Page 1

Sterisonic™ GxP
Series Cell Culture
Incubators
CO2 Incubators
MCO-19AIC(UVH)
MCO-19AIC(UV)
MCO-19AIC
CO
/ O2 Incubators
2
MCO-19M(UVH)
MCO-19M(UV)
MCO-19M
Features:
• The industry’s most complete cell culture
solution for highly regulated applications or
conventional incubation.
• Safe, effective and documented two-hour in situ
decontamination for the fastest turn-around.
H
2O2
www.sanyobiomedical.com
My Life. My Work. My Choice.
Page 2
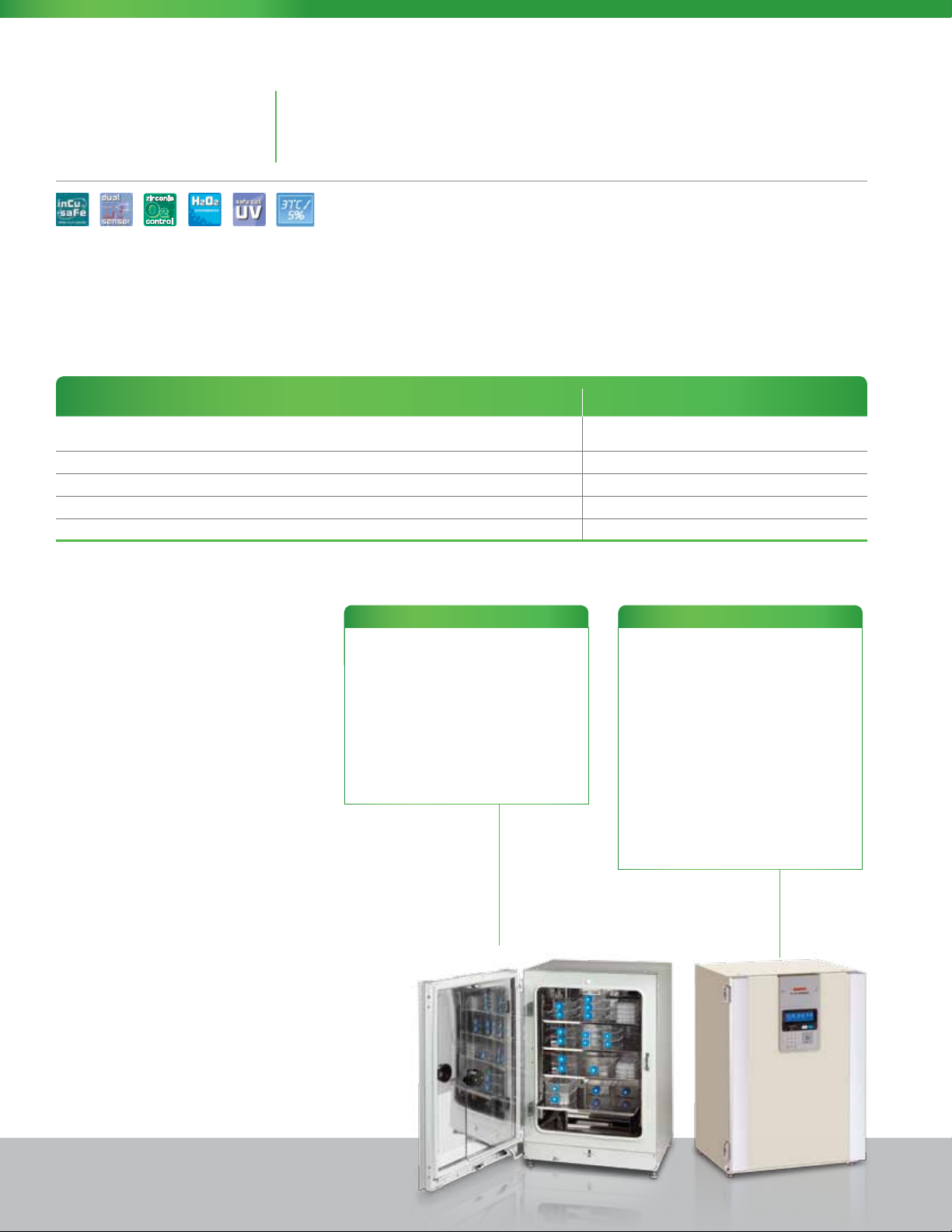
MCO-19AIC
MCO-19M
MCO-19AIC(UV)
MCO-19AIC(UVH)
MCO-19M(UV)
MCO-19M(UVH)
Sterisonic™GxP Series Cell Culture CO2 and CO2 / O2 Incubators
The industry’s most complete cell culture solution for highly regulated applications or conventional
incubation. Now with safe, effective and documented two-hour in situ H
est turn-around and maximum availability.
Sterisonic™ GxP Selection
Feature
Vapor Decontamination Standard Optional Optional Standard Optional Optional
H
2O2
™
SafeCell
UV Decontamination Standard Standard Optional Standard Standard Optional
Single Beam, Dual Detector IR CO
Oxygen Concentration Control, Zirconia Control N/A N/A N/A Standard Standard Standard
Sensor Standard Standard Standard Standard Standard Standard
2
MCO-
19AIC(UVH)
CO2 Incubators CO2/O2 Incubators
MCO-
19AIC(UV)
MCO19AIC
decontamination for the fast-
2O2
MCO-
19M(UVH)
MCO-
19M(UV)
MCO-
19M
Product Applications
The combination of Sterisonic™ GxP
incubator performance functions permit
use with condence in high-value cell
protocols among hard-to-grow cell lines,
cells highly sensitive to contamination,
ultra-sensitive media and reagents, or
protocols that require a strict isolation
and decontamination between processes. These include but are not limited to:
• Stem cell research
• Autologous tissue regeneration
and regenerative medicine
• In vitro fertilization
1
• Genomic and proteomic expression
• Esoteric plant and amphibian
cell culture
• Hypersensitive and transgenic
cell culture
• Low media volume microplate work
1
501(k) clearance applied for. Contact SANYO for status. MCO-19AIC
and MCO-20AIC CO2 incubators have received U.S. Food and Drug
Administration 510(k) clearance for in vitro fertilization applications in
accordance with the FDA Safe Medical Devices Act of 1990 and the Medical
Device Amendments of 1992. Reference: Number K013703. Regulation
Number: 21 CFR 884-6120, Assisted Reproduction Accessories, Regulatory
Class II, Product Code 85MOG, October 30, 2001
Designed for use with a variety
of standard cell culture vessels
and protocols.
• Interior components and adjustable
shelves are congured for easy access,
in situ decontamination and exible arrangement for a variety of applications.
• Four adjustable shelves are included,
standard; maximum shelf capacity
is 15 shelves.
MC O -19AIC (UV H)
MC O -19 M
Multi-Gas Incubator, Oxygen
and CO2 Control
• Ideal for in vitro fertilization (IVF), genetic
research, regenerative medicine and
other protocols that require CO
sub-ambient (hypoxic) or above-ambient
oxygen control.
• Cabinet based on MCO-19 platform
with inCu safe
stainless steel chamber, continuous
contamination control, patented D.H.A.
multi-point, air-jacketed temperature
control system, elevated relative humidity
with integral water-level sensor.
™
copper-enriched
and
2
2 Sterisonic™ GxP Series Cell Culture Incubators
Page 3

Sterisonc™ GxP Features and Benefits
The SANYO Sterisonic™ GxP is designed for a wide array of demanding and highly regulated applications in the biomedical, pharmaceutical,
medical research and clinical laboratory. Representing years of research, development and component testing, the Sterisonic™ GxP incorporates a
collective of mutually functional systems and design attributes to offer a holistic solution to cell culture protocols, from the most sophisticated to
more familiar and conventional processes.
Decontamination System
• The unique Sterisonic™ GxP H2O2
decontamination system limits downtime to less than three hours when
total chamber decontamination with
verication is desired.
• All interior components and CO
sampling loop are sterilized in situ;
no need for removal and autoclaving.
• Active Background Contamination
Control
™
ghts contamination while
cell culture protocols are in process.
The patented SafeCell
system scrubs interior airow
to destroy airborne and humidity pan
contaminants.
Exclusive inCu saFe
™
enriched stainless steel interior
surfaces assure constant germicidal
protection.
Shelf brackets are formed
with an exaggerated angle
to minimize surface contact
with flat shelves*.
™
UV
copper-
2
Control and Monitoring
• The Sterisonic™ GxP control and
information center includes an intuitive
pop-up menu, high resolution LCD
for inputs, outputs and performance
at-a-glance.
• Multi-point data logging offers
push-button graphical display. An
optional PC interface permits remote
transmission for GMP/GLP protocols
as required.
• Precise PID logic controls and
adjusts to all temperature, CO
set-
2
points and alarm parameters.
CO2 Control
SANYO proprietary single-beam,
dual detector infrared (IR2) CO
sensor delivers precise CO
control,
2
quick recovery following door openings,
and auto sampling with no moving parts.
• Continuous zero calibration is standard.
• An optional semi-automatic, one-point
calibration system is available. Catalog
No. MCO-SG; see Accessories.
2
Zirconia O2 Control System,
MCO-19M Series
A zirconia oxygen sensor main-
tains sub-ambient O
1% to 18%. Additionally, enriched O
levels from 22% to 80% are enabled
with proper safety precautions.
• Concurrently the MCO-19M permits
a CO
range 0% to 20% via infrared
2
sensor.
• Nitrogen gas bubbler accelerates
recovery of chamber humidity levels
following door openings.
• An electronic P.I.D. control maintains
accurate temperature and gas setpoints over the entire system range.
• The MCO-19M includes an automatic
gas switchover system that changes
from the primary to a secondary gas
cylinder for either oxygen or nitrogen;
an optional second gas switchover
system is available for CO
• Cabinet based on MCO-19 platform
with inCu saFe
™
copper-enriched
stainless steel chamber, continuous
contamination control, patented D.H.A.
multi-point, air-jacketed temperature
control system, elevated relative humidity with integral water-level sensor.
levels from
2
.
2
2
MC O-19 M
MCO-19M shown with
four separate inner
doors with gaskets.
3
Page 4
Sterisonc™ GxP Features and Benefits
Temperature
and Humidity Control
• The patented Direct Heat and Air™
conditioning system manages setpoint
temperature through multiple, variable
warming points under microprocessor
control.
• The humidity pan is easy to ll, easy
to clean; the automatic optical sensor
advises of low water level.
H2O2 Decontamination
The use of H2O2 decontamination
in biological safety cabinets and barrier
isolators is a popular alternative to ethylene oxide (EtO) as a safer, more efcient
decontamination method. H
been widely used in the pharmaceutical
industry. In aerospace research, H
used to sterilize satellites.
The FDA has recently granted 510(k)
clearance to use H
in individual medi-
2O2
cal device manufacturing applications.
EtO criteria outlined in ANSI/AAMI/ISO
has long
2O2
2O2
is
14937 may be used as a validation guideline. For references online visit
www.sanyobiomedical.com/sterisonic.
Unlike conventional incubators, unique
features of the SANYO Sterisonic
GxP incubator permit use of the H
process in situ with complete safety,
zero impact on adjacent equipment or
the environment, and speed to return
the incubator to service.
• The H
functions with the patented SANYO
SafeCell
decontamination process
2O2
™
UV system. Following a
seven-minute H
circulation and dwell cycle, vaporization
is stopped and the SafeCell
turned ON for up to ninety minutes.
• When exposed to UV light, the H
vapor breaks down into water and
oxygen, leaving only traces of water
droplets. These droplets automatically condense onto a naturally cooler
section of the interior oor for easy
wipe-up.
• Throughout the entire cycle the
Sterisonic
™
GxP airow system continues to gently circulate interior air
assuring 100% vapor contact with all
interior surfaces, ultimately creating
a serial dilution of H
over the UV lamp.
vaporization,
2O2
as it passes
2O2
™
2O2
™
UV lamp
2O2
• Orientation of interior sample ports
of the single beam, dual detector IR
CO
sensor creates a slight Venturi
2
ow through the sample chamber, permitting total decontamination of the
CO
system at the same time.
2
• Shape and location of interior components such as shelves, shelf brackets,
plenum covers and the humidity pan
permit the components to remain
in the chamber during the decontamination process, conveniently bypassing the need for a separate autoclave
cycle.
• Once the cycle is complete, the door
locking system is released; the inner
door can be opened, interior components repositioned and the incubator
is returned to service.
Ergonomic Cabinet Design
With reversible inner and outer doors,
a single SANYO incubator offers the
industry's most exible installation
options available.
• Low prole cabinet with door-mounted
control panel permits easy access and
viewing.
• The outer door latch and door heater
cable is easily switched if a reverse
opening is required.
Sterisonic™ GxP Series H2O2 Decontamination Performance Value
2 Hours
SANYO
Sterisonic™
H2O
decontamination vs. high heat decontamination
2
= Uptime (Hours)
The documented two-hour in situ H2O2 sequence puts the fully sterilized Sterisonic™ GxP available and
ready for use quicker than any other incubator worldwide.
4 Sterisonic™ GxP Series Cell Culture Incubators www.sanyobiomedical.com
Brand X
=
Downtime (Hours)
14 Hours
Page 5

Sterisonc™ GxP Features and Benefits
• Cabinet knock-outs are pre-drilled
and tapped to eliminate drilling and to
simplify re-mounting of door hardware.
• The outer door closes against a
soft, easily cleaned magnetic gasket
designed to eliminate ambient air
shear across the glass inner door,
minimizing condensation.
• A door ajar alarm provides an audible
and visual warning if the outer door
is left open.
• A center-mounted key lock is located
beneath the door for added security;
model MCO-19AIC(UVH) only.
Shelves and Inventory
Management
Convenient space efcient inventory
management is simplied through a system of adjustable, extendable shelves.
• Inventory shelves and brackets are
formed from polished copper-enriched
stainless steel, removable without
tools, and can remain inside the
incubator during the H
nation cycle or autoclaved separately if
desired.
decontami-
2O2
• Shelves are perforated to permit natural vertical air convection through and
around labware.
• Shelf brackets are formed with an
exaggerated angle to minimize surface
contact with at shelves (patent pending). Brackets slip easily into vertical
supports that attach to interior chamber walls with clearance sufcient to
permit proper air circulation.
Inner Door and Gasket
The inner design is critical to successful
contamination control technique.
• The inner gasket body forms an
effective thermal transition between
the ambient air and warm, humidied
incubator atmosphere, minimizing
condensation and eliminating moisture
traps which can harbor contaminants.
• The inner door gasket is a dual
durometer extrusion (two levels of
softness) from closed-cell silicone
to inhibit contamination.
• The gasket feather-edge allows
the inner glass door to close gently
against the chamber opening for
a tight peripheral seal.
• The entire inner door gasket is removable for cleaning and/or replacement
if required.
• Radiant heat from the outer door,
apportioned by the microprocessor
MCO-38AIC(UVH)
Stacking Doubles Capacity
in Same Footprint.
• The Sterisonic™ GxP cabinet is
designed for stacking, allowing one
unit to be positioned on top of
another, doubling interior volume
without additional oor space.
• The combination of stacking and
reversible doors offers the most
installation options possible.
• An optional roller base is recommended for stacked installations
to permit mobility if required;
see Accessories.
control as a function of the patented
Direct Heat and Air Jacket
™
, automatically warms the inner door glass
in proportion to total heat demand
and condensation control.
Field Reversible Door
The eld-reversible door allows
universal installation using the left-hand
hinge (standard) or a right-hand hinge
modication
• The outer door includes a universal
nger grip at each side.
• Mounting holes for hinge hardware
are pre-drilled and capped with easily
removable trim plugs.
• The door heater cable plugs into the
alternate connection to complete
the change.
Elevated Humidity,
Low Water Level Warning
To avoid cell culture desiccation, the
SANYO Sterisonic
maintains ~95% RH at 37°C.
• Humidication is achieved by a combined forced-air and natural evaporation method enhanced by the Direct
Heat and Air Jacket
protected by an optical water level
indicator to warn of low water in the
removable humidity pan.
• The humidity pan removes easily for
regular cleaning and rell.
• When lled, the pan slides into place.
The optical sensor returns to position
and the SafeCell
any contaminants introduced during
the process.
• For the H
cycle the humidity pan is repositioned
against the chamber wall to eliminate
the need for separate autoclaving.
™
GxP CO2 incubator
™
base heater, and
™
UV lamp destroys
in situ decontamination
2O2
5
Page 6

Sterisonic™ GxP Control System
Intelligent Interface Through Integrated LCD Control with Graphical Display—The Sterisonic™ GxP incubator is managed by an integrated microprocessor controller with LCD graphical display to simplify all incubator functions. Stable temperature and humidity conditions are achieved through
a combination of performance systems supervised by the controller complete with alarm, programming, calibration and diagnostic protocols.
Control Features
• The Sterisonic™ GxP control and
information center includes an intuitive
pop-up menu, high resolution LCD
for inputs, outputs and performance
at-a-glance.
• Multi-point data logging offers pushbutton graphical display. An optional PC
interface permits remote transmission
for GMP/GLP protocols as required.
• Selected performance histories can
be acquired in graphical format for
easy interpretation.
• Precise PID logic controls and adjusts
to all temperature and CO
setpoints
2
and alarm parameters.
• A range of setpoint, alarm and programmable inputs are established through
pop-up menus and function keys.
• Standard parameters are factory-set for
quick start-up, and all parameters can
be changed as required.
• Logged parameters can be exported
to remote databases, off-site alarm or
data capture systems through optional
communications board for compliance
monitoring; see Accessories.
• A remote alarm terminal mounted
at the rear of the cabinet can be
connected to an external alarm system.
• Tactile feedback, touch pad data shift
and entry keys simplify operation.
• The control panel is center mounted
in the outer door for easy access and
viewing.
Decontamination Cycle
The H2O2 decontamination cycle is
monitored for safety and cycle status.
A physical interlock and neutralization
sequence assures total decontamination
and operator safety.
1
Start Cycle: When the H2O2 button is pressed
a confirming message prompts the user to proceed
with the decontamination cycle or cancel.
2
H2O2 Vapor Cycle: Once the door locks automatically, the cycle starts. The flashing H2O2 display
confirms the process and counts down remaining
H2O2 vaporization time.
3
UV Resolution: The H2O2 atomizer automatically
completes after a 7 minute cycle. UV lamp comes ON.
The flashing UV Resolve display counts down remaining
time in the UV cycle as H2O2 is reduced to water and
trace oxygen.
4
Cycle Complete: When the cycle is complete the
door lock releases automatically. The H2O2 atomizer and
cable can be disconnected and removed and all interior
components restored to their normal position.
Sterisonic™ GxP H202
Decontamination Cycle
(3 Hours, total)
Elapsed Time:
Conventional High Heat
Decontamination
(24 Hours, total)
Sterilize: 7 min.
SANYO H2O2 atomizer creates vapor which is
circulated throughout chamber by interior blower.
PREP: 15 min.
Reposition shelves, humidity
pan and plenum inside
SANYO chamber. Interior
surfaces are exposed.
Start Cycle: 30 min.
Press H2O2 start button.
Chamber warms to 45˚C.
15 min. 30 min. 45 min. 1 2
Resolve: 90 min.
UV lamp glows for 90 minutes,
reducing H
to harmless water droplets.
2O2
Finish.
Shelves, humidity pan and plenum
are returned to operating position.
3
4 5 6 7 8 9 10 11 12 13 14 15 16 17 18 19 20 21 22 23 24
PREP: 15 min.
Remove interior components
sensitive to high heat.
Start Cycle: 90 min.
Interior chamber elevates to high heat.
Sterilize: 14 hours
Interior chamber remains at high heat.
6 Sterisonic™ GxP Series Cell Culture Incubators www.sanyobiomedical.com
Page 7

Sterisonic™ GxP Control System
• Digital alphanumeric LCD display.
• Message display
• Pop-up menu
1
2
3
4
• Visual alarm indicator
decontamination sequence
• H
2O2
start key
• Menu call button
• Display contrast adjustment
• Positive feedback tactile
input buttons
• Positive feedback tactile
entry and function keys
Finish.
Incubator must cool from high heat
temperatures to near ambient.
7
Page 8

Sterisonic™ GxP Design and Technology
All SANYO CO2 incubators feature patented Direct Heat and Air Jacket™ temperature control for accurate, uniform temperature control and
inCu saFe™ for continuous contamination control. SANYO laboratory CO2 incubators feature selected SafeCell™ UV with exclusive, patented
Active Background Contamination Control™.
Direct Heat and Air Jacket™ Heating System
Zone Location Function Energy Microprocessor Controller
Main
(Red)
Base
(Yel low )
Side, top and rear walls Dominant heat source Variable
Floor
Base heater elevates the humidity reservoir
to achieve 95% RH at 37 °C
Variable
Energizes any, all or
in combination of heating
elements as required.
Front
(Green)
Air Jacket
(White)
Insulation
(Gray)
Outer door
Side, top and rear walls
Side, top and rear walls, door
Direct Heat and Air Jacket™
Heating System
The patented Direct Heat and Air
™
Jacket
surrounds the inner walls with a
natural convection airow that converts
to radiant wall heat through thermal conduction to achieve accurate, uniform and
highly responsive temperature control
within the chamber.
• The microprocessor controller directs
proportional distribution of power to
independent heating sources surrounding the chamber.
• Arranged in three zones, these
sources include the side, top and
rear walls, the chamber base and
the outer door.
• Each zone is controlled by the micro-
processor which manages continuous
feedback from the incubator chamber
sensors.
Warms the inner glass in response to ambient conditions;
eliminates condensation on glass and around the opening
and promotes temperature uniformity
Sealed, surrounds interior chamber with natural
air convection and promotes chamber wall uniformity
Promotes energy efciency, mitigates effect of
ambient temperature uctuations on air jacket
The patented Direct Heat and Air Jacket™ heating
system distributes proportional energy to the interior
chamber through a natural convection air jacket
surrounded by a high density insulation to protect
against ambient temperature fluctuations
Variable
– –
– –
H2O2 Decontamination
The unique Sterisonic™ GxP H2O2
decontamination system limits
downtime to less than three hours when
total chamber decontamination is desired.
• All interior components and CO
sampling
2
loop are sterilized in situ; no need for
removal and autoclaving.
• Available as standard on the
MCO-19AIC(UVH) and MCO-19M(UVH);
optional on the MCO-19AIC(UV) and
MCO-19M(UV).
• Following decontamination, SANYO Active
Background Contamination Control
™
ghts
contamination while cell culture protocols
are in process.
SafeCell™ UV System
Sterisonic GxP Models –UVH
and –UV include SafeCell
contamination control technology based on
an integrated combination of narrow bandwidth, ozone-free ultraviolet light, ceramic
infrared CO
control, inCu saFe™ copper-
2
enriched stainless steel alloy interiors and
Direct Heat and Air Jacket
™
aged by a microprocessor controller.
™
UV
heating man-
8 Sterisonic™ GxP Series Cell Culture Incubators www.sanyobiomedical.com
Page 9

Sterisonic™ GxP Design and Technology
These incubators offer the industry's
most stable cell culture environment
and are useful for the most critical
applications where continuous contamination control is essential to cell viability.
SANYO SafeCell
™
UV Series CO2 incubators offer signicant economic benets
by minimizing interruptions for decontamination, by improving cell culture
growth and expression under stable,
repeatable conditions, and by minimizing
the potential for product loss.
• SafeCell
™
UV includes a programmable
ultraviolet lamp, isolated from cell
cultures, that sterilizes conditioned air
and humidity reservoir water to prevent contamination without affecting
cell cultures in vitro.
• SafeCell
™
UV inhibits the growth of
mycoplasma, bacteria, molds, spores,
yeasts and fungi without costly HEPA
lter air scrubbers which accumulate
contaminants in the chamber air.
• High temperature decontamination
systems, which can actually encourage in vitro growth of heat resistant
thermophilic and hyperthermophilic
microorganisms, are avoided.
• inCu saFe
™
interior surfaces provide
natural resistance to contamination.
• The SafeCell
™
UV lamp reduces H2O2
to trace sterile water condensate
following the seven-minute vapor
decontamination process.
Dual Wavelength Infrared
CO2 Control System
The SANYO single beam, dual
detector infrared CO
unprecedented control accuracy and
stability by simultaneously measuring
two wavelengths.
The chamber sample and a reference
is congured around a ceramic-based
sensor linked to the microprocessor
controller with a sophisticated P.I.D./R
(proportional, integral and derivative) algorithm. Benets include ultra-fast recovery
without overshoot and accurate CO
ages during periods of frequent incubator
access with multiple door openings.
system offers
2
™
aver-
2
• The ceramic-based technique is impervious to changes in temperature and
relative humidity, highly stable during
door openings, and sensitive to restoration of setpoint equilibrium when the
door is closed.
• The sensor maintains a continuous
auto-zero function for accuracy.
• Actual CO
is displayed on the main
2
control panel.
• A CO
sample port mounted on the
2
incubator front permits convenient
conrmation of chamber CO
• An optional automatic CO
density.
2
switchover
2
system is available; see Accessories.
A two-stage regulator from the supply
cylinder to the incubator is required;
see Accessories.
MCO-19M Series
Multi-Gas Performance
O2 Control System
In addition to precise CO
MCO-19M Series incubator delivers
exceptional O
control in support of cell
2
culture processes which require belowambient or above-ambient oxygen levels.
These include in vitro fertilization (IVF),
gene research, regenerative medicine
and emerging applications that are sensitive to oxygen levels other than ambient.
• A zirconia oxygen sensor maintains
sub-ambient O
levels from 1% to
2
18%. Additionally, enriched O
from 22% to 80% are enabled with
proper safety precautions.
• Concurrently the MCO-19M permits
a CO
range 0% to 20% via infrared
2
sensor.
• Nitrogen gas bubbler accelerates
recovery of chamber humidity levels
following door openings.
• An electronic P.I.D. control maintains
accurate temperature and gas setpoints over the entire system range.
• The MCO-19M includes an automatic
gas switchover system that changes
from the primary to a secondary gas
cylinder for either oxygen or nitrogen;
control, the
2
levels
2
an optional second gas switchover
system is available for CO
.
2
• Cabinet based on MCO-19 platform
with inCu safe™ copper-enriched
stainless steel chamber, continuous contamination control, patented
D.H.A. multi-point, air-jacketed temperature control system, elevated relative
humidity with integral water-level
sensor.
inCu saFe™ Construction
for Germicidal Protection
SANYO offers exclusive use
of inCu saFe
steel alloy interior surfaces within
a technical design created to eliminate
contamination sources and to mitigate
the effect of airborne contaminates
introduced through normal use.
• Selected to provide natural germicidal
protection without rust or corrosion,
inCu saFe
micidal attribute to inhibit the growth
of molds, fungi, mycoplasma and
bacteria.
• All interior components, including
the air management plenum, shelf
supports, humidity pan and blower
wheel assembly are easily removable
without tools if required.
• During the H
cycle interior components can be
repositioned within the chamber for in
situ decontamination.
• When components are removed, all
interior surfaces are exposed for conventional wipe down.
• Large curve corners and electropol-
ished surfaces are easy to clean.
• Pass thru ports accommodate probes
or instrumentation leads as required
for specialized cell culture protocols.
Each chamber includes a port positioned in the interior, rear wall, upper
left, with dual, non-VOC silicone stoppers inside and outside the cabinet for
added protection.
™
copper-enriched stainless
™
expresses a natural ger-
decontamination
2O2
9
Page 10

Sterisonic™ GxP Design and Technology
Active Background
Contamination Control
A continuous Active Background Contamination Control
contamination without downtime.
At the base of the plenum an isolated
beam of high intensity, ozone-free
ultraviolet light destroys contaminants
in the air and in the humidity water reservoir, away from active cell cultures.
™
process eliminates
™
SafeCell™ UV Lamp Program Cycles
Mode Function
After H
2O2
Vaporization
After Door
Opening
OFF If UV protection is not desired.
24 Hour
Continuous ON
The UV lamp automatic ally cycles ON for up to 90 minutes following the seven-minute
H2O2 vapor cycle, reducing the H2O2 to water droplets. These droplets automatically
condense onto a naturally cooler section of the interior oor for easy wipe -up.
UV lamp automatically ON for ve minutes after door is closed decontaminates
incoming room air.
Useful for overnight decontamination prior to rst use, clinical decontamination protocols
between patients, or following total chamber wipe-out af ter maintenance or ser vice.
• Contaminants trapped within the
distilled water pan are destroyed by
ultraviolet light.
• Sterile, humidied air is released
from the lower plenum for vertical convection through and around the perforated shelves. Interior air motion is
suspended when the door is opened,
minimizing movement of room air
contaminants into the chamber.
• UV light is isolated by the plenum
cover to protect cell cultures
• Airborne contaminants are eliminated
by an automatic UV cycle that automatically turns ON for a specied
period after each door opening.
• Trace contaminants that attach to
interior surfaces are destroyed by the
passive germicidal properties of the
inCu saFe
™
surfaces.
The SafeCell™ UV lamp cycle is factory set for normal use, and can be re-programmed as desired by entering
parameters through the central microprocessor control panel. Program parameters for the H2O2 decontamination cycle
are non-adjustable for operator safety.
Mycoplasma Survival Results
Mycoplasma
Strain
Mycoplasma
fermentans PG18
Mycoplasma
orale CH19299
Mycoplasma
arginini G230
Mycoplasma
hominis PG21
Chart summarizes test results with four strains of mycoplasma. Results demonstrate how SANYO InCu saFe™
copper enriched stainless steel alloy offers germicidal properties of conventional C1100 copper while maintaining
both corrosion-proof and discoloration-resistant properties of conventional Type 304 stainless steel.
Detailed test results are available from SANYO.
Positive
Control
survival no survival survival survival
survival no survival survival survival
survival no survival survival survival
survival no survival survival survival
Conventional Type
304 Stainless Steel
SANYO
InCu saFe
™
Conventional
Copper C1100
10 Sterisonic™ GxP Series Cell Culture Incubators www.sanyobiomedical.com
Page 11

Sterisonic™ GxP Specifications
Sterisonic™ GxP Series Energy, Electrical and Utilties
Description
MCO-19AIC
MCO-19AIC(UV)
MCO-19AIC(UVH)
MCO-19M
MCO-19M(UV)
MCO-19M(UVH)
Maximum Power Consumption 310W 310W
Maximum Heat Discharge 1062 BTU 1062 BTU
Electrical Connection
Gas Connection 4 to 6 mm inner diameter tubing 4 to 6 mm inner diameter tubing
CO
2
CO
Gas Input Pressure Nominal 4.3 psi from two- stage CO2 regulator Nominal 4.3 psi from two- stage CO2 regulator
2
CO
Gas Cylinder Switchover System Optional Optional
2
O
Control System N/A Microprocessor PID, Zirconia Sensor
2
O
Range and Variation N/A 1-18%, 22-8 0%
2
N
Inlet Connect/ Pressure N/A Nominal 7 psi from two-stage regulator
2/O2
N
Switchover System N/A Standard
2/O2
115V,60Hz, 1 phase, NEMA 5 -15P plug provided;
requires NEMA 5-15R grounded receptacle.
115V,60Hz, 1 phase, NEMA 5 -15P plug provided;
requires NEMA 5-15R grounded receptacle.
Sterisonic™ GxP Series Dimensions, Weights and Capacities
Model Number
MC O-19AI C
MCO-19AIC(UV)
MCO-19AIC(UVH)
MC O-19 M
MC O-19 M(UV )
MC O-19 M(UV H)
Volume
(cu.ft.)
6.0
170 L
6.0
170 L
Exterior Dimensions
(w x f-b x h)
24.4" x 27.9" x 35.4"
620 x 710 x 900 mm
24.4" x 27.9" x 35.4"
620 x 710 x 900 mm
Interior Dimensions
(w x f-b x h)
19.3" x 20.6" x 26 .2"
490 x 523 x 665 mm
19.3" x 20.6" x 26 .2"
490 x 523 x 665 mm
Shelves Net Weight
15 max / 4 supplied std.
17.7 " x 17.7 " (15.4 lbs capacity)
450 x 450 mm (7 kg capacity)
15 max / 3 supplied std.
17.7 " x 17.7 " (15.4 lbs capacity)
450 x 450 mm (7 kg capacity)
(nominal)
205 lbs
93 kg
207 lbs
94 kg
4.5"
(115 mm)
4.9"
(125 mm)
FRONT SIDE
24.4" (620 mm)
19.3" (490 mm)
22.8" (580 mm)
26.2" (665 mm)
34.6" (880 mm)
0.8"
(20 mm)
24.8" (630 mm)
20.6" (523 mm)
18.4" (469 mm)
3.1"
(80 mm)
1.9" (49 mm)
1.3" (34 mm)
1.7" (44 mm)
11
Page 12

Sterisonic™ GxP CO2 Series Specifications
Sterisonic™ GxP CO2 Incubators
Description
Single Chamber Model Number MCO-19AIC(UVH) MCO-19AIC(UV) MCO-19AIC
Dual Chamber Model Number MCO-38AIC(UVH) MCO-38AIC(UV) MCO-38AIC
Major Operating Systems
H2O2 Decontamination System Standard Optional Optional
™
SafeCell
UV System Standard Standard Optional
Single Beam, Dual Detector IR CO
inCu saFe
™
Copper Enriched Stainless Steel Interior Standard Standard Standard
LCD Graphical Controller/ Display, Door Mounted Standard Standard Standard
Direct Heat, Air
™
(DHA ) Air Jacket Standard Standard Standard
Decontamination
H
Decontamination System Vaporization in situ Optional Optional
2O2
Interior UV Lamp, Programmable, Ozone Free Standard Standard Optional
Copper Enriched Stainless Steel Interior
with Germicidal Protection
Sensor Standard Standard Standard
2
Standard Standard Standard
Environmental Performance
Temperature Control Range +5ºC above ambient to 50°C (in a 5ºC to 35ºC ambient)
Temperature Control Uniformity Deviation ±0.25ºC (in 25ºC ambient, setting 37 ºC, 5% CO
CO
Control Range and Deviation 0% to 20%, ±0.15% in 25ºC ambient, set ting 37 ºC, 5% CO2, no load
2
CO
Sensor Platform
2
Calibration Single point zero automatic; semi- automatic one point span @ reference optional.
CO
2
Ceramic based, single beam, dual wavelength measurement
of actual vs. contrast, with continuous auto-zero calibration.
, no load)
2
Airow Gentle vertical air ow, continuous with inner door closed.
Interior Humidity 95%RH @ 37°C through evaporation via DHA
™
heating system; reective optical low water sensor.
Control, Monitoring, Alarm
Temperature and CO
Control P.I.D., setpoint resolution 0.1% and 0.1°C
2
Display Alphanumeric LCD digital display messaging.
Data Acquisition Data Acquisition Automatic log function of temperature and CO
Communications
Optional PC interface, Catalog No. MTR- 4 80 with RS232/RS4 85 data ports available
Remote alarm contacts standard. Optional 4-20mA connection.
.
2
Cabinet Design and Construction
Interior, Shelves 4, Copper-enriched stainless steel.
Inner Door Tempered glass.
Insulation Rigid foam polyurethane.
Outer Door Reversible, heated.
Access Port Single opening with interior and exterior 1.18" (30 mm) non -VOC silicone stoppers.
Leveling Feet 4, adjustable.
12 Sterisonic™ GxP Series Cell Culture Incubators www.sanyobiomedical.com
Page 13

Sterisonic™ GxP CO2/O2 Series Specifications
Sterisonic™ GxP CO2/O2 Incubators
Description
Single Chamber Model Number MCO-19M(UVH) MCO-19M(UV) MCO-19 M
Dual Chamber Model Number MCO-38M(UVH) MCO- 38M(UV ) MCO -38M
Major Operating Systems
H2O2 Decontamination System Standard Optional Optional
™
SafeCell
Single Beam, Dual Detector IR CO
inCu saFe
LCD Graphical Controller/ Display, Door Mounted Standard Standard Standard
Direct Heat, Air
Decontamination
H
Interior UV Lamp, Programmable, Ozone Free Standard Standard Optional
Copper Enriched Stainless Steel Interior
with Germicidal Protection
UV System Standard Standard Optional
Sensor Standard Standard Standard
2
™
Copper Enriched Stainless Steel Interior Standard Standard Standard
™
(DHA ) Air Jacket Standard Standard Standard
Decontamination System Vaporization in situ Optional Optional
2O2
Standard Standard Standard
Environmental Performance
Temperature Control Range +5ºC above ambient to 50°C (in a 5ºC to 35ºC ambient)
Temperature Control Uniformity Deviation ±0.25ºC (in 25ºC ambient, setting 37 ºC, 5% CO
CO
Control Range and Deviation 0% to 20%, ±0.15% in 25ºC ambient, set ting 37 ºC, 5% CO2, no load
2
CO
Sensor Platform
2
Calibration Single point zero automatic; semi-automatic one point span at reference (optional).
CO
2
O
Control Range and Deviation 1-18%, 22-80%, ±0.2% in 25º C ambient, setting 37º C, no load
2
O
Sensor Platform Zirconia sensor, microprocessor P.I.D. control
2
Ceramic based, single beam, dual wavelength measurement
of actual vs. contrast, with continuous auto-zero calibration.
, no load)
2
Airow Gentle vertical air ow, continuous with inner door closed.
Interior Humidity 95%RH @ 37°C through evaporation via DHA
™
heating system; reective optical low water sensor.
Control, Monitoring, Alarm
Temperature and CO
Control P.I.D., setpoint resolution 0.1% and 0.1°C
2
Display Alphanumeric LCD digital display messaging.
Data Acquisition Data Acquisition Automatic log function of temperature and CO
Communications
Optional PC interface, Catalog No. MTR- 4 80 with RS232/RS4 85 data ports available
Remote alarm contacts standard. Optional 4-20mA connection.
.
2
Cabinet Design and Construction
Interior, Shelves 3, Copper-enriched stainless steel.
Inner Door 4 separate, gasketed inner doors, tempered glass
Insulation Rigid foam polyurethane.
Outer Door Reversible, heated.
Access Port Single opening with interior and exterior 1.18" (30 mm) non -VOC silicone stoppers.
Leveling Feet 4, adjustable.
13
Page 14

Sterisonic™ GxP CO2 Series Accessories
SANYO Biomedical products include a broad range of accessories to meet specific application requirements. For accessories or options
not listed herein, contact SANYO or your authorized SANYO sales representative.
Sterisonic™ GxP CO2 Series Incubators
Single Chamber Model Number
Dual Chamber Model Number
H
Decontamination K it Built-in MCO-HL N / A
2O2
H
Vapor Atomizer MCO-HP N/A N / A
2O2
H
Reagent (Formulated for SAN YO Sterisonic™ GxP) MCO- H2O2 N/A N / A
2O2
Automatic CO
Gas Calibration System, semi-automatic one point calibration function. MCO-SG MCO-SG MCO-SG
CO
Cylinder Regulator, CGA tting 320 M CO -10 0L M CO -10 0L MCO-10 0 L
2
Roller Base. For use in single or stacked installations. MCO-18 RB MC O -18R B MCO -18 RB
inCu saFe
inCu saFe
Integrated Cooling Option MCO-CL MCO-CL MCO- CL
Communications Port. Located at rear of chamber.
Connector, cable and software not supplied.
Communications Port. Located at rear of chamber, analog 4 -20 mA . MCO-420MA MCO-420MA MCO- 420MA
SafeCell
Cylinder Switchover System M CO - 21GC MCO-21G C M CO- 21G C
2
™
Shelf and Brackets. Includes two shelf brackets. Full shelf MCO- 47S T M CO -47ST MCO- 47S T
™
Half Tray System MCO-25ST MCO-25ST MCO -25ST
™
UV System Kit Narrow-bandwidth 253.7nm lamp and assembly. Built-In Built- In MCO -19 UV S
MCO-19AIC(UVH)
MCO-38AIC(UVH)
Catalo g No. Catalog No. C atalog No.
MTR-480 MTR- 480 MTR-480
MCO-19AIC(UV)
MCO-38AIC(UV)
MCO-19AIC
MCO-38AIC
H2O2 Reagent (MCO-H2O2)
SANYO H2O2 solution is specially formulated for optimal
use with the MCO-HP atomizer. Each pre-measured
bottle is sufficient for a complete H2O2 decontamination
sequence. Unit of issue: six per carton.
H2O2 Vapor Atomizer (MCO-HP)
Shown with connecting cable, standard.
Integrated Cooling Coil (MCO-CL)
Factory installed; specify when ordering. Water bath/
circulator not included. Permits stable operation at
ambient or below ambient temperatures.
Extends performance specifications as follows:
• Temperature Range: +18°C to +50°C, distribution
+/-0.25°C, variation +/-0.1°C.
• Relative Humidity: 5°C to +50°C, 95% +/- 5%RH; 20°C
to 25°C >80%RH; 18°C >70%RH
Includes temperature mapping results for individual
unit per serial number.
Relative Temperature Performance
+50ºC +50ºC
With
Cooling Coil
+18 ºC
Without
Cooling Coil
+29ºC over
Ambient
Ambient
Temperature
+24ºC
14 Sterisonic™ GxP Series Cell Culture Incubators www.sanyobiomedical.com
Page 15

Sterisonic™ GxP CO2/O2 Series Accessories
Sterisonic™ GxP CO2/O2 Series Incubators
Single Chamber Model Number
Dual Chamber Model Number
H
Decontamination K it Built-in N /A N / A
2O2
H
Vapor Atomizer MCO-HP N/A N / A
2O2
H
Reagent (Formulated for SAN YO Sterisonic™ GxP) MCO- H2O2 MCO- H2O2 N / A
2O2
Automatic CO
Cylinder Switchover System M CO - 21GC MCO-21G C M CO- 21G C
2
Gas Calibration System, semi-automatic one point calibration function. MCO-SG MCO-SG MCO-SG
CO
Cylinder Regulator, CGA tting 320 M CO -10 0L M CO -10 0L MCO-10 0 L
2
N
Cylinder Regulator, CGA tting 5 80 (for low oxygen applications) MC O -100N MCO -10 0N MCO -10 0N
2
O
Cylinder Regulator, CGA tting 5 40 (for high oxygen applications) MCO-10 0 M M CO -10 0 M MCO-100 M
2
Roller Base. For use in single or stacked installations. MCO-18 RB MC O -18R B MCO -18 RB
inCu saFe
inCu saFe
™
Shelf and Brackets. Includes two shelf brackets. Full shelf MCO- 47S T M CO -47ST MCO- 47S T
™
Half Tray System MCO-25ST MCO-25ST MCO -25ST
Integrated Cooling Option MCO-CL MCO-CL MCO- CL
Communications Port. Located at rear of chamber.
Connector, cable and software not supplied.
Communications Port. Located at rear of chamber, analog 4-20 mA . MCO-420MA MCO-420MA MCO- 420MA
™
SafeCell
UV System Kit Narrow-bandwidth 253.7nm lamp and assembly. Built-In Built- In MCO -19 UV S
MCO-19M(UVH
MCO-38M(UVH)
MCO-19M(UV)
MCO-38M(UV)
MCO-19M
MCO-38M
Catalo g No. Catalog No. C atalog No.
MTR-480 MTR- 480 MTR-480
Integrated Cooling Option
Feature
Temperature Range +18ºC to +50 ºC, distribution + / -0.25 ºC, variation +/ - 0.1ºC
Relative Humidity 5ºC to +50ºC, 9 5% +/ - 5%RH; 20ºC to 25ºC > 80%RH; 18ºC > 70%RH
Includes temperature mapping results for individual unit per serial number
Components Stainless Steel Cooling Coil, Interconnection Lines, 6 L, Refrigerated Water Bath & Circulator (Optional / Not Provided )
15
Page 16

Service and Technical Support
Unique SANYO Services
• On-site consultation
• Specialized documentation for each
individual unit
• Customized testing procedures
based on personalized customer
requirements
SANYO Connect
SANYO’s customer-driven biomedical service program guarantees local
attention from qualied SANYO service
representatives, whenever and wherever you need it.
• New Unit Installation and Training
• Preventative Maintenance
• Warranty and Non-Warranty Repairs
• Calibration/Validation Services
• Refurbishment and Reconditioning
• Customized Service and Warranty
Programs
• In-Stock Parts for Immediate Delivery
Predelivery and On-Site Services
Predelivery services include factory
acceptance testing, calibration, and
temperature mapping. On-site services
include installation qualication,
operational qualication, performance
qualication, calibration and temperature
mapping.
Product conforms to RoHS
(European Restriction of Hazardous
Substance directives)
SANYO Electric Co.,Ltd., Biomedical Division, Gumma is certied for quality management
system:ISO9001/medical devices quality management system:ISO13485/environmental
management system:ISO14001
™
UV U.S. Patent 6255103; Direct Heat and Air Jacket™ U.S. Patent 5519188;
SafeCell
SafeCell™ UV, inCu saFe™, Direct Heat and Air Jacket™, P.I.D./R™ and Active Background
Contamination Control™, are trademarks of SANYO Electric Biomedical Co., Ltd.
© 2010 Specifications subject to change without notice.
SANYO North America Corporation
1300 Michael Drive, Suite A, Wood Dale, IL 60191
Toll free USA (80 0) 858-8442, Fax (630) 238-0074
Biomedical Solutions Division
www.sanyobiomedical.com
101910 V6
 Loading...
Loading...