Page 1

SR-L6111W, SF-L6111W
Application Specific Preservation,
Vaccine Storage
MPR-1410/1410R
MPR-721/721R
MPR-214F, MPR-414F
Ideally suited for vaccine storage in clinics, hospitals, retail
pharmacies and laboratories. Size range includes compact
undercounter freezers and refrigerators to large capacity
combination units in upright configurations. SANYO provides
purpose-built equipment to meet your requirements of precise and
stable temperature control and monitoring of your vaccine supply.
Model: SR-L6111W & MPR-414F
Page 2
Precise and Stable Vaccine Storage Environment
Function creates form
Enviro-Center
Polyester resin finish baked on zinc galvanized steel
CFC-free rigid polyurethane foamed in place
Microprocessor control
small: 3(15kg/shelf)
4 casters with 2 adjustable leveling feet
Self diagnostics, remote alarm contact (DC30V,2A), Door key lock
2-pen type circular recorder (MTR-G3504), Mounting kit (MPR-S7)
SANYO DAQ System (MTR-480 & MTR-2000)
Function creates form
Enviro-Center
MPR-214F MPR-414F/MPR-414FS
Refrigerator Freezer Refrigerator Freezer
540 x 557 x 1.790 mm 800 x 600 x 1.805 mm
21.2x21.9x70.4(inch) 31.5x23.6x71.0 (inch)
176 liters (6.2 cu.ft.) 39 liters (1.4 cu.ft.) 340 liters (12.0 cu.ft.) 82 liters (2.9 cu.ft.)
80 kg 414F: 126 kg / 414FS: 119 kg
Polyester resin finish baked on zinc galvanized steel
Styrol resin Colored aluminium plate Stainless steel Colored aluminium plate
CFC-free rigid polyurethane foamed in place
2 to 14˚C -20 to -30˚C 2 to 14˚C -20 to -30˚C
Ambient temp. : 35ºC Ambient temp. : 30ºC Ambient temp. : 35ºC Ambient temp. : 30ºC
Microprocessor control
Fan-Forced air circulation Direct cooling Fan-Forced air circ. Direct cooling
60W 60W 160W 160W
R-134A (HFC) R-134A (HFC) R-407D (HFC)
3(20kg/shelf) 1(10kg/shelf) large: 2(25kg/shelf) 1(15kg/shelf)
small: 3(15kg/shelf)
ø 30mm (left) ø 30mm (left) ø 30mm (rear) ø 30mm (rear)
4 casters with 2 adjustable leveling feet
High/low temperature alarm, Door ajar alarm, Memory back up during power failure
Self diagnostics, remote alarm contact (DC30V,2A), Door key lock
2-pen type circular recorder (MTR-G3504), Mounting kit (MPR-S7)
Drawers for the bottom left compartment (MPR-41R): MPR-414F/FS only
SANYO DAQ System (MTR-480 & MTR-2000)
MPR-414F/414FS MPR-214F
Yes Yes
Yes Yes
Yes Yes
Yes Yes
Yes Yes
Yes Yes
Globally, inadequate refrigeration and inadequate storage
conditions have attributed to depleting our already diminishing
vaccine supply. This decrease in critical vaccine levels can be
particularly high when multi dose vials are used and where there
is poor stock and clinic management. WHO and UNICEF have
estimated that vaccine wastage rates in developing countries
can reach as high as 50% for specific vaccines such as the 1020 dose lyophilised vaccines. According to the CDC, improper
storage is the most common vaccine delivery problem they
encounter. Cold chain errors affect up to 44 million doses, costing
between $433 and $481 million.
• Large temperature uctuations
• Inefcient use of cabinet and shelving space
• Strict loading requirements
• No alarms
Application Specific Preservation
SANYO offers a selection of Pharmaceutical Refrigerators
and Freezers that include signicant design and performance
properties which differentiate them from conventional household
refrigerators that are not suitable for pharmacy use.
Accurate and uniform
temperature distribution in a
refrigerator plays a key role
in ensuring the life of your
Household Refrigeration
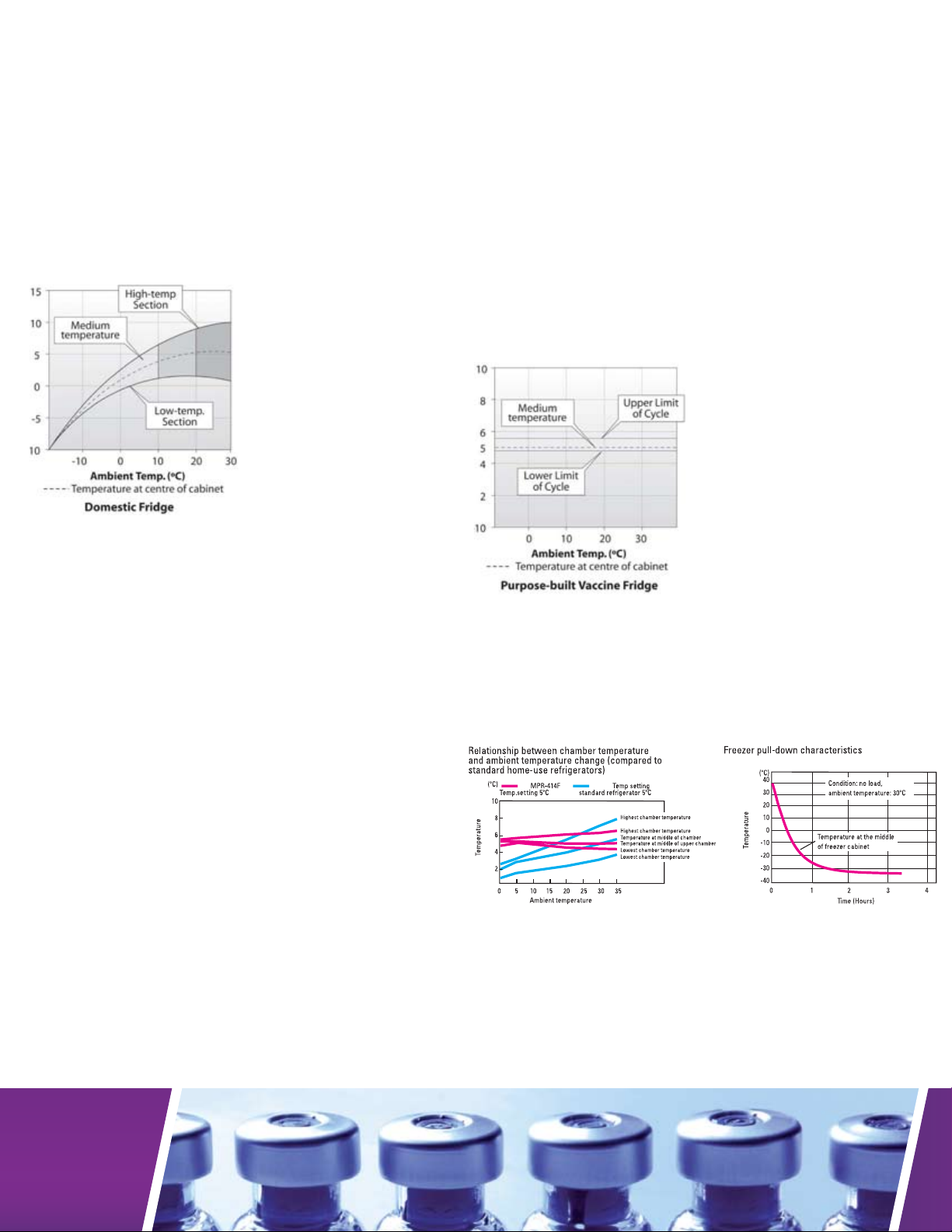
Household refrigerators for the storage of vaccines, reagents
and biologicals are no longer deemed adequate by medical
professionals. These types of refrigerators do not offer precise
temperature setpoint, hold, uniformity or recovery after door
openings. So, while household refrigerators appear to be cost
effective, initially, the long-term costs associated with variable
temperature and lost biologicals can be significant.
Key Disadvantages of Household
Refrigerators
vaccines, reagents and other
biologicals. Research has
shown that minor variances in
temperatures such as those in
a household refrigerator can
compromise the effectiveness
of your biologicals, risking
up to thousands of dollars in
valuable contents.
• Thermal mass is required to stabilize temperatures
• Numerous temperature zones restricts vaccine storage to
certain areas of the refrigerator
• Air from evaporator is below 0°C, so any vaccines near air vents
can be exposed to these temperatures. Air vent locations differ
between manufacturers and models.
• Type and location of thermostat differs between manufacturers
and models.
• Generally slow to react to increases in temperature and have a
wide temperature tolerance.
• Temperature controller dial has arbitrary scale. Difcult to
accurately set desired temperature.
• Change in ambient temperature affects internal temperatures.
• Poor temperature monitoring capabilities
• Defrost function can cause temperature uctuations
• Poor temperature recovery.
Key Advantages of SANYO Application
Specific Refrigeration
• Digital temperature
sensors inside the fridge and
controllers built specifically to
maintain a set temperature
within the 2°C to 8°C
temperature range
• Evaporator operates at 2°C,
therefore preventing vaccines
from freezing
• Alarms and temperature
monitoring for quick
notification of adverse
operating conditions
• Separate temperature
monitor probe
• Fan-forced air circulation for precise temperature uniformity
• Efcient temperature recovery properties.
• Built to handle changes in ambient temperatures
• External digital temperature display
• Minimal temperature uctuations around set point
Regulations and Requirements
Variations in temperature and failure to follow storage and
monitoring requirements can drastically affect the effectiveness
of vaccines through non-compliance of regulatory standards
of governing agencies. Sanyo designs equipment for specific
applications while meeting the standards of the Food and
Page 3

Drug Administration (FDA), American Association of Blood
Banks (AABB), Joint Commission on Accreditation of
Healthcare Organizations (JCAHO), International Committee
for Harmonization (ICH), and United States Department of
Agriculture (USDA).
efficient, cost-effective cabinet design to deliver both
refrigeration and freezer capacity in a single unit. With increasing
federal regulations, storing your valuable vaccines, reagents and
other biologicals will not suffice; see the comparison on the table
below.
Vaccine Storage Requirements
H1N1 2°-8°C (35°-46°F). Do not freeze.
DTaP, TdDt
Hepatitis A & B Vaccines
H. inuenza type B
Haemphilus (HiB)
Inuenza Vaccine
IPV
MMR, MR
Measles Virus
Rubella Virus
Mumps Virus
Pneumoccal 2°-8°C (35°-46°F). Do not freeze
Varicella Vaccine Freeze immediately upon arrival.
2°-8°C (35°-46°F). Do not freeze.
2°-8°C (35°-46°F). Do not freeze.
2°-8°C (35°-46°F). Do not freeze.
2°-8°C (35°-46°F). Do not freeze.
2°-8°C (35°-46°F). Do not freeze.
2°-8°C (35°-46°F). Do not freeze.
2°-8°C (35°-46°F). Protect from light.
(MMR Virus may be frozen.)
2°-8°C (35°-46°F). Protect from light.
2°-8°C (35°-46°F). Protect from light.
2°-8°C (35°-46°F). Protect from light.
Store at or below -15°C (+5°F).
Diluent can be stored at room
temperature. Do not freeze diluent.
May be stored at refrigerator
temperature (2°-8°C - 35°45°F) for up to 72 hours prior to
reconstitution. Vaccine stored at
2°-8°C which is not used within 72
hours of removal from -15°C storage
must be discarded.
Vaccine Storage Requirements
• Maintain required temperature range throughout the year
• Separate doors for refrigerator and freezer
• Large enough to hold year’s largest vaccine inventory
• Dedicated to biologics*
*Recommended by the Centers of Disease Control
Effect of Temperature on Vaccines
Vaccines need to be kept in refrigerators or freezers at certain
temperature ranges to remain potent.
Live vaccines
• Tolerate freezing
• Deteriorate rapidly after removal from freezer
Inactivated vaccines
• Damaged by exposure to freezing temperatures
• Tolerate short time out of refrigeration
Domestic
Features MPR Series
CFC-Free, reliable temp.
control not affected by
ambient temp.
Digital Display of chamber
temp.
Precise temperature setting of
chamber with microprocessor
control
Variable temp. control of
Refrigerator (2-4°C)
Variable temp. control of
freezer (-20°C to -30°C)
Uniform temp. at all shelf
levels
Separate operation of
Refrigerator and Freezer
Window for viewing yes no
Racks (SUS-304, MPR-41R) yes no
Monitoring hole/port yes some models yes
Temperature recorder (option) yes no
Door ajar option with indicator
light and audible alarm
High/low alarm and under
cooling and overheating
protection
Remote alarm terminal yes no
Set temp. deviation yes no
Self diagnostic function yes no
Unique automatic defrost
system
Condensate evaporator yes yes
Necessary function, construction or performance for preservation of reagents
and pharmaceuticals.
yes no
yes no
yes no
yes no
yes Max/Mid/Min
yes no
yes 18°C
yes some models yes
yes no
yes no
Refrigerator
MPR-Series Pharmacy Refrigerator
With the growing emphasis on proper storage of laboratory and
pharmacy materials, SANYO MPR-Series Pharmacy Refrigerators
with Freezer combine high performance refrigeration, control and
alarm/monitoring systems with energy-
Refrigerators and Freezers Designed
for Precise and Stable Vaccine Storage
MPR-1014
MPR-514
MPR-214F
MPR-414F
Page 4

Specifications
GREEN
product
Function creates form
Enviro-Center
Polyester resin finish baked on zinc galvanized steel
CFC-free rigid polyurethane foamed in place
Microprocessor control
small: 3(15kg/shelf)
4 casters with 2 adjustable leveling feet
Self diagnostics, remote alarm contact (DC30V,2A), Door key lock
2-pen type circular recorder (MTR-G3504), Mounting kit (MPR-S7)
SANYO DAQ System (MTR-480 & MTR-2000)
Function creates form
MPR-414F / 414FS performance data
Name
Model
External dimensions
WxDxH
Effective capacity
Weight
Exterior
Interior
Insulation
Temperature range
Temperature control
Cooling method
Compressor
Refrigerant
Shelves
Access port
Casters
Alarm and safety
Options
Enviro-Center
MPR-214F MPR-414F/MPR-414FS
Refrigerator Freezer Refrigerator Freezer
540 x 557 x 1.790 mm 800 x 600 x 1.805 mm
21.2x21.9x70.4(inch) 31.5x23.6x71.0 (inch)
176 liters (6.2 cu.ft.) 39 liters (1.4 cu.ft.) 340 liters (12.0 cu.ft.) 82 liters (2.9 cu.ft.)
80 kg 414F: 126 kg / 414FS: 119 kg
Polyester resin finish baked on zinc galvanized steel
Styrol resin Colored aluminium plate Stainless steel Colored aluminium plate
CFC-free rigid polyurethane foamed in place
2 to 14˚C -20 to -30˚C 2 to 14˚C -20 to -30˚C
Ambient temp. : 35ºC Ambient temp. : 30ºC Ambient temp. : 35ºC Ambient temp. : 30ºC
Microprocessor control
Fan-Forced air circulation Direct cooling Fan-Forced air circ. Direct cooling
60W 60W 160W 160W
R-134A (HFC) R-134A (HFC) R-407D (HFC)
3(20kg/shelf) 1(10kg/shelf) large: 2(25kg/shelf) 1(15kg/shelf)
small: 3(15kg/shelf)
ø 30mm (left) ø 30mm (left) ø 30mm (rear) ø 30mm (rear)
4 casters with 2 adjustable leveling feet
High/low temperature alarm, Door ajar alarm, Memory back up during power failure
Self diagnostics, remote alarm contact (DC30V,2A), Door key lock
2-pen type circular recorder (MTR-G3504), Mounting kit (MPR-S7)
Drawers for the bottom left compartment (MPR-41R): MPR-414F/FS only
SANYO DAQ System (MTR-480 & MTR-2000)
Temperature alarm
Overheating protection
Memory back-up function
Door Ajar
Self-diagnostic function
Key lock switch
MPR-414F/414FS MPR-214F
Yes Yes
Yes Yes
Yes Yes
Yes Yes
Yes Yes
Yes Yes
Quiet, Energy Efficient Performance
• Two specially designed SANYO hermetically sealed
compressors operate with quiet efficiency; two compressors
allow independent cooling of both refrigerator and freezer
compartments.
• Four door design reduces cold air loss during door openings;
makes most efficient use of available space.
• Hot gas heated mullion prevents condensation and icing,
maintains integrity of door gasket seal.
• SANYO developed CFC-free refrigerants are specially
formulated for laboratory use.
• CFC-free foamed-in-place insulation minimizes performance
variations due to ambient temperature changes.
Built-in Under the Counter Scientific
Grade Laboratory Refrigerator,
SR-L/SF-L Series
SANYO undercounter refrigerators and freezers feature stable
temperature and uniformity with convenient door mounted
temperature controls. Doors have key locks for added security.
• Superior temperature control and uniformity via a door
mounted microprocessor controller and interior forced air
circulation.
• Safe and secured storage behind a keyed locking door and
optional padlock hasp.
• Laboratory-Ready™ with integrated alarm functions, remote
alarm contacts and monitoring probe access port.
• Meets JCAHO standards for controlling medication access.
The cabinet offers superior temperature control and uniformity
required for short-term and long-term storage of biologicals,
reagents and other temperature sensitive materials. Compact
and space efcient, the 6.1 cu.ft. undercounter refrigeration
and the 5.4 cu.ft. undercounter freezer cabinets include a door-
mounted microprocessor temperature control and digital display
module with integrated alarm, monitoring and remote data
functions.
Model Type Capacity Temperature
SR-L6111W Undercounter
SF-L6111W Undercounter
MPR-1410/1410R Biomedical
MPR-721/721R Biomedical
MPR-514/514R Pharmacuetical Freezer 17.2 cu.ft. 2 to 14°C
MPR-1014/1014R Pharmacuetical Freezer 36.5 cu.ft. 2 to 14°C
MPR-214F Refrigerator/Freezer
MPR-414F Refrigerator/Freezer
Cabinet features include positive chamber air circulation for
maximum temperature uniformity, and an access port for
independent monitoring probe(s). The at prole inner door
enhances interior storage volume on adjustable open wire
shelves.
Security considerations for protection of high value items include
a controller lock-out function to prohibit unauthorized setpoint
changes, and a keyed locking door with optional padlock hasp.
Door Mounted Controller
• Digital input of temperature with setpoint range from 1°C to
14°C (SR), -15°C to -25°C allows end user temperature exibility
• Automatic tracking alarm +/-3°C around setpoint monitors
critical temperature variances
• Door ajar alarm with alarm delay timer eliminates nuisance
alarms
• Easy to read, angled L.E.D. display and keypad
• Remote alarm contacts for connecting to a centralized alarm
monitoring system.
Vaccine Storage Unit Capacities
6.1 cu.ft. -1 to -14°C
Refrigerator
5.4 cu.ft. -13 to -25°C
Freezer
48.4 cu.ft. 2 to 23°C
Freezer
24.2 cu.ft. 2 to 23°C
Freezer
Comination
Comination
(R) 6.2 cu.ft.
(F) 1.4 cu.ft.
(R) 12.0 cu.ft.
(F) 2.9 cu.ft.
2 to 14°C
-20 to -30°C
2 to 14°C
-20 to -30°C
SANYO is committed to developing green technologies that provide energy
efficiency resulting in lower operational costs with less impact on the environment.
Product conforms to RoHS (European Restriction
of Hazardous Substance directives)
Biomedical and Environmental Solutions Division
201 Creditview Road, Woodbridge, Ontario L4L 9T1
SANYO North America Corporation
1300 Michael Drive, Wood Dale, IL 60191 USA
SANYO Canada, Inc.
LR-VACSTRGE-11.09
 Loading...
Loading...