Page 1

Technical Note TN-106 05/15/VK
A GUIDELINE FOR PID INSTRUMENT RESPONSE
CORRECTION FACTORS AND IONIZATION ENERGIES*
RAE Systems PIDs can be used for the detection of a wide variety of
gases that exhibit different responses. In general, any compound with
ionization energy (IE) lower than that of the lamp photons can
be measured.* The best way to calibrate a PID to different compounds
is to use a standard of the gas of interest. However, correction factors
have been determined that enable the user to quantify a large number
of chemicals using only a single calibration gas, typically isobutylene.
In our PIDs, correction factors can be used in one of three ways:
1. Calibrate the monitor with isobutylene in the usual fashion to
read in isobutylene equivalents. Manually multiply the reading
by the correction factor (CF) to obtain the concentration of
the gas being measured.
2. Calibrate the unit with isobutylene in the usual fashion to read
in isobutylene equivalents. Call up the correction factor from the
instrument memory or download it from a personal computer
and then call it up. The monitor will then read directly in units
of the gas of interest.
3. Calibrate the unit with isobutylene, but input an equivalent,
“corrected” span gas concentration when prompted for this value.
The unit will then read directly in units of the gas of interest.
Example 1:
With the unit calibrated to read isobutylene equivalents, the reading
is 10 ppm with a 10.6 eV lamp. The gas being measured is butyl
acetate, which has a correction factor of 2.6. Multiplying 10 by 2.6
gives an adjusted butyl acetate value of 26 ppm. Similarly, if the
gas being measured were trichloroethylene (CF = 0.54), the adjusted
value with a 10 ppm reading would be 5.4 ppm.
Example 2:
With the unit calibrated to read isobutylene equivalents, the reading
is 100 ppm with a 10.6 eV lamp. The gas measured is m-xylene
(CF = 0.43). After downloading this factor, the unit should read about
43 ppm when exposed to the same gas, and thus read directly in
m-xylene values.
Example 3:
The desired gas to measure is ethylene dichloride (EDC). The CF is 0.6
with an 11.7 eV lamp. During calibration with 100 ppm isobutylene,
insert 0.6 times 100, or 60 at the prompt for the calibration gas
concentration. The unit then reads directly in EDC values.
Conversion to mg/m
To convert from ppm to mg/m3, use the following formula:
3
* The term “ionization energy” is more scientifically correct and
replaces the old term “ionization potential.” High-boiling (“heavy”)
compounds may not vaporize enough to give a response even when
their ionization energies are below the lamp photon energy. Some
inorganic compounds like H
when their ionization energies are well below the lamp photon energy.
RAE Systems by Honeywell 877-723-2878 raesystems.com
2O2
and NO
give weak response even
2
For air at 25°C (77°F), the molar gas volume is 24.4 L/mole and the
formula reduces to:
1
Page 2

Technical Note TN-106 05/15/VK
For example, if the instrument is calibrated with a gas standard in
ppmv, such as 100 ppm isobutylene, and the user wants the display
3
to read in mg/m
of hexane, whose m.w. is 86 and CF is 4.3, the
overall correction factor would be 4.3 x 86 x 0.041 equals 15.2.
Correction Factors for Mixtures
The correction factor for a mixture is calculated from the sum
of the mole fractions Xi of each component divided by their
respective correction factors CFi:
Thus, for example, a vapor phase mixture of 5% benzene
and 95% n-hexane would have a CFmix of
CFmix = 1 / (0.05/0.53 + 0.95/4.3) = 3.2. A reading of 100
would then correspond to 320 ppm of the total mixture,
comprised of 16 ppm benzene and 304 ppm hexane.
For a spreadsheet to compute the correction factor and TLV of a
mixture see the appendix at the end of the CF table.
TLVs and Alarm Limits for Mixtures
The correction factor for mixtures can be used to set alarm limits
for mixtures. To do this one first needs to calculate the exposure
limit for the mixture. The Threshold Limit Value (TLV) often defines
exposure limits. The TLV for the mixture is calculated in a manner
similar to the CF calculation:
In the above example, the 8-h TLV for benzene is 0.5 ppm and
for n-hexane 50 ppm. Therefore the TLV of the mixture is
TLVmix = 1 / (0.05/0.5 + 0.95/50) = 8.4 ppm, corresponding to
8.0 ppm hexane and 0.4 ppm benzene. For an instrument
calibrated on isobutylene, the reading corrsponding to the TLV is:
2. Pressurized gas cylinder (Demand-flow regulator):
A demand-flow regulator better matches pump speed
differences, but results in a slight vacuum during calibration
and thus slightly high readings.
3. Collapsible gas bag: The instrument will draw the
calibration gas from the bag at its normal flow rate, as
long as the bag valve is large enough. The bag should
be filled with enough gas to allow at least one minute
of flow (~ 0.6 L for a MiniRAE, ~0.3 L for MultiRAE).
4. T (or open tube) method: The T method uses a T-junction
with gas flow higher than the pump draw. The gas supply is
connected to one end of the T, the instrument inlet is connected
to a second end of the T, and excess gas flow escapes through
the third, open end of the T. To prevent ambient air mixing,
a long tube should be connected to the open end, or a high
excess rate should be used. Alternatively, the instrument
probe can be inserted into an open tube slightly wider
than the probe. Excess gas flows out around the probe.
The first two cylinder methods are the most efficient in terms
of gas usage, while the bag and T methods give slightly more
accurate results because they match the pump flow better.
B. Pressure. Pressures deviating from atmospheric pressure
affect the readings by altering gas concentration and pump
characteristics. It is best to calibrate with the instrument and
calibration gas at the same pressure as each other and the
sample gas. (Note that the cylinder pressure is not relevant
because the regulator reduces the pressure to ambient.) If
the instrument is calibrated at atmospheric pressure in one
of the flow configurations described above, then 1) pressures
slightly above ambient are acceptable but high pressures
can damage the pump and 2) samples under vacuum may
give low readings if air leaks into the sample train.
A common practice is to set the lower alarm limit to half the TLV,
and the higher limit to the TLV. Thus, one would set the alarms
to 1.3 and 2.6 ppm, respectively.
CALIBRATION CHARACTERISTICS
A. Flow Configuration. PID response is essentially
independent of gas flow rate as long as it is sufficient to
satisfy the pump demand. Four main flow configurations
are used for calibrating a PID:
1. Pressurized gas cylinder (Fixed-flow regulator):
The flow rate of the regulator should match the flow
demand of the instrument pump or be slightly higher.
RAE Systems by Honeywell 877-723-2878 raesystems.com
C. Temperature. Because temperature effects gas density and
concentration, the temperature of the calibration gas and
instrument should be as close as possible to the ambient
temperature where the unit will be used. We recommend
that the temperature of the calibration gas be within the
instrument’s temperature specification (typically 14° to 113° F
or -10° to 45° C). Also, during actual measurements, the
instrument should be kept at the same or higher temperature
than the sample temperature to avoid condensation in the unit.
D. Matrix. The matrix gas of the calibration compound and
VOC sample is significant. Some common matrix components,
such as methane and water vapor can affect the VOC signal.
2
Page 3

Technical Note TN-106 05/15/VK
PIDs are most commonly used for monitoring VOCs in air,
in which case the preferred calibration gas matrix is air.
For a MiniRAE, methane, methanol, and water vapor reduce
the response by about 20% when their concentration is
15,000 ppm and by about 40% at 30,000 ppm. Despite
earlier reports of oxygen effects, RAE PID responses with
10.6 eV lamps are independent of oxygen concentration,
and calibration gases in a pure nitrogen matrix can be
used. H
and CO2 up to 5 volume % also have no effect.
2
E. Concentration. Although RAE Systems PIDs have electronically
linearized output, it is best to calibrate in a concentration range
close to the actual measurement range. For example, 100 ppm
standard gas for anticipated vapors of 0 to 250 ppm, and 500 ppm
standard for expected concentrations of 250 to 1000 ppm. The
correction factors in this table were typically measured at 50 to
100 ppm and apply from the ppb range up to about 1000 ppm.
Above 1000 ppm the CF may vary and it is best to calibrate
with the gas of interest near the concentration of interest.
F. Filters. Filters affect flow and pressure conditions and therefore
all filters to be used during sampling should also be in place
during calibration. Using a water trap (hydrophobic filter)
greatly reduces the chances of drawing water aerosols or
dirt particles into the instrument. Regular filter replacements
are recommended because dirty filters can adsorb VOCs
and cause slower response time and shifts in calibration.
G. Instrument Design. High-boiling (“heavy”) or very reactive
compounds can be lost by reaction or adsorption onto materials
in the gas sample train, such as filters, pumps and other
sensors. Multi-gas meters, including EntryRAE, MultiRAE
and AreaRAE have the pump and other sensors upstream of
the PID and are prone to these losses. Compounds possibly
affected by such losses are shown in green in the table, and
may give slow response, or in extreme cases, no response at
all. In many cases the multi-gas meters can still give a rough
indication of the relative concentration, without giving an
accurate, quantitative reading. The ppbRAE and MiniRAE series
instruments have inert sample trains and therefore do not
exhibit significant loss; nevertheless, response may be slow for
the very heavy compounds and additional sampling time up to
a minute or more should be allowed to get a stable reading.
TABLE ABBREVIATIONS
CF = Correction Factor (multiply by reading to get corrected
value for the compound when calibrated to isobutylene)
NR = No Response
IE = Ionization Energy (values in parentheses are not well
established)
C = Confirmed Value indicated by “+” in this column; all others
are preliminary or estimated values and are subject to change
ne = Not Established ACGIH 8-hr. TWA
C## = Ceiling value, given where 8-hr.TWA is not available
DISCLAIMER
TN-106 is a general guideline for Correction Factors (CF) for use
with PID instruments manufactured by RAE Systems. The CF may
vary depending on instrument and operation conditions. For the best
accuracy, RAE Systems recommends calibrating the instrument to
target gas. Actual readings may vary with age and cleanliness of
lamp, relative humidity, and other factors as well. For accurate work,
the instrument should be calibrated regularly under the operating
conditions used. The factors in this table on the following pages
were measured in dry air (40 to 50% RH) at room temperature,
typically at 50 to 100 ppm. CF values may vary above about 1000 ppm.
Updates
The values in this table on the following pages are subject to change
as more or better data become available. Watch for updates of this
table on the Internet at http://www.raesystems.com.
IE data are taken from the CRC Handbook of Chemistry and Physics,
73rd Edition, D.R. Lide (Ed.), CRC Press (1993) and NIST Standard Ref.
Database 19A, NIST Positive Ion Energetics, Vers. 2.0, Lias, et.al.,
U.S. Dept. Commerce (1993). Exposure limits (8-h TWA and Ceiling
Values) are from the 2005 ACGIH Guide to Occupational Exposure
Values, ACGIH, Cincinnati, OH 2005. Equations for exposure limits
for mixtures of chemicals were taken from the 1997 TLVs and BEIs
handbook published by the ACGIH (1997).
RAE Systems by Honeywell 877-723-2878 raesystems.com
3
Page 4
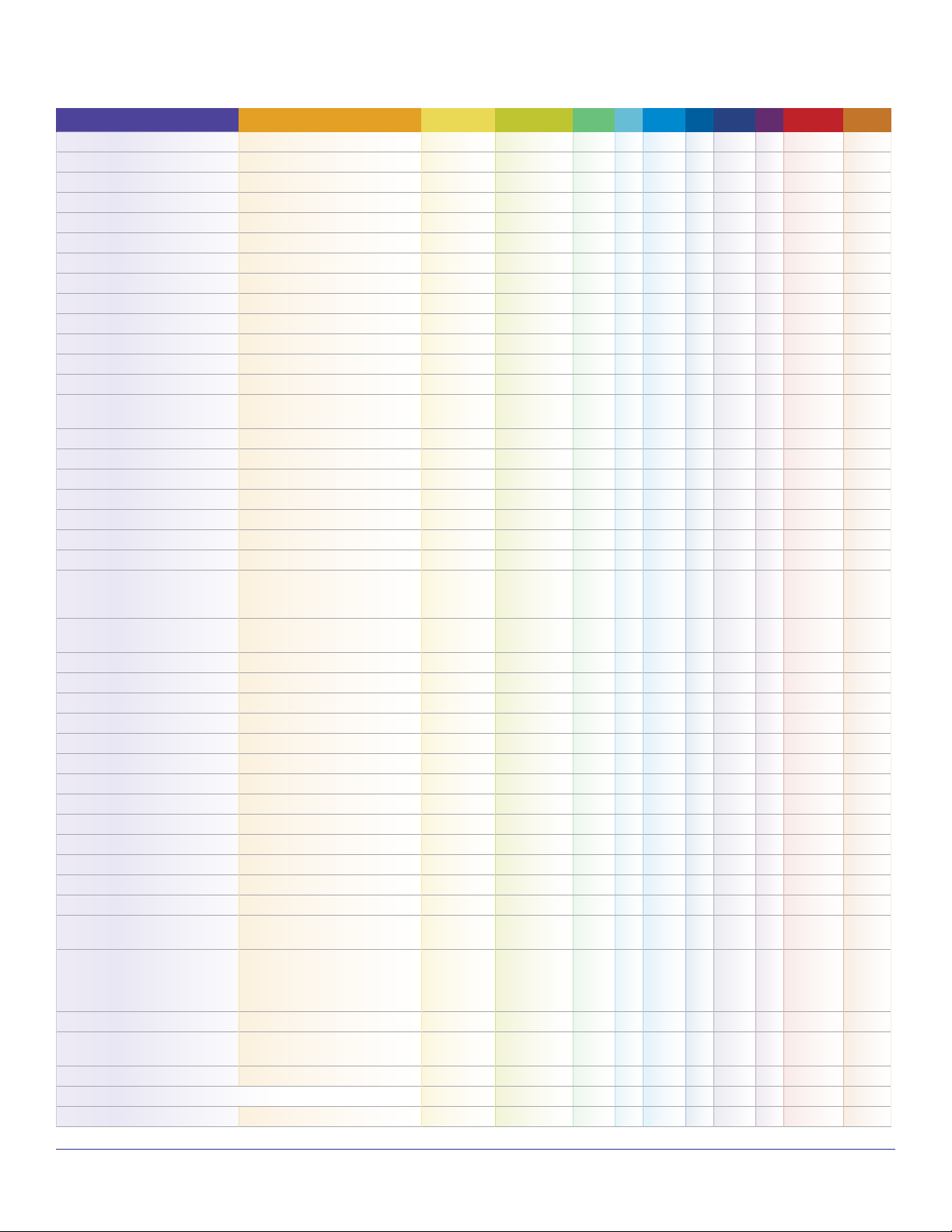
Technical Note TN-106 05/15/VK
Compound Name Synonym/Abbreviation CAS No. Formula 9.8 C 10.6 C 11.7 C IE (eV)TWA
Acetaldehyde 75-07-0 C2H4O NR + 6 + 3.3 + 10.23 C25
Acetic acid Ethanoic Acid 64-19-7 C2H4O
Acetic anhydride Ethanoic Acid Anhydride 108-24-7 C4H6O
Acetone 2-Propanone 67-64-1 C3H6O 1.2 + 0.9 + 1.4 + 9.71 500
Acetone cyanohydrin 2-Hydroxyisobutyronitrile 75-86-5 C4H7NO 4 + 11.1 C5
Acetonitrile Methyl cyanide, Cyanomethane 75-05-8 C2H3N 100 12.19 40
Acetylene Ethyne 74-86-2 C2H
2
Acrolein Propenal 107-02- 8 C3H4O 42 + 3.9 + 1.4 + 10.10 0 .1
Acrylic acid Propenoic Acid 79-10-7 C3H4O
Acrylonitrile Propenenitrile 107-13 -1 C3H3N NR + 1.2 + 10.91 2
Allyl alcohol 107-18 -6 C3H6O 4.5 + 2.4 + 1.6 + 9.67 2
Allyl chloride 3-Chloropropene 107-05-1 C3H5Cl 4.3 0.7 9.9 1
Ammonia 76 64-41-7 NH
Amyl acetate mix of n-Pent yl acetate &
628-63-7 C7H14O
3
2-Methylbutyl acetate
Amyl alcohol 1-Pentanol 75-85-4 C5H12O 5 10.00 ne
Aniline Aminobenzene 62-53-3 C6H7N 0.50 + 0.48 + 0.47 + 7.72 2
Anisole Methoxybenzene 100-66-3 C7H8O 0.89 + 0.58 + 0.56 + 8.21 ne
Arsine Arsenic trihydride 77 84-42-1 AsH
3
Benzaldehyde 100-52-7 C7H6O 1 9.49 ne
Benzene 71-4 3-2 C6H
6
Benzonitrile Cyanobenzene 100-47-0 C7H5N 1.6 9.62 ne
Benzyl alcohol
α-Hydrox ytoluene,
100 -51-6 C7H8O 1.4 + 1.1 + 0.9 + 8.26 ne
Hydroxymethylbenzene,
Benzenemethanol
Benzyl chloride
α-Chlorotoluene,
100- 4 4-7 C
Cl 0.7 + 0.6 + 0.5 + 9 .14 1
7H7
Chloromethylbenzene
Benzyl formate Formic acid benzyl ester 104-57-4 C
Boron trifluoride 7637-07-2 BF
Bromine 7726-95-6 Br
8H8O2
3
2
Bromobenzene 108-86 -1 C6H5Br 0.6 0.5 8.98 ne
2-Bromoethyl methyl ether 6482-24-2 C3H7OBr 0.84 + ~10 ne
Bromoform Tribromomethane 75-25-2 CHBr
3
Bromopropane,1- n-Propyl bromide 106-94-5 C3H7Br 150 + 1.5 + 0.6 + 10.18 ne
Butadiene 1,3-Butadiene, Vinyl ethylene 106-99-0 C4H
6
Butadiene diepoxide, 1,3- 1,2,3,4-Diepoxybutane 298-18-0 C4H6O
Butane 106-97-8 C4H
10
Butanol, 1- Butyl alcohol, n-Butanol 71-36-3 C4H10O 70 + 4.7 + 1.4 + 9.99 20
Butanol, t- tert-Butanol, t-Butyl alcohol 75-65-0 C4H10O 6.9 + 2.9 + 9.90 100
Butene, 1- 1-Butylene 106-98-9 C4H
Butoxyethanol, 2- But yl Cellosolve, Ethylene
111-76-2 C6H14O
8
glycol monobutyl ether
Butoxyethyl Acetate, 2- 2-Butoxyethyl acetate; 2-Butoxy-
112- 07-2 C8H16O
ethanol acetate; But yl Cellosolve
acetate; Butyl glycol acetate;
EGBE A; Ektasolve EB acetate
Butyl acetate, n- 123-86-4 C6H12O
Butyl acrylate, n- Butyl 2-propenoate,
141-32-2 C7H12O
Acrylic acid but yl ester
Butylamine, n- 109-7 3-9 C4H11N 1.1 + 1.1 + 0.7 + 8.71 C5
Butyl cellosolve see 2-Butoxyethanol 111-76-2
Butyl hydroperoxide, t- 75-91-2 C4H10O
NR + 22 + 2.6 + 10.66 10
2
NR + 6.1 + 2.0 + 10.14 5
3
2.1 + 11.40 ne
2
12 + 2.0 + 10.60 2
NR + 10.9 + 5.7 + 10 .16 25
11 + 2.3 + 0.95 + <9.9 100
2
1.9 + 9.89 0.05
0.55 + 0.47 + 0.6 + 9.25 0.5
0.9 + 0.73 + 0.66 + ne
NR NR NR 15 .5 C1
NR + 1. 30 + 0.74 + 10.51 0.1
NR + 2.7 + 0.5 + 10.48 0.5
0.8 0.6 + 1.1 9.07 2
25 + 3.5 + 1.2 ~10 ne
2
67 + 1.2 10.53 800
0.9 9.58 ne
1.8 + 1.2 + 0.6 + <10 25
2
3
2
2
2.0 + 1.6 + <10 1
2
1.27 + 20
2.6 + 10 150
1.6 + 0.6 + 10
RAE Systems by Honeywell 877-723-2878 raesystems.com
4
Page 5

Technical Note TN-106 05/15/VK
Compound Name Synonym/Abbreviation CAS No. Formula 9.8 C 10.6 C 11.7 C IE (eV)TWA
Butyl mercaptan 1-Butanethiol 109-79-5 C4H10S 0.55 + 0.52 + 9.14 0.5
Butyraldehyde Butanal 123 -72-8 C4H8O 1.87 + 9.82 20
CamelinaI HRJ 1.1 + 0.32 +
CamelinaI HRJ/JP-8 50/50 0.89 + 0 .41 +
CamelinalHRJ 1.15 +
CamelinalHRJ/JP-8 1.07 +
Carbon disulfide 75-15-0 CS
Carbon tetrachloride Tetrachloromethane 56-23-5 CCl
2
4
Carbonyl sulfide Carbon oxysulfide 4 6 3 -58-1 COS 11.18
Cellosolve see 2-Ethoxyethanol
CFC -14 see Tetrafluoromethane
CFC-113 see 1,1,2-Trichloro-1,2,2-trifluoroethane
Chlorine 7782-50-5 Cl
Chlorine dioxide 10049-04-4 ClO
2
2
Chlorobenzene Monochlorobenzene 10 8-9 0-7 C6H5Cl 0.44 + 0.55 + 0.39 + 9.06 10
Chlorobenzotrifluoride, 4- PCBTF, OXSOL 100
98-56-6 C7H4ClF
p-Chlorobenzotrifluoride
Chloro-1,3-butadiene, 2- Chloroprene 126-9 9-8 C4H5Cl 3 10
Chloro-1,1-difluoroethane, 1- HCFC-142B, R-142B 75-68-3 C2H3ClF
Chlorodifluoromethane HCFC-22, R-22 75-45-6 CHClF
Chloroethane Ethyl chloride 75-00-3 C2H5Cl NR + NR + 1.1 + 10.97 100
Chloroethanol Ethylene chlrohydrin 107-07-3 C2H5ClO 10.52 C1
Chloroethanol, 2- 2-Chloroethanol; 2-Chloroethyl
107-07-3 C2H5ClO 2.88 + 10.5 5
alcohol; Ethylene chlorhydrin
Chloroethyl ether, 2- bis (2-chloroethyl) ether 111-44-4 C4H8Cl2O 8.6 + 3.0 + 5
Chloroethyl methyl ether, 2- Methyl 2-chloroethyl ether 627-42-9 C3H7ClO 3 ne
Chloroform Trichloromethane 67-66-3 CHCl
Chloro-2-methylpropene, 3- Methallyl chloride, Isobutenyl
563- 47-3 C4H7Cl 1.4 + 1.2 + 0.63 + 9.76 ne
3
chloride
Chloropicrin 76-06-2 CCl3NO
Chlorotoluene, o- o-Chloromethylbenzene 95-49-8 C7H7Cl 0.5 0.6 8.83 50
Chlorotoluene, p- p-Chloromethylbenzene 106-43-4 C7H7Cl 0.6 8.69 ne
Chlorotrifluoroethene CTFE, Chlorotrifluoroethylene
79-38-9 C2ClF
3
Genet ron 1113
Chlorotrimethylsilane 75-77-4 C3H9ClSi NR NR 0.82 + 10.83 ne
Cresol, m- m-Hydroxytoluene,
108-39-4 C7H8O 0.57 + 0.50 + 0.57 + 8.29 5
3-Methylphenol
Cresol, o- ortho-Cresol; 2-Cresol; o-Cresylic
95 -48-7 C7H8O 1 + 8.14 5
acid; 1-Hydroxy-2-methylbenzene;
2-Hydroxytoluene; 2-Methyl
phenol
Cresol, p- para-Cresol; 4-Cresol; p-Cresylic
106-4 4-5 C7H8O 1.4 + 8.34 5
acid; 1-Hydroxy-4-methylbenzene;
4-Hydroxy toluene; 4-Methyl
phenol
Crotonaldehyde trans-2-Butenal 123-73-9
C4H6O 1.5 + 1.1 + 1.0 + 9.73 2
4170-30-3
Cumene Isopropylbenzene 98-82-8 C
9H12
Cyanogen bromide 506-68-3 CNBr NR NR NR 11. 84 ne
Cyanogen chloride 506-77-4 CNCl NR NR NR 12.34 C0.3
Cyclohexane 110-82 -7 C6H
12
Cyclohexanol Cyclohexyl alcohol 108-93-0 C6H12O 1.5 + 0.9 + 1.1 + 9.75 50
Cyclohexanone 108-9 4-1 C6H10O 1.0 + 0.9 + 0.7 + 9 .14 25
4 + 1.2 + 0.44 10.0 7 10
NR + NR + 1.7 + 11.4 7 5
1.0 + 11. 48 0.5
NR + NR + NR + 10.57 0 .1
0.74 + 0.63 + 0.55 + <9.6
3
NR NR NR 12 .0 ne
2
NR NR NR 12 .2 1000
2
NR + NR + 3.5 + 11.37 10
NR + ~400 + 7 + 0.1
2
6.7 + 3.9 + 1.2 + 9.76 5
0.58 + 0.54 + 0.4 + 8.73 50
3.3 + 1.4 + 0.64 + 9.86 300
RAE Systems by Honeywell 877-723-2878 raesystems.com
5
Page 6

Technical Note TN-106 05/15/VK
Compound Name Synonym/Abbreviation CAS No. Formula 9.8 C 10.6 C 11.7 C IE (eV)TWA
Cyclohexene 110-83-8 C6H
10
Cyclohexylamine 108 -91-8 C6H13N 1.2 8.62 10
Cyclopentane 85%
287-92-3 C5H
10
NR + 15 + 1.1 10.3 3 600
2,2-dimethylbutane 15%
Cyclopropylamine Aminocyclpropane 765-30-0 C3H7N 1.1 + 0.9 + 0.9 + ne
Decamethylcyclopentasiloxane 541-02-6 C10H30O5Si50.16 + 0 .13 + 0.12 + ne
Decamethyltetrasiloxane 141-62-8 C10H30O3Si40.17 + 0 .13 + 0.12 + <10.2 ne
Decane 124-18-5 C10H
22
Diacetone alcohol 4-Methyl-4-hydroxy-2-pentanone 123-42-2 C6H12O
4.0 + 1.4 + 0.35 + 9.65 ne
2
Dibromochloromethane Chlorodibromomethane 124-48-1 CHBr2Cl NR + 5.2 + 0.7 + 10.59 ne
Dibromo-3-
DBCP 96-12- 8 C3H5Br2Cl NR + 1.7 + 0.43 + 0.001
chloropropane, 1,2-
Dibromoethane, 1,2- EDB, Ethylene dibromide,
106-93-4 C2H4Br
NR + 1.7 + 0.6 + 10.37 ne
2
Ethylene bromide
Dichlorobenzene, o- 1,2-Dichlorobenzene 95-50-1 C6H4Cl
Dichlorodifluoromethane C FC-12 75-71-8 CCl2F
2
0.54 + 0.64 + 0.38 + 9.08 25
2
Dichlorodimethylsilane 75-78 -5 C2H6Cl2Si NR NR 1.1 + >10.7 ne
Dichloroethane, 1,2- EDC, 1,2-DCA, Ethylene
107-06-2 C2H4Cl
2
dichloride
Dichloroethene, 1,1- 1,1-DCE , Vinylidene chloride 75-35-4 C2H2Cl
Dichloroethene, c-1,2- c-1, 2-DCE , cis-Dichloroethylene 156-5 9-2 C
Dichloroethene, t-1,2- t-1,2- DC E, trans-Dichloroethylene 156-60-5 C
Dichloro-1-fluoroethane, 1,1- R -141B 1717-00-6 C
2
2H2Cl2
2H2Cl2
F NR + NR + 2.0 + ne
2H3Cl2
Dichloromethane see Methylene chloride
Dichloropentafluoropropane AK-225, mix of ~4 5% 3,3-
dichloro-1,1,1,2,2-pentafluoro-
442-56-0
507-55-1
C
HCl2F
3
NR + NR + 25 + ne
5
propane (HCFC-22 5ca) & ~55%
1,3 -Dichloro-1,1,2,2 ,3-pentafluoropropane (HCFC-225cb)
Dichloropropane, 1,2- 78-87-5 C3H6Cl
Dichloro-1-propene, 1,3- 54 2-7 5-6 C3H4Cl
Dichloro-1-propene, 2,3- 78-88-6 C3H4Cl
Dichloro-1,1,1- trifluoroethane, 2,2- R-123 306-83-2 C2HCl2F
Dichloro-2,4,6- trifluoropyridine,
DCTFP 1737-9 3-5 C5Cl2F3N 1.1 + 0.9 + 0.8 + ne
2
1.3 + 0.96 + <10 1
2
1.9 + 1.3 + 0.7 + <10 ne
2
NR + NR + 10.1 + 11.5 ne
3
3,5-
Dichlorvos
**
Vapona; O,O-dimethyl
62-73 -7 C4H7Cl2O4P 0.9 + <9.4 0.1
O-dichlorovinyl phosphate
Dicyclopentadiene DCPD, Cyclopentadiene dimer 77-73-6 C10H
Diesel Fuel
Diesel Fuel #2 (Automotive)
**
**
68334-30-5 m.w. 226 0.9 + 11
68334-30-5 m.w. 216 1.3 0.7 + 0.4 + 11
12
0.57 + 0.48 + 0.43 + 8.8 5
Diethylamine 109-89-7 C4H11N 1 + 8.01 5
Diethylaminopropylamine, 3- 104-7 8-9 C7H18N
2
Diethylbenzene see Dowtherm J
Diethyl ether Diethyl ether; Diethyl oxide; Ethyl
60-29-7 C4H10O 1.74 + 9.51 400
oxide; Ether; Solvent ether
Diethylene glycol butyl ether 2-(2-Butoxyethoxy)ethanol, BDG,
112- 34-5 C8H18O
3
Butyldiglycol, DB Solvent
Diethylene glycol monobutyl
ether acetate
But yldiglycol acet ate, DB Acetate,
Diethylene glycol monobutyl ether
124-17- 4 C10H20O
4
acetate
Diethylmaleate 141-05-9 C8H12O
4
Diethyl sulfide see Ethyl sulfide
Diglyme see Methoxyethyl ether 111-9 6-6 C
6H14O3
0.8 + 8.95 300
0.7 50
NR + NR + 11.75 1000
NR + 0.6 + 11. 0 4 10
0.82 + 0.8 + 9.79 5
0.8 9.66 200
0.45 + 0.34 + 9.65 200
0.7 10.87 75
1.3 ne
4.6 + 5
5.62 + ne
4 ne
RAE Systems by Honeywell 877-723-2878 raesystems.com
6
Page 7

Technical Note TN-106 05/15/VK
Compound Name Synonym/Abbreviation CAS No. Formula 9.8 C 10.6 C 11.7 C IE (eV)TWA
Diisobutyl ketone DIBK, 2,2-dimethyl-4-heptanone 108-83-8 C9H18O 0.71 + 0.61 + 0.35 + 9.04 25
Diisopropylamine 108-18-9 C6H15N 0.84 + 0.74 + 0.5 + 7.7 3 5
Diisopropylcarbodiimide,N,N’- DIPC 693-13-0 C7H14N
Diisopropylethylamine ‘Hünig’s base’,
7087-68-5 C8H19N 0.7 + ne
2
N-Ethyldiisopropylamine, DIPEA ,
Ethyldiisopropylamine
Diketene Ketene dimer 674-82-8 C4H4O
2.6 + 2.0 + 1.4 + 9.6 0.5
2
Dimethylacetamide, N,N- DMA 127-19-5 C4H9NO 0.87 + 0.8 + 0.8 + 8.81 10
Dimethylamine 124-4 0- 3 C2H7N 1.5 8.23 5
Dimethyl carbonate Carbonic acid dimethyl ester 616-38-6 C3H6O
Dimethyl disulfide DMDS 624-92-0 C2H6S
NR + ~7 0 + 1.7 + ~10. 5 ne
3
0.2 + 0.20 + 0.21 + 7.4 ne
2
Dimethyl ether see Methyl ether
Dimethylethylamine DMEA 5 9 8-5 6-1 C4H11N 1.1 + 1.0 + 0.9 + 7. 74 ~3
Dimethylformamide, N,N- DMF 68-12-2 C3H7NO 0.7 + 0.7 + 0.8 + 9.13 10
Dimethylhydrazine, 1,1- UDMH 57-14 -7 C2H8N
Dimethyl methylphosphonate DMMP, methyl phosphonic
756-79-6 C3H9O3P NR + 4.3 + 0.74 + 10.0 ne
2
acid dimethyl ester
Dimethyl sulfate 77-78 -1 C2H6O4S ~23 ~2 0 + 2.3 + 0 .1
Dimethyl sulfide see Methyl sulfide
Dimethyl sulfoxide DMSO, Methyl sulfoxide 67-68-5 C2H6OS 1. 4 + 9 .10 ne
Dioxane, 1,4- 12 3-91-1 C4H8O
Dioxolane, 1,3- Ethylene glycol formal 646-06-0 C3H6O
®
Dowtherm A see Therminol
Dowtherm J (97% Diet hylbenzene)
DS-108F Wipe Solvent Ethyl lactate/Isopar H/
**
**
Propoxypropanol ~7:2:1
25340-17-4 C10H
97-64 -3
64742-48-9
2
4.0 + 2.3 + 1.6 + 9.9 20
2
14
m.w. 118 3.3 + 1.6 + 0.7 + ne
1569- 01- 3
Epichlorohydrin ECH Chloromethyloxirane,
106-89-8 C2H5ClO ~20 0 + 8.5 + 1.4 + 10.2 0.5
1-chloro2,3-epoxypropane
Ethane 74-84-0 C2H
6
Ethanol Ethyl alcohol 64-17- 5 C2H6O 9.6 + 3.1 + 10.47 1000
Ethanolamine
Ethene Ethylene 74-8 5-1 C2H
Ethoxyethanol, 2- Ethyl cellosolve, Ethylene
**
MEA, Monoethanolamine 141-4 3-5 C2H7NO 5.6 + 1.6 + 8.96 3
4
110-80-5 C4H10O
2
glycol monoethyl ether
Ethyl acetate Acetic ester; Acetic ether;
141-78- 6 C4H8O
2
Ethyl es ter of acetic acid; Ethyl
ethanoate
Ethyl acetoacetate 141-97-9 C
6H10O3
Ethyl acrylate 140-88-5 C5H8O
Ethylactate Acetic ester; Acetic ether;
141-78- 6 C4H8O
1.4 + 1.2 + 1.0 + <10 ne
2
2
Ethyl es ter of acetic acid; Ethyl
ethanoate
Ethylamine 75-04 -7 C2H7N 0.8 8.86 5
Ethylbenzene 100-41-4 C8H
10
Ethyl caprylate Ethyl octanoate 106 -32 -1 C10H20O
Ethylenediamine 1,2-Ethanediamine;
107-15 -3 C2H8N
0.52 + 0.65 + 0.51 + 8.77 100
2
0.9 + 0.8 + 1.0 + 8.6 10
2
1,2-Diaminoethane
(Ethylenedioxy)diethanethiol,
2,2’-
Ethylene glycol
Ethylene glycol, Acrylate
**
**
Ethylene glycol dimethyl ether 1,2-Dimethoxyethane, Monoglyme 110-71-4 C4H10O
1,2-Bis(2-mercaptoethoxy)ethane,
1497 0-8 7-7 C6H14O2S
3,6-Dioxa-1,8-octane-dithiol
1,2-Ethanediol 10 7-21-1 C2H6O
2-hydroxyethyl Acrylate 818-61-1 C5H8O
2
2
3
1.1 1.1 0.7 9.2 ne
2
0.42 + ne
0.8 + 0.8 + 7.2 8 0.01
1.3 9.19 25
0.5
NR + 15 + 11. 52 ne
9 + 4.5 + 10.51 ne
1.3 9.6 5
3.8 + 10.01 400
2.4 + 1. 0 + <10.3 5
2.18 + 10.01 400
+ 0.52 + 0.51 +
1.3 + ne
16 + 6 + 10.16 C100
8.2 ≤10.6
RAE Systems by Honeywell 877-723-2878 raesystems.com
7
Page 8

Technical Note TN-106 05/15/VK
Compound Name Synonym/Abbreviation CAS No. Formula 9.8 C 10.6 C 11.7 C IE (eV)TWA
Ethylene glycol monobutyl
ether acetate
Ethylene glycol, monothio 60-24-2 C2H6OS 1.5 9.65
Ethylene oxide Oxirane, Epoxyethane 75-21-8 C
Ethyl ether Diethyl ether 60-29-7 C4H10O 1.1 + 9.51 400
Ethyl 3-ethoxypropionate EEP 763-69-9 C7H14O
Ethyl formate 109-9 4-4 C3H6O
Ethyl-1-hexanol, 2- Isooct yl alcohol 104-7 6-7 C8H18O 1.9 + ne
Ethyl hexyl acrylate, 2- Acrylic acid 2-ethylhexyl ester 103-11-7 C11H20O
Ethylidenenorbornene 5-Ethylidene bicyclo(2,2,1)
Ethyl (S)-(-)-lactate
see also DS-108F
Ethyl mercaptan Ethanethiol 7 5-08-1 C2H6S 0.60 + 0.56 + 9.29 0.5
Ethyl sulfide Diethyl sulfide 352-93-2 C4H10S 0.5 + 8.43 ne
Formaldehyde Formalin 50-00-0 CH2O NR + NR + 1.6 + 10.87 C0.3
Formamide 75-12-7 CH3NO 6.9 + 4 10.16 10
Formic acid 64-18-6 CH2O
Furfural 2-Furaldehyde 98 -01-1 C5H4O
Furfuryl alcohol 98-00-0 C5H6O
Gasoline #1 8006-61-9 m.w. 7 2 0.9 + 300
Gasoline #2, 92 octane 8006-61-9 m.w. 93 1.3 + 1.0 + 0.5 + 300
Glutaraldehyde 1,5-Pentanedial, Glutaric
Glycidyl methacrylate 2,3-Epoxypropyl methacrylate 106-91-2 C7H10O
Halothane 2-Bromo-2-chloro-1,1,1-
HCFC-22 see Chlorodifluoromethane
HCFC-12 3 see 2, 2-Dichloro-1,1,1-trifluoroethane
HCFC-141B see 1,1-Dichloro-1-fluoroethane
HCFC-14 2B see 1-Chloro-1,1-difluoroethane
HCFC-13 4A see 1,1,1,2-Tetrafluoroethane
HCFC-225 see Dichloropentafluoropropane
Heptane, n- 142-82-5 C7H
Heptanol, 4- Dipropylcarbinol 589-55-9 C7H16O 1.8 + 1.3 + 0.5 + 9.61 ne
Hexamethyldisilazane,
1,1,1,3, 3, 3-
**
Hexamethyldisiloxane HMDSx 107-46-0 C
Hexane, n- 110-5 4-3 C6H
Hexanol, 1- Hexyl alcohol 111-27-3 C6H14O 9 + 2.5 + 0.55 + 9.89 ne
Hexene, 1- 592-41-6 C
HFE-7100 see Methyl nonafluorobutyl ether
Histoclear (Histo-Clear) Limonene/corn oil reagent m.w. ~136 0.5 + 0.4 + 0.3 + ne
Hydrazine
**
Hydrazoic acid Hydrogen azide HN
Hydrogen Synthesis gas 1333-74-0 H
Hydrogen cyanide Hydrocyanic acid 74-90-8 HCN NR + NR + NR + 13.6 C4.7
Hydrogen iodide
**
Hydrogen peroxide 77 2 2-8 4-1 H2O
Hydrogen sulfide 7783-06-4 H2S NR + 3.3 + 1. 5 + 10.45 10
Hydroxyethyl acrylate, 2- Ethylene glycol monoacrylate 818 -61-1 C5H8O
1,2-Dimethoxyethane, Monoglyme 110-71-4 C4H10O
2H4
16219-75-3 C9H
12
O 13 + 3.5 + 10.57 1
1.1 1.1 0.7 9.2
2
1.2 + 0.75 + ne
3
2
2
1.1 + 0.5 + ne
1.9 10.61 100
0.4 + 0.39 + 0.34 + ≤8.8 ne
hept-2-ene
Ethyl lactate, Ethyl (S)- (-) hydroxypropionate
687-47-8
C5H10O
97-64 -3
111-30 -8 C5H8O
13 + 3.2 + 1.6 + ~10 ne
3
2
NR + NR + 9 + 11. 33 5
2
2
1.1 + 0.8 + 0.6 + C0.05
2
0.92 + 0.8 + 9.21 2
0.80 + <9.5 10
dialdehyde
2.6 + 1.2 + 0.9 + 0.5
3
151-6 7-7 C2HBrClF
3
0.6 11.0 50
trifluoroethane
16
HMDS 999-97-3 C6H19NSi
OSi20.33 + 0.27 + 0.25 + 9.64 ne
6H18
14
6H12
302-01-2 H4N
2
3
2
45 + 2.8 + 0.60 + 9.92 400
2
0.2 + 0.2 + ~8.6 ne
350 + 4.3 + 0.54 + 10 .13 50
0.8 9.44 30
>8 + 2.6 + 2.1 + 8.1 0.01
10.7
NR + NR + NR + 15.4 3 ne
Hydriodic acid 1003 4-85-2 HI ~0.6 10.39
2
NR + NR + NR + 10.54 1
3
8.2 + ne
ne
RAE Systems by Honeywell 877-723-2878 raesystems.com
8
Page 9

Technical Note TN-106 05/15/VK
Compound Name Synonym/Abbreviation CAS No. Formula 9.8 C 10.6 C 11.7 C IE (eV)TWA
Hydroxypropyl methacrylate 27813-02-1
923-26-2
**
Iodine
7553-56-2 I
Iodomethane Methyl iodide 74-88-4 CH
Isoamyl acetate Isopentyl acetate 123-92-2 C
Isobutane 2-Methylpropane 75-28-5 C
Isobutanol 2-Methyl-1-propanol 7 8-8 3-1 C
Isobutene Isobutylene, Methyl butene 115 -11-7 C
Isobutyl acetate 2-methylpropyl ethanoate,
110-19-0 C
β-methylpropyl acetate
Isobutyl acrylate Isobutyl 2-propenoate,
106-63-8 C7H12O
Acrylic acid Isobutyl ester
Isoflurane 1-Chloro-2, 2,2-trifluoroethyl
26675-46-7 C
difluoromethyl ether, forane
Isooctane 2,2,4-Trimethylpentane 540-84-1 C8H
Isopar E Solvent Isoparaffinic hydrocarbons 64741-66-8 m.w. 121 1.7 + 0.8 + ne
Isopar G Solvent Photocopier diluent 64742-48-9 m.w. 14 8 0.8 + ne
Isopar K Solvent Isoparaffinic hydrocarbons 64742- 48-9 m.w. 156 0.9 + 0.5 + 0.27 + ne
Isopar L Solvent Isoparaffinic hydrocarbons 6 4742-4 8-9 m.w. 163 0.9 + 0.5 + 0.28 + ne
Isopar M Solvent Isoparaffinic hydrocarbons 64742-47-8 m.w. 191 0.7 + 0.4 + ne
Isopentane 2-Methylbutane 78-78-4 C5H
Isophorone 78-59 -1 C9H14O 3 9.07 C5
Isoprene 2-Methyl-1,3-butadiene 78-79-5 C5H
Isopropanol Isopropyl alcohol, 2-propanol, IPA 67-63-0 C3H8O 500 + 4.6 + 2.7 10 .12 200
Isopropyl acetate 108-21- 4 C5H10O
Isopropyl ether Diisopropyl ether 108-20-3 C6H14O 0.8 9.20 250
Jet fuel JP-4 Jet B, Turbo B, F-40
Wide cut type aviation fuel
Jet fuel JP-5 Jet 5, F-44, Kerosene t ype
aviation fuel
80 08-2 0-6 +
64741-4 2-0
80 08-2 0-6 +
64747-77-1
Jet fuel JP-8 F -3 4, Kerosene type aviation fuel 8008-20-6 +
64741-7 7-1
Jet fuel A-1 F-34, Kerosene t ype aviation fuel 8008 -20- 6 +
64741-7 7-1
Jet Fuel TS Thermally Stable Jet Fuel,
Hydrotreated kerosene fuel
80 08-2 0-6 +
64742-47-8
JP-10 0.7 + 0.5 +
JP5, Petroleum/camelinal 1.05 +
JP5/Petroleum 0.98 +
Limonene, D- (R)-(+)-Limonene 5989-2 7-5 C10H
Kerosene C10-C16 petro.distillate see Jet Fuels 8 008-20-6
MDI see 4,4’-Methylenebis (phenylisocyanate)
Maleic anhydride 2,5-Furandione 10 8-31-6 C4H2O
Mercapto-2-ethanol
β-Mercaptoethanol,
60-24-2 C2H6OS 1. 5 + 9.65 0.2
2-Hydroxyethylmercaptan, BME,
Thioethylene glycol
Mesitylene 1,3,5-Trimethylbenzene 108-67-8 C9H
Methallyl chloride see 3-Chloro-2-methylpropene
Methane Natural gas 74-82-8 CH
Methanol Methyl alcohol, carbinol 6 7-56-1 CH4O NR + NR + 2.5 + 10.85 200
Methoxyethanol, 2- Methyl cellosolve, Ethylene glycol
109-86-4 C3H8O
monomethyl ether
C7H12O
2
I 0.21 + 0.22 + 0.26 + 9.54 2
3
7H14O2
4H10
O 19 + 3.8 + 1.5 10.02 50
4H10
4H8
6H12O2
ClF5O NR + NR + 48 + ~11.7 ne
3H2
18
12
8
9.9 + 2.3 + 1.1 + ne
3
0.1 + 0.1 + 0 .1 + 9.40 C0 .1
10.1 2.1 1.0 <10 100
100 + 1.2 + 10.57 ne
1.00 + 1.0 0 + 1.00 + 9.24 ne
2.1 + 9.97 15 0
2
1.5 + 0.60 + ne
1.2 9.86 ne
8.2 ne
0.69 + 0.63 + 0.60 + 8.85 ne
2
2.6 9.99 100
m.w. 115 1.0 + 0.4 + ne
m.w. 16 7 0.6 + 0.5 + 29
m.w. 16 5 0.94 + 0.3 + 30
m.w. 14 5 0.67 34
m.w. 16 5 0.9 + 0.6 + 0.3 + 30
16
3
12
4
0.36 + 0.35 + 0.3 + 8.41 25
NR + NR + NR + 12.61 ne
4.8 + 2.4 + 1.4 + 10 .1 5
2
0.33 + ~8.2 ne
~10. 8 0.1
RAE Systems by Honeywell 877-723-2878 raesystems.com
9
Page 10

Technical Note TN-106 05/15/VK
Compound Name Synonym/Abbreviation CAS No. Formula 9.8 C 10.6 C 11.7 C IE (eV)TWA
Methoxyethoxyethanol, 2- 2-(2-Methoxyethoxy)ethanol
Diethylene glycol monomethyl
ether
Methoxyethyl ether, 2- bis(2-Methoxyethyl) ether,
Diethylene glycol dimethyl ether,
Diglyme
Methyl acetate 79-20-9 C3H6O
Methyl acrylate Methyl 2-propenoate,
Acrylic acid methyl ester
Methylamine Aminomethane 74 -8 9-5 CH
Methyl amyl ketone MAK, 2-Heptanone,
Methyl pentyl ketone
Methylaniline, N- MA; (Methylamino) benzene;
N-Methyl aniline;
Methylphenylamine;
N-Phenylmethylamin
Methyl bromide Bromomethane 74-8 3-9 CH3Br 110 + 1.7 + 1.3 + 10.5 4 1
Methyl-2-butanol, 2- tert-Amyl alcohol,
tert-Pentyl alcohol
Methyl t-butyl ether MTBE, tert-Butyl methyl ether 1634-04-4 C
Methyl cellosolve see 2-Methoxyethanol
Methyl chloride Chloromethane 74- 87- 3 CH3Cl NR + NR + 0.74 + 11.2 2 50
Methylcyclohexane 107-87-2 C7H
Methylene bis
(phenyl-isocyanate), 4,4’-
MDI, Mondur M C15H10N2O2Very slow ppb level response 0.005
**
Methylene chloride Dichloromethane 75-09-2 CH
Methyl ether Dimethyl ether 115-10-6 C2H6O 4.8 + 3.1 + 2.5 + 10.0 3 ne
Methyl ethyl ketone MEK, 2-Butanone 78-93-3 C4H8O 0.86 + 1.0 + 1.1 + 9.51 200
Methylhydrazine Monomethylhydrazine,
Hydrazomethane
Methyl isoamyl ketone MIAK, 5-Methyl-2-hexanone 110-12- 3 C7H14O 0.8 + 0.76 + 0.5 + 9.28 50
Methyl isobutyl ketone MIBK, 4-Methyl-2-pentanone 108 -10-1 C6H12O 0.9 + 0.8 + 0.6 + 9.30 50
Methyl isocyanate 624-83-9 C2H3NO NR + 4.6 + 1.5 10.67 0.02
Methyl isothiocyanate 551-61-6 C2H3NS 0.5 + 0.45 + 0.4 + 9.25 ne
Methyl mercaptan Methanethiol 74 -93-1 CH4S 0.65 0.54 0.66 9.44 0.5
Methyl methacrylate 80-62-6 C5H8O
Methyl nonafluorobutyl ether HFE -7100 DL 16 3702-08-7,
Methyl-1,5-pentanediamine, 2-
(coats lamp)
**
Dytek-A amine, 2-Methyl
pentamethylenediamine
Methyl propyl ketone MPK, 2-Pentanone 107-87-9 C5H12O 0.93 + 0.79 + 9.38 200
Methyl-2-pyrrolidinone, N- NMP, N-Methylpyrrolidone,
1-Methyl-2-pyrrolidinone,
1-Methyl-2-pyrrolidone
Methyl salicylate
Methylstyrene, α-
**
Methyl 2-hydroxybenzoate 119- 3 6-8 C8H8O
2-Propenylbenzene 98-83-9 C9H
Methyl sulfide DMS, Dimethyl sulfide 75 -18-3 C
Methyl vinyl ketone MVK, 3-Buten-2-one 78-94-4 C4H6O 0.93 + 9.65 ne
Methyltetrahydrofuran 2- MeTH F, Tet rahyd ro-2 -
methylfuran, Tetrahydrosilvan
Mineral spirits Stoddard Solvent, Varsol 1,
White Spirits
Mineral Spirits Viscor 120B Calibration Fluid,
b.p. 15 6-207°C
111-77-3 C7H16O 2.3 + 1.2 + 0.9 + <10 ne
111-9 6-6 C6H14O
96-33-3 C4H6O
N 1.2 8.97 5
5
0.64 + 0.54 + 0.44 + <9.8 ne
3
NR + 6.6 + 1.4 + 10.27 200
2
2
3.7 + 1.2 + (9.9) 2
110-43-0 C7H14O 0.9 + 0.85 + 0.5 + 9.30 50
100 -61-8 C7H9N 0.68 + 7.3 2 2
75-85-4 C5H12O 1.62 + 10.16 100
O 0.9 + 9.24 40
5H12
14
2Cl2
60-34-4 C2H6N
1.6 + 0.97 + 0.53 + 9.64 400
NR + NR + 0.89 + 11. 32 25
1.4 + 1.2 + 1.3 + 7.7 0.01
2
2.7 + 1.5 + 1.2 + 9.7 100
2
C5H3F9O NR + ~35 + ne
163702-07-6
15520-10-2 C6H16N
2
~0.6 + <9.0 ne
872-50-4 C5H9NO 1.0 + 0.8 + 0.9 + 9.17 ne
1.3 + 0.9 + 0.9 + ~9 ne
3
10
S 0.49 + 0.44 + 0.46 + 8.69 ne
2H6
0.5 8.18 50
96-47-9 C5H10O 2.44 + 9.22 ne
8020-83-5
m.w. 14 4 1.0 0.69 + 0.38 + 100
8052-41-3
685 51-17-7
8052-41-3 m.w. 142 1.0 + 0.7 + 0.3 + 10 0
RAE Systems by Honeywell 877-723-2878 raesystems.com
10
Page 11

Technical Note TN-106 05/15/VK
Compound Name Synonym/Abbreviation CAS No. Formula 9.8 C 10.6 C 11.7 C IE (eV)TWA
Monoethanolamine see Ethanolamine
Mustard HD, Bis (2-chloroethyl) sulfide 505-60-2
39 472- 4 0-7
68157-62-0
Naphtha see V M & P Naphtha
Naphthalene Mothballs 91-20-3 C10H
Nickel carbonyl (in CO) Nickel tetracarbonyl 13463-39-3 C4NiO
Nicotine 3-(1-Methyl-2-pyrrolidyl)pyridine 54 -11-5 C10H14N
Nitric oxide 10102-4 3-9 NO ~6 5.2 + 2.8 + 9.26 25
Nitrobenzene 98-95-3 C6H5NO
Nitroethane 79-24 -3 C2H5NO
Nitrogen dioxide 10102-44- 0 NO
Nitrogen trifluoride 778 3-54 -2 NF
Nitromethane 75-52-5 CH3NO
Nitropropane, 2- 79-46-9 C3H7NO
Nonane 111-8 4 -2 C9H
Norpar 12 n-Paraf fins, mostly C10-C
Norpar 13 n-Paraf fins, mostly C13-C
13
14
64 771-72- 8 m.w. 161 3.2 + 1.1 + 0.28 + ne
64 771-72- 8 m.w. 18 9 2.7 + 1.0 + 0.3 + ne
Octamethylcyclotetrasiloxane 556-67-2 C8H24O4Si40.21 + 0.17 + 0.14 + ne
Octamethyltrisiloxane 107-51-7 C8H24O2Si30.23 + 0.18 + 0.17 + <10.0 ne
Octane, n- 111-65 -9 C8H
Octene, 1- 111-66-0 C8H
Pentachloropropane 1,1,1,3,3-pentachloropropane 23153-23-3 C3H3Cl
Pentane 109-66-0 C5H
Peracetic acid
**
Perox yacetic acid,
79-21-0 C2H4O
Acetyl hydroperoxide
Peracetic/Acetic acid mix
**
Perox yacetic acid,
79-21-0 C2H4O
Acetyl hydroperoxide
Perchloroethene PCE, Perchloroethylene,
127-18-4 C2Cl
Tetrachloroethylene
Propylene glycol methyl ether,
PGME 107-9 8-2 C6H12O
1-Methoxy-2-propanol
Propylene glycol methyl ether
PGMEA 108-65-6 C
acetate,
1-Methoxy-2-acetoxypropane,
1-Methoxy-2-propanol acetate
Phenol Hydroxybenzene 108-95-2 C6H6O 1.0 + 1.0 + 0.9 + 8.51 5
Phosgene Dichlorocarbonyl 75-44-5 CCl2O NR + NR + 8.5 + 11.2 0 .1
Phosgene in Nitrogen Dichlorocarbonyl 75-44-5 CCl2O NR + NR + 6.8 + 11.2 0 .1
Phosphine (coats lamp) 7803 -51-2 PH
Photocopier Toner Isoparaffin mix 0.5 + 0.3 + ne
Picoline, 3- 3-Methylpyridine 108-99-6 C6H7N 0.9 9.04 ne
Pinene, α-
Pinene, β-
2437-95-8 C10H
18172-67-3 C
Piperylene, isomer mix 1,3-Pentadiene 504-60-9 C
Propane 74-98-6 C3H
Propanol, n- Propyl alcohol 71-23-8 C3H8O 5.5 1.7 10.22 200
Propene Propylene 115- 07-1 C3H
Propionaldehyde Propanal 123-38-6 C3H6O 1. 9 9.95 ne
Propyl acetate, n- 109-60-4 C5H10O
Propyl acetate Propylacetate; n-Propyl ester of
109-60-4 C5H10O
acetic acid
C4H8Cl2S 0.6 0.0005
8
2
3
20
18
16
12
4
6H12O3
3
16
10H16
5H8
8
6
0.45 + 0.42 + 0.40 + 8 .13 10
4
2
2.6 + 1.9 + 1.6 + 9.81 1
2
2
0.18 <8.8 0.001
1.98 + ne
3 10.88 100
23 + 16 + 6 + 9.75 3
NR NR NR 13.0 10
2
2
4 11.02 20
2.6 10.71 10
1.4 9.72 200
13 + 1.8 + 9.82 300
0.9 + 0.75 + 0.4 + 9.43 75
5
1.25 + 0 .1
80 + 8.4 + 0.7 + 10.35 600
NR + NR + 2.3 + ne
3
3
50 + 2.5 + ne
0.69 + 0.57 + 0.31 + 9.32 25
2.4 + 1.5 + 1.1 + 100
3
1.65 + 1.0 + 0.8 + ne
28 3.9 + 1.1 + 9.87 0.3
0.31 + 0.47 8.07 ne
0.38 + 0.37 + 0.37 + ~8 100
0.76 + 0.69 + 0.64 + 8.6 100
NR + 1.8 + 10.95 2500
1.5 + 1.4 + 1.6 + 9.73 ne
2
2
3.5 10.04 200
2.27 + 10.04 200
RAE Systems by Honeywell 877-723-2878 raesystems.com
11
Page 12

Technical Note TN-106 05/15/VK
Compound Name Synonym/Abbreviation CAS No. Formula 9.8 C 10.6 C 11.7 C IE (eV)TWA
Propylamine, n- 1-Propylamine,
1-Aminopropane
Propylene carbonate
**
Propylene glycol 1,2-Propanediol 57-55-6 C3H8O
Propylene glycol propyl ether 1-Propoxy-2-propanol 156 9-01-3 C6H14O
Propylene oxide Methyloxirane 75 -5 6-9
Propyleneimine 2-Methylaziridine 75-55-8 C3H7N 1.5 + 1.3 + 1.0 + 9.0 2
Propyl mercaptan, 2- 2-Propanethiol, Isopropyl
mercaptan
Pyridine 110 -86 -1 C5H5N 0.78 + 0.7 + 0.7 + 9.25 5
Pyrrolidine (coats lamp) Azacyclohexane 12 3-75-1 C4H9N 2.1 + 1.3 + 1.6 + ~8.0 ne
RR730 0 (PGME /PGMEA) 70:30 PGME:PGME A
(1-Methoxy-2-propanol:
1-Methoxy-2-acetoxypropane)
Sarin GB, Isopropyl
methylphosphonofluoridate
Shell SPK 1.26 +
Shell SPK 1.29 + 0.4 +
Shell SPK 50/50 1.02 + 0.41 +
Shell SPK/JP-8 1.11 +
Stoddard Solvent see Mineral Spirits 8020-83-5
Styrene 100-42-5 C8H
Sulfur dioxide 7446-09-5 SO
Sulfur hexafluoride 2551-62-4 SF
Sulfuryl fluoride Vikane 2699-79-8 SO2F
**
Tabun
Ethyl N, Ndimethylphosphoramidocyanidate
Tallow HRJ 1.09 +
Tallow HRJ 0.95 + 0.36 +
Tallow HRJ/JP-8 1.14 +
Tallow HRJ/JP-8 50/50 0.9 + 0.39 +
Tetrachloroethane, 1,1,1,2- 630-20-6 C2H2Cl
Tetrachloroethane, 1,1,2,2- 79-34-5 C2H2Cl
Tetrachlorosilane 10023-04-7 SiCl
Tetraethyllead TEL 78-00 -2 C8H20Pb 0.4 0.3 0.2 ~11.1 0.008
Tetraethyl orthosilicate Ethyl silicate, TEOS 7 8-10 -4 C8H20O4Si 0.7 + 0.2 + ~9.8 10
Tetrafluoroethane, 1,1,1,2- HFC -134 A 811- 97-2 C2H2F
Tetrafluoroethene TFE, Tetrafluoroethylene,
Perfluoroethylene
Tetrafluoromethane CFC-14, Carbon tetrafluoride 75-73-0 CF
Tetrahydrofuran THF 109-99-9 C4H8O 1.9 + 1.7 + 1.0 + 9.41 200
Tetramethyl orthosilicate Methyl silicate, TMOS 681-8 4-5 C4H12O4Si 10 + 1. 9 + ~10 1
Therminol® D-12
Therminol® VP-1
**
**
Hydrotreated heav y naphtha 64742-4 8-9 m.w. 160 0.8 + 0.51 + 0.33 + ne
Dowtherm A, 3:1 Diphenyl oxide:
Biphenyl
Toluene Methylbenzene 108-88-3 C
Tolylene-2,4-diisocyanate TDI, 4-Methyl-1,3-phenylene-2,4 -
diisocyanate
Trichlorobenzene, 1,2,4- 1, 2,4-TC B 120- 82-1 C6H3Cl
Trichloroethane, 1,1,1- 1,1,1-TCA, Methyl chloroform 71-55 -6 C2H3Cl
107-10-8 C3H9N 1.1 + 1.1 + 0.9 + 8.78 ne
108-32-7 C4H6O
3
18 4.2 + 1.6 + <10.2 ne
2
1.3 + 1.0 + 1.6 + ne
2
62 + 1 + 10.5 ne
C3H6O ~240 6.6 + 2.9 + 10.22 20
16088-62-3
154 48-47-2
75-33-2 C3H8S 0.64 + 0.66 + 9 .15 ne
107-9 8-2 C4H10O2/
C6H12O
107-44 -8
C4H10FO2P ~3
3
1.4 + 1.0 + ne
50642-23-4
8
2
6
2
0.45 + 0.43 + 0.4 + 8.43 20
NR NR + NR + 12.32 2
NR NR NR 15 .3 1000
NR NR NR 13.0 5
77-81-6 C5H11N2O2P 0.8 15ppt
1.3 ~11.1 ne
4
4
116-14 -3 C2F
101-84-8
92-52-4
4
4
C12H10O
C12H
10
7H8
584-84-9 C9H6N2O
4
NR + NR + 0.60 + ~11.1 1
4
NR NR 15 + 11.7 9 ne
NR NR ne
~15 10.12 ne
NR + NR + >15. 3 ne
0.4 + 1
0.54 + 0.45 + 0.51 + 8.82 50
1.4 + 1.4 + 2.0 + 0.002
2
0.7 + 0.9 + 9.04 C5
3
3
NR + 1 + 11 350
RAE Systems by Honeywell 877-723-2878 raesystems.com
12
Page 13

Technical Note TN-106 05/15/VK
Compound Name Synonym/Abbreviation CAS No. Formula 9.8 C 10.6 C 11.7 C IE (eV)TWA
Trichloroethane, 1,1,2- 1,1,2-TCA 79-00-5 C2H3Cl
Trichloroethene TCE, Trichoroethylene 79-01-6 C2HCl
3
Trichloromethylsilane Methyltrichlorosilane 75-79-6 CH3Cl3Si NR NR 1.8 + 11.36 ne
Trichlorotrifluoroethane, 1,1,2- C FC-113 76-13 -1 C2Cl3F
Triethylamine TEA 121-44-8 C6H15N 0.95 + 0.9 + 0.65 + 7. 3 1
Triethyl borate T EB; Boric acid triethyl ester,
150-4 6-9 C6H15O3B 2.2 + 1.1 + ~10 ne
Boron ethoxide
Triethyl phosphate Ethyl phosphate 78-40-0 C6H15O4P ~50 + 3 .1 + 0.60 + 9.79 ne
Trifluoroethane, 1,1,2- 430-66-0 C2H3F
3
Trimethylamine 75-50-3 C3H9N 0.9 7. 8 2 5
Trimethylbenzene, 1,3,5- see Mesitylene 108-67-8 25
Trimethyl borate TMB; Boric acid trimethyl ester,
121- 4 3-7 C3H9O3B 5.1 + 1.2 + 10.1 ne
Boron methoxide
Trimethyl phosphate Methyl phosphate 512-5 6-1 C3H9O4P 8.0 + 1.3 + 9.99 ne
Trimethyl phosphite Methyl phosphite 121- 45-9 C3H9O3P 1.1 + + 8.5 2
Turpentine Pinenes (85%) + other diisoprenes 8006-64-2 C10H
Undecane 1120-21-4 C11H
16
24
Varsol see Mineral Spirits
Vinyl actetate 108-05-4 C4H6O
Vinyl bromide Bromoethylene 593-60-2 C2H3Br 0.4 9.80 5
Vinyl chloride Chloroethylene, VCM 7 5-01-4 C2H3Cl 2.0 + 0.6 + 9.99 5
Vinyl-1-cyclohexene, 4- Butadiene dimer,
100-40-3 C8H
12
4-Ethenylcyclohexene
Vinylidene chloride see 1,1-Dicholorethene
Vinyl-2-pyrrolidinone, 1- NVP, N-vinylpyrrolidone,
88-12- 0 C6H9NO 1.0 + 0.8 + 0.9 + ne
1-ethenyl-2-pyrrolidinone
Viscor 120B see Mineral Spirits —Viscor 120B Calibration Fluid
V. M. & P. Naphtha L igroin; Solvent naph tha; Varnish
maker’s & painter’s naphtha
Xylene, m- 1,3 -Dimethylbenzene 108-38-3 C8H
Xylene, o- 1,2-Dimethylbenzene 95-47-6 C8H
Xylene, p- 1,4-Dimethylbenzene 106-42-3 C8H
64742-89-8 m.w. 111
(C8-C9)
10
10
10
NR + NR + 0.9 + 11.0 10
3
0.62 + 0.54 + 0.43 + 9.47 50
3
NR NR 11.99 1000
34 12.9 ne
0.37 + 0.4 + 0.29 + ~8 20
2 9.56 ne
1.5 + 1.2 + 1.0 + 9.19 10
2
0.6 + 0.56 + 9.83 0.1
1.7 + 0.97 + 300
0.50 + 0.44 + 0.40 + 8.56 100
0.56 + 0.45 + 0.43 8.56 100
0.48 + 0.39 + 0.38 + 8.44 100
* The term “ionization energy” is more scientifically correct and replaces the old term “ionization potential.” High-boiling (“heavy”) compounds may not vaporize enough
to give a response even when their ionization energies are below the lamp photon energy. Some inorganic compounds like H
and NO2 give weak response even when
2O2
their ionization energies are well below the lamp photon energy.
** Compounds indicated in green can be detected using a MiniRAE 3000, UltraRAE 3000 or ppbRAE 3000 with slow response, but may be lost by adsorption on a
MultiRAE, EntryRAE and AreaRAE. Response on multi-gas meters can give an indication of relative concentrations, but may not be quantitative and for some chemicals
no response is obser ved.
Therminol® is a registered Trademark of Solutia, Inc.
RAE Systems by Honeywell 877-723-2878 raesystems.com
13
 Loading...
Loading...