Ri Witness User Manual
User Manual
R I W I T N E S S ™
Embryology Heated Plate
+45 46 79 02 02 | customerservice@origio.com | coopersurgical.com
Research Instruments Ltd, Bickland Industrial Park, Falmouth, Cornwall TR11 4TA, UK |
0120 |
Only |
Document 6-70-807UM(6) | DRF 4740 | 29 April 2019 |
|

Contents Research Instruments Ltd
CONTENTS |
|
SECTION1-PREFACE |
1 |
SECTION2-INTRODUCTION TO RI WITNESS |
2 |
Indications for Use for RI Witness Embryology Heated Plate |
2 |
Contraindications |
2 |
Applicable Part Numbers |
2 |
Related Documents |
2 |
Compatibility |
2 |
Installation |
2 |
SECTION3-SAFETY WARNINGS |
3 |
Glossary of Safety/Information Symbols |
4 |
Safety and Reliability |
6 |
Temperature Safety |
6 |
RFID Reader Environment |
6 |
Guidance and Manufacturer’s Declaration (Part 15 of FCC) — Electromagnetic Emissions |
7 |
Guidance and Manufacturer’s Declaration (IEC 60601-1-2) — Electromagnetic Emissions |
7 |
Guidance and Manufacturer’s Declaration — Electromagnetic Immunity |
8 |
Guidance and Manufacturer’s Declaration — Electromagnetic Immunity |
9 |
SECTION4-PRODUCT OVERVIEW |
10 |
Embryology Heated Plate |
10 |
RI Witness Embryology Heated Plate Specification Table |
11 |
SECTION5-RI WITNESS BASIC OPERATION |
12 |
Startup Procedure |
12 |
Shutdown Procedure |
12 |
Connecting to the Software |
12 |
User Interface |
12 |
Operator Position |
13 |
Achieving the Correct Sample Temperature |
13 |

Research Instruments Ltd Contents
CONTENTS
Changing the Temperature Setpoint Using the Device |
14 |
Changing the Temperature Setpoint Using a PC and RI Witness WorkArea Software |
14 |
Temperature Calibration |
15 |
ITO Glass Window Calibration Using Built-In User Interface |
15 |
Full 5-channel Calibration Using PC and RI Witness WorkArea Software |
16 |
Tube Reader Antenna Accessory |
17 |
SECTION6-TROUBLESHOOTING |
18 |
RFID SYSTEM |
18 |
Temperature Control: Alarms and System Status |
19 |
Audible Alarms |
19 |
Alarm System Testing |
20 |
Alarm Conditions Codes |
20 |
SECTION7-CARE AND MAINTENANCE |
24 |
Cleaning |
24 |
Transport |
24 |
Storage |
24 |
SECTION8-WARRANTY INFORMATION AND LIMITS ON LIABILITY |
25 |
SECTION9-RETURNING PRODUCT TO RI FOR REPAIR |
26 |
Customer Service Contact details: |
27 |
US only customers contact details: |
27 |
Section 1
Preface Research Instruments Ltd
1 |
SECTION1-PREFACE |
|
Thank you for choosing RI Witness. |
This manual provides all necessary information to use RI Witness Embryology Heated Plate and should be read in conjunction with any manuals provided with other RI Witness hardware or software components that are being used. The system should be operated by trained personnel only. All sections of this manual should be read and understood fully before any operation of the system. Please see the Intended Use for more information.
If the operator is unsure of any of the information contained in this manual they should contact Research Instruments or an appointed representative before attempting to use this equipment.
In no event does Research Instruments Ltd (RI) assume the liability for any technical or editorial errors of commission, or omission; nor is RI liable for direct, indirect, incidental, or consequential damages arising out of the use or inability to use this manual.
The information in this manual is current at the time of publication. Our commitment to product improvement requires that we reserve the right to change equipment, procedures and specifications at any time. The latest version of the User Manual can be downloaded from software.research-instruments.com. The RI Witness manual belongs with the RI Witness system and should be passed on with the system if relocated to another clinic.
The use of ™ in this manual indicates a trademark of Research Instruments Ltd. Any other brand names,
referred to in this manual, are trademarks of their respective owners.
© This manual is protected by copyright, all rights reserved, and no part here of may be photocopied or reproduced in any form without the prior written consent of RI.
Thisindicatescautionarytextwhichshouldbefollowedtoavoidinjurytousersordamage to samples.
 The system should be operated by qualified and trained personnel only.
The system should be operated by qualified and trained personnel only.
|
Section 2 |
|
|
Research Instruments Ltd |
Introduction |
|
|
|
|
|
|
SECTION2-INTRODUCTION TO RI WITNESS |
|
|
|
Indications for Use for RI Witness Embryology Heated Plate |
|
|
|
To maintain the temperature of human reproductive tissue such as oocytes and embryos through an |
2 |
||
assisted reproduction (AR) cycle. |
|
|
|
0120 |
Only |
|
|
Contraindications |
|
This device is not intended to be exposed to known sources of electromagnetic interference (EMI) with medical devicessuch as diathermy,CT,MRI, RFID (except other RI Witness RFID components) and electromagnetic security systems, eg metal detectors and electronic article surveillance systems.
Applicableindicationsforusearesubjecttotheregulationsofthecountryintowhichthedeviceissold. AvailabilityofRIWitnessforclinicaluseisdependentontheregulatoryapprovalstatusofRIWitnessin the country where the device is sold.
Applicable Part Numbers
Part Number |
Description |
6-70-807* |
RI Witness Embryology Heated Plate |
|
|
6-70-809 |
RI Witness Tube Reader |
|
|
*6-70-807canbesuppliedinseveralconfigurationsdependingontherequiredmountingtype,egflush fitted or sit-on-top.
Related Documents
6-7-121UM RI Witness WorkArea Software Manual
6-7-122UM RI Witness Manager Software Manual
Compatibility
RI Witness is used in conjunction with the following:
•Essential medical devices, eg dishes and tubes, maybe AR or non-AR specific.
•Non-essential medical devices, eg safety cabinets, incubators, micromanipulators, lasers.
•Non-medical devices (general laboratory equipment), eg work benches, microscopes, PCs.
This device has RFID reader capability. If it is the intention that it be employed in a clinical lab, we recommenditsusealongsideothermedicaldevicesandthattheperformanceofthesemedicaldevices be monitored for potential effects of EMI disturbances, and reported when appropriate.
Installation
Installations of the RI Witness Embryology Heated plate should be carried out by a RI technician or other RI authorised personnel. Incorrect installation could result in overall poor performance.
1 |
2 |

|
|
|
Section 3 |
|
|
||
Research Instruments Ltd |
Safety Warnings |
|
|
||||
|
|
|
|
|
|
|
|
SECTION3-SAFETY WARNINGS |
|
|
|
|
|
||
|
|
|
|
|
|||
|
|
This symbol indicates cautionary text which should be followed to avoid injury to users |
|
|
|
|
|
|
|
|
|
|
|
||
|
|
or damage to samples. |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
The system should be operated by qualified and trained personnel only. |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
3 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|||
|
|
DO NOT disassemble or modify any part of the RI Witness Embryology Heated plate, or |
|
|
|
||
|
|
substitute any component for any other. Doing so may result in damage to samples. This |
|
|
|
||
|
|
voids the warranty and/or service contract. |
|
|
|
|
|
|
|
|
|
|
|
||
|
|
|
|
|
|||
|
|
ONLY use the power cable and power supply adaptor supplied with the system. |
|
|
|
|
|
|
|
The cable to the power supply is the ‘disconnect device’ for this equipment. To remove all |
|
|
|
|
|
|
|
|
|
|
|
||
|
|
electrical power from this product, disconnect the power cable from the electrical outlet. |
|
|
|
||
|
|
Equipmentshouldbepositionedsoastoalloweasyaccesstothepowercable.Theappliance |
|
|
|
||
|
|
coupler or mains plug is used as the disconnect and must remain readily operable. |
|
|
|
||
|
|
|
|
|
|
||
|
|
|
|
|
|||
|
|
WARNING To avoid the risk of electric shock, this equipment must only be connected |
|
|
|
|
|
|
|
|
|
|
|
||
|
|
to a supply mains with protective earth. |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
WARNING Not to be used in a patient environment. |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|||
|
|
WARNING Refer to Guidance and Manufacturer’s Declaration Tables in this section of the |
|
|
|
|
|
|
|
|
|
|
|
||
|
|
User Manual for guidance on the environment suitable for this device. |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
||
|
|
|
|
|
|||
|
|
WARNING The temperature of the plate should not be more than 1.5ºC from the |
|
|
|
|
|
|
|
displayed temperature at any time. A temperature of more than 1.5ºC will cause the |
|
|
|
|
|
|
|
temperature inside the dish to change more rapidly and samples are at risk of over- |
|
|
|
|
|
|
|
heating. In this instance samples should be removed from the plate immediately. |
|
|
|
||
|
|
We recommend the plate temperature be monitored periodically using a calibrated |
|
|
|
||
|
|
thermocouple thermometer. |
|
|
|
|
|
|
|
|
|
|
|
||
|
|
|
|
|
|||
|
|
WARNING Use of this equipment adjacent to or stacked with other equipment should |
|
|
|
|
|
|
|
be avoided because it could result in improper operation. If such use is necessary, this |
|
|
|
|
|
|
|
equipment and the other equipment should be observed to verify that they are operating |
|
|
|
|
|
|
|
normally. |
|
|
|
|
|
|
|
|
|
|
|
||
|
|
|
|
|
|||
|
|
WARNING There are no replaceable parts supplied with this device. Should any parts |
|
|
|
|
|
|
|
|
|
|
|
||
|
|
need to be replaced, contact RI or your distributor. |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
||
|
|
|
|
|
|||
|
|
WARNING Use of accessories, transducers and cables other than those specified or |
|
|
|
|
|
|
|
provided by the manufacturer of this equipment could result in increased electromagnetic |
|
|
|
|
|
|
|
emissions or decreased electromagnetic immunity of this equipment and result in |
|
|
|
|
|
|
|
improper operation. |
|
|
|
|
|
|
|
|
|
|
|
||
|
|
|
|
|
|||
|
|
WARNING Portable RF communications equipment (including peripherals such as antenna |
|
|
|
|
|
|
|
cables and external antennas) should be used no closer than 30 cm (12 inches) to any |
|
|
|
|
|
|
|
part of the Embryology Heated Plate, including cables specified by the manufacturer. |
|
|
|
|
|
|
|
Otherwise, degradation of the performance of this equipment could result. |
|
|
|
|
|
|
|
|
|
|
|
|
|
3
Section 3 |
|
|
Section 3 |
Safety Warnings |
Research Instruments Ltd |
Research Instruments Ltd |
Safety Warnings |
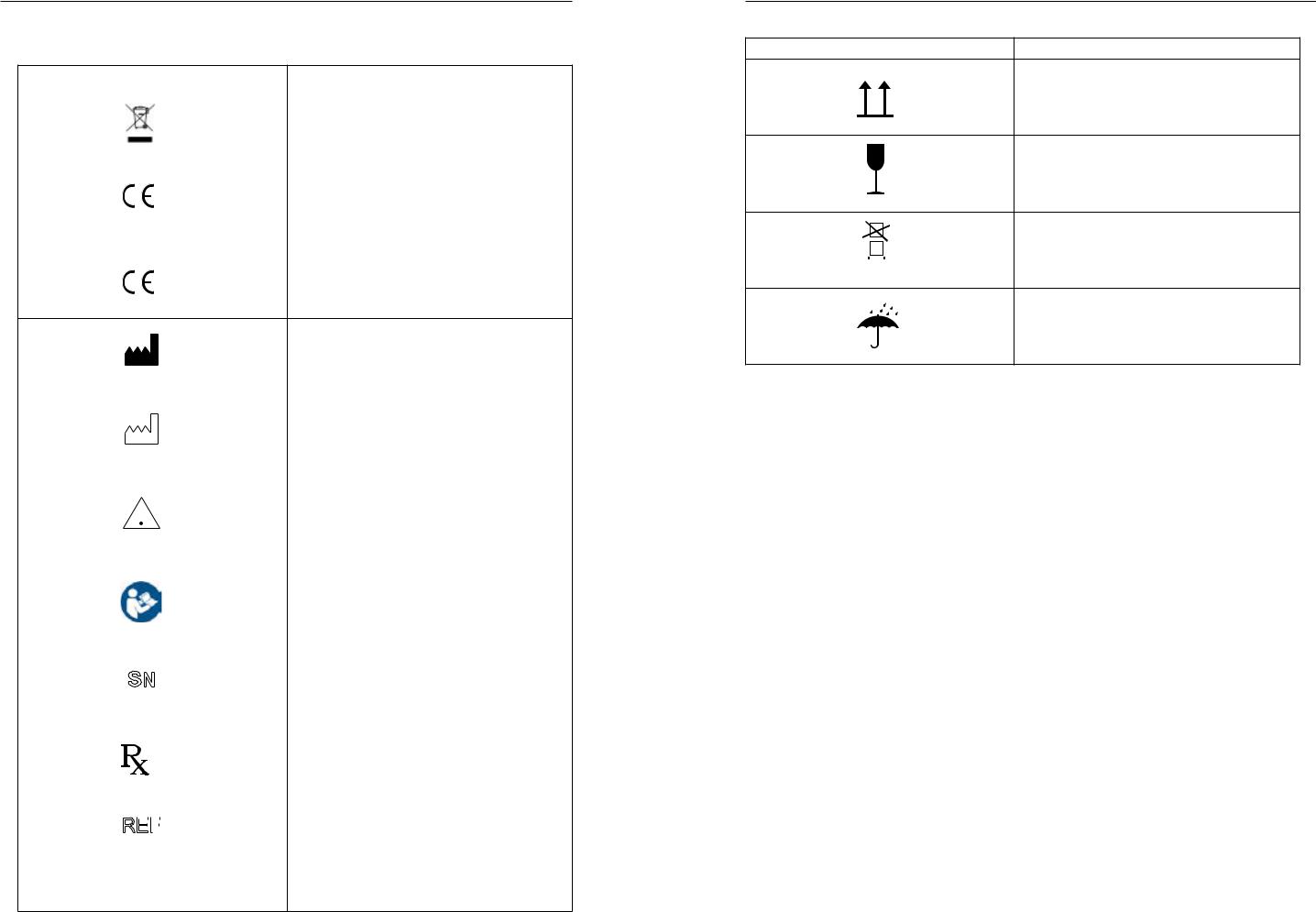
Glossary of Safety/Information Symbols
Source: ISO 15223-1
BS EN 60601-1
|
|
Symbol |
Meaning |
|
|
|
Do not dispose of product with normal waste |
|
|
|
Disposal of according to EU WEEE Directive |
|
|
|
|
|
3 |
|
In accordance with Annex II of the European |
|
|
|
Medical Device Directive 93/42/EEC, as amended |
|
0120 |
by Directive 2007/47/EC under the supervision of |
|
|
notified body No.0120, SGS, UK Ltd. |
||
|
|||
|
|
|
|
|
|
|
|
|
|
|
In accordance with Radio Equipment Directive |
|
|
|
(RED) 2014/53/EU |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Indicates the medical device manufacturer |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Indicates the date of manufacture |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Indicates the need for the user to consult the |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
instructions for use for important cautionary |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
informationsuchaswarningsandprecautionsthat |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
cannot, for a variety of reasons, be presented on |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
the medical device itself. |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Consult instructions for use |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Thefivedigitnumberisauniqueidentifierassigned |
|
|
SN |
|
|||||||||||
|
|
|
to the product |
|||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Caution: US Federal law restricts this device for |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
sale to or on the order of a licensed healthcare |
|
|
|
|
|
|
|
|
|
|
|
Only |
practitioner |
||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Indicates the reference number |
||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
REF |
|
|
|
|||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Direct current (DC) |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
4
Symbol |
Meaning |
This way up
|
Fragile, handle with care |
3 |
|
|
|
|
|
|
|
|
|
|
|
|
|
3 |
Stacking limited to 3 units |
|
|
|
|
|
|
Keep dry
5
 Loading...
Loading...