Page 1

WS80A
Service Manual
Page 2

Page 3

SAMSUNG MEDISON
DIAGNOSTIC ULTRASOUND SYSTEM
WS80A
Service Manual
English
SM-WS80A-ENG-02
Page 4

Page 5

Safety Requirements
■ Categorization
− Type of protection against electric shocks: Class I
− Degree of protection against electric shocks (when the patient is in
physical contact): Type BF or type CF applied part
− Degree of protection against the ingress of harmful liquids: General
equipment
− Degree of safety of use in the presence of flammable anesthetic agent
mixed with air, oxygen, or nitrous oxide: Not suitable for use near
flammable anesthetic agent mixed with air, oxygen, or nitrous oxide
− Mode of operation: Continuous operation
■ Safety standards the device conforms to
− Medical electrical equipment, part 1: General requirements for basic safety
and essential performance IEC 60601-1:2005
− Medical electrical equipment, part 1-2: General requirements for basic
safety and essential performance - Collateral standard: Electromagnetic
compatibility - Requirements and tests IEC 60601-1-2:2007
− Medical electrical equipment, part 1-6: General requirements for basic
safety and essential performance - Collateral standard: Usability IEC
60601-1-6:2010
− Medical electrical equipment, part 2-37: Particular requirements for the
basic safety and essential performance of ultrasonic medical diagnostic
and monitoring equipment IEC 60601-2-37:2007
− Medical electrical equipment, part 1: General requirements for safety IEC
60601-1:1988, A1:1991, A2:1995
− Medical electrical equipment, part 1-1: General requirements for safety -
Collateral standards: General requirements for medical electrical systems
IEC 60601-1-1:2000
− Medical electrical equipment, part 1-2: General requirements for safety -
Collateral standards: Electromagnetic compatibility - Requirements and
tests IEC 60601-1-2:2001, A1:2004
− Medical electrical equipment, part 1-4: General requirements for safety -
Collateral standards: Programmable electrical medical systems IEC
60601-1-4:1996, A1:1999
Page 6

− Medical electrical equipment, part 2-37: Particular requirements for the
basic safety and essential performance of ultrasonic medical diagnostic
and monitoring equipment IEC 60601-2-37:2001, A1:2004, A2:2005
− Medical devices - Application of risk management to medical devices ISO
14971:2007
− Medical electrical equipment, part 1: General requirements for safety UL
60601-1:2003
− Medical electrical equipment - part 1: General requirements for safety
CAN/CSA C22.2 No. 601.1-M90:1990, R2003, R2005
− Biological evaluation of medical devices – part 1: evaluation and testing
ISO 10993-1: 2009
− Standard means for reporting the acoustic output of medical diagnostic
ultrasonic equipment IEC 61157:2007
■
Statements
This is the CSA symbol used in Canada and the
United States.
This mark certifies that the product conforms to
applicable EEC standards and has been certified by
the European certification agency.
This mark certifies that the product conforms to
applicable EEC standards.
GMP symbol represents the good manufacturing
practice and quality standards in accordance with
the Korean Quality Standards.
Page 7

Precautions for Use
Be sure to read this operation manual thoroughly, to familiarize yourself with the
operation of the product and the relevant safety information, before attempting to
use the product.
■ Keep this manual near the product and refer to it when using the product.
■ Please familiarize yourself with the safety precautions in 'Chapter 1. Safety' and
'Chapter 8. Maintenance' in particular.
■ This operation manual does not contain clinical opinions or diagnoses. Also,
please consult the reference for each study area before evaluating the
measurement result of an application.
■ This product is an ultrasound diagnostic system, and cannot be used with your
personal computer. If you use this product in such an environment, we cannot
be held responsible for any resulting problems.
■ This product must be used by a person who possesses clinical pathology
training and/or certification; use by unqualified persons is prohibited.
■ The manufacturer is not responsible for any damage to this product caused by
user carelessness and/or neglect.
■ The contents of this operation manual may be changed without notice.
■ Products that are not manufactured by Samsung Medison are indicated with the
trademarks of their respective owners.
■ The following terms are used to highlight safety precautions that the user must
be aware of:
DANGER
WARNING
CAUTION
NOTE
Disregarding this instruction may result in death, serious injury, or
other dangerous situations.
Follow these instructions to prevent a serious accident or damage
to property.
Follow these instructions to prevent a minor accident or damage
to property.
The accompanying information covers an installation, operation, or
maintenance procedure that requires careful attention from the
user, but has little chance of leading directly to a dangerous
situation.
Page 8

Page 9

Revision History
The revision history of this manual is as follows.
VERSION DATE NOTE
V3.00.00-00 2015.10.30
Initial Release
System Upgrades and Manual Set Updates
Samsung Medison Ultrasound is committed to innovation and continued
improvement. Upgrades may be announced that consist of hardware or software
improvements. Updated manuals will accompany those system upgrades.
Verify that Check if this version of the manual is correct for the system version. If
not, please contact the Customer Service Department.
If You Need Assistance
If you need any assistance with the equipment, like the service manual, please
contact the Samsung Medison Customer Service Department or one of their
worldwide customer service representatives, immediately.
Page 10

Table of Contents 1
Table of Contents
Chapter 1. Introduction
Product Specifications .......................................................................................................... 2
Product Configuration ........................................................................................................... 5
The Monitor ..................................................................................................................... 7
The Control Panel ........................................................................................................... 9
The Console .................................................................................................................... 16
Peripheral Devices .......................................................................................................... 18
Probes ............................................................................................................................. 21
Accessories ..................................................................................................................... 22
Optional Functions .......................................................................................................... 23
Chapter 2. Safety
Purpose of Use ....................................................................................................................... 2
Safety Information .................................................................................................................. 3
Safety Symbols ................................................................................................................ 3
Symbols ........................................................................................................................... 6
LABEL ............................................................................................................................. 7
Electrical Safety ..................................................................................................................... 8
Prevention of Electric Shocks.......................................................................................... 8
ECG-related Information ................................................................................................. 10
ESD ................................................................................................................................. 10
EMI .................................................................................................................................. 11
EMC ................................................................................................................................. 11
Biological Safety .................................................................................................................... 23
ALARA Principle .............................................................................................................. 23
Environmental Protection ..................................................................................................... 38
Correct Disposal of This Product(Waste Electrical & Electric Equipment) ..................... 38
Page 11

2 WS80A Service Manual
Chapter 3. Installing the Product
Unpacking the Product ......................................................................................................... 3
Installation Environment ....................................................................................................... 4
Installing the Product ............................................................................................................ 5
Power Cord Connection .................................................................................................. 6
Probe Connection ........................................................................................................... 6
Connecting Peripherals .................................................................................................. 7
System Power ........................................................................................................................ 9
Turning the Power On ..................................................................................................... 9
Shutting Down the System .............................................................................................. 10
System Settings ..................................................................................................................... 11
System General Setting .................................................................................................. 12
Scan Mode ...................................................................................................................... 17
Display ............................................................................................................................ 21
Annotate .......................................................................................................................... 26
Peripheral Device Settings ............................................................................................. 31
User Defined Keys .......................................................................................................... 38
MPR Menu ...................................................................................................................... 40
Network ........................................................................................................................... 41
Options ............................................................................................................................ 43
DICOM Setup (optional) .................................................................................................. 45
Auto Calc ......................................................................................................................... 59
System Information ......................................................................................................... 60
Chapter 4. Product Inspection
Inspecting Functions ............................................................................................................ 2
Basic Inspections ............................................................................................................ 2
Detailed Inspections ........................................................................................................ 4
Page 12

Table of Contents 3
Chapter 5. Product Structure
Overview ................................................................................................................................. 3
System Block Diagram .......................................................................................................... 5
Basic Structure of UGEO WS80A ......................................................................................... 7
Overview .......................................................................................................................... 7
Ultrasound System Part .................................................................................................. 8
PC Part ....................................................................................................................... ..... 9
User Interface Part .......................................................................................................... 10
AC to Power Module ....................................................................................................... 10
Ultrasound System Part ........................................................................................................ 11
PSA ................................................................................................................................. 11
Analog Control ................................................................................................................. 13
Beam Former Board ........................................................................................................ 15
Back End Board .............................................................................................................. 1 9
PC Part ....................................................................................................................... ............. 24
PC Module ....................................................................................................................... 24
Software DSC .................................................................................................................. 25
Rear Board ...................................................................................................................... 27
Power Supply .................................................................................................................. 28
User Interface Part ................................................................................................................. 31
Control Panel ................................................................................................................... 31
Docking CP Board ........................................................................................................... 33
Touch Panel .................................................................................................................... 34
Monitor ............................................................................................................................. 35
Chapter 6. Service Mode
System Information ................................................................................................................ 2
Windows Mode ....................................................................................................................... 3
Page 13

4 WS80A Service Manual
Admin Mode ........................................................................................................................... 4
Admin Mode Functions ................................................................................................... 5
Adding and Deleting Options ............................................................................................... 11
Adding an Option ............................................................................................................ 12
Chapter 7. Troubleshooting
Power Issues .......................................................................................................................... 2
Power Does Not Turn On ............................................................................................... 2
Power Does Not Turn Off................................................................................................ 2
Power Turns Off by Itself ................................................................................................ 3
Monitor .................................................................................................................................... 4
Nothing Is Displayed on the Screen ............................................................................... 4
Screen is Discolored ....................................................................................................... 4
Error Messages ...................................................................................................................... 5
Error Occurs during Booting ........................................................................................... 5
Image ...................................................................................................................................... 6
2D Mode: There is No IMAGE ECHO or IMAGE FORMAT ........................................... 6
Lines (Noise) Appear in 2D Mode Image ....................................................................... 6
M, C, PW, CW Mode Trouble ......................................................................................... 6
Error Code .............................................................................................................................. 7
Chapter 8. Disassembly and Assembly
Caution ................................................................................................................................... 2
Preparation ..................................................................................................................... 2
Disassembling the Product .................................................................................................. 3
Front Cover Disassembly ............................................................................................... 3
Rear Cover Disassembly ................................................................................................ 5
Control Panel Disassembly ............................................................................................. 7
Monitor Disassembly ....................................................................................................... 8
Page 14

Table of Contents 5
Monitor Arm Disassembly ............................................................................................... 9
Assembling the Product ........................................................................................................ 11
Chapter 9. Probes
Probe ....................................................................................................................................... 2
Ultrasound Transmission Gel .......................................................................................... 15
Using Sheaths ................................................................................................................. 16
Probe Safety Precautions ................................................................................................ 17
Cleaning and Disinfecting the Probe ............................................................................... 19
Chapter 10. Maintenance
Product Maintenance ............................................................................................................. 2
Cleaning and disinfecting ................................................................................................ 2
Air Filter Management ..................................................................................................... 4
Accuracy Checks ............................................................................................................. 5
Information Maintenance ...................................................................................................... 6
Backing up User Setting .................................................................................................. 6
Backing Up Patient Information ....................................................................................... 6
Software .......................................................................................................................... 6
Chapter 11. Service Part List
Body Cover Parts .............................................................................................................. 2
System Parts ..................................................................................................................... 7
Control Panel Parts ........................................................................................................... 11
System Cable Parts ........................................................................................................... 14
Page 15

Chapter 1
Introduction
Product Specifications .................................................................................................. 2
Product Configuration .................................................................................................. 5
The Monitor ............................................................................................................................... 7
The Control Panel ..................................................................................................................... 9
The Console ........................................................................................................................... 16
Peripheral Devices .................................................................................................................. 18
Probes ..................................................................................................................................... 21
Accessories ............................................................................................................................ 22
Optional Functions .................................................................................................................. 23
Page 16

1 - 2 WS80A Service Manual
Product Specifications
Height: 1,430 – 1,710mm (with monitor)
Width: 557mm
Physical
Dimensions
Imaging
modes
Depth: 791 – 860mm
Weight: 105.4kg (with monitor)
Weight: Approx. 130kg (with Safe Working Load)
2D-Mode
M-Mode
Color Doppler
Pulsed Wave (PW) Spectral Doppler
Continuous Wave (CW) Spectral Doppler
Tissue Doppler Imaging (TDI)
Tissue Doppler Wave (TDW)
Power Doppler (PD)
Directional Power Doppler (S-Flow)
Color M-Mode
Anatomical M mode
3D imaging Mode
4D imaging Mode
ElastoScan Mode
Gray Scale
Focusing
Probes
(Type BF / IPX7)
256 (8 bits)
Transmit focusing, maximum of eight points (four points simultaneously
selectable)
Digital dynamic receive focusing (continuous)
Linear Array
L3-12A, L5-13, LA2-9A, LA3-16A, LA3-16AI, LA4-18B
Convex
C2-6, CA1-7A, CA2-8A, CA2-9A, CA3-10A, CF4-9, SC1-6
Endocavity
E3-12A, EA2-11B, VR5-9
Phased Array
PA3-8B, PE2-4, PM1-6A
Page 17

3D
CV1-8A, LV3-14A, V4-8, V5-9
Chapter 1. Introduction 1 - 3
Probe
connections
Monitor
ECG
Rear Panel
Input / Output
Connections
Five Active Probe Ports (include one CW probe port)
Main Monitor
23 inch Full HD LCD monitor (LED backlight unit)
called "LCD monitor" henceforth
Touch Screen Monitor
10.1 inch LCD monitor (LED backlight unit)
called "LCD monitor" henceforth
USB Type (Type CF)
Audio in / out
Microphone
External Trigger in / out
External monitor DVI-I
Network
USB
Foot Switch
Image
Storage
Application
Electrical
Parameters
Measurement
Packages
Signal processing
(Pre-processing)
Maximum 12,700 frames for Cine memory
Maximum 8,192 lines for LOOP memory
Image filing system
Obstetrics, Gynecology, Urology, Abdomen, Vascular, Small Part, MSK,
Pediatric, Cardiac, TCD, Intraoperative
100-240V~, 1100VA, 50/60Hz
OB, Gynecology, Cardiac, Vascular, Fetal Heart, Urology, Abdomen, Small
Parts, MSK, TCD, Pediatric Hips
* Refer the Chapter 9 for additional information
TGC Control (Digital / Slider)
Mode-independent gain control
Acoustic power control (adjustable)
Page 18
1 - 4 WS80A Service Manual
Dynamic aperture
Dynamic apodization
Dynamic range control (adjustable)
Image view area control
M-mode sweep speed control
Frame average
Edge Enhancement / Blurring
Signal processing
(Post-processing)
Measurement
Gamma-scale windowing
Image orientation (left/right and up/down, rotation)
White on black/black on white
Zoom
Trackball operation of multiple cursors
2D mode: Linear measurements and area measurements using elliptical
approximation or trace
M mode: Continuous readout of distance, time, and slope rate
Doppler mode: Velocity and trace
Auxiliary
User Interface
Pressure Limits
Humidity Limits
Temperature Limits
USB Video Printer
USB to RS-232 Serial Cable
Foot Switch(IPX8)
USB Flash Memory Media
USB HDD
Monitor
English, German, French, Spanish, Italian, Russian, Chinese
Operating: 700 – 1,060hPa
Storage: 700 – 1,060hPa
Operating: 30 – 75%
Storage & Shipping: 20 – 90%
Operating: 10 – 35OC
Storage & Shipping: -25 – 60
O
C
Page 19

Chapter 1. Introduction 1 - 5
Product Configuration
This Product consists of monitor, control panel, console, peripheral devices and probes.
① Monitor
② Monitor arm
③ DVD drive
④ Speaker
⑤ Control panel
⑥ Probe holder
⑦ Keyboard
Lift
USBport
[Figure 1.1 Front of the product]
CW probe port
Probe port
Air filter
Brake
Wheels
Touch panel
Gel Warmer
(Option)
Page 20

1 - 6 WS80A Service Manual
① Handle
② Storage compartments
③ Ventilation
④ Rear panel
⑤ Cable hook
⑥ Power terminal
⑦ ID Label
[Figure 1.2 Back of the product]
Page 21
Chapter 1. Introduction 1 - 7
The Monitor
Ultrasound images and other information are displayed on the color LCD monitor.
▐ Screen Layout
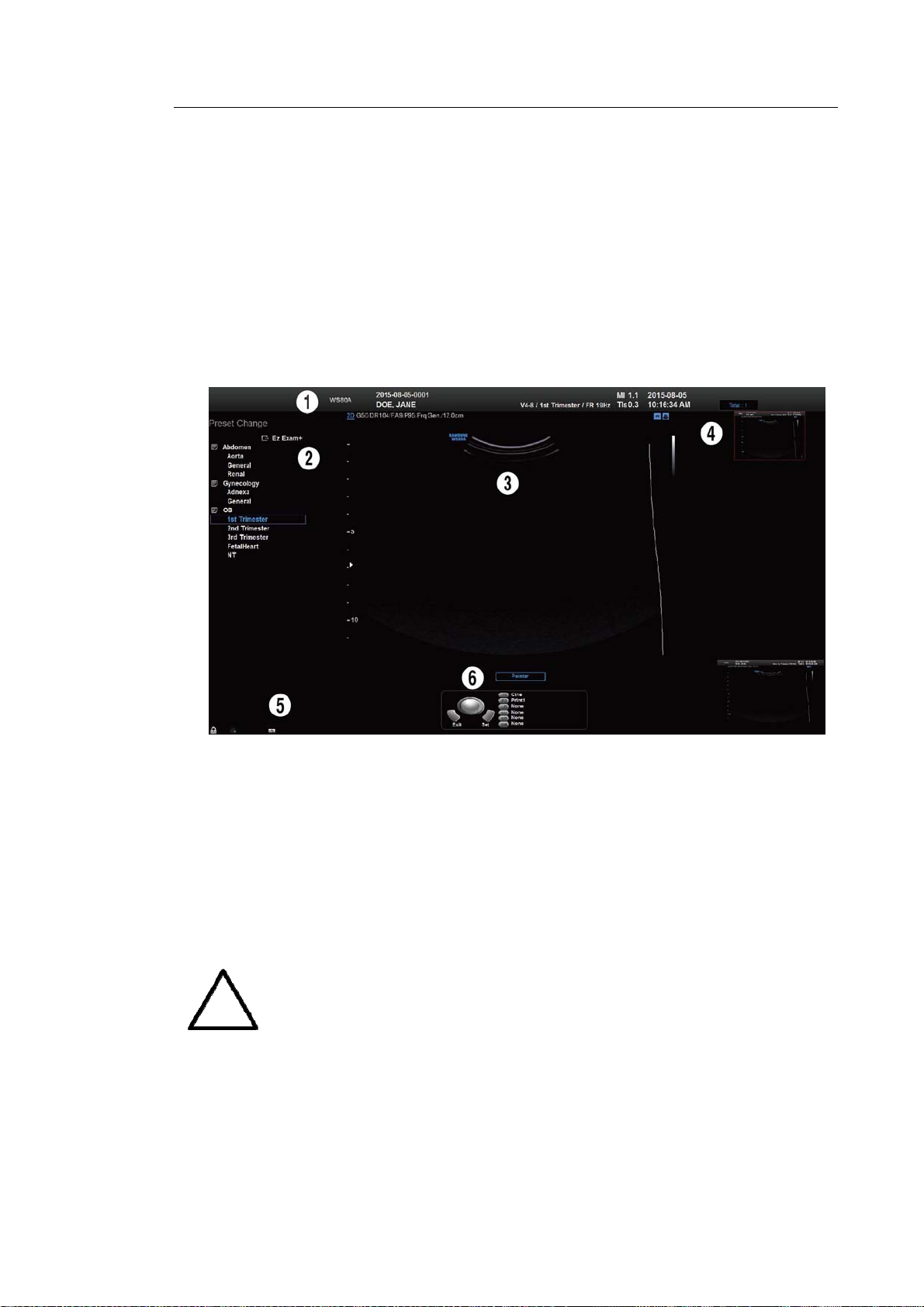
The screen displays ultrasound images, operation menus and a variety of other information.
The main areas of the screen are ① Title Area, ② Preset Change and EZ Exam Area, ③
Image Area, ④ Thumbnail Area, ⑤ User Information Area, and ⑥ User Defined Key Area,
as shown below.
[Figure 1.3 Screen Layout]
① Title Area
Displays patient name, hospital name, application, frame rate and depth, probe information,
acoustic output information, and date and time.
② Preset Change and Ez Exam+ Area
Displays Preset Change. You can quickly change the preset of a probe. The Ez Exam+
menu will also appear if being used.
You can set up the Ez Exam+ at Ez Exam+ Setup; please refer to ‘Chapter 3.
Utilities’ for information on Ez Exam+ Setup.
NOTE
Page 22

1 - 8 WS80A Service Manual
③ Image Area
Displays ultrasound images, TGC, image information, annotation, and measurement
information are also displayed.
④ Thumbnail Area
Images saved by selecting Save are displayed as thumbnails. If saving Single screens, up
to 5 images are shown in a list; for Quad screens, up to 16 images are displayed. Clicking
with the pointer will enlarge the selected thumbnail in the Image area.
⑤ User Information Area
Information that is useful to the user, such as current system status, image information,
selectable items, etc., is displayed.
⑥ User Defined Key Area
Settings for User Defined Keys, including the positions of Set and Exit, are displayed.
You can change the setting of each button in Setup > User Defined Key.
NOTE
TIP Principles of Operation of the Diagnostic Ultrasound System
Medical ultrasound images are created when the computer's digital memory
converts the high-frequency wave signals that are transmitted and received by the
probe.
As ultrasound waves propagate through the human body, they generate reflected
signals whenever they encounter a change in density. For example, reflected
signals are generated when signals pass from fatty tissues to muscle tissues.
Reflected signals return to the probe where they are converted into electronic
signals. The reflected signals are amplified and processed by analog and digital
circuits that have filters for various frequencies and response time options. Then
they are again converted into high-frequency electronic signals, and saved as a
series of digital image signals. The monitor displays the image signals stored on
the storage device in real time.
The entire process of transmitting, receiving, and processing signals is controlled
by the computer.
For information on User Key Setup, please refer to 'Chapter 3.
Utilities'.
Page 23

The Control Panel
The system can be controlled by using the control panel.
Chapter 1. Introduction 1 - 9
[Figure 1.4 Control Panel]
The control panel consists of a keyboard, soft menus, buttons, dials, dial-buttons, a slider, and a
trackball.
The dial-button can be used both as a dial and a button.
▐ Functions of the Control Panel
The followings describe each control in the control panel and show how to use them.
Controls with multiple functions are described in detail in the following chapters of Chapter 3
in this manual.
Button Turns the system on/off.
On/Off
Page 24
1 - 10 WS80A Service Manual
2D
M / x
PD / y
PW / z
C / Ref. Slice Dial-button
3D
4D
CW / TB
Dial-button
Dial-button
Dial-button
Dial-button
Button
Button
Dial-button
Button: Starts 2D mode.
Dial: Adjusts the 2D gain.
Button: Starts or ends M mode.
Dial: Adjusts M gain. Also, turning this dial-button when in 3D
View rotates the image along the x-axis.
Button: Starts or ends Power Doppler mode.
Dial: Adjusts the PD gain. Also, turning this dial-button, when in
3D View rotates the image along the y-axis.
Button: Starts or ends PW Spectral Doppler mode.
Dial: Adjusts the PW gain. Also, turning this dial-button when in
3D View rotates the image along the z-axis.
Button: Starts or ends Color Doppler mode.
Dial: Adjusts the C gain. Moves the reference slice horizontally in
3D View.
Starts or ends 3D mode.
Starts or ends 4D mode.
Button: Starts or ends CW Spectral Doppler mode.
Dial: Adjusts the CW gain. Adjusts top and bottom margins of
ROI in 3D View-MPR. TB is an abbreviation for “Top-Bottom”.
Angle / LR
Depth
Focus
Zoom
Q Scan
Freeze
Save
Button: Adjusts the angle of the sample volume in Spectral
Doppler mode. It is also used to adjust the BodyMarker’s probe
Dial-button
Switch
Switch
Dial-button You can magnify an image.
Button
Button
Button
cursor or indicator angle.
Adjusts left and right margins of ROI in 3D View-MPR. LR is
Dial:
an abbreviation for “Left-Right”.
Adjusts the scanning depth of the image.
Changes location and number of focus on the target location you
wish to study.
Press this button to turn the Quick Scan function on. The ‘Q
Scan’ mark will appear at the top of an image.
Pauses/resumes scanning.
Saves an image or a report displayed on the screen to the
database.
Page 25

U1, 2, 4
P 1~2
Button
Button
Chapter 1. Introduction 1 - 11
Stands for ‘User Key.’ This button allows users to select a
function to apply to the button. The function of each button can
be set in Setup > User Defined Key. The selected settings will be
displayed in the User Defined Key area of the monitor.
Stands for ‘Peripheral Key.’ This button allows users to select a
function to apply to the button. The function of each button can
be set in Setup > User Defined Key. The selected settings will be
displayed in the User Defined Key area of the monitor.
BodyMarker
Text
EZ Exam
Set / Exit
Button
Button
Dial-button Use the EZ Exam and Preset Change features.
Button
Button
Button
Button
Allows the user to enter a BodyMarker over an image.
Allows the user to place text on an image.
In this mode, only the image is displayed on the screen.
Compares two independent images.
Compares four independent images.
This button is used to assign user-defined functions. The
function of each button can be set in Utility > Setup > User
Defined Key.
Set: Selects an item or value using the trackball or changes the
function of the trackball.
Exit: Exits the function currently being used and returns to the
previous state.
Pointer
Clear
Change
Calculator
Caliper
Trackball
Button
Button
Button This is used to change the current trackball function.
Button
Button
Trackball
When this is pressed, an arrow marker appears to point to parts
of the displayed image.
Deletes text, indicator, BodyMarker, and measurement result,
etc. displayed on an image.
Starts measurements by application.
Starts to measure distance, circumference, area, and volume.
Moves the cursor on the screen and scrolls through Cine
images.
Page 26

1 - 12 WS80A Service Manual
Keyboard
The keyboard is used to type in text.
[Figure 1.5 Keyboard]
▐ Touch Panel
These control tools are located on both sides of the touch screen. Available buttons are as
follows:
[Figure 1.6 Touch Panel]
Page 27

Chapter 1. Introduction 1 - 13
Patient
Displays the Patient Information screen, which is used for selecting a patient
ID from the list or entering new patient information.
End Exam
Probe
Report
SonoView Runs SonoView, which is the image filing program.
U3
Utility The Utility Menu appears on the touch screen.
Finishes the exam of the currently selected patient and resets the related
data.
Displays the Probe Selection screen to select or change the probe and
application.
Displays the Report screen that shows the measurement results of the
current application and other information.
Stands for User Key; functions can be assigned to these buttons as desired.
The function of each button can be set in Setup > User Defined Key. The
settings are displayed in the User Defined Key area in the monitor.
S-Flow Initiates or terminates the S-Flow (Directional Power Doppler) Mode.
ADVR Initiates recording feature.
TGC
The TGC screen will be displayed on the touch screen. TGC stands for Time
Gain Compensation.
Page 28
1 - 14 WS80A Service Manual
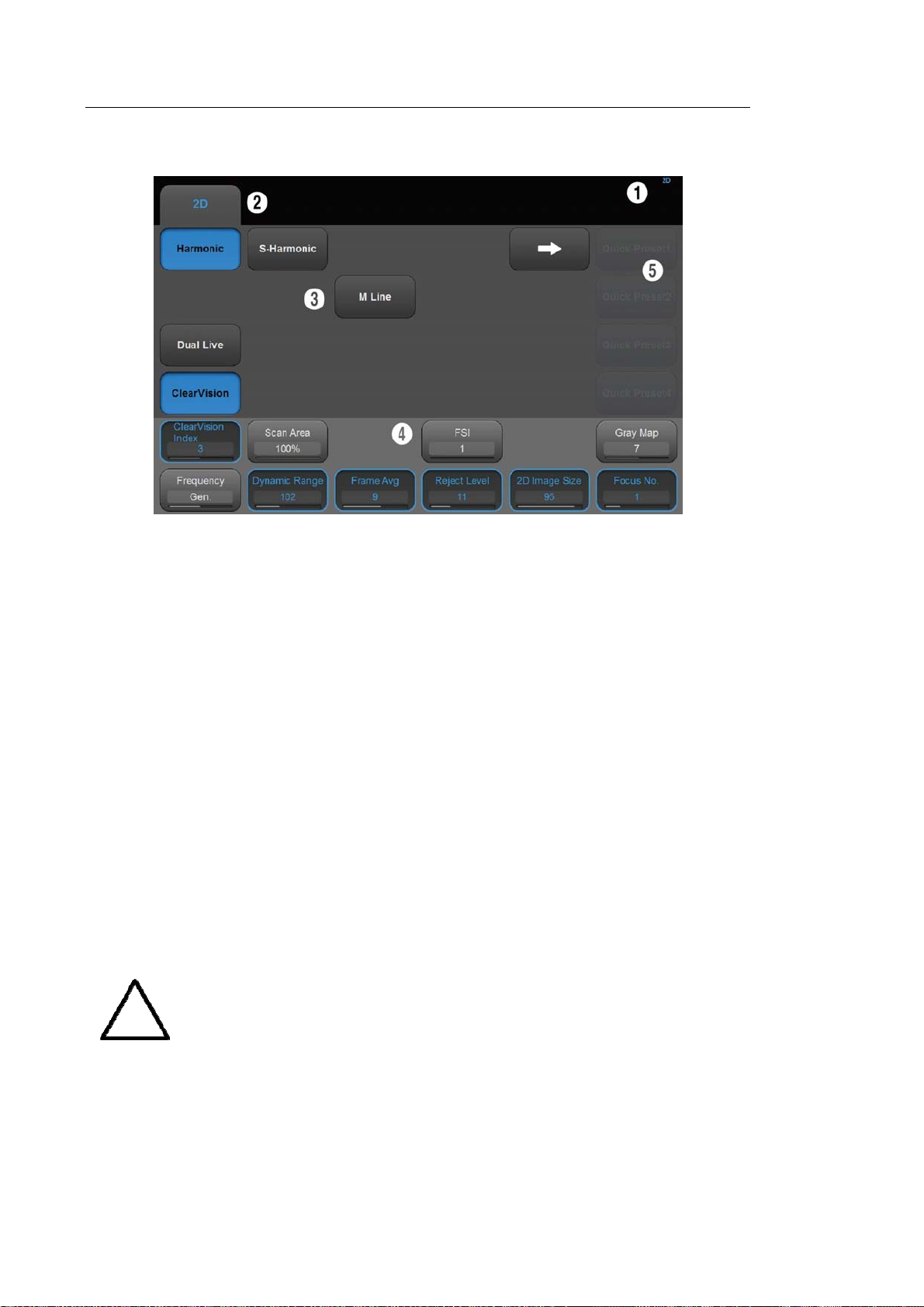
▐ Touch Screen
[Figure 1.7 Touch Screen]
The touch screen is an operating tool that can be touched by the user to input data. The
functions that are available in the current mode are shown in the form of buttons or a
dial-button.
■ Touch screen display
① Information Area: Shows the title of the touch screen currently displayed.
② Tab Area: Shows diagnostic modes and utilities under different tabs. The touch screen
can be changed by pressing one of the tabs.
③ Menu Area: The menu items that are available in the current input mode are shown in
the form of buttons. The user can access the desired menu item by pressing the
corresponding button. The menu currently in use is shown in blue.
④ Soft Menu Area: The soft menu items that are available in the current input mode are
shown.The menus in use are shown with blue borders. Press or rotate the dial-buttons
right below each menu.
⑤ Quick Preset: With predefined diagnosis mode and presets of probes frequently used by
the user, this function provides quick and easy access to frequently used probe in each
diagnosis mode
For further details about setting up Quick Preset, please refer to ‘Setup >
General > Quick Preset > Quick Preset Setup’ in ‘Chapter 3 Utilities’.
NOTE
Page 29

Chapter 1. Introduction 1 - 15
※Tip! When there are two Soft Menus
When there are two menus available – upper and lower, both menus can be adjusted with the
corresponding dial-button. Or tap the button for the menu you want to use on the touch screen
and then use the dial-button.
▐ Adjusting the Control Panel
Do not apply excessive force to the control panel.
CAUTION
Adjust right/left
Hold the control panel handle and move it carefully to the right or left.
Adjust up/down
Use the handle at the back of the product when moving it.
Press the lever on the control panel handle and move it carefully up or down.
Page 30
1 - 16 WS80A Service Manual
① ② ③ ④ ⑤
⑥
⑦ ⑧
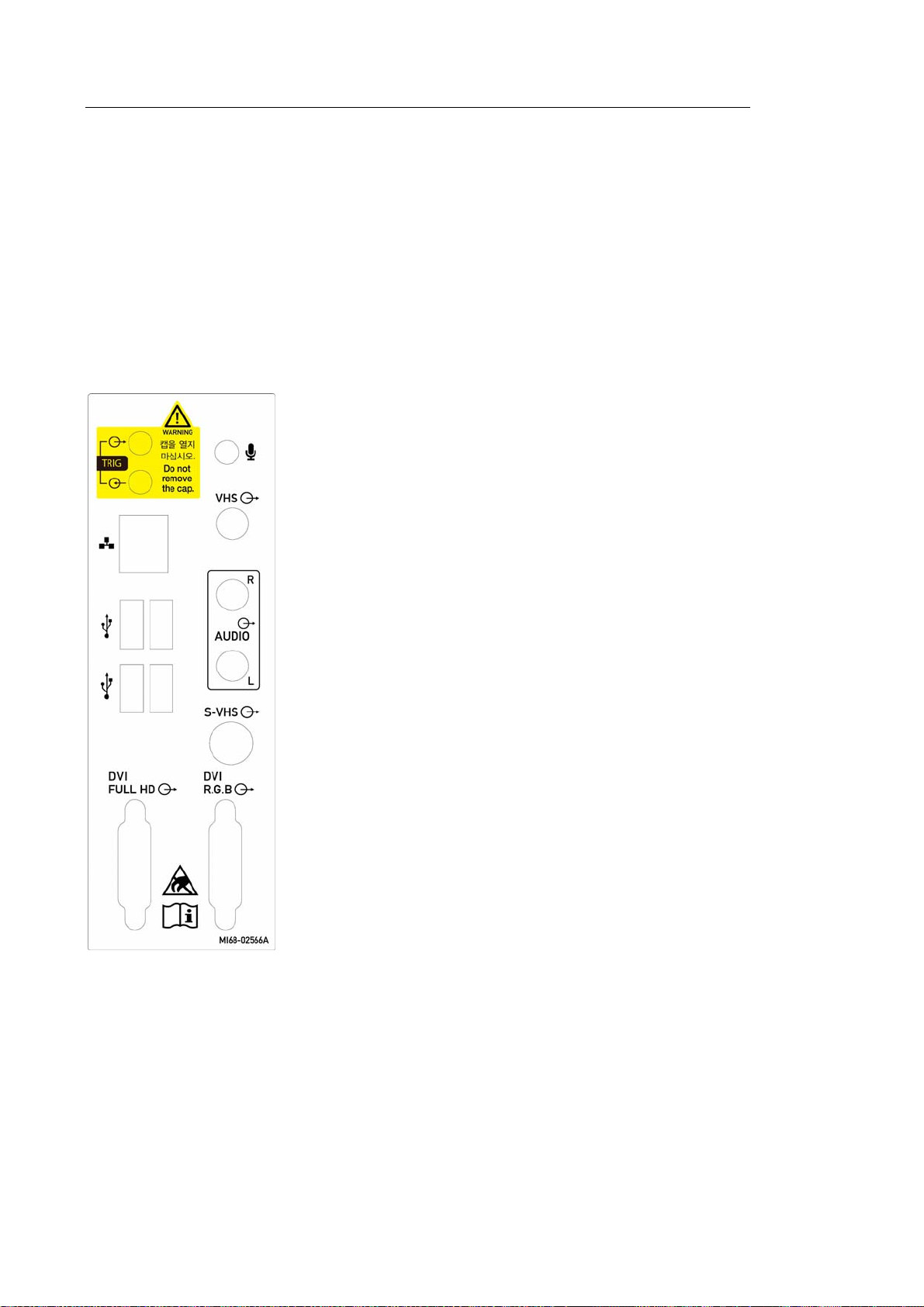
The Console
The console consists of two parts – the inner and outer units. The inside of the console contains
ultrasound imaging components. On the exterior of the console are various connectors, probe
holders, storage compartments, handles, and wheels, etc.
▐ Rear Panel
Various peripheral devices including monitors are connected via the rear panel at the back of
the system.
① Trig port (In/Out): Not used.
② Microphone port (Input): Connect a microphone to
this port.
③ VHS port (Output): Outputs composite image to the
monitor.
④ Audio port (Output): Used to output audio signals.
⑤ S-VHS port (Output): Outputs S-VHS image to the
monitor.
⑥ DVI port (Output): Outputs the digital signal (DVI
Full HD) and analog signal (DVI R.G.B) to the
monitor.
[Figure 1.9 Rear panel]
⑦ USB port: Used to connect to USB peripheral
devices..
⑧ Network port: Used to connect to a network. You
can transfer patient information to another server
via the DICOM network.
Page 31

▐ Power Connection Part
③ ②
①
The power connection part is located at the bottom on the rear panel.
④
[Figure 1.10 Power Connection Part]
① Power switch: Supplies or blocks power to the entire system.
② Power inlet: For the power cable to connect to external power
③ Power outlet: Provides power to the external peripheral devices from the internal
power supply of the system.
Chapter 1. Introduction 1 - 17
④ Equipotential terminal: Must be connected to the equipotential bonding network in a
treatment room.
▐ Probe Holder
Probe holders are mounted at the left and right-hand sides of the control panel.
Page 32

1 - 18 WS80A Service Manual
Peripheral Devices
Peripheral devices can be connected to their corresponding ports on the left/right or rear sides of
the console, as needed.
Do not install a peripheral device that is not listed in this operation manual
in the patient environment. If you install an unlisted device in the patient
CAUTION
environment, it may cause an electrical hazard
Do not connect additional external peripheral devices to the auxiliary
socket outlet. Doing so may decrease safety level..
[Figure 1.11 Patient Environment]
NOTE
For instructions on using a specific peripheral device, refer to the device's
operation manual.
▐ Internal Peripheral Devices
These are peripheral devices mounted in the system.
DVD-Multi
DVD-RW, DVD+RW, DVD-R, DVD+R, CD-R, CD-RW, CD-ROM
Hard Disc Drive
At least 500 GB
Page 33

Chapter 1. Introduction 1 - 19
▐ External Peripheral Devices
These are peripheral devices that can be connected for use when needed and are connected
via the USB port located at the rear panel.
When using a peripheral device via a USB port, always turn the
CAUTION
NOTE
power off before connecting/disconnecting the device.
Connection/disconnection of USB devices during power-on may
lead to malfunction of the system and the USB devices.
■ When removing the removable disk, use Utility > Storage
manager.
■ USB ports are located both on the control panel and the rear panel
of the console.
We recommend that you connect USB storage devices (flash
memory media, etc.) to the ports on the control panel, and other
USB peripheral devices to the rear panel for convenience.
The following products are recommended:
USB Video Printer
- BW: Mitsubishi P95DE, Sony UP-D897
- Color: Mitsubishi CP-30DW, Sony UP-D25MD
USB Line Printer
- BW: Samsung ML-2955DW
- Color: Samsung CLP-615ND
■ You must install a printer and drivers that are compatible with
the English version of Microsoft Windows 7TM. Contact
Samsung Medison’s customer service department for inquiries
about printer driver installation.
CAUTION
■ When installing a printer, make sure that the printer is the same
printer selected in Microsoft WindowsTM or Setup.
■ Please check the port that the printer uses before connecting.
Printers should be connected to the printer port while the USB
printer should be connected to the USB port.
Page 34

1 - 20 WS80A Service Manual
USB to RS-232C Serial Cable
USB to Serial (RS-232C) Converter with FTDI Chipset (FTDI FT232BM Compatible)
Foot Switch
– 3 Pedals HID Type
To configure the foot switch function, go to Utility > Setup > Peripherals > Foot Switch.
You can select one function from the following: Freeze, Update, Record, Print1, Save,
Store Clip, Volume Start, PD Mode, Color Mode, M Mode, PW Mode, CW Mode,
Elastoscan Mode, TDI Mode, TDW Mode, Biopsy, Save/Send, or Store Clip/Send.
WARNING
Misc.
Flash Memory Media
NOTE
For more information about the Open Line Transfer, refer to
`Chapter 9. Measurements and Calculations’.
Foot Switch cannot be used in the operating room
NOTE
■ The system cannot recognize USB 1.1 flash memory. Remove the flash
memory from the console and equip again with an appropriate device.
■ Regarding file formats that are not ordinarily saved: Please check first to see if
it is possible to save the file format on a desktop PC before trying to save the
file on flash memory.
■ Do not use flash memory media which contain anti-virus programs or are
defective. Otherwise, the product may fail to work properly.
■ The system cannot recognize USB 1.1 flash memory. Remove
the flash memory from the console and equip again with an
appropriate device.
■ Regarding file formats that are not ordinarily saved: Please
NOTE
check first to see if it is possible to save the file format on a
desktop PC before trying to save the file on flash memory.
■ Do not use flash memory media which contain anti-virus
programs or are defective. Otherwise, the product may fail to
work properly
Page 35

Chapter 1. Introduction 1 - 21
Probes
Probes are devices that generate ultrasound waves and process reflected wave data for the
purpose of image formation.
NOTE
For more information on probes, refer to ‘Chapter 5. Probes’ and the
‘Reference Manual’.
▐ Connecting probes
To ensure the safety of the product and the probe, turn off the power before connecting the
probe to, or disconnecting it from, the probe port.
1. Connect probes to the probe ports on the front panel of the system. A maximum of five
probes including the CW probe can be connected at one time. The CW probe should
CW only be connected to its own port.
2. To install, turn the connector turning handle clockwise.
[Figure 1.11 Probe Connector]
Page 36

1 - 22 WS80A Service Manual
Accessories
An accessory box containing the items below is supplied with the product.
CAUTIO
N
NOTE
Main cord set, separately certified according to the relevant standards, is to be
used when supplied to EU and USA/CAN.
Accessories can be different according to the country.
[Figure 1.12 Accessories]
Page 37

Optional Functions
This product has the following S/W optional functions
Chapter 1. Introduction 1 - 23
■ 4D
■ 3D XI
■ CW Function
■ Cardiac Measurement
■ DICOM
■ XI STIC
■ Elastoscan
■ Panoramic
■ 3DMXI
■ HDVI
■ ADVR
■ E-Thyroid
■ Realistic Vue
■ AutoIMT+
■ 5D LB
■ 5D NT
■ Mobile Export
■ 5D Heart
■ 5D Follicle
■ 5D CNS
■ Elite
■ MPI
■ Software Trial
■ DICOM Q/R
■ S-Detect
■ 5D Limb
■ Crystal Vue
■ 5D Heart Color
■ E-Breast
■ 2D NT
For further information about the options listed above, please refer to the relevant chapters in the
operation manual.
■ Elite is a package for WS80A v3.00 and does not refer to any particular
function.
NOTE
■ Software Trial does not refer to any particular function.
■ 5D CNS+
Page 38

Chapter 2
Safety
Purpose of Use .............................................................................................................. 2
Safety Information ......................................................................................................... 3
Safety Symbols ......................................................................................................................... 3
Symbols .................................................................................................................................... 6
Labels ....................................................................................................................................... 7
Electrical Safety ............................................................................................................. 8
Prevention of Electric Shock ..................................................................................................... 8
ECG-related Information ......................................................................................................... 10
ESD ......................................................................................................................................... 10
EMI .......................................................................................................................................... 11
EMC ........................................................................................................................................ 11
Mechanical Safety ....................................................................................................... 19
Moving the Equipment ............................................................................................................ 19
To Use the Product Safely ...................................................................................................... 20
Biological Safety ......................................................................................................... 23
ALARA Principle ..................................................................................................................... 23
Environmental Protection ........................................................................................... 38
Correct Disposal of This Product (Waste Electrical & Electronic Equipment) ........................ 38
Page 39

2 - 2 WS80A Service Manual
Purpose of Use
The WS80A Diagnostic Ultrasound System and transducers are intended for diagnostic
ultrasound imaging and fluid analysis of the human body.
The clinical applications include: Fetal/Obstetrics, Abdominal, Gynecology, Pediatric, Small
Organ, Neonatal Cephalic, Adult Cephalic, Trans-rectal, Trans-vaginal, Muscular-Skeletal
(Conventional, Superficial), Urology, Cardiac Adult, Cardiac Pediatric and Peripheral vessel.
NOTE
For detailed information on applications and presets, please refer to
‘Chapter 2. Introduction’ and ‘Chapter 5. Probes’ in this operation manual.
▌ Contraindications
This system is not intended for ophthalmic use or any use causing the acoustic beam to pass
through the eye.
■ Federal law restricts this device to sale by or on the order of a physician.
CAUTION
■ For information on the use or clinical application of this product, please
refer to ‘Chapter 6. Starting Diagnosis’ and ‘Chapter 7. Diagnosis Mode’ in
this operation manual.
Page 40

Chapter 2. Safety 2 - 3
Safety Information
Please read this chapter before using the Samsung Medison ultrasound system. It is relevant to
the ultrasound system, the probes, the recording devices, and any of the optional equipment.
This system is intended for use by, or by the order of, and under the supervision of, a licensed
physician who is qualified for direct use of the medical device.
Safety Symbols
The International Electrotechnical Commission (IEC) has established a set of symbols for
medical electronic equipment, which classify a connection or warn of potential hazards. The
classifications and symbols are shown below:
Symbols Description
WARNING: The accompanying information must be followed to
prevent serious accidents and/or damage to property.
CAUTION: The accompanying information helps to prevent minor
accidents and/or damage to property.
Refer to the operation manual.
Follow the operation manual.
CAUTION: Risk of electric shock
Type BF applied part (Classification based on degree of protection
against electric hazard)
Page 41

2 - 4 WS80A Service Manual
Symbols Description
Defibrillation-proof type CF applied part (Classification based on
degree of protection against electric hazard)
Power on/off
Power on
Power off
V~
Power ON for part of the product
Power Off for part of the product
Alternating current voltage source
Direct current voltage source
Dangerous voltage (Indicates dangerous voltages over 1000V AC
or 1500V DC)
Protective earth (ground)
Equipotentiality
Page 42

Chapter 2. Safety 2 - 5
Symbols Description
Data output port
Data input port
Data Input/Output port
Input port
Output port
Print remote output
Foot Switch Port
IPX 1
ECG port
USB port
Network port
Microphone Port
Probe port
IPX 1: Protected against vertically falling water drops
Page 43

2 - 6 WS80A Service Manual
Symbols Description
IPX 7
IPX 8
IPX 7: Protected against the effects of temporary immersion in
water
IPX 8: Protected against the effects of continuous immersion in
water
CAUTION: Electrostatic sensitive devices (ESD)
Do not sit on the product.
Do not push the product.
Do not lean against the product.
Symbols
Be mindful of the space. Do not place a finger, and or any part of
your body in the space.
Symbols Description
Authorised Representative In The European Community
Manufacturer
Page 44

Chapter 2. Safety 2 - 7
Labels
Phrases containing the words ‘warning’ and/or ‘caution’ are displayed on the product's surface in
order to protect it.
Page 45

2 - 8 WS80A Service Manual
Electrical Safety
This equipment is categorized as a Class I device with Type BF or Type CF (ECG) applied parts.
■ As for US requirement, the LEAKAGE CURRENT might be measured from
a center-tapped circuit when the equipment connects in the United States
CAUTION
Prevention of Electric Shock
In a hospital environment, hazardous current can form due to potential differences between
exposed conductive parts and connected devices. The solution to the problem is consistent
equipotential bonding. Medical equipment is connected with connecting leads made up of
sockets which are angled to the equipotential bonding network in medical rooms.
to 240V supply system.
■ To help assure grounding reliability, connect to a “hospital grade” or
“hospital only” grounded power outlet.
[Figure 1.1 Equipotential Bonding]
Additional equipment connected to medical electrical equipment must comply with the
respective IEC standards (e.g., IEC 60950/EN 60950 for data processing equipment, IEC
60601-1/EN 60601-1 for medical devices). Furthermore, all components of the product shall
comply with the requirements for medical electrical systems IEC 60601-1-1/EN 60601-1-1. Any
person connecting additional equipment to the signal input and output ports of medical electrical
equipment must verify that the equipment complies with IEC 60601-1-1/EN 60601-1-1.
Page 46

WARNING
Chapter 2. Safety 2 - 9
■ Electric shock may result if this system, including all of its externally
mounted recording and monitoring devices, is not properly grounded.
■ Never remove the cover from the product. Hazardously high voltage
flows through the product. All internal adjustments and replacements
must be made by a qualified Samsung Medison Customer Service
Department.
■ Always check the product's casing, cables, cords, and plugs for damage
before using the product. Disconnect and do not use the power source if
the face is cracked, chipped, torn, the housing is damaged, or if the
cable is abraded.
■ Always disconnect the system from the wall outlet prior to cleaning it.
■ All patient contact devices, such as probes and ECG leads, must be
removed from the patient prior to the application of a high voltage
defibrillation pulse.
■ The use of flammable anesthetic gas or oxidizing gases (N2O) should be
avoided. Doing so may cause an explosion.
■ Avoid placing the system where it is likely to be difficult to operate, or
disconnect.
CAUTION
■ Do not use HF surgical equipment with the system. Any malfunctions in
the HF surgical equipment may result in burns to the patient.
■ The System must only be connected to a supply mains with protective
earth to avoid risk of electric shock.
■ The system has been designed for 100-240VAC; you should select the
input voltage of any connected printer and VCR. Prior to connecting a
peripheral power cord, verify that the voltage indicated on the power cord
matches the voltage rating of the peripheral device.
■ An isolation transformer protects the system from power surges. This
continues to operate when the system is on standby.
■ Do not immerse the cable in liquids. Cables are not waterproof.
■ Make sure that the inside of the system is not exposed to or flooded with
liquids. In such cases, fire, electric shock, injury, or damage to the
product may occur.
■ The auxiliary socket outlets installed on this system are rated 100240VAC, with a maximum total load of 150VA. Only use these outlets for
supplying power to equipment that is intended to be part of the
ultrasound system. Do not connect additional multiple-socket outlets or
extension cords to the system.
■ Do not connect any peripheral devices not listed in this manual to the
auxiliary socket outlets of the system.
■ Do not touch SIP/SOP and the patient simultaneously. There is a risk of
electric shock from current leakage.
Page 47

2 - 10 WS80A Service Manual
ECG-related Information
■ This device is not intended to provide a primary ECG monitoring
function, and therefore does not have means of indicating an
inoperative electrocardiograph.
■ Do not use ECG electrodes with HF surgical equipment. HF surgical
WARNING
equipment may be damaged, which may result in fire.
■ Do not use ECG electrodes during cardiac pacemaker procedures or
any procedures that involve other types of electrical stimulators.
■ Do not use ECG leads and electrodes in an operating room.
ESD
Electrostatic discharge (ESD), commonly referred to as a static shock, is a naturally occurring
phenomenon. ESD is most prevalent during conditions of low humidity, which can be caused by
heating or air conditioning. The static shock, or ESD, is a discharge of the electrical energy
build-up from a charged individual to a lesser or uncharged individual or object. An ESD occurs
when an individual with an electrical energy build-up comes in to contact with conductive objects
such as metal doorknobs, file cabinets, computer equipment, and even other individuals.
■ The level of electrical energy discharged from a system user or patient to
an ultrasound system can be significant enough to cause damage to the
system or probes.
■ Always perform the pre-ESD preventive procedures before using
connectors marked with the ESD warning label.
CAUTION
- Apply anti-static spray to carpets or linoleum.
- Use anti-static mats.
- Ground the product to the patient’s table or bed.
■ It is highly recommended that the user be given training on ESD-related
warning symbols and preventive procedures.
Page 48

Chapter 2. Safety 2 - 11
EMI
This product complies with EMI (Electromagnetic Interference) standards. However, using the
system inside an electromagnetic field can lower the quality of ultrasound images and even
damage the product.
If this occurs often, Samsung Medison suggests a review of the environment in which the
system is being used, to identify possible sources of radiated emissions. These emissions could
be from other electrical devices used within the same room or an adjacent room.
Communication devices, such as cellular phones and pagers, can cause these emissions. The
existence of radios, TVs, or microwave transmission equipment nearby can also cause
interference.
CAUTION
In cases where EMI is causing disturbances, it may be necessary to relocate
this system.
EMC
Testing of the EMC (Electromagnetic Compatibility) of this system has been performed
according to the international standard for EMC with medical devices (IEC 60601-1-2). This IEC
standard was adopted in Europe as the European norm (EN 60601-1-2).
▌ Guidance and Manufacturer’s Declaration - Electromagnetic
Emissions
This product is intended for use in the electromagnetic environment specified below. The
customer or the user of this product should assure that it is used in such an environment.
Emission Test
Compliance
Status
Electromagnetic Environment - Guideline
RF Emission
CISPR 11
RF Emission
CISPR 11
Group 1
Class A
The Ultrasound System uses RF energy only for its internal
function. Therefore, its RF emissions are very low and are not
likely to cause any interference in nearby electronic equipment.
The Ultrasound System is suitable for use in all establishments
other than domestic, and may be used in domestic
Page 49

2 - 12 WS80A Service Manual
Harmonic
Emission
IEC 61000-3-2
Flicker Emission
IEC 61000-3-3
Class A
Complies
establishments and those directly connected to the public low-
voltage power supply network that supplies buildings used for
domestic purposes, provided the following warning is heeded:
Warning: This system is intended for use by healthcare
professionals only. This system may cause radio interference or
may disrupt the operation of nearby equipment. It may be
necessary to take mitigation measures, such as re-orienting or
relocating the Ultrasound System or shielding the location.
▌ Approved Cables, Probes and Accessories for EMC
Cables
Cables connected to this product may affect its emissions.
Refer to the table below for recommended cable types and lengths:
Cable Type Length
VGA Shielded Normal
RS232C Shielded Normal
USB Shielded Normal
LAN(RJ45) Twisted pair Any
S-Video Shielded Normal
Foot Switch Shielded 2.99m
B/W Printer Unshielded Coaxial Normal
MIC Unshielded Any
Printer Remote Unshielded Any
Audio R.L Shielded Normal
VHS Shielded Normal
ECG AUX input Shielded < 3m
Parallel Shielded Normal
Probes
The image probe used with this product may affect its emission. The probe listed in
‘Chapter 5. Probes’ when used with this product, have been tested to comply with the
group1 Class A emission as required by International Standard CISPR 11.
Page 50

Peripherals
Peripherals used with this product may affect its emissions.
When connecting other customer-supplied accessories to the system, it is the
CAUTION
WARNING
user’s responsibility to ensure the electromagnetic compatibility of the
system.
The use of cables, transducers, and accessories, other than those specified,
may result in increased emissions or decreased immunity of the Ultrasound
System..
Chapter 2. Safety 2 - 13
Page 51

2 - 14 WS80A Service Manual
Immunity Test
Electrostatic
Discharge (ESD)
IEC 61000-4-2
Electronic fast
excess/burst
IEC 61000-4-4
Surge
IEC 61000-4-5
Voltage drop,
short blackout,
and voltage
fluctuation in
power supply
device
IEC 61000-4-11
Commercial
frequency
(50/60Hz)
magnetic field
IEC 61000-4-8
Note : Uт is the main voltage (AC) prior to the application of the test level.
IEC 60601
Test Level
±6KV contact
±8KV wait
±2KV
(for lines of power
supply device)
±1KV
(for lines of
input/output)
±1KV differential mode
±2KV common mode
in 0.5 cycle <5%Uт
(>95% drop, unit: Uт)
in 5 cycles, 40%Uт
(60% drop, unit: Uт)
in 25 cycles, 70%Uт
(30% drop, unit: Uт)
for 5 seconds <5%Uт
(<95% drop, unit: Uт)
3A/m 3A/m
Standard Level
±6KV contact
±8KV wait
±2KV
(for lines of power
supply device)
±1KV
(for lines of
input/output)
±1KV differential mode
±2KV common mode
in 0.5 cycle <5%Uт
(>95% drop, unit: Uт)
in 5 cycles, 40%Uт
(60% drop, unit: Uт)
in 25 cycles, 70%Uт
(30% drop, unit: Uт)
for 5 seconds <5%Uт
(<95% drop, unit: Uт)
Electromagnetic
Environment - Guideline
Wooden, concrete, or
porcelain tiles must be used
for floor materials.
If the floor materials are
synthetic materials, the
relative humidity level must
be maintained at least 30%
at all times.
The quality of main power
must be same as the power
quality used in common
commercial or medical
environment.
The quality of main power
must be same as the power
quality used in common
commercial or medical
environment.
The quality of main power
must be same as the power
quality used in common
commercial or medical
environment.
uninterrupted operation is
required when there is a
malfunction to the main
power, it is recommended
that you use the
uninterruptible power supply
or the battery to supply the
power.
The magnetic field of power
frequency must be same as
the magnetic field used in
common commercial or
medical environment.
If
Page 52

Chapter 2. Safety 2 - 15
Immunity
Test
RF Conduction
IEC 61000-4-6
RF discharge
IEC 61000-4-3
IEC 60601 Test
Level
3Vrms
150kHz
~ 80MHz
3V/m
80MHz
~ 2.5GHz
Standard
Level
3V Portable and mobile RF communications
3V/m Where P is the transmitter’s maximum output
Electromagnetic Environment -
Guideline
equipment should be used no closer to any
part of the Ultrasound System, including
cables, than the recommended separation
distance. This is calculated using the equation
applicable to the frequency of the transmitter.
Recommended separation distance
80MHz to 800MHz
800MHz to 2.5GHz
power rating in watts (W) according to the
transmitter’s manufacturer, and d is the
recommended separation distance in meters
(m).
Field strengths from fixed RF transmitters, as
determined by an electromagnetic site survey,
a should be less than the compliance level in
each frequency range.
Interference may occur in the vicinity of
equipment marked with the following symbol:
b
NOTE 1) At 80MHz and 800MHz, the higher frequency range applies.
NOTE 2) These guidelines may not apply in all situations. Electromagnetic propagation is affected by
absorption and reflection from structures, objects and people.
a
Field strengths from fixed transmitters, such as base stations for radio, (cellular/cordless) telephones
and land mobile radios, amateur radio, AM and FM radio broadcasts and TV broadcasts cannot be
predicted with accuracy. To assess the electromagnetic environment due to fixed RF transmitters, an
electromagnetic site survey should be considered. If the measured field strength, in the location in
which the Ultrasound System is used, exceeds the applicable RF compliance level above, the
Ultrasound System should be observed to verify normal operation. If abnormal performance is
observed, additional measures may be necessary, such as reorienting or relocating the Ultrasound
System or using a shielded location with a higher RF shielding effectiveness and filter attenuation.
b
Over the frequency range 150kHz to 80MHz, field strengths should be less than 3V/m.
Page 53

2 - 16 WS80A Service Manual
▌ Recommended distance between wireless communication
device and this product
This product is intended for use in an electromagnetic environment in which radiated RF
disturbances are controlled. The user can help prevent electromagnetic interference by
maintaining a minimum distance between portable and mobile RF communications
equipment (transmitters) and this product as recommended below, according to the
maximum output power of the communications equipment.
Rated maximum
output power of
transmitter
[W]
0.01
0.1
1
10
100
For the maximum power rated output of transmitters not on the above list, the recommended
separation distance, d(m), can be calculated by using the equation applicable to the transmitter's
frequency. p is the maximum power rated output (in Watts) of the transmitter.
Note 1) At 80MHz and 800MHz, a separation distance for a higher frequency range applies.
Note 2) This guideline may not be applicable to every situation. The electromagnetic wave can be
absorbed or reflected by structures, objects, or humans.
Separation distance, according to frequency of transmitter [m]
150kHz to 80MHz
0.12 0.12 0.23
0.38 0.38 0.73
1.2 1.2 2.3
3.8 3.8 7.3
12 12 23
80MHz to 800MHz
800MHz to 2.5GHz
Page 54

Chapter 2. Safety 2 - 17
▌ Electromagnetic environment – guidance
It is recommended to use ultrasound systems in shielded locations offering RF shielding
effectiveness, with shielded cables. Field strengths outside the location shielded from fixed
RF transmitters, as determined by an electromagnetic site survey, should be less than 3V/m.
It is essential to verify that the actual shielding effectiveness and filter attenuation of the
shielded location meet the minimum specifications.
If the system is connected to customer-supplied equipment, such as a local
CAUTION
area network (LAN) or a remote printer, Samsung Medison cannot
guarantee that the remote equipment will work correctly in the presence of
electromagnetic emission phenomena.
▌ Avoiding Electromagnetic Interference
Typical interference on Ultrasound Imaging Systems varies depending on Electromagnetic
phenomena. Please refer to the following table:
Imaging
mode
2D When changing the
operation mode or
system
configuration, or
reconfiguring the
system,
a short blinking
appears on the
image of the screen
or on the recorded
M Background noise for the
Color The color blinks,
image.
ESD1 RF2 Power Line3
For sector image probes, a
white radial band or blinking
appears on the center line of
the image.
For linear imaging probes, a
white radial band appears or
sometimes the image edge
will appear brighter.
image gets louder or a white
M mode line is displayed.
radial/vertical band appears,
background noise gets
louder, or color image
changes.
White dot, line table, or
diagonal line is displayed
or a diagonal line is
displayed near the image
center.
White dot, line table, or
diagonal line is displayed
or background noise for
the image gets louder.
The color blinks, dot or
line table appears, or color
noise level changes.
Page 55

2 - 18 WS80A Service Manual
Doppler
(Doppler)
1. ESD which occurs when charges accumulated on an insulated floor or human is discharged.
2. RF energy created from the RF transmission devices such as mobile phone, portable radio,
wireless device, commercial radio and TV.
3. Malfunction which occurs due to switch of power supply to power cable or connected cable,
electronic control, or natural phenomenon such as lightning.
A horizontal line is displayed
on the spectrum display or
tone, abnormal noise is
created, or both occur
together.
A vertical line is displayed
on the spectrum display or
a bursting noise is
created, or both occur
together.
A medical device can either generate or receive electromagnetic interference. The EMC
standards describe tests for both emitted and received interference.
Samsung Medison’s ultrasound systems do not generate electromagnetic interference in
excess of the referenced standards.
An Ultrasound System is designed to receive signals at radio frequency and is therefore
susceptible to interference generated by RF energy sources. Examples of other sources of
interference are medical devices, information technology products, and radio and television
transmission towers. Tracing the source of radiated interference can be a difficult task.
Users should consider the following in an attempt to locate the source.
- Is the interference intermittent or constant?
- Does the interference show up only with one probe operating at the same frequency or
with several probes?
- Do two different probes operating at the same frequency have the same problem?
- Is the interference present if the system is moved to a different location in the facility?
The answers to these questions will help to determine if the problem resides with the
system or the scanning environment. After you answer the questions, contact your local
Samsung Medison customer service department.
Page 56

Chapter 2. Safety 2 - 19
Mechanical Safety
Moving the Equipment
The product weighs more than 100 kg; be careful when you
WARNING
Make sure that the brakes on wheels have been unlocked before you move it. Also, make
sure to retract the monitor arm completely so that it is secured in a stationary position.
Always use the handles at the back of the console and move the product slowly.
This product is designed to be resistant to physical shocks. However, subjecting the product to
excessive shocks, such as dropping it, may seriously damage the product.
If the system operates abnormally after repositioning, please contact the Samsung Medison
move it. Careless transporting may cause injury to the user or
may damage the product.
Customer Service Department.
▌ Foot Lock
You can use the brakes to control the movement of the product. The brakes are mounted on
each wheel of the main body with interlinked on/off buttons. This is how you engage or
release the brakes:
- Engaged: To engage the brakes, press the On button with your foot. The Off button will
rise.
- Released: To release the brakes, press the Off button with your foot. The On button will
rise.
We recommend that you lock the brakes when using the product.
Page 57

2 - 20 WS80A Service Manual
▌ Cautions on an inclined surface
Always make sure that the control panel is facing the direction of movement.
Pay attention to the movement of wheels when transporting the product.
WARNING
Leaving the CART unattended on an inclined surface may cause the CART to topple, even
if you engage the foot lock. Avoid an inclined surface to station the product.
Practice is recommended before transporting the product on an inclined
surface.
To Use the Product Safely
■ Do not press the control panel excessively.
■ Never attempt to modify the product in any way.
■ Read the instructions on safe operation of the product if using the
product after a prolonged period of non-use.
■ Make sure that other objects, such as metal pieces, do not enter the
system.
■ Do not block the ventilation slots.
CAUTION
■ To prevent damage to the power cord, be sure to grip the plug head –
not the cord – when unplugging.
■ Excessive bending or twisting of cables on patient-applied parts may
cause failure or intermittent operation of the system.
■ Improper cleaning or sterilization of a patient-applied part may cause
permanent damage.
■ Assuming that the product is used in accordance with the guidelines
contained in this manual and maintained by qualified service personnel,
the expected lifespan of the product is approximately 7 years.
Please refer to 'Chapter 10. Maintenance' for detailed information on protecting, cleaning and
disinfecting the equipment.
Page 58

Chapter 2. Safety 2 - 21
▌ Precautions for Using Monitor
When adjusting the height or position of the monitor, be careful not to leave your fingers or
hand in area between the monitor arms - they may get trapped and hurt.
[Figure 2.2 Safety note for monitor]
▌ Caution for Using Control Panel
■ Do not push the control panel with excessive force or lean on it.
CAUTION
■ Do not sit on the control panel or exert excessive force on it.
When adjusting the height or position of the control panel, be careful not to leave your
fingers or hand in area between the control panel and the lift - they may get trapped and
hurt.
[Figure 2.3 Cautions on Using Control Panel]
When using the handle of the control panel, be careful of the space between the handle
and the keyboard. The keyboard may come out to collide with the hand.
Page 59

2 - 22 WS80A Service Manual
[Figure 2.4 Cautions on Using Handle]
Page 60

Chapter 2. Safety 2 - 23
Biological Safety
For safety information on the probe and biopsy, refer to 'Chapter 5. Probes'.
■ Ultrasound waves may have damaging effects on cells, and
therefore may be harmful to the patient. If there is no medical
benefit, minimize the exposure time and maintain a low
ultrasound wave output level. Please refer to the ALARA
principle.
■ Do not use the product if an error message or a warning
message about a dangerous situation is displayed on the
screen. Write down the message displayed on the screen, turn
WARNING
the power off, and contact the service department of Samsung
Medison.
■ Do not use a system that exhibits erratic or inconsistent
behavior. Discontinuities in the scanning sequence are an
indication of a hardware failure that must be repaired before
use.
■ The system limits the maximum contact temperature to 43℃,
and the ultrasonic waves output observes American FDA
regulations.
ALARA Principle
Performing diagnoses using an ultrasound device is defined by the “As Low As Reasonably
Achievable” (ALARA) principle. The decision as to what is reasonable has been considered and
defined by many people. However, no set of rules can be formulated that would be sufficiently
complete to dictate the correct response for every circumstance. By keeping ultrasound
exposure as low as possible, while obtaining diagnostic images, users can minimize ultrasonic
bioeffects.
Since the threshold for diagnostic ultrasound bioeffects is undetermined, it is the sonographer’s
responsibility to control the total energy transmitted into the patient. The sonographer must
reconcile exposure time with diagnostic image quality. To ensure diagnostic image quality and
limit exposure time, the ultrasound system provides controls that can be manipulated during the
exam to optimize the results of the exam.
The user's knowledge of, and the ability to abide by, the ALARA principle is very important.
Advances in diagnostic ultrasound, not only in the technology, but also in the applications of the
technology, have resulted in the need for more and better information to guide the user. This
important information is based on a variety of ultrasound output data, and plays an important
Page 61

2 - 24 WS80A Service Manual
role in putting the ALARA principle into effect.
Numerous variables affect the output data that forms the basis of the provided information.
These variables include mass, body size, location of the bone relative to the focal point,
attenuation in the body, and ultrasound exposure time. Among these, exposure time is the
variable that one must pay the most attention to. For, unlike other variables, exposure time is
entirely controlled by the operator of the ultrasound system.
▌ Applying ALARA
The system-imaging mode used depends upon the information needed. 2D-mode and M-
mode imaging provide anatomical information, while Doppler, Power, and Color imaging
provide information about blood flow. Scanned modes like 2D-mode, Power, or Color,
disperse or scatter the ultrasonic energy over an area, while unscanned modes like M-mode
or Doppler concentrate ultrasonic energy. Understanding the nature of the imaging mode
being used allows the sonographer to apply the ALARA principle with informed judgment. The
probe frequency, system set-up values, scanning techniques, and operator experience aid the
sonographer in meeting the definition of the ALARA principle. The decision as to the amount
of acoustic output is, in the final analysis, up to the system operator. This decision must be
based on the following factors: type of patient, type of exam, patient history, ease or difficulty
of obtaining diagnostically useful information, and the potential localized heating of the patient
due to probe surface temperatures. Prudent use of the system occurs when patient exposure
is limited to the lowest index reading for the shortest amount of time necessary to achieve
acceptable diagnostic results.
Although a high index reading does not necessarily mean that a bioeffect is occurring, it
should still be taken seriously. Every effort should be made to reduce the possible effects of a
high index reading. Limiting exposure time is an effective way to accomplish this goal.
There are several system controls that the operator can use to adjust the image quality and
limit the acoustic intensity. These controls are related to the techniques that an operator might
use to implement ALARA. These controls can be divided into three categories: direct, indirect,
and receiver controls.
▌ Direct Controls
Application selection and the output intensity control directly affect acoustic intensity. There
are different ranges of allowable intensity or output based on your selection. Selecting the
Page 62

Chapter 2. Safety 2 - 25
correct range of acoustic intensity for the application is one of the priorities required during
any exam. For example, peripheral vascular intensity levels are not recommended for fetal
exams. Some systems automatically select the proper range for a particular procedure, while
others require manual selection. Ultimately, the user bears the responsibility for proper clinical
use. Samsung Medison's systems provide both automatic and user-definable settings.
Output has a direct impact on acoustic intensity. Once the application has been established,
the output control can be used to increase or decrease the intensity output. The output control
allows you to select intensity levels lower than the defined maximum. Prudent use ensures
good image quality while employing the lowest output intensity.
▌ Indirect Controls
The indirect controls are those that have an indirect effect on the acoustic intensity. These
controls affect the imaging mode, pulse repetition frequency, focus depth, pulse length, and
probe selection.
The choice of imaging mode determines the nature of the ultrasound beam. 2D-mode is a
scanned mode; Doppler is a stationary or unscanned mode. A stationary ultrasound beam
concentrates energy on a single location. A moving or scanned ultrasound beam disperses
the energy over a wide area and the beam is only concentrated on a given area for a fraction
of the time necessary in unscanned mode.
The pulse repetition frequency or rate refers to the number of ultrasound bursts of energy over
a specific period of time. The higher the pulse repetition frequency, the more pulses of energy
in a given period of time. Several controls affect pulse repetition frequency: Focal depth,
display depth, sample volume depth, color sensitivity, number of focal zones, and sector width
controls.
The focus of the ultrasound beam affects the image resolution. Maintaining or increasing the
resolution at a different focal zone involves the adjustment of numerous outputs from the focal
zone. This output adjustment is one of the system’s optimization features. Different exams
require different focal depths. Setting the focus to the proper depth improves the resolution of
the structure of interest.
Pulse length is the time during which the ultrasonic burst is turned on. The longer the pulse,
the greater the time-average intensity value. The greater the time-average intensity, the
greater the likelihood of temperature increase and cavitation. Pulse length, burst length, and
pulse duration refer to the output pulse duration in pulsed Doppler mode. In addition,
increasing the Doppler sample volume increases the pulse length.
Page 63

2 - 26 WS80A Service Manual
Probe selection affects intensity indirectly. Tissue attenuation changes with frequency. The
higher the probe operating frequency, the greater the attenuation of the ultrasonic energy.
Higher probe operating frequencies require greater output intensity to scan at a deeper depth.
To scan deeper at the same output intensity, a lower probe frequency is required. Using more
gain and output beyond a point, without corresponding increases in image quality, can mean
that a lower frequency probe is needed.
Receiver Controls
Receiver controls are used by the operator to improve image quality. These controls
have no effect on output. Receiver controls only affect how the ultrasound echo is
received. These controls include gain, TGC, dynamic range, and image processing. The
important thing to remember concerning output is that the receiver controls should be
optimized before increasing it. For example; before increasing output, gain should be
optimized to improve image quality.
▌ Additional Considerations
Ensure that scanning time is kept to a minimum, and ensure that only medically required
scanning is performed. Never compromise quality by rushing an exam. A poor exam will
require a follow-up, which ultimately increases the scanning time. Diagnostic ultrasound is
an important tool in medicine, and, like any tool, should be used efficiently and effectively.
▌ Output Display Features
The system output display comprises two basic indices: a mechanical index and a thermal
index. The thermal index consists of the following indices: soft tissue (TIs), bone (TIb) and
cranial bone (TIc). One of these three thermal indices will be displayed at all times. One of
the indices will be displayed according to the application currently in use, depending on the
system settings or user choice.
The mechanical index is continuously displayed over the range of 0.0 to 1.9, in increments
of 0.1. The thermal index consists of the three indices, and only one of these is displayed all
the time. Each probe application has an appropriate default selection for the combination.
The TIb or TIs is continuously displayed over the range of 0.0 to maximum output, based on
the probe and application, in increments of 0.1.
Page 64

Chapter 2. Safety 2 - 27
The application-specific nature of the default setting is also an important factor of index
behavior. The default setting is the system control state, which is preset by the manufacturer
or the operator. The system has default index settings for the probe application. The default
settings are applied automatically by the ultrasound system when the power is turned on,
new patient data is entered into the system database, or a change of application takes place.
The decision as to which of the three thermal indices is displayed is based on the following
criteria:
Appropriate index for the application: TIs is used for imaging soft tissue, and TIb for a focus
at or near a bone. Elements such as fluid, bone, and blood flow may act as artifacts that
increase or decrease the TI. A highly attenuating tissue path, for example, may cause the
potential for local zone heating to be lower than the thermal index displays.
In comparison with the probe mode, unscanned modes of operation also affects the thermal
index. For scanned modes, heating tends to be near the surface; for unscanned modes, the
potential for heating tends to be deeper in the focal zone.
Always limit ultrasound exposure time. Do not rush the exam. Ensure that the indices are
kept to a minimum, and that exposure time is limited without compromising diagnostic
sensitivity.
Mechanical Index (MI) Display
Mechanical bioeffects are threshold phenomena that occur when a certain level of
output is exceeded. The threshold level varies, however, with the type of tissue. The
potential for mechanical bioeffects varies with peak pressure and ultrasound frequency.
The MI accounts for these two factors. The higher the MI value, the greater the
likelihood of mechanical bioeffects occurring, but there is no specific MI value that
means that a mechanical effect will definitely occur. The MI should only be used as a
guide for implementing the ALARA principle.
Thermal Index (TI) Display
The TI informs the user of the potential for temperature increase occurring at the body
surface, within body tissue, or at the point of focus of the ultrasound beam on bone. The
TI is an estimate of the temperature increase in specific body tissues. The actual
amount of any temperature rise is influenced by factors such as tissue type, vascularity,
and mode of operation. The TI should be used only as a guide for implementing the
Page 65

2 - 28 WS80A Service Manual
ALARA principle.
The bone thermal index (TIb) informs the user about potential heating at or near the
focus after the ultrasound beam has passed through soft tissue or fluid, such as the
skeletal structure of a 2-3 month old fetus. The cranial bone thermal index (TIc) informs
the user about the potential heating of bone at or near the surface, for example, the
cranial bone. The soft tissue thermal index (TIs) informs the user about the potential for
heating within soft homogeneous tissue. TIc is displayed when you select a transcranial
application.
You can select the TI to display at Setup > Imaging > Display.
Mechanical and Thermal indices Display Precision and Accuracy
The Mechanical and Thermal Indices on the system are precise to 0.1 units.
The MI and TI display accuracy estimates for the system are given in the Acoustic
Output Tables section of this operation manual. These accuracy estimates are based on
the variability ranges of probes and systems, inherent acoustic output modeling errors,
and the measurement variability, as described below.
The displayed values should be interpreted as relative information to help the system
operator achieve the ALARA principle through prudent use of the system. The values
should not be interpreted as actual physical values of investigated tissue or organs. The
initial data that is used to support the output display is derived from laboratory
measurements based on the AIUM measurement standard. The measurements are
then put into algorithms to calculate the displayed output values.
Many of the assumptions used in the process of measurement and calculation are
conservative in nature. Over-estimation of actual in situ exposure is built into the
measurement and calculation process for the vast majority of tissue paths. For example,
the acoustic output values measured underwater are de-rated using a conservative,
industry standard, attenuation coefficient of 0.3dB/cm-MHz.
Conservative values for tissue characteristics were selected for use in the TI models.
Conservative values for tissue or bone absorption rates, blood perfusion rates, blood heat
capacity, and tissue thermal conductivity were selected.
Steady state temperature rise is assumed in the industry standard TI models, and the
assumption is made that the ultrasound probe is held steady in one position long
Page 66

Chapter 2. Safety 2 - 29
enough for a steady state to be reached.
A number of factors are considered when estimating the accuracy of display values;
hardware variations, algorithm accuracy estimation, measurement variability, and
variability among probes and systems are significant factors. Probe deviation results
from piezoelectric crystal efficiencies, process-related impedance differences, and
sensitive lens focusing parameter variations. Differences in the system pulse voltage
control and efficiencies also contribute to variability. There are inherent uncertainties in
the algorithms used to estimate acoustic output values over the range of possible
system operating conditions and pulse voltages. Inaccuracies in laboratory
measurements are related to differences in hydrophone calibration and performance,
positioning, alignment and digitization tolerances, and variability among test operators.
The conservative assumptions of the output estimation algorithms of linear propagation,
at all depths, through a 0.3dB/cm-MHz attenuated medium are not taken into account in
the calculation of the accuracy estimate displayed. Neither linear propagation nor
uniform attenuation at the 0.3dB/cm-MHz rate occurs in underwater measurements, or
in most tissue paths in the body. In the body, different tissues and organs have
dissimilar attenuation characteristics. In water, there is almost no attenuation. In the
body, and particularly in underwater measurements, non-linear propagation and
saturation losses occur as pulse voltages increase.
The display accuracy estimates take into account the variability ranges of probes and
systems, inherent acoustic output modeling errors, and measurement variability. Display
accuracy estimates are measured according to AIUM measurement standards but not
based on errors caused during the measurement. They are also independent of the
effects of non-linear loss on the measured values.
Page 67

2 - 30 WS80A Service Manual
▌ Control Effect- Controls Affecting the Indices
As various system controls are adjusted, the TI and MI values may change. This will be
most apparent as the Power control is adjusted; however, other system controls will also
affect the on-screen output values.
Power
The power controls the system’s acoustic output. Two real-time output values are on the
screen: a TI and an MI. They change as the system responds to power adjustments.
In combined modes, such as simultaneous Color, 2D-mode, and pulsed Doppler, the
individual modes each add to the total TI. Each mode is a vital contributor to this total;
the displayed MI will be from the mode with the largest peak pressure.
▌ 2D Mode Controls
2D Mode Size
Narrowing the sector angle may increase the frame rate. This will increase the TI. The
pulse voltage may be automatically adjusted down by the software controls to keep the
TI below the system maximum. A decrease in pulse voltage will decrease MI.
Zoom
Magnifying the image increases frame rate. This will also increase the TI. The number
of focal zones may automatically increase to enhance the resolution. In such
circumstances, the maximum intensity may occur at a different depth, which may
change the MI.
Persistence
A lower persistence will decrease the TI. Pulse voltage may be automatically increased.
An increase in pulse voltage will increase MI.
Focal no.
Increasing the number of focal zones may change both the TI and MI by changing the
Page 68

frame rate or focal depth automatically. Lower frame rates decrease the TI. The MI
displayed will correspond to the focal zone with the largest peak intensity.
Focus
Changing the focal depth will change the MI. Generally, higher MI values will occur
when the focal depth is near the natural focus of the transducer.
▌ Color and Power Controls
Color Sensitivity
Increasing the color sensitivity increases the TI and the time spent to scan color images.
Color pulses are the dominant pulse type in this mode.
Color Sector Width
Narrower color sector width will increase the color frame rate, and so the TI will increase.
Chapter 2. Safety 2 - 31
The system may automatically decrease the pulse voltage to stay below the system
maximum. A decrease in pulse voltage will decrease the MI. If pulsed Doppler is also
enabled, then pulsed Doppler will remain the dominant mode and the TI change will be
small.
Color Sector Depth
Deeper color sector depth may automatically decrease the color frame rate, or select a
new color focal zone or color pulse length. The TI will change because of the combination
of these effects. Generally, the TI will decrease with the increased color sector depth. The
MI will correspond to the peak intensity of the dominant pulse type, which is a color pulse.
However, if pulsed Doppler is also enabled, then pulsed Doppler will remain the dominant
mode and the TI change will be small.
Scale
Using the scale control to increase the color velocity range may increase the TI. The
system will automatically adjust the pulse voltage to stay below the system maximum. A
decrease in pulse voltage will also decrease the MI.
Page 69

2 - 32 WS80A Service Manual
Sec Width
A narrower 2D-mode sector width in Color imaging will increase the color frame rate.
The TI will increase. The MI will not change. If pulsed Doppler is also enabled, then
pulsed Doppler will remain as the primary mode and the TI change will be small.
▌ M Mode and Doppler Controls
Speed
M-mode and Doppler sweep speed adjustments will not affect the MI. When M-mode
sweep speed changes, TI changes.
Simultaneous and Update Methods
Use of combination modes affects both the TI and MI through the combination of pulse
types. During Simultaneous mode, the TI is an additive element. During Auto-update
and Duplex, the TI will display the dominant pulse type. The displayed MI will be from
the mode with the largest peak pressure.
Sample Volume Depth
When the Doppler sample volume depth is increased, the Doppler PRF may
automatically decrease. A decrease in PRF will decrease the TI. The system may also
decrease the pulse voltage to remain below the system maximum. A decrease in pulse
voltage will decrease the MI.
▌ Doppler, CW, M-mode, and Color Imaging Controls
When a new imaging mode is selected, both the TI and the MI will revert to their default settings.
Each mode has a corresponding pulse repetition frequency and maximum intensity point. In
combined or simultaneous modes, the TI is the sum of the contribution from the modes enabled,
and the MI is the value for the focal zone of the mode with the largest de-rated intensity. If a
mode is turned off and then reselected, the system will return to the previously selected settings.
Probes
Each probe model available has unique specifications for the contact area, beam shape,
and center frequency. Settings are reset when you select a probe. Samsung Medison's
Page 70

Chapter 2. Safety 2 - 33
factory defaults vary with probe, application and mode. Defaults that are below the FDA
limits have been chosen for intended use.
Depth
An increase in the 2D-mode depth will automatically decrease the 2D-mode frame
rate. This would decrease the TI. The system may also automatically choose a
deeper 2D-mode focal depth. A change of focal depth may change the MI. The MI
displayed is that of the zone with the largest peak intensity.
Application
Acoustic output defaults are set when you select an application. Samsung Medison's
factory defaults vary with probe, application, and mode. Defaults that are below the
FDA limits have been chosen for intended use.
▌ Related Guidance Documents
For more information about ultrasonic bioeffects and related topics, refer to the following;
- AIUM Report, January 28, 1993, “Bioeffects and Safety of Diagnostic Ultrasound”
- Bioeffects Considerations for the Safety of Diagnostic Ultrasound, J Ultrasound Med.,
Sept. 1998: Vol. 7, No. 9 Supplement
- Acoustic Output Measurement Standard for Diagnostic Ultrasound Equipment. (AIUM,
NEMA. 1998)
- Acoustic Output Labeling Standard for Diagnostic Ultrasound Equipment (AIUM, 1998)
- Second Edition of the AIUM Output Display Standard Brochure, Dated March 10, 1994.
(A copy of this document is shipped with each system.)
- Information for Manufacturer Seeking Marketing Clearance of Diagnostic Ultrasound
Systems and Transducers. FDA. September 1997. FDA.
- Standard for Real-Time Display of Thermal and Mechanical Acoustic Output Indices on
Diagnostic Ultrasound Equipment. (Revision 1, AIUM, NEMA. 1998)
- WFUMB. Symposium on Safety of Ultrasound in Medicine: Conclusions and
Recommendations on Thermal and Non-Thermal Mechanisms for Biological Effects of
Ultrasound, Ultrasound in Medicine and Biology, 1998: Vol. 24, Supplement1.
Page 71

2 - 34 WS80A Service Manual
▌ Acoustic Output and Measurement
Since the first use of diagnostic ultrasound, the possible human biological effects
(bioeffects) of ultrasound exposure have been studied by various scientific and medical
institutions. In October 1987, the American Institute of Ultrasound in Medicine(AIUM) ratified
a report prepared by its Bioeffects Committee (Bioeffects Considerations for the Safety of
Diagnostic Ultrasound, J Ultrasound Med., Sept. 1988: Vol.7, No.9 Supplement), sometimes
referred to as the Stowe Report, which reviewed available data on possible effects of
ultrasound exposure. Another report, “Bioeffects and Safety of Diagnostic Ultrasound”,
dated January 28, 1993, provides more up-to-date information.
The acoustic output for this system has been measured and calculated in accordance with
the December 1985 “510(K) Guide for Measuring and Reporting Acoustic Output of
Diagnostic Ultrasound Medical Devices”, except for the hydrophone, which meets the
requirements of “Acoustic Output Measurement Standard for Diagnostic Ultrasound
Equipment” (NEMA UD 2-1992).
▌ In Situ, De-rated, and Water Value Intensities
All intensity parameters are measured in water. Since water does not absorb acoustic energy,
these water measurements represent the largest possible value. Biological tissue absorbs
acoustic energy. The true value of the intensity at any point depends on the amount and type of
tissue, and the frequency of the ultrasound that passes through the tissue. The intensity value
in the tissue, In Situ, has been estimated using the following formula:
)23.0( alf
In Situ = Water
where: In Situ = In Situ Intensity Value
Water = Water Value Intensity
e = 2.7183
a = Attenuation Factor
Tissue a(dB/cm-MHz)
Brain .53
Heart .66
Kidney .79
Liver .43
Muscle .55
e
Page 72

Chapter 2. Safety 2 - 35
l = skin line to measurement depth (cm)
f = Center frequency of the transducer/system/mode combination(MHz)
Since the ultrasonic path during an examination is likely to pass through varying lengths and
types of tissue, it is difficult to estimate the true In Situ intensity. A de-rating factor of 0.3 is
used for general reporting purposes; therefore, the In Situ value which is commonly reported
uses the formula:
In Situ (derated) = Water
e
)069.0( lf
Since this value is not the true in situ intensity, the term “de-rated” is used.
The maximum de-rated and maximum water values do not always occur under the same
operating conditions. Therefore, the reported maximum water and de-rated values may not be
related to the In Situ (de-rated) formula. For example, a multi-zone array transducer has the
greatest water value intensities in its deepest zone. The same transducer may have its largest de-
rated intensity in one of its shallowest focal zones.
▌ Acoustic Output and Measurement
The terms and symbols used in the acoustic output tables are defined in the following paragraphs.
ISPTA.3 The derated spatial-peak temporal-average intensity (milliwatts per square
centimeter).
ISPPA.3 The derated spartial-peak pulse-average intensity (watts per square
centimeter). The value of IPA.3 at the position of global maximum MI (IPA.3@MI)
may be reported instead of ISPPA.3 if the global maximum MI is reported.
MI The Mechanical Index. The value of MI at the position of ISPPA.3,
(MI@ISPPA.3) may be reported instead of MI (global maximum value) if ISPPA.3
is 190W/cm
The derated peak rarefactional pressure (megapascals) associated with the transmit pattern
giving rise to the reported Pr.3 MI value.
WO Ultrasonic power(milliwatts). For operating conditions giving rise to ISPTA.3, WO
is the total time-average power. For operating conditions subject to reporting
under ISPPA.3, WO is the Ultrasonic power associated with the transmit pattern
giving rise to the value reported under ISPPA.3.
Fc Center frequency (MHz). For MI and ISPPA.3, Fc is the Center frequency
associated with the transmit pattern giving rise to the global maximum value of
the respective parameter. For ISPTA.3, for combined modes involving beam types
of unequal Center frequency, Fc is defined as the overall ranges of center
frequencies of the respective transmit patterns.
ZSP The axial distance at which the reported parameter is measured (centimeters)
2
.
Page 73

2 - 36 WS80A Service Manual
x-6,y-6 These are respectively the in-plane (azimuth) and out-of-plane (elevation) -6
dimensions in the x-y plane where ZSP is found (centimeters).
PD The Pulse duration (microseconds) associated with the transmit pattern giving
rise to the reported value of the respective parameter
PRF The Pulse repetition frequency (Hz) associated with the transmit pattern giving
rise to the reported value of the respective parameter.
EBD The Entrance beam dimensions (centimeters) for the azimuth and elevation
planes.
EDS The Entrance dimensions of the scan (centimeters) for the azimuth and
elevation planes.
▌ Acoustic Measurement Precision and Uncertainty
The Acoustic Measurement Precision and Acoustic Measurement Uncertainty are described below.
Quantity Precision Total Uncertainty
PII.3 (derated pulse intensity integral) 3.2 % +21 % to - 24 %
Wo (acoustic power) 6.2 % +/- 19 %
Pr.3 (derated rarefaction pressure) 5.4 % +/- 15 %
Fc (center frequency) < 1 % +/- 4.5 %
Systematic Uncertainties.
For the pulse intensity integral, derated rarefaction pressure Pr.3, center frequency and pulse
duration, the analysis includes considerations of the effects on accuracy of:
Hydrophone calibration drift or errors.
Hydrophone / Amp frequency response.
Spatial averaging.
Alignment errors.
Voltage measurement accuracy, including.
- Oscilloscope vertical accuracy.
- Oscilloscope offset accuracy.
- Oscilloscope clock accuracy.
- Oscilloscope Digitization rates.
- Noise.
The acoustic power is measured using a Radiation Force for systematic uncertainties through
Page 74

Chapter 2. Safety 2 - 37
the use of calibrated NIST acoustic power sources.
We also refer to a September 1993 analysis conducted by the working group of the IEC
technical committee 87 and prepared by K. Beissner, as a first supplement to IEC publication
1161 .
The document includes analysis and discussion of the sources of error / measurement effects
due to:
Balance system calibration.
Absorbing (or reflecting) target suspension mechanisms.
Linearity of the balance system.
Extrapolation to the moment of switching the ultrasonic transducer (compensation for
ringing and thermal drift).
Target imperfections.
Absorbing (reflecting ) target geometry and finite target size.
Target misalignment.
Ultrasonic transducer misalignment.
Water temperature.
Ultrasonic attenuation and acoustic streaming.
Coupling or shielding foil properties.
Plane-wave assumption.
Environmental influences.
Excitation voltage measurement.
Ultrasonic transducer temperature.
Effects due to nonlinear propagation and saturation loss.
The overall findings of the analysis give a rough Acoustic Power accuracy figure of +/- 10% for
the frequency range of 1 - 10 MHz.
▌ Training
The users of this ultrasound system must familiarize themselves with the ultrasound system
to optimize the performance of the device and to detect possible malfunctions. It is
recommended that all users receive proper training before using the device. You can
receive training on the use of the product from the Samsung Medison service department,
or any of the customer support centers worldwide.
Page 75

2 - 38 WS80A Service Manual
Environmental Protection
■ To dispose of the system or accessories that have come to the end of
their lifespan, contact the vendor or follow appropriate disposal
procedures.
CAUTION
■ You are responsible for complying with the relevant regulations for waste
disposal.
■ The lithium ion battery used in the product must be replaced by
aSamsung Medison service engineer or an authorized dealer.
Correct Disposal of This Product
(Waste Electrical & Electronic Equipment)
Applicable in countries with separate collection systems
This marking on the product, accessories or literature indicates that the product and its
electronic accessories (e.g. charger, headset, USB cable) should not be disposed of with other
household waste at the end of their working life. To prevent possible harm to the environment
or human health from uncontrolled waste disposal, please separate these items from other
types of waste and recycle them responsibly to promote the sustainable reuse of material
resources.
Household users should contact either the retailer where they purchased this product, or their
local government office, for details of where and how they can take these items for
environmentally safe recycling.
Business users should contact their supplier and check the terms and conditions of the
purchase contract. This product and its electronic accessories should not be mixed with other
commercial wastes for disposal.
For information on Samsung’s environmental commitments and product specific regulatory
obligations e.g. REACH visit:
Samsung.com/uk/aboutsamsung/samsungelectronics/corporatecitizenship/data_corner.html
State of California Proposition 65 Warning (US only)
This product contains chemicals known to the State of California to
WARNING
cause cancer, birth defects or other reproductive harm.
Page 76

Chapter 3
Installing the Product
Unpacking the Product ................................................................................................. 3
Installation Environment ............................................................................................... 4
Installing the Product .................................................................................................... 5
Power Cord Connection ............................................................................................................. 6
Probe Connection ...................................................................................................................... 6
Connecting Peripherals ............................................................................................................. 7
System Power ................................................................................................................ 9
Turning the Power On ................................................................................................................ 9
Shutting Down the System ...................................................................................................... 10
System Settings ........................................................................................................... 11
System General Setting ........................................................................................................... 12
Scan Mode ............................................................................................................................... 17
Display ..................................................................................................................................... 21
Annotate ................................................................................................................................... 26
Peripheral Device Settings ...................................................................................................... 31
User Defined Keys ................................................................................................................... 38
MPR Menu ............................................................................................................................... 40
Network .................................................................................................................................... 41
Options ..................................................................................................................................... 43
DICOM Setup (optional) .......................................................................................................... 45
Auto Calc ................................................................................................................................. 59
System Information .................................................................................................................. 60
Page 77

3 - 2 WS80A Service Manual
Transporting
This product is a finely tuned piece of medical electronic equipment; careful attention is required
when transporting it.
▌ Precautions when transporting the product
The package box is designed to protect the product from physical impacts. Nevertheless,
exercise caution to protect the product from external knocks.
▌ Humidity and Temperature
"[Table 3-1. The Product's Humidity and Temperature Tolerance]" shown below illustrates
the temperature and humidity ranges for transporting, storing, and operating the product.
Category Temperature OC Humidity %
Transporting -25 ~ 60 20 ~ 90
Storage -10 ~ 50 20 ~ 90
Operating 10 ~ 35 30 ~ 75
[Table 3-1. The Product’s Humidity and Temperature Tolerance]
Page 78

Chapter 3. Installing the Product 3 - 3
Unpacking the Product
▌ Dismantling the Product Box
1. Open the box.
2. Remove the protection cover.
3. Take out the probe box and accessory box and store them in a safe place.
4. Unlock the brakes on wheels.
5. Grab the rear handle on the product and move it to a place to install.
[Figure 3.1 Dismantling the Product Box]
▌ Accessory
This product includes a box containing various accessories. If the accessory box is not
appropriate for your purchase, contact the vendor from which you made the purchase.
Page 79

3 - 4 WS80A Service Manual
Installation Environment
▌ Caution
When installing the product, please pay attention to the following information. For more
information on using and setting up the WS80A, refer to the accompanying manual.
If the product is used near a generator, an X-ray system, or a
CAUTION
■ Optimal conditions for the system are a temperature of 10-35 and a humidity of 30-75%.
■ Avoid excess humidity.
■ Avoid direct sunlight.
broadcasting cable, noise may cause the screen to display images
incorrectly. Sharing the power source with another electric appliance may
also cause noise.
■ Avoid excessive fluctuations in temperature.
■ Optimal conditions for the system are a temperature of 10-35and a humidity of 30-75%.
■ Avoid installing the product near a heating appliance.
■ Avoid dusty and/or poorly ventilated locations.
■ Avoid locations that are subject to vibration.
■ Avoid a location where chemical substances or harmful gases are present.
Page 80

Chapter 3. Installing the Product 3 - 5
Installing the Product
▌ Installation Safety
In a hospital environment, dangerous electrical current may occur as a result of the potential
difference between a contactable conductive part and connected equipment in treatment
rooms. The solution to the problem is consistent equipotential bonding. Equipotential
terminals on medical equipment should be connected to the equipotential bonding network
in medical rooms as shown in the picture.
[Figure 3.3 Equipotential bonding]
If the product needs to be transported or stored for an
extended duration, the temperature and humidity of the
CAUTION
Refer to "[Table 3-2. Operational Temperature of Product]" before turning the product on
environment must be checked.
A sudden change in temperature may cause condensation
and lead to product failure.
Temperature -20 -15 -10 -5 0 5 10 ~ 35 45 50 55 60
Waiting time 16 10 8 6 4 2
[Table 3-2. Operational Temperature of Product]
Use
immediately
2 4 6 10
.
Page 81

3 - 6 WS80A Service Manual
Power Cord Connection
Prior to connecting a power cord, verify that the voltage indicated on the power cord matches th
e voltage rating of the place to install.
NOTE
The product may ship with the power cable connected to the
console.
[Figure 3.4 Power Connection Part]
Probe Connection
Be sure to connect or disconnect probes when the power is off to ensure the safety of the
system and the probes.
1. Connect probes to the probe connectors on the front panel of the system. Up to four
(five including CW) probes may be connected. However, the CW probe should only be
connected to its own connector.
2. To install, turn the connector turning handle clockwise.
[Figure 3.5 Connecting the Probe]
Page 82

Connecting Peripherals
Do not install a peripheral device not listed in this operation manual in the
CAUTION
patient environment. Installation of such a device may cause an electrical
hazard.
Chapter 3. Installing the Product 3 - 7
[Figure 3.5 Patient Environment]
NOTE
For more information on the recommended peripheral devices, refer to
"Chapter 1. Introduction".
▌ Internal Peripheral Devices
These are peripheral devices mounted in the system.
DVD-Multi
Hard Disc Drive
Page 83

3 - 8 WS80A Service Manual
▌ External Peripheral Devices
These are peripheral devices that can be connected for use when needed and are connected
via the USB port located at the rear panel.
When installing or uninstalling a peripheral device that uses a USB
port on the console, be sure to turn the console’s power off. If the
CAUTION
NOTE
power is not turned off completely, the system or the USB peripheral
device may fail to work correctly.
Do not connect additional external peripheral devices to the auxiliary
socket outlet. Doing so may decrease safety level.
When remove the removable disk, use Utility > Storage manager.
If you are using USB 1.1 flash memory, the system may fail to recognize
the device. In such circumstances, remove the flash memory from the
console and then reconnect it.
USB Video Printer
USB Line Printer
USB to RS-232C Serial Cable
Foot Switch
Other Flash Memory Media
Page 84

Chapter 3. Installing the Product 3 - 9
System Power
Boot up the system for use.
Before turning the power on, connect the probes and peripheral devices
CAUTION
Turning the Power On
While the power is turned off, press the On/Off button. Booting will start, and the product logo
will be displayed on the screen. When booting is complete, a 2D mode screen will be displayed
in End Exam state.
you want to use. Connecting them while you are using the product may
injure the patient or severely damage the product.
CAUTION
NOTE
Before starting the diagnosis, you must register the patient information.
If the power switch near the power connection port on the rear panel
of the product has been switched off, wait for 10 seconds before
turning on the product.
While the system is booting, do not press buttons on the keyboard,
and make sure that there are no objects sitting on the keyboard
buttons; otherwise the system may not work correctly.
If you forcefully turn off the power and then turn on the product
again, the system may momentarily turn on and then turn off again.
This is a characteristic of the Intel® PC main board contained in the
product, and not a system error.
Page 85

3 - 10 WS80A Service Manual
Shutting Down the System
Press the On/Off button while using the system to initiate shutdown.
Pressing the On/Off button for longer than five seconds will
immediately turn the power off and may damage the hard disk; do
not turn off the power by using this method unless absolutely
CAUTION
necessary.
To ensure that the product is safely cut off from electrical power,
set the power switch at the rear of the product to Off position after
using the product.
Page 86

Chapter 3. Installing the Product 3 - 11
System Settings
This mode is used for system settings. It does not affect image output. The setup may be
modified depending on specific needs or preferences.
1. Press the Setup button on the touch panel, or tap Utility > Setup button on the touch screen.
2. The Settings screen will appear. Select a tab that has items to specify.
TIP Selecting a tab
You can select a desired tab in either one of two ways. Select the method that suits
you.
– Use the trackball and the Set button to select a tab.
– Tap a corresponding button on the touch screen.
3. Specify settings for each item.
4. Save the settings and exit. Either click the Close button on the monitor screen, tap the Exit
button on the touch screen, or press the Exit button on the control panel to switch to Scan
mode.
5. Tap Return on the touch screen to go back to the Utility menu.
[Figure 3.6 Setup - Touch Screen
Page 87

3 - 12 WS80A Service Manual
System General Setting
On Setup screen, touch the General tab. Or tap General on the touch screen. You can specify
general settings such as title settings.
[Figure 3.7 Setup - General]
▌ Title
You can specify the information that is displayed in the title area on the screen.
Institution
Enter the name of the hospital/institution where the product is installed.
NOTE
Department
Enter details about the medical institution or the organization. This information is used
to identify information transferred via DICOM.
Date
Today's date is displayed. To change the date, tap
NOTE
You cannot input the following characters: #, [, ", :, ?, |, \, "
.
You cannot change the date and time when a patient ID has
been registered. To change the date and time, you should finish
the current examination by pressing the End Exam button on the
control panel.
You can select a year from 2006 to 2027.
Page 88

Chapter 3. Installing the Product 3 - 13
※Tip! How to set the date and time
1. Click
2. Set the date and time by using the trackball and the Set button on the control
panel.
3. When the date and time have been properly set, click Apply to apply
changes. Click OK to close the Date and Time window. Click Cancel or the
Exit button on the control panel to cancel.
Date Format
next to the Date (or Time) field.
[Figure 3.8 Date & Time]
Select the date display format. Press the combo button to select the preferred display
format. The date display format you select will also apply to various entry fields in
Patient Information.
Time
The current time is displayed.
Time Format
Select the time display format. Press the combo button to select the preferred display
format (12 Hour or 24 Hour).
Page 89

3 - 14 WS80A Service Manual
▌ Account
Register a user ID and password.
User Log-in
Set the user account (log-in) function. If the user log-in is set to on, it can be used for the
following areas:
– Screen Saver
– Accessing SonoView/Patient
– Search window for patient
Set ID and Password
This is the exclusive administrator function for approval and management of accounts.
Account List window will be enabled.
– Add: Fill out the User ID, Password, and Name fields. Then click the Add button to
create a new ID.
– Modify: Save the changes.
– Delete: Delete the selected ID.
– Close: Close the settings.
Log-in
You can set the User Account ID after logging in to the Admin account. Please contact a
service engineer for more information on the Admin account.
■ The Admin account cannot be deleted.
■ Once the user account function is activated, you cannot load other exams
NOTE
without logging in.
■ The password must be 6 to 15 characters and composed of at least three of
the following:
– English alphabet upper case
– English alphabet lower case
– Numbers
– Special characters
Page 90

Chapter 3. Installing the Product 3 - 15
▌ Screen Keyboard
Auto Pop-up on Patient Information
Set this to On or Off by using the trackball. If it is set to on, the on-screen keyboard
appears automatically when entering patient information.
▌ Control
Trackball Speed on Scan Mode
Specify the trackball speed as Slow, Normal, or Fast.
Trackball Speed on Measurement
Specify the trackball speed as Slow, Normal, or Fast. Slower speeds allow more precise
measurements.
Zoom In
Selects the direction in which to rotate Zoom dial to zoom in on an image.
– Clockwise: Rotating the dial-button clockwise zooms in on an image.
– Counterclockwise: Rotating the dial-button counter-clockwise zooms in on an image.
Set/Exit Key
Set the functions of the buttons to the left and right of the trackball on the control panel.
– L: Set/R: Exit: The left button is set to Set and the right button is set to Exit.
– L: Exit/R: Set: The left button is set to Exit and the right button is set to Set.
– L: Set/R: Set: Both the left and the right buttons are assigned the Set function. When
this button is selected, a warning message appears, stating that Exit function needs to
be established.
Indicator Location Selection
You can set the position to use the Indicator. Select touch screen or control panel.
▌ Sound
Generate a buzzer sound when a button or dial-button is used.
Page 91

3 - 16 WS80A Service Manual
Button Sound
Set this to On or Off using the trackball. When this is set to on, the buzzer sounds each
time a button or dial-button is used.
▌ Patient Information
Save Patient Page as First Images
Set this to On or Off by using the trackball. If it is On, the Patient Information Entry screen
will be saved when creating an ID.
▌ Zoom Box Setup
Zoom Box Reference Position
Select the position of the Zoom Box Reference. You may select either Image or Zoom
Box.
▌ Quick Preset
Quick Preset Setup
Press the button to display Quick Preset Setup screen. After selecting the Probe,
Application, and Preset connected to the port, press the + button to add them to Quick
Preset. Up to 4 sets can be saved. The Probe, Application, and Preset saved will be
shown on the touch screen in the diagnosis mode.
Page 92

Chapter 3. Installing the Product 3 - 17
Scan Mode
Select the Scan Mode tab on the Setup screen. Or tap Scan Mode on the touch screen.
[Figure 3.9 Setup – Scan Mode]
▌ Store Clip
Store Clip Method
Specify the method and range in which an image is acquired and saved.
You can select ECG Beat, Time or Manual. Note that ECG Beat can be selected only
when ECG is on.
– ECG Beat: Specify the heart beat as 1-8 beats.
– Time: Specify it as 1-50 seconds.
– Manual: Pressing the button on the control panel that has been designated as Store
Clip automatically starts saving the images; pressing the same button again stops
saving.
Cine Loop Period
– Retrospective: When the Store Clip button is pressed during scanning, the previous
images are saved.
– Prospective: When the Store Clip button is pressed during scanning, the subsequent
images are saved.
Page 93

3 - 18 WS80A Service Manual
To configure the Store Clip button, go to Utility > Setup > User Defined Key >
User Key Setup.
NOTE
▌ Combination Action
Freeze with
Select a function to automatically execute when the Freeze button on the control panel is
pressed. It can be set up for each preset using the combo button. Available options are
BodyMarker, Caliper, AutoCalc, Measure, Text, and None.
– D or M Modes Only: When ‘Freeze with’ is set to Measure, Measure Freeze Action will
function only in Doppler and M Modes.
End Exam with
– None: Tapping the End Exam button on the touch screen exits Exam Mode and
switches the screen to the B Mode Scan screen.
– Patient Window: Tapping the End Exam button on the touch screen switches the
screen to the Patient Information screen.
– Reset Preset: The established preset has been initialized.
▌ Multiple Mode
Simultaneous Mode
You can decide whether to enable Simultaneous Mode in Spectral Doppler Mode, using
the following three options:
– Off: Select this if you do not wish to use Simultaneous Mode.
– Allow B/PW: Select this if you do not wish to use Simultaneous Mode in 2D/C/PW
Modes, but do wish to use it in 2D/PW Mode.
– Allow B/C/PW: Select this if you wish to use Simultaneous Mode for both 2D/PW
Mode and for 2D/C/PW Modes.
Color Mode Position on Dual Live
Select the position of the Color Doppler Mode in Dual Live Mode.
– Left or Top: Color Doppler Mode is located in the left or upper part of the screen.
Page 94

Chapter 3. Installing the Product 3 - 19
– Right or Bottom: Color Doppler Mode is located in the right or lower part of the screen.
– Left-Right Dual Live Mode Only: The Top-Bottom Dual button disappears when you
check this checkbox.
Dual Mode
Show ‘Change Window’ button: Select whether to activate the Change window in Dual
Mode.
Option
You can specify more than one item. Select an item with the trackball or the Set button to
check or uncheck an item.
– Active HPRF on PW mode: Select whether to activate HPRF (High Pulse Repetition
Frequency), which is supported in PW Spectral Doppler Mode. Check the checkbox to
use the HPRF function.
– Color Map Auto Invert (on linear): Check this checkbox to automatically highlight the
Color Map. This is only applied when you change Steer in 2D/C/D Mode, C Mode, or
DPDI Mode in PD Mode.
– M/PW Loop Side By Side: Add Loop Side By Side display in M Mode or Power
Spectral Doppler Mode.
– Width Scale: Automatically fit the image size to the screen size, when the depth of a
2D image is adjusted. This function can be used only with a Linear Probe.
– Active PW mode through M-Line: In 2D Mode, pressing the Set button when M Line is
“On” will take you directly to PW Mode.
– M-Line with sample volume gate: When M Line is in use, a sample volume Gate is
shown together with the M Line.
– Hide mode info.of saved cine: Hide mode info of the saved cine and increase the
image size.
▌ Default Text
Show Operator Name on Scan Image
Type: Select Diag. Physician, Ref. Physician, or Sonographer. Turn this option Off to hide
it from the screen.
▌ Auto Calc.
Auto Calc. is a Spectral Doppler Mode feature that automatically performs specific
Page 95

3 - 20 WS80A Service Manual
calculations based on measured values.
The specified items will appear on the screen only when the Auto Calc. button
on the touch screen is tapped in Spectral Doppler Mode.
NOTE
Enable or disable the following items for automatic calculation by checking their checkbox.
You can select up to eight values.
When the Peak Systolic Velocity and End Diastolic Velocity values are 0, not all results for
the items will be displayed on the screen. In addition, the result value for Time Averaged
Mean Velocity is displayed only when Mean Trace is ‘On’.
Page 96

Chapter 3. Installing the Product 3 - 21
Display
Select the Display tab in the Setup screen. Or tap Display on the touch screen. Specify
display-related options.
[Figure 3.10 Setup – Display]
▌ Power Saving
Screen Saver
Select whether or not to display the screen saver. When this is On, you can set the
screensaver to be activated in 1 to 30 minutes.
Auto Freeze in 10 Minutes
Scan mode will freeze automatically if the product is not used for 10 minutes.
TIP
The Scan Mode is frozen automatically, regardless of Auto Freeze setting, when the
product is not used for 1 hour.
In 3D mode, Auto Freeze is activated when the product is not used for 20
minutes.
NOTE
Page 97

3 - 22 WS80A Service Manual
▌ Font
Specify the target for which you want to set the font. Choose from Document Font and
Measure Result Font. The selected font can be previewed.
Reset
This sets the system’s default fonts. The default settings are as follows:
Document Font Measure Result Font
Font Name Helvetica Verdana
Font Size 20 11
Font Color White Yellow
Type
Select the font type to use.
Size
Select the font size to use.
Color
Select the font color to use.
Certain fonts may not appear correctly on the screen.
NOTE
▌ S-Detect
S-Detect: BI-RADs Description
Show or hide the on-screen call text based on the BI-RADs score in S-Detect. Turn this
option off to hide the call text from the screen.
Page 98

Chapter 3. Installing the Product 3 - 23
▌ Information
Direction Marker
Set the Direction Marker. Select between SAMSUNG WS80A or WS80A.
Doppler Axis
Select the units of measurement for the axis scale in Spectral Doppler Mode.
– cm/s: Specify the Doppler axis scale unit as cm/s.
– m/s: Specify the Doppler axis scale unit as m/s.
– kHz: Specify the Doppler axis scale unit as kHz.
▌ Wide Screen Mode
The Wide Screen mode can be turned On or Off by using the trackball in Single, Dual, or
Quad mode. Turn this option off to set the screen to 4:3 ratio.
▌ Display
TGC Line
This sets whether to display the TGC line. When TGC Line is Off, the TGC line appears
when you set the TGC line, and then disappears after three seconds.
Image Info
This sets whether to display image information. When the image information interferes
with an image and is turned off, it will not be displayed.
TI Type
Specify the TI to display on the screen as TIs (Soft tissue Thermal Index), TIb (Bone
Thermal Index), or TIc (Cranial bone Thermal Index).
Patient Name with
– Age: Select whether to display the name and age under the patient ID.
– Birthday: Select whether to display the name and date of birth underneath the patient
ID.
Page 99

3 - 24 WS80A Service Manual
‘Age’ and ‘Birthday’ cannot be used simultaneously.
NOTE
OB Display
Specify how the LMP, GA and EDD entered in the Patient Information screen will be
displayed on the monitor screen. Select two from LMP, GA, and EDD.
– Replace Patient ID (on Top info bar): Replace the ID in the title area.
– Replace Patient Name (on Top info bar): Replace the patient name in the title area.
– Replace App. (on Top info bar): Replace the application in the title area.
– Measure Result: Display the measurement result along with the selected LMP, GA or
EDD.
– Off: None of the options are displayed on the screen.
▌ Patient Name Formatting
This function is to display patient names in Asian languages, such as Korean, Chinese, and
Japanese.
This setting is initialized when you click Reset.
This button only appears on the screen in a product that supports Asian patient
names.
NOTE
Name Formatting
Set the order in which patient names are displayed.
– Default “Last, First Middle”: Names are displayed in the order of last name, first
name and middle name (E.g. Smith, Robert L).
– Custom: For Item, specify the order of the last name, first name and middle name. For
“Separator”, specify a symbol, such as a comma, colon or a space, to separate each
name.
Page 100

Chapter 3. Installing the Product 3 - 25
Representation Priority
Patient Information
Specify how patient names are displayed. Select the precedence of Roman, Ideographic
and Phonetic letters.
 Loading...
Loading...