Portapres 2 Finger Blood Pressure System User manual
Portapres Model-2
Non--invasive continuous finger blood pressure measurement and recording system
User’s Guide
TNO TPD Biomedical Instrumentation
Amsterdam, The Netherlands
c 1999 TNO TPD Biomedical Instrumentation. All rights reserved. This document is for information purposes only.
warranty, disclaimer of warranties and limitations
tno makes no warranty or representation, either express or implied, with respect to the portapres device, its quality, merchantability, or fitness for a particular purpose. The equipment is provided as is, no oral or written information or advice given by either party or its employees shall create a warranty or make any modification, extension or addition to the warranty
tno shall not be liable for any direct, indirect, incidental or consequential damages, including lost profits and damages for personal injury or property damage, arising from or in connection with the licensed rights or its use whatsoever.
In no case shall tno tpd biomedical instrumentation’s liability exceed the purchase price for the device.
Information in this document is subject to change without notice and does not represent a commitment on the part of TNO TPD Biomedical Instrumentation. The Full license version of the BeatScope software is furnished under a license agreement. The software may be used only in accordance with that agreement.
Modelflow, Portapres and Finometer are trademarks of TNO TPD Biomedical Instrumentation. Finapres is a registered trademark of Ohmeda Monitoring Systems.
No part of this publication may be reproduced, transmitted, transcribed, stored in a retrieval system, or translated into any language in any form by any means, for any other purpose than the purchaser’s personal use, without prior written permission of TNO TPD Biomedical Instrumentation.

Caution I This Portapres Model--2 User’s Guide describes the Portapres Model--2 device with serial number greater than 180 (June 1999). Every Portapres Model-- 2 unit has an identification label with a specific identification string. The last three digits of this string contain the serial number of the Portapres Model--2 system. If, for example, an identification label of a Portapres Model--2 control unit (located on the bottom) displays ‘99.09.M2.CU.234’ then the serial number of the Portapres Model--2 system is ‘234’.
Caution II The Portapres Model--2 system may only be used in the combination of units as delivered by TNO TPD Biomedical Instrumentation.
If, however, you want to use units of a Portapres Model--2 system in combination with units of another Portapres Model--2 system, please note that:
•To maintain designed operator and patient safety, you are not allowed to combine the units of a Portapres Model--2 device with serial number 001 to 180 with the units of a system with a serial number above 180.
Note that this also applies to the Portapres batteries, AC adapter and battery charger. Batteries delivered with a Portapres Model--2 system with a serial number greater than 180 can be identified by the ‘Portapres Model--2’ label.
If you are in doubt, contact TNO TPD Biomedical Instrumentation.
•Finger cu s however can be used and exchanged with other Portapres Model-- 2 systems without any limitations.
Contents
1Introduction
1.1 |
Portapres Model--2 |
3 |
1.2 |
Related publications |
4 |
1.3 |
Warranty |
4 |
1.4 |
Technical support |
5 |
2Safety information
2.1 |
Warnings, patient safety |
7 |
2.2 |
Cautions |
7 |
2.3 |
Precautions |
8 |
2.4 |
Symbols and icons |
8 |
2.5 |
Protective measures |
9 |
3System description
3.1 |
Checklist of carrying case contents |
13 |
3.2 |
Waist belt |
14 |
3.3 |
Frontend unit |
16 |
3.4 |
Height correction unit |
17 |
3.5 |
Finger cu s |
17 |
3.6 |
Control unit |
19 |
3.7 |
Batteries |
19 |
3.8 |
Portapres AC adapter |
21 |
3.9 |
Portapres NiCd battery charger |
22 |
4Setting up Portapres Model--2
4.1 |
Connecting a DC power source |
23 |
4.2 |
Loading a Portapres battery |
24 |
4.3 |
Connecting the Portapres AC adapter |
27 |
4.4 |
Placing the Waist belt, Frontend and Height correction unit |
28 |
4.5 |
Connecting the Control unit |
30 |
5Using the Control unit
5.1 |
Control unit keypad |
33 |
5.2 |
Portapres modes |
33 |
portapres |
1 |
5.3 Error-mode |
38 |
6Configuring Portapres
6.1 |
Set finger switching interval |
41 |
6.2 |
Null the Height correction unit |
42 |
6.3 |
Enter patient data |
45 |
6.4 |
Check the Flash memory card |
46 |
6.5 |
Change date and time |
50 |
6.6 |
Connect the Analog output unit |
50 |
6.7 |
Start on--line PC monitoring with BeatScope |
53 |
7Performing a measurement
7.1 |
Using finger cu s |
55 |
7.2 |
Checklist |
58 |
7.3 |
Starting a measurement |
58 |
7.4 |
Measurement options |
60 |
7.5 |
Run time messages |
62 |
7.6 |
Stopping a measurement |
63 |
7.7 |
Transferring data to a PC |
64 |
8Error messages and troubleshooting
8.1 |
Audible indicators |
65 |
8.2 |
Error messages |
65 |
9Maintenance, calibration and storage
9.1 |
Cleaning |
71 |
9.2 |
Storage |
72 |
9.3 |
Pressure calibration check |
73 |
10 |
Specifications |
|
10.1 |
Performance |
75 |
10.2 |
Mechanical |
77 |
10.3 |
Electrical |
78 |
10.4 |
Environmental |
79 |
2 |
portapres |

1 Introduction
1.1 Portapres Model--2
Portapres Model--2 is a battery operated portable instrument to monitor finger arterial pressure continuously for 24 hours or longer under ambulatory conditions. The cu based measurement of blood pressure in a finger uses the arterial vol- ume--clamp method of the Czech physiologist J. Penázˇ of Brno, and the Physiocal —physiological calibration— criteria for the proper unloading of the finger arteries of K.H. Wesseling. With the finger changing position when ambulant, hydrostatic pressure level changes and these changes are measured by a so--called Height correction unit and compensated. For prolonged finger pressure monitoring as is possible with Portapres, the device automatically switches the measurement between two adjacent fingers at selectable time intervals, usually every half hour. When a measurement is started with finger switching activated Portapres becomes fully automatic and will continue monitoring without further operator intervention.
Portapres stores the full, height corrected, finger pressure waveform, the height correction value and run time messages on a built--in Flash memory card. After transferring the data from the Flash memory card to a PC beat--to--beat results are computed with the BeatScope software package.
 PORTAPRES
PORTAPRES
Figure 1.1 Portapres Model--2
portapres |
3 |
Introduction
1.2 Related publications
This User’s Guide was written for use by the operators of the Portapres Model--2 device. It contains installation and operating instructions, it explains warnings and operational hazards, it describes proper finger cu procedures, and ends with some routine maintenance procedures and a full device specification. Other documents relevant for Portapres Model--2 operation are:
•Portapres Model--2 Carrying Instructions
Instruction guide for the patient carrying Portapres.
•Portapres Model--2 Reference Guide
The Reference Guide contains background information about the Portapres, its measurement principles, some hints and pitfalls, and a bibliography on finger arterial pressure measurement with FinapresTM and Portapres.
•Portapres Model--2 NiCd Battery Charger
Instructions for the use of the Portapres Model--2 NiCd battery charger.
•BeatScope 1.0 User’s Guide
Guide to the Windows 9x/NT BeatScope beat viewing and analysis software package.
1.3 Warranty
The Portapres Model--2 device and its accessories are constructed of high quality materials and great care has been taken in its manufacture. We stand behind our product and will do what is in our power to have you as a satisfied customer and Portapres user. The Portapres system is guaranteed by TNO TPD Biomedical Instrumentation (BMI) for a period of one year after the date of purchase. During this warranty period BMI will, without charge for labor or parts, repair or replace defective parts.
4 |
portapres |
Introduction
The warranty does not include the following:
•Finger cu s. Still, finger cu s are reusable items which can, given proper care and handling, often be used for several years.
•Transport costs and insurance of the shipment of the Portapres to BMI.
•Defects caused by repairs through unauthorized personnel, or the use of accessories not obtained from, or approved by BMI.
•Periodic check--ups.
•Damage through misapplication, misuse, or failure to follow the instruction in this User’s Guide, in the Related publications (section 1.2), or in other accompanying documents.
•Accidents that a ected Portapres or its accessories.
1.4 Technical support
If the product fails to function properly, or when assistance, or service, or recalibration, or spare parts are required, please contact:
Dr ir G.J. Langewouters
at:
TNO TPD Biomedical Instrumentation
Academic Medical Center, Suite K2-228
Meibergdreef 9
NL -- 1105 AZ Amsterdam
The Netherlands
Phone |
+ 31 20 566 3343 |
Fax |
+ 31 20 697 6424 |
E--mail |
bmi@tpd.tno.nl |
portapres |
5 |

Introduction
Portapres contains no field serviceable parts. Servicing to any component of this device, therefore, is to be performed by TNO TPD Biomedical Instrumentation only. Unauthorized repairs or modifications will void the warranty and may violate the conformity of Portapres Model--2 with the requirements of the Medical Device Directive 93/42/EEC set forth by TNO TPD Biomedical Instrumentation.
6 |
portapres |
2 Safety information
2.1 Warnings, patient safety
•The data produced by Portapres or the accompanying software is intended as an adjunct in patient assessment and should not be used as the sole means for determining a patient’s diagnosis.
•Portapres is a finger blood pressure monitor. Do not use the finger cu s on other members of the body, such as a toe or the wrist of an infant. Performance on a toe is undocumented. Use on the wrist of a neonate or small infant substantially reduces flow to the hand and can be maintained only for very short periods of less than one (1) min.
•Portapres can only be used on adult humans and on children over the age of 7 years. Performance in younger subjects is undocumented.
•To maintain the designed operator and patient safety only use accessories, such as finger cu s, batteries, power supply, and battery charger, that are provided by TNO TPD Biomedical Instrumentation.
•(U.S.A.) Federal law restricts this device to sale by or on the order of a physician.
•To maintain the designed operator and patient safety, peripheral equipment that is connected to Portapres or one of its components, must be certified according to EN 60601-1 for electromedical equipment.
•Portapres is class B equipment according to EN 60601-1. Use the Portapres AC adapter only with a properly grounded (100 – 240 V, 50 or 60 Hz) AC power outlet. If no such properly grounded outlet is available, for maximal safety, power Portapres from its Portapres batteries only.
2.2 Cautions
•Portapres cannot be used inside, or in the vicinity of, magnetic resonance imaging (MRI) equipment. Strong magnetic fields may damage some Portapres components.
•To prevent possible damage to the keys of the Control unit, do not use sharp or hard objects to press the keys. Only use your fingertips to press the keys.
portapres |
7 |
Safety information
•Never clean the device or its components by submersion in a liquid. Follow the cleaning instructions in section 9.1,
•No field serviceable parts are inside the device. Any repair or modification to any component of this device is to be carried out by TNO TPD Biomedical Instrumentation personnel only. Unauthorized repairs will void the warranty and possibly the CE--mark requirements.
•Do not apply air pressure to a finger cu unless it is wrapped around a finger or other solid object.
•Do not attempt to repair defective finger cu s. This may substantially a ect measurement accuracy.
•Do not unroll finger cu s to a flat shape but maintain its rolled up conical shape as much as possible and as far as compatible with proper cu application (See section 7.1). Unrolling to a flat shape will damage finger cu s.
2.3 Precautions
•To obtain finger blood pressure measurements of the highest accuracy the movement of the hand and fingers should be minimized and the hand should be kept near heart level whenever possible, even though hydrostatic level errors are compensated.
•Finger blood pressure accuracy depends on the correct application and proper sizing of the finger cu s. Always verify that you are using a proper size cu (as described in section 7.1)
•To obtain correction for hydrostatic e ects on finger blood pressure, make sure that the Height correction unit is connected and properly nulled. Check that the reference part of the Height correction unit is really fixed at heart level and the transducer part at finger cu level.
2.4 Symbols and icons
On the Portapres device, its components and accessories the following icons may
8 |
portapres |

Safety information
be found.
!Read accompanying documents
Rechargeable battery
Non--rechargeable battery

 0344 Indicates compliance to Medical Device Directive 93/42/EEC
0344 Indicates compliance to Medical Device Directive 93/42/EEC
Type B Equipment
Dispose of as hazardous waste
2.5 Protective measures
Cu pressures applied to the finger of up to 350 mmHg are practically painless and do no harm unless applied for long periods. Portapres electrical circuits do not touch the skin, and are not in galvanic contact with body fluids. The following measures are taken for the safety and comfort of the patient monitored and for the convenience of the operator.
portapres |
9 |
Safety information
Electrical protective measures
•Low cu LED voltage (<1.8 V) and power dissipation (<50 mW) reduce electrical hazard and prevent undue heating which might cause skin irritation.
•An electrical short circuit in the cu or in the instrument cuts o cu pressure within 1 s.
•An interrupted frontend or cu cable cuts o cu pressure within 1 s.
•All analog signal outputs are short circuit proof.
•A self test of essential instrumental functions and parameters is performed every second of time.
Cu pressure protective measures
• An electrical circuit in the Pump unit limits pressure to 400 mmHg.
• Compressed air fed to the Frontend unit is pressure regulated to 380 mmHg (0.5 bar) by an air pressure controller in combination with a solid state pressure transducer.
•A watch dog timer cuts o cu pressure and resets the Portapres computer and software in case of internal computer malfunction.
•A cu pressure greater than 250 mmHg sustained for 2.5 s cuts o cu pres-
sure.
• During the start procedure cu pressure is limited to a maximum of 295 mmHg to last less than 2 s.
General system protective measures
•If cu pressure oscillates during a measurement the software takes action to remove the oscillation, although such oscillations present no hazard or discomfort to the patient.
•When fully contracted finger arteries are detected during the start procedure, allowing no pressure monitoring, Portapres issues a warning display to the Control unit, shuts o cu pressure, waits 100 s, and tries to start again.
10 |
portapres |
Safety information
•Increases in the contractional state of the finger arteries to such a degree that a correct measurement is dubious are flagged in the Control unit display by showing question marks (?). Before this situation actually occurs the operator is alerted of full contraction being near, by exclamation marks (!) in the display. In addition, these warnings are stored in the data files on the Flash memory card for later evaluation.
portapres |
11 |
12 |
portapres |
3 System description
3.1 Checklist of carrying case contents
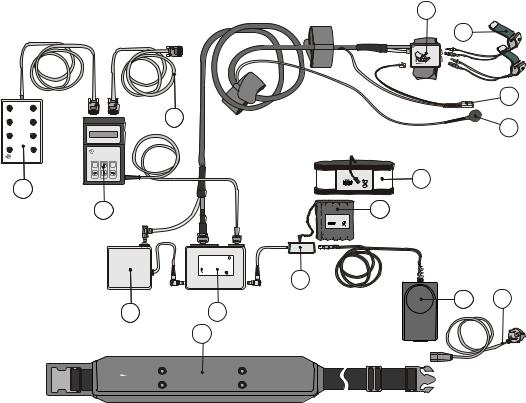
When a Portapres Model--2 device is shipped its component parts are contained in an aluminium carrying case. This section describes and lists the standard and optional items of the Portapres Model--2 system, so you may check the case contents upon arrival. For a system overview see Figure 3.1.
Standard Portapres device
a.Waist belt containing a Pump unit, a Main unit, and a battery compartment with a DC Power connector block.
b.Main unit within the Waist belt containing the electronics printed circuit boards of the Portapres Model--2 device.
c.Pump unit within the Waist belt containing a two--cylinder air compressor with its associated filters, and control and power conserving electronics.
d.Battery compartment within the Waist belt to hold a battery connected to the device through a DC Power connector block.
e.Control unit equipped with a keypad and an LCD 2 line by 16 character display.
f.Frontend unit containing an air pressure control valve, a pressure transducer, sensitive signal preamplifiers and various connectors for cu s and Height correction unit.
g.Height correction unit liquid filled tube partly integrated in the frontend cable surrounding sheet, transducer part at finger level.
h.Height correction unit liquid filled tube partly integrated in the frontend cable surrounding sheet, zero pressure reference part at heart level.
i.Finger cu s: 2 S(mall) white colored cu s, 2 M(edium) beige colored cu s, and 2 L(arge) blue colored cu s.
j.Analog output unit with 8 BNC connectors providing analog signal outputs.
k.Lithium battery optional.
l.NiCd battery optional.
m.Portapres AC adapter and adapter cable of 1.5 m length with 4-pin Lemo DC connector.
portapres |
13 |
System description
n.Serial interface cable from the Control unit to a remote PC.
o.Power cord (country dependent type) for Portapres AC adapter.
p.Screwdriver special type for small, partially hidden screws.
q.Diskettes (3) containing a version of the Beatscope software with limited possibilities. The full version is an optional extra.
Standard documentation
1.Portapres Model--2 User’s Guide The user’s guide to Portapres (this guide).
2.Portapres Model--2 Reference Guide Background information on the measurement of finger arterial pressure.
3.Portapres Model--2 Carrying Instructions Information for the patient carrying Portapres.
4.BeatScope 1.0 User’s Guide The BeatScope software user’s guide.
Optional accessories
•Portapres Lithium batteries, non--rechargeable.
•Portapres NiCd batteries, rechargeable.
•Portapres NiCd battery charger, for Portapres rechargeable NiCd batteries, includes user’s guide.
•Full-license software, complete system BeatScope software (set of 3 diskettes), including the limited-license plus beat--to--beat analysis software.
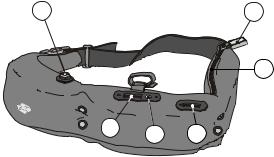
3.2 Waist belt
The Waist belt is made of flexible Neoprene and has three compartments to hold, from left to right (Figure 3.2) the Pump unit, the Main unit, and the battery.
The highly e cient air compressor maintains su cient air pressure to inflate the finger cu to finger arterial pressure continuously. It is electronically controlled
14 |
portapres |
Portapres |
Height |
|
waveform |
level |
n |
Systolic |
Heart |
|
pressure |
Rate |
pressure |
Interval |
READY |
MODE |
12:34 |
STRT |
C1/15min |
+16 |
||
Mean |
Interbeat |
|
|
|
Diastolic |
Marker |
|
|
|
pressure |
signal |
|
PORTAPRES CONTROL UNIT |
|
TPD Biomedical Instrumentation
PORTAPRES ANALOG OUTPUTS
TPD Biomedical Instrumentation
j |
e
System description
f
i
PORTAPRES FRONTEND UNIT
C2
C1
g
h
TNO-TPD |
k |
Biomedical Instrumentation |
|
PORTAPRES NiCd BATTERY PACK |
|
Non-rechargeable |
|
l |
Rechargeable
|
Bat1 |
Bat2 |
Adapter |
|
|
|
10 ..15 VDC |
|
|
|
Portapres Power Connector |
|
||
|
Portapres Model-2 |
|
|
|
|
TNO-TPD Biomedical Instrumentation |
|
|
|
|
Academic Medical Centre, Suite K2-228 |
|
|
|
|
Meibergdreef 9, 1105 AZ Amsterdam |
|
|
|
|
The Netherlands |
|
|
|
|
0344 |
d |
|
|
|
|
|
|
|
c |
b |
|
m |
o |
|
|
|
||
a

 PORTAPRES
PORTAPRES
a. |
Waist belt |
f. |
Frontend unit |
k. |
lithium battery |
b. |
Main unit |
g. |
Height correction transducer |
l. |
NiCd battery |
c. |
Pump unit |
h. |
Height correction reference |
m. AC adapter |
|
d. |
Power connector block |
i. |
finger cu |
n. serial interface cable |
|
e. |
Control unit |
j. |
Analog output unit |
o. AC adapter power cord |
|
Figure 3.1 Portapres Model--2 system overview
to conserve battery power. The electronics unit comprises a proprietary computer board, amplifiers and signal conditioners, signal conversion chips, and a Flash memory card for signal storage, mostly in low power CMOS technique. The bat-
portapres |
15 |
System description
tery compartment can be opened with a zipper and houses a DC Power connector block with several parallel inputs to connect two batteries and AC adapter power simultaneously, plus room for one Portapres battery.
a |
|
|
f |
|
|
|
|
e |
|
PO |
|
|
|
|
RTAP |
|
|
|
|
|
RES |
|
|
|
|
b |
c |
d |
|
|
|
|
||
a. quick connect type air outlet |
c. event marker button |
battery compartment |
||
for the frontend hose |
|
d. receptacle for the Control |
f. Power connector block to |
|
b. receptacle for the frontend |
unit cable connector |
accept DC power |
||
cable connector |
|
e. a zipper giving access to the |
|
|
Figure 3.2 The Portapres Model--2 Waist belt
3.3 Frontend unit
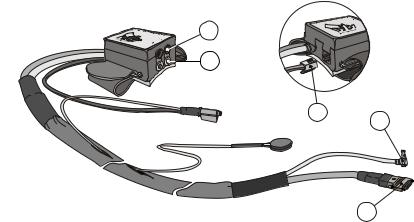
The Frontend unit (Figure 3.3) contains a high speed air pressure control valve, an air pressure transducer, electronics to drive the infrared plethysmograph in the finger cu , a two--position valve to switch between finger cu s, and a receptacle and electronics for the Height correction unit transducer. The liquid filled height correction tube runs partly within the sheet of the frontend cable.
The Frontend unit is connected to the Main unit with a multipole electrical connector and to the Pump unit via the pump air connector. The height correction electronics is connected to the frontend box rear side. Each finger cu connects to an electrical connector and to an air outlet on the frontend box front side. All connectors fit in only one receptacle and can be wired in only one way. Only the finger cu connectors can be inserted in one of two positions.
16 |
portapres |
System description
c
d
e
a
|
|
b |
a. pump air connector |
c. finger cu receptacle |
e. height correction receptacle |
b. frontend connector |
d. finger cu air outlet |
|
Figure 3.3 Portapres Model--2 Frontend unit
3.4 Height correction unit
The hydrostatic Height correction unit (Figure 3.4) consists of a liquid filled tube terminated at the measurement end in a sti pressure transducer membrane and at the reference end in a compliant plastic bag. The transducer part must be placed at finger cu level. The reference part must be at the blood pressure reference level, at the vertical level (height) of the tricuspid valve of the heart. The height correction pressure transducer is driven from the frontend via its electrical connector inserted at the rear of the frontend box. The liquid filled plastic tube runs partly within the sheet of the frontend cable.
3.5 Finger cu s
A finger cu (Figure 3.5) consists of an air bladder, electrical shielding, and several layers of plastic and rubber with Velcro at the outside. The cu contains the
portapres |
17 |

System description
b |
c |
a
a. |
reference part |
connector |
b. |
height correction unit |
c. transducer part |
Figure 3.4 Portapres Model--2 Height correction unit
infrared LEDand photodiode (also called ‘electronics’) of a photoelectric plethysmograph. A cu cable connector and an air hose connector plug into the frontend box. Cu s are available in three sizes: small (white), medium (beige) and large (blue). Please be sure to select a properly sized cu for each finger (Figure 7.1).
a
d
c
b
a. |
air hose connector |
c. |
cu air bladder |
b. |
cu cable connector |
d. |
infrared plethysmograph |
Figure 3.5 Finger Cu
18 |
portapres |
System description
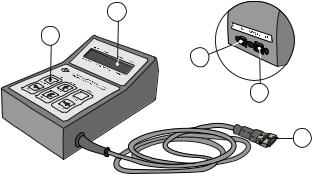
3.6 Control unit
The Control unit (Figure 3.6) has a 2×16 character LCD display and a six key keypad. The Control unit can be connected to or disconnected from the Portapres Main unit without a ecting Portapres operation. The Control unit is typically used at the start of a measurement to setup, to configure, and to start a Portapres measurement. During an ambulatory session the Control unit would be disconnected from the Main unit. If still connected, however, it can also be used during a measurement to online monitor Portapres output, on its display, or via separate analog outputs, or through a remote PC running Beatscope.
a
b
d
c
e
a. 2 × 16 character LCD display |
labelled (‘Analog outputs’) |
e. Control unit cable connector |
b. key pad with six keys |
d. receptacle for the serial |
to the Portapres Main unit |
c. receptacle for the cable |
interface cable to a remote |
|
from the Analog output unit |
PC labelled (‘RS--232 to PC’) |
|
Figure 3.6 Portapres Model--2 Control unit
3.7 Batteries
For true ambulatory measurements batteries are used. Two types of batteries are available: non--rechargeable Lithium batteries and rechargeable NiCd batteries.
portapres |
19 |

System description
Warning, explosion hazard! Never recharge non--rechargeable Portapres Lithium batteries since they may then explode.
3.7.1 Portapres non--rechargeable Lithium batteries
Portapres Lithium batteries (Figure 3.7) are used for long--term ambulatory measurements. The 13 Ah capacity of these packs su ces for at least 24 h of uninterrupted monitoring, even in hypertensive patients, and for up to 36 h in normotensive patients, without changing the battery.
TNO-TPD |
Biomedical Instrumentation |
PORTAPRES NiCd BATTERY PACK |
Non-rechargeable |
Figure 3.7 Portapres non--rechargeable Lithium battery
3.7.2 Portapres rechargeable NiCd batteries
Portapres rechargeable NiCd batteries (Figure 3.8) have a 2.4 Ah capacity when fully charged. This su ces for a minimum of 5 hof monitoring without changing the battery (at 25◦C).
Only use the Portapres NiCd battery charger that is supplied with the device to recharge Portapres NiCd batteries. Never use a di erent kind of charger. Refer to the accompanying Portapres NiCd battery charger documentation for detailed information.
20 |
portapres |
System description
TNO-TPD
Biomedical Instrumentation
PORTAPRES NiCd BATTERY PACK
Rechargeable
Figure 3.8 Portapres rechargeable NiCd battery
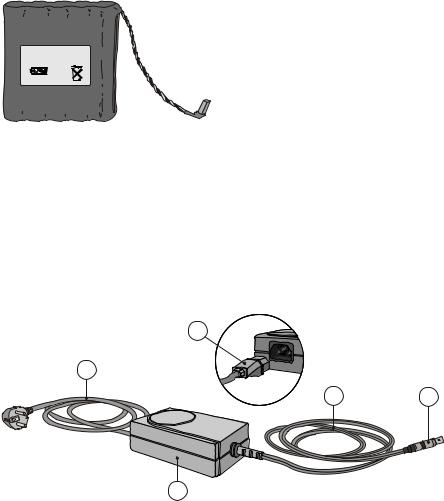
3.8 Portapres AC adapter
The Portapres AC adapter (Figure 3.9) may replace a battery for non--ambulatory monitoring periods such as in the cardiovascular laboratory or at home at night. The AC adapter can be used in all countries having line voltages between 100 and 240 VAC, at 50 or 60 Hz.
b
a
e |
d |
c
a. power cord (country |
b. |
power cord connector |
d. |
AC adapter cable connector |
dependent type) |
c. |
Portapres AC adapter |
e. |
Portapres AC adapter cable |
Figure 3.9 Portapres AC adapter
portapres |
21 |
 Loading...
Loading...