Ramsey Electronics LEAD ACID BATTERY CHARGER KIT, LABC1 User Manual

LEAD ACID BATTERY
CHARGER KIT
Ramsey Electronics Model No. LABC1
An educational kit that will come in handy around the shop and
garage. Build your own charger instead of shelling out big bucks
for a “store bought” unit. You might learn more than you ever
wanted to know about batteries and battery charging. Amaze
your friends with your new found knowledge. Wait . . .is that
Regis on the phone?
• No more fried gel cells!
• Extends the life of your 12 volt lead acid batteries.
• Automatic ambient temperature compensation.
• Automatically adjusts charge voltage depending on battery status.
• Bright front panel charge indicator.
• Saves spending money on costly replacement batteries; pays for
itself in no time!
• Add our matching case and knob set for a professional appearance.
LABC1 • 1

RAMSEY TRANSMITTER KITS
• FM100B Professional FM Stereo Transmitter
• FM25B Synthesized Stereo FM Transmitter
• MR6 Model Rocket Tracking Transmitter
• TV6 Television Transmitter
• Cube Television Transmitters
RAMSEY RECEIVER KITS
• FR1 FM Broadcast Receiver
• AR1 Aircraft Band Receiver
• SR2 Shortwave Receiver
• SC1 Shortwave Converter
RAMSEY HOBBY KITS
• SG7 Personal Speed Radar
• SS70A Speech Scrambler
• BS1 “Bullshooter” Digital Voice Storage Unit
• AVS10 Automatic Sequential Video Switcher
• WCT20 Cable Wizard Cable Tracer
• ECG1 Heart Monitor Kit
• TFM3 Tri-Field Meter
RAMSEY AMATEUR RADIO KITS
• DDF1 Doppler Direction Finder
• HR Series HF All Mode Receivers
• QRP Series HF CW Transmitters
• CW7 CW Keyer
• CPO3 Code Practice Oscillator
• QRP Power Amplifiers
RAMSEY MINI-KITS
Many other kits are available for hobby, school, Scouts and just plain FUN. New
kits are always under development. Write or call for our free Ramsey catalog.
LABC1 KIT INSTRUCTION MANUAL
Ramsey Electronics publication No. MLABC1 Rev 1.2
First printing: November 2001
COPYRIGHT 2001 by Ramsey Electronics, Inc. 590 Fishers Station Drive, Victor, New York
14564. All rights reserved. No portion of this publication may be copied or duplicated without the
written permission of Ramsey Electronics, Inc. Printed in the United States of America.
LABC1 • 2

Ramsey Publication No. MLABC1
Price $5.00
KIT ASSEMBLY
AND INSTRUCTION MANUAL FOR
LEAD ACID BATTERY
CHARGER KIT
TABLE OF CONTENTS
Quick Battery Theory ..................4
Circuit Description .......................6
Schematic Diagram ....................8
Parts Layout Diagram .................9
Parts List ....................................10
Kit Assembly ...............................12
Custom Case Assembly .............15
Adjusting your LABC1 .................17
Safety Considerations .................18
Troubleshooting Guide ...............19
Warranty ..................................... 23
RAMSEY ELECTRONICS, INC.
590 Fishers Station Drive
Victor, New York 14564
Phone (585) 924-4560
Fax (585) 924-4555
www.ramseykits.com
LABC1 • 3

Quick Battery Theory
To begin, we should cover a few facts about lead acid batteries in general.
Most traditional historians date the invention of batteries to the early 1800’s
when experiments by Alessandro Volta generated electrical current from
chemical reactions between dissimilar metals. Volta’s original ‘voltaic pile’
consisted of zinc and silver disks separated by a porous nonconductive
material saturated with seawater. When stacked in a particular manner, a
voltage could be measured across each silver and zinc disk.
Other more radical thinkers, however, believe that lead acid battery
technology has been around since the early days of the Egyptian Pharaohs!
Whether they discovered the electro-chemical process on their own or if the
‘Space Aliens’ using their pyramids as an intergalactic spaceport taught them
still requires a bit more clarification. We’ll leave that one for you to follow up
on!
While advances in construction and materials have come a long way over the
years, the basic principles still apply. Lead acid cells of all types (‘Wet’ or
‘VRLA’ ) undergo a specific set of chemical reactions while charging and
discharging. They are also formed from similar types of active materials. For
the most part, lead acid batteries are made up of lead plates submerged in a
sulfuric acid solution. The positive electrode plates are formed from lead
dioxide (PbO
) while the negative electrodes are made of sponge metallic lead
2
(Pb). The porous nature of the lead plates allows the electrolyte, a dilute
mixture of 35% sulfuric acid and 65% water, to efficiently contact the
maximum surface area and obtain the most charge carriers. The electrolyte
solution provides the sulfate ions formed during the discharge chemical
reaction process giving us the electrons needed for current flow into the load.
One of the byproducts created during the discharge process of freeing sulfate
ions is lead sulfate (PbSO
). As the battery discharges, the lead sulfate
4
attaches to the electrode plates raising the internal resistance of the battery
which in turn lowers its working terminal voltage.
To determine the SOC (State Of Charge) of a lead acid battery, the classic
voltmeter approach does not work well. The terminal voltage will vary widely
between batteries as a function of things like ambient temperature and the
relative age of the battery. A full set of temperature profile tables would show
big differences in the open circuit terminal voltage over a wide temperature
range. This is why a good charger must incorporate a temperature
compensation network to avoid ‘over’ or ‘under’ charging the battery at
different ambient temperatures. To test a lead acid battery’s SOC, the best
indicator is a hydrometer. When you test a battery’s SOC with a hydrometer,
you are actually measuring the amount of sulfuric acid left in the electrolyte
LABC1 • 4
solution. As more energy is drained from the battery, the ratio of sulfuric acid to
water decreases and the created lead sulfate byproduct begins forming on the
electrode plates. A low hydrometer reading means the chemical makeup that
generates the free electrons is diminished so not as much energy is stored for
use.
The term ‘specific gravity’ is often used to benchmark a lead acid battery’s
SOC. The specific gravity of a substance is a comparison of its density to that
of water (1.000). Imagine a one gallon bottle filled with water and a second
filled with feathers. There are equal volumes of material present in both but the
bottle with the feathers will weigh less than that containing the water. The
resultant specific gravity value of the bottle of feathers would be less than that
of the bottle of water. With lead acid batteries, the sulfur atoms break down and
leach out of the electrolyte solution as it discharges. The breakdown of the
electrolyte reduces its overall ‘weight’ as the sulfur is removed from the solution
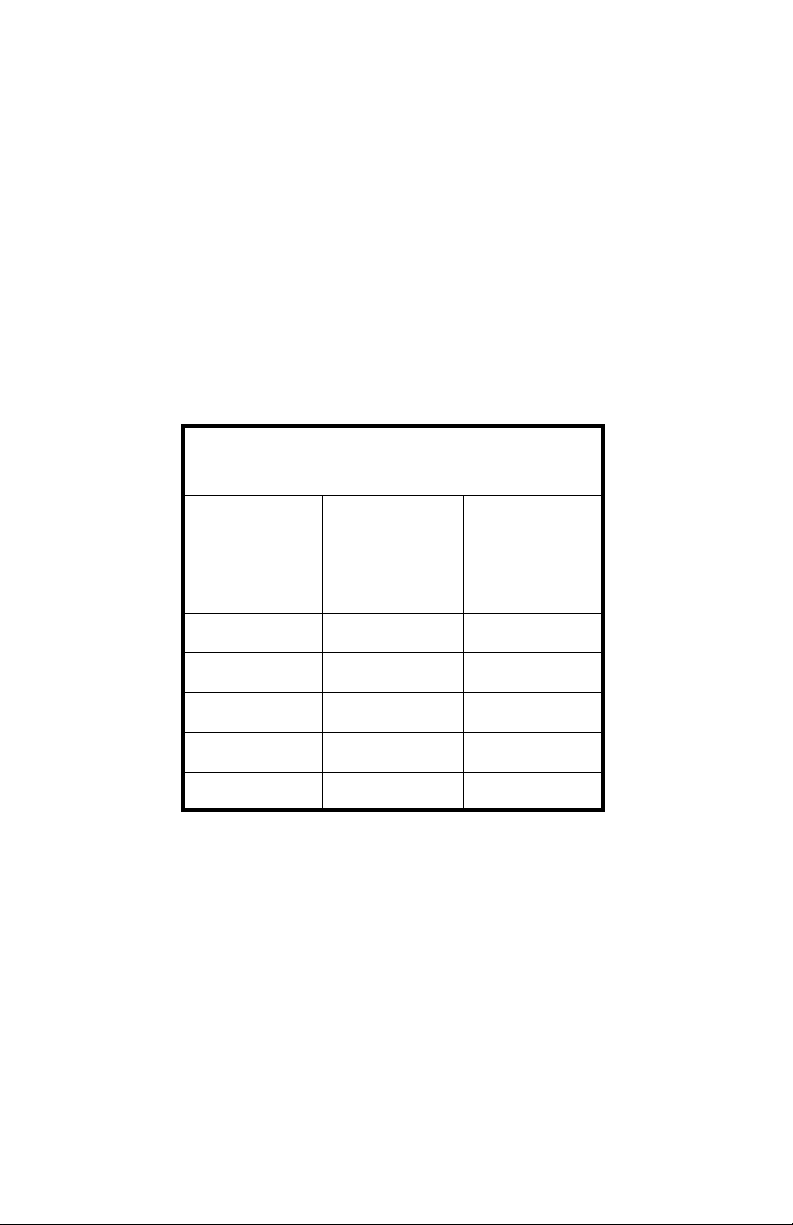
thus reducing the specific gravity measurement. Take a look at Table 1.
State of Charge as related to Specific
Gravity and Open-Circuit Voltage
State Of
Charge
100% 1.265 12.63
75% 1.210 12.30
50% 1.160 12.00
25% 1.120 11.76
Table 1.
Great care should be taken to avoid discharging a battery beyond the 75%
SOC point. Once the specific gravity drops below the 1.210 level, excessive
sulfate deposits form on the electrode plates. This process is called ‘sulfation’
and leads to the hardening of the electrode plates. If the battery is kept in a low
charge state for long a period of time, the sulfation process will eventually
reduce the ability of the battery to generate ion charge carries to the point that
it no longer provides the needed power. This point is otherwise known as a
DEAD BATTERY!
When you recharge the battery, the process is reversed and the sulfur returns
to the electrolyte solution. Proper cycling of the battery will ensure a long and
functional life. If the battery is abused by allowing sulfation of the electrode
0% 1.100 11.64
Specific
Gravity
Open-
Circuit
Voltage
(approximate)
LABC1 • 5

plates on a regular basis or over an extended period of time, the charging
process will not be able to restore the battery to its former full potential. Time
to make a costly battery replacement!
Circuit Description
The LABC1 has been designed as a dependable workhorse to charge and
hold your 12 Volt lead acid batteries at their peak level, insuring a long life and
maximum performance. The charging procedure used when working with a
flooded ‘wet’ cell battery or one of the newer VRLA (Valve Regulated Lead
Acid – ‘Gel’ or ‘AGM’) batteries is the same. The battery being charged will
automatically set the LABC1 in one of two charging modes upon hookup. The
circuit design takes into account the battery’s current SOC (State Of Charge)
and adjusts the terminal voltage at J2 accordingly. The main charging circuit is
very simple because as we discussed before, the concept of lead acid
batteries has been around for centuries (give or take a few thousand years if
you don’t believe in the ‘Space Alien’ theory). The real secret to correctly
charging a lead acid battery system is to use a temperature compensated
voltage source that automatically varies its output in accordance with the
batteries SOC. ‘Frying’ your battery occurs when the charging unit fails to
sense that the electro-chemical rejuvenation (or charging) process has slowed
to the point that the higher voltage charging mode should end. Continual high
voltage charging will decrease the overall life of the battery.
Let’s take a closer look at the LABC1 schematic and see what’s happening.
The power supply inlet for the LABC1 is J1. The input voltage is immediately
presented to a full wave bridge rectifier consisting of diodes D1 to D4 and then
filtered by C1 to reduce the voltage ripple. Using a bridge configuration on the
voltage input allows the user more options to power their LABC1. The use of a
14 VAC or 20 VDC (positive tip) power supply will do nicely with any 12 Volt
lead acid battery. Varying your power supplies current capacity will allow you
to charge any type of lead acid battery without a problem. Most of the
standard cells require a charging current of 650mA or greater. For these
systems a 14 VAC (2 Amps or so) transformer will work very well. If your
application is to charge very small capacity batteries with a maximum charge
current of only a few hundred milliamps, using a 14 VAC @ 500mA ‘wall wart’
supply or a current limited bench-top power supply set for 20 VDC will avoid
excessive current draw that could damage a heavily discharged battery.
Internal heating from excessive charge current will also degrade your overall
battery life.
Moving on, VR1 is a voltage regulator that provides the precision terminal
voltage we need to charge the lead acid cells. Unlike a standard voltage
regulator that is designed for a fixed level output, VR1 lends itself well as a
variable voltage source. With a maximum current source capability of about
1.3 amps, VR1 gives the user the flexibility to charge even very large capacity
LABC1 • 6

batteries. Granted, that might take a while.
The other support components on the board help VR1 to know when to adjust
its output voltage up or down to ensure the proper charging rate of the battery.
These other components are grouped into two major sections, the SOC
feedback loop and the ambient temperature compensation used during the
‘Float’ mode after the battery has been fully charged.
The SOC feedback loop consists mainly of U1 and R6 together to form a low
voltage comparator in conjunction with R1 and R4 to set the range of the
charging voltage. Here’s how the loop functions. Assume for starters that the
battery under charge, or BUC (not to be confused with your BUT, or Battery
Under Test) is discharged and drawing enough current to set the LABC1 in
charge mode. After the current drawn by the battery drops below a certain
point, the need for ‘high’ voltage charging has ended. U1 monitors the voltage
drop across R6 to determine when to switch VR1’s output at J2 from 14.4V
(‘Charge’ mode) to 13.4V (‘Float’ mode). As the battery comes to a full charge,
the charging current it draws drops below about 150mA. The voltage across
R6 (0.47 ohms) will then fall below 0.07V thanks to Ohm’s Law, V=IxR. This
trigger point causes the V+ pin (U1:1) to toggle from its ‘Charging’ mode ‘high’
value of about 12.8V to a charged ‘Float’ mode ‘low’ value of about 0.7V.
When V+ (U1:1) toggles low, R4 is switched into the reference feedback
circuit of VR1 causing its output voltage drop back to 13.4V. The ‘Charged’
LED (D15) is turned on when the Base-Emitter junction of Q1 is thus forward
biased indicating that the battery is charged and is being ‘topped-off’ by the
‘Float’ mode operation.
Now that the battery is charged, the ambient temperature compensation circuit
comes into play. The effects of this circuit, formed by R2, R3 and diodes D5 to
D14, are used only during the ‘Float’ mode operation to adjust the terminal
voltage in accordance with the ambient temperature. If the temperature is not
factored in, you would run the risk of over-charging the battery when it’s hot or
under-charging the battery when it’s cold. Taking advantage of the thermal
characteristics of a PN diode (
raises or lowers the reference terminal of VR1 by 22mV (10 x 2.2mV/°C) for
every 1°C change. This is just the right negative temperature compensation
we needed to properly charge our lead acid batteries!
At the start of the charge cycle, you’ll notice that the heatsink used with VR1
can get very warm if you are charging a large capacity battery. The fact that
the temperature sensor matrix is on the same circuit board and in the same
case will not negatively affect the compensation network because there will be
very little dissipated heat by the board components once the unit switches into
‘Float’ mode. The drop in charge current drawn by the battery is so low by the
time ‘Float’ mode is entered, the air cavity around the temperature sensor
diodes will re-acclimate to the surrounding ambient temperature.
∆2.2mV/°C), the diode matrix (D5 to D14)
LABC1 • 7

LABC1 • 8
 Loading...
Loading...