Page 1
Arnold
ARCADIS Varic
Replacements of Parts
System
SP
Replacement of Parts
08080017
Print No.:
Replaces: SPR2-310.841.01.06.02
SPR2-310.841.01.07.02
© Siemens AG
The reproduction, transmission or use
of this document or its contents is not
permitted without express written
authority. Offenders will be liable for
damages. All rights, including rights
created by patent grant or registration
of a utility model or design, are
reserved.
English
Doc. Gen. Date: 08.07
2005
Page 2

2 Revision / Disclaimer
1Revision / Disclaimer
Document revision level
The document corresponds to the version/revision level effective at the time of system
delivery. Revisions to hardcopy documentation are not automatically distributed.
Please contact your local Siemens office to order current revision levels.
Disclaimer
The installation and service of equipment described herein is to be performed by qualified
personnel who are employed by Siemens or one of its affiliates or who are otherwise
authorized by Siemens or one of its affiliates to provide such services.
Assemblers and other persons who are not employed by or otherwise directly affiliated
with or authorized by Siemens or one of its affiliates are directed to contact one of the
local offices of Siemens or one of its affiliates before attempting installation or service procedures.
ARCADIS Varic SPR2-310.841.01.07.02 Siemens AG
08.07 CS PS SP
Page 2 of 98
Medical Solutions
Page 3

Table of Contents 3
0Table of Contents
1 _______ Prerequisites ___________________________________________________ 6
Required documents . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 6
Required tools, measurement devices, and accessories . . . . . . . . . . . . . . . . . . . . . . 6
Text emphasis. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 7
Symbols . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 8
Service, shutdown, hibernation, handover to the customer . . . . . . . . . . . . . . . . . . . . . . . 9
Safety information and protective measures . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 10
General safety information (in existing documents) . . . . . . . . . . . . . . . . . . . . . . . . . 10
General electrical safety information. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 10
Radiation safety information . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 11
Safety information, mechanical . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 11
Safety information - risk of infection . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 12
Laser light localizer option . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 12
Information on the protective conductor resistance test . . . . . . . . . . . . . . . . . . . . . . 13
System leakage current measurement information . . . . . . . . . . . . . . . . . . . . . . . . . 15
Information on navigation systems installed as options . . . . . . . . . . . . . . . . . . . . . . 19
Cover panels . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 20
Main system, rear cover . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 20
Main system, SIREPHOS cover panel . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 20
Monitor trolley . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 21
Concluding steps . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 22
2 _______ Main System - Replacing Components _____________________________ 23
D1 control board. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 23
Replacement and additional work steps . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 23
D2 power board . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 25
D3 interface board . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 26
D40 board for downward movement of the lifting column. . . . . . . . . . . . . . . . . . . . . . . . 27
M14 power supply, +5 V/+15 V/-15 V . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 28
M13 power supply 230V/13V . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 29
I.I. mini-voltage supply . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 30
Roederstein I.I. mini voltage supply . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 30
Spellman I.I. mini voltage supply . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 31
Collimator . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 33
Exchanging the SIREPHOS . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 34
Replacing the camera and the I.I. optics (camera version ≥ 2.0) . . . . . . . . . . . . . . . . . . 36
Removing/replacing the camera . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 36
Removing/replacing the I.I. optics. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 37
Adjusting the camera and I.I. optics (camera version ≥ 2.0). . . . . . . . . . . . . . . . . . . . . . 38
Centering the camera . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 38
Reproduction scale adjustment. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 38
Camera focus . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 39
Replacing the camera and the I.I. optics (camera version < 2.0) . . . . . . . . . . . . . . . . . . 41
Removing/replacing the camera . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 42
Siemens AG SPR2-310.841.01.07.02 ARCADIS Varic
Medical Solutions
08.07 CS PS SP
Page 3 of 98
Page 4

4 Table of Contents
Removing/replacing the I.I. optics . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 43
Adjusting the camera and I. I. optics (camera version < 2.0) . . . . . . . . . . . . . . . . . . . . . 44
Centering the camera . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 45
Reproduction scale adjustment . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 45
Camera focus . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 46
Replacing the I. I. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 48
Checking the temperature indicator of the new I.I. . . . . . . . . . . . . . . . . . . . . . . . . . . 48
Removing the I.I. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 48
Installing the new I.I. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 49
Checks and adjustments . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 49
Dose area product measuring device . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 51
Laser targeting device (SIREPHOS-side). . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 52
Integrated Laser light localizer (I.I. -side) . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 53
Replacing the C-arm rollers . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 54
Securing the basic unit against tilting . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 54
Set the C-arm and lifting column to the working position . . . . . . . . . . . . . . . . . . . . . 55
Removing the SIREPHOS. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 55
Removing the C-arm . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 55
Replacing the rollers and reinstalling the C-arm . . . . . . . . . . . . . . . . . . . . . . . . . . . . 56
Attaching the covers . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 56
Checks. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 56
3 _______ Monitor Trolley - Replacing Components ___________________________ 58
Replacing the power supply assembly . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 58
Replacing the UPS . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 61
Replacing the UPS battery . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 63
UPS MGE Type 800 . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 63
MGE UPS Type 850 . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 65
Replacing the keyboard. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 67
Replacing the PC . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 68
Replacing PC covers. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 70
Opening the casing . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 70
Removing the front panel . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 72
Replacing the drive . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 74
Replacing the lithium battery. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 76
Restoring BIOS settings (necessary after battery replacement) . . . . . . . . . . . . . . . . 78
Replacing a PCI board PC M420 and M430 (e. g. CIPP board). . . . . . . . . . . . . . . . . . . 80
Replacing a PCI board PC M450 (e. g. CIPP board) . . . . . . . . . . . . . . . . . . . . . . . . . . . 83
Replacing or installing a double USB slot PC M420 and M430 (optional for CAN con-
verter) . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 86
4 _______ Voltages ______________________________________________________ 89
Monitor trolley voltages . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 89
Main system voltages . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 90
I.I. voltages . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 91
ARCADIS Varic SPR2-310.841.01.07.02 Siemens AG
08.07 CS PS SP
Page 4 of 98
Medical Solutions
Page 5

Table of Contents 5
Roederstein I.I. mini voltage supply . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 91
Spellman I.I. mini voltage supply . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 91
5 _______ Brake Force/Lubrication _________________________________________ 94
Brake force calibration . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 94
Adjustment of the angulation brake . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 94
Lubricating the vertical column . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 95
Supplement, measuring the tube current . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 95
6 _______ Changes to the previous version __________________________________ 97
Siemens AG SPR2-310.841.01.07.02 ARCADIS Varic
Medical Solutions
08.07 CS PS SP
Page 5 of 98
Page 6
6 Prerequisites
Required documents 0
1- 1Prerequisites
• ARCADIS Varic wiring diagram
• For the laser targeting device as applica-
ble:
Required tools, measurement devices, and accessories 0
NOTE
• Standard tool kit*
• Digital multimeter, e.g. Fluke 8060 APart no. 97 02 101 Y4290
• Oscilloscope > 50 MHz, e.g. Fluke
CombiScope PM 3390 A
All tools, measurement devices, and accessories with the exception of those marked with a "*" are listed along with their specifications in the STC (Service Tools Catalog).
Part no. 99 00 861 Y3155
Adjustment instructions/laser targeting
device RXR2-130.032.01..
• Dose measurement device
e. g. DALI PTW*
or NOMEX PTW*
or DIADOS PTW
no longer in ARTD
no longer in ARTD
Part no. 97 17 612 Y0388
• Ground wire test meter
e.g. Unimed 1000 tester Part no. 51 38 727 Y0766
• 1 set of resolution tests e.g. part no. 28 71 820 RE999
• 1 set of radiation filters e.g. part no. 97 98 596 G5321
• Centering cross e.g. part no. 96 60 051 RE999
• WPS heat conducting paste e.g. part no. 20 48 650 SRN 6400
• 200 N spring scale e.g. part no. 44 15 113 RH090
• Torque wrench 20 Nm to 100 Nm e.g. part no. 80 86 159 RE999
• Optimol Viscogen KL 300, 50 ml Part no. 72 79 107
• Sealing compound* Part no. 20 49 716 SRN 6002
ARCADIS Varic SPR2-310.841.01.07.02 Siemens AG
08.07 CS PS SP
Page 6 of 98
Medical Solutions
Page 7
Prerequisites 7
Text emphasis 1.1
DANGER
WARNING
CAUTION
NOTICE
DANGER indicates an immediate danger of death or serious physical injury.
¹ n.a.
WARNING indicates a danger that may lead to death or serious
physical injury.
¹ n.a.
CAUTION used with the safety alert symbol indicates a risk of
minor or moderate physical injury and/or damage to property.
¹ n.a.
NOTICE used without the safety alert symbol indicates a risk that
if disregarded will lead or may lead to a situation which may result
in an undesirable result or state other than death, physical injury
or damage to property.
NOTE
¹ n.a.
NOTE contains information provided with special emphasis to
facilitate proper use of the equipment or proper execution of a
procedure, i.e. hints, tips.
Siemens AG SPR2-310.841.01.07.02 ARCADIS Varic
Medical Solutions
08.07 CS PS SP
Page 7 of 98
Page 8

8 Prerequisites
Symbols 1.2
X Checks and adjustments that must be performed with radiation ON are identified by the
radiation warning symbol.
V This symbol means "Dangerous voltage".
C This symbol means "Attention, refer to the documentation".
E This symbol indicates components sensitive to electrostatic discharge (ESD).
ARCADIS Varic SPR2-310.841.01.07.02 Siemens AG
08.07 CS PS SP
Page 8 of 98
Medical Solutions
Page 9
Prerequisites 9
Service, shutdown, hibernation, handover to the customer 1.3
WARNING
NOTE
Switch off via hibernation (version VB13C and later) after service
or before handover to the customer is not sufficient.
Various error messages can appear after the next system boot
and configuration changes are not adopted.
¹ For correct saving of the changed configurations, a shut-
down must be performed. Shut down the system via the
upper monitor menu bar <Options>-<End Session>-<Shut Down System> and then press the off key on
the monitor trolley.
Before the system is handed over to the customer, the system
must be shut down via the menu bar <Options>-<End Session>-<Shut Down System>.
Switch off via hibernation (on/off button on the monitor trolley) is
not sufficient.
Siemens AG SPR2-310.841.01.07.02 ARCADIS Varic
Medical Solutions
08.07 CS PS SP
Page 9 of 98
Page 10
10 Prerequisites
Safety information and protective measures 1.4
General safety information (in existing documents) 0
WARNING
Danger of injuries, death or material damage.
Non-compliance can lead to death, injury or material damage.
Please note:
¹ The product-specific safety notes in these instructions,
¹ The general safety information in TD00-000.860.01... and
¹ The safety information in accordance with ARTD Part 2.
General electrical safety information 0
WARNING
Electrical safety!
Non-compliance can lead to severe injury or even death, as well
as material damage.
¹ Parts under electrical voltage are accessible when the
covers are open. To avoid danger, disconnect the system
from the power supply before opening the covers. Disconnect the power plug.
CAUTION
¹ If an uninterruptible power supply (UPS) is installed in
the system, the voltage output of the UPS must also be
deenergized or the voltage output plug must be disconnected.
¹ If work steps must be performed using electrical power,
the general safety information according to
TD00-000.860.01 must be observed.
Electrical voltage!
Non-compliance can result in material damage.
¹ When working on the system, ESD regulations must be
observed.
ARCADIS Varic SPR2-310.841.01.07.02 Siemens AG
08.07 CS PS SP
Page 10 of 98
Medical Solutions
Page 11

Prerequisites 11
Radiation safety information 0
WARNING
X ¹ Checks requiring the release of radiation are identified by
X-ray radiation!
Non-compliance can lead to illness, irreversible damage to body
cells and the genotype, severe injury and even death.
During work on the system in which radiation must be released,
the radiation protection directives and the rules for radiation protection according to ARTD-002.731.02.. must be complied with.
Please note:
¹ Use available radiation protection devices.
¹ Wear radiation protection clothing (lead apron).
¹ Stay as far away as possible from the radiation source.
¹ Release radiation only if necessary.
¹ Set the radiation activity as low as possible. (Low kV and
mA values, short radiation time)
¹ Release radiation for as short a time as possible.
the radiation warning symbol shown on the left.
Safety information, mechanical 0
CAUTION
Danger of burns from hot parts or components!
Non-observance can lead to mild to moderately severe burns, particularly on the hands.
¹ When the covers are opened, parts and components (e.g.
power supply components, heat sinks, electromagnetic
brakes) are accessible which during operation can reach
temperatures of > 50 degrees Celsius. To avoid burns,
switch off the system and let it cool down for at least 5
minutes before touching any parts or components.
Siemens AG SPR2-310.841.01.07.02 ARCADIS Varic
Medical Solutions
08.07 CS PS SP
Page 11 of 98
Page 12
12 Prerequisites
CAUTION
Danger of injuries from mechanical parts!
Non-observance can lead to mild to moderately severe injuries,
particularly to the hands.
¹ When the cover panels are opened, parts such as plugs,
threaded bolts, cut-off cable ties, and sharp component
edges are exposed. Inattention can lead to skin injuries
(crushing, scrapes, cuts), particularly to the hands.
¹ Work steps carried out on such parts and components
must be performed with particular attention and caution.
¹ Wear work gloves if necessary.
Safety information - risk of infection 0
WARNING
Risk of infection due to pathogens!
Non-compliance can lead to severe injury and even death.
¹ This product can be contaminated with infected blood or
other bodily fluids.
¹ Avoid all contact with blood or other bodily fluids!
¹ Strictly observe the safety information in
ARTD-002.731.37.. regarding prevention of infectious diseases during customer service calls.
Laser light localizer option 0
CAUTION
NOTE
Laser emissions!
This product contains class 2 lasers. (USA: Laser class 2)
Non-observance can lead to injury, particularly to the retina of the
eye, and can thus lead to irreversible visual impairment.
¹ Observe the safety information in ARTD-002.731.03...
When using the laser light localizer, do not look directly
into the laser beam.
Laser emissions!
There is no immediate danger to the eye (blinking reflex). Nevertheless, do not look directly into the laser beam.
ARCADIS Varic SPR2-310.841.01.07.02 Siemens AG
08.07 CS PS SP
Page 12 of 98
Medical Solutions
Page 13
Prerequisites 13
Information on the protective conductor resistance test 0
Observe the instructions in the "Safety Rules for Installation and Repair"
(ARTD-002.731.17 ...).
The protective conductor resistance must be measured after every intervention in the system.
However, documentation of the measured values is required only during periodic safety
checks.
If parts/components that can significantly influence the protective conductor resistance
(e.g., replacement of the power cable, replacement of the power-up module, replacement
of multi-pole connection cables which also create the protective conductor connection
between system parts (e.g., monitor cable or C-arm cable)) are replaced or if protective
conductor connections have been repaired, the protective conductor resistance must be
measured. The values must be documented and evaluated in the protective conductor
resistance protocol.
NOTE
For evaluation purposes, the first measured value and the values
documented during maintenance or safety checks must be compared to the measured values. A sudden or unexpected increase
in the measured values may indicate a defect in the protective
conductor connections - even if the limit value of 0.2 ohms is not
exceeded. (Protective conductor or contacts).
The measurement must be performed according to DIN VDE 0751, Part 1 (see ARTD Part
2). The protective conductor resistance for all touchable conductive parts must be measured during the normal operating state of the system.
Make sure that control cables or data cables between the components of the system are
not mistaken for protective conductor connections.
During the measurement, the power cable and additional connection cables which also
create the protective conductor connection between system parts (e.g. monitor cable
between the basic unit and monitor trolley) must be moved section by section to detect
cable breaks.
The protective conductor resistance must not exceed 0.2 Ohms.
Siemens AG SPR2-310.841.01.07.02 ARCADIS Varic
Medical Solutions
08.07 CS PS SP
Page 13 of 98
Page 14

14 Prerequisites
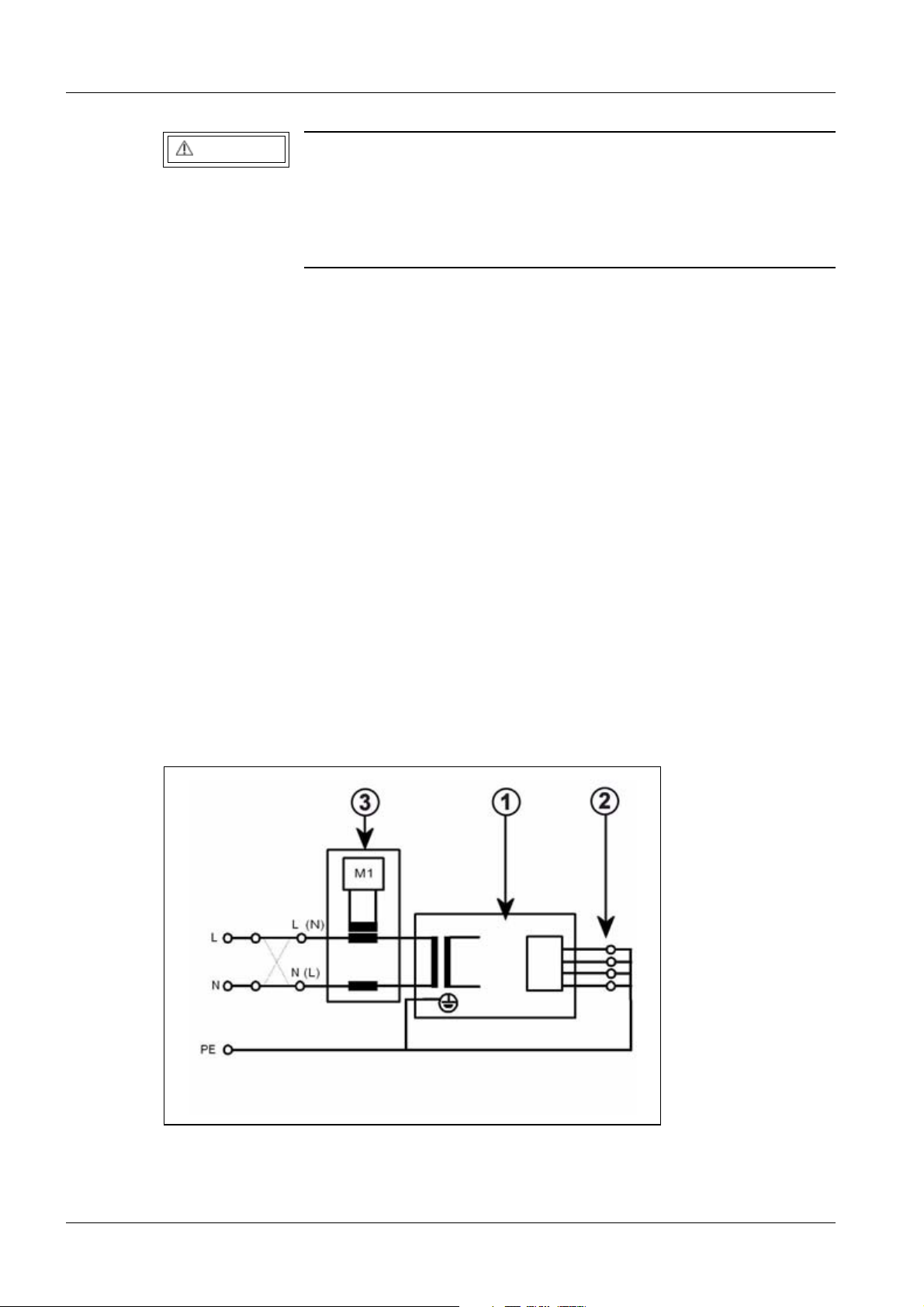
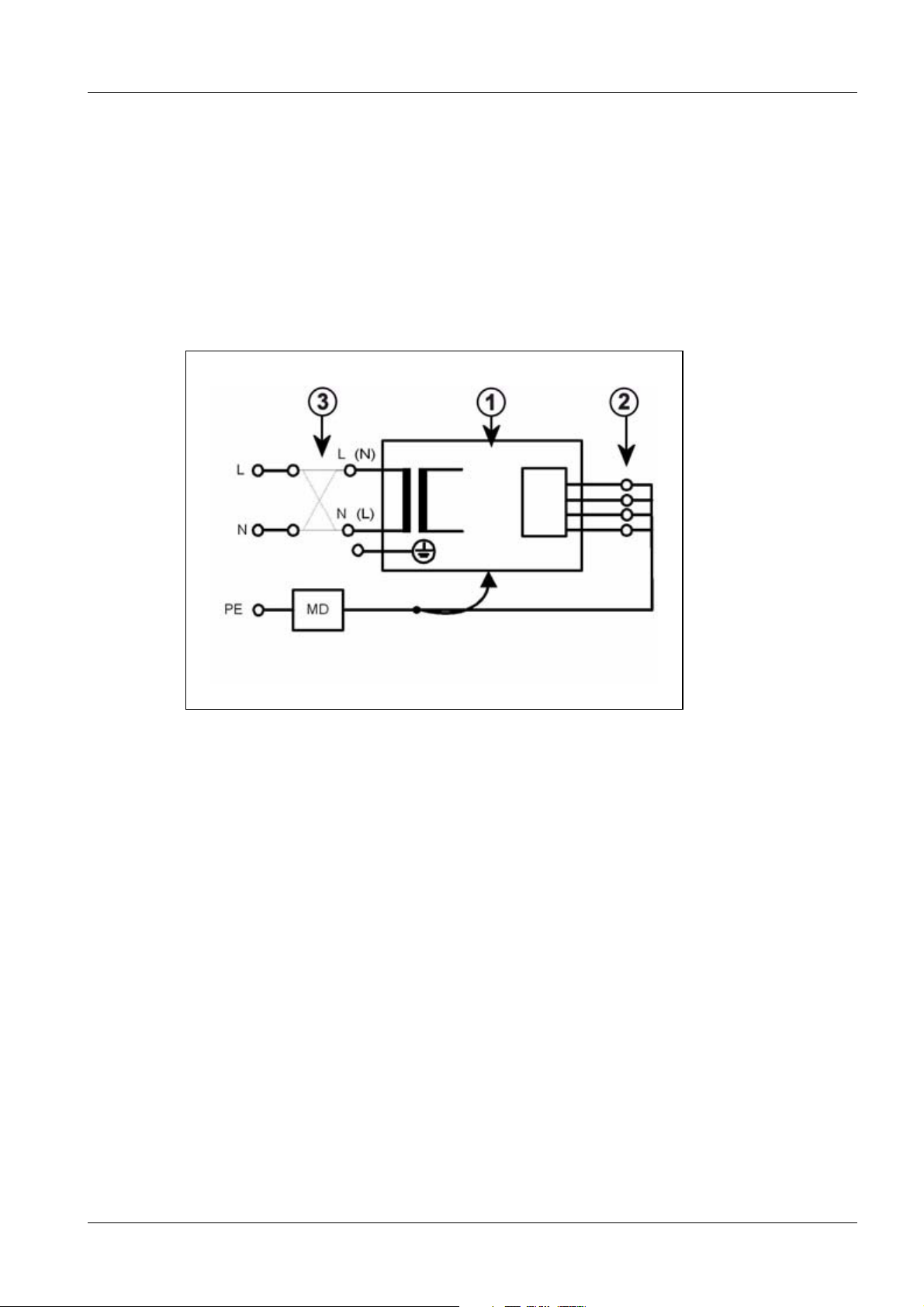
Fig. 1: Measuring circuit for measuring the protective conductor resistance for units that are
disconnected from power, in compliance with DIN VDE 0751-1/2001-10, Fig. C2.
Pos. 1 = Syste m
Pos. 2 = Application part type B (if available)
Pos. 3 = Measurement setup (integrated into measuring device)
ARCADIS Varic SPR2-310.841.01.07.02 Siemens AG
08.07 CS PS SP
Page 14 of 98
Medical Solutions
Page 15

Prerequisites 15
System leakage current measurement information 0
NOTE
If parts in the primary circuit (e.g. power cable, line filter, power
transformers, or complete ON/OFF assemblies) are replaced during service work, the system leakage current measurement must
be subsequently performed and recorded as a repeat measurement.
However, the first measured value must be newly determined and
a new protocol be must created under the following conditions:
- Lack of system leakage current measurement documentation.
- When local line voltage or line frequency deviates from the line
voltage and line frequency values documented in the protocol
(e.g., in the event of a site/operator change)
- When a different procedure for measuring the system leakage
current than the one documented in the protocol is used.
For the purpose of traceability, reference to the new protocol must
be written in the old protocol. The reason for newly determining
the first measured value must be documented and confirmed with
a name and signature.
Observe the instructions in the "Safety Rules for Installation and Repair"
(ARTD-002.731.17 ...).
Siemens AG SPR2-310.841.01.07.02 ARCADIS Varic
Medical Solutions
08.07 CS PS SP
Page 15 of 98
Page 16
16 Prerequisites
WARNING
Electrical voltage!
Non-compliance can lead to severe injury and even death.
¹ The leakage current measurement may be performed on
systems of protection class I only after the protective
conductor test has been passed.
First measured value
The first measured value was already determined and documented in the system leakage
current protocol. The measuring procedure was also recorded.
The measurement was performed with the recorded line voltage, line frequency and with
the recorded measuring equipment.
Measurement
Perform the measurement according to DIN VDE 0751, Part 1 (see ARTD-002.731.17....),
and record the determined value.
The measuring procedure indicated in the protocol must be used.
If the first measured value has to be newly determined (see previous information), a measuring procedure can be selected (direct measurement or differential measurement).
Measurement of the system leakage current according to the differential current method
(measurement setup according to (Fig.2/p.16)) must be given preference, since this
method is not dangerous to the person performing the measurement and other persons.
However, please note the minimum resolution of the leakage current measuring instrument and any additional manufacturer's data restricting the use of the measuring device.
Fig. 2: Measuring circuit for measuring the system leakage current according to the differential
current method in compliance with DIN VDE 0751-1/2001-10, Fig. C6 for protection
ARCADIS Varic SPR2-310.841.01.07.02 Siemens AG
08.07 CS PS SP
Page 16 of 98
Medical Solutions
Page 17
Prerequisites 17
class I.
Pos. 1 = Syste m
Pos. 2 = Application part type B (if available)
Pos. 3 = Measurement setup (integrated into measuring device)
If the direct measurement of the system leakage current is used (measurement setup
according to (Fig.3/p.17)), the system must be insulated during the measurement and
must not be touched.
Fig. 3: Measuring circuit for direct measurement of the system leakage current in compliance
with DIN VDE 0751-1/2001-10, Fig. C5 for protection class I.
Pos. 1 = Syste m
Pos. 2 = Application part type B (if available)
Pos. 3 = Measurement setup (integrated into measuring device)
Siemens AG SPR2-310.841.01.07.02 ARCADIS Varic
Medical Solutions
08.07 CS PS SP
Page 17 of 98
Page 18
18 Prerequisites
WARNING
Electrical voltage!
Non-compliance can lead to severe injury and even death.
¹ No housing parts of the system may be touched during
direct measurement of the leakage current (measurement setup according to (Fig.3/p.17)).
¹ Third-person access to the system must be prevented.
The system must be switched on during measurement. Measuring devices with automated measuring sequences must therefore be set to manual measurement.
Enter the highest value in the system leakage current protocol.
This value must not exceed the permissible system leakage current values according to
DIN VDE 0751-1/2001-10, Table F.1, line "system leakage current for units according to
remarks 1 and 3" of 2.5 mA.
Do not exceed 2.5 mA.
Measure and record the current line voltage. If the measured line voltage deviates from
the nominal voltage, correct the measured value to the value corresponding to a measurement at the nominal value of the line voltage. This must also be documented.
Document the measuring procedure (differential measurement or direct measurement)
and the measuring device used (designation and serial number).
In the case of repeat measurements, the measured value must also be evaluated.
NOTE
For evaluation purposes, the first measured value and the values
documented during maintenance or safety checks must be compared to the measured values. A sudden or unexpected increase
in the measured values may indicate that a fault has occurred in
the primary power supply circuit (insulation damage, damage
from moisture, defective interference suppressor, etc.) - even if
the limit value of 2.5 mA is not exceeded.
.
The evaluation is not necessary in the case of a new determination.
File the protocol sheet in the system binder or log book.
ARCADIS Varic SPR2-310.841.01.07.02 Siemens AG
08.07 CS PS SP
Page 18 of 98
Medical Solutions
Page 19
Prerequisites 19
Information on navigation systems installed as options 0
This ARCADIS system can be used in combination with navigation systems supplied by
various navigation system manufacturers.
The use of the navigation system lies within the responsibility of the customer.
Some service tasks can affect the accuracy of the navigation system.
WARNING
Service work steps that can affect the accuracy of the installed navigation system:
Some service work steps performed on the ARCADIS system will
lead to inaccuracy in a navigation system installed optionally.
Failure to take the necessary steps to address this situation can
lead to severe injuries in the patient.
¹ If service work steps are performed on the ARCADIS sys-
tem that can affect the accuracy of optionally installed
navigation systems (see list below), the customer must
be notified verbally and/or in writing that the accuracy of
the installed navigation system is no longer guaranteed
after such service work steps have been performed, and
that the accuracy of the navigation system must be
checked and certified before it is used again.
• All work steps that affect the geometry of the C-arm and its components, such as:
¹ Removing or installing the I.I.
¹ Removing or installing the I.I. housing
¹ Removing or installing the I.I. mounting ring on the I.I.
¹ Removing or installing the I.I. grid
¹ Removing or installing the I.I. optics
¹ Removing or installing the CCD camera
¹ Removing or installing any mechanical components of the C-arm
• Any adjustments that alter the geometry of the imaging components, such as:
¹ Adjustment of camera optics (optical sharpness, image size)
¹ Adjustment of camera rotation (positioning with respect to I.I. optics)
¹ Adjustment of I.I. geometry and sharpness (I.I. mini voltage supply)
• Any subsequent installation of released options that affect the geometry of the C-arm
and its components, such as:
¹ Integrated laser light localizer option (geometric alteration)
¹ I.I.laser light localizer option (weight alteration)
¹ 2D Navigation Option (Attachment of altered mounting ring to I.I.)
Siemens AG SPR2-310.841.01.07.02 ARCADIS Varic
Medical Solutions
08.07 CS PS SP
Page 19 of 98
Page 20
20 Prerequisites
Cover panels 1.5
NOTE
Observe the safety information in this chapter!
Main system, rear cover 0
Removing the rear cover
1. Engage the foot brake.
2. Remove the screws from the rear cover.
3. Pull the cover back approximately 25 cm.
4. Unscrew the ground wire from the cover panel.
5. Pull the cover completely off and tilt it down.
6. Lift up the cover and raise both lateral metal brackets from the guide rails.
Installing the back cover
1. Fit the lateral metal brackets back into the guide rail.
2. Reattach the protective conductor to the rear cover.
3. Lift the cover and push it forward. Be careful with the EMC spring contact.
4. Reattach the cover and tighten the screws.
Main system, SIREPHOS cover panel 0
NOTE
1. Loosen the cover screws of the SIREPHOS cover.
2. Use a sharp knife to cut open the sealing compound all around the cover.
3. Lift off the SIREPHOS cover.
4. Remove the sealing compound residue from the cover and from the SIREPHOS.
5. On completing service, place the SIREPHOS cover on the SIREPHOS and completely
seal it with sealing compound.
6. Use a paper towel to wipe off excess sealing compound.
7. Once again, use sealing compound 2049716 to seal the SIREPHOS cover completely.
8. Refasten the SIREPHOS cover with the cover screws. If necessary, apply side pressure to the SIREPHOS cover until the sealing compound hardens.
The SIREPHOS cover panel is sealed all around against dirt contamination using a sealing compound (part no. 20 49 716).
ARCADIS Varic SPR2-310.841.01.07.02 Siemens AG
08.07 CS PS SP
Page 20 of 98
Medical Solutions
Page 21

Prerequisites 21
Monitor trolley 0
Removing the cover panels
1. Remove the 4 screws from the middle back cover to access the imaging system (rear).
2. Remove the upper back cover plate of the log book compartment.
3. Remove the lower back cover to access the power supply.
4. Remove the front cover to access the imaging system (front).
Installing the cover panels
1. Install the covers in reverse order.
Siemens AG SPR2-310.841.01.07.02 ARCADIS Varic
Medical Solutions
08.07 CS PS SP
Page 21 of 98
Page 22

22 Prerequisites
Concluding steps 1.6
NOTE
Observe the guidelines on the protective conductor test and the
leakage current measurement in this chapter.
• If necessary, perform the leakage current measurement.
• After completing all work steps and closing all the cover panels, perform the protective
conductor test in accordance with ARTD-002.731.17...
¹ The protective conductor resistance must not exceed 0.2 Ohms.
ARCADIS Varic SPR2-310.841.01.07.02 Siemens AG
08.07 CS PS SP
Page 22 of 98
Medical Solutions
Page 23
Main System - Replacing Components 23
D1 control board 0
2- 2Main System - Replacin g Components
WARNING
Replacement and additional work steps 0
1. Make sure that a current backup of the main system is available.
2. Read out the load counter of the Sirephos (Path: <Service>-<Main System>-<Adjustment>-<Load Counter>).
3. Document this value because it is necessary for a Sirephos replacement. If the D1 is
defective, the load count is available in the offline report (path:<Service>-<Reports>-<Main System>-<Configuration Offline").
4. Disconnect and replace the D1 board.
Electrical voltage!
See chapter 1, Safety Information.
¹ Make sure that LED V400 on the D2 board is off prior to
performing any work on boards D1 and D2. This should
occur approximately 3 minutes after switching OFF the
ARCADIS system.
5. Install the new D1 board and reconnect all cable connections. Be sure that the shielding and ground wire connections are connected correctly.
6. Set the jumpers and switches according to the wiring diagram.
7. Perform the download procedure for the D1 control board (path: <Service>-<Main System> -<Download>-<C-Arm>).
NOTE
If the EE-PROM of the replacement D1 is not completely deleted, it
will be automatically cleared after the host software has been
downloaded. The delete procedure is indicated as "d" on the
7-segment display and requires approximately 5 minutes. During
the delete procedure, do not switch off the system or perform additional programming. After successful deletion, the 7-segment display returns to the normal status display.
8. After download, wait for the system to reboot.
NOTE
With software version VB11A, the system serial number (1xxxx)
must be entered and saved manually under <Main System>-<Configuration>-<Main System> before a restore of the main system is
carried out.
9. Restore the main system parameters (path: <Service>-<Backup & Restore> "Packages" - "Main System").
10. Perform a generator adjustment (path: <Service>-<Main System>-<Adjustment>-<Generator Adjust.>).
Siemens AG SPR2-310.841.01.07.02 ARCADIS Varic
Medical Solutions
08.07 CS PS SP
Page 23 of 98
Page 24

24 Main System - Replacing Components
11. Check the dose rate control and adjust it as necessary (path: <Service>-<Main System>-<Adjustment>-<Dose rate>).
12. Ensure that camera rotation is functioning properly. Adjust, if necessary.
13. Ensure that the collimator is functioning properly. Adjust, if necessary.
14. Check the display functions and the setting of the blades on the monitor. Adjust, if necessary.
15. Ensure that the area dose measurement device (if present) is functioning correctly.
16. Test the FL/PFL/DR and direct exposure functions of the system.
WARNING
When these service tasks have been performed, the accuracy of
an optionally installed navigation system is no longer guaranteed.
See the chapter "Prerequisites," section "Safety information and
protective measures."
¹ If an optionally installed navigation system is present,
the customer must be notified that the accuracy of the
installed navigation system is no longer guaranteed, and
that the navigation system must be checked and certified
before it is used again.
ARCADIS Varic SPR2-310.841.01.07.02 Siemens AG
08.07 CS PS SP
Page 24 of 98
Medical Solutions
Page 25

Main System - Replacing Components 25
D2 power board 2.1
WARNING
1. Disconnect D1 and D2 boards completely.
2. Disconnect the cables between boards D1 and D2.
3. Remove boards D1 and D2.
4. Apply heat conducting paste to the heat sink for the power semiconductor on the new
D2 board.
5. Reinstall boards D2 and D1 and reattach all connections.
6. Ensure that the shielding and ground connections are positioned correctly.
7. Perform the kV offset adjustment and the generator adjustment (filament learning).
8. Test the FL/PFL/DR and direct exposure functions, if there is a cassette holder.
Electrical voltage!
See chapter 1, Safety Information.
¹ Make sure that LED V400 is off prior to performing any
work on boards D1 and D2. This should occur approximately 3 minutes after switching OFF the ARCADIS system.
Siemens AG SPR2-310.841.01.07.02 ARCADIS Varic
Medical Solutions
08.07 CS PS SP
Page 25 of 98
Page 26

26 Main System - Replacing Components
D3 interface board 2.2
WARNING
1. Replace the D3 interface board.
2. Check the +26.75 V voltage for the I.I. mini-voltage supply. Adjust it, if necessary. Refer
to chapter 3 of these instructions, Checking the Operating Voltages.
3. Check the radiation release/format switchover/vertical column movement functions.
Electrical voltage!
See chapter 1, Safety Information.
¹ Switch system power supply off and disconnect the
power plug.
ARCADIS Varic SPR2-310.841.01.07.02 Siemens AG
08.07 CS PS SP
Page 26 of 98
Medical Solutions
Page 27

Main System - Replacing Components 27
D40 board for downward movement of the lifting column 2.3
WARNING
1. Replace the D40 board.
2. Using the operating instructions for the basic system, check both the movements of the
C-arm and the signal messages.
Electrical voltage!
See chapter 1, Safety Information.
¹ Switch system power supply off and disconnect the
power plug.
Siemens AG SPR2-310.841.01.07.02 ARCADIS Varic
Medical Solutions
08.07 CS PS SP
Page 27 of 98
Page 28

28 Main System - Replacing Components
M14 power supply, +5 V/+15 V/-15 V 2.4
WARNING
1. Replace the power supply.
2. Check the power supply voltage and adjust it if necessary. Refer to chapter
(Voltages / p. 89).
Electrical voltage!
See chapter 1, Safety Information.
¹ Switch the system power supply off and disconnect the
power plug.
ARCADIS Varic SPR2-310.841.01.07.02 Siemens AG
08.07 CS PS SP
Page 28 of 98
Medical Solutions
Page 29

Main System - Replacing Components 29
M13 power supply 230V/13V 2.5
WARNING
1. Replace the power supply.
2. Check the power supply voltage according to chapter (Voltages / p. 89).
Electrical voltage!
See chapter 1, Safety Information.
¹ Switch the system power supply off and disconnect the
power plug.
Siemens AG SPR2-310.841.01.07.02 ARCADIS Varic
Medical Solutions
08.07 CS PS SP
Page 29 of 98
Page 30

30 Main System - Replacing Components
I.I. mini-voltage supply 2.6
WARNING
WARNING
Electrical voltage!
See chapter 1, Safety Information.
¹ Switch the system power supply off and disconnect the
power plug.
Electrical voltage!
See chapter 1, Safety Information.
¹ Prior to removing the mini-voltage supply, the system
must be switched off for at least 3 minutes, until the high
voltage in the system and in the I.I. mini-voltage supply
dissipates.
Roederstein I.I. mini voltage supply 0
Fig. 4: I. I. mini-voltage supply
1. Remove the cylindric cover on the I.I..
2. Replace the I.I. mini-voltage supply (Fig.4/p.30).
3. Refer to the I.I. test certificate 1 for the E1/E2/E3 and A voltages. Check and adjust
them as necessary according to the "Voltages" chapter.
4. Check the functions and adjustments of the collimator. Readjust them, if necessary.
5. Check the display functions and the setting of the blades on the monitor. Adjust them, if
necessary.
ARCADIS Varic SPR2-310.841.01.07.02 Siemens AG
08.07 CS PS SP
Page 30 of 98
Medical Solutions
Page 31

Main System - Replacing Components 31
6. Check the overall resolution according to the IQ test.
7. Perform the IQ test.
WARNING
When these service tasks have been performed, the accuracy of
an optionally installed navigation system is no longer guaranteed.
See the chapter "Prerequisites," section "Safety information and
protective measures."
¹ If an optionally installed navigation system is present,
the customer must be notified that the accuracy of the
installed navigation system is no longer guaranteed, and
that the navigation system must be checked and certified
before it is used again.
Spellman I.I. mini voltage supply 0
Fig. 5: 9 inch / 23 cm I.I. with Spellman I.I. mini power supply
Pos. 1 Mounting screws
Pos. 2 Plugs E1 to E3, Penning and anode
Pos. 3 Ribbon cables
Pos. 4 DIP switches
Pos. 5 Test points
1. Remove the cover on the I.I.
2. Replace the I.I. mini-voltage supply.
3. Refer to the I.I. test certificate 1for the E1/E2/E3 and A voltages. Check and adjust them
as necessary according to the "Voltages" chapter.
4. Check the functions and adjustments of the collimator. Readjust them, if necessary.
5. Check the display functions and the setting of the blades on the monitor. Adjust them, if
necessary.
6. Check the overall resolution according to the IQ test.
7. Perform the IQ test.
Siemens AG SPR2-310.841.01.07.02 ARCADIS Varic
Medical Solutions
08.07 CS PS SP
Page 31 of 98
Page 32

32 Main System - Replacing Components
WARNING
When these service tasks have been performed, the accuracy of
an optionally installed navigation system is no longer guaranteed.
See the chapter "Prerequisites," section "Safety information and
protective measures."
¹ If an optionally installed navigation system is present,
the customer must be notified that the accuracy of the
installed navigation system is no longer guaranteed, and
that the navigation system must be checked and certified
before it is used again.
ARCADIS Varic SPR2-310.841.01.07.02 Siemens AG
08.07 CS PS SP
Page 32 of 98
Medical Solutions
Page 33

Main System - Replacing Components 33
Collimator 2.7
1. Replace the collimator.
2. Ensure that the collimator is functioning properly and is set correctly. If necessary,
adjust it.
3. Ensure that the collimator blades of the iris diaphragm are visible during fluoroscopy in
survey format and zoom format on at least 2 sides of the monitor image.
4. Check collimation for direct exposure if a cassette holder is being used.
5. Check the display of the blades on the monitor and adjust it if necessary.
6. Remove the old set of collimator labels near the SIREPHOS and attach the included
new labels in the same position.
7. Check that the original collimator labels are the same as the new ones.
Siemens AG SPR2-310.841.01.07.02 ARCADIS Varic
Medical Solutions
08.07 CS PS SP
Page 33 of 98
Page 34

34 Main System - Replacing Components
Exchanging the SIREPHOS 2.8
1. Read out and note the number of resets and the current load counts from the SIREPHOS tank. Path: <Service>-<Main System>-<Adjustment>-<Load Counter>.
2. Open the SIREPHOS cover. See chapter 1, (Cover panels / p. 20).
3. Remove the laser targeting device, if present.
4. Remove the dose measurement chamber, if present.
5. Remove the collimator.
6. Disconnect the SIREPHOS plug.
7. Remove the ground screw for the protective conductor.
8. Remove the rubber stop of the C-arm on the SIREPHOS side. The screws that secure
the SIREPHOS are now visible.
9. Remove the SIREPHOS screws. When doing this, have a second person hold the
X-ray tube unit.
10. Place the new X-ray tube unit on the guide bolts and secure it with the two Allen screws.
11. Ensure that all cables are routed properly.
12. Reconnect the SIREPHOS plug and secure it.
13. Reconnect the protective conductor using the ground screw.
14. Do not install the rubber stop yet.
15. Reinstall the collimator and connect it.
16. Reinstall the dose measurement chamber/laser targeting device and connect them.
WARNING
17. Enter the new serial no. of the new single tank. Path: <Service>-<Main System>-<Configuration>-<Load <Counter>.
X 18. Adjust the generator. Path: <Service>-<Main System>-<Adjustment>-<Generator
Adjustment>.
19. Reset the load counter. Path: <Service>-<Main System>-<Adjustment>-<Load
<Counter>.
20. Ensure that the collimator is functioning properly and is set correctly.
21. Ensure that the collimator blades are centered in the blanking circle.
X-ray radiation!
See chapter 1, Safety Information.
¹ Protect against radiation exposure. Wear a lead apron.
22. If possible, loosen the Sirephos screws slightly and tilt the SIREPHOS to adjust it.
23. Retighten the SIREPHOS screws.
24. Ensure that the collimator blades of the iris diaphragm are visible during fluoroscopy in
the survey format and zoom format on at least 2 sides of the monitor image.
25. Check the display of the blades on the monitor screen and adjust it as necessary.
26. Check collimation for direct exposure if there is a cassette holder.
27. Reinstall the rubber stop.
ARCADIS Varic SPR2-310.841.01.07.02 Siemens AG
08.07 CS PS SP
Page 34 of 98
Medical Solutions
Page 35

Main System - Replacing Components 35
28. Ensure that the area/dose product measurement device is functioning properly (if
present).
29. Check the setting of the laser targeting device and adjust it as necessary according to
the Adjustment Instructions for the Laser Targeting Device, RXR2-130.815.01.
30. Reattach the SIREPHOS cover.
31. Seal the SIREPHOS cover using sealing compound. See chapter 1, (Cover
panels / p. 20).
Siemens AG SPR2-310.841.01.07.02 ARCADIS Varic
Medical Solutions
08.07 CS PS SP
Page 35 of 98
Page 36

36 Main System - Replacing Components
Replacing the camera and the I.I. optics (camera version ≥ 2.0) 2.9
Relevant systems: ARCADIS Varic system serial no. 10218, 10219, 10222 and systems
with serial no. > 10223; camera version ≥ 2.0 (see ID label).
Fig. 6: II _ Connection
Fig. 7: Replacing the camera and I.I. optics
Removing/replacing the camera 0
1. Switch the system off.
2. Remove the upper I.I. cover (already removed in (Fig.6/p.36)).
3. Unplug the connectors (1/Fig.6/p.36), (2/Fig.6/p.36) and disconnect the fan wires
(3/Fig.6/p.36).
4. Loosen the clamping screw (1/Fig.7/p.36).
5. Rotate the clamping ring (2/Fig.7/p.36)a half rotation in the counterclockwise direc-
tion to loosen the camera. For this purpose, insert an Allen key (2.5 mm) into the hole
(2/Fig.7/p.36) and push the clamping ring in the counterclockwise direction.
6. Remove the 3 Allen screws (3/Fig.7/p.36).
7. Turn the entire camera (4/Fig.6/p.36) counterclockwise until it is no longer attached.
For subsequent reinstallation, count the number of turns when removing the camera.
The connector (2/Fig.6/p.36) should be in the position shown in (Fig.6/p.36).
8. Install the (new) camera in reverse order.
9. Adjust the camera and I.I. optics.
10. Perform the IQ test.
11. Complete the country-specific acceptance (§16 partial acceptance... /DHHS...).
ARCADIS Varic SPR2-310.841.01.07.02 Siemens AG
08.07 CS PS SP
Page 36 of 98
Medical Solutions
Page 37

Main System - Replacing Components 37
WARNING
When these service tasks have been performed, the accuracy of
an optionally installed navigation system is no longer guaranteed.
See the chapter "Prerequisites," section "Safety information and
protective measures."
¹ If an optionally installed navigation system is present,
the customer must be notified that the accuracy of the
installed navigation system is no longer guaranteed, and
that the navigation system must be checked and certified
before it is used again.
Removing/replacing the I.I. optics 0
Prerequisites: Camera must already be removed.
1. Remove the 3 M4 screws (4/Fig.7/p.36).
2. Remove the I.I. optics.
3. Install the (new) optics and camera in reverse order.
¹ When screwing in the 3 M4 screws (4/Fig.7/p.36), make sure that the optic is
pressed against the centering bolts.
4. Adjust the camera and I.I. optics.
5. Perform the IQ test.
WARNING
When these service tasks have been performed, the accuracy of
an optionally installed navigation system is no longer guaranteed.
See the chapter "Prerequisites," section "Safety information and
protective measures."
¹ If an optionally installed navigation system is present,
the customer must be notified that the accuracy of the
installed navigation system is no longer guaranteed, and
that the navigation system must be checked and certified
before it is used again.
Siemens AG SPR2-310.841.01.07.02 ARCADIS Varic
Medical Solutions
08.07 CS PS SP
Page 37 of 98
Page 38

38 Main System - Replacing Components
Adjusting the camera and I.I. optics (camera version ≥ 2.0) 2.10
Relevant systems: ARCADIS Varic system serial no. 10218, 10219, 10222 and systems
with serial no. >10223; camera version ≥ 2.0 (see ID label).
Fig. 8: Adjusting the camera
Centering the camera 0
NOTE
WARNING
The camera can be centered by moving the optics. Centering is
performed in the factory and should be acceptable. The camera
optics must be moved toward the centering bolts.
When these service tasks have been performed, the accuracy of
an optionally installed navigation system is no longer guaranteed.
See the chapter "Prerequisites," section "Safety information and
protective measures."
¹ If an optionally installed navigation system is present,
the customer must be notified that the accuracy of the
installed navigation system is no longer guaranteed, and
that the navigation system must be checked and certified
before it is used again.
Reproduction scale adjustment 0
Prerequisites: Camera centering should be OK.
1. Loosen the clamping screw (4/Fig.8/p.38).
2. Loosen the lock ring (5/Fig.8/p.38).
3. Unplug the connectors (1/Fig.6/p.36), (2/Fig.6/p.36) and disconnect the fan wires
(3/Fig.6/p.36).
ARCADIS Varic SPR2-310.841.01.07.02 Siemens AG
08.07 CS PS SP
Page 38 of 98
Medical Solutions
Page 39

Main System - Replacing Components 39
4. Remove the 3 Allen screws from the camera flange (1/Fig.8/p.38).
5. You can increase/decrease the image size by turning the entire camera. The camera
plugs must not be in the same position.
¹ Turn clockwise = larger image
¹ Turn counterclockwise = smaller image
T 6. Rotate the camera until the plugs are at the same location as prior to the adjustment
and retighten the 3 M2 Allen screws (1/Fig.8/p.38).
¹ Tightening torque 14 ± 1Ncm
7. Tighten the lock ring (5/Fig.8/p.38).
8. Retighten the clamping screw (4/Fig.8/p.38).
9. Reattach the connectors (1/Fig.6/p.36), (2/Fig.6/p.36) and the fan wires
(3/Fig.6/p.36).
X 10. Release fluoro and check the image size. Repeat the adjustment if necessary.
11. Open the service application and adjust the 0 degree position of the image.
Path: <Service>-<Main System>-<Adjustment>-<Image Rotation>.
12. Perform the IQ test.
WARNING
When these service tasks have been performed, the accuracy of
an optionally installed navigation system is no longer guaranteed.
See the chapter "Prerequisites," section "Safety information and
protective measures."
¹ If an optionally installed navigation system is present,
the customer must be notified that the accuracy of the
installed navigation system is no longer guaranteed, and
that the navigation system must be checked and certified
before it is used again.
Camera focus 0
Prerequisites: Camera reproduction scale should be OK.
1. Loosen the focus ring clamping screw (2/Fig.8/p.38).
2. You can adjust the optimum sharpness by turning the focus ring (3/Fig.8/p.38).
X 3. Release fluoro and check the sharpness. Repeat the adjustment if necessary.
4. Retighten the focus clamping screw (2/Fig.8/p.38).
5. Perform the IQ test.
NOTE
Adjusting the camera focus also has a slight effect on the reproduction scale. The reproduction scale adjustment may have to be
repeated.
Siemens AG SPR2-310.841.01.07.02 ARCADIS Varic
Medical Solutions
08.07 CS PS SP
Page 39 of 98
Page 40

40 Main System - Replacing Components
WARNING
When these service tasks have been performed, the accuracy of
an optionally installed navigation system is no longer guaranteed.
See the chapter "Prerequisites," section "Safety information and
protective measures."
¹ If an optionally installed navigation system is present,
the customer must be notified that the accuracy of the
installed navigation system is no longer guaranteed, and
that the navigation system must be checked and certified
before it is used again.
ARCADIS Varic SPR2-310.841.01.07.02 Siemens AG
08.07 CS PS SP
Page 40 of 98
Medical Solutions
Page 41

Main System - Replacing Components 41
Replacing the camera and the I.I. optics (camera version < 2.0) 2.11
Relevant systems: ARCADIS Varic serial no. < 10218 and 10220, 10221, 10223; or the
camera was already replaced with the new camera version ≥ 2.0 (see ID label).
Fig. 9: Replacing the camera and the I. I. optics_1
Siemens AG SPR2-310.841.01.07.02 ARCADIS Varic
Medical Solutions
08.07 CS PS SP
Page 41 of 98
Page 42

42 Main System - Replacing Components
Fig. 10: Replacing the camera and the I. I. optics_2
Removing/replacing the camera 0
1. Remove the I.I. cover (already removed in (Fig.9/p.41)).
2. Unplug the connectors (1/Fig.9/p.41), (2/Fig.9/p.41) and disconnect the fan wires
(3/Fig.9/p.41).
3. Loosen the clamping screw (4/Fig. 10 / p. 42).
4. Rotate the clamping ring (5/Fig. 10 / p. 42) a half rotation in the counterclockwise direc-
tion to loosen the camera. For this purpose, insert an Allen wrench (2.5 mm) into the
hole (5/Fig. 10 / p. 42) and push the clamping ring in the counterclockwise direction.
5. Turn the entire camera (4/Fig.9/p.41) counterclockwise until it is no longer attached.
For subsequent reinstallation, count the number of turns when removing the camera.
The connector (2/Fig.9/p.41) should be in the position shown in (Fig.9/p.41).
6. Install the (new) camera in reverse order.
7. Adjust the camera and I.I. optics.
8. Perform the IQ test.
9. Complete the country-specific acceptance (§16 partial acceptance... /DHHS...).
ARCADIS Varic SPR2-310.841.01.07.02 Siemens AG
08.07 CS PS SP
Page 42 of 98
Medical Solutions
Page 43

Main System - Replacing Components 43
WARNING
When these service tasks have been performed, the accuracy of
an optionally installed navigation system is no longer guaranteed.
See the chapter "Prerequisites," section "Safety information and
protective measures."
¹ If an optionally installed navigation system is present,
the customer must be notified that the accuracy of the
installed navigation system is no longer guaranteed, and
that the navigation system must be checked and certified
before it is used again.
Removing/replacing the I.I. optics 0
Prerequisites: Camera must already be removed.
1. Remove the 3 M4 screws (1/Fig. 10 / p. 42).
2. Remove the I.I. optics.
3. Install the (new) optics and camera in reverse order.
4. Adjust the camera and I.I. optics.
5. Perform the IQ test.
WARNING
When these service tasks have been performed, the accuracy of
an optionally installed navigation system is no longer guaranteed.
See the chapter "Prerequisites," section "Safety information and
protective measures."
¹ If an optionally installed navigation system is present,
the customer must be notified that the accuracy of the
installed navigation system is no longer guaranteed, and
that the navigation system must be checked and certified
before it is used again.
Siemens AG SPR2-310.841.01.07.02 ARCADIS Varic
Medical Solutions
08.07 CS PS SP
Page 43 of 98
Page 44

44 Main System - Replacing Components
Adjusting the camera and I. I. optics (camera version < 2.0) 2.12
Relevant systems: ARCADIS Varic serial no. < 10218 and 10220, 10221, 10223; or the
camera was already replaced with the new camera version ≥ 2.0 (see ID label).
Fig. 11: Replacing the camera and the I. I. optics_2
ARCADIS Varic SPR2-310.841.01.07.02 Siemens AG
08.07 CS PS SP
Page 44 of 98
Medical Solutions
Page 45

Main System - Replacing Components 45
Fig. 12: Monitor orientation
Centering the camera 0
NOTE
WARNING
The camera can be centered by moving the optics. Centering is
performed in the factory and should be acceptable. The camera
optics must be moved toward the centering bolts.
When these service tasks have been performed, the accuracy of
an optionally installed navigation system is no longer guaranteed.
See the chapter "Prerequisites," section "Safety information and
protective measures."
¹ If an optionally installed navigation system is present,
the customer must be notified that the accuracy of the
installed navigation system is no longer guaranteed, and
that the navigation system must be checked and certified
before it is used again.
Reproduction scale adjustment 0
Prerequisites: Camera centering should be OK.
1. Loosen the clamping screw (4/Fig. 11 / p. 44).
2. Loosen the lock ring (5/Fig. 11 / p. 44).
Siemens AG SPR2-310.841.01.07.02 ARCADIS Varic
Medical Solutions
08.07 CS PS SP
Page 45 of 98
Page 46

46 Main System - Replacing Components
3. Unplug the connectors (1/Fig.9/p.41), (2/Fig.9/p.41) and disconnect the fan wires
(3/Fig.9/p.41).
4. You can increase/decrease the image by turning the entire camera (Fig. 12 / p. 45).
Always turn it a full turn because the camera plugs should be in the same position.
¹ Turn clockwise = larger image
¹ Turn counterclockwise = smaller image
5. Reattach the connectors (1/Fig.9/p.41), (2/Fig.9/p.41) and the fan wires
(3/Fig.9/p.41).
6. Release fluoro and check the image size. Repeat the adjustment if necessary.
7. Retighten the clamping screw (4/Fig. 11 / p. 44).
8. Open the service application and adjust the 0-degree position of the image.
Path: <Service>-<Main System>-<Adjustment>-<Image Rotation>.
9. Perform the IQ test.
WARNING
When these service tasks have been performed, the accuracy of
an optionally installed navigation system is no longer guaranteed.
See the chapter "Prerequisites," section "Safety information and
protective measures."
¹ If an optionally installed navigation system is present,
the customer must be notified that the accuracy of the
installed navigation system is no longer guaranteed, and
that the navigation system must be checked and certified
before it is used again.
Camera focus 0
Prerequisites: Camera centering and camera reproduction scale should be OK.
1. Loosen the focus ring clamping screw (2/Fig. 11 / p. 44).
2. You can adjust the optimum sharpness by turning the focus ring (3/Fig. 11 / p. 44).
X 3. Release fluoro and check the sharpness. Repeat the adjustment if necessary.
4. Retighten the focus clamping screw (2/Fig. 11 / p. 44).
5. Perform the IQ test.
NOTE
Adjusting the camera focus also has a slight effect on the reproduction scale. The reproduction scale adjustment may have to be
repeated.
ARCADIS Varic SPR2-310.841.01.07.02 Siemens AG
08.07 CS PS SP
Page 46 of 98
Medical Solutions
Page 47

Main System - Replacing Components 47
WARNING
When these service tasks have been performed, the accuracy of
an optionally installed navigation system is no longer guaranteed.
See the chapter "Prerequisites," section "Safety information and
protective measures."
¹ If an optionally installed navigation system is present,
the customer must be notified that the accuracy of the
installed navigation system is no longer guaranteed, and
that the navigation system must be checked and certified
before it is used again.
Siemens AG SPR2-310.841.01.07.02 ARCADIS Varic
Medical Solutions
08.07 CS PS SP
Page 47 of 98
Page 48

48 Main System - Replacing Components
Replacing the I. I. 2.13
Checking the temperature indicator of the new I.I. 0
1. Prior to removing the I.I., check the temperature indicator of the new I. I.
2. If the inner square field of the indicator is white, the I.I. did not exceed the temperature
range. Remove the temperature indicator.
3. If the indicator is discolored (inner field black), proceed according to IQ document
RXD-000.038.01.
Removing the I.I. 0
Fig. 13: Removing_the I. I.
CAUTION
1. Rotate the C-arm so that the I.I. is on top.
2. Remove the I. I. cover (see (1/Fig. 13 / p. 48)) and unsolder the cassette switch.
ARCADIS Varic SPR2-310.841.01.07.02 Siemens AG
Electrical voltage!
See chapter 1, Safety Information.
¹ Switch OFF the ARCADIS system and wait approximately
3 minutes for the voltage in the I.I. mini-voltage supply to
drop.
Page 48 of 98
08.07 CS PS SP
Medical Solutions
Page 49

Main System - Replacing Components 49
3. Disconnect all cables from the camera.
4. Remove the I.I. optics (incl. camera) refer to "Removing the I.I. Optics"
NOTE
Do not loosen the eccentric screws on the edge of the optics!
These are used to center the optics with respect to the I.I. output
screen. When removing the optics, make sure that no dust or dirt
contaminates the I.I. output window or the optics.
5. Disconnect the cables from the I.I. mini-power supply.
6. Rotate the C-arm so that the I. I. is underneath and the tube assembly is on top.
CAUTION
Risk of crushing!
See chapter 1, Safety Information.
¹ Apply all C-arm brakes.
7. Remove the attachment screws from the I.I. mounting. When doing this, hold onto the
I.I. Remove the I.I. from the mounting.
8. Remove and disconnect the mini-power supply from the I. I.
Installing the new I.I. 0
NOTE
When installing the optics make sure that no dust or dirt contaminates the I.I. output window or the optics.
1. Install and connect the mini-power supply.
2. Install (new) I. I., optic and camera in reverse order.
3. Connect the camera cables in reverse order.
Checks and adjustments 0
1. Check the I.I. electrode voltages according to the test protocol for the I.I.
2. Do not readjust in cases where there are only slight deviations from the values on the
test protocol (measurement device tolerances).
3. Check the centering, reproduction scale and camera focusing. Refer to the previous
description "Adjusting the Camera and I. I.Optics".
4. Check the setting of the X-iris and readjust it as necessary.
5. Check the display of the blades on the monitor and readjust it if necessary.
6. Check the dose rate and readjust it if necessary.
7. Perform the IQ test. Check the resolution first and readjust the optical resolution of the
I.I. optics if necessary. Refer to the previous description "Adjusting the Camera and I.I.
Optics".
8. Complete the country-specific acceptance (§16 partial acceptance... /DHHS...).
Siemens AG SPR2-310.841.01.07.02 ARCADIS Varic
Medical Solutions
08.07 CS PS SP
Page 49 of 98
Page 50

50 Main System - Replacing Components
9. Solder the 2 wires to the cassette switch and remount the I. I. cover.
WARNING
When these service tasks have been performed, the accuracy of
an optionally installed navigation system is no longer guaranteed.
See the chapter "Prerequisites," section "Safety information and
protective measures."
¹ If an optionally installed navigation system is present,
the customer must be notified that the accuracy of the
installed navigation system is no longer guaranteed, and
that the navigation system must be checked and certified
before it is used again.
ARCADIS Varic SPR2-310.841.01.07.02 Siemens AG
08.07 CS PS SP
Page 50 of 98
Medical Solutions
Page 51

Main System - Replacing Components 51
Dose area product measuring device 2.14
1. Switch the system off.
2. Remove the single-tank cover.
3. Replace the dose area product measuring device.
4. Open the local service and select <Main System>-<Adjustment>-<DAP/Air Kerma>
and check the accuracy of the dose area product measuring device.
¹ Perform a DAP accuracy check if DAP display is configured on the monitor.
¹ Perform an Air Kerma accuracy check if Air Kerma display is configured on the
monitor.
Siemens AG SPR2-310.841.01.07.02 ARCADIS Varic
Medical Solutions
08.07 CS PS SP
Page 51 of 98
Page 52

52 Main System - Replacing Components
Laser targeting device (SIREPHOS-side) 2.15
CAUTION
1. Remove the SIREPHOS cover. See chapter (Cover panels / p. 20).
2. Replace the laser diodes after removing the bracket and unsolder the wires.
3. Adjust the laser diodes using the adjustment device supplied with the laser targeting
device.
4. Reattach the SIREPHOS cover.
5. Seal the SIREPHOS cover using sealing compound. See chapter 1.
Laser emissions!
See the chapter "Prerequisites," section "Safety information and
protective measures."
¹ Do not look directly into the laser beam!
ARCADIS Varic SPR2-310.841.01.07.02 Siemens AG
08.07 CS PS SP
Page 52 of 98
Medical Solutions
Page 53

Main System - Replacing Components 53
Integrated Laser light localizer (I.I. -side) 2.16
CAUTION
1. Remove the I.I. ring at the I.I. input screen.
2. Remove the integrated I.I. laser light localizer and disconnect the small voltage plug,
after removing the I.I. ring.
3. Remove the grid from the defective integrated I.I. laser light localizer and insert it at the
new integrated I.I. laser light localizer .
4. Connect the voltage plug of the new integrated I.I. laser light localizer to the voltage
plug of the I.I.
5. Attach the laser light localizer including the grid and the I.I. ring to the I.I. and fasten it
with the screws.
6. Adjust the laser diode beams to the center point of the single tank. The I.I. should be on
top, the SIREPHOS should be at the bottom, the C-arm angulation should be in the 0
degree position.
WARNING
Laser emissions!
See the chapter "Prerequisites," section "Safety information and
protective measures."
¹ Do not look directly into the laser beam!
When these service tasks have been performed, the accuracy of
an optionally installed navigation system is no longer guaranteed.
See the chapter "Prerequisites," section "Safety information and
protective measures."
¹ If an optionally installed navigation system is present,
the customer must be notified that the accuracy of the
installed navigation system is no longer guaranteed, and
that the navigation system must be checked and certified
before it is used again.
Siemens AG SPR2-310.841.01.07.02 ARCADIS Varic
Medical Solutions
08.07 CS PS SP
Page 53 of 98
Page 54

54 Main System - Replacing Components
Replacing the C-arm rollers 2.17
CAUTION
Prerequisite:
- 2 persons for moving the C-arm in and out.
- Table with sufficient stability for holding the C-arm.
- Complete set of rollers (4 single rollers and 1 roller support).
Risk of crushing!
Risk of minor to medium physical injuries.
¹ As soon as the C-arm is relieved, the unit tilts easily to
the rear.
¹ Before the C-arm is relieved, secure the basic unit
against tilting to the rear.
Securing the basic unit against tilting 0
1. Push a wooden block or other stable object of sufficient height under the middle of the
basic unit (1/Fig. 14 / p. 54).
Fig. 14: Securing the basic unit
ARCADIS Varic SPR2-310.841.01.07.02 Siemens AG
08.07 CS PS SP
Page 54 of 98
Medical Solutions
Page 55

Main System - Replacing Components 55
Set the C-arm and lifting column to the working position 0
1. The ARCADIS is plugged into the mains and switched on for a short time.
2. Move the vertical lifting column into its highest position.
3. Switch the ARCADIS system off and separate it from the mains by pulling out the mains
plug.
4. Remove the SIREPHOS cover.
5. Remove the covers (3/Fig. 14 / p. 54) of the C-arm support.
6. Unscrew the cable holder on the C-arm support (2/Fig. 14 / p. 54).
7. Turn the C-arm to +90 degrees or -90 degrees; the C-arm is horizontal as shown in
(Fig. 14 / p. 54).
8. Plug the system into the mains and switch it on for a short time.
9. Position the C-arm over a table with sufficient stability.
10. Move the vertical lifting column so that the C-arm including the image intensifier and
SIREPHOS rest lightly on the table.
11. Switch system off and separate it from the mains. Pull out the mains plug.
Removing the SIREPHOS 0
1. Apply the angulation brake and the orbital brake of the C-arm.
2. Remove the end stop (rubber buffer) on the SIREPHOS side. The bolt with the mounting screws of the SIREPHOS is visible.
3. Remove all connection cables from the C-arm cable to the SIREPHOS.
4. Remove all connection cables to the collimator.
5. If a laser targeting device is present, unsolder the cables.
6. If a DIAMENTOR is present, disconnect the cable from the dose measuring chamber.
7. The image intensifier and C-arm must rest on the table.
8. One person holds the SIREPHOS, while the second person loosens the mounting
screws of the SIREPHOS.
9. Remove the SIREPHOS from the table and set it on a suitable base.
Removing the C-arm 0
1. The C-arm can now be turned completely out of the C-arm support.
2. Make sure that the image intensifier and C-arm always remain on the table.
3. All rollers are now accessible.
Siemens AG SPR2-310.841.01.07.02 ARCADIS Varic
Medical Solutions
08.07 CS PS SP
Page 55 of 98
Page 56

56 Main System - Replacing Components
Replacing the rollers and reinstalling the C-arm 0
1. Replace the 4 rollers for lateral guidance. To do this, remove the locking ring, replace
the rollers and refit the locking ring.
2. Replace the complete roller support .
3. Then thread the C-arm between the inner and outer rollers.
4. Mount the SIREPHOS back on the C-arm using Allen screws. Be careful not to crush
the cables.
5. Fasten the protective ground wire back to the SIREPHOS.
6. Reinstall all cable connections to the SIREPHOS, collimator and possibly available
laser targeting device and Diamentor ionization chamber options.
7. Plug the system into the mains and switch it on for a short time.
8. Move the vertical lifting column out completely and remove the table.
9. Switch the system off and separate it from the mains. Pull out the mains plug.
10. Remove the wooden block.
11. Fasten the cable holder of the C-arm cable back to the C-arm support.
Attaching the covers 0
1. Once again, use sealing compound 2049716 to seal the SIREPHOS cover completely.
2. Refit the SIREPHOS cover. If necessary, apply side pressure to the SIREPHOS cover
until the sealing compound hardens.
3. Refit the rubber buffer (orbital movement end stop) on the SIREPHOS side.
4. Refit the lateral covers of the C-arm support.
5. Refit the rear cover of the basic unit. Make sure the protective ground wire makes good
contact.
6. Refit any other removed covers and make sure that the protective ground wires are in
good contact.
Checks 0
1. Check the orbital movement in the vertical and horizontal angulation position of the
C-arm.
2. Check the orbital movement forces in an unbraked and braked condition according to
the service instructions.
3. Plug the ARCADIS system into the mains and switch it on.
4. Check the collimator settings (iris diaphragm and slot diaphragms) according to the service instructions.
5. If a cassette holder is present, check the cassette exposure collimation.
6. If a laser targeting device is present, check the function of the laser targeting device.
7. If the optional Diamentor is present, check its functioning.
ARCADIS Varic SPR2-310.841.01.07.02 Siemens AG
08.07 CS PS SP
Page 56 of 98
Medical Solutions
Page 57

Main System - Replacing Components 57
8. Check the image quality according to the IQ test.
9. Perform the protective ground wire test according to TD00-000.860.01.... The protec-
tive ground wire resistance must not exceed 0.2 ohms.
WARNING
When these service tasks have been performed, the accuracy of
an optionally installed navigation system is no longer guaranteed.
See the chapter "Prerequisites," section "Safety information and
protective measures."
¹ If an optionally installed navigation system is present,
the customer must be notified that the accuracy of the
installed navigation system is no longer guaranteed, and
that the navigation system must be checked and certified
before it is used again.
Siemens AG SPR2-310.841.01.07.02 ARCADIS Varic
Medical Solutions
08.07 CS PS SP
Page 57 of 98
Page 58

58 Monitor Trolley - Replacing Components
Replacing the power supply assembly 0
3- 3Monitor Trolley - Replacing Compo nents
WARNING
NOTE
Electrical voltage!
See chapter 1, Safety Information.
¹ Switch system power supply off, disconnect the power
plug and disconnect all power plugs from the UPS.
The power supply assembly is programmed to 230V AC mains
voltage. Ensure that the new power supply is programmed to the
local mains voltage value and the fuses F1 and F2 are changed to
the correct value, as printed in the circuit diagram of the system.
Fig. 15: Power supply
1. Switch off the system.
2. Remove the system power plug.
3. Disconnect all UPS power plugs (input and output plugs).
4. Unscrew the 4 screws from the rear cover and remove the cover (1/Fig. 15 / p. 58)
5. Unscrew the 6 screws from the lower rear cover and remove the cover (cover already
removed in (Fig. 15 / p. 58)).
6. Unscrew the 4 Allen screws (2/Fig. 15 / p. 58) for the power supply component.
ARCADIS Varic SPR2-310.841.01.07.02 Siemens AG
08.07 CS PS SP
Page 58 of 98
Medical Solutions
Page 59

Monitor Trolley - Replacing Components 59
7. Pull the power supply component about 10cm out of the trolley.
Fig. 16: D 50
8. Disconnect the power line at connector D50 X 11
¹ Live Monitor - X 11.2 blue / X11.5 brown
¹ Imaging System (PC) - X 11.3 blue / X11.6 brown
¹ UPS output - X 11.1 blue / X 11.4 brown
9. Disconnect the power line at connector D50 X 2
¹ Reference Monitor - X 2.1 blue / X 2.2 brown
¹ Paper Printer (option) - X 2.1 blue / X 2.2 brown
10. Disconnect the power line at connector D50 X 4
¹ UPS input - X 4.2 blue / X 4.3 brown
11. Disconnect the display unit cable at connector D50 X 3
¹ X 3.1 = green / x 3.2 = red / X 3.3 = yellow / X 3.4 = black / X 3.5 = violet / X 3.6 =
blue
12. Disconnect the ground wires coming from the monitor trolley and the monitor cable .
13. Unplug D50 X6, X7, X8, X9, X12 and the X13 connector.
14. Unplug the PC connectors D 66 X4 (BNC) and D66 X5 (Fig. 22 / p. 68) and pull these
cables down.
15. Remove the cable clamps from the SG-cable and the power cable.
Siemens AG SPR2-310.841.01.07.02 ARCADIS Varic
Medical Solutions
08.07 CS PS SP
Page 59 of 98
Page 60

60 Monitor Trolley - Replacing Components
16. Disconnect and pull the SG-cable and the power cable out from the power supply
assembly.
17. Remove the power supply component from the monitor trolley.
18. Program the mains voltage of the new power supply assembly (transformer T1 and T2)
to the local mains voltage value.
19. Check the fuse values of the fuses F1 and F2.
¹ If needed, replace them with fuses with the correct values for the programmed
local mains voltage value (100V AC to 127V AC: 20A slow blow / 200V AC to
240V AC: 15A slow blow, see wiring diagram of the system).
20. Install the new power supply component in reverse order.
21. Reconnect all power plugs to the UPS.
22. Switch on the system and carry out a functional test.
ARCADIS Varic SPR2-310.841.01.07.02 Siemens AG
08.07 CS PS SP
Page 60 of 98
Medical Solutions
Page 61

Monitor Trolley - Replacing Components 61
Replacing the UPS 3.1
WARNING
Electrical voltage!
See chapter 1, Safety Information.
¹ Switch system power supply off, disconnect the power
plug and disconnect all power plugs from the UPS.
Fig. 17: Replacing the UPS_2
1. Remove the rear cover from the monitor trolley.
2. Disconnect all power plugs from the UPS.
3. Loosen the tension band (1/Fig. 17 / p. 61) and remove the UPS from the monitor trol-
ley. Side covering was already removed in (Fig. 17 / p. 61).
4. Switch off the UPS by pressing the power switch.
NOTE
UPS MGE type 850: To switch the UPS off or on, the power switch
needs to be pressed for > 2 seconds.
5. Switch on the new UPS at its power switch.
6. Install the new UPS component in reverse order.
7. Switch on the system and carry out a functional test.
Siemens AG SPR2-310.841.01.07.02 ARCADIS Varic
Medical Solutions
08.07 CS PS SP
Page 61 of 98
Page 62

62 Monitor Trolley - Replacing Components
NOTE
For correct functionality of the UPS, the new battery should be
charged for approx. 8 hours. If possible and after all service tasks
are performed, switch the system off, but leave the monitor trolley
connected to the mains voltage wall socket. As long as the power
plug of the monitor trolley is plugged in the mains voltage wall
socket, the UPS battery will be charged.
ARCADIS Varic SPR2-310.841.01.07.02 Siemens AG
08.07 CS PS SP
Page 62 of 98
Medical Solutions
Page 63

Monitor Trolley - Replacing Components 63
Replacing the UPS battery 3.2
WARNING
CAUTION
Electrical voltage!
See chapter 1, Safety Information.
¹ Switch system power supply off, disconnect the power
plug and disconnect all power plugs from the UPS.
Risk of burns and destroying the battery component due to possible high short-circuit current!
Wearing metal objects like rings, watches bracelets and using
tools with uninsulated handles during procedures can lead to a
short-circuit of the battery contacts. This can destroy the battery
component and can heat up the metal objects, causing a
short-circuit at very high temperatures.
¹ Before servicing the battery components, remove
watches, rings, bracelets and all other metal objects from
the hands and arms, Use tools with insulated handles.
UPS MGE Type 800 0
Fig. 18: Replacing the UPS_battery_1
Siemens AG SPR2-310.841.01.07.02 ARCADIS Varic
Medical Solutions
08.07 CS PS SP
Page 63 of 98
Page 64

64 Monitor Trolley - Replacing Components
Fig. 19: Replacing the UPS_battery_2
1. Disconnect all power plugs from the UPS.
2. Remove the UPS from the monitor trolley.
3. Switch off the UPS by pressing the power switch on the UPS.
4. Remove the plastic cover “MGE UPS Systems”” (1/Fig. 18 / p. 63) from the UPS.
5. Unscrew the 2 slotted grub screws (2/Fig. 18 / p. 63) from the UPS and remove the
front cover.
6. Disconnect the connector (1/Fig. 19 / p. 64) and pull the battery out of the slot.
7. Insert the new battery into the UPS.
8. Connect the battery connector in front of the battery.
NOTE
Make sure that the battery connector contacts are not bent when
plugged in! Check the contact (1/Fig. 19 / p. 64) if the system does
not switch on .
9. Install the UPS front cover and fasten it with the screws.
10. Install the UPS plastic cover “MGE UPS Systems”.
11. Switch on the UPS by pressing the power switch on the UPS.
12. Insert the UPS component in reverse order into the monitor trolley.
ARCADIS Varic SPR2-310.841.01.07.02 Siemens AG
08.07 CS PS SP
Page 64 of 98
Medical Solutions
Page 65

Monitor Trolley - Replacing Components 65
NOTE
For correct functionality of the UPS, the new battery should be
charged for approx. 8 hours. If possible and after all service tasks
are performed, switch the system off, but leave the monitor trolley
connected to the mains voltage wall socket. As long as the power
plug of the monitor trolley is plugged in the mains voltage wall
socket, the UPS battery will be charged.
MGE UPS Type 850 0
Fig. 20: UPS MGE 850 Battery change
1. Disconnect all power plugs from the UPS.
2. Remove the UPS from the monitor trolley.
3. Switch off the UPS by pressing the power switch at the UPS for more than 2 seconds.
4. Lay the UPS sideward, as shown in (Fig. 20 / p. 65).
5. Remove the 2 screws on the bottom of the UPS, as shown in A, (Fig. 20 / p. 65).
6. Lift up and pull up the front cover, as shown in B, (Fig. 20 / p. 65).
7. Disconnect the battery connector in front of the battery and pull the battery out of the
slot.
8. Insert the new battery into the UPS.
9. Connect the battery connector in front of the battery.
Siemens AG SPR2-310.841.01.07.02 ARCADIS Varic
Medical Solutions
08.07 CS PS SP
Page 65 of 98
Page 66

66 Monitor Trolley - Replacing Components
NOTE
Make sure that the battery connector contacts are not bent when
plugged in! Check the contact (1/Fig. 19 / p. 64) if the system does
not switch on .
10. Install the UPS front cover and fasten it with the screws.
11. Switch on the UPS by pressing the power switch on the UPS for more than 2 seconds.
12. Insert the UPS component in reverse order into the monitor trolley.
NOTE
For correct functionality of the UPS, the new battery should be
charged for approx. 8 hours. If possible and after all service tasks
are performed, switch the system off, but leave the monitor trolley
connected to the mains voltage wall socket. As long as the power
plug of the monitor trolley is plugged in the mains voltage wall
socket, the UPS battery will be charged.
ARCADIS Varic SPR2-310.841.01.07.02 Siemens AG
08.07 CS PS SP
Page 66 of 98
Medical Solutions
Page 67

Monitor Trolley - Replacing Components 67
Replacing the keyboard 3.3
Fig. 21: Replacing the keyboard_3
1. Remove the 4 screws (1/Fig. 21 / p. 67).
2. Lift the keyboard upward out of the monitor trolley.
3. Unplug the keyboard connectors.
4. Install the new keyboard in reverse order.
5. Carry out a functional test.
Siemens AG SPR2-310.841.01.07.02 ARCADIS Varic
Medical Solutions
08.07 CS PS SP
Page 67 of 98
Page 68

68 Monitor Trolley - Replacing Components
Replacing the PC 3.4
Connection
1 UPS control
2 Keyboard
3 Mouse
4 Printer connect parallel
5 COM 1
6 Dongle
7 Printer connect USB
8 LAN connector
9 Monitor B
10 Monitor A
11 M50 D20 X9
12 D66 X5
13 D66 X4 (Gigalink)
14 Adapter plug COM1
15 Net plug (only M420 / 430)
16 On/off switch (only M420/430)
17 CAN converter (option)
Fig. 22: PC rear
1. Save patient data and create a backup if possible.
2. Switch off the system and remove the system power plug.
3. Unscrew the 6 screws from the rear cover (1/Fig. 15 / p. 58) and remove the cover.
4. Unplug all cable connections from the PC (Fig. 22 / p. 68).
5. Loosen the tension belt from the PC
6. Remove the old PC and insert the new PC.
7. Reconnect all PC plugs (Fig. 22 / p. 68).
NOTE
All plugs must be connected at shown in the figure above for the
software installation to be successful (see (Fig. 22 / p. 68)).
8. Switch on the system and install the software according to the "System Software Installation" instructions.
9. Carry out a functional test and an IQ Quick test.
ARCADIS Varic SPR2-310.841.01.07.02 Siemens AG
08.07 CS PS SP
Page 68 of 98
Medical Solutions
Page 69

Monitor Trolley - Replacing Components 69
WARNING
When these service tasks have been performed, the accuracy of
an optionally installed navigation system is no longer guaranteed.
See the chapter "Prerequisites," section "Safety information and
protective measures."
¹ If an optionally installed navigation system is present,
the customer must be notified that the accuracy of the
installed navigation system is no longer guaranteed, and
that the navigation system must be checked and certified
before it is used again.
Siemens AG SPR2-310.841.01.07.02 ARCADIS Varic
Medical Solutions
08.07 CS PS SP
Page 69 of 98
Page 70

70 Monitor Trolley - Replacing Components
Replacing PC covers 3.5
Opening the casing 0
PC M420 and 430
Fig. 23: Remove PC covers
1. Switch off the system.
2. Pull the power plug out of the mains outlet.
3. Replace the PC from monitor trolley (Replacing the PC / p. 68).
4. Press the green unlocking button on the rear of the casing (1/Fig. 23 / p. 70).
5. Hold the green unlocking button depressed and slide the casing side cover upwards in
the direction of the arrow (2/Fig. 23 / p. 70).
6. Pull the side cover out of the casing.
PC M450
ARCADIS Varic SPR2-310.841.01.07.02 Siemens AG
08.07 CS PS SP
Page 70 of 98
Medical Solutions
Page 71

Monitor Trolley - Replacing Components 71
Fig. 24: Remove PC cover_a M450_
Fig. 25: Remove PC cover_b M450_
1. Switch off the system.
2. Pull the power plug out of the power outlet.
3. Replace the PC from monitor trolley (Replacing the PC / p. 68).
4. Unscrew the two knurled screws (1/Fig. 24 / p. 71) on the back of the casing.
5. Slide the side cover approximately 2cm (a/Fig. 25 / p. 71) in the direction of the arrow
(1/Fig. 25 / p. 71), until it reaches the stop.
Siemens AG SPR2-310.841.01.07.02 ARCADIS Varic
Medical Solutions
08.07 CS PS SP
Page 71 of 98
Page 72

72 Monitor Trolley - Replacing Components
6. Pull the side cover in the direction of the arrow (2/Fig. 25 / p. 71) of the casing.
Removing the front panel 0
PC M420 and M430
Fig. 26: Removing the front panel
1. Replace the PC from monitor trolley (Replacing the PC / p. 68).
2. Open the casing (Opening the casing / p. 70).
3. Detach the unlocking lever (2/Fig. 26 / p. 72) and open the front panel
(1/Fig. 26 / p. 72).
4. Detach the plastic hook from the front panel of the casing and carefully remove the front
panel. If you pull too hard, you may loosen or damage the LCD cable.
5. The TCD cable is long enough so that you can carefully place it to one side with the
front panel. You do not need to unplug the cable before removing the front panel.
PC M450
ARCADIS Varic SPR2-310.841.01.07.02 Siemens AG
08.07 CS PS SP
Page 72 of 98
Medical Solutions
Page 73

Monitor Trolley - Replacing Components 73
Fig. 27: Opening the front panel M450_
1. Replace the PC from monitor trolley (Replacing the PC / p. 68).
2. Open the casing (Opening the casing / p. 70).
3. Detach the three locking tabs on the left side of the front panel (1/Fig. 27 / p. 73).
4. Fold open the front in the direction of the arrow (2/Fig. 27 / p. 73).
5. If necessary, detach the pivot axle on the right-hand side of the front panel from the casing and carefully remove the front panel.
Siemens AG SPR2-310.841.01.07.02 ARCADIS Varic
Medical Solutions
08.07 CS PS SP
Page 73 of 98
Page 74

74 Monitor Trolley - Replacing Components
Replacing the drive 3.6
PC M420 and M430
Fig. 28: Replacing the drive
1. Replace the PC from monitor trolley (Replacing the PC / p. 68).
2. Open the casing (Opening the casing / p. 70).
3. Remove the front (Removing the front panel / p. 72).
4. Disconnect the data and the power supply connectors from the drive.
5. Press the rails (1/Fig. 28 / p. 74) together and pull the drive out of the casing
(2/Fig. 28 / p. 74).
6. Push the new drive into the casing until the rails engage.
7. Plug the data and the power supply connectors into the drive. Make sure the polarity is
correct.
8. Attach the front panel in reverse order.
E 9. Close the casing in reverse order.
10. Reinstall the PC in reverse order and reconnect the PC plugs (see (Fig. 22 / p. 68)).
11. Carry out a functional test.
PC M450
ARCADIS Varic SPR2-310.841.01.07.02 Siemens AG
08.07 CS PS SP
Page 74 of 98
Medical Solutions
Page 75

Monitor Trolley - Replacing Components 75
Fig. 29: Replacing the drive M450_
1. Replace the PC from monitor trolley (Replacing the PC / p. 68).
2. Open the casing (Opening the casing / p. 70).
3. Remove the front (Removing the front panel / p. 72).
4. Disconnect the data and the power supply connectors from the drive.
5. Loosen the screws (1/Fig. 29 / p. 75).
6. Slide the drive out of the bay in the direction of the arrow (2/Fig. 29 / p. 75) from behind.
The drive now jumps out slightly from the casing.
7. Slide the drive out of the casing (3/Fig. 29 / p. 75).
8. Push the new drive into the casing until the rails engage.
9. Plug the data and the power supply connectors into the drive. Make sure the polarity is
correct.
10. Attach the front panel in reverse order.
E 11. Close the casing in reverse order.
12. Reinstall the PC in reverse order and reconnect the PC plugs (see (Fig. 22 / p. 68)).
13. Carry out a functional test.
Siemens AG SPR2-310.841.01.07.02 ARCADIS Varic
Medical Solutions
08.07 CS PS SP
Page 75 of 98
Page 76

76 Monitor Trolley - Replacing Components
Replacing the lithium battery 3.7
Prerequisite: The software installation CD/DVD for the system (includes BIOS installa-
tion) must be available.
PC M420 and M430
Fig. 30: Replacing the lithium battery_1
PC M450
ARCADIS Varic SPR2-310.841.01.07.02 Siemens AG
08.07 CS PS SP
Page 76 of 98
Medical Solutions
Page 77

Monitor Trolley - Replacing Components 77
Fig. 31: Components M450_
Fig. 32: Replacing the lithium battery
Siemens AG SPR2-310.841.01.07.02 ARCADIS Varic
Medical Solutions
08.07 CS PS SP
Page 77 of 98
Page 78

78 Monitor Trolley - Replacing Components
NOTE
In order to permanently save the system information, a lithium
battery is installed to provide the CMOS memory with current. An
appropriate error message notifies the user when the charge is
too low or the battery is empty. The lithium battery must be
replaced.
Incorrect replacement of the lithium battery may lead to a risk of
explosion!
The lithium battery may be replaced only by an identical battery or
a type recommended by the manufacturer.
Make sure that you insert the battery correctly. The plus pole must
be on top!
Do not touch the minus pole of the battery with your finger. Use
gloves or a cotton cloth to insert the battery.
1. Replace the PC from monitor trolley (Replacing the PC / p. 68).
2. Open the casing (Opening the casing / p. 70).
3. Press the locking lug in the direction of the arrow (1/Fig. 32 / p. 77); the battery jumps
slightly out of the holder (2/Fig. 32 / p. 77).
E 4. Remove the battery M420/M430 (Fig. 30 / p. 76) or M450 (Fig. 31 / p. 77) and follow
the "Disposal Instructions" for disposing of the old battery.
5. Push the new lithium battery of the identical type into the holder (3/Fig. 32 / p. 77) and
press it downward until it engages (4/Fig. 32 / p. 77).
6. Reinstall the casing in reverse order.
7. Reinstall the PC in reverse order and reconnect the PC plugs (see (Fig. 22 / p. 68)).
Restoring BIOS settings (necessary after battery replacement) 0
NOTE
When the battery is empty, the BIOS is set to default and the first
boot device is the CD-ROM drive.
In addition to activating the system on/off switch, the following
may be necessary ->
- Turn off the PC via the power switch at the rear panel of the PC.
- Turn on the PC via the rear power switch and press the push button on the front panel of the PC.
- If the system is unintentionally switched off during BIOS installation, it may be necessary to press the reset key (2/Fig. 21 / p. 67)
with a pin shaped object.
ARCADIS Varic SPR2-310.841.01.07.02 Siemens AG
08.07 CS PS SP
Page 78 of 98
Medical Solutions
Page 79

Monitor Trolley - Replacing Components 79
NOTE
At this time a restore of the BIOS settings is only possible
together with the complete software installation procedure.
--> Perform software installation according to instruction
SPR2-310.816.02...
Siemens AG SPR2-310.841.01.07.02 ARCADIS Varic
Medical Solutions
08.07 CS PS SP
Page 79 of 98
Page 80

80 Monitor Trolley - Replacing Components
Replacing a PCI board PC M420 and M430 (e. g. CIPP board) 3.8
Fig. 33: Replacing a PCI board_1
ARCADIS Varic SPR2-310.841.01.07.02 Siemens AG
08.07 CS PS SP
Page 80 of 98
Medical Solutions
Page 81

Monitor Trolley - Replacing Components 81
Fig. 34: Replacing a PCI board_2
Fig. 35: Replacing a PCI board_3
Siemens AG SPR2-310.841.01.07.02 ARCADIS Varic
Medical Solutions
08.07 CS PS SP
Page 81 of 98
Page 82

82 Monitor Trolley - Replacing Components
NOTE
Work steps 1 through 6 are only necessary for a long design of a
PCI board (e.g. CIPP board ) (see (Fig. 30 / p. 76)
See ARCADIS spare parts list for which boards are released for
field exchange.
1. Replace the PC from monitor trolley (Replacing the PC / p. 68).
E 2. Open the casing (Opening the casing / p. 70).
3. Loosen the screw (1/Fig. 33 / p. 80).
4. Fold out the cover (2/Fig. 33 / p. 80).
5. Disconnect the data and power supply cables to the hard disk drive.
6. Press the locking bar (1/Fig. 34 / p. 81) downward and pull the hard drive casing
(2/Fig. 34 / p. 81) out of the PC.
7. Press the unlocking mechanism (1/Fig. 35 / p. 81) downward and open the locking rail
(2/Fig. 35 / p. 81). The word "PRESS" is embossed on the unlocking mechanism.
8. Remove the locking screws from the relevant slot.
9. Disconnect the connectors from the PCI board.
E 10. Remove the board from the slot (3/Fig. 35 / p. 81).
11. Take the new board out of its packaging and install it in reverse order.
12. Reinstall the casing in reverse order.
13. Reinstall the PC in reverse order and reconnect the PC plugs (see (Fig. 22 / p. 68)).
14. Carry out a functional test and an IQ Quick test.
WARNING
When these service tasks have been performed, the accuracy of
an optionally installed navigation system is no longer guaranteed.
See the chapter "Prerequisites," section "Safety information and
protective measures."
¹ If an optionally installed navigation system is present,
the customer must be notified that the accuracy of the
installed navigation system is no longer guaranteed, and
that the navigation system must be checked and certified
before it is used again.
ARCADIS Varic SPR2-310.841.01.07.02 Siemens AG
08.07 CS PS SP
Page 82 of 98
Medical Solutions
Page 83

Monitor Trolley - Replacing Components 83
Replacing a PCI board PC M450 (e. g. CIPP board) 3.9
Fig. 36: Removing the side fan M450_
Fig. 37: Components M450_
Siemens AG SPR2-310.841.01.07.02 ARCADIS Varic
Medical Solutions
08.07 CS PS SP
Page 83 of 98
Page 84

84 Monitor Trolley - Replacing Components
Fig. 38: Replacing a PCI board M450_
1. Replace the PC from monitor trolley (Replacing the PC / p. 68).
E 2. Open the casing (Opening the casing / p. 70).
3. Release the side fan by pressing the locking hook ((a/Fig. 36 / p. 83) in the direction of
the arrow (1/Fig. 36 / p. 83).
4. Pull the bottom edge of the side fan in the direction of the arrow (2/Fig. 36 / p. 83) out of
the casing.
5. Disconnect the cable of the fan from the main board and remove the side fan.
6. Remove the mounting block (mounting block/Fig. 37 / p. 83)
7. Press on the clips (1/Fig. 38 / p. 84) in the direction of the arrow and unhook them from
the casing rear panel.
8. Fold open the locking rail in the direction of the arrow (2/Fig. 38 / p. 84).
9. Disconnect the connectors from the PCI board.
10. Remove the board from the slot (3/Fig. 38 / p. 84)
11. Take the new board out of its packaging and install it in reverse order.
12. Reinstall the casing in reverse order (Fig. 23 / p. 70).
13. Reinstall the PC in reverse order and reconnect the PC plugs (see (Fig. 22 / p. 68)).
14. Carry out a functional test and an IQ Quick test.
ARCADIS Varic SPR2-310.841.01.07.02 Siemens AG
08.07 CS PS SP
Page 84 of 98
Medical Solutions
Page 85

Monitor Trolley - Replacing Components 85
WARNING
When these service tasks have been performed, the accuracy of
an optionally installed navigation system is no longer guaranteed.
See the chapter "Prerequisites," section "Safety information and
protective measures."
¹ If an optionally installed navigation system is present,
the customer must be notified that the accuracy of the
installed navigation system is no longer guaranteed, and
that the navigation system must be checked and certified
before it is used again.
Siemens AG SPR2-310.841.01.07.02 ARCADIS Varic
Medical Solutions
08.07 CS PS SP
Page 85 of 98
Page 86

86 Monitor Trolley - Replacing Components
Replacing or installing a double USB slot PC M420 and M430 (optional for CAN converter)
3.10
Fig. 39: Replacing or installing a double USB slot_1
ARCADIS Varic SPR2-310.841.01.07.02 Siemens AG
08.07 CS PS SP
Page 86 of 98
Medical Solutions
Page 87

Monitor Trolley - Replacing Components 87
Fig. 40: Replacing or installing a double USB slot_2
Fig. 41: Replacing or installing a double USB slot_3
E 1. Open the casing (Opening the casing / p. 70).
2. Press the unlocking mechanism (1/Fig. 35 / p. 81) downward and open the locking rail
(2/Fig. 35 / p. 81). The word "PRESS" is embossed on the unlocking mechanism. .
NOTE
Slot 3 should be used for the double USB assembly.
3. Replace the DVP3 board (Fig. 30 / p. 76) according to (Replacing a PCI board PC
M420 and M430 (e. g. CIPP board) / p. 80).
4. Disconnect the connector USB G/H (16/Fig. 39 / p. 86) from the main board
(Fig. 39 / p. 86).
5. Reinstall the double USB connector in the slot assembly.
6. Position and connect the USB cable as shown in (Fig. 41 / p. 87).
Siemens AG SPR2-310.841.01.07.02 ARCADIS Varic
Medical Solutions
08.07 CS PS SP
Page 87 of 98
Page 88

88 Monitor Trolley - Replacing Components
NOTE
NOTE
The cable position must be as shown in Fig. (Fig. 40 / p. 87) and
Fig. (Fig. 41 / p. 87).
Make sure that the distance between the graphic board fan and the
USB cable is fixed.
7. Reinstall the DVP3 board in reverse order.
8. Reinstall the casing in reverse order (Fig. 23 / p. 70).
9. Reinstall the PC in reverse order and reconnect the PC plugs (see (Fig. 22 / p. 68)).
10. Carry out a functional test.
ARCADIS Varic SPR2-310.841.01.07.02 Siemens AG
08.07 CS PS SP
Page 88 of 98
Medical Solutions
Page 89

Voltages 89
Monitor trolley voltages 0
4- 4Voltages
Secondary voltages for the line voltage; table 1
Voltage for Test point Voltage Comment
Generator X3.1 -- X3.2 200 V ~ to 215 V ~ To main system
Generator X3.5 -- X3.6 230 V ~ to 246 V ~ To main system
ASPIA PC, live
monitor
Reference monitor,
Paper printer
UPS input X4.2 -- X4.3 230 V ~ to 246 V ~ Supply voltage
X11.1 -- x11.4 230 V ~ to 246 V ~ From UPS output
X2.1 -- X2.2 230 V ~ to 246 V ~ Paper printer = option
Siemens AG SPR2-310.841.01.07.02 ARCADIS Varic
Medical Solutions
08.07 CS PS SP
Page 89 of 98
Page 90

90 Voltages
Main system voltages 4.1
Operating voltages; table 2.
Voltage Tolerance Test point Potentiometer LED
+5V + 0.2 V D1 TP Vcc -- D1 TP ⊥ D M14. +5 V/adj. V80
+15V ± 0.1 V D1 TP +15V -- D1 TP ⊥ A M14. +15 V/adj. n.a.
-15V ± 0.5 V D1 TP -15V -- D1 TP ⊥ A M14. -15 V/adj. n.a.
+13V ± 0.2 V M13 +13V -- M13 GND n.a. n.a.
+27V - 0.5 V D3X3.2 -- D3 X3.3 n.a. n.a.
+24V + 5.5V D3X1.9 -- D3 X1.7 n.a. n.a.
200V~ +15V Generator line filter
Z1.3 -- Z1.4
230V~ +16V M14 N.2 -- M14 N.1 n.a. n.a.
n.a. n.a.
ARCADIS Varic SPR2-310.841.01.07.02 Siemens AG
08.07 CS PS SP
Page 90 of 98
Medical Solutions
Page 91

Voltages 91
I.I. voltages 4.2
NOTE
The needed electrode voltages of the I.I. are printed in the I.I. test
certificate 1, delivered with the I.I.
Roederstein I.I. mini voltage supply 0
The E1/E2/E3 and A voltages may be taken from the I.I. test protocol and checked or
adjusted on the basis of the control test points listed in table 3.
Voltage Test point Ground
point
(0V)
E1 UE1 ⊥ P10 P11 1:1
E2 UE2 ⊥ P6 P7 1:1
E3 UI 15 ⊥ P2 P3 1:10000
30 kV, anode UI 30 ⊥ P1 P1 1:10000
WARNING
After the electrode voltages are adjusted, the accuracy of an
optionally installed navigation system is no longer guaranteed.
Potentiometer for full
format
Potentiometer
for zoom format
Vol tag e
divider ratio
See the chapter "Prerequisites," section "Safety Information and
protective measures."
¹ If an optionally installed navigation system is present,
the customer must be notified that the accuracy of the
installed navigation system is no longer guaranteed, and
that the navigation system must be checked and certified
before it is used again.
Spellman I.I. mini voltage supply 0
The E1/E2/E3 and A voltages may be taken from the I.I. test protocol and checked or
adjusted on the basis of the control test points listed in table 3.
Tab. 1 Test points and voltage divider ratio
Voltage Test point Test point
Ground (0V)
E1 TP1 TP G 1:100
E2 TP2 TP G 1:200
Voltage divider
ratio
Siemens AG SPR2-310.841.01.07.02 ARCADIS Varic
Medical Solutions
08.07 CS PS SP
Page 91 of 98
Page 92

92 Voltages
Voltage Test point Test point
Ground (0V)
Voltage divider
ratio
E3 TP3 TP G 1:3333
Penning TP P TP G 1:1000
Anode TP A TP G 1:10000
Adjustment
Tab. 2 DIP switches
DIP switches 1 2 3 4
Normal status ON OFF OFF OFF
Adjustment E1 (U1) ON OFF ON OFF
Adjustment E2 (U2) ON ON OFF OFF
Adjustment E3 (U3) ON ON ON OFF
NOTE
DIP switch 1:
Pos. OFF = 25kV anode voltage, not adjustable Pos. ON = 30 kV
anode voltage, not adjustable.
For the ARCADIS I.I. types, dip switch 1 must always be set to the
position “ON” (= 30 kV anode voltage).
1. Before performing the voltage adjustment for one of the electrodes, set the corresponding DIP switches to the position described in the“DIP switches” table and connect a digital multimeter to the correct test points, described in the” Test points and voltage divider
ratio” table.
2. According to the activated DIP switch, adjust the E1, E2 or E3 value by using the
“Adjustment” potentiometer.
3. After the correct voltage is adjusted, switch off the corresponding DIP switches.
¹ The adjusted voltage value for this electrode is stored.
• If needed, set the DIP switches for the next electrode adjustment and adjust the next
electrode voltage.
• If needed, select the next I.I. zoom format and repeat the voltage adjustments for the
electrodes E, E2 and E3 for the I.I. zoom format.
ARCADIS Varic SPR2-310.841.01.07.02 Siemens AG
08.07 CS PS SP
Page 92 of 98
Medical Solutions
Page 93

Voltages 93
WARNING
After the electrode voltages are adjusted, the accuracy of an
optionally installed navigation system is no longer guaranteed.
See the chapter "Prerequisites," section "Safety information and
protective measures."
¹ If an optionally installed navigation system is present,
the customer must be notified that the accuracy of the
installed navigation system is no longer guaranteed, and
that the navigation system must be checked and certified
before it is used again.
Siemens AG SPR2-310.841.01.07.02 ARCADIS Varic
Medical Solutions
08.07 CS PS SP
Page 93 of 98
Page 94

94 Brake Force/Lubrication
Brake force calibration 0
5- 5Brake Force/Lubrication
1. Position prerequisites for measuring the forces: angulation, orbital, and horizontal
swivel movements must be at zero on the scale and horizontal movement should be at
20 on the scale.
2. All measurements should be made using a spring balance on the rail near the image
intensifier with the DHHS spacer, if present, installed on the X-ray tube.
Movement
Brake
released:
Horizontal travel 10 N to 40 N 160 N to 200 N n.a.
Angulation movement 10 Nm to 20 Nm 60 Nm to 80 Nm n.a.
Orbital movement 10 Nm to 18 Nm 55 Nm to 70 Nm Measure with the C-arm
Horizontal pivot 5 Nm to 10 Nm 90 Nm to 150 Nm n.a.
Nominal values
Comments
Brake applied:
in the vertical position.
Adjustment of the angulation brake 0
Systems without optional angulation drive
Fig. 42: Angulation brake_1
Fig. 43: Angulation brake_2
• Remove the plug (1/Fig. 42 / p. 94) and adjust the angulation brake with a 6 mm Allen
key (1/Fig. 43 / p. 94).
ARCADIS Varic SPR2-310.841.01.07.02 Siemens AG
08.07 CS PS SP
Page 94 of 98
Medical Solutions
Page 95

Brake Force/Lubrication 95
Systems with optional angulation drive (in conjunction with VARIOSTAR)
Fig. 44: Angulation brake_3
• The angulation brake is adjusted with a grub screw (4 mm Allen key) that can be
reached through a hole on the bottom of the horizontal carriage
(2/Fig. 43 / p. 94),(1/Fig. 44 / p. 95).
Lubricating the vertical column 0
1. The spindle in the vertical column should be lubricated during annual maintenance.
2. Raise the vertical column approximately 50 cm. A drilled hole is visible in the column.
3. Fill this hole with approximately 2 cm
3
of oil.
4. Oil to be used: Special purpose oil (Optimol GmbH; Viscogen KL300; 40 g), item number 73 95 353 RH090.
Supplement, measuring the tube current 0
NOTE
1. Switch the system OFF.
2. Remove jumper X97 from board D1.
3. Connect the mA measuring device at D1.X39 and D1.X40.
When measuring the tube current, the distribution current must
be derived from the measured value. The distribution current
depends on the kV and is calculated according to Ohm‘s law. The
distribution resistance is 400 MOhm.
4. Switch the system ON.
X 5. Release radiation and read the overall current.
Siemens AG SPR2-310.841.01.07.02 ARCADIS Varic
Medical Solutions
08.07 CS PS SP
Page 95 of 98
Page 96

96 Brake Force/Lubrication
6. Read out the kV value produced while the current is being measured.
Calculate the tube current as follows:
high voltage [kV]
tube current [mA] = overall current [mA] - ___________________________________
distribution resistance [MOhm]
e.g.:
At 110 kV, an overall current of 5.275 mA is measured.
110 [kV]
tube current [mA] = 5.275 [mA] - ___________ = 5.275 [mA] - 0.275 [mA] = 5.0 [mA]
400 [MOhm]
ARCADIS Varic SPR2-310.841.01.07.02 Siemens AG
08.07 CS PS SP
Page 96 of 98
Medical Solutions
Page 97

Changes to the previous version 97
6- 6Changes to the previous version
Section “Adjustment of the angulation brake” added.
Siemens AG SPR2-310.841.01.07.02 ARCADIS Varic
Medical Solutions
08.07 CS PS SP
Page 97 of 98
Page 98

98 Changes to the previous version
ARCADIS Varic SPR2-310.841.01.07.02 Siemens AG
08.07 CS PS SP
Page 98 of 98
Medical Solutions
 Loading...
Loading...