USER MANUAL
FLOWMETER
MODELS:
1MFA, 4MFA, 6MFA and
8MFA Series
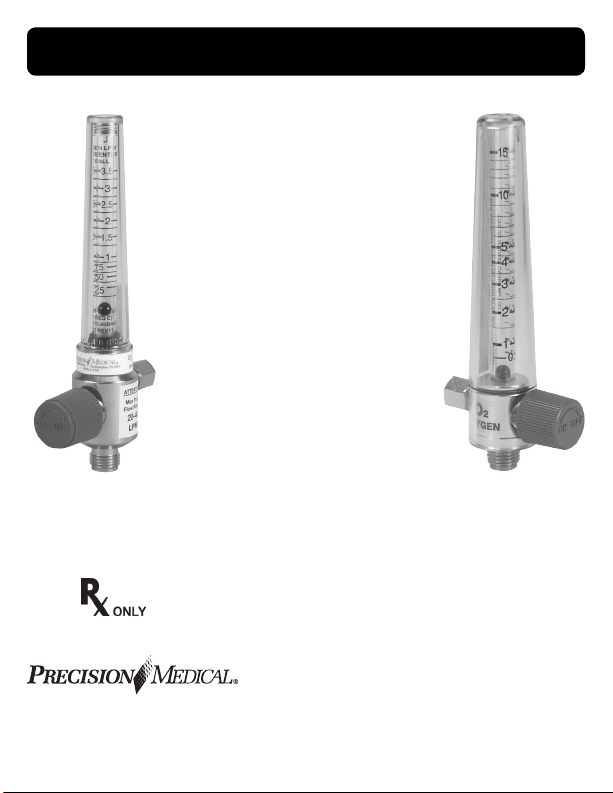
1MFA3001 (Shown)
SAVE THESE INSTRUCTIONS
Federal (USA) law restricts this device to sale
by or on the order of a physician.
300 Held Drive Tel: (+001) 610-262-6090
Northampton, PA 18067 USA Fax: (+001) 610-262-6080
ISO 13485 Certied www.precisionmedical.com
8MFA1001 (Shown)

RECEIVING / INSPECTION
Remove the Precision Medical, Inc. Flowmeter from the packaging and
inspect for damage. If there is any damage, DO NOT USE and contact
your Provider.
INTENDED USE
The Flowmeter is intended for use by physicians, respiratory therapists
and other authorized hospital personnel to administer selected doses
of medical gases to a patient.
READ ALL INSTRUCTIONS BEFORE USING
This manual instructs a Professional to install and operate the Flowmeter.
This is provided for your safety and to prevent damage to the Flowmeter.
If you do not understand this manual, DO NOT USE the Flowmeter and
contact your Provider.
SAFETY INFORMATION - WARNINGS AND CAUTION
WARNING
CAUTION
CAUTION
Indicates a potentially hazardous situation which, if
not avoided, could result in death or serious injury.
Indicates a potentially hazardous situation which, if
not avoided, may result in minor or moderate injury.
Used without the safety alert symbol indicates
a potentially hazardous situation which, if not
avoided, may result in property damage.
Operating Instructions
Symbol for “USE NO OIL”
Symbol indicates the device complies with the
requirements of Directive 93/42/EEC concerning
medical devices and all applicable International
Standards. (On CE marked Devices ONLY)
WARNING
• Use Flowmeters only for their “Intended Use” as described in this
manual.
• ALWAYS conrm prescribed ow before administering to patient
and monitor ow on a frequent basis.
• Flowmeters may contain magnetic, ferrous material that may
affect the results of an MRI.
To Reduce the Risk of Fire or Explosion:
• ALWAYS follow ANSI and CGA standards for Medical Gas
Products and Flowmeters and Oxygen Handling.
• DO NOT use or store oils, greases, organic lubricants or any
combustible materials on or near this Flowmeter.
• DO NOT use near any type of ame or ammable/explosive
substances, vapors or atmosphere.
•
DO NOT smoke in an area where oxygen is being administered.
CAUTION
• Flowmeters must be operated with the Flow Tube in a vertical,
upright position.
• Only personnel instructed and trained in its use should operate
this Flowmeter.
• Ensure all connections are tight and leak free.
• Only use oxygen-safe leak detector.
• DO NOT autoclave.
• DO NOT gas sterilize with EtO (Ethylene Oxide).
• DO NOT clean with aromatic hydrocarbons.
• DO NOT immerse Flowmeter in any kind of liquid. This will void
the warranty.
• The 1MFA3001, 4MFA1001 & 6MFA1001 Flowmeters may have a
factory installed restrictor. Prior to use, check Flowmeter labeling
for ow restrictions.
•
The 1MFA3001, 4MFA1001 & 6MFA1001 Flowmeters contain a
glass Flow Tube which is fragile. Special care should be observed
to avoid breaking the Flow Tube.
(Continued on Inside)

SPECIFICATIONS
Flow Range Graduations Accuracy
0 – 200 cc 20 cc (0-200)
0 – 1 l/min .1 (0-1) l/min 0-1 ±.05 l/min
0 – 3.5 l/min
0 – 5
l/min .25 (0-5) l/min 0-5 ±.20 l/min
0 – 6 l/min .5 (0-6) l/min 0-6 ±.50 l/min
0 – 8 l/min .5 (0-8) l/min 0-8 ±.25 l/min
0 – 15 l/min
0 – 26 l/min
0 – 70 l/min 5 (0-70) l/min 0-70
.125 (0-1) l/min
.25 (1-3.5)
.5 (0-5) l/min
1 (5-15)
1 (2-26)
l/min
l/min
l/min
Flush Flow is the output of the owmeter when the ow indicator is
beyond the highest calibrated graduation. The Flush Flow range is as
indicated on the owmeter labeling.
Transport / Storage
Requirements
-40˚F (-40˚C) to 140˚F (60˚C)
The gas and inlet pressures are indicated on the Flow Tube or Flowmeter body.
NOTE: Storage / Transport outside the specied range may cause
damage to the owmeter.
The effect on accuracy of ow due to variations in ambient temperature is
standard accuracy +7.3% @ 32°F (0°C) and -3.0% @ 104°F (40°C).
The above Flowmeter models are calibrated at specied inlet pressure, 70°F
(21°C), standard atmospheric pressure. International models are calibrated
per specications marked on Flow Tube or Flowmeter body.
Specications are subject to change without prior notice.
0-100 cc ±10 cc
101-200 cc ±14 cc
0-3.5 ±.15 l/min
0-5 ±.25 l/min
6-15 ±.50
2-4 ±.50 l/min
5-26 ±10% of reading
l/min
±10% of reading
For the most current manual revision
please visit our website: www.precisionmedical.com
Tell us how we are doing!
Visit us at www.precisionmedical.com

OPERATING INSTRUCTIONS
WARNING
Read this User Manual before installing or operating the Flowmeter.
CAUTION
Inspect the Flowmeter for visual damage before use, DO NOT USE
if damaged.
NOTE: Precision Medical, Inc. strongly recommends the use of kink
proof Cannula.
1. Turn Knob to the “OFF” position.
2. Connect the Flowmeter to the appropriate gas source. The
appropriate gas and pressure are specied on the Flow Tube or
Flowmeter body.
3. Verify that the Float Ball is at the very bottom of the Flow Tube.
NOTE: If the Float is not resting at the bottom of the Flow Tube, the
product is leaking; consult the “TROUBLESHOOTING” Guide.
4. Adjust Flow:
To increase - Turn Knob counterclockwise
To decrease - Turn Knob clockwise
5.
Set ow by aligning center of Float Ball with indicator lines on the Flow
Tube.
6. Adjusting ow beyond the last calibrated indicator line will result in
an undetermined ow.
7. To obtain maximum ush ow, turn Knob fully Counterclockwise.
NOTE: Flush ow is any ow above the last calibrated line on the
Flow Tube with an unrestricted ow, as indicated on owmeter
labeling.

CAUTION
• DO NOT over tighten Knob when turning off. This will cause damage to
the Flowmeter.
• Pressures other than those indicated on the Flow Tube or Flowmeter
body may affect the accuracy of the indicated ow.
• Gas Temperatures other than 70° F (21°C) may affect the accuracy of
the indicated ow.
• Attaching accessories to the outlet (which may increase resistance to outlet
ow) may change indicated ow but will not affect the accuracy of the ow.
• ONLY use appropriate gas specic indexed ttings to connect Flowmeter
to gas source. Use Oxygen connections for oxygen Flowmeters; use air
connections for air Flowmeters.
•
DO NOT attempt to repair the 8MFA Flowmeters. There are no serviceable parts.
CLEANING INSTRUCTIONS
1. Disconnect all connections before cleaning.
2. Clean exterior surfaces of the Flowmeter with a cloth dampened with a mild
detergent and water.
3. Wipe dry with a clean cloth.
TROUBLESHOOTING
If the Flowmeter fails to function, consult your Provider or Precision Medical, Inc.
Problem Probable Cause Remedy
Will not shut off • Leak
Sticking Float Ball
Unable to set
desired ow
Knob will not turn
8MFA Models DO NOT have serviceable parts.
• Defective Valve
• Debris in Flow Tube
• Blocked Inlet • Replace Body Assembly
• Valve seized • Replace Body Assembly
•
Replace Tetraseal and/or Housing
• Replace Body Assembly
• Clean Flow Tube & Float Ball
RETURNS
Returned products require a Returned Goods Authorization (RGA) number.
Any product returned to Precision Medical, Inc. must be packaged in a sealed
container to prevent damage. Precision Medical, Inc. will not be responsible
for goods damaged in transit. Return Policy available on the Internet,
www.precisionmedical.com.

REPLACEMENT PARTS *
PART
Spring NA 1575 NA
Washer NA 1787 NA
Disc NA 1009 1114 1009 1114 1009
Housing 1143
Tetraseal™
Flow Tube
Float Ball 1576 1005 1029 1005 1154 1154 1005 1029
Body
Assembly
Knob 1007 1008
Holder 506176 NA
*
International parts specications and specic ratings are available upon request.
2513 BH, The Hague
The Netherlands
Phone: +31 (0) 70.345.8570 Fax: +31 (0) 70.346.7299
Classication: IIa
Classication criteria:
We hereby declare that an examination of the under mentioned production quality assurance system has
been carried out following the requirements of the UK national legislation to which the undersigned is
subjected, transposing Annex II, 3 of the Directive 93/42/EEC and Directive 2007/47/EC on medical devices.
We certify that the production quality system conforms to the relevant provisions of the aforementioned
legislation, and the result entitles the organization to use the CE 0473 marking on those products listed above.
Applied Standards: EN 1041, EN 14971, EN ISO 13485, ISO 15001, ISO 15002, ISO 15223-1
Notied Body:
Address: Davy Avenue Knowlhill Milton Keynes MK5 8NL, UK
Certication Registration No’s:
Devices already manufactured: S/N traceability Device History Records
Validity of DOC: 04 August 2012 to Date of Expiry
Manufacture Representative:
Position: Quality Systems/ISO Representative
Date of Issue: 04 August 2012
6MFA 4MFA 1MFA
1001 3001 3501 4001 1001 8001 5001 1101 1211 2001 9001
Kit #
Kit #
Kit #
503213
503214
1891 1897 502053 505271 504407 504823 504407 504434 504824
8MFA Models DO NOT have serviceable parts.
DECLARATION OF CONFORMITY
Precision Medical, Inc.
Flowmeters 1MFA, 4MFA, 6MFA & 8MFA Series
300 Held Drive
Northampton, PA 18067, USA
Emergo Europe (European Ofce)
Molenstraat 15
1012 1152 1010 1021 502459 507573 502117 1011 1031
503215
Clause 3.2 Rule 11 of Annex IX of MDD
AMTAC Certication Services Limited
1126 CE Date of Expiry: 03 August 2017
Quality Manager
MODEL #
1123

LIMITED WARRANTY
AND LIMITATION OF LIABILITY
Precision Medical, Inc. warrants that the Medical Gas Flowmeter (the Product)
will be free of defects in workmanship and/or material for the following period:
(a) Flow Tube and Housing Lifetime of the product
(b) Needle Valve Five (5) years from shipment
(c) All other parts of the Medical One (1) year from shipment
Gas Flowmeter not identied
in (a) or (b) above
Warranty does not cover breakage / abuse.
Should any failure to conform to this warranty appear within the applicable
period, Precision Medical, Inc. shall, upon written notication thereof and
substantiation that the goods have been stored, installed, maintained and
operated in accordance with Precision Medical, Inc.’s instructions and standard
industry practice, and that no modications, substitutions, or alterations have
been made to the goods, correct such defect by suitable repair or replacement
at its own expense.
ORAL STATEMENTS DO NOT CONSTITUTE WARRANTIES.
The representative of Precision Medical, Inc. or any retailers are not authorized
to make oral warranties about the merchandise described in this contract, and
any such statements shall not be relied upon and are not part of the contract
for sale. Thus, this writing is a nal, complete and exclusive statement of the
terms of that contract.
THIS WARRANTY IS EXCLUSIVE AND IS IN LIEU OF ANY WARRANTY OF
MERCHANTABILITY, FITNESS FOR A PARTICULAR PURPOSE OR OTHER
WARRANTY OF QUALITY, WHETHER EXPRESS OR IMPLIED.
Precision Medical, Inc. shall not under any circumstances be liable for special,
incidental or consequential damages including but not limited to lost prots,
lost sales, or injury to person or property. Correction of non-conformities as
provided above shall constitute fulllment of all liabilities of Precision Medical,
Inc. whether based on contract, negligence, strict tort or otherwise. Precision
Medical, Inc. reserves the right to discontinue manufacture of any product or
change product materials, designs, or specications without notice.
Precision Medical, Inc. reserves the right to correct clerical or typographical
errors without penalty.
503349 rev5 [t4] 8/14 (?M) Printed in USA
 Loading...
Loading...