Samsung LABGEOPA20 Users Manual

IVD
PA20
User Manual
Version 1.0
BCA-PA20
SAMSUNG ELECTRONICS CO., LTD.
129, Samsung-ro, Yeongtong-gu, Suwon-si, Gyeonggi-do, Korea
Distribution in USA : Nexus Dx, Inc., A Subsidiary of Samsung Electronics Co., Ltd., 6759 Mesa Ridge Road,
San Diego, CA 92121 USA / Tel : 858-410-4600, Fax : 858-410-4700, www.nexus-dx.com
For proper use of the analyzer, please refer to the product manual.

Copyright
© Copyright Reserved 2012 Samsung Electronics Co., Ltd
The hardware/software mentioned in this user manual is protected by copyright law.
Except for a copy of the User Manual for normal use, making a partial or whole copy of it
without written permission by Samsung Electronics is strictly prohibited by copyright law.
The content and specications of this user manual are subject to change without prior
notice.
Correct Disposal of This Product
(Waste Electrical & Electronic Equipment)
(Applicable in countries with separate collection systems)
This marking on the product, accessories or literature indicates that the product and its
electronic accessories (e.g. charger, headset, USB cable) should not be disposed of with
other household waste at the end of their working life. To prevent possible harm to the
environment or human health from uncontrolled waste disposal, please separate these
items from other types of waste and recycle them responsibly to promote the sustainable
reuse of material resources. Household users should contact either the retailer where they
purchased this product, or their local government oce, for details of where and how they
can take these items for environmentally safe recycling. Business users should contact their
supplier and check the terms and conditions of the purchase contract. This product and its
electronic accessories should not be mixed with other commercial wastes for disposal.
2

Table of Contents
1. Safety Warnings and Caution ........................7
User’s Manual and Labeled Symbols ................................. 9
Cautions for system installation .......................................11
Cautions for system move ................................................. 12
Cautions before use ............................................................ 12
Cautions during use ............................................................13
Precautions Before Testing ................................................ 14
Preparing to Test Samples .........................................................................14
Cautions for storage and maintenance after use .......... 15
2. Introduction .................................................17
Intended Use ........................................................................ 18
Introduction ......................................................................... 19
Operating Principle ............................................................. 20
What’s in the box ................................................................. 20
Box contents...................................................................................................20
Sold separately ..............................................................................................21
Introduction of features ..................................................... 22
Front (Right) ...................................................................................................22
Front (Left) ......................................................................................................23
Back ...................................................................................................................24
3. Installation ....................................................25
Installation ............................................................................ 26
Replacing printer paper ..................................................... 27
3

Samsung LABGEO
4. Analyzing ......................................................35
PA20
Table of Contents
Disc ......................................................................................... 29
Handling and Storage of the Disc .................................... 30
Adding the sample to the Disc .......................................... 31
Analyzing .............................................................................. 36
Turning power on .........................................................................................36
Preparing analysis ........................................................................................40
Canceling analysis in progress ................................................................43
Analysis completed ....................................................................................45
Viewing analysis results .............................................................................47
Viewing results ..................................................................... 48
Viewing results of patient’s analysis ......................................................50
Viewing QC results .......................................................................................52
Searching results ..........................................................................................54
Printing results ..............................................................................................56
Transferring results ......................................................................................61
Deleting results .............................................................................................63
Quality control ..................................................................... 65
Starting quality control ..............................................................................65
Analyzer settings (General User Settings) ...................... 69
Display settings ................................................................... 71
Calibration (touch screen calibration) ..................................................72
LCD Brightness .............................................................................................73
Screensaver settings....................................................................................74
LCD O settings ...........................................................................................75
Volume settings .................................................................. 76
Analysis settings ................................................................. 77
4
Unit settings ...................................................................................................78
Change order .................................................................................................80
Reference range settings ..........................................................................82

Samsung LABGEO
PA20
Table of Contents
QC material ........................................................................... 84
Registering QC Material .............................................................................84
Changing QC material ...............................................................................89
Deleting QC material ..................................................................................91
Print settings ........................................................................ 93
Barcode settings ................................................................. 95
Language settings .............................................................. 97
Self-test ................................................................................. 99
Analyzer settings (Administrator Settings) ..................103
Lock settings ......................................................................105
Setting security levels for each function ........................................... 105
Locking quality control ..........................................................................107
Changing password ................................................................................. 109
Network settings ...............................................................112
Connecting to a network ........................................................................ 112
Setting network connections ..............................................................114
Setting EMR/LIS .......................................................................................... 116
Lock settings.......................................................................117
Changing Date&time format ..........................................119
System settings .................................................................121
System Reset .............................................................................................. 123
USB Backup ................................................................................................. 127
System updates ......................................................................................... 129
Turning o power ..............................................................131
5. Using the LABGEO Data Manager .............133
Introduction to the LABGEO Data Manager .................134
Installation of the LABGEO Data Manager ...................135
5

Samsung LABGEO
6. Maintenance ...............................................155
PA20
Table of Contents
Running the LABGEO Data Manager ............................. 139
Home screen .......................................................................140
Registering a blood analyzer device ..................................................141
Viewing analysis results ...................................................147
Editing analysis results .....................................................148
Selecting and editing multiple analysis results .............................. 150
Printing analysis results ...................................................151
Removing the LABGEO Data Manager ..........................153
Maintenance ....................................................................... 156
Maintenance ............................................................................................... 156
Cleaning the exterior ...............................................................................156
Cleaning the Disc Tray .............................................................................157
Transportation and Storage...................................................................157
7. Troubleshooting .........................................159
Troubleshooting ................................................................ 160
Check before requesting repair ............................................................ 160
Restoring factory settings ......................................................................160
Error List ..............................................................................161
8. Other Information ......................................165
Quaility control policy ......................................................166
Westgard multi-rule technique ............................................................ 166
System information ...........................................................167
Other information .............................................................168
6
Product Service ..................................................................169
Index ....................................................................................170

1
Safety Warnings and Caution
User’s Manual and Labeled Symbols ................................................. 9
Cautions for system installation ....................................................... 11
Cautions for system move ................................................................. 12
Cautions before use ............................................................................ 12
Cautions during use ............................................................................ 13
Precautions Before Testing ................................................................ 14
Preparing to Test Samples ............................................................................................. 14
Cautions for storage and maintenance after use .......................... 15

Safety Warnings and Caution
Please follow the safety warnings and cautions in this manual for safe and appropriate use
of the product.
Repairs and maintenance work not explained in this User Manual are to be performed by a
designated Samsung service provider. Any attempt to repair the product by the user may
result in injury. Do not use the product in an area with combustible gas or high levels of
contamination for an extended period of time.
Malfunctions and damage caused by any other use than specied in the User Manual is
excluded from the manufacturer’s warranty.
If the equipment is used in a manner not specied by the manufacturer, the protection
provided by the equipment may be impaired.
8
Samsung LABGEO
PA20
Safety Warnings and Caution
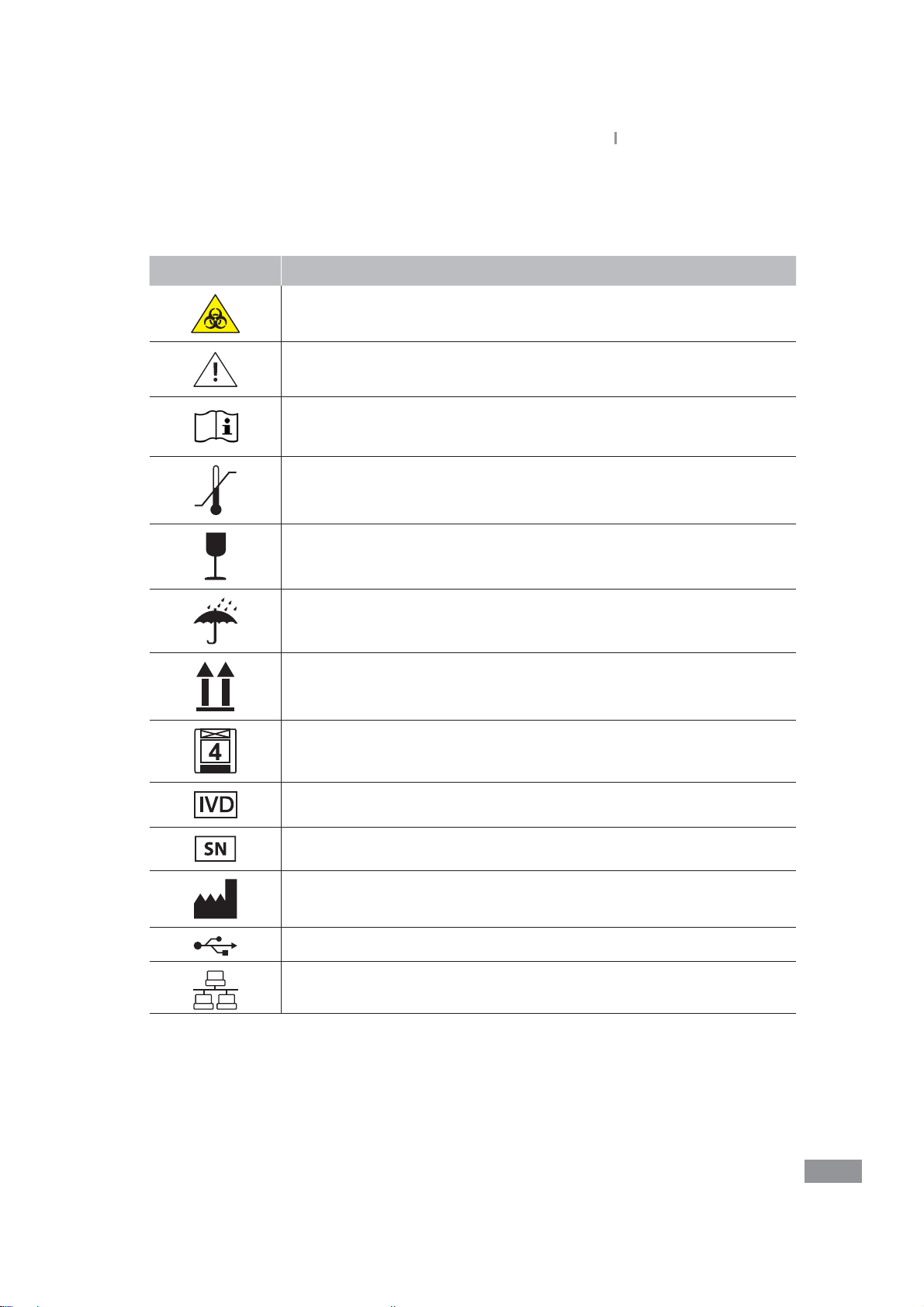
Ŷ User’s Manual and Labeled Symbols
Symbol Explanation
Biohazard. A sample as well as a used cartridge containing a sample are
potentially hazardous. Handle with care.
Caution. In order to safely and properly use this product, safety
precautions and cautions in this manual must be followed.
Consult instruction for use.
Temperature limitation/ Allowed Temperature Range Shows temperature
limit for storage or transport
Fragile/Fragile contents; handle with care
Keep dry/Package should avoid wetting
This way up
Loading Unit/Avoid loading more than the marked number of units
In Vitro diagnostic device
Serial number
Manufacturer
USB port
Ethernet port
9
Samsung LABGEO
PA20
Safety Warnings and Caution
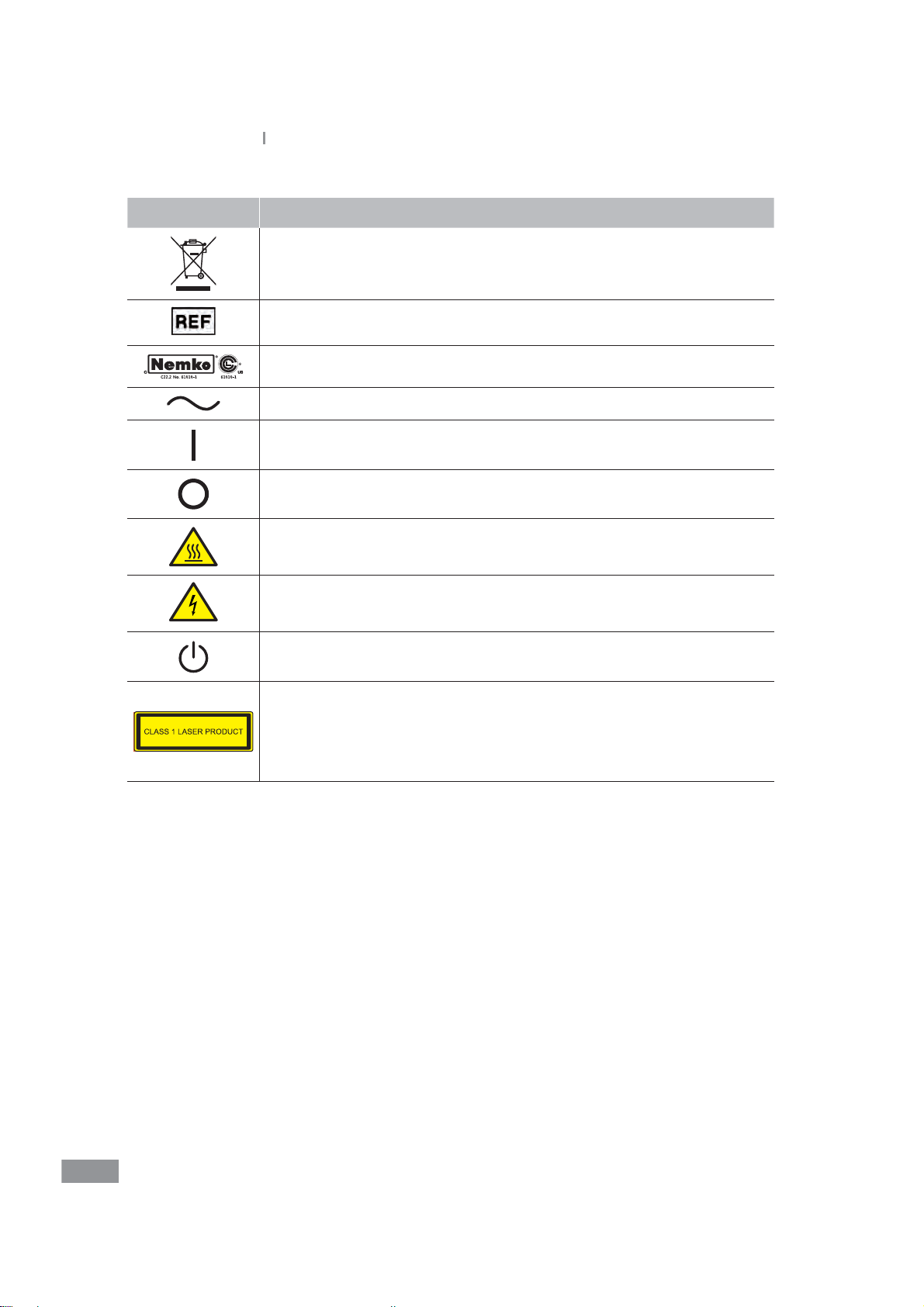
Symbol Explanation
‘New’ waste The bar can be replaced by the date of manufacture
Catalog number
North American Safety Certication Mark
Alternating current
Power On
Power O
Hot Surface
Risk of electronic shock
Stand-by switch
LASER CLASSIFICATION
This Clinical Chemistry and Immunology Analyzer is conrmed as the
class 1 laser product in IEC60825-1:2007
- Wavelength: 808 nm
- Maximum output of laser radiation: Max. 1.5 W
10

Samsung LABGEO
Ŷ Cautions for system installation
Ŷ
Keep the analyzer at least 6 inches (15cm) away from any wall to provide
adequate ventilation and prevent overheating.
Ŷ
Be careful not to expose the analyzer to sources of vibration such as placing
it near a centrifuge.
Ŷ
Do not use the analyzer where liquids or chemicals are used or where gas
may be produced. Insure that no liquids are spilled into the Unit if there
is any spillage disconnect mains immediately and return to product for
serving.
Ŷ
The analyzer must be used at an operating temperature of 10 - 32 °C
(50- 89 °F). The analyzer might be aected by:
t Condensing humidity and water
t Heat and extreme temperature variations
t Electromagnetic radiation
t Movement of the analyzer during sample processing
Ŷ
Only use the cable provided with the analyzer.
Ŷ
To avoid risk of re or electric shock, do not use a damaged power cable.
Ŷ
Always use the correct voltage supply. Incorrect voltage can cause damage
to the analyzer or result in re. Be sure that the electrical outlet is properly
grounded.
Ŷ
Do not attempt to disassemble, repair or modify the analyzer as damage
could occur. Opening the unit will void the warranty. Contact Samsung
Technical Support.
Ŷ
Do not place any objects on the analyzer.
Ŷ
Do not cover the rear ventilation panel. This may damage the internal parts,
resulting in a re or electric shock.
Ŷ
Do not introduce foreign substances into the servicing hole.
Ŷ
Turn on the power switch at the rear of the Analyzer. (Grounding Proper
grounding is required when installing the system. Check the wall outlet
ground (Earth) for proper grounding to the facilities electrical ground. If you
are unsure of the outlet grounding, contact your facilities engineer to verify
the proper outlet ground). Next, turn the power switch on the front of the
Analyzer on and check that the program starts normally.
Ŷ
Do not touch the power plug with wet hands. This may result in an electric
shock.
PA20
Safety Warnings and Caution
11

Samsung LABGEO
PA20
Safety Warnings and Caution
Ŷ Cautions for system move
Ŷ
Dropping the product while moving or carrying may cause damage to the
product.
Ŷ
Be careful of impact when moving the product at all times.
Ŷ
When moving the product by car, please use the original packaging
material.
When moving the analyzer, rst turn o the power and disconnect the power cable from
the product.
Ŷ Cautions before use
Ŷ
When the blood is not used immediately, be sure to store it appropriately to
prevent it from contamination.
Ŷ
Check for dust or other impurities on a regular basis.
Ŷ
Always install the product in a well-ventilated, clean, and dry area.
Ŷ
Keep the product away from heat-emitting products (such as heaters). It
may cause deformation of the product, re, or adversely inuence analysis
results.
12

Ŷ Cautions during use
Ŷ
This system is for professional use only. The user must be a physician, clinical
laboratory personnel, or healthcare professionals.
Ŷ
Avoid external shock or vibration during analysis.
Ŷ
Do not use expired discs.
Ŷ
Do not open the tray while the disc is being analyzed. During the analysis,
LED lamp or laser radiation may cause hazard due to improper operation.
Ŷ
The Analyzer contains UV/Visible LED Light source and laser. The users are
not exposed to Optical radiation in normal conditions. Because it is blocked
with analyzer cover cabinet. Do not open the tray during analysis. Do not
look into the analyzer during analysis. Your vision will be damaged due to
UV-lamp.
Ŷ
Powder-free gloves must be worn when operating the product.
Ŷ
Do not move the analyzer during operation.
Ŷ
When inserting a disc into the tray, check the orientation of the disc. It must
be inserted with the label side up. Consult test Instructions for Use.
Ŷ
Always use designated power cable. Do not unplug the device in the middle
of an analysis.
Ŷ
If the product produces smoke or strange odors, discontinue use, unplug
the power cord from the outlet, and contact your local distributor for service
information.
Ŷ
If operation has been aborted due to abnormal circumstances such as a
power outage during operation, discard the disc and start over with a new
disc.
Ŷ
Do not turn o the power during analysis. Press Cancel to abort a test if
needed. Discard the disc and start over with a new disc.
Ŷ
Be careful not to damage the barcode on the disc. If the barcode is
damaged, the test will not perform accurately.
Ŷ
Do not use the analyzer where liquids or chemicals are used or where gas
may be produced.
Ŷ
Do not allow liquid to spill into the analyzer. If there is any spillage,
disconnect power immediately and contact your local distributor.
Ŷ
If you wish to stop the analysis in progress without turning the analyzer o ,
press the Cancel button and stop the analysis. After stopping an analysis in
progress, throw away the cartridge and replace it with a new one.
Ŷ
The operating temperature of the analyzer is 10 - 32 °C (50 - 89 °F); the
analyzer automatically maintains an internal temperature of 37°C. (The
device shuts o automatically if it exceeds normal operating temperature.)
Samsung LABGEO
PA20
Safety Warnings and Caution
13
Samsung LABGEO
PA20
Safety Warnings and Caution
Ŷ Precautions Before Testing
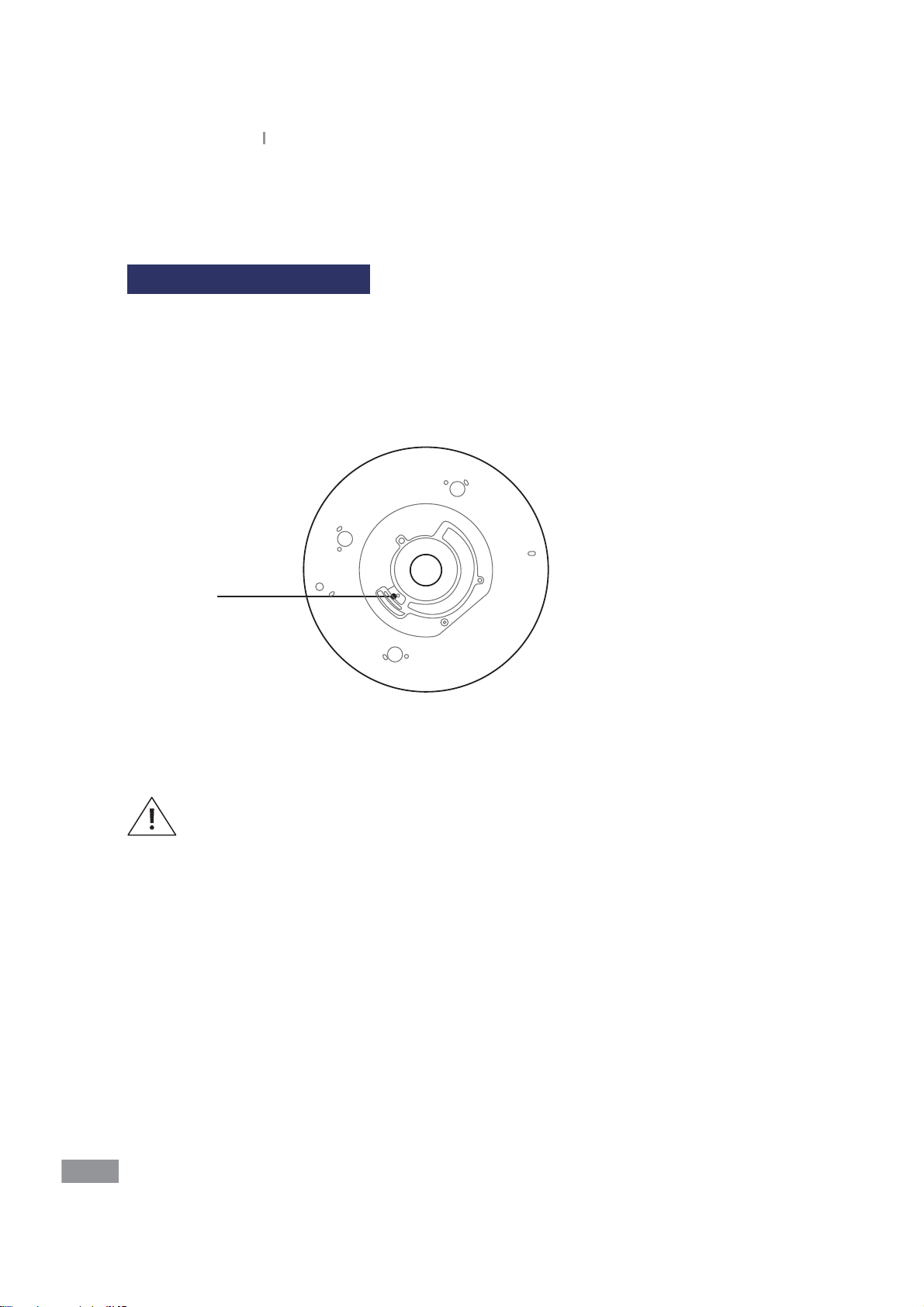
Preparing to Test Samples
Prepare a disc, sample, and injecting tool (pipette) to be used for the test.
1
Consult Test Instructions for Use.
2
Use a pipette to inject the sample into the disc as described in the Test Instructions for
3
Use.
Inlet
Inject into the inlet shown in the gure above as described in the Test Instructions for
4
Use.
Refer to the Test Instructions for Use for the correct sample volume to be injected
into the disc.
14
Samsung LABGEO
PA20
Safety Warnings and Caution
Ŷ Cautions for storage and maintenance after use
Ŷ
Do not store the product in humid areas or areas inuenced by
temperature, humidity or wind.
Ŷ
Store the device on a at surface and keep it away from vibration or impact.
Ŷ
Do not store the product with chemicals or gas.
Ŷ
Operating conditions and temperature: 10 - 32 °C (50 - 89 °F),
Humidity: 0% - 80%
Ŷ
Storage conditions and temperature: -10°C - +40°C , Humidity: 0% - 80%
Ŷ
If there is blood residue from a specimen or any contaminants in the disc
tray and the interior or exterior of the analyzer, remove the contaminant
using a soft cloth while wearing gloves.
Wet the cloth using 30% isopropyl alcohol when removing contaminants.
Do not use highly corrosive agents such as benzene or acetone.
These agents may damage the analyzer.
Ŷ
When moving or transporting the product, follow the conditions for using
and storing the product specied in this manual. Follow all cautions when
moving the product.
Ŷ
A specimen may be contaminated with pathogens or viruses that cause
infectious illnesses such as Hepatitis B. All specimens must be considered
and handled as potential contaminants.
Ŷ
Used discs must be processed using Medical Waste Treatment Laws after
use.
15

Samsung LABGEO
PA20

Introduction
Intended Use ........................................................................................ 18
Introduction ......................................................................................... 19
Operating Principle ............................................................................. 20
What’s in the box .................................................................................20
Box contents ...................................................................................................................... 20
Sold separately .................................................................................................................. 21
Introduction of features ..................................................................... 22
Front (Right) ....................................................................................................................... 22
Front (Left) .......................................................................................................................... 23
2
Back ....................................................................................................................................... 24

Introduction
Ŷ Intended Use
The Samsung LABGEO
analytes and immunological reactions in lithium-heparinized whole blood, plasma, or
serum in a clinical or point-of-care setting. It is designed to use a disposable reagent disc
which contains reagents and buers for a complete test. The Analyzer unit contains the
Laser which is for controlling microuidics. UV LED is light source is for detection chemical
reaction.
For use in moderate complexity labs with whole blood, plasma or serum.
If the Samsung LABGEO
manual, the analyzer may not operate as intended, may produce inaccurate or
no results, and may pose a safety harzard.
Use only Samsung LABGEO
PA20
provides quantitative in-vitro measurements of clinical chemistry
PA20
is used in any way other than described in this
PA20
reagent disc with the Samsung LABGEO
PA20
.
18

Ŷ Introduction
Samsung LABGEO
PA20
Introduction
The Samsung LABGEO
PA20
is a fully integrated photometric device that can perform both
clinical chemistry analyses and immunoassays simultaneously. Innovative laser-actuated
microvalves together with the centrifugal microuidics makes the total process of plasma
separation, metering, mixing, incubation, washing, and detection fully automatic.
A lithium-heparinized whole blood, plasma, or serum sample of 450uL is applied directly
to a disposable reagent disc and the disc is inserted into the analyzer by an operator. Each
reagent disc is self-contained clear plastic with diameter of 12 cm and thickness of 0.8 cm,
which consists of dierent kinds of reagents and buers.
The entire process is activated once the operator touches the “Run” button on the display.
The ‘self-test’ function is initiated, whereby the main power, main motor, laser motor,
temperature IC, photometer, laser, heater and barcode reader are checked. The ‘self-test’
function is conducted automatically before each test or control run. This built-in self
testing function minimizes maintenance costs. After inserting a reagent disc into a tray,
the analyzer automatically detects the disc type and identies the clinical chemistry and
immunoassay tests before performing the assays. The analysis is completed in about 12 to
25 minutes depending on the disc type.
The results are displayed on the LCD screen along with the reference ranges, can be printed
out by an internal thermal printer, and can be transferred to a laboratory information
system and electronic medical record systems (LIS/EMR) via an Ethernet and wireless LAN
ports. Data storage is up to 5,000 patient results and 3,000 control results in the instrument.
The system provides user-friendly and easy interface for analysis that minimizes user
manipulation. Handy compact design (246(W) x 375(D) x 289(H) mm
3
) allows maximized
space utilization and the light weight (~ 9.2 kg) provides portability.
Compared to conventional blood analyses done in clinical laboratories, the analyzer system
is suitable for point-of-care applications because it requires a smaller amount of blood
takes less time, and does not require specially trained operators, or expensive instruments
to run biochemical assays and immunoassays separately.
19
Samsung LABGEO
PA20
Introduction
Ŷ Operating Principle
The analyzer is equipped with two optical detection modules. One is a photometer and
the other is a uorometer. The photometer can measure absorbance at 10 dierent
wavelengths (340, 405, 450, 500, 550, 570, 600, 630, 660, and 700 nm) to accommodate
various chemical and immunological reaction protocols. The measured absorbance is
converted into the concentration of the analytes.
For endpoint reactions, the nal absorbance is used for the calculation of the concentration
of the analyte.For kinetic reactions, the absorbance change during a predetermined time is
utilized for the calculation of the concentration instead of the nal absorbance.
The uorometer utilizes a time-resolved uorescence method to support high-sensitivity
immunoassay based on europium nanoparticle lables. A 365 nm LED is used as an
excitation light source and a red-sensitive photomultiplier tube is used as a photodetector.
The measured uorescence intensity is converted into the concentration of the anlayte.
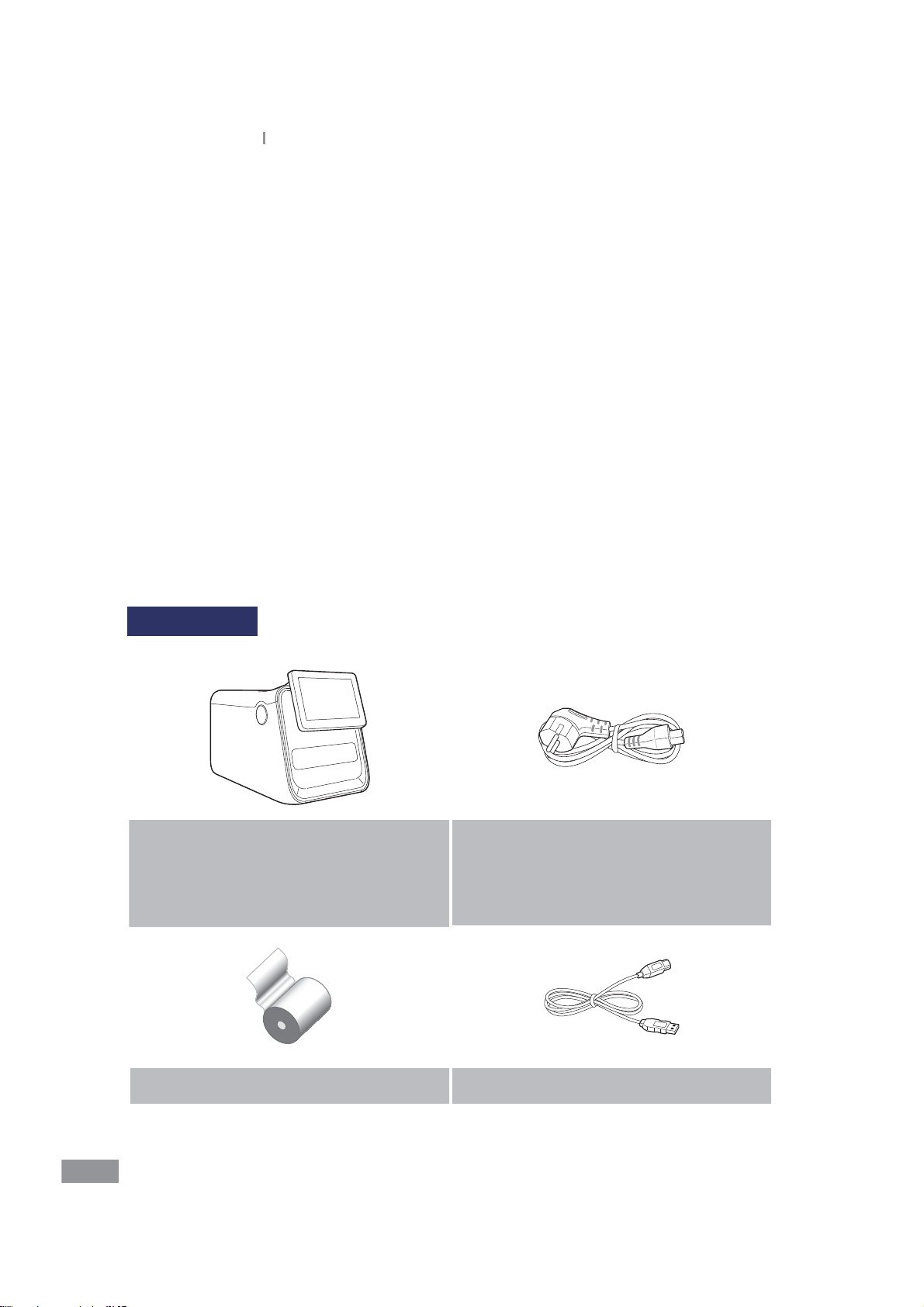
Ŷ What’s in the box
Box contents
Analyzer Samsung
LABGEO
BCA-PA20
PA20
Power Cable
A 1.8m long cable used to supply power to
the system. The eective voltage range is
between 100-240 VAC
(50-60Hz).
20
Printer paper USB Cable
User Manual Software installation CD
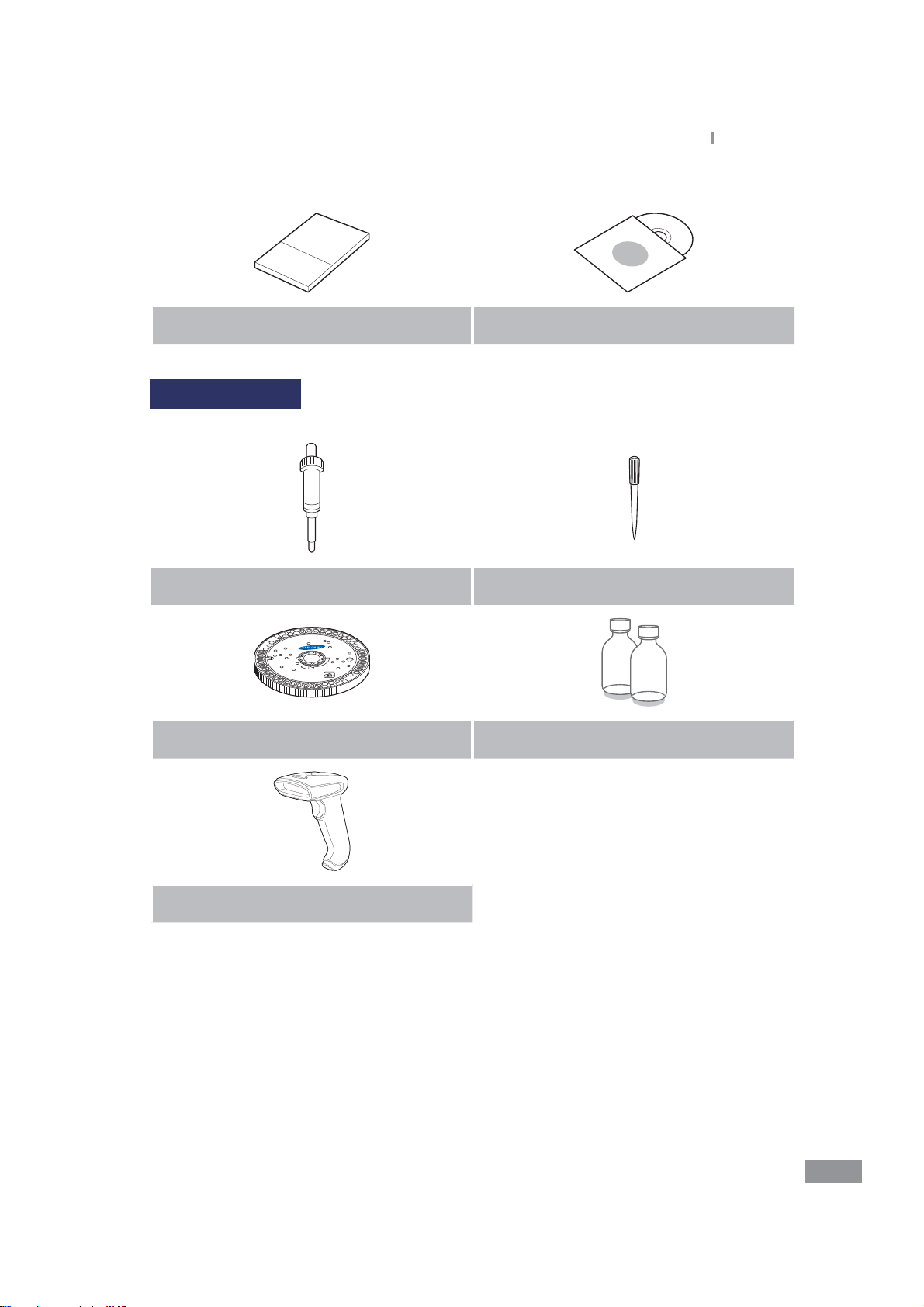
Sold separately
Samsung LABGEO
PA20
Introduction
Pipette tips
Disc Control Serum
Barcode scanner (optional)
t Please check the above contents of the box after purchase.
t If any of the contents are missing or damaged. Do not use the product and contact your
retailer for a replacement.
21
Samsung LABGEO
PA20
Introduction
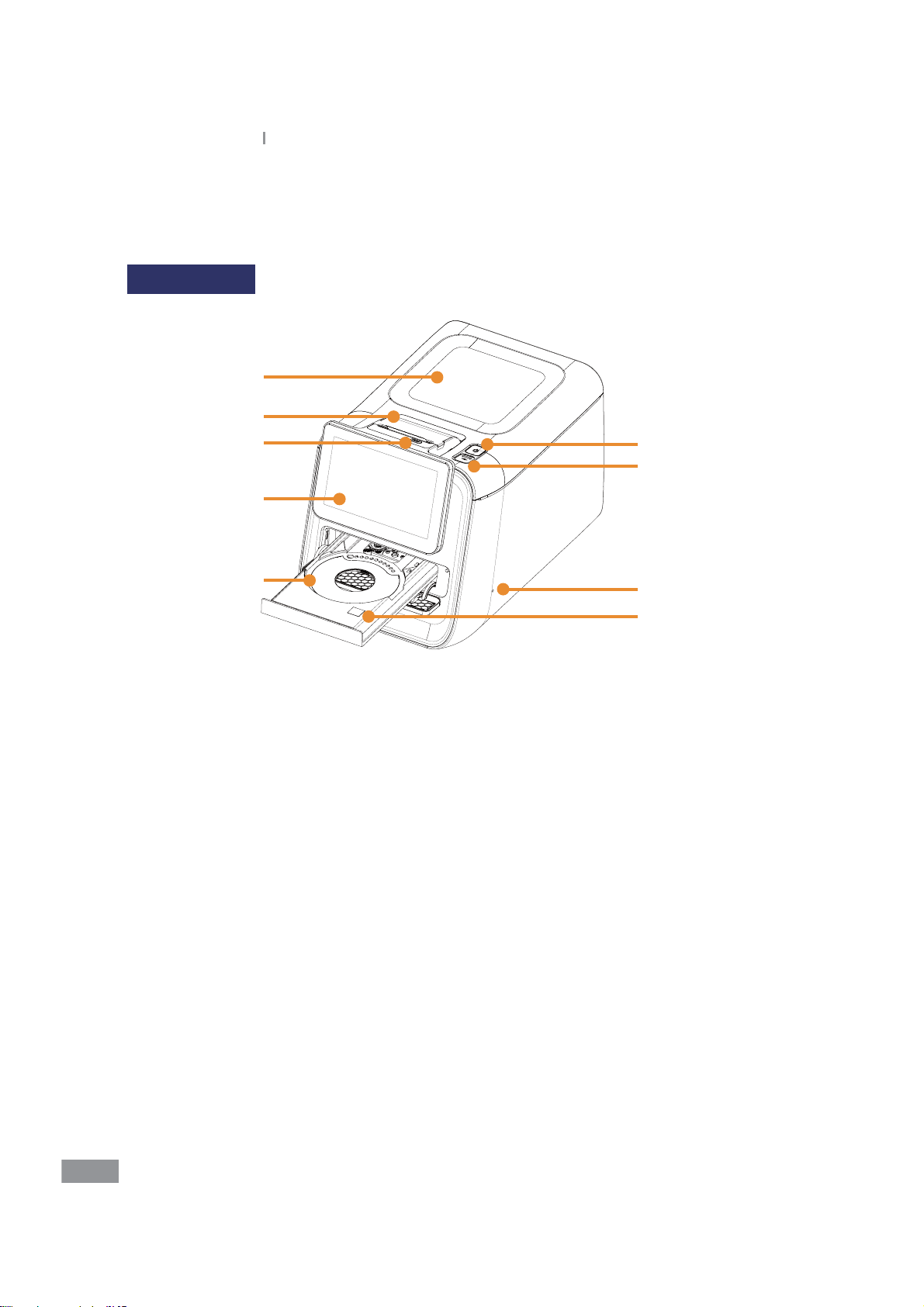
Ŷ Introduction of features
Front (Right)
1
2
3
6
7
4
5
8
9
1. Main Unit
2. Printer
3. Printer feed button
4. 7-inch Color TFT LCD Monitor
5. Tray
6. Power Button: Used to turn the analyzer on or o
7. USB Port: Used to update the software and to back up the analysis results in the USB
memory
8. Servicing Hole: For qualied Samsung Technical Support personnel only
9. HOT SURFACE label
22
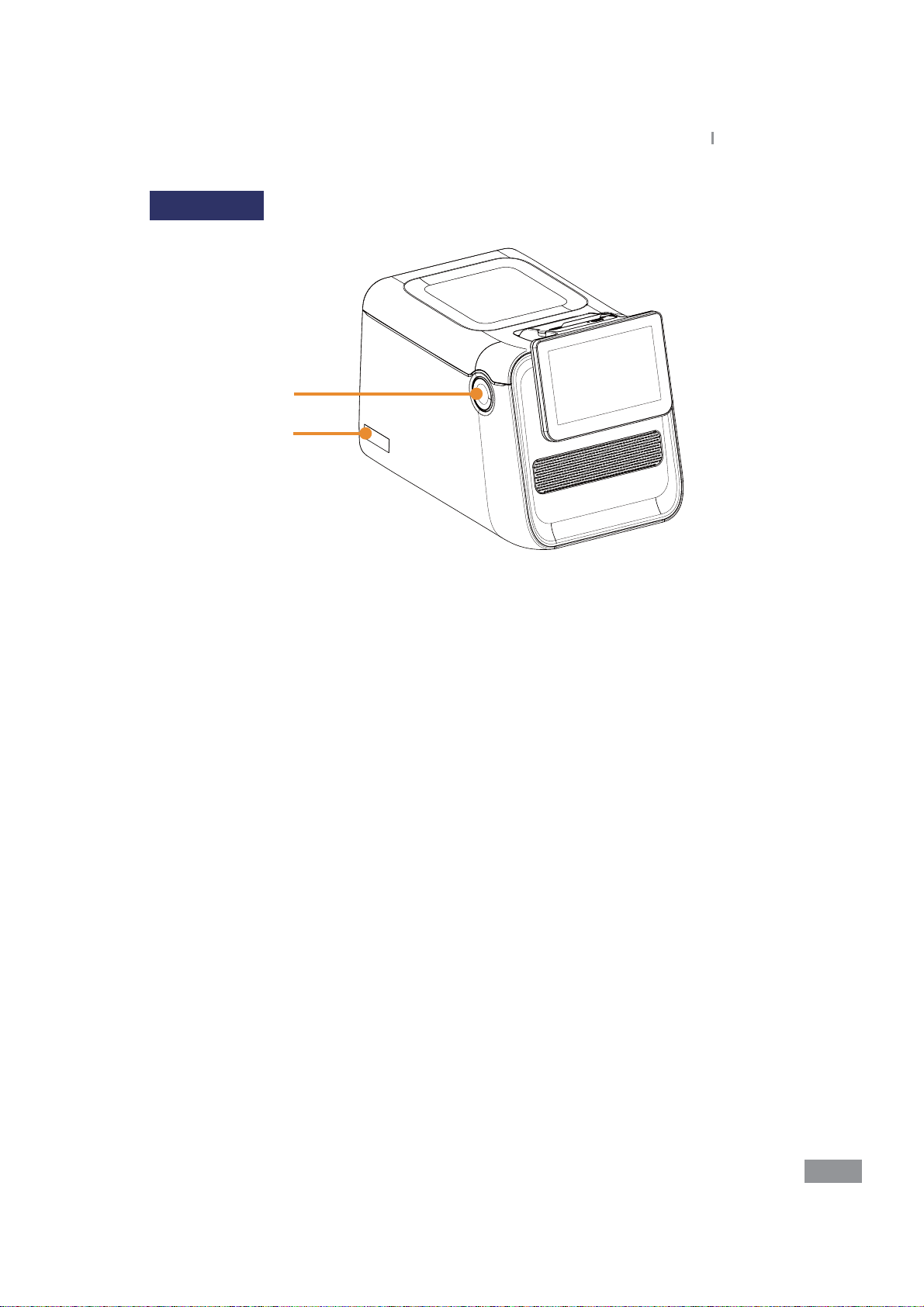
Front (Left)
1
2
Samsung LABGEO
PA20
Introduction
1. Class 1 laser product label
2. Monitor tilting button
23
Samsung LABGEO
PA20
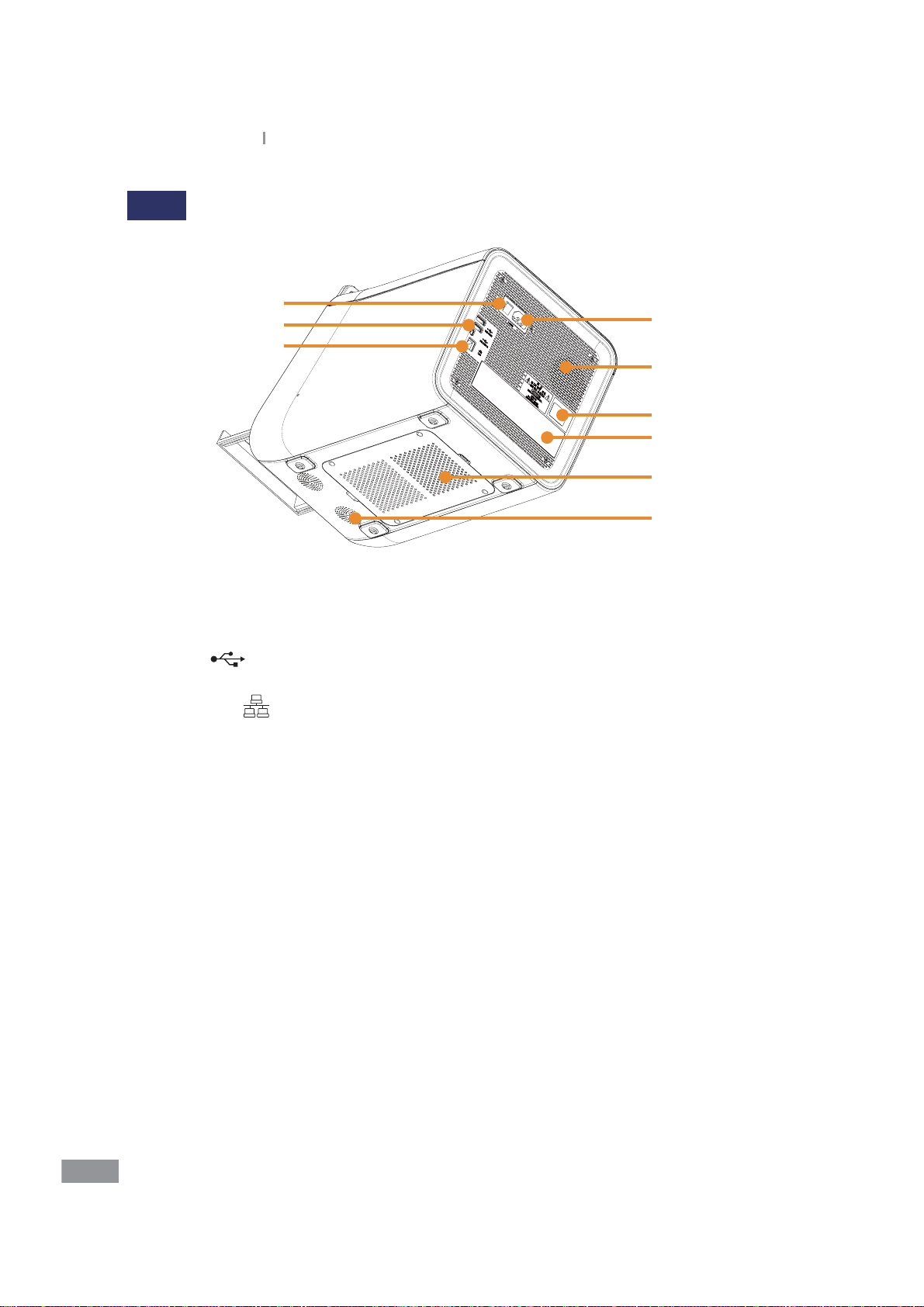
Back
1
3
2
4
5
6
7
8
9
1. Power Switch: Used to turn the power to the analyzer on or o, turning o this switch
completely cuts the power o.
2. Power Input: Connect the Power Cable here
3. USB Port
for connecting to a computer
4. Ethernet Port
5. Cooling Fan
6. Biological Hazard Warning
7. Displays basic information of the analyzer including product name, manufacturer,
manufactured date and serial number
8. Motor Cover: To be removed for maintenance and only by authorized Samsung
Technical Support personnel
9. Speaker
: Used to update the software and to back up. The smaller port is provided
: Used to connect to LAN or the Internet
24

Installation
Installation ............................................................................................ 26
Replacing printer paper ..................................................................... 27
Disc ......................................................................................................... 29
Handling and Storage of the Disc .................................................... 30
Adding the sample to the Disc .......................................................... 31
3

Installation
Ŷ Installation
Carefully remove the analyzer from the packaging, and check for any damage that
1
may have occurred during transport. If it is damaged, contact your courier or retailer
immediately. Check and make sure that the contents of the box match the contents
described in the User Manual. Contact your retailer if any component is missing.
Place the analyzer on a level surface near an appropriate AC electrical outlet.
2
Connect the power cable to the power input on the rear panel of the analyzer.
3
Plug the power cable into the AC power outlet. The analyzer uses a switching-mode
4
power supply (100-240 V).
LAN and USB cables are used for PC software (LABGEO Data Manager) connections.
5
26
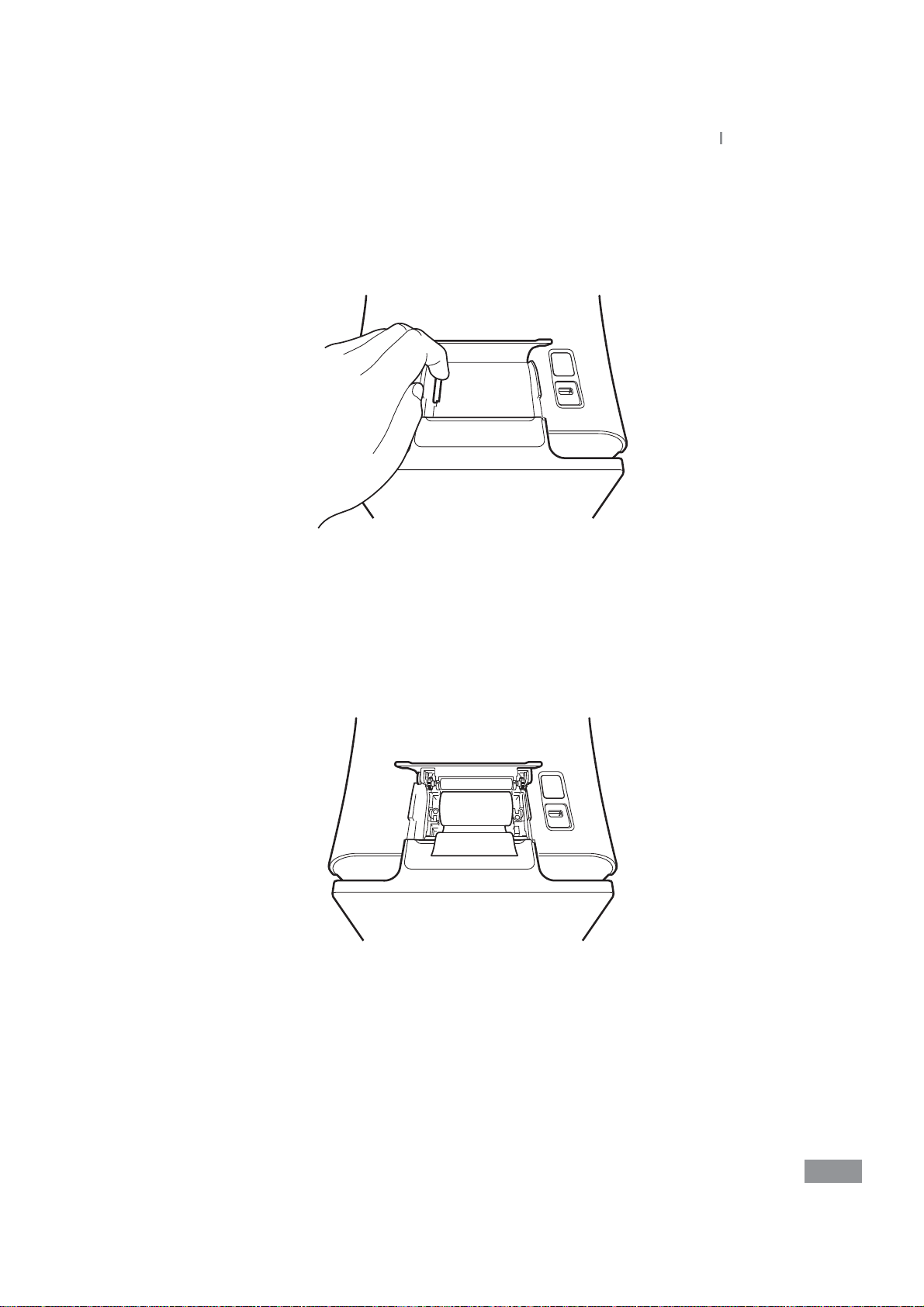
Ŷ Replacing printer paper
Open the paper lid (pull the lid upward by the handle).
1
Remove the used roll.
2
Samsung LABGEO
PA20
Installation
Unwind a new paper roll so that the leading edge comes out from the bottom, facing
3
the user.
Gently insert a new roll into the receptacle, and conrm that the paper comes out
4
through the front of the printer while holding the leading edge.
27
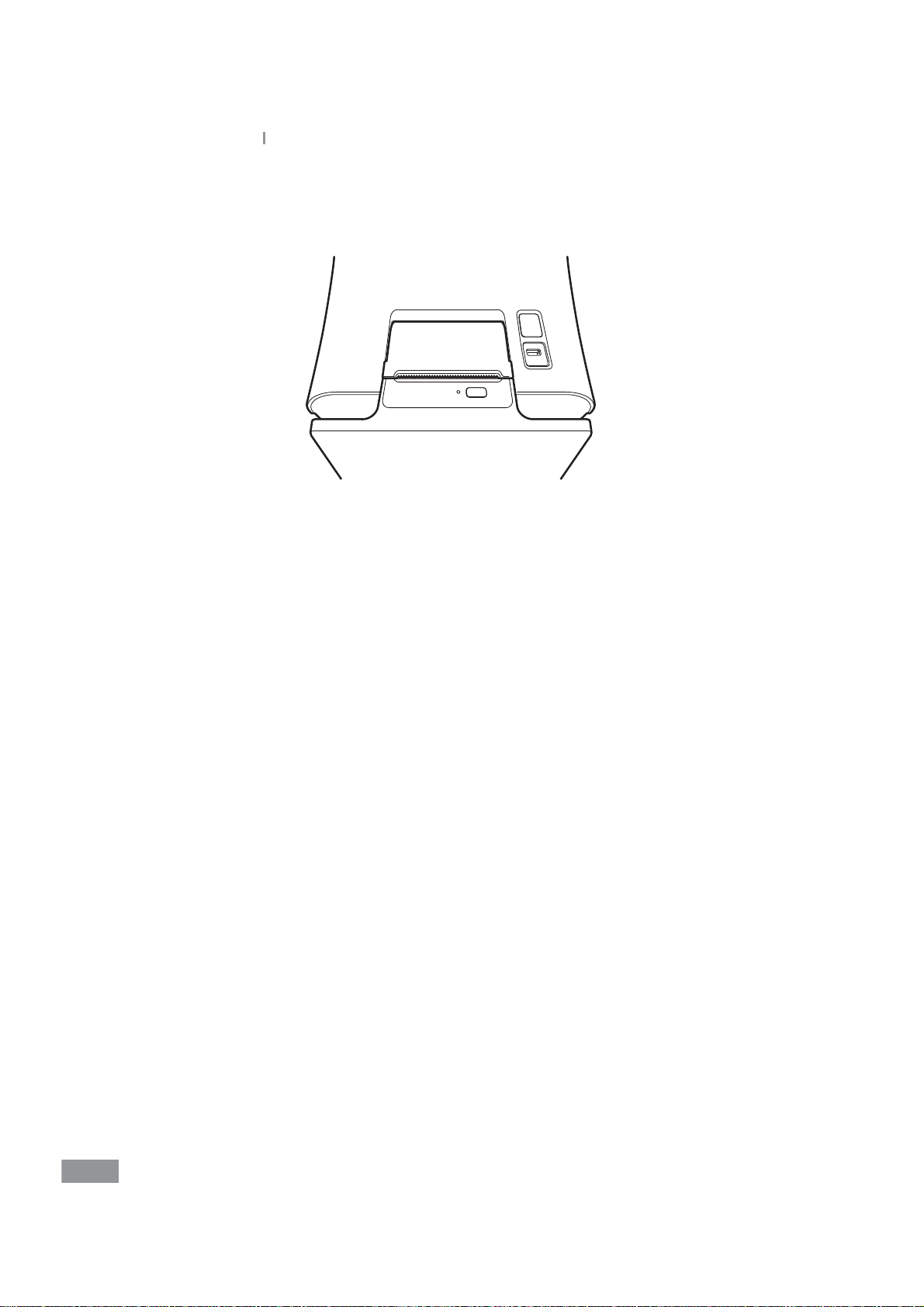
Samsung LABGEO
Close the lid and conrm that the paper is positioned between the lid and the front of
5
PA20
Installation
the printer.
28
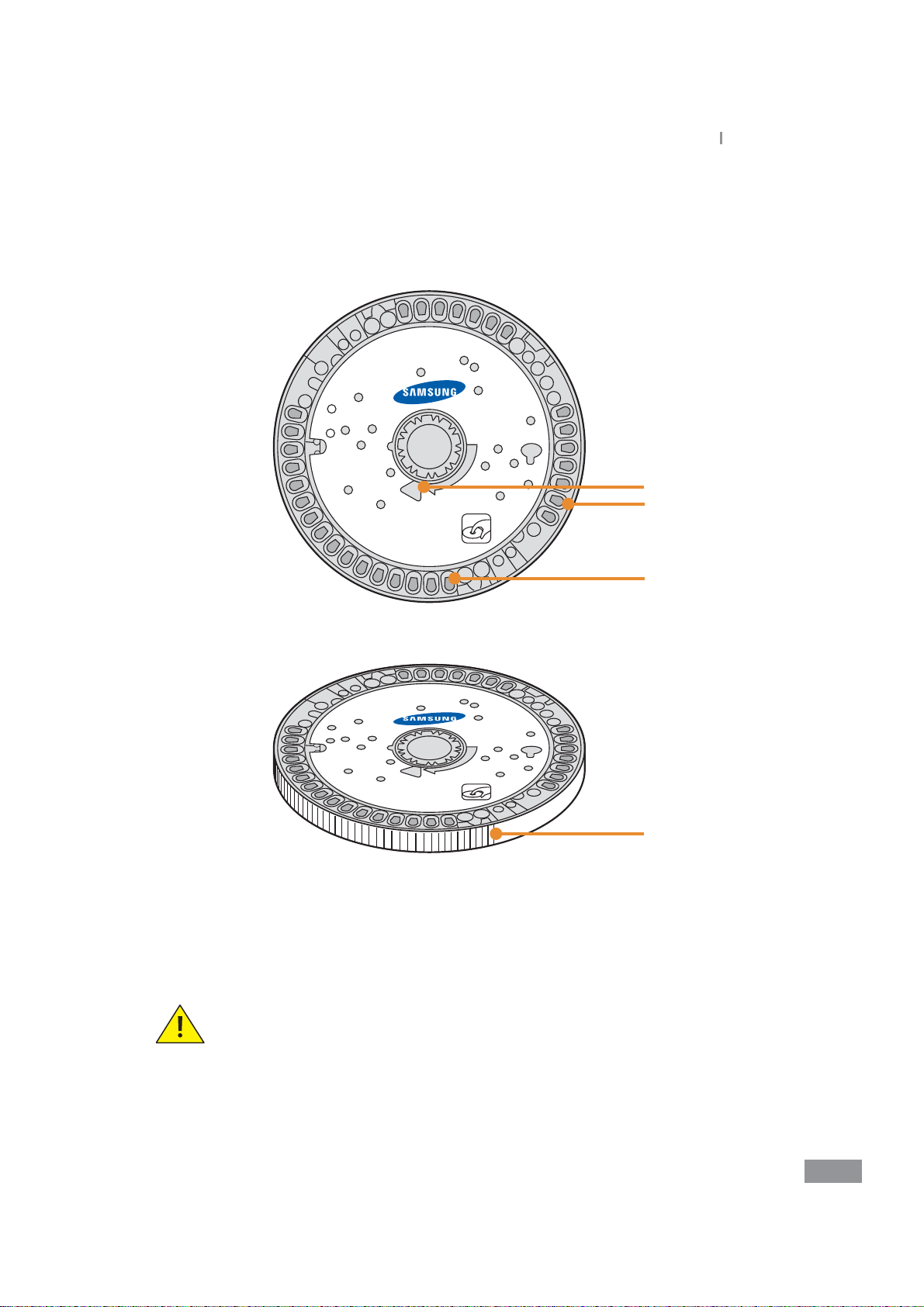
Ŷ Disc
The actual disc may dier from the images shown below.
Samsung LABGEO
1
2
4
PA20
Installation
1. Sample injection Hole
2. Reagent
3. Disc Barcode
4. Detection area (gray-colored zone)
Care must be taken to keep the detection area clear of ngerprints or other
foreign substances.
Contamination of the detection area may aect the analysis results.
3
29
Samsung LABGEO
PA20
Installation
Ŷ Handling and Storage of the Disc
Handle the discs carefully to avoid damaging them.
The disc is a high-technology integrated product but is vulnerable to impacts.
Be careful not to bump the disc when transporting, handling or loading it.
Do not use the disc if it has been dropped.
t Always use powder-free gloves when handling the product.
t Store the disc LEVEL between 2 - 8 °C (36 - 46 °F).
t Use by the expiry date marked on the disc pouch or the package label. The analyzer
detects and rejects a disc that has expired.
t Bring the disc in its unopened pouch to room temperature (18 - 25 °C or 64 - 77 °F) for at
least 10 minutes before using it on the analyzer.
t The disc can be left at room temperate (18 - 25 °C or 64 - 77 °F) for a total of up to 12
hours if the pouch remains sealed. Do not use the disc if left at room temperature more
than 12 hours.
t Do not expose the open disc to direct sunlight.
t Do not expose the disc to temperatures over 32 °C (89 °F).
t Do not use a disc if the pouch is cut or torn as the reagents inside may be damaged by
moisture.
t Tear open the pouch at the notch located at the top edge. Once the pouch is opened,
the disc must be used within approximately 10 minutes or discarded. It cannot be put
back in the refrigerator for use at a later time.
t Be careful not to leave ngerprints or other contaminants in the detection areas of the
disc.
t Keep the disc level at all times!
30
BIOHAZARD! Operator health and safety regulations require that Universal
Precautions are to be observed whenever handling human blood samples
PA20
or working with the Samsung LABGEO
. For additional information refer to
OSHA 29 CFR Part 1910, Standard number 1910.1030 (Toxic and Hazardous
Substances: Bloodborne Pathogens”). This can be found on the internet at
http://www.osha.gov and going to “1910.1030.” Used discs contain human
blood. Care must be taken in handling used discs as the blood may have been
infected by microbes that may cause infectious diseases such as hepatitis
B. The disc, once used, should be disposed of according to medical waste
disposal rules.
For additional guidelines on handling and disposing of hazardous medical
wastes, refer to “Clinical Laboratory Waste Management, Approved
Guideline—Second Edition (GPS-A2) issued by the Clinical and Laboratory
Standards Institute (previously known as NCCLS).
This can be found at http://clsi.org.
 Loading...
Loading...