) to change settings or
select No (2) to leave as programmed.
8. To test in duplicate, select Yes (1)
or No (2) for single testing, press <
↵↵↵↵
>.
9. Enter the maximum difference allowed,
where:
Max difference (%) = (difference
between values divided
by the mean value) x 100
Program INR:
Incubation Time (sec) :
Default Value :
ISI-Value :
Max. Difference (%) :
Modify? Yes 1 No 2
INR – Program
Double Test?
Yes 1 N o 2
Max. Difference (%) :
to continue
↵↵↵↵
Press
Max. Difference (%): 10
New entry press
Press ESC to continue
to store
↵↵↵↵
10. Program the incubation time referenced
in the test application.
11. Default Value is the PT Mean Normal*
12. ISI Value for the thromboplastin in
use.
13. Max. Difference as determined above.
14. Select No (2) when modification is
complete. The cycle will return to the
Incubation Time (sec) : 60
Default Value : 12,2
ISI-Value : 1.78
Max. Difference (%) : 10
Modify? Yes 1 No 2
Program INR:
MAIN MENU when all modifications
are complete.
The KC4
∆
™ will automatically calculate the INR using the programmed values entered
for the mean of the Reference Range and the ISI value for the thromboplastin in use.
* Prior to entering values, a reference range should be determined for the lot number of
thromboplastin that will be used. Because PT reference ranges can vary between
laboratories and between different reagent formulations, determination of the reference
range on the population being served is recommended. For accurate INR reporting, the
determination of the geometric mean is recommended. When the geometric mean has
been determined, values may be programmed into the KC4
∆
™.
5-2 EN October 2001
Page 53

KC4 ∆∆∆∆™
Test Programming
5.2 INR Flow Chart
5
Program INR:
Incubation Time (sec) :
Default Value :
ISI-Value :
Max. Difference (%) :
Modify? No 2
Yes 1
INR – Program
Double Test?
Yes 1 No 2
Max. Difference (%) :
to continue
↵
Press ↵
↵↵
Program INR
Incubation time (sec): ?
New entry press
Press ESC to continue
Program INR
Default value:
New entry press
Press ESC to continue
Program INR
ISI-Value:
to store
↵↵↵↵
to store
↵↵↵↵
Max. Difference (%): 10
New entry press
Press ESC to continue
INR – Program
Double Test?
Yes 1 No 2
Max. Difference (%) :
to continue
↵↵↵↵
Press
to store
↵↵↵↵
New entry press
Press ESC to continue
Program INR:
Incubation Time (sec) : 60
Default Value : 12,2
ISI-Value : 1.78
Max. Difference (%) : 10
Modify? Yes 1 No 2
to store
↵↵↵↵
NEXT TEST
October 2001 5-3 EN
Page 54

KC4 ∆∆∆∆™
5
Test Programming
5.3 Prothrombin Time (Percent Activity Curve)
Allow the instrument to reach 37°C prior to testing.
S I G M A - A M E L U N G
KC 4 DELTA
DATE: 12 Sep 2001 1
TIME: 10 : 08 2
PROGRAM SELECTION 3
PRINT PARAMETERS 4
Prior to testing, a Test Program or profile needs to be established. The three programs
available are discussed in Section 4. The Individual Program will be used here.
At the MAIN MENU:
1. Press 3
2. Enter 4
3. Press <
, Program Selection.
digit Password:_ _ _ _;
↵↵↵↵
>.
4. Select Individual Program Modify 4
5. Choose tests from the keypad.
6. Press <
↵↵↵↵
> to store.
Enter Password:
Press
.
Password Modify 1
Routine Program Modify 2
Emergency Program Modify 3
Individual Program Modify 4
Delete all programs 5
Tests
In individual program
New entry press ENTER
Press ESC to cancel
to continue.
↵↵↵↵
Press ENTER to continue
PT
to store
↵↵↵↵
5-4 EN October 2001
Page 55

KC4 ∆∆∆∆™
Test Programming
5
7. Select Yes (1 ) to change settings or select No (2)
to leave as programmed.
8. Press Yes ( 1) if testing is to be done in duplicate,
or No (2) if in single.
Program PT:
Incubation Time (sec) : ?
Calibration Curve PT : ?
Point 1 (%) : ?
(sec) : ?
Point 2 (%) : ?
(sec) : ?
Point 3 (%) : ?
(sec) : ?
PT MAX % : ?
Difference Check PT : No
Modify? Yes 1 No 2
PT – Program
Double Test?
Yes 1 No 2
Max. Difference (%) :
to continue (more
↵↵↵↵
Press
parameters)
9. Enter the maximum difference allowed, where:
Max difference (%) = (difference between
values divided by the mean value) x 100
10. Program the incubation time referenced in the
test application.
11. Enter the reference value of the PT calibration
curve. This is the first point, the highest point of
the curve. This does not have to be 100%, it may
vary depending on the source of the reference
plasma.
12. Enter the clotting time in seconds of the first
point of the PT calibration curve.
Max. Difference (%): ?
New entry press
Press ESC to continue
Program PT:
Incubation Time (sec) : 60
Calibration Curve PT : ?
Point 1 (%) : 100
Point 2 (%) :
Point 3 (%) :
PT MAX % :
Max. Difference (%) : 10
Modify? Yes 1 No 2
to store
↵↵↵↵
(sec) : 12
(sec) :
(sec) :
October 2001 5-5 EN
Page 56

5
KC4 ∆∆∆∆™
Test Programming
13. Enter the second value of the P T calibration
curve. The second point, the middle point of the
curve is a dilution of the reference plasma.
Example: 30% is used.
14. Enter the clotting time in seconds for the middle
point of the PT calibration curve.
15. Enter the third value of the PT calibration curve.
The third point, the lowest point of the curve is a
dilution of the reference plasma.
Example: 12.5% is used:
16. Enter the clotting time in seconds of the third
point of the PT calibration curve.
17. Enter the Maximum % P T value.
Values exceeding maximum value
entered are not printed out.
Example: 130% is used.
Program PT:
Incubation Time (sec) : 60
Calibration Curve PT : ?
Point 1 (%) : 100
(sec) : 12
Point 2 (%) : 30
(sec) : 27
Point 3 (%) :
(sec) :
PT MAX % :
Max. Difference (%) : 10
Modify? Yes 1 No 2
Program PT:
Incubation Time (sec) : 60
Calibration Curve PT : ?
Point 1 (%) : 100
(sec) : 12
Point 2 (%) : 30
(sec) : 27
Point 3 (%) : 12.5
(sec) : 62
PT MAX % : 130
Max. Difference (%) : 10
Modify? Yes 1 No 2
18. Select No (2), when modification is complete. The cycle will return to the MAIN
MENU when all modifications are complete.
5-6 EN October 2001
Page 57

KC4 ∆∆∆∆™
Test Programming
5.4 PT (Percent Activity) Flow Chart
5
Program PT:
Incubation Time (sec) : ?
Calibration Curve PT : ?
Point 1 (%) : ?
(sec) : ?
Point 2 (%) : ?
(sec) : ?
Point 3 (%) : ?
(sec) : ?
PT MAX % : ?
Difference Check PT : No
Modify? Yes 1
PT – Program
Double Test?
Yes 1 No 2
Max. Difference (%)
to continue
Press
↵↵↵↵
No 2
NEXT TEST
Program PT:
Incubation Time (sec) : 60
Calibration Curve PT : ?
Point 1 (%) : 100
(sec) : 12
Point 2 (%) : 30
(sec) : 27
Point 3 (%) : 12.5
(sec) : 62
PT MAX % : 130
Max. Difference (%) : 10
Modify? Yes 1
No 2
NEXT TEST
Max. Difference (%) ?
New entry press
Press ESC to continue
PT – Program
Double Test?
Yes 1 No 2
Difference Check: No
to continue
Press
↵↵↵↵
to store
↵↵↵↵
October 2001 5-7 EN
Page 58

5
5.5 Ratio Flow Chart
KC4 ∆∆∆∆™
Test Programming
PROGRAM RATIO:
Incubation time (sec): 0
Default Value: 0,0
Max. Difference (%): ?
RATIO: No
Modify? Yes 1 No 2
RATIO – Program
Double Test?
Yes 1 No 2
Max. Difference (%): ?
to continue
↵↵↵↵
Press
Press ESC to continue
PROGRAM RATIO:
Incubation time (sec): 0
Default Value: 0,0
Difference Check RATIO: No
Modify? Yes 1 No 2
Program RATIO
Incubation time (sec): 0
New entry press
Press ESC to continue
Program RATIO
Default Value:
to store
↵↵↵↵
Max. Difference (%):
New entry press
Press ESC to continue
RATIO – Program
Double Test?
Yes 1 No 2
Max. Difference (%):
to continue
Press
↵↵↵↵
to store
↵↵↵↵
New entry press
Press ESC to continue
PROGRAM RATIO:
Incubation time (sec): 0
Default Value: 0,0
Max. Difference (%): 5%
RATIO: No
Modify? Yes 1 No 2
to store
↵↵↵↵
NEXT TEST
5-8 EN October 2001
Page 59

KC4 ∆∆∆∆™
5
Test Programming
5.6 Activated Partial Thromboplastin Time
Allow the instrument to reach 37°C prior to testing.
S I G M A - A M E L U N G
KC 4 DELTA
DATE: 12 Sep 2001 1
TIME: 10 : 08 2
PROGRAM SELECTION 3
PRINT PARAMETERS 4
Prior to testing, a Test Program or profile needs to be established. The three programs
available are discussed in Section 4. The Individual Program will be used here.
At the MAIN MENU:
1. Press 3
, Program Selection.
2. Enter 4 digit Password:_ _ _ _;
3. Press <
↵↵↵↵
>.
4. Press Individual Program Modify 4
5. Choose tests from the keypad.
6. Press <
↵↵↵↵
>.
Enter Password:
Press
.
Password Modify 1
Routine Program Modify 2
Emergency Program Modify 3
Individual Program Modify 4
Delete all programs 5
Tests
In individual program
New entry press ENTER
Press ESC to cancel
to continue.
↵↵↵↵
Press ENTER to continue
APTT
to store
↵↵↵↵
7. Select Yes (1) to change settings or
select No (2) to leave as programmed.
Program – PTT
Incubation time (sec): 240
Max. Difference (%): ?
Modify? Yes 1 No 2
October 2001 5-9 EN
Page 60

5
KC4 ∆∆∆∆™
Test Programming
8. To test in duplicate, select Yes (1) or
Select No (2) to test in single.
9. Enter the maximum value allowed,
PTT – program
Double Test?
No 2
Yes 1
Max. Difference (%): ?
to continue
Press
↵↵↵↵
Max. Difference (%): 10
where:
Max difference (%) = (difference between
values divided by the Mean value) x 100.
New entry press
Press ESC to continue
to store
↵↵↵↵
10. Program the incubation time referenced in the test application.
11. Select No (2) when modifications are complete. The cycle will return to the Main
Menu when all modifications are complete.
5-10 EN October 2001
Page 61

KC4 ∆∆∆∆™
Test Programming
5.7 APTT Flow Chart
5
Program – PTT
Incubation time(sec): ?
Max. Difference(%): ?
Modify? Yes
PTT – program
Double Test?
Yes 1 No 2
Max. Difference(%): ?
Press
1No 2
to continue
↵↵↵↵
Program – PTT
Incubation time (sec): 240
Max. Difference (%): 10
Modify? Yes 1 No 2
Program – PTT
Incubation time (sec): 240
Max. Difference (sec): 10
Modify? Yes 1 No 2
Max. Difference(%): ?
New entry press
Press ESC to continue
PTT – program
Double Test?
Yes 1 No 2
Difference Check: No
to continue
↵↵↵↵
Press
to store
↵↵↵↵
Next Test
October 2001 5-11 EN
Page 62

KC4 ∆∆∆∆™
5
Test Programming
5.8 FIBRINOGEN
Prior to programming Fibrinogen, the concentration of the calibration curve points and
the clotting time for each point must be known.
Allow the instrument to reach 37°C prior to testing.
S I G M A - A M E L U N G
KC 4 DELTA
DATE: 12 Sep 2001 1
TIME: 10 : 08 2
PROGRAM SELECTION 3
PRINT PARAMETERS 4
Prior to testing, the test must be programmed into a testing format as discussed in
Section 4. The Individual Program will be used here.
At the MAIN MENU:
1. Press 3
, for Program Selection.
2. Enter 4 digit Password:_ _ _ _.
3. Press <
↵↵↵↵
>.
4. Press Individual Program Modify 4
5. Choose tests from the keypad.
6. Press <
↵↵↵↵
>.
Enter Password:
Press
.
Password Modify 1
Routine Program Modify 2
Emergency Program Modify 3
Individual Program Modify 4
Delete all programs 5
Tests
In individual program
New entry press ENTER
Press ESC to cancel
to continue.
↵↵↵↵
Press ENTER to continue
FIB
to store
↵↵↵↵
5-12 EN October 2001
Page 63

KC4 ∆∆∆∆™
Test Programming
5
7. To modify, select Yes (1) to change or, No (2) to
test in leave as programmed.
8. Press Yes ( 1) if testing is to be done in duplicate
or Press No (2) if in single.
Program – FIB:
Incubation Time (sec) :
Calibration Curve FIB:
Point 1 (G/L) :
(sec) :
Point 2 (G/L) :
(sec) :
Point 3 (G/L) :
(sec) :
Dilution 1:5
Max. Difference (%) :
Modify? Yes 1 No 2
FIB – Program
Double Test?
Yes 1 No 2
Max. Difference (%) :
to continue
Press
↵↵↵↵
9. Enter the maximum difference allowed, where:
Max difference (%) = (difference between values
divided by the Mean value) x 100
10. Press <
↵↵↵↵
> to continue changing parameters.
Max. Difference (%):
New entry press
Press ESC to continue
FIB – Program
Double Test?
Yes 1 No 2
Max. Difference (%) :
to continue
↵↵↵↵
Press
to store
↵↵↵↵
October 2001 5-13 EN
Page 64

5
Test Programming
11. Choose the incubation time referenced in the test application.
KC4 ∆∆∆∆™
12. Enter the reference value of the
Fibrinogen reference plasma in G/L
for the first point of the curve. This
can vary depending on the source
of the reference plasma. When
using the KC4
∆
™ Coagulation
Analyzer, care should be taken that
the clot time of the highest point is
more than the minimum read time
(4.5 sec.) of the instrument. This
can be achieved by utilizing the
optimal dilutions for the reference
plasma referred to in the reagent
applications.
Program – FIB:
Incubation Time (sec) :
Calibration Curve FIB:
Point 1 (G/L) :
(sec) :
Point 2 (G/L) :
(sec) :
Point 3 (G/L) :
(sec) :
Dilution 1:5
Max. Difference (%) :
Modify? Yes 1 No 2
Program – FIB:
Incubation Time (sec) :
Calibration Curve FIB:
Point 4 (G/L) :
(sec) :
Point 5 (G/L) :
(sec) :
Dilution 1:5
Difference Check FIB : No
Modify? Yes 1 No 2
13. Enter the clotting time in seconds for the first point of the Fibrinogen reference
curve.
14. Enter the second value for the Fibrinogen reference curve. The second point is a
dilution of the reference plasma.
15. Enter the clotting time in seconds for the second point of the Fibrinogen reference
curve.
16. Enter the third value for the Fibrinogen reference curve. The third point is a
dilution of the reference plasma.
17. Enter the clotting time in seconds for the third point of the Fibrinogen reference
curve.
18. Enter the fourth value of the Fibrinogen reference curve. The fourth point is a
dilution of the reference plasma.
19. Enter the clotting time in seconds for the fourth point of the Fibrinogen reference
curve.
20. Enter the fifth value of the Fibrinogen reference curve. The fifth point is a dilution
of the reference plasma.
21. Enter the clotting time in seconds for the fifth point of the Fibrinogen reference
curve.
22. Enter the dilution for the patient or control samples to be analyzed. Fibrinogens
use a 5 point reference curve.
23. Select No (2) when modifications are complete. The cycle will return to the Main
Menu when all modifications are complete.
Note: Suggested dilutions and volumes used are found in the KC4
∆
™ Fibrinogen
Application.
5-14 EN October 2001
Page 65

KC4 ∆∆∆∆™
Test Programming
5.9 Fibrinogen Flow Chart
Program – FIB:
Incubation Time (sec) :
Calibration Curve FIB:
Point 1 (G/L) :
(sec) :
Point 2 (G/L) :
(sec) :
Point 3 (G/L) :
(sec) :
Dilution 1:5
Program FIB
Incubation time (sec):
New entry press
Press ESC to cancel
Program FIB
Calibration curve FIB :
New entry press
Press ESC to cancel
to store
↵↵↵↵
to store
↵↵↵↵
5
Max. Difference (%) :
Modify? Yes 1
FIB – program
Double Test?
Yes 1 No 2
Max. Difference(%):
to continue
Press
↵↵↵↵
Max. Difference (%):
New entry press
Press ESC to continue
FIB – program
Double Test?
Yes 1 No 2
to store
↵↵↵↵
No 2
Program FIB
Point 1 (G/L) :
New entry press
Press ESC to cancel
Continue through all parameters
of the program.
Program – FIB:
Incubation Time (sec) :
Calibration Curve FIB:
Point 1 (G/L) :
(sec) :
Point 2 (G/L) :
(sec) :
Point 3 (G/L) :
(sec) :
Dilution 1:5
to store
↵↵↵↵
Program – FIB:
Incubation Time (sec) :
Calibration Curve FIB:
Point 4 (G/L) :
(sec) :
Point 5 (G/L) :
(sec) :
Dilution 1:5
Difference Check FIB : No
Modify? Yes 1 No 2
Max. Difference(%):
to continue
↵↵↵↵
Press
Difference Check FIB : No
Modify? Yes 1 No 2
NEXT TEST
October 2001 5-15 EN
Page 66

KC4 ∆∆∆∆™
5
Test Programming
5.10 FACTORS
Prior to programming Factors, the concentration of the calibration curve points must be
known and the clotting time for each point must be established.
Allow the instrument to reach 37°C prior to testing.
S I G M A - A M E L U N G
KC 4 DELTA
DATE: 12 Sep 2001 1
TIME: 10 : 08 2
PROGRAM SELECTION 3
PRINT PARAMETERS 4
Prior to testing, a Test Program or profile needs to be established. The three programs
available are discussed in Section 4. The Individual Program will be used here.
At the MAIN MENU:
1. Press 3
, Program Selection.
2. Enter 4 digit Password:_ _ _ _.
3. Press <
↵↵↵↵
>.
4. Select Individual Program Modify 4
5. Choose desired tests from the keypad.
6. Press <
↵↵↵↵
>.
Enter Password:
Press
.
Password Modify 1
Routine Program Modify 2
Emergency Program Modify 3
Individual Program Modify 4
Delete all programs 5
Tests
In individual program
New entry press ENTER
Press ESC to cancel
to continue.
↵↵↵↵
Press ENTER to continue
FAC
to store
↵↵↵↵
5-16 EN October 2001
Page 67

KC4 ∆∆∆∆™
Test Programming
5
7. Select Yes (1) to change settings or select No (2)
to go to the next test.
8. To test in duplicate, select Yes (1) or No (2) for
single.
PROGRAM FAC Factor = 0
Incubation Time (sec) :
Count Points :
Calibration Curve F ?
Point 1 (%) : 0,0
(sec) : 0,0
Point 2 (%) : 0,0
(sec) : 0,0
Point 3 (%) : 0,0
(sec) : 0,0
Modify? Yes 1 No 2 (next test)
FAC – Program
Double Test?
Yes 1 No 2
Max. Difference: ?
to continue
↵↵↵↵
Press
9. Enter the maximum difference allowed, where:
Maximum value (%) = (difference between
values)/mean value x 100.
10. Press <
↵↵↵↵
> to continue.
Max. Difference(%): 0
New entry press
Press ESC to continue
FAC - Program
Double Test?
Yes 1 No 2
Max. Difference: ?
Press
Note: Only one factor curve may be entered at a time.
to continue
↵↵↵↵
to store
↵↵↵↵
October 2001 5-17 EN
Page 68

5
KC4 ∆∆∆∆™
Test Programming
11. Enter the Factor that will be used.
12. Enter an incubation time referenced in the test
application.
13. Choose as many Curve Points (Count Points) as
appropriate for testing requirements (9 points max).
14. Enter the reference value of the Factor calibration
curve. This is the first point, the highest point of the
curve. This does not always have to be 100%, it can
vary depending on the source of the reference plasma.
15. Enter the clotting time in seconds of the first point of
the Factor calibration curve.
16. Continue entering percentage points and seconds to
complete the calibration curve.
17. Select No (2) when modifications are complete. The cycle will return to the Main
Menu when all modifications are complete.
PROGRAM FAC Factor = 0
Incubation Time (sec) :
Count Points :
Calibration Curve F ?
Point 1 (%) : 0,0
(sec) : 0,0
Point 2 (%) : 0,0
(sec) : 0,0
Point 3 (%) : 0,0
(sec) : 0,0
Modify? Yes 1 No 2 (next test)
5-18 EN October 2001
Page 69

KC4 ∆∆∆∆™
Test Programming
5.11 Factors Flow Chart
5
PROGRAM FAC Factor = 0
Incubation Time (sec) :
Count Points :
Calibration Curve F ?
Point 1 (%) : 0,0
(sec) : 0,0
Point 2 (%) : 0,0
(sec) : 0,0
Point 3 (%) : 0,0
(sec) : 0,0
Modify? Yes 1
FAC – Program
Double Test?
Yes 1 No 2
Max. Difference: ?
Press ENTER
Max. Difference (%):
New entry press
Press ESC to continue
No 2 (next test)
to continue
↵↵↵↵
to store
↵↵↵↵
Program FAC
Incubation time (sec):
New entry press
Press ESC to cancel (through remaining
parameters)
After final parameter has been entered.
PROGRAM FAC Factor = 0
Incubation Time (sec) :
Count Points :
Calibration Curve F ?
Point 1 (%) : 0,0
Point 2 (%) : 0,0
Point 3 (%) : 0,0
Modify? Yes 1 No 2
to store
↵↵↵↵
(sec) : 0,0
(sec) : 0,0
(sec) : 0,0
(next test)
FAC – Program
Double Test?
Yes 1 No 2
Difference Check: No
Press ENTER
to continue
↵↵↵↵
NEXT TEST
October 2001 5-19 EN
Page 70

5
Test Programming
5.12 STATS
STATS are programmed in Section 4.2 Emergency Program.
KC4 ∆∆∆∆™
1. From the MAIN MENU or if ROUTINE testing is in progress, press <
the Operating screen:
2. From the keypad press <RUN>.
3. At the Test Selection Screen, choose Emergency Program (2
Operating Screen. If 2 beeps sound, nothing has been programmed; see
Section 4.
↵↵↵↵
4. If multiple tests are grouped in the STAT Mode; press <
in the group.
> to change to other tests
) to bring up the
↵↵↵↵
> to bring up
5-20 EN October 2001
Page 71

KC4 ∆∆∆∆™
6
Quality Control
6Quality Control
Regularly performed quality control is the best monitor of test performance. To assure
that control and unknown sample results are evaluated under the same test conditions,
control material should be included with each run.
The reagent manufacturer's QC recommendations should be used as a guide for
establishing a QC protocol. Control results deviating from established ranges are
indicative of a system failure and should be investigated immediately. The more
common sources of error and the corrective action to take are presented in the
Troubleshooting section, Section 8.
October 2001 6--1 EN
Page 72

Page 73

KC4 ∆∆∆∆™
7
Maintenance
7Maintenance
Introduction
There is no routine mechanical maintenance associated with the KC4∆™. The KC4∆™
was factory calibrated for rotational speed, magnetic sensor strength and temperature.
General housekeeping is the only maintenance that need be performed with any
regularity. Occasional cleaning with a damp paper towel is recommended to remove
accumulated dust or other material. Spills should be cleaned up as they occur.
Reagents can be corrosive and any spillage into the reagent incubation well should be
cleaned up immediately. All sample spills should be considered to have created a
potentially biohazardous environment and should be cleaned up immediately using
appropriate safeguards to avoid personal contamination. If decontamination is
required, wipe area with a paper towel moistened with a mild disinfectant.
Any balls that inadvertently find their way into the bottom of any of the wells can be
removed using a magnet.
October 2001 7-0 EN
Page 74

Page 75

KC4 ∆∆∆∆™
8
Troubleshooting
8Troubleshooting
Contents
8.1 TROUBLESHOOTING FLOW DIAGRAM ................................................... 8-1
8.2 TROUBLESHOOTING PROCEDURES TABLE............................................ 8-1
October 2001 8-0 EN
Page 76

Page 77

KC4 ∆∆∆∆™
Troubleshooting
8.1 Troubleshooting Flow Diagram
8
8.2 Troubleshooting Procedures Table
Symptoms Possible Causes Corrective Action
After pressing On/
Off, the display is
empty; measurement
well is not rotating.
After pressing On/
Off, temperature fails
to stabilize at 37
measurement well is
rotating.
Controls are in
range
Unexpected results
on patient samples.
°
C;
Instrument Error
∆
KC4
power supply or power supply
not connected to outlet.
Instrument Error
Nonfunctional sensor or
thermostat overheating.
Pre-Analytical error
Overfill or underfill of
collection tube.
Pre-Analytical error
Incorrect volume, type (e.g.,
EDTA, heparin), concentration
or lack of anticoagulant.
™ not connected to
Assure that the connecting
cable is seated firmly in the
power supply socket.
Assure that the power supply
cord is connected to the
appropriate outlet.
Place approximately 3 ml water
into a 15 mm test tube. Insert a
thermometer. Observe
temperature after a 10–15 minute
equilibration period.
Call Sigma Diagnostics
Technical Service
1-800-325-0250.
Full draw in commercial
vacuum tubes assures correct
blood/anticoagulant ratio.
Use anticoagulant as
recommended by the reagent
manufacturer.
October 2001 8-1 EN
Page 78

KC4 ∆∆∆∆™
8
Troubleshooting
Symptoms Possible Causes Corrective Action
(continued)
Controls are in
range
Unexpected results
on patient samples.
Pre-Analytical error
Ratio of anticoagulant to blood
is inappropriate.
Pre-Analytical error
Clotted specimen.
Failure to correct citrate volume
for patients with high (>55%) or
low (<21%) hematocrit.
Testing should never be
performed on specimens
containing micro or macro clots.
Controls out of
range
Unexpected results.
Pre-Analytical error
Inadequate or too vigorous
mixing of specimen.
Pre-Analytical error
Contamination with heparin.
Pre-Analytical error
Delay in transporting or
processing or the use of
nonstandardized procedures
for transporting, processing,
storing or testing the
specimen.
Pre-Analytical error
Contact with glass.
Sample related
Loss of Factors V and VIII
Sample related
Incorrect volume being used.
Reagent related
Contaminated reagents.
Invert gently to mix well without
mechanical trauma.
Do not draw blood from a
heparin lock or any other
heparinized line.
Follow manufacturer’s
instructions.
Centrifuge immediately.
Centrifuge at correct rcf for
correct time interval.
Store no longer than 4 hours
at room temperature or
refrigerated temperatures.
Transfer plasma to plastic
storage tube using plastic
transfer pipettes.
Avoid heating at 37
than 5 minutes.
Follow manufacturer’s
instructions.
Reconstitute new reagent or
open new bottle.
°
C for longer
Reagent related
Wrong reagent being used.
Reagent related
Incorrect volume of reagent
being used.
Reagent related
Reconstitution with incorrect
diluent volume.
Reagent related
Reconstitution with other
than the recommended
diluent.
8-2 EN October 2001
Follow manufacturer’s
instructions.
Follow manufacturer’s
instructions.
Follow manufacturer’s
instructions.
Follow manufacturer’s
instructions.
Page 79

KC4 ∆∆∆∆™
Troubleshooting
Symptoms Possible Causes Corrective Action
(continued)
Controls out of
range
Unexpected results.
Reagent related
New lot number of reagent
with different reactivity.
Reagent related
Reagent defective due to
mishandling in shipping or
storage.
Lot-to-lot variation in reagent
reactivity is not unusual. Reverify reference range. Prepare
new reference curves as
appropriate.
First use of reagent in this
shipment? Temperature of
storage area appropriate?
8
Reagent related
Reagent deteriorated.
Reagent related
Reagent deteriorated because
of extended warming in
reagent well.
Reagent related
Reagent contaminated.
Control related
Deteriorated or contaminated
control material.
Analytical error
Incorrect incubation time.
Do not use reagent beyond
stated reconstituted stability
time or beyond expiration date
of un-reconstituted reagent.
Do not store reagent on
instrument. At the completion of
testing, remove reagent from
instrument, cap and store
according to the manufacturer’s
instructions.
Avoid contact of pipette tips with
previously pipetted sample or
reagent.
Prepare fresh controls
Incorrectly reconstituted control
material(s). Reconstitute
according to the manufacturer’s
instructions. Use only fresh
deionized water for
reconstitution.
Follow manufacturer’s
instructions.
Analytical error
Incorrect reagent
temperature.
Analytical error
Incorrect testing sequence.
October 2001 8-3 EN
15 mm tube must be used. No
more than 3.5 ml of reagent
should be placed in tube. Allow
15–20 minutes for reagent to
come to temperature in the
reagent well. Some reagents,
(Thrombin Reagent for
Fibrinogen) should not be
warmed but must be
equilibrated to room temperature
prior to use. Follow reagent
manufacturer’s instructions.
Follow manufacturer’s
instructions.
Page 80

KC4 ∆∆∆∆™
8
Troubleshooting
Symptoms Possible Causes Corrective Action
Erratic within-run
test results.
Controls may be in
or out of range.
Analytical error
Imprecision in manual
pipetting of sample and
reagent.
Perform pipette maintenance.
A pipette Operating Manual is
included in the box of the
automatic pipette provided with
∆
the KC4
Practice pipetting technique.
Refer to the pipetting section for
guidance.
Incorrect dispense position.
Consistent location for reagent
dispense is important. Refer to
Pipetting section for guidance.
Failure to mix particulate
reagent prior to use. Cover top
of tube with cap or Parafilm
and invert gently to mix.
™.
Reagent related
Inconsistent or inaccurate
reconstitution of reagent or
control material.
Reagent related
Reagent deterioration caused
by extended heating in
reagent well.
Reagent related
Reagent concentration caused
by evaporation.
Failure to mix sample and first
reagent. After dispense of
sample and reagent, pick the
cuvette up and swirl gently
5–6 times to evenly disperse the
mixture in the bottom of the
cuvette.
Reconstitute new reagent and/
or control material.
Remove reagent from instrument
at the completion of testing.
8-4 EN October 2001
Page 81

KC4 ∆∆∆∆™
Troubleshooting
Symptoms Possible Causes Corrective Action
(continued)
Erratic within-run
test results.
Controls may be in
or out of range.
Sample related
Improper specimen collection
and handling.
Sample related
No sample added.
Reagents should be capped
when not in use.
Check sample integrity looking
for microclots, hemolysis or
other irregularities.
Assure that ratio of
anticoagulant to sample is
correct (full draw).
Draw a new specimen. If erratic
results are again obtained,
inquire about the clinical
condition of the patient. Results
on patients in DIC are
characteristically erratic.
Observe recommended sample
storage conditions.
8
No clot formation Sample related
Low fibrinogen.
Reagent related
No or incorrect reagent added.
Analytical error
No ball in cuvette.
Clot formed but not
detected. Timer does
not stop.
Analytical error
Cuvette not seated in well.
Sample related.
Clot formed before 4.6 seconds.
Assure that sample has been
added.
A deficiency of Fibrinogen will
greatly prolong the results of
many coagulation tests.
Assure that correct reagents are
being used.
Assure that the ball has not
fallen out of the cuvette prior to
insertion into measuring well.
Ball is positioned above the
sensor. Assure that there are no
balls or other obstructing
material in the bottom of the
well.
If performing Fibrinogen, use
next higher dilution.
To stop the timer, insert a new
cuvette with ball in the measuring
well. After 10 seconds, lift the
cuvette out of the well.
Parafilm® is a registered trademark of American Can Company, Greenwich, CT.
October 2001 8-5 EN
Page 82

Page 83

KC4 ∆∆∆∆™
A
APPENDIX
AAPPENDIX
Contents
A.1 INR FAST TRACK................................................................................... A-1
A.2 APTT FAST TRACK ................................................................................ A-2
A.3 FIBRINOGEN FAST TRACK .................................................................... A-3
A.4 FIBRINOGEN CALIBRATION CURVE DILUTION....................................... A-4
A.5 EXTRINSIC FACTORS II, V, VII AND X FAST TRACK............................... A-5
A.6 EXTRINSIC FACTOR STANDARD CURVE DILUTIONS .............................. A-5
A.7 INTRINSIC FACTORS VIII, IX, XI AND XII FAST TRACK ......................... A-7
A.8 INTRINSIC FACTOR STANDARD CURVE DILUTIONS ............................... A-8
October 2001 A-0 EN
Page 84

Page 85

KC4 ∆∆∆∆™
A
APPENDIX
A.1 INR Fast Track
Note: Use the Multipette. See Section 5 for information regarding programming.
>.
↵↵↵↵
>.
↵↵↵↵
>.
1. From the MAIN MENU, press <
2. Press <RUN>.
3. Select Individual Program.
4. Select <INR> and press <
5. Enter Samples per rack (the test must be set up for duplicates; so the count
would be 1 for a single sample or 2 for 2 samples).
↵↵↵↵
Press <
6. Enter Patient ID and press <
7. Warm thromboplastin reagent to 37°C.
8. Place cuvettes into rack positioned in the unheated preparation area.
9. Dispense one ball into each cuvette if not using a Tetravette.
10. Pipette 50 µl sample into bottom of each cuvette.
11. Close flap, remove rack from preparation area and swirl gently 4–5 times.
12. Transfer rack into heated incubation area or into the rotating test positions.
13. Incubate for a minimum of 60 seconds up to a maximum of 180 seconds.
14. Press to prepare Incubation Timers and then press for each channel with
sample in cuvette.
>.
↵↵↵↵
15. Upon completion of incubation Timing for the last well being tested, place rack into
rotating test positions, and open Flap.
16. Press <START> and begin dispensing PT reagent into wells. With the Multipette,
the timer starts automatically as reagent is dispensed.
17. Record results.
October 2001 A-1 EN
Page 86

KC4 ∆∆∆∆™
A
APPENDIX
A.2 APTT Fast Track
Note: Use the Multipette. See Section 5 for information regarding specific
programming.
↵↵↵↵
>.
↵↵↵↵
>.
2
↵↵↵↵
>.
to 37°C.
incubation time for the test; it is a
1. From the MAIN MENU, press <
2. Press <RUN>.
3. Select Individual Program.
4. Select <APTT> and press <
5. Enter Samples per rack (the test must be set up for duplicates; the count would be
1 for a single sample or 2 for 2 samples).
Press <
6. Enter Patient ID and press <
7. Warm APTT reagent and CaCl
8. Place cuvettes into rack positioned in the unheated preparation area.
9. Dispense one ball into each cuvette if not using a Tetravette.
10. Pipette 50 µl sample into bottom of each cuvette.
11. Close flap, remove rack from preparation area, and swirl 4–5 times.
12. Transfer rack to heated incubation area or into the rotating test positions.
13. Incubate for 240 seconds (This is the TOTAL
combination of the sample warm-up plus the warming of the APTT reagent).
↵↵↵↵
>.
14. Press to prepare Incubation Timers and then press for each channel with
sample in cuvette.
15. At 185 seconds add 50 µl APTT reagent.
16. Upon completion of incubation Timing for the last well being tested, place rack into
rotating test positions, and open flap.
17. Press <START>. Begin dispensing 50 µl CaCl
measurement timing begins automatically.
18. Record results.
into cuvettes. Using the Multipette,
2
A-2 EN October 2001
Page 87

KC4 ∆∆∆∆™
A
APPENDIX
A.3 FIBRINOGEN Fast Track
Note: Use the Multipette. See Section 5 for information regarding programming.
>.
↵↵↵↵
>.
↵↵↵↵
>.
1. From the MAIN MENU, press <
2. Press <RUN>.
3. Select Individual Program.
4. Select <FIB> and press <
5. Enter Samples per rack (the test must be set up for duplicates; the count would be
1 for a single sample or 2 for 2 samples).
↵↵↵↵
Press <
6. Enter Patient ID and press <
7. Warm Thrombin Reagent to room temperature (18–25°C).
8. Prepare 1:10 sample dilutions.
9. Dispense one ball into each cuvette if not using a Tetravette.
10. Pipette 100 µl diluted sample (patient test plasmas are prepared by diluting
plasma 1:10 with Imidazole Buffer*) into bottom of each cuvette.
11. Close flap, remove rack from preparation area and swirl gently 4–5 times.
12. Transfer rack into heated incubation area or into the rotating test positions.
13. Incubate for a minimum of 60 seconds and up to a mzximum of 180 seconds.
>.
↵↵↵↵
14. Press to prepare Incubation Timers and then press for each channel with
sample in cuvette.
15. Upon completion of incubation timing for the last well being tested place rack into
rotating test positins, and open flap.
16. Press <START> and begin dispensing 100 µl Thrombin Reagent into each well.
Using the Multipette, the timer starts automatically as reagent is dispensed.
17. Record results.
*Refer to test application.
October 2001 A-3 EN
Page 88

KC4 ∆∆∆∆™
A
APPENDIX
A.4 FIBRINOGEN CALIBRATION CURVE DILUTION
Fibrinogen Reference Buffer
Dilution (ml) (ml) Dilution Factor
1:7 0.1 0.6 1.43
1:8 0.1 0.7 1.25
1:10 0.1 0.9 1
1:20 0.1 1.9 0.5
1:25 0.1 2.4 0.4
1:30 0.1 2.9 0.33
1:40 0.1 3.9 0.25
A-4 EN October 2001
Page 89

KC4 ∆∆∆∆™
A
APPENDIX
A.5 EXTRINSIC FACTORS II, V, VII and X FAST TRACK
Note: Use the Multipette. See Section 5 for information regarding programming.
1. From the MAIN MENU, press <
2. Press <RUN>.
3. Select Individual Program.
4. Select <FAC> and press <
5. Enter Samples per rack (the test must be set up for duplicates; the count would be
1 for a single sample or 2 for 2 samples).
↵↵↵↵
Press <
6. Enter Patient ID and press <
7. Warm thromboplastin Reagent to 37°C.
8. Prepare 1:10 sample dilutions.
9. Dispense one ball into each cuvette if not using a Tetravette.
10. Pipette 50 µl diluted sample (patient test plasmas are prepared by diluting plasma
1:10 with Imidazole Buffer)* into bottom of each cuvette.
11. Add 50 µl appropriate Factor Deficient Plasma.
12. Close flap, remove rack from preparation area and swirl gently 4–5 times.
13. Transfer rack into heated incubation area or into the rotating test positions.
14. Incubate for a minimum of 60 seconds and up to a maximum of 180 seconds.
>.
↵↵↵↵
>.
↵↵↵↵
>.
↵↵↵↵
>.
15. Press to prepare Incubation Timers and then press for each channel with
sample in cuvette.
16. Upon completion of incubation timing for the last well being tested, place rack into
rotating test positions, and open flap.
17. Press <START> and begin dispensing 100 µl thromboplastin into each well.
Using the Multipette, the timer starts automatically as reagent is dispensed.
18. Record results.
* Refer to test application.
October 2001 A-5 EN
Page 90

KC4 ∆∆∆∆™
A
APPENDIX
A.6 EXTRINSIC FACTOR STANDARD CURVE DILUTIONS
Reference
Dilution
1:10 0.2 1.8 1
1:20 1.0 of 1:10 1.0 0.5
1:40 1.0 of 1:20 1.0 0.25
1:80 1.0 of 1:40 1.0 0.125
1:160 1.0 of 1:80 1.0 0.0625
Plasma (ml) Buffer (ml) Dilution Factor
A-6 EN October 2001
Page 91

KC4 ∆∆∆∆™
A
APPENDIX
A.7 INTRINSIC FACTORS VIII, IX, XI and XII FAST TRACK
Note: Use the Multipette. See Section 5 for information regarding programming.
↵↵↵↵
>.
↵↵↵↵
↵↵↵↵
>.
>.
to 37°C.
2
1. From the MAIN MENU, press <
2. Press <RUN>.
3. Select Individual Program.
4. Select <FAC> and press <
5. Enter Samples per rack (the test must be set up for duplicates; the count would be
1 for a single sample or 2 for 2 samples).
↵↵↵↵
Press <
6. Enter Patient ID and press <
7. Warm APTT reagent and CaCl
8. Prepare 1:5 sample dilutions.
9. Place cuvettes into rack positioned in the unheated preparation area.
10. Dispense one ball into each cuvette if not using a Tetravette.
11. Pipette 50 µl diluted sample (patient test plasmas are prepared by diluting plasma
1:10 with Imidazole Buffer)* into bottom of each cuvette.
12. Add 50 µl appropriate Factor Deficient Plasma.
13. Close flap, remove rack from preparation area and swirl gently 4–5 times.
14. Transfer rack into heated incubation area or into the rotating test positions.
>.
15. Incubate for 240 seconds (This is the TOTAL
combination of the sample warm-up plus the warming of the APTT reagent).
16. Press to prepare Incubation Timers and then press for each channel with
sample in cuvette.
17.
At 185 seconds add 50 µl APTT reagent.
18. Upon completion of incubation timing for the last well being tested, place rack into
rotating test positions, and open flap.
19. Press <START>. Begin dispensing
measurement timing begins automatically.
20. Record results.
* Refer to test application
October 2001 A-7 EN
50
µµµµ
CaCl
l
incubation time for the test, a
into cuvettes. Using the Multipette,
2
Page 92

KC4 ∆∆∆∆™
A
APPENDIX
A.8 INTRINSIC FACTOR STANDARD CURVE DILUTIONS
Dilution Reference Plasma (ml) Buffer (ml) Dilution Factor
1:5 0.4 1.6 1
1:10 1.0 of 1:5 1.0 0.5
1:20 1.0 of 1:10 1.0 0.25
1:40 1.0 of 1:20 1.0 0.125
1:80 1.0 of 1:40 1.0 0.0625
A-8 EN October 2001
 Loading...
Loading...