Page 1
Customer Service Information
United Kingdom
+44 0800 212 438 (free call)
Rayovac Europe Ltd,
Watermans House, Kingsbury Crescent,
The Causeway, Staines, Middlesex,
TW18 3BA, UNITED KINGDOM
www.remington.co.uk
Model No.
09/UK/ Version 0 /09 Part No.
REMINGTON
®
is a Registered Trade Mark of Spectrum Brands, Inc.,
or one of its subsidiaries
VARTA Consumer Batteries GmbH & Co. KGaA
Alfred-Krupp-Str. 9
73479 Ellwangen
Germany
www.remington-europe.com
© 2009 SBI
This product is not suitable for use in bath or shower.
IPL5000
IPL5000
6
T22-27289
www.remington-ilight.com
Page 2

IPL5000
Page 3
GB
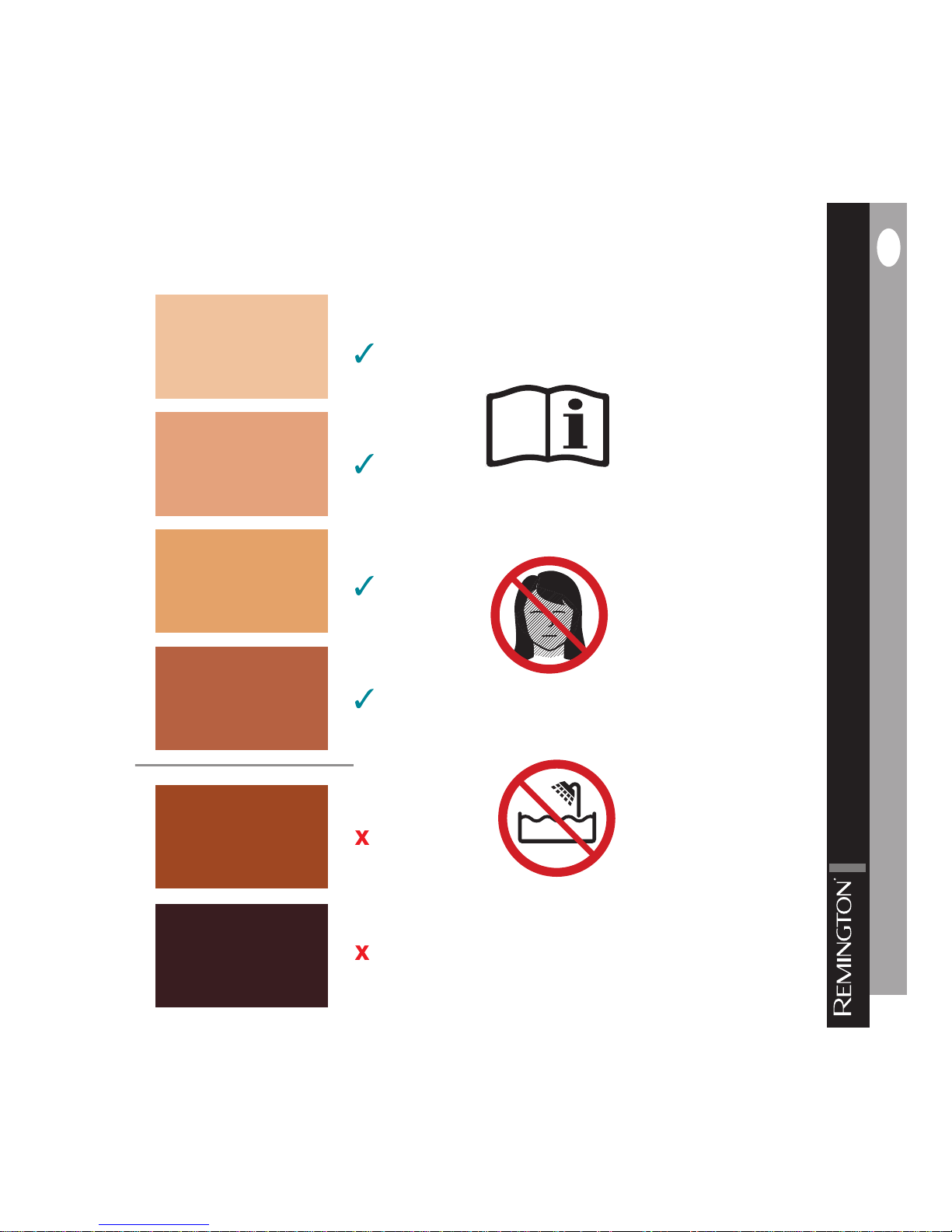
Skin Chart
Skin Chart
Do not use on the face.
Do not use near water.
Read instructions manual before use.
2
Page 4
3
1
]
Page 5

Getting Acquainted With i-Light
4
What is i-Light?
i-Light is a home-use device for the
of body hair using Intense
Pulsed Light (IPL) technology. This is the same technology used in professional
hair
remova
l salons and clinics. If used correctly it can provide long-lasting
hair reduction.
What is Intense Pulse Light (IPL) and how does the i-Light
work?
i-Light works by directing an extremely short, intense pulse of light into the skin.
The light energy is absorbed by the melanin in the hair follicle temporarily disabling the growth mechanism in the hair and delaying hair growth.
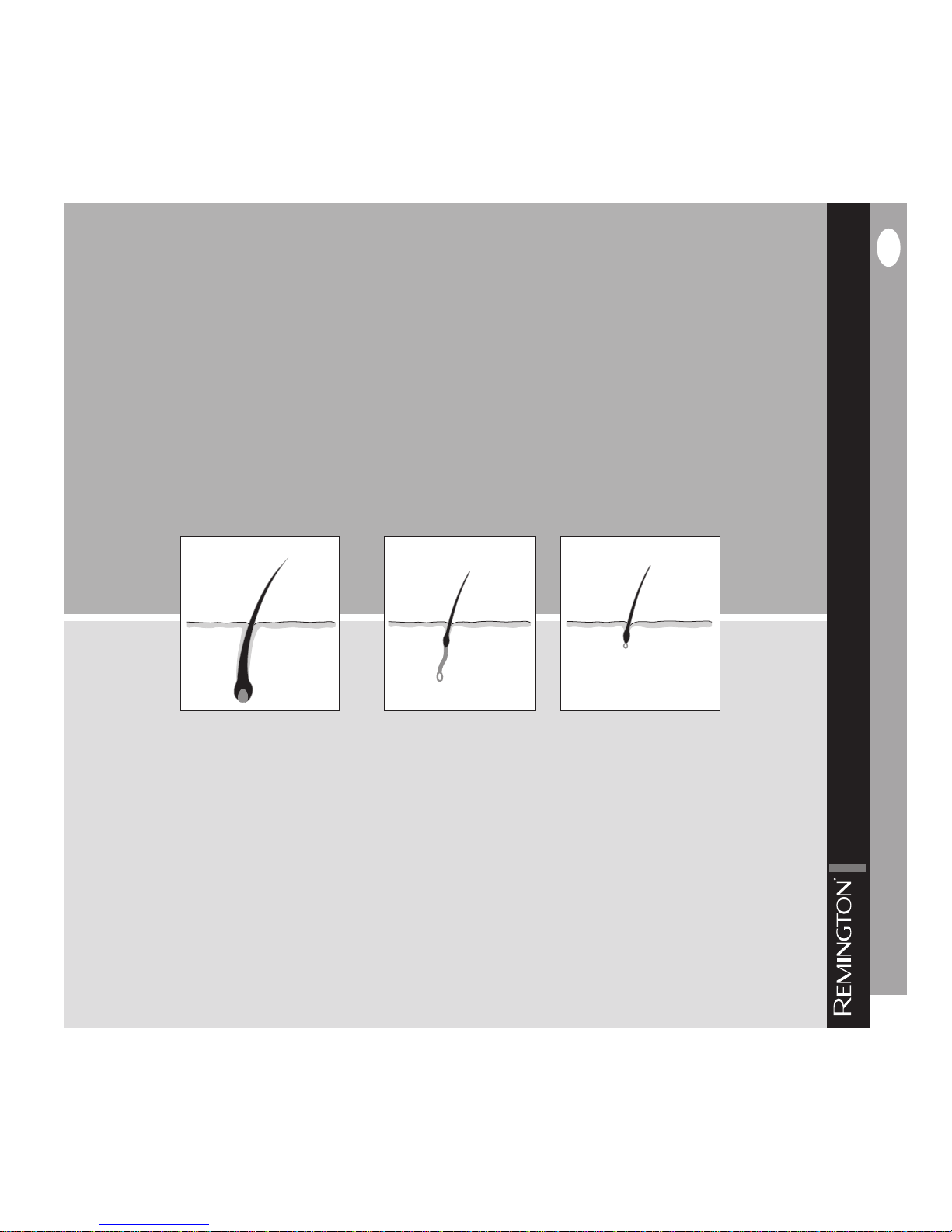
Hair follicles typically pass through three phases throughout the hair growth
cycle. These phases are:
Anagen Phase (growing phase) – the active growth phase of the hair follicles.
Melanin concentration is at its highest as it is responsible for pigmentation of the
hair. Only hairs in the anagen phase are susceptible to treatment with IPL (fig. 2).
Catagen Phase (degradation phase) – this is a short transition stage, which follows the anagen phase and signals the end of active growth of hair. It typically
lasts 2-3 weeks (fig. 3).
Telogen Phase (resting phase) – the hair follicle is completely at rest during this
phase, which is the longest phase and lasts about 100 days. During this time, the
new hairs push out the old hairs, allowing the growth cycle to begin again (fig. 4).
What to expect from i-Light
Our studies have shown significant hair reduction after only a single treatment.
However, optimal results are generally not obtained with a single session. For
best results, repeat a treatment session whenever you notice hair starting to
regrow. Results vary by individual, however, most will re-treat areas every two
weeks for three treatments until desired results are achieved.
A few days after the first treatment you may start to notice hair has fallen out.
Some hair growth will occur that is less dense, finer and lighter in color than the
original hair. This regrowth is perfectly normal and should be expected.
remova
l
Page 6
5
2
]
Anagen Phase
3
]
Catagen Phase
4
]
Telogen Phase
GB
Getting Acquainted With iLight
Page 7
6
Warnings and Safety Precautions
IMPORTANT SAFETY CAUTIONS
Before you start using i-Light:
Be sure to read all Warnings and Safety Information
Before you begin, check to see if i-Light is suitable for you.
Use the skin tone chart, provided on the box and at the front of this manual,
to determine if this device is right for you.
Skin Type
■ See skin color chart on page .
■ Do not use on naturally dark skin (Fitzpatrick type V and VI), as it may result in burns,
blisters and changes in skin color.
■ Do not use on tanned skin or after recent sun exposure, as it may cause burns or
skin injury.
■ i-Light is not effective on naturally white, gr y, blond or red body hair.
Areas not to treat
■ Do not use on the face or the neck.
■ Do not use on nipples, areola, or genitals.
■ Do not use if you have tattoos or permanent makeup in the area to be treated.
■ Do not use on dark brown or black spots such as moles, birth marks, or freckles.
■ Do not use on an area of recent surgery, deep peel, laser resurfacing, scars, or skin
that has been damaged with burns or scalds.
When not to use/When to avoid using i-Light.
■ Do not use if you are pregnant or breast feeding.
■ Do not use if you were exposed to sun or artificial tanning within the past 4 weeks.
■ Do not use on dry or fragile skin caused by the use of chemical peels, glycolic peels,
Alpha Hydroxy Acids (AHAs).
■ Do not flash more than once on the same area as this may cause burns.
■ Do not use on the same area of your skin more than once a week.
■ Do not use for at least 14 days following microdermabrasion treatment.
■ Do not use if you are already undertaking permanent hair removal treatments.
e
2
Page 8

GB
7
Warnings and Safety Precautions
■ Do not use if you have a skin disease such as active skin cancer, if you have a
history of skin cancer or any other localized cancer in the areas to be treated, or if
you have pre-cancerous lesions or multiple atypical moles in the areas to be treated.
■ Do not use if you have epilepsy with flashlight sensitivity.
■ Do not use if you have a history of collagen disorder, including a history of keloid
scar formation or a history of poor wound healing.
■ Do not use if you have a history of vascular disorder, such as the presence of
varicose veins or vascular ectasia in the areas to be treated.
■ Do not use if your skin is sensitive to light and causes a rash or an allergic reaction.
If you are taking photosensitizing agents or medications, check the package insert
of the medicine. Never use the unit if it can cause photo-allergic reactions or phototoxic reactions, or if you should avoid sun while taking a medication.
■ Do not use if you have diabetes, lupus erythematodes, porphyria or congestive heart
disease.
■ Do not use on areas of your skin which are currently being treated with or have
recently been treated with Alpha Hydroxy Acids (AHAs), Beta Hydroxy Acids (BHAs),
topical isotretinoin and azelaic acid.
■ Do not use if you have taken oral isotretinoin Accutane or Roaccutane in the last
six months. This treatment can make skin more susceptible to tears, wounds and
irritations.
■ Do not use if you have any bleeding disorder or take anticoagulation medications,
including heavy use of aspirin, in a manner which does not allow for a minimum
1-week washout period prior to each treatment.
■ Do not use if you have infections, eczema, burns, inflamed follicles, open lacerations,
abrasions, surgeries, herpes simplex, wounds or lesions and haematomas in the
areas to be treated.
■ Do not use if you have a history of immunosuppressive disease (including HIV
infection or AIDS) or if you take immunosuppressive medications.
■ Do not use when you are on painkillers, which reduce the sensitivity to heat.
■ Do not use if you use long-lasting deodorants. This can result in skin reactions.
■ Do not use over or near anything artificial such as silicon implants, Implanon
contraceptive implants, pacemakers, subcutaneous injection ports (insulin dispenser)
or piercings.
Page 9

8
Warnings and Safety Precautions
As with most electrical appliances, electrical parts are electrically live even when the
switch is off. To reduce the risk of injury or death by electric shock:
■ Always unplug the unit from the electrical outlet immediately after using.
■ Do not use near water.
■ Do not place or store this appliance where it can fall or be pulled into a tub or sink.
■ Do not place or drop into water or other liquid.
■ Do not reach for the unit if it has fallen into water or other liquid. Unplug unit
immediately.
■ Do not reach for the unit if it has become wet. Unplug unit immediately.
■ Unplug the unit before cleaning it.
■ Keep the unit dry at all times.
■ If you move the unit from a very cold to a very warm environment, wait approximately
2 hours before using it.
■
An appliance should never be left unattended when plugged into a power outlet.
■
Keep the po wer plug and cord a way from heated surfaces.
■
Make sure the power plug and cord do not get wet.
■
Do not plug or unplug the unit with wet hands.
■
Do not use the product with a damaged
cord. A replacement can be obtained
via the
Remington
®
Service Centre.
■
Use and store the product at a temperature between 15°C and 35°C.
■
Always unplug from the mains when cleaning.
■
Only use the parts supplied with the appliance.
■
Keep this product out of reach of children. The use of this appliance by persons with
reduced physical, sensory or mental capabilities or lack of experience and knowledge
can give cause to hazards. Persons responsible for their safety should giv e explicit
instructions or supervise the use of the appliance.
IMPORTANT SAFETY INSTRUCTIONS
WARNING – TO REDUCE THE RISK OF BURNS, ELECTROCUTION,
FIRE, OR INJURY TO PERSONS:
power
Page 10

GB
9
Preparing for Use
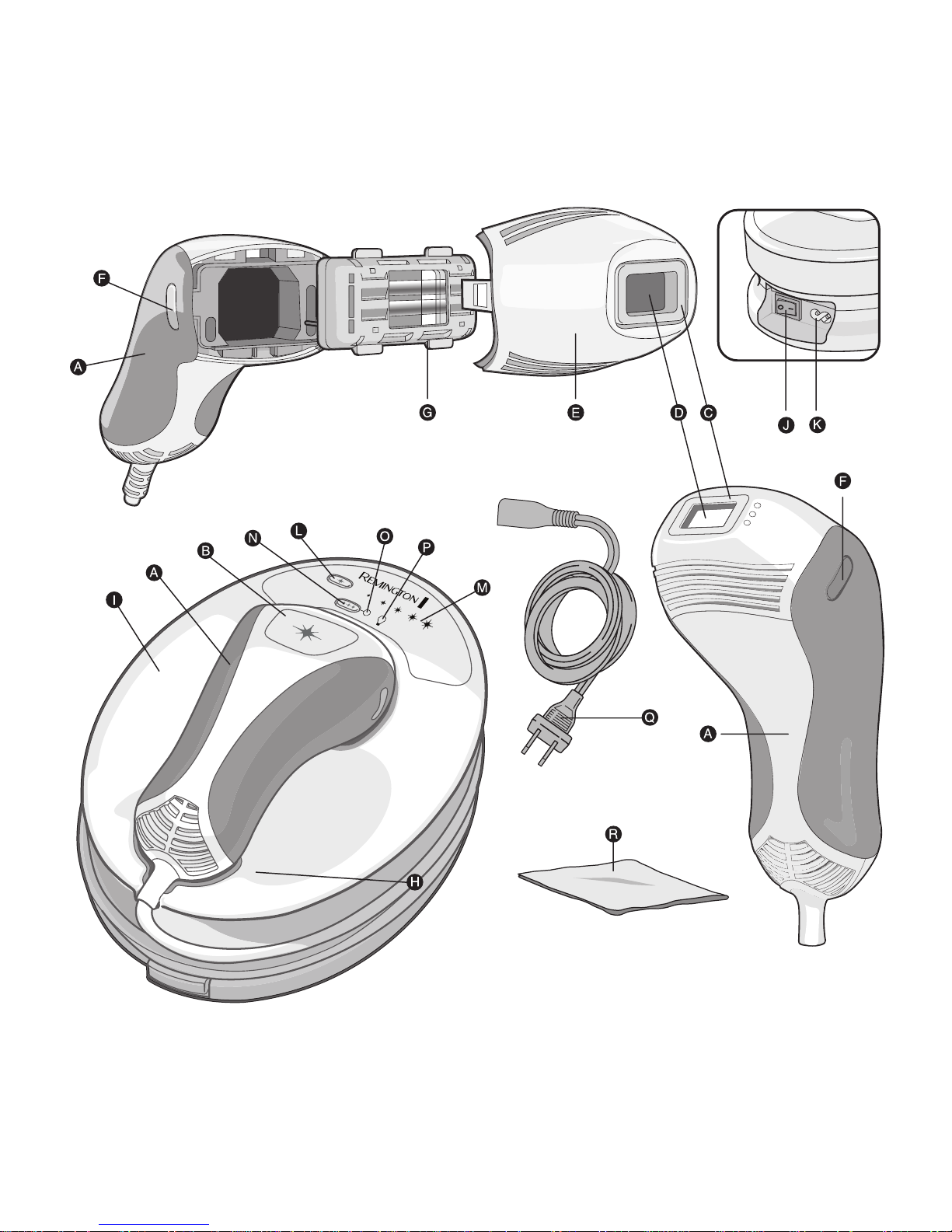
i-Light Unit Diagram Reference (page 3)
Familiarize yourself with the features of your new i-Light device.
Flash Window (fig. 1-D)
The Flash Window is a filtered glass window with built-in UV protection that
allows specific wavelengths of light to pass from the hand piece to your skin
and hair follicles.
WARNING: Always inspect the Flash Window before use to ensure there is no dam-
age to the lens.
WARNING: Always clean the Flash Window before use with the lint-free cloth pro-
vided to ensure there is no oil or debris on the lens.
Skin Contact Sensor
(fig. 1-C)
The Skin Contact Sensor is a safety mechanism that prevents the device
from accidental activation. In order for the device to activate, the Skin
Contact Sensor must be fully depressed against the skin.
A
i-Light Handpiece
B
Flash Button
C
Skin Contact Sensor
D
Flash Window
E
Nose Cone
F
Nose Cone Release Buttons
G
Light Cartridge
H
Handpiece Cord
I
i-Light Base Unit
J
Power Switch
K
Power Inlet
L
Intensity Level Selection Button
M
Intensity Level Selection Display
N
Flash Mode Selection
O
Flash Mode Selection Display
P
Bulb Status Indicator Display
Q
Power Cord
R
Lint Free Cloth
Page 11

Getting Acquainted With i-Light
10
Flash Button (fig. 1-B)
The Flash Button is located on the hand piece. To activate the flash bulb,
ensure the Skin Contact Sensor is fully engaged and press the Flash Button.
Bulb Status Indicator Display
(fig. 1-P)
The device is ready to flash when the Bulb Status Indicator Display is
illuminated green.
NOTE:
If the Flash Button is pressed AND the Skin Contact Sensor is not fully engaged OR
the Bulb Status Indicator Display is not illuminated, an audible “beep” will sound.
When the Bulb Status Indicator Display illuminates yellow, there are 150
flashes remaining in the flash bulb. When the Bulb Status Indicator Display
flashes yellow, the bulb cartridge has been used up and will no longer
operate. You must replace the bulb cartridge to continue using the device.
Nose Cone Release Buttons
(fig. 1-F)
Press both buttons and gently pull to remove the nose cone.
WARNING: AL WAYS ensure the unit is OFF and the pow er cord is disconnected
before removing the nose cone. If the nose cone is removed while the
unit is po wered ON, all indicator lights on the base unit will flash and
audible “beeps” will sound.
Preparing for Use
When the power switch is turned ON, the system will perform a self-test to ensure
the device is functioning properly. This test will only take 1-2 seconds. When the test
has completed, you may notice a quick flash from the output window.
This is completely normal and indicates the device is ready to use.
When in use, this device can draw a moderate current level. Remington® recommends
that you avoid using this product on the same electrical circuit at the same time as
other high current draw devices (such as a hair dryer). Doing so could result in a
blown fuse or tripped circuit breaker.
Page 12

GB
11
Getting Acquainted With i-Light
Light Cartridge (fig. 1-G)
Each light cartridge has a life of 1,500 flashes. When all flashes have been
used, the cartridge must be replaced.
Replacement model: SP-IPL
Intensity Level Selection
(fig. 1-L)
The i-Light device is equipped with 5 intensity levels. Level 1 is the lowest setting and level 5 is the highest setting.
TIP: For the most eff ective results, alwa ys use the highest intensity level that does not
cause discomfort on the skin. To determine the intensity level being used, observe
the number of lights illuminated on the Intensity Level Selection Display.
Your i-Light device will automatically be set to Intensity Level 1 each time the
device is powered ON. To change the level, press the Intensity Level Selection
Button.
Flash Mode Selection (fig. 1-N)
The i-Light device is equipped with two operating modes: Single Flash Mode
and Multi-Flash Mode. Your i-Light device will automatically be set to Single
Flash Mode each time the device is powered ON.
Single Flash Mode: The i-Light device will flash once when the Flash Button is
pressed AND the skin contact sensor is engaged.
Multi-Flash Mode: The i-Light device will flash once ev ery two seconds when the
Flash Button is pressed AND the skin contact sensor is fully engaged.
The Multi-Flash Mode allows you to quickly treat large areas such as the legs, chest
or back by simply gliding the hand piece to a new location after each flash.
To turn Multi-Flash Mode ON, press the Multi-Flash Mode Selector Button.
The Multi-Flash Mode Selector Display will illuminate. Press again to turn
Multi-Flash Mode OFF and return to Single-Flash Mode.
Page 13

12
Getting Acquainted With i-Light
7
]
Test the i-Light device on your skin (fig. 5)
1. Review the Warnings and Safety Precautions.
2. Familiarize yourself with the features of the i-Light device.
3. Consult the Skin Tone Chart to ensure your skin color is in the
acceptable range.
4.
Test the i-Light device on a small patch of skin and wait 48
hours to ensure there are no adverse reations.
Treat desired area(s) with the i-Light device
Prepare your skin for treatment
1.
Ensure the area to be treated is clean and free from oils,
deodorants, perfume, make up, lotions and creams. Shave the
hair from the area to be treated.
2.
Never use wax, epilation, tweezers, or depilation products to
remove the hair because they counteract the IPL process.
Prepare the device for treatment
3.
Unwrap the hand piece cord from the i-Light base and rest
the hand piece in the base cradle. Locate the power switch
on the i-Light base and ensure the unit is OFF. Connect the
power cord to the i-Light base at the power inlet. Connect the
power cord to the wall outlet. Turn the power switch ON
(fig 6).
4.
Select the desired Intensity Level. If the Multi-Flash Mode is
desired, turn ON using the Flash Mode Selector Button (fig 7).
48h
5
]
6
]
Page 14

GB
13
Getting Acquainted With i-Light
Treat desired area(s) with the i-Light device –
Single Flash Mode
1.
Place the i-Light hand piece against your skin so the Flash
Window is flush with the skin surface (fig. 8).
2.
Ensure the Skin Contact Sensor is fully engaged and the Bulb
Status Indicator Display is illuminated.
3.
Press the Flash Button to activate the device (fig. 9).
4. Move the hand piece to a new treatment area and repeat
steps 1-3 (fig. 10).
Treat desired area(s) with the i-Light device –
Multi Flash Mode
1.
Place the i-Light hand piece against your skin so the Flash
Window is flush with the skin surface (fig. 8).
2.
Ensure the Skin Contact Sensor is fully engaged and the Bulb
Status Indicator Display is illuminated.
3.
Press AND HOLD the Flash Button down to activate the
device (fig. 9).
4.
Immediately after the device has flashed, slide the hand
piece to a new location. After a short delay (approximately 2
seconds) the device will flash again (fig. 10).
NOTE:
■ During Multi-Flash Mode, the Skin Contact Sensor
AND the Flash Button must remain fully depressed. If
either becomes disengaged, the device will not operate. To resume treatment, repeat steps 1-4.
■ During Multi-Flash Mode, the Bulb Status Indicator
Display will remain illuminated while the Skin Contact
Sensor and Flash Button are engaged.
8
]
9
]
10
]
Page 15

14
Post Treatment Care / Treatment Tips
Treatment Tips
■ For best results, avoid overlapping flashes. This helps prevent exposure to
more energy than is necessary to suppress hair growth. It also ensures that
you get the maximum use of the light cartridge.
■ For the most effective results, always use the highest intensity level that does
not cause discomfort on the skin. The level you use should feel warm on your
skin, but should never cause discomfort.
■ Optimal results are generally not obtained with a single session. For best
results, repeat a treatment session whenever you notice hair starting to
regrow. Results vary by individual, however, most people will treat areas every
two weeks for the first three treatments, until desired results are achieved.
■ You may notice that bony areas, such as elbows, shins, and ankles, are more
sensitive during treatment. This is normal and should not be cause for alarm.
To avoid this sensitivity, try stretching the skin away from the bony area
during treatment.
Post-Treatment Care
After treatment, you may experience slight redness or a warm sensation on your
skin. This is normal and will disappear quickly. To avoid irritation to your skin
after a treatment, take the following precautions:
■ Avoid sun exposure for 24 hours after a treatment. Protect the skin with SPF
30 for 2 weeks after each treatment.
■ After treatment, keep the area clean and dry and drink plenty of water to
keep skin hydrated.
■ Do not handle the treated area roughly.
■ Do not take hot baths, showers, or use steam rooms and saunas for 24 hours
after treatment.
■ Do not swim for 24 hours after treatment.
■ Do not take part in contact sports for 24 hours after treatment.
■ Do not wear tight-fitting clothing over the treated area.
■ Do not prolong sun exposure such as sunbathing, using a tanning bed, or self-
tan for at least 2 weeks after the last treatment.
Page 16

GB
15
Cleaning
Your Device
■ Do not depilate (waxing, plucking, threading or creams) during the treatment
– shaving is acceptable as long as you avoid shaving 24 hours after each
treatment.
■ Do not use bleaching creams or perfumed products for 24 hours after
treatment.
■ Do not scratch or pick at the treated area.
Cleaning Your i-Light Device
CAUTION: Before cleaning your i-Light device, make sure that the power switch is OFF and the
power cord is disconnected from the base unit.
■ Regular cleaning helps to ensure optimal results and a long life for the i-Light
device. The exterior surface of the base unit and hand piece may be wiped
clean with a slightly damp cloth.
■ To clean the Flash Window, use only the lint-free cloth included with your
i-Light device. Take care not to scratch or chip the Flash Window. Scratches
and chips can reduce the effectiveness of the unit.
■ For stubborn stains, use a dampened cotton swab to apply a small amount
of water to the Flash Window and clean with the lint-free cloth provided.
■ Use a small hand-held vacuum to remove dust and debris from the hand
piece vents.
WARNING: If the Flash Window is cracked or broken, the unit must not be used.
Nev er scratch the filter glass or the metallic surface inside the Nose
Cone.
CAUTION: The i-Light is a high voltage device . Never immerse in w ater . Ne v er clean
the unit or any of its parts under the tap or in the dishwasher .
Do not use petroleum-based or flammable cleaning agents because of
the risk of fire. Nev er use scouring pads , abrasive cleaning agents or
aggressive liquids such as oil or acetone to clean the unit.
Page 17

16
Troubleshooting / Storage / Maintenance
i-Light Device Maintenance
CAUTION:
Before performing maintenance on your i-Light de vice , ensure that the power
switch is OFF and the power cord is disconnected from the base unit.
Replacing the bulb
1.
Press the nose cone release buttons and gently pull to remove the nose cone.
2. Gently pull out the old bulb cartridge.
3. Replace with a new bulb cartridge. Replacement model: SP-IPL
CAUTION: When replacing the bulb cartridge, do not touch the flash bulbs directly as
this leaves oils and residue. Doing so could reduce the effectiveness of
the bulbs or cause them to crack during treatment.
4.
Replace the nose cone, making sure it snaps into place.
Storage
■ Switch off the unit, unplug it and let it cool down for 10 minutes before storage.
■ Store the unit in a dry place at a temperature between 15º C and 35º C.
Troubleshooting
Always read these instructions fully before using i-Light.
Refer to this troubleshooting guide if you experience any problems with i-Light,
as this section addresses the most common problems you could encounter with
i-Light.
If you have followed the instructions in this section and continue to experience
problems, please contact the Remington
®
Service Center for further assistance.
I turn the power switch ON, but the unit is not working.
■ Make sure the unit is plugged into a working electrical outlet.
■ Try switching to a different outlet.
The unit appears to have cracks or is broken.
■ Do not use if the unit is damaged. If you have concerns about using the unit,
discontinue use and contact the Remington
®
Service Center for further assistance.
Page 18

GB
17
Frequently Asked Questions
I have switched the unit ON, but I cannot increase or decrease the light intensity.
■ Try resetting the unit by turning it off and waiting several seconds before turning it
back on.
The Bulb Status Indicator Light turns green but the unit does not flash when the
button is pressed.
■ Make sure the Skin Contact Sensor is in full contact with the skin.
■ Try resetting the unit by turning it off and waiting several seconds before turning it
back on.
There is a strange smell.
■ Be sure the area is completely shaved before treatment.
The treated areas become red after treatment.
■ This is normal and the redness should subside. If not, try using a lower light intensity.
I have not seen optimal results or hair has begun to grow back.
■ Hair may begin to grow back after your initial treatment. This is perfectly normal. For
optimal results, repeat the treatment when you notice hair regrowth.
Intensity/Flash Mode LED
All currently selected
LEDs flashing
Intensity LEDs flashing
i
n sequenc
e
All LEDs flashing with
warning beep
Indication
U
nit is overheated and is disabled momentarily to cool down.
Unit is malfunctioning. Turn the unit off, wait a few moments and try
again. If problem persists, the unit should be returned for repair.
Nose cone is removed or loose.
Audible Tones
Not Full Contact
Replace Light Cartridge
Contact Sensors Stuck
Indication
Flash button was pressed while the skin contact sensor was not
fully engaged.
Light cartridge is missing or needs to be replaced.
Flash button was pressed after the contact sensor had been
engaged since unit start up. Contact sensor may be stuck.
Page 19

18
Frequently Asked Questions
Q. What areas of my body can I treat with i-Light?
A. i-Light is designed to be used on areas below the neck, including the legs,
underarms, bikini line, arms, chest and back.
Q. What can I expect from i-Light?
A.
i-Light provides safe and effective salon-grade hair removal using IPL
technology.
Q. What are the risks involved with i-Light? Is it safe?
A. i-Light is safe to use, but like any electronic device it is important that you
read and follow the operating instructions.
Q. How often should I use i-Light?
A. You should use i-Light whenever you start to see hair regrowth.
Q. How long do treatments take?
A. Time varies based on the size of the area being treated, but one full leg
should take no more than 15 minutes.
Q. When will I begin to see results?
A. Results are not immediate. Hair may sometimes appear to be growing back
after treatment but many of these hairs will begin to fall out after two weeks.
Hair grows in a cycle of 3 different phases which lasts 18-24 months. Only
-
Q. What is i-Light? What is Intense Pulse Light (IPL)?
A. IPL works by directing an extremely short, intense pulse of filtered light into
the skin. The light is absorbed by the colored pigments in and surrounding
the hair and disables the hair follicle temporarily, preventing hair regrowth.
Q. Who can use i-Light?
A. Both men and women can use i-Light to remove unwanted hair anywhere
below the neck. i-Light has been designed for individuals with light to medium skin tones and dark hair. Safe skin tones include white, ivory, tan, beige,
and light brown only. Safe natural hair colors include black, dark brown, and
medium brown.
(www.remington-ilight.com)
Frequently Asked Questions
Page 20

GB
19
Frequently Asked Questions
Q. What are the warnings against using i-Light?
A.
Certain conditions may limit your ability to use the unit. Please read the
Warnings and Safety Precautions section in the User Manual in its entirety
before using i-Light.
Q. How often do I need to replace the bulb?
A.
The bulb needs to be replaced after 1500 flashes. The Bulb Status Indicator
Light will illuminate green for the first 1350 flashes of the cartridge life. It will
turn to a yellow light to designate that only 150 flashes remain. When the
light begins to flash the cartridge life is up and you must replace the bulb.
Replacement model: SP-IPL
Q. Can I use i-Light on my face?
A.
No. i-Light is not recommended for use on the face or the neck.
Q. How do I care for treated areas following treatment?
A.
Avoid unprotected sun exposure to the treated areas.
Q. Should I suspend normal activity after using i-Light?
A. There is no need to suspend normal activity following treatment assuming
no abnormal complications occur. It is recommended that you perform the
treatment prior to going to bed so that any resulting redness fades by morning.
Q. Why is my hair growing despite treatments?
A. Hair continues to grow for up to 2 weeks after treatment, at which time you
will notice the hair beginning to fall out. Another reason for continued growth
could be that the area was missed during a treatment. Continue to treat the
area whenever you notice regrowth.
Q. Why can’t I use i-Light after recent sun exposure?
A. Sun exposure causes high levels of melanin to be present and exposes the
skin to higher risk of burns or blisters following treatment.
hairs in the anagen phase are susceptible to treatment, which is why multiple
treatments are required for optimal results. The results are generally notice
able within a few weeks of the first treatment. Continuous regular weekly (or
every 2 weeks for three treatments) use will give good results within 6 to 12
weeks (darker skin may take longer).
Page 21

20
Frequently Asked Questions
Q. Can I use i-Light if I have blonde, red, gray or white hairs?
A. i-Light works best on darker hair types because they contain more mela-
nin, the pigment that gives hair and skin its color. Melanin is what absorbs
the light energy used during i-Light treatment. Black and dark brown hairs
respond the best. Brown and light brown hairs will also respond, but typically
require more treatments. Red hairs may show some response. White, grey or
blonde hairs usually don’t respond to i-Light treatments, although some users
have noted results after multiple treatments.
Q. Can I use i-Light if I have naturally dark skin?
A. No. i-Light is designed to react with the dark pigment of the hair. As a result,
dark brown and black skin may absorb too much of the device’s energy
(heat), which may cause skin damage. Do not use i-Light on naturally dark
skin, as it contains too much melanin. Treating dark skin with i-Light can
result in burns, blisters and skin color changes (hyper- or hypo-pigmentation).
Review the skin color chart on page to determine if i-Light is right for you.
Q. Do I need eye protection while using i-Light?
A. No, it is not harmful to the eyes, unless it is directed to the face. i-Light fea-
tures a safety system which prevents unintentional flashing when the device
is not in contact with the skin. The small amount of light emitted during treatment is similar to that of a camera flash and is not harmful to the eyes, unless
it is directed to the face.
Q. Can I use i-Light if I am pregnant or nursing?
A. No. i-Light has not been tested on pregnant women, therefore we do not
recommend using i-Light if you are pregnant or breast feeding. Hormonal
changes could increase sensitivity and the risk of injury to the
skin.
2
Q. How often should I treat with i-Light?
A. An interval of 2 weeks for the initial treatment, is proven to be the most
effective in hair reduction. You should avoid treating the same area multiple
times in one session, as it will not improve efficacy but increases the risk of
skin irritation.
Q. Is i-Light dangerous for the skin after long term use?
A. There have not been any reported side effects or skin damage from long-
term use of intense pulse light.
Page 22

GB
21
Applicable Skin Tones, Skin tone chart –
Fitzpatrick skin types 1 - 4
You can use this skin-type chart for self-assessment, by adding up the score for
each of the questions you’ve answered. At the end there is a scale providing a
range for each of the six skin-type categories. Following the scale is an explanation of each of the skin types. You can quickly and easily determine which skin
type you are.
Genetic Disposition
Total score for Genetic Disposition: _____
Score 0 1 2 3 4
What is the
colour of
your eyes?
Light blue,
Grey,
Green
Blue, Grey
or Green
Blue Dark
Brown
Brownish
Black
What is
the natural
colour of
your hair?
Sandy Red Blond Chestnut
Dark
Blond
Dark
Brown
Black
What is
the colour
of your
skin (non
exposed
areas)?
Reddish Vary Pale Pale with
Beige tint
Light
Brown
Dark
Brown
Do you
have
freckles on
unexposed
areas?
Many Several Few Incidental None
The Fitzpatrick Skin-Type Chart
Page 23

22
The Fitzpatrick Skin-Type Chart
Reaction to Sun Exposure
Score 0 1 2 3 4
What
happens
when you
stay in the
sun too
long?
Painful
redness,
blistering,
peeling
Blistering
followed
by peeling
Burns
sometimes
followed
by peeling
Rare burns Never
had
burns
To what
degree do
you turn
brown?
Hardly or
not at all
Light color,
tan
Reasonable
tan
Tan very
easily
Turn dark
brown
quickly
Do you
turn brown
within
several
hours
after sun
exposure?
Never Seldom
Sometimes
Often Always
How does
your face
react to the
sun?
Very
sensitive
Sensitive Normal Very
reisitant
Never
had a
problem
Total score for Reaction to Sun Exposure: _____
Page 24

GB
23
The Fitzpatrick Skin-Type Chart
Tanning Habits
Score 0 1 2 3 4
When did
you last
expose your
body to sun
(or artificial
sunlamp/
tanning
cream)?
More than
3 months
ago
2-3
months
ago
1-2
months
ago
Less than
a month
ago
Less than
2 weeks
ago
Did you
expose the
area to be
treated to
the sun?
Never Hardly
ever
Sometimes
Often Always
Total score for Tanning Habits: _____
Add up the total scores for each of the three sections for your Skin Type Score.
Page 25

24
The Fitzpatrick Skin-Type Chart
Skin Type Score - Fitzpatrick Skin Type
TYPE 1: Highly sensitive, always burns, never tans.
Example: Red hair with freckles
TYPE 2: Very sun sensitive, burns easily, tans minimally.
Example: Fair skinned, fair haired Caucasians
TYPE 3: Sun sensitive skin, sometimes burns, slowly tans to light brown.
Example: Darker Caucasians
TYPE 4: Minimally sun sensitive, burns minimally, always tans to moderate
brown.
Example: Mediterranian type Caucasians, some Hispanics
TYPE 5: Sun insensitive skin, rarely burns, tans well.
Example: Some Hispanics, some Blacks
TYPE 6: Sun insensitive, never burns, deeply pigmented.
Example: Darker Blacks
0-7 I
8-16 II
17-25 III
25-30 IV
over 30 V-VI
Do Not Use i-Light
Page 26

GB
25
Disposal / Service and Warranty
PROTECT THE ENVIRONMENT
Do not dispose the product in household waste at the end of its useful lif e.
Disposal can tak e place at the Remington
®
Service Centre or appropriate
collection sites.
For further information on recycling see www.remington-europe.com
SERVICE & WARRANTY
This product has been checked and is free of defects. We warrant this product
against any defects that are due to the f aulty material or workmanship for a 2
year period from the original date of consumer purchase. If the product should
become defective within the w arranty period, we will repair any such defect or
elect to replace the product or any part of it without charge provided there is
proof of purchase . This does not mean an extension of the warr anty period.
In the case of a warranty simply call the Remington
®
Service Centre in your
region.
This warranty is off ered ov er and above your normal statutory rights .
The warranty shall apply in all countries in which our product was sold via an
authorised dealer.
This warranty does not include replacement bulbs which are consumable parts .
Also, not covered is damage to the product by accident or misuse, abuse, alteration to the product or use inconsistent with the technical and/or safety instructions
required.
This warranty shall not apply if the product has been dismantled or repaired by a
person not authorised by us.
 Loading...
Loading...