Page 1

Coaching-Gerät
Dispositif d’assistance
INSTRUCTIONS FOR USE
Gebrauchsanweisung
Instructions d’utilisation
Page 2

Page 3

Contents
English . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 1
Introduction. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 1
Intended Use . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 1
Operating Principle . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 1
Safety Information. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 1
Basic Orientation . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 3
Recommended Training . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 4
Using the TrueCPR Device . . . . . . . . . . . . . . . . . . . . . . . . . . . 5
Troubleshooting Tips . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 12
Operator’s Checklist . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 13
Data Management . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 14
Cleaning the Device . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 15
Storing the Device . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 15
Maintaining and Recycling the Device . . . . . . . . . . . . . . . . . 16
Symbols . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 17
Product Specifications . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 18
Safety Classifications . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 19
Electromagnetic Compatibility Guidance . . . . . . . . . . . . . . . 19
Regulatory Compliance Information . . . . . . . . . . . . . . . . . . . 23
German/Deutsch . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 25
Einführung. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 25
Verwendungszweck . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 25
Funktionsweise . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 25
Sicherheitsinformationen . . . . . . . . . . . . . . . . . . . . . . . . . . . 25
Grundlagen . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 27
Empfohlene Schulung. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 29
Verwenden des TrueCPR-Geräts . . . . . . . . . . . . . . . . . . . . . 29
Hinweise zur Fehlersuche/Fehlerbehebung . . . . . . . . . . . . . 38
Bedienerprüfliste. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 39
Datenverwaltung. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 40
Reinigung des Geräts . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 42
Page 4

Contents
Aufbewahrung des Geräts . . . . . . . . . . . . . . . . . . . . . . . . . . 42
Wartung und Recycling des Geräts . . . . . . . . . . . . . . . . . . . 42
Symbole . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 44
Technische Daten zum Produkt . . . . . . . . . . . . . . . . . . . . . . 45
Sicherheitsklassifikationen . . . . . . . . . . . . . . . . . . . . . . . . . . 46
Hinweise zur elektromagnetischen Verträglichkeit . . . . . . . . 46
Konformitätserklärung . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 51
French/Français . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 53
Introduction. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 53
Utilisation prévue . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 53
Principe de fonctionnement . . . . . . . . . . . . . . . . . . . . . . . . . 53
Informations relatives à la sécurité . . . . . . . . . . . . . . . . . . . . 53
Indications de base. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 55
Formation recommandée . . . . . . . . . . . . . . . . . . . . . . . . . . . 57
Utilisation de l’appareil TrueCPR . . . . . . . . . . . . . . . . . . . . . 57
Conseils de dépannage . . . . . . . . . . . . . . . . . . . . . . . . . . . . 65
Liste de contrôle de l’utilisateur . . . . . . . . . . . . . . . . . . . . . . 66
Gestion des données . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 67
Nettoyage de l’appareil. . . . . . . . . . . . . . . . . . . . . . . . . . . . . 68
Stockage de l’appareil . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 69
Maintenance et recyclage de l’appareil . . . . . . . . . . . . . . . . 69
Symboles . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 70
Spécifications du produit . . . . . . . . . . . . . . . . . . . . . . . . . . . 72
Classifications de sécurité . . . . . . . . . . . . . . . . . . . . . . . . . . 72
Guide de compatibilité électromagnétique. . . . . . . . . . . . . . 73
Informations de conformité règlementaire . . . . . . . . . . . . . . 77
Page 5

Introduction
English
The Physio-Control TrueCPR coaching device provides rescuers with real-time
feedback on chest compressions during cardiopulmonary resuscitation (CPR) in
accordance with current CPR guidelines.
IMPORTANT! Read these instructions carefully before use, and keep for future
reference.
These operating instructions provide the procedural steps for proper use of the
TrueCPR device, and can be used for training purposes. Operators should read
these instructions and be thoroughly familiar with the device before use.
Intended Use
The TrueCPR device is intended to provide feedback to assist rescuers to
perform CPR. Rescuers must be trained in CPR and use of the device.
The TrueCPR device is intended for use on patients eight years of age and older.
Indications
The TrueCPR device is indicated for use on patients in cardiac arrest
(unconscious, pulseless, not breathing normally).
Operating Principle
The TrueCPR device consists of a chest pad, which is placed on the sternum,
and a back pad, which is placed beneath the patient. The device determines the
depth of chest compressions during CPR by measuring the distance between the
chest pad and back pad. The device measures distance by sending and
receiving electromagnetic signals between the chest pad and back pad.
Safety Information
The following terms are used in these operating instructions:
Warning: Hazards or unsafe practices that may result in serious personal
injury or death.
Caution: Hazards or unsafe practices that may result in minor personal injury,
product damage, or property damage.
©2013 Physio-Control, Inc. 1
Page 6
Safety Information
WARNINGS
POSSIBLE PATIENT INJURY:
• The TrueCPR device is not intended for use on children or infants. Only
use the device on patients who are 8 years of age or older.
POSSIBLE INEFFECTIVE CPR:
• The TrueCPR device does not indicate complete compression release. Be
sure to release each compression completely.
• After use, inspect device and cable for wear or damage. Remove from
service if damaged.
• Do not use the TrueCPR device over very large metal surfaces. Perform
CPR unaided instead.*
• The TrueCPR device may provide incorrect feedback if not positioned
properly.
• Check back pad and chest pad position after patient movement and
during transport.
• If patient is on a mattress, place a backboard beneath the patient
according to standard protocols. Then insert the device back pad
between the patient and backboard.
• If the device cannot be positioned correctly on the patient or fails to
operate, remove the device and perform CPR unaided.
POSSIBLE DEVICE FAILURE: Do not modify the TrueCPR device.
POSSIBLE ELECTRICAL INTERFERENCE WITH DEVICE
PERFORMANCE: Equipment operating in close proximity may emit strong
electromagnetic or radio frequency interference (RFI), which could affect the
performance of this device. Avoid operating the device near cauterizers,
diathermy equipment, or other portable and mobile RF communications
equipment. See “Separation Distances” on page 22 for recommended
distances of equipment. Contact Physio-Control Technical Support if
assistance is required.
POSSIBLE EXPLOSION HAZARD: Do not use this device in the presence of
flammable gases or anesthetics.
* The TrueCPR device has been designed for use on hospital beds,
stretchers, gurneys, and in ambulances. The device is compatible with
common patient-worn or implanted metal, such as jewelry, ICDs, or
pacemakers.
IMPORTANT! Check the battery readiness indicator regularly. If indicator is not
flashing, replace batteries immediately.
2 TrueCPR Device Instructions for Use
Page 7
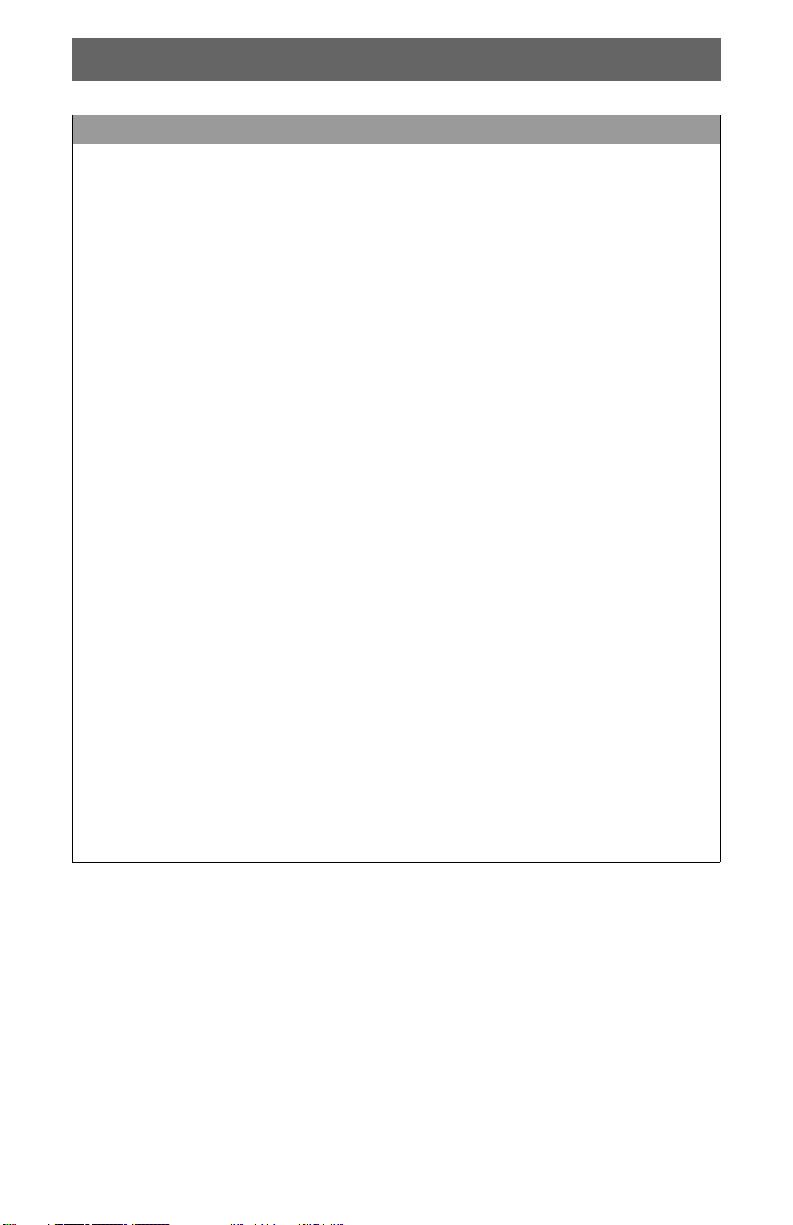
Basic Orientation
5
1
2
4
3
11
6
7
8
10
12
9
Chest Pad Screen
English
Item Description
Power Button: Press green button to turn device on. Press and hold for
1
2 seconds to turn device off.
Mute Button: Press to turn metronome on or off.
2
In Event Review mode, press to switch between screens.
3 Palm Pad: Hand placement area.
Airway Button: Press to switch metronome between Airway
4
(continuous compressions) mode and No Airway mode.
5 Screen: Displays compression feedback and other indicators.
Recoil Reminder: Reminder to release each compression completely.
6
Device does not indicate complete release. Icon is always present
during compression feedback.
Low Battery Indicator: Appears when remaining battery capacity is
7
less than 25 minutes of operation.
©2013 Physio-Control, Inc. 3
Compression Rate Meter: Displays rate as compressions per minute.
8
Changes to Inactivity Timer when compressions stop.
9 Elapsed Time Meter: Displays elapsed time since beginning of event.
10 Airway Indicator: Appears when device is in Airway mode.
Compression Target Zone: Changes from light green to dark green
11
when compression reaches target depth of 5–6 cm (2–2.5 in).
Page 8
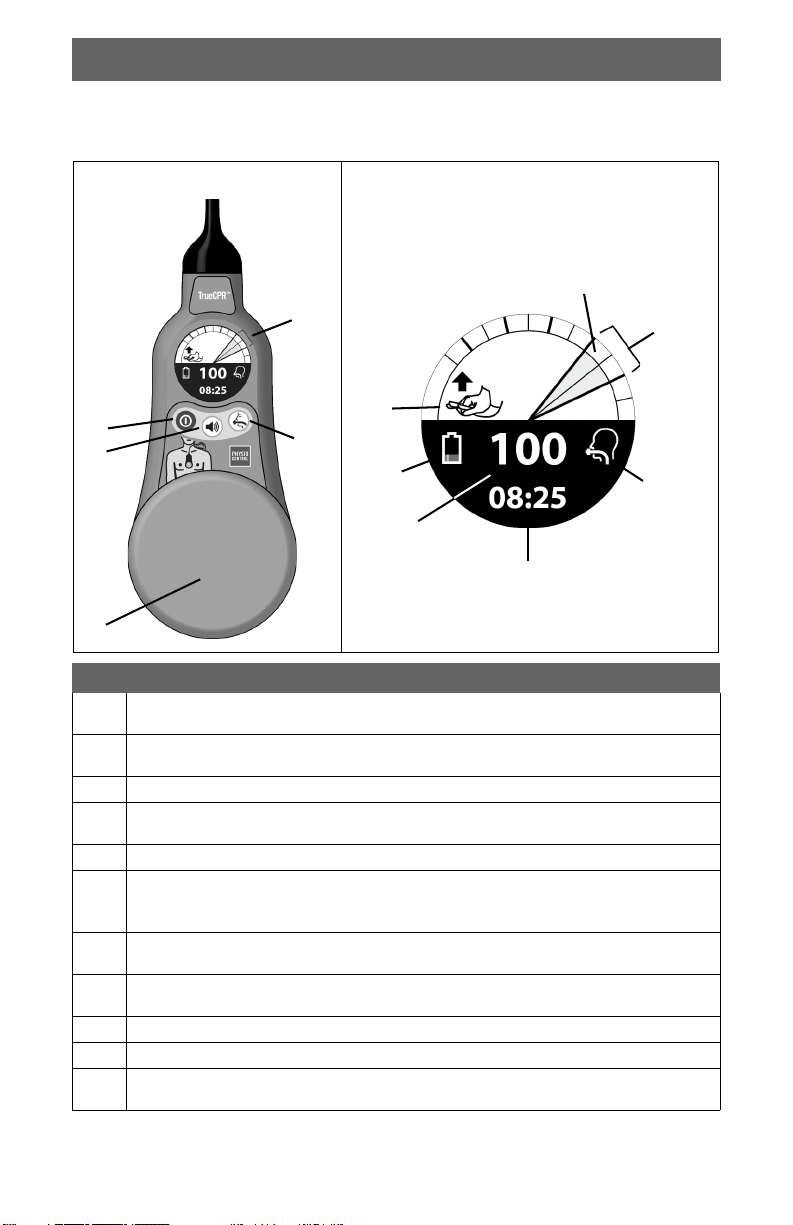
Recommended Training
1
2
3
Item Description
Compression Depth History: Dark green or gray segment in outer
12
circle shows depth of previous compression. Each tick mark represents
0.5 cm (0.2 in) of depth.
Back Pad
Item Description
Battery Readiness Indicator: Flashing LED on back pad handle
1
indicates battery capacity is sufficient for at least 25 minutes of
operation. Note: LED flashes approximately once every 4 seconds.
USB Port Cover: Provides access to USB port. Use a coin or similar
2
tool to open.
Battery Compartment: Holds two nonrechargeable Duracell
3
batteries. Use a coin or similar tool to open.
Recommended Training
Before using the TrueCPR device on a patient, the operator should be trained in
the proper technique for performing CPR with the TrueCPR device. It is
recommended that CPR performance metrics be included in your TrueCPR
device training program.
4 TrueCPR Device Instructions for Use
®
DL123
Page 9
English
AND
Using the TrueCPR Device
Performing CPR
To use the TrueCPR device:
1 Confirm patient is in cardiac arrest.
2 Remove the device from the carrying bag.
3 Separate the chest pad and back pad.
4 Press the (POWER) button to turn the device on. The calibration screen
appears.
IMPORTANT! Do not press on the chest pad until calibration is complete
and feedback screen appears (approximately 3 seconds).
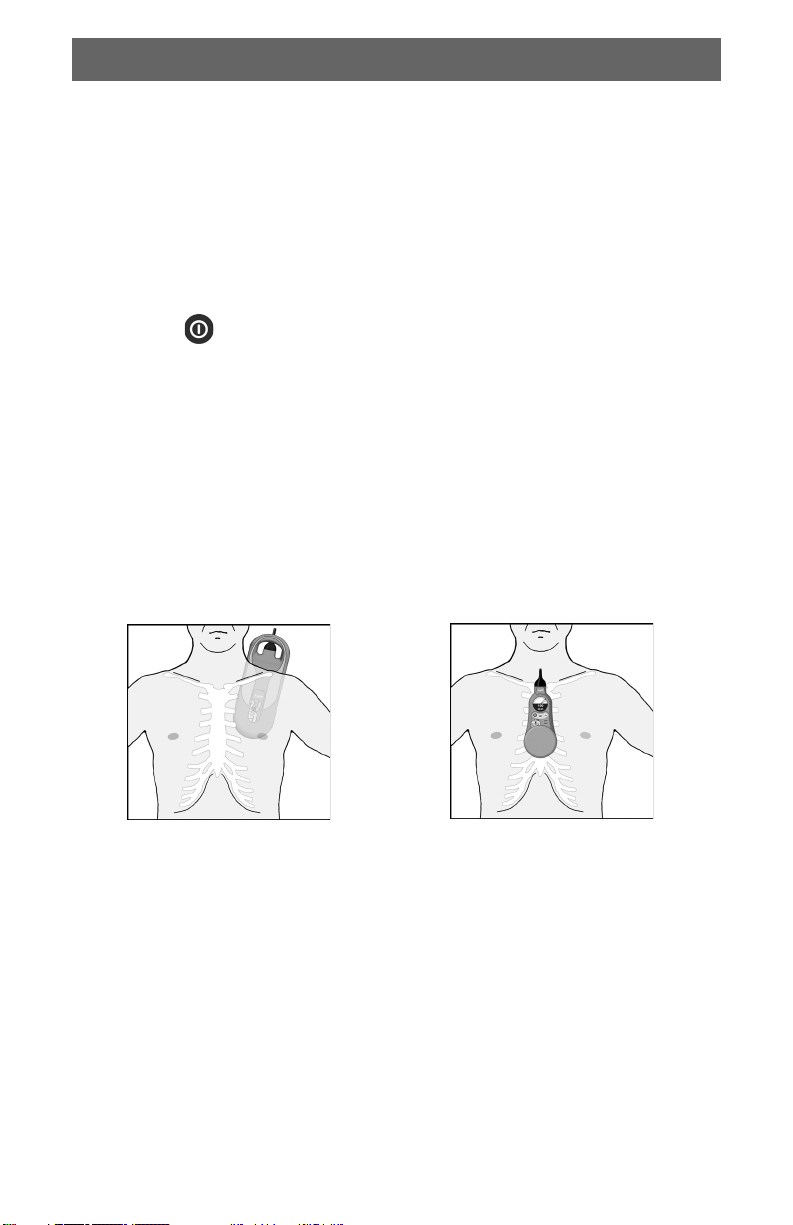
5 Position the back pad beneath the patient as shown in Figure 1. This helps
the back pad stay in position. The back pad may be placed on either side of
the patient.
6 Dry chest if necessary to prevent the chest pad from slipping.
7 Place the chest pad so that the palm pad is in the middle of the chest, on the
lower half of the sternum.
Figure 1 Recommended Device Position
Back Pad in Vertical Position Chest Pad in Vertical Position
Notes:
• If the Reposition Back Pad alert indicator appears, adjust the back pad
position. See “Alert Indicators” on page 11 for more information. If the
Reposition Back Pad alert persists, perform CPR unaided.
• If the device cannot be placed in the recommended position, the
alternative position shown in Figure 2 may be used. When the back pad is
placed in the side position, the chest pad must be rotated 180°. Keep the
palm pad in the middle of the chest on the lower half of the sternum. If the
device cannot be positioned properly, perform CPR unaided.
©2013 Physio-Control, Inc. 5
Page 10
Using the TrueCPR Device
AND
Figure 2 Alternative Device Position
Back Pad in Side Position Chest Pad in Rotated Position
8 Place the heel of your hand on the palm pad and begin compressions in time
with the metronome. Be sure to maintain correct CPR hand position.
IMPORTANT! Be sure to release each compression completely. The device
indicates compression depth and rate. It does not indicate recoil.
WARNING
POSSIBLE INEFFECTIVE CPR: If the screen on the TrueCPR device is
unreadable, unresponsive, or otherwise visibly damaged, do not use the
device. Perform CPR unaided instead.
9 Follow the device metronome to maintain your compression rhythm.
Note: To silence the metronome, press the (MUTE) button.
10 Observe the screen for depth feedback and any alert indications. Adjust your
technique based on the feedback. See the screen illustrations beginning on
page 9 for descriptions of the feedback.
11 Provide rescue breaths when prompted by the ventilation screens and tones.
Three short tones indicate when you should prepare to ventilate. Two longer
tones indicate when ventilations should occur. Resume chest compressions
immediately after the two longer tones.
Note: When in No Airway mode, the device provides ventilation prompts at a
6 TrueCPR Device Instructions for Use
Page 11
English
Inactivity timer
Airway indicator
ratio of 30 compressions to 2 ventilations (30:2). When in Airway mode,
ventilation prompts are not provided. See “Using the TrueCPR Device with
Secured Airway” on page 7 for more information.
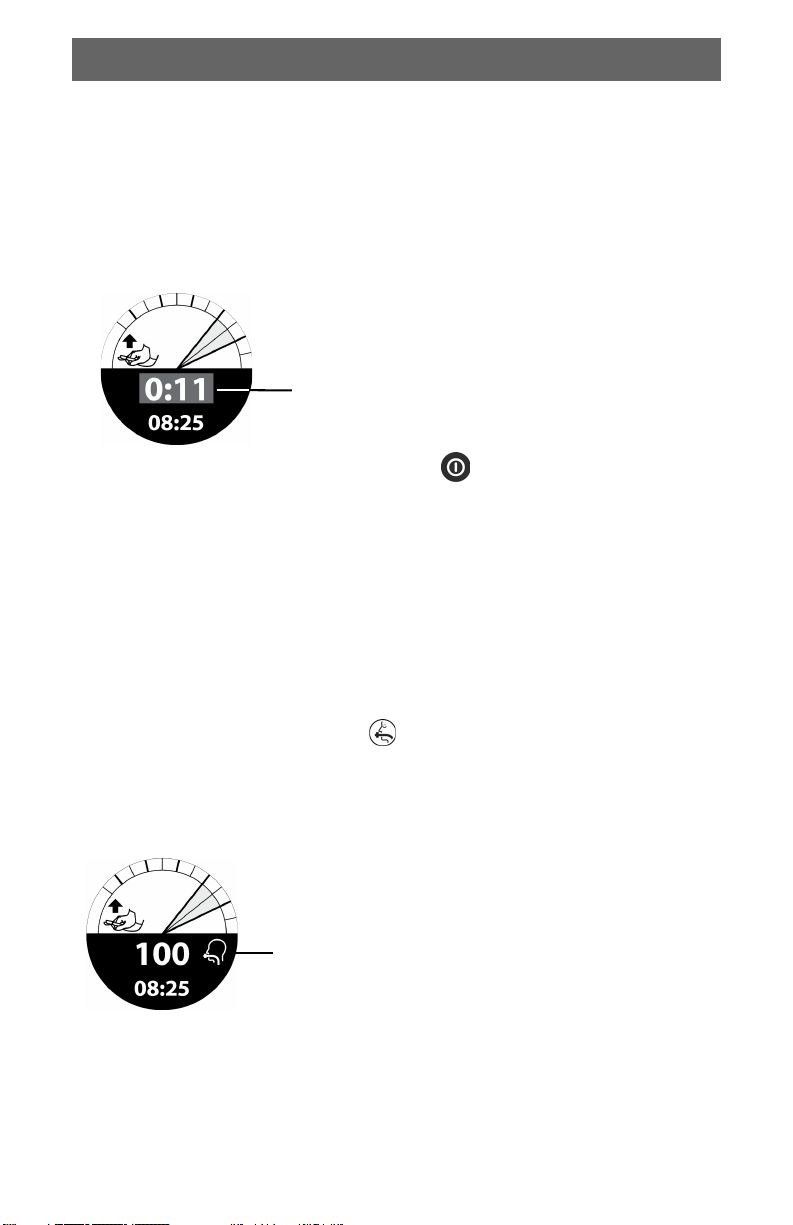
12 Continue CPR as long as indicated by your local protocols. When
compressions stop, the inactivity timer appears in place of the compression
rate meter. If compressions resume within 10 minutes, compression
feedback resumes. The device turns off automatically after 10 minutes with
no activity.
13 To turn the device off, press and hold the (POWER) button for 2 seconds.
Using the TrueCPR Device on a Mattress
If the patient is on a mattress, place a backboard beneath the patient according
to standard protocols. Then insert the TrueCPR device back pad between the
patient and backboard.
Using the TrueCPR Device with Secured Airway
When the TrueCPR device turns on, it defaults to No Airway mode. Ventilation
prompts are provided at a ratio of 30 compressions to 2 ventilations (30:2). If an
artificial airway is in place, press the (AIRWAY) button to switch the device to
Airway (continuous compressions) mode.
The following two changes occur when the device is switched to Airway mode.
• The airway indicator appears on the screen.
• Ventilation prompts are not provided.
Each time you press the AIRWAY button, the device switches between Airway
mode and No Airway mode.
©2013 Physio-Control, Inc. 7
Page 12

Using the TrueCPR Device
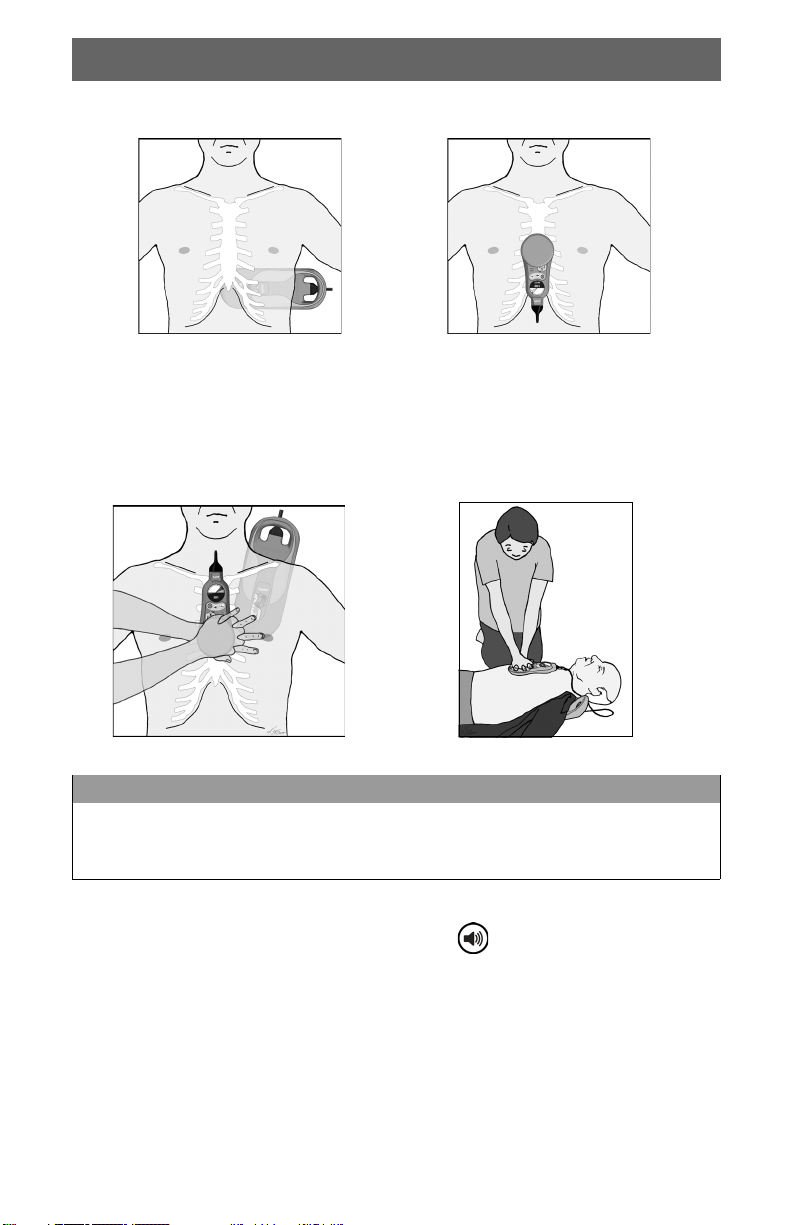
Using the TrueCPR Device During Defibrillation
It is acceptable to keep the TrueCPR device on the patient during defibrillation.
Observe the following precautions:
• Position the chest pad and defibrillator electrodes
as shown.
• Do not touch the TrueCPR device during defibrillation.
• Do not apply defibrillator electrodes on top of the
TrueCPR device cable.
Using the TrueCPR Device on Patients with Sternal Incisions
Establish and follow local protocols for use of the TrueCPR device on patients
with sternal incisions or injuries.
Using the TrueCPR Device on Patients with Implantable Devices
The magnetic fields used by the TrueCPR device have passed all interaction
testing and known safety standards for implanted pacemaker and ICD systems.
Using the TrueCPR Device for Training
The TrueCPR device may be used on a manikin for training purposes. Some
manikins may require updates to reach compression depths of 5 to 6 cm (2 to
2.5 in), consistent with AHA and ERC guidelines. You can use a manikin that
does not allow these depths, but the TrueCPR device will not reach the
compression target zone.
Note: The Reposition Back Pad indicator (see page 11) may appear if the
manikin contains large metal plates.
8 TrueCPR Device Instructions for Use
Page 13
Screen Illustrations
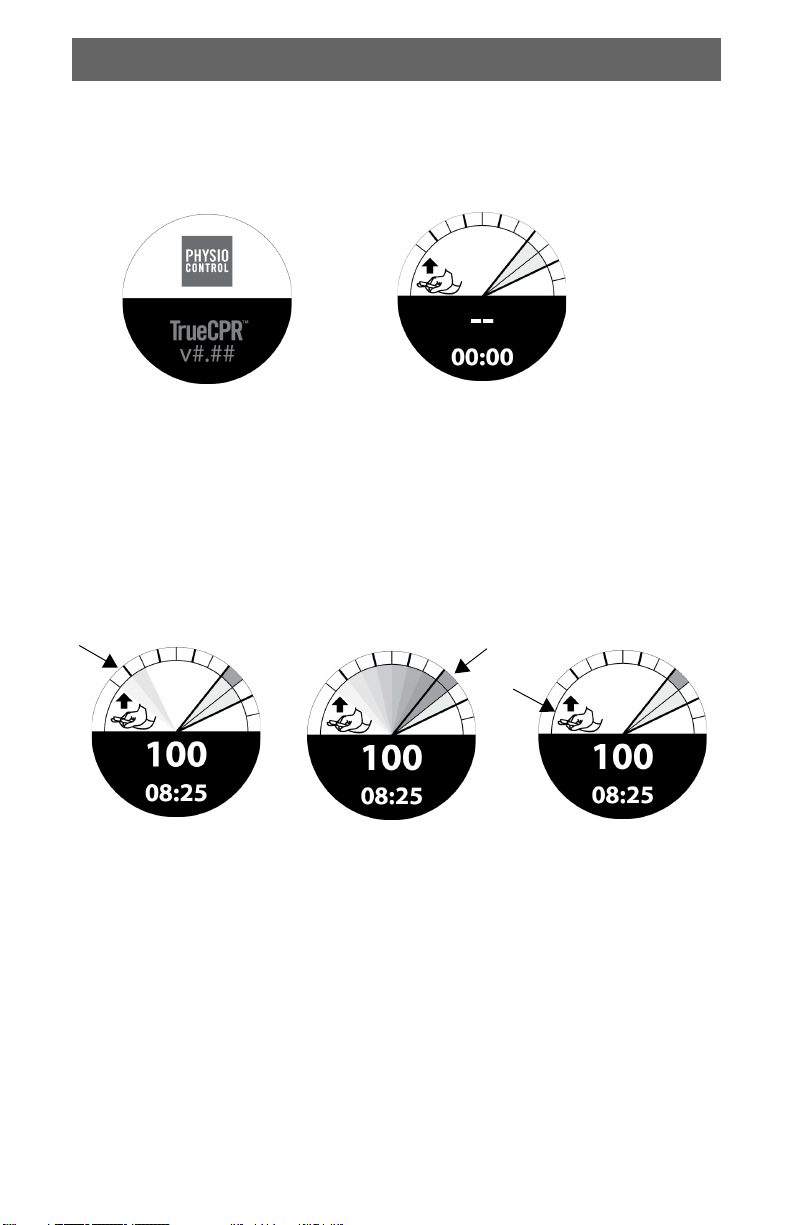
Calibration Screen
When you turn on the TrueCPR device, the calibration screen appears.
English
Calibration in progress.
• Do not apply pressure to
chest pad.
• Ensure chest pad and back
pad are separated during
calibration.
Calibration complete.
Begin chest compressions.
Compression Indicators
During compressions, the gray fan moves between the baseline and
compression zone as shown.
Compression in progress:
Gray fan representing
compression depth
moves across screen.
Depth indication begins
when compression depth
reaches 1.5 cm (0.6 in),
and moves across screen
as depth increases.
Sufficient depth:
Dark green wedge in
compression target
zone.
Recoil reminder:
Continue lifting your
hands off patient’s chest
after gray fan disappears.
Device does not indicate
complete release.
©2013 Physio-Control, Inc. 9
Page 14
Using the TrueCPR Device
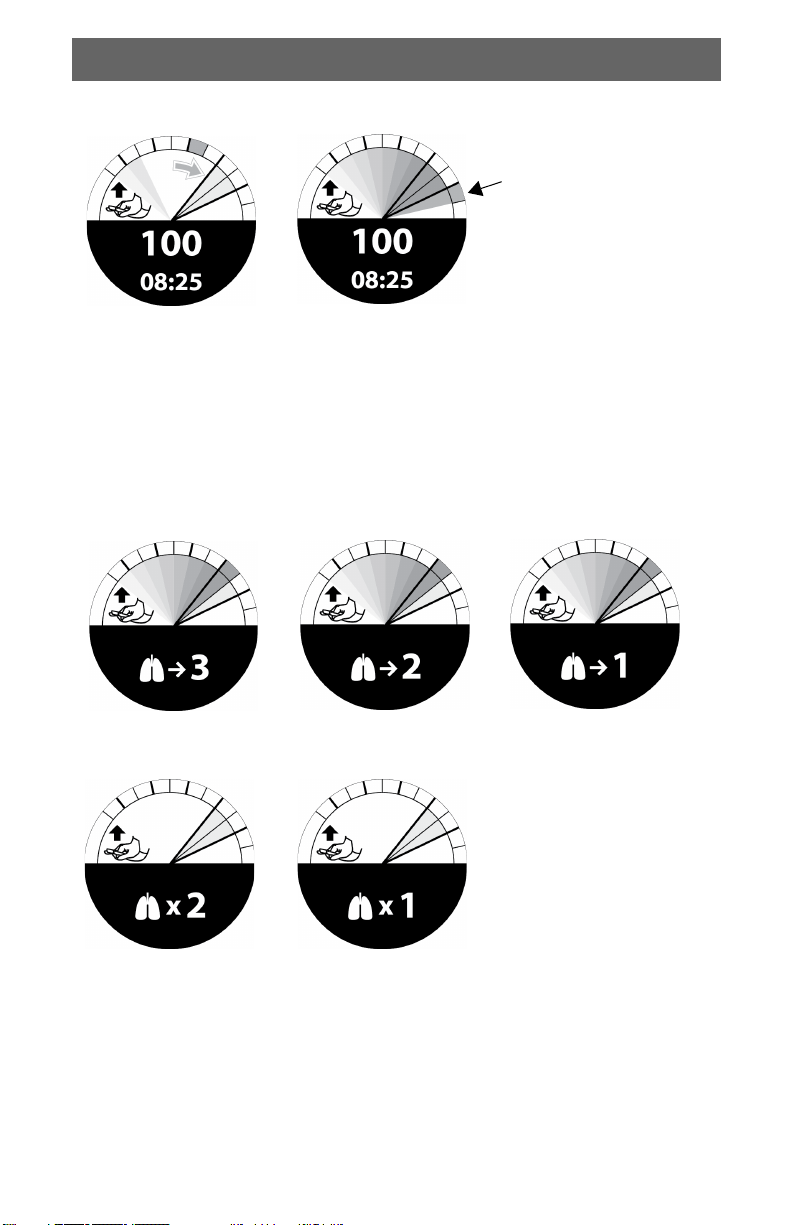
Incorrect Compression Indicators
Compression too
shallow: Arrow
appears.
Compression > 6 cm:
Orange wedge appears
outside compression target
zone.
Rescue Breath Indicators and Tones
In No Airway mode, the device provides a countdown screen and tones to
indicate when you should prepare to give rescue breaths. The countdown is
followed by two ventilation prompts. The compression to breath ratio is 30:2.
Countdown to ventilation prompts. Three short tones sound.
Ventilation prompts. Two long tones indicate
when ventilations should occur. Resume chest
compressions immediately after the two tones.
10 TrueCPR Device Instructions for Use
Page 15
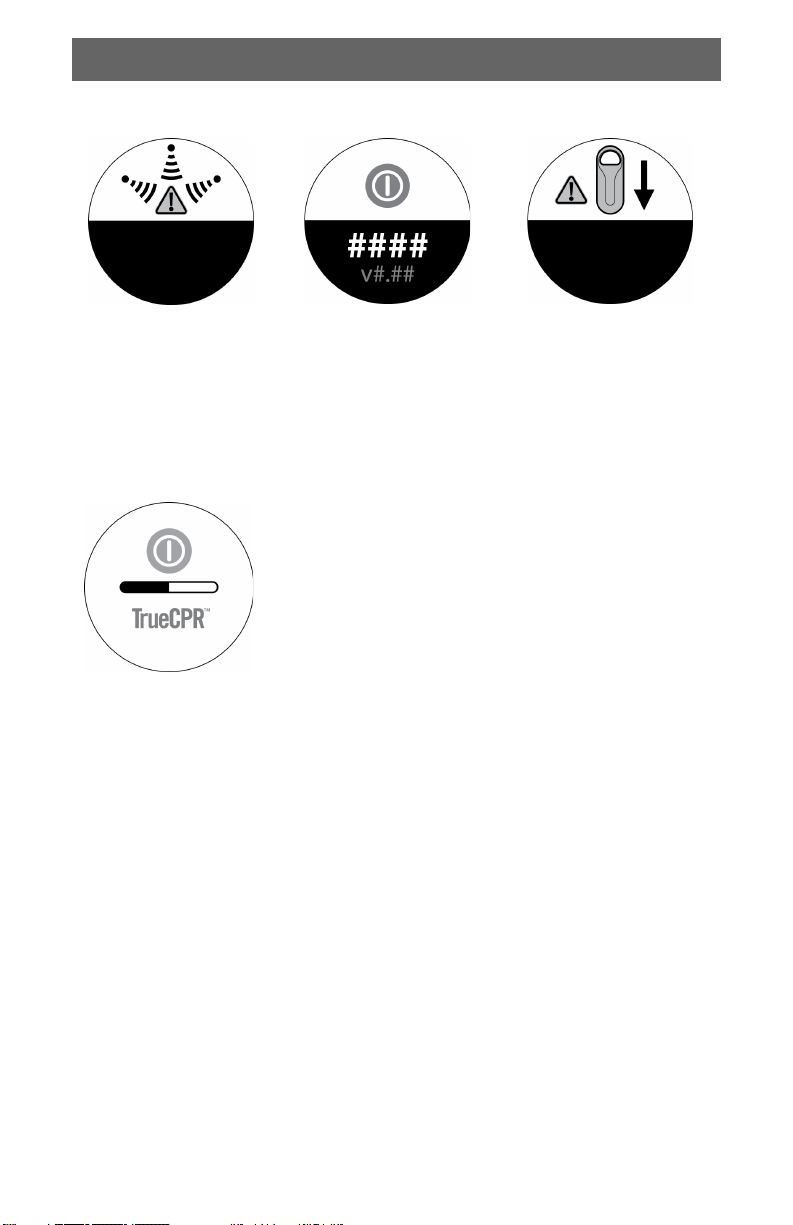
Alert Indicators
English
Electronic noise is
interfering with device.
Locate and remove
source of interference.
If indicator persists,
perform CPR unaided.
Shut-down Screen
Device cannot provide
feedback. Turn device off
and back on. If indicator
persists, perform CPR
unaided.
Progress bar appears during shut-down.
Back pad is incorrect
distance from chest pad.
Reposition back pad. If
indicator persists,
perform CPR unaided.
Note: Shut-down may take several minutes.
Do not remove batteries while shut-down is in
progress.
©2013 Physio-Control, Inc. 11
Page 16
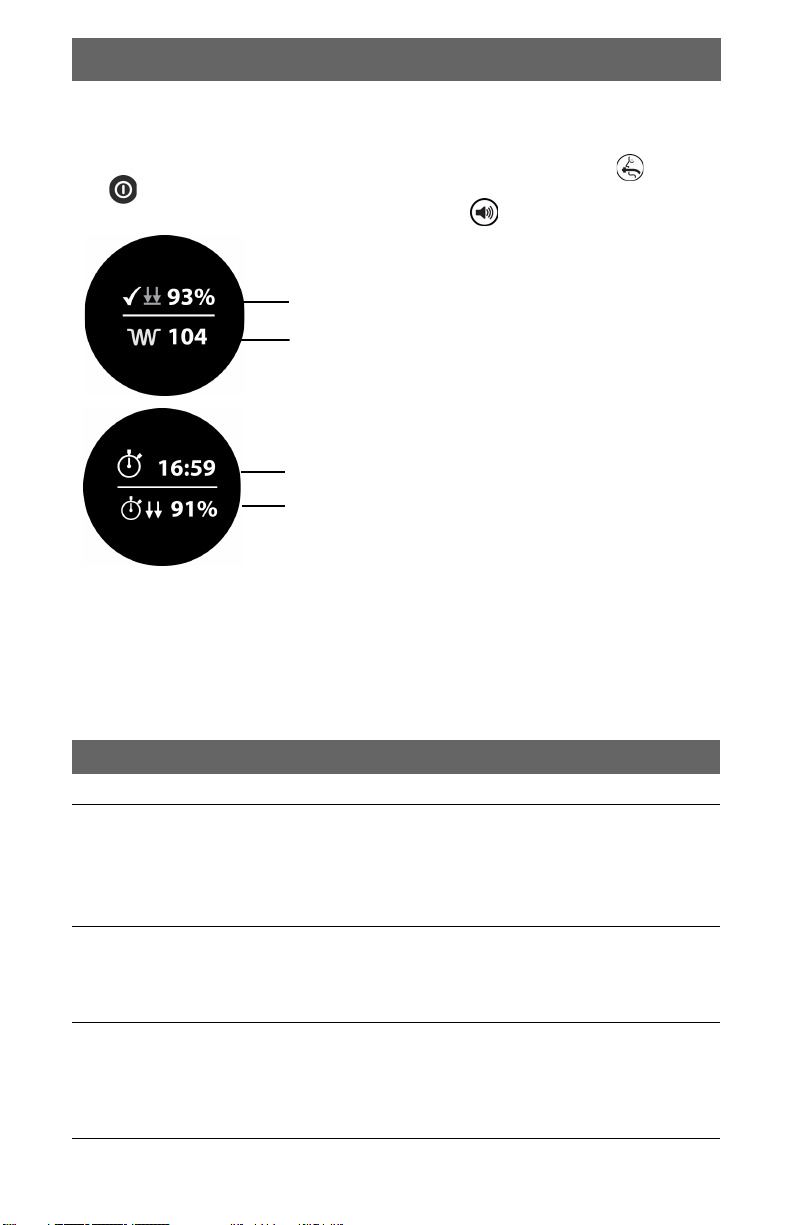
Troubleshooting Tips
Event Review Screens
Two event review screens display data for the most recent device use. To display
the event review screens, start with the device turned off. Press the (AIRWAY)
and (POWER) buttons simultaneously to turn the device on in Event Review
mode. To switch between the screens, press the (MUTE) button.
Percent of compressions with depth > 5 cm
(2 in).
Average compression rate (compressions per
minute).
Total event duration.
Percent of total event with compressions.
Troubleshooting Tips
These troubleshooting tips provide immediate corrective actions for possible
problems. If a problem persists, contact your local Physio-Control representative
for assistance. In the USA, call Technical Support at 800.442.1142.
OBSERVATION CORRECTIVE ACTION
No audible metronome. • Use compression rate meter for guidance.
Device does not function. • Only use device within specified operating
Display is visible, but does not
provide compression feedback.
Alert indicator persists. • Turn device off and back on. If indicator
12 TrueCPR Device Instructions for Use
temperature range of 0°–40°C (32°–104°F).
• Remove batteries, wait 30 seconds, and
then re-install batteries.
• Perform CPR unaided.
• Turn device off and back on. Do not apply
pressure to chest pad until calibration is
complete and feedback screen appears.
• Perform CPR unaided.
persists, perform CPR unaided.
• Contact Technical Support or your local
Physio-Control representative for
assistance.
Page 17
Check each box after completing
Year: ______ Unit Serial Number: ______________ Location: ________________
Date
Initials
DL123 batteries.
®
Coaching Device Operator’s Checklist
™
Operator’s Checklist
TrueCPR
Use this checklist to inspect the TrueCPR device. This form may be reproduced.
Instruction and Recommended Corrective Action
Check that battery readiness indicator on back pad
is flashing.
If not, replace batteries with two new,
nonrechargeable Duracell
Check device and cable for wear or damage.
If present, remove device from service and contact
your Physio-Control representative for assistance.
Page 18
Data Management
Data Management
The TrueCPR device can store compression data for three 60-minute sessions,
or up to six sessions totaling 180 minutes. When all available memory has been
used, the data from the oldest use is overwritten automatically.
Data can be transferred to a computer with TrueCPR device-compatible software
via USB connection. Event reports can be printed directly from the software. For
information on TrueCPR device reports and full event review functionality,
contact your local Physio-Control representative.
WARNING
SHOCK HAZARD:
• To avoid potential shock hazard, any device connected to the TrueCPR
device through the USB connector should be battery powered or certified
to IEC 60601-1.
• Do not use the USB connector if the TrueCPR device is damaged.
POSSIBLE ELECTRICAL INTERFERENCE: Using cables or accessories
that are not specified for use with the TrueCPR device may result in increased
emissions or decreased immunity from electromagnetic or radio frequency
interference (RFI) for this device. This could affect the performance of the
TrueCPR device or equipment in close proximity. Use only cables and
accessories specified in these instructions for use.
To initiate data transfer:
1 Connect a USB cable between the TrueCPR device and a computer with
compatible software.
Note: Use only a USB cable that meets the following specifications: USB 2.0
A-Male to Mini-B 5-pin Male, 28/24 AWG cable with ferrite core (gold plated),
1 m (3 ft).
2 Turn on the TrueCPR device. If the software on the computer does not start
automatically, open the program manually.
3 Follow the instructions in the software on the computer to select the TrueCPR
device and initiate data transfer. Data transfer may take up to 5 minutes if
memory is full. The following screens may appear on the TrueCPR device:
Data transfer in
progress.
14 TrueCPR Device Instructions for Use
Data transfer successful. Data transfer failed.
Page 19
English
Troubleshooting Tips for Data Management
OBSERVATION CORRECTIVE ACTION
Feedback screen appears on
TrueCPR device and
metronome sounds.
Data Transfer Failed screen
appears.
• Confirm USB cable is properly connected.
• Confirm computer is on.
• Replace USB cable.
• Use recommended USB cable (see Step 1
on page 14).
• Turn TrueCPR device off and check
connections. Then turn device on and
attempt data transfer again.
• If problem persists, contact Technical
Support.
Cleaning the Device
The TrueCPR device should be cleaned and inspected after each use.
1 Clean the device with a sponge or cloth that has been dampened with one of
the cleaning agents shown below:
• Quaternary ammonium compounds
• Hydrogen peroxide, 3% solution
• Sodium dichloroisocyanurate (NaDCC), 3000 ppm solution
• Chlorine bleach, 1:10 dilution with water
2 Inspect the device and cable for wear or damage. Remove from service if
damaged.
3 After cleaning, wipe off solution with a clean, damp cloth.
IMPORTANT! Do not immerse or soak the chest pad or back pad.
Storing the Device
Place chest pad onto back pad. Store in carrying bag so that battery readiness
indicator is visible.
CAUTION
POSSIBLE DEVICE DAMAGE: If the TrueCPR device will be stored for an
extended period of time, remove the batteries to prevent possible damage
due to battery leakage.
©2013 Physio-Control, Inc. 15
Page 20

Maintaining and Recycling the Device
Maintaining and Recycling the Device
Maintenance
The TrueCPR device and cable should be inspected regularly as part of your
routine equipment inspections. See the Operator’s Checklist on page 13 for
instructions.
Battery Replacement
Use only Duracell DL123 batteries for reliable performance.
WARNING
POSSIBLE LEAK, FIRE, OR EXPLOSION:
• Do not disassemble, puncture, crush, heat above 100°C (212°F), or
incinerate the batteries.
• Do not use rechargeable batteries in the TrueCPR device. Use only
nonrechargeable Duracell DL123 batteries.
• Replace both batteries at the same time with new Duracell DL123
batteries. Do not mix partially discharged and new batteries.
To replace the batteries, use a coin or similar tool to open the battery
compartment door. Remove the old batteries, wait 30 seconds, and then insert
new batteries as shown in the diagram on the compartment door. Close the
compartment door securely.
Note: If the battery readiness indicator does not flash after the batteries are
replaced, perform the following corrective actions.
• Verify batteries are installed correctly.
• Remove batteries, wait 30 seconds, and then re-install batteries.
• Turn device on and look for low battery indicator on screen. If low battery
indicator does not appear, battery readiness indicator may be faulty.
Contact Technical Support or your local Physio-Control representative for
assistance.
Service and Repair
The TrueCPR device contains no serviceable parts. If the device does not
function correctly, contact your local Physio-Control representative for
assistance.
16 TrueCPR Device Instructions for Use
Page 21
English
IP55
Service Life
During product development, the TrueCPR coaching device is subjected to
rigorous life testing. However, both rapid technological changes and the
availability of replacement parts limit the useful life of all modern medical devices.
The routine cleaning and inspection program recommended in this manual will
assist in reliable operation.
Recycling the Device and Batteries
Recycle the device and batteries according to national and local regulations. For
instructions on disposing of this product, see www.physio-control.com/recycling
or contact your local Physio-Control representative.
Symbols
The following symbols may be found on the TrueCPR device or packaging.
SYMBOL DESCRIPTION
Power button
Mute button
Airway button
Device requires two Duracell DL123 batteries (nonrechargeable)
Operating temperature 0° to 40°C (32° to 104°F)
Defibrillation proof, type BF applied part
Attention, consult accompanying documents. (Symbol has blue
background and graphical symbol is white.)
Do not step on the device.
Do not sit on the device.
Do not dispose of this product in the unsorted municipal waste stream.
Dispose of this product according to local regulations. For instructions
on disposing of this product, see www.physio-control.com/recycling.
Enclosure ingress protection code per IEC 60529. This rating provides
for a specified degree of protection against the ingress of particles and
the harmful ingress of water.
Device includes RF transmitter.
Mark of conformity according to the European Medical Device
Directive 93/42/EEC
©2013 Physio-Control, Inc. 17
Page 22

Product Specifications
or
or
YYYY
SYMBOL DESCRIPTION
Mark of conformity to Canadian and US standards
Mark of conformity to ACA standards
Alert sign associated with a Class 2 device under the R&TTE Directive
(Radio and Telecommunications Terminal Equipment)
Manufacturer’s identification number (part number)
Catalog number
Serial number
Date of manufacture
Manufacturer
Authorized EC representative
Product Specifications
SPECIFICATION DESCRIPTION
Power Requirements Batteries: 2 Duracell nonrechargeable DL123 cells
Operating Time 180 minutes at room temperature with new batteries
Operating Temperature Device can be used at 0° to 40°C (32° to 104°F).
Applied part temperature may reach 43°C (109°F) during
extended use at ambient temperature of 40°C (104°F).
Shelf Life Provides 30 minutes of CPR feedback after 24 months without
use at 25°C (77°F), starting with new batteries installed.
Storage Temperature -30° to 70°C (-22° to 158°F)
Relative Humidity 5 to 90%, non-condensing
Altitude -382 to 4,572 m (-1,253 to 15,000 ft)
Object and Water
Resistance
Weight Less than 0.75 kg (1.65 lb) with batteries installed
Ventilation Prompts 2 ventilation prompts every 30 compressions in No Airway mode.
Compression Depth Target depth range of 5 to 6 cm (2 to 2.5 in)
Metronome Rate 104.4
Model 80596-000003
IP55 per IEC 60529 and EN 1789
No ventilation prompts in Airway mode.
± 1 compressions per minute, consistent with AHA and
ERC guidelines.
18 TrueCPR Device Instructions for Use
Page 23
English
Safety Classifications
CATEGORY CLASSIFICATION
Protection Against Electric Shock Internally powered ME equipment.
Type BF defibrillation proof applied parts.*
Protection Against Solid Object and
Liquid Ingress
Mode of Operation Continuous operation
* Type BF defibrillation proof applied parts are the underside of the chest pad and the top
of the back pad. See page 17 for symbol illustration.
IP55
Electromagnetic Compatibility Guidance
Electromagnetic Emissions
Table 1 Guidance and Manufacturer’s Declaration - Electromagnetic Emissions
The TrueCPR coaching device, model 80596-000003, is intended for use in the
electromagnetic environment specified below. The customer or user should ensure that
the TrueCPR device is used in such an environment.
Emissions Test Compliance Electromagnetic Environment - Guidance
RF emissions
CISPR 11
RF emissions
CISPR 11
Harmonic emissions
IEC 61000-3-2
Voltage fluctuations/
flicker emissions
IEC 61000-3-3
Group 1 The TrueCPR device uses RF energy only for its
Class B The TrueCPR device is suitable for use in all
Not applicable
Not applicable
internal function. Therefore, its RF emissions are
very low and are not likely to cause any interference
in nearby electronic equipment.
establishments including domestic and those
directly connected to the public low-voltage power
supply network that supplies buildings used for
domestic purposes.
The TrueCPR device is battery-powered.
The TrueCPR device emits a continuous wave signal at 12 ± 0.1 kHz. The maximum
Effective Isotropic Radiated Power (EIRP) and Effective Radiated Power (ERP) levels are
-40 dBm, measured at a distance of 3 m (10 ft).
Essential Performance
The TrueCPR device maintains safe and effective performance (accurate depth) when
operated in the electromagnetic environment specified in Table 2 through Table 4 .
©2013 Physio-Control, Inc. 19
Page 24
Electromagnetic Compatibility Guidance
Electromagnetic Immunity
Table 2 Guidance and Manufacturer’s Declaration - Electromagnetic Immunity
The TrueCPR device, model 80596-000003, is intended for use in the electromagnetic
environment specified below. The customer or user should ensure that the TrueCPR
device is used in such an environment.
Immunity Test
Electrostatic
discharge (ESD)
IEC 61000-4-2
Electrical fast
transient/burst
IEC 61000-4-4
Surge
IEC 61000-4-5
Voltage dips,
short
interruptions
and voltage
variations on
power supply
input lines
IEC 61000-4-11
Power
frequency (50/
60 Hz) magnetic
field
IEC 61000-4-8
Note: U
is the AC Mains voltage prior to application of the test level.
T
IEC 60601
Test Level
±6 kV contact
±8 kV air
±2 kV for power
supply lines
±1 kV for input/
output lines
±1 kV line(s) to
line(s)
±2 kV line(s) to
earth
<5% U
T
(>95% dip in UT)
for 0.5 cycle
40% U
T
(60% dip in UT)
for 5 cycles
70% U
T
(30% dip in UT)
for 25 cycles
<5% U
T
(>95% dip in UT)
for 5 sec
3 A/m 3 A/m Power frequency magnetic fields
Compliance
Level
±6 kV contact
±8 kV air
Electromagnetic Environment -
Guidance
Floors should be wood,
concrete, or ceramic tile. If floors
are covered with synthetic
material, the relative humidity
should be at least 30%.
Not applicable The TrueCPR device is battery-
powered.
Not applicable The TrueCPR device is battery-
powered.
Not applicable The TrueCPR device is battery-
powered.
should be at levels characteristic
of a typical location in a typical
commercial or hospital
environment.
20 TrueCPR Device Instructions for Use
Page 25
English
d 1.2 P=
d 1.2 P=
d 2.3 P=
Table 3 Guidance and Manufacturer’s Declaration - Electromagnetic Immunity
The TrueCPR device, model 80596-000003, is intended for use in the electromagnetic
environment specified below. The customer or user should ensure that the TrueCPR
device is used in such an environment.
Immunity Test
Conducted RF
IEC 61000-4-6
Radiated RF
IEC 61000-4-3
IEC 60601
Test Level
3 Vrms
150 kHz to
80 MHz
3 V/m
80 MHz to
2.5 GHz
Compliance
Electromagnetic Environment -
Level
Portable and mobile RF
communications equipment should be
used no closer to any part of the
TrueCPR device, including cables, than
the recommended separation distance
calculated from the equation applicable
to the frequency of the transmitter.
Recommended separation distance
3 Vrms
3 V/m
Where P is the maximum output power
rating of the transmitter in watts (W)
according to the transmitter
manufacturer and d is the
recommended separation distance in
meters (m).
Field strengths from fixed RF
transmitters, as determined by an
electromagnetic site survey,
less than the compliance level in each
frequency range.
Interference may occur in the vicinity of
equipment marked with the following
symbol:
Guidance
80 MHz to 800 MHz
800 MHz to 2.5 GHz
a
should be
b
Note 1: At 80 MHz and 800 MHz, the higher frequency range applies.
Note 2: These guidelines may not apply in all situations. Electromagnetic propagation is
affected by absorption and reflection from structures, objects and people.
©2013 Physio-Control, Inc. 21
Page 26
Electromagnetic Compatibility Guidance
PPP
Table 3 Guidance and Manufacturer’s Declaration - Electromagnetic Immunity
(Continued)
a Field strengths from fixed transmitters, such as base stations for radio (cellular/cordless) telephones
and land mobile radios, amateur radio, AM and FM radio broadcast and TV broadcast cannot be
predicted theoretically with accuracy. To assess the electromagnetic environment due to fixed RF
transmitters, an electromagnetic site survey should be considered. If the measured field strength in
the location in which the TrueCPR device is used exceeds the applicable RF compliance level above,
the TrueCPR device should be observed to verify normal operation. If abnormal performance is
observed, additional measures may be necessary, such as re-orienting or relocating the TrueCPR
device.
b Over the frequency range 150 kHz to 80 MHz, field strengths should be less than 3 V/m.
Separation Distances
Table 4 Recommended separation distances between portable and mobile RF
communications equipment and the TrueCPR device, model 80596-000003.
The TrueCPR device is intended for use in an electromagnetic environment in which
radiated RF disturbances are controlled. The customer or user of the TrueCPR device can
help prevent electromagnetic interference by maintaining a minimum distance between
portable and mobile RF communications equipment (transmitters) and the TrueCPR
device as recommended below, according to the maximum output power of the
communications equipment.
Rated maximum
output power of
transmitter
W
0.01 0.12 0.12 0.23
0.1 0.38 0.38 0.73
1 1.2 1.2 2.3
10 3.8 3.8 7.3
100 12 12 23
For transmitters rated at a maximum output power not listed above, the recommended
separation distance d in meters (m) can be determined using the equation applicable to
the frequency of the transmitter, where P is the maximum output power rating of the
transmitter in watts (W) according to the transmitter manufacturer.
Note 1: At 80 MHz and 800 MHz, the separation distance for the higher frequency range
applies.
Note 2: These guidelines may not apply in all situations. Electromagnetic propagation is
affected by absorption and reflection from structures, objects and people.
Separation distance according to frequency of transmitter
m
150 kHz to
80 MHz
d = 1.2
80 MHz to
800 MHz
d = 1.2
800 MHz to 2.5 GHz
d = 2.3
22 TrueCPR Device Instructions for Use
Page 27

English
Regulatory Compliance Information
Federal Communications Commission (FCC) Compliance
This device complies with part 15 of the FCC rules. Operation is subject to the following
two conditions: (1) This device may not cause harmful interference, and (2) this device must
accept any interference received, including interference that may cause undesired
operation.
WARNING
Changes or modifications not expressly approved by the party responsible for
compliance could void the user’s authority to operate the equipment.
Radio and Telecommunications Terminal Equipment Directive (R&TTE) Compliance
This equipment is a Class 2 device under the R&TTE Directive:
BE BG CZ DK DE
FR ES EL IE EE
IT CY LV LT LU
PL AT NL MT HU
PT RO SI SK FI
SE UK
©2013 Physio-Control, Inc. 23
Page 28

Page 29

Einführung
German/Deutsch
Das Physio-Control TrueCPR-Coaching-Gerät bietet Rettungspersonal während
der Herz-Lungen-Wiederbelebung (HLW) Feedback in Echtzeit gemäß den
aktuellen HLW-Richtlinien.
WICHTIG! Lesen Sie diese Gebrauchsanweisung sorgfältig durch und
bewahren Sie sie für später auf.
In dieser Gebrauchsanweisung werden die Schritte für die ordnungsgemäße
Verwendung des TrueCPR-Geräts beschrieben. Sie kann auch zu
Schulungszwecken verwendet werden. Bediener müssen diese
Gebrauchsanweisung lesen und mit dem Gerät vollkommen vertraut sein, bevor
sie es verwenden.
Verwendungszweck
Das TrueCPR-Gerät liefert Rettungspersonal Feedback, um es bei der HLW zu
unterstützen. Rettungspersonal muss in HLW und der Verwendung des Geräts
geschult sein.
Das TrueCPR-Gerät ist für Patienten ab acht Jahren vorgesehen.
Indikationen
Das TrueCPR-Gerät ist zur Verwendung bei Patienten mit Herzstillstand
(bewusstlos, kein Puls, keine Spontanatmung) bestimmt.
Funktionsweise
Das TrueCPR-Gerät besteht aus einem Brustsensor, der auf das Brustbein gelegt
wird, und einer Rückenplatte, die unter den Patienten gelegt wird. Das Gerät
bestimmt während der HLW die Tiefe der Thoraxkompressionen durch Messung
des Abstands zwischen Brustsensor und Rückenplatte. Zur Abstandsmessung
sendet und empfängt das Gerät elektromagnetische Signale zwischen
Brustsensor und Rückenplatte.
Sicherheitsinformationen
In dieser Gebrauchsanweisung werden die folgenden Begriffe verwendet:
Warnhinweis: Gefahrenquelle oder falsche Vorgehensweise, die zu
ernsthaften Verletzungen oder zum Tod führen kann.
©2013 Physio-Control, Inc. 25
Page 30

Sicherheitsinformationen
Vorsichtshinweis:Gefahrenquelle oder falsche Vorgehensweise, die zu
leichteren Verletzungen, zu Schäden am Produkt oder zu Sachschäden
führen kann.
WARNHINWEIS
MÖGLICHE VERLETZUNG DES PATIENTEN:
• Das TrueCPR-Gerät ist nicht zur Anwendung bei Kindern oder
Kleinkindern vorgesehen. Das Gerät darf nur für Patienten von
mindestens 8 Jahren verwendet werden.
MÖGLICHE INEFFEKTIVE HLW:
• Das TrueCPR-Gerät zeigt nicht das vollständige Entlasten nach der
Kompression an. Nach jeder Kompression vollständig entlasten.
• Das Gerät und Kabel nach der Anwendung auf Abnutzung und
Beschädigung überprüfen. Bei Beschädigung außer Betrieb nehmen.
• Das TrueCPR-Gerät nicht auf sehr großen Metallflächen verwenden.
Stattdessen HLW ohne Geräteunterstützung durchführen.*
• Wenn das TrueCPR-Gerät nicht ordnungsgemäß positioniert ist, liefert es
möglicherweise falsches Feedback.
• Nach Bewegung des Patienten und während des Transports Position
von Rückenplatte und Brustsensor überprüfen.
• Wenn der Patient auf einer Matratze liegt, gemäß Standardprotokollen
ein Rückenbrett unter den Patienten legen. Dann die Rückenplatte
zwischen Patient und Rückenbrett platzieren.
• Wenn das Gerät nicht ordnungsgemäß auf dem Patienten positioniert
werden kann oder wenn das Gerät nicht funktioniert, entfernen Sie es
und führen Sie die HLW ohne das Gerät durch.
MÖGLICHE STÖRUNGEN DES GERÄTS: Das TrueCPR-Gerät nicht
modifizieren.
MÖGLICHE BEEINTRÄCHTIGUNG DER GERÄTEFUNKTION DURCH
ELEKTRISCHE STÖRUNGEN: In unmittelbarer Nähe betriebene Geräte
können starke elektromagnetische Störsignale oder Hochfrequenzsignale
aussenden, die sich möglicherweise negativ auf die Funktionsfähigkeit dieses
Gerätes auswirken. Das Gerät sollte nicht in der Nähe von
Kauterisationsgeräten, Diathermiegeräten oder anderen tragbaren bzw.
mobilen Funkkommunikationsgeräten verwendet werden. In
„Schutzabstände“ auf Seite 50 sind die empfohlenen Schutzabstände
zwischen Geräten aufgeführt. Wenden Sie sich bei Bedarf an den
technischen Kundendienst von Physio-Control.
MÖGLICHE EXPLOSIONSGEFAHR: Dieses Gerät nicht in Gegenwart
entzündlicher Gase oder Anästhetika verwenden.
* Das TrueCPR-Gerät ist für die Verwendung in Krankenhausbetten, auf
Tragen, Liegen und in Rettungswagen vorgesehen. Das Gerät ist mit üblichen
am Patienten befindlichen oder implantierten Metallobjekten wie Schmuck,
Defibrillatoren oder Herzschrittmachern kompatibel.
26 Gebrauchsanweisung für TrueCPR-Gerät
Page 31

German/Deutsch
5
1
2
4
3
11
6
7
8
10
12
9
WICHTIG! Die Batteriebereitschaftsanzeige regelmäßig überprüfen. Wenn die
Anzeige nicht blinkt, die Batterien sofort ersetzen.
Grundlagen
Brustsensor Bildschirm
Nr. Beschreibung
Netztaste: Grüne Taste drücken, um das Gerät einzuschalten. Zum
1
Ausschalten des Geräts Taste 2 Sekunden lang gedrückt halten.
Ton-Aus-Taste: Drücken, um Metronom ein- oder auszuschalten.
2
Im Modus „Ereignisüberprüfung“ drücken, um zwischen Bildschirmen
zu wechseln.
3 Handflächenfeld: Fläche zum Platzieren der Hand.
Atemweg-Taste: Drücken, um Metronom zwischen Modus „Atemweg“
4
(kontinuierliche Kompressionen) und Modus „Kein Atemweg“
©2013 Physio-Control, Inc. 27
umzuschalten.
5 Bildschirm: Zeigt Kompressionsfeedback und weitere Daten an.
Entlastungserinnerung: Erinnerung, nach jeder Kompression
vollständig zu entlasten. Das Gerät gibt nicht die vollständige Entlastung
6
an. Das Symbol wird immer während des Kompressionsfeedbacks
angezeigt.
Anzeige für niedrigen Batteriestand: Wird angezeigt, wenn
7
Batterieladung für weniger als 25 Minuten Betrieb ausreicht.
Page 32

Grundlagen
1
2
3
Nr. Beschreibung
Kompressionsratenmessung: Zeigt die Anzahl der Kompressionen pro
8
Minute an. Wechselt zu Inaktivitäts-Timer, wenn keine Kompressionen
erfolgen.
Messung der abgelaufenen Zeit: Zeigt die seit Beginn des Ereignisses
9
abgelaufene Zeit an.
Atemweg-Anzeige: Wird angezeigt, wenn sich Gerät im Modus
10
„Atemweg“ befindet.
Kompressionszielzone: Wechselt von Hellgrün zu Dunkelgrün, wenn
11
Solltiefe der Kompression von 5-6 cm erreicht wird.
Verlauf der Kompressionstiefe: Das dunkelgrüne oder graue Segment
12
im äußeren Kreisbogen zeigt die Tiefe der vorherigen Kompression an.
Jeder Teilstrich stellt 0,5 cm Tiefe dar.
Rückenplatte
Nr. Beschreibung
Batteriebereitschaftsanzeige: Blinkende LED am Griff der
Rückenplatte gibt an, dass die Batterieladung für mindestens
1
25 Minuten Betrieb ausreicht.
Hinweis: LED blinkt einmal etwa alle 4 Sekunden.
Abdeckung des USB-Anschlusses: Bietet Zugang zu USB-Anschluss.
2
Mit Münze oder ähnlichem Gegenstand öffnen.
Batteriefach: Enthält zwei nicht aufladbare Duracell® DL123 Batterien.
3
Mit Münze oder ähnlichem Gegenstand öffnen.
28 Gebrauchsanweisung für TrueCPR-Gerät
Page 33

German/Deutsch
UND
Empfohlene Schulung
Der Bediener sollte vor der ersten Verwendung des TrueCPR-Geräts an einem
Patienten in der korrekten Ausführung der HLW mit dem TrueCPR-Gerät
geschult werden. Es wird empfohlen, die Messung der HLW-Leistung in das
Schulungsprogramm für das TrueCPR-Gerät aufzunehmen.
Verwenden des TrueCPR-Geräts
Ausführen einer HLW
Das TrueCPR-Gerät wird wie folgt verwendet:
1 Vergewissern, dass beim Patienten Herzstillstand vorliegt.
2 Das Gerät aus der Tragetasche nehmen.
3 Brustsensor und Rückenplatte trennen.
4Taste (NETZTASTE) drücken, um das Gerät einzuschalten. Der
Kalibrierungsbildschirm wird angezeigt.
WICHTIG! Erst auf den Brustsensor drücken, wenn Kalibrierung
abgeschlossen ist und Feedback-Bildschirm angezeigt wird (nach
ca. 3 Sekunden).
5 Rückenplatte wie in Abbildung 1 gezeigt am Patienten positionieren.
Hierdurch wird das Verrutschen der Rückenplatte erschwert. Die
Rückenplatte kann auf der linken oder rechten Seite des Patienten platziert
werden.
6 Brust ggf. abtrocknen, um Verrutschen des Brustsensors zu verhindern.
7 Den Brustsensor so positionieren, dass sich das Handflächenfeld in der Mitte
des Brustkorbs in der unteren Hälfte des Brustbeinbereichs befindet.
Abbildung 1 Empfohlene Position des Geräts
Rückenplatte in vertikaler Position Brustsensor in vertikaler Position
©2013 Physio-Control, Inc. 29
Page 34

Verwenden des TrueCPR-Geräts
AND
Hinweise:
• Wenn die Warnanzeige für die Neupositionierung der Rückenplatte
erscheint, die Rückenplatte neu positionieren. Weitere Informationen
finden Sie unter „Warnanzeigen“ auf Seite 36. Wenn dann die Warnung
zur Neupositionierung der Rückenplatte immer noch angezeigt wird, die
HLW ohne das Gerät ausführen.
• Wenn das Gerät nicht an die empfohlene Position gebracht werden kann,
kann die in Abbildung 2 gezeigte alternative Position verwendet werden.
Wenn die Rückenplatte an der seitlichen Position platziert wird, muss der
Brustsensor um 180° gedreht werden. Das Handflächenfeld muss in der
Mitte des Brustkorbs in der unteren Hälfte des Brustbeinbereichs bleiben.
Wenn das Gerät nicht ordnungsgemäß positioniert werden kann, die HLW
ohne das Gerät ausführen.
Abbildung 2 Alternative Position des Geräts
Rückenplatte in seitlicher Position Brustsensor in gedrehter Position
8 Handballen auf das Handflächenfeld legen und Kompressionen im Takt des
Metronoms beginnen. Die korrekte HLW-Handposition muss beibehalten
werden.
WICHTIG! Nach jeder Kompression vollständig entlasten. Das Gerät zeigt
die Kompressionstiefe und -rate an. Es zeigt nicht die Entlastung an.
30 Gebrauchsanweisung für TrueCPR-Gerät
Page 35

German/Deutsch
Inaktivitäts-Timer
WARNHINWEIS
MÖGLICHE INEFFEKTIVE HLW: Wenn der Bildschirm des TrueCPR-Geräts
unleserlich ist, nicht reagiert oder sichtlich beschädigt ist, das Gerät nicht
verwenden. Stattdessen HLW ohne Geräteunterstützung durchführen.
9 Die Kompressionen im Takt des Metronoms ausführen.
Hinweis: Zur Unterbrechung des Metronoms die Taste (TON AUS)
drücken.
10 Auf dem Bildschirm auf Feedback zur Kompressionstiefe und Warnanzeigen
achten. Die Kompression an das Feedback anpassen. Das Feedback wird in
den Abbildungen ab Seite 33 beschrieben.
11 Beatmung durchführen, wenn auf Bildschirm entsprechende Aufforderung
erscheint und Beatmungssignale ertönen. Drei kurze akustische Signale
geben an, wann die Vorbereitung auf die Beatmung erfolgen soll. Zwei
längere akustische Signale geben an, wann die Beatmung erfolgen soll.
Führen Sie Thoraxkompressionen sofort nach den beiden längeren
akustischen Signalen durch.
Hinweis: Im Modus „Kein Atemweg“ erfolgen die Aufforderungen zur
Beatmung im Verhältnis von 30 Kompressionen zu 2 Beatmungen (30:2). Im
Modus „Atemweg“ erfolgen keine Aufforderungen zur Beatmung. Weitere
Informationen siehe „Verwenden des TrueCPR-Geräts mit künstlichem
Atemweg“ auf Seite 32.
12 HLW so lange fortsetzen, wie in den lokalen Protokollen vorgeschrieben. Bei
Unterbrechung der Kompressionen wird statt der
Kompressionsratenmessung der Inaktivitäts-Timer angezeigt. Wenn
Kompressionen innerhalb von 10 Minuten fortgesetzt werden, erfolgt wieder
Kompressionsfeedback. Nach 10 Minuten Inaktivität wird das Gerät
automatisch ausgeschaltet.
13 Zum Ausschalten des Geräts die Taste (NETZTASTE) 2 Sekunden lang
gedrückt halten.
Verwenden des TrueCPR-Geräts auf einer Matratze
Wenn der Patient auf einer Matratze liegt, gemäß Standardprotokollen ein
Rückenbrett unter den Patienten legen. Dann die Rückenplatte des TrueCPRGeräts zwischen Patient und Rückenbrett platzieren.
©2013 Physio-Control, Inc. 31
Page 36

Verwenden des TrueCPR-Geräts
100
08:25
Atemweg-Anzeige
Verwenden des TrueCPR-Geräts mit künstlichem Atemweg
Das TrueCPR-Gerät ist beim Einschalten im Standardmodus „Kein Atemweg“.
Die Aufforderungen zur Beatmung erfolgen im Verhältnis von 30 Kompressionen
zu 2 Beatmungen (30:2). Wenn ein künstlicher Atemweg gelegt ist, die Taste
(ATEMWEG) drücken, um das Gerät in den Modus „Atemweg“ (kontinuierliche
Kompressionen) zu schalten.
Wenn das Gerät in den Modus „Atemweg“ geschaltet wird, treten die folgenden
beiden Änderungen ein.
• Auf dem Bildschirm erscheint die Atemweg-Anzeige.
• Es erfolgen keine Aufforderungen zur Beatmung.
Bei jedem Drücken der Taste ATEMWEG wechselt das Gerät zwischen dem Modus
„Atemweg“ und dem Modus „Kein Atemweg“.
Verwenden des TrueCPR-Geräts während der Defibrillation
Das TrueCPR-Gerät kann während der Defibrillation am Patienten bleiben.
Folgende Vorsichtsmaßnahmen treffen:
• Den Brustsensor und die Defibrillatorelektroden wie
in der Abbildung positionieren.
• Während der Defibrillation das TrueCPR-Gerät nicht
berühren.
• Defibrillatorelektroden nicht über dem Kabel des
TrueCPR-Geräts anbringen.
Verwenden des TrueCPR-Geräts bei Patienten mit sternalen Inzisionen
Die lokalen Protokolle für die Verwendung des TrueCPR-Geräts bei Patienten mit
sternalen Inzisionen oder Verletzungen ermitteln und befolgen.
Verwenden des TrueCPR-Geräts bei Patienten mit implantierten Geräten
Die vom TrueCPR-Gerät verwendeten Magnetfelder haben alle Interaktionstests
und Sicherheitsstandards für implantierte Herzschrittmacher und
Herzschrittmachersysteme erfüllt.
32 Gebrauchsanweisung für TrueCPR-Gerät
Page 37

German/Deutsch
Verwenden des TrueCPR-Geräts zu Schulungszwecken
Das TrueCPR-Gerät kann zu Schulungszwecken an einer Übungspuppe
verwendet werden. Bei manchen Übungspuppen ist möglicherweise eine
Aktualisierung erforderlich, um gemäß den AHA- und ERC-Richtlinien eine
Kompressionstiefe von 5 bis 6 cm zu erreichen. Übungspuppen, die diese
Kompressionstiefe nicht ermöglichen, können verwendet werden. In diesem Fall
erreicht das TrueCPR-Gerät jedoch nicht die Kompressionszielzone.
Hinweis: Wenn die Übungspuppe große Metallplatten enthält, erscheint
möglicherweise die Anzeige zur Neupositionierung der Rückenplatte (siehe
Seite 36).
Bildschirmabbildungen
Kalibrierungsbildschirm
Beim Einschalten des TrueCPR-Geräts wird der Kalibrierungsbildschirm
angezeigt.
Kalibrierung wird ausgeführt.
• Drücken Sie nicht auf den
Brustsensor.
• Stellen Sie sicher, dass
Brustsensor und Rückenplatte
während der Kalibrierung
voneinander getrennt sind.
©2013 Physio-Control, Inc. 33
Kalibrierung abgeschlossen.
Beginnen Sie mit den
Thoraxkompressionen.
Page 38

Verwenden des TrueCPR-Geräts
Kompressionsanzeigen
Während der Kompressionen bewegt sich der graue Fächer zwischen der
Grundlinie und der Kompressionszone (siehe Abbildung).
Kompression wird
ausgeführt: Der graue
Fächer stellt die
Kompressionstiefe dar
und bewegt sich über
den Bildschirm. Die
Anzeige der Tiefe
beginnt, wenn eine
Kompressionstiefe von
1,5 cm erreicht ist, und
bewegt sich mit
zunehmender Tiefe
entlang dem Bildschirm.
Ausreichende Tiefe:
Dunkelgrüner Keil in
Kompressionszielzone.
Anzeigen für falsche Kompression
Kompression zu gering:
Pfeil wird angezeigt.
Kompression > 6 cm:
Außerhalb der
Kompressionszielzone wird
oranger Keil angezeigt.
Entlastungserinnerung:
Heben Sie weiter die
Hände von der Brust des
Patienten, nachdem der
graue Fächer nicht mehr
angezeigt wird. Das
Gerät gibt nicht die
vollständige Entlastung
an.
34 Gebrauchsanweisung für TrueCPR-Gerät
Page 39

German/Deutsch
Indikatoren und akustische Signale für Beatmung
Im Modus „Kein Atemweg“ geben ein Countdown-Bildschirm und akustische
Signale an, wann die Beatmung verabreicht werden soll. Nach Ablauf des
Countdowns erfolgen zwei Aufforderungen zur Beatmung. Das Verhältnis von
Kompression zu Beatmung beträgt 30:2.
Countdown für Aufforderungen zur Beatmung. Drei kurze akustische Signale
ertönen.
Aufforderungen zur Beatmung. Zwei längere
akustische Signale geben an, wann die Beatmung
erfolgen soll. Setzen Sie Thoraxkompressionen
sofort nach den beiden akustischen Signalen fort.
©2013 Physio-Control, Inc. 35
Page 40

Verwenden des TrueCPR-Geräts
Warnanzeigen
Elektronische
Interferenzen stören
den Betrieb des Geräts.
Die Quelle der Störung
ermitteln und
entfernen. Wenn die
Anzeige weiterhin
erscheint, HLW ohne
Gerät durchführen.
Gerät liefert kein
Feedback. Das Gerät
aus- und wieder
einschalten. Wenn die
Anzeige weiterhin
erscheint, HLW ohne
Gerät durchführen.
Bildschirm für Herunterfahren
Falscher Abstand der
Rückenplatte zum
Brustsensor. Position der
Rückenplatte ändern.
Wenn die Anzeige
weiterhin erscheint, HLW
ohne Gerät durchführen.
Während des Herunterfahrens wird ein
Fortschrittsbalken angezeigt.
Hinweis: Das Herunterfahren kann mehrere
Minuten dauern. Während des
Herunterfahrens Batterien nicht entfernen.
36 Gebrauchsanweisung für TrueCPR-Gerät
Page 41

German/Deutsch
Bildschirme für Ereignisüberprüfung
Auf zwei Ereignisüberprüfungsbildschirmen werden Daten zur letzten
Verwendung des Geräts angezeigt. Zum Anzeigen der
Ereignisüberprüfungsbildschirme muss das Gerät zunächst ausgeschaltet sein.
Die Tasten (ATEMWEG) und (NETZTASTE) gleichzeitig drücken, um das
Gerät im Modus „Ereignisüberprüfung“ einzuschalten. Zum Wechseln zwischen
den Bildschirmen die Taste (TON AUS) drücken.
Prozentsatz der Kompressionen mit Tiefe > 5 cm.
Durchschnittliche Kompressionsrate
(Kompressionen pro Minute).
Gesamte Ereignisdauer.
Prozentsatz der Ereignisdauer mit
Kompressionen.
©2013 Physio-Control, Inc. 37
Page 42

Hinweise zur Fehlersuche/Fehlerbehebung
Hinweise zur Fehlersuche/Fehlerbehebung
Diese Hinweise zur Fehlersuche/Fehlerbehebung bieten Informationen zu
unmittelbaren Abhilfemaßnahme für mögliche Probleme. Wenn ein Problem
weiterhin auftritt, wenden Sie sich an einen Vertreter von Physio-Control vor Ort.
PROBLEM ABHILFE
Metronom nicht hörbar. • Zur Orientierung die
Gerät funktioniert nicht. • Gerät nur im angegebenen
Anzeige ist sichtbar, liefert
jedoch kein
Kompressionsfeedback.
Warnanzeige erscheint
weiterhin.
Kompressionsratenmessung verwenden.
Betriebstemperaturbereich von 0 °C - 40 °C
verwenden.
• Batterien herausnehmen und nach 30 Sekunden
erneut einlegen.
• HLW ohne Geräteunterstützung durchführen.
• Das Gerät aus- und wieder einschalten. Erst auf
den Brustsensor drücken, wenn Kalibrierung
abgeschlossen ist und Feedback-Bildschirm
angezeigt wird.
• HLW ohne Geräteunterstützung durchführen.
• Das Gerät aus- und wieder einschalten. Wenn
die Anzeige weiterhin erscheint, HLW ohne Gerät
durchführen.
• Wenden Sie sich zur Unterstützung an den
technischen Kundendienst oder an einen
Vertreter von Physio-Control vor Ort.
38 Gebrauchsanweisung für TrueCPR-Gerät
Page 43

abhaken
™
Bedienerprüfliste
-Coaching-Geräts
Prüfliste für Bediener des TrueCPR
Diese Prüfliste zum Überprüfen des TrueCPR-Geräts verwenden. Diese Prüfliste darf kopiert werden.
Jahr: ______ Seriennummer des Geräts: ______________
Standort: ________________
Datum
Namenskürzel
Nach Durchführung der jeweiligen Maßnahme das betreffende Kästchen
DL123 Batterien ersetzen
®
Überprüfen, ob Batteriebereitschaftsanzeige auf
Rückenplatte blinkt.
Wenn sie nicht blinkt, die Batterien durch zwei nicht
aufladbare Duracell
Das Gerät und Kabel auf Abnutzung und
Anleitung und empfohlene Maßnahmen
Beschädigung überprüfen.
Wenn Abnutzung oder Beschädigung festgestellt
wird, das Gerät außer Betrieb nehmen und Kontakt
mit dem zuständigen Vertreter von Physio-Control
aufnehmen.
Page 44

Datenverwaltung
Datenverwaltung
Das TrueCPR-Gerät kann Daten für drei Fälle von jeweils 60 Minuten oder für bis
zu sechs Fälle von insgesamt 180 Minuten speichern. Wenn kein Speicher mehr
frei ist, werden die ältesten Daten automatisch überschrieben.
Mithilfe von Software, die mit dem TrueCPR-Gerät kompatibel ist, können Daten
über eine USB-Verbindung auf einen Computer übertragen werden.
Ereignisberichte lassen sich direkt über die Software drucken. Informationen zu
Berichten des TrueCPR-Geräts und sämtlichen Funktionen der
Ereignisüberprüfung sind von der örtlichen Physio-Control-Vertretung erhältlich.
WARNHINWEIS
STROMSCHLAGGEFAHR:
• Um möglichen Stromschlaggefahren vorzubeugen, muss jedes Gerät, das
über den USB-Anschluss mit dem TrueCPR-Gerät verbunden ist,
batteriebetrieben oder nach IEC 60601-1 zertifiziert sein.
• Den USB-Anschluss nicht verwenden, wenn das TrueCPR-Gerät
beschädigt ist.
MÖGLICHE ELEKTRISCHE STÖREINFLÜSSE: Die Verwendung von
Kabeln oder Zubehörteilen, die nicht für die Verwendung mit dem TrueCPRGerät spezifiziert sind, kann zu höheren Abstrahlungen oder geringerer
Störfestigkeit bezüglich elektromagnetischer Störungen oder
Hochfrequenzstörsignalen des Geräts führen. Dies kann den Betrieb des
TrueCPR-Geräts oder von Geräten in unmittelbarer Nähe beeinträchtigen.
Nur Kabel und Zubehörteile verwenden, die in dieser Gebrauchsanweisung
angegeben sind.
Die Datenübertragung wird wie folgt gestartet:
1 Ein USB-Kabel zwischen dem TrueCPR-Gerät und einem Computer mit
kompatibler Software anschließen.
Hinweis: Nur ein USB-Kabel verwenden, das folgenden Spezifikationen
entspricht: USB 2.0 A-Stecker zu 5-pol. Mini-B-Stecker, Kabel 28/24 AWG
mit Ferritkern (vergoldet), 1 m.
2 Das TrueCPR-Gerät einschalten. Wenn die Software auf dem Computer nicht
automatisch gestartet wird, das Programm manuell öffnen.
3 Befolgen Sie die Anweisungen der Software auf dem Computer, um das
TrueCPR-Gerät auszuwählen und die Datenübertragung zu starten. Wenn der
40 Gebrauchsanweisung für TrueCPR-Gerät
Page 45

German/Deutsch
Speicher voll ist, kann die Datenübertragung bis zu 5 Minuten dauern. Auf
dem TrueCPR-Gerät werden eventuell die folgenden Bildschirme angezeigt:
Datenübertragung wird
ausgeführt.
Datenübertragung
erfolgreich.
Datenübertragung
fehlgeschlagen.
Hinweise zur Fehlersuche/Fehlerbehebung bei der Datenverwaltung
PROBLEM ABHILFE
Auf dem TrueCPR-Gerät wird
der Feedback-Bildschirm
angezeigt und das Metronom
ertönt.
Bildschirm „Datenübertragung
fehlgeschlagen“ wird
angezeigt.
• Sicherstellen, dass USB-Kabel
ordnungsgemäß angeschlossen ist.
• Sicherstellen, dass Computer
eingeschaltet ist.
• USB-Kabel auswechseln.
• Empfohlenes USB-Kabel verwenden
(siehe Schritt 1 auf Seite 40).
• Das TrueCPR-Gerät ausschalten und
Anschlüsse überprüfen. Dann Gerät
einschalten und Datenübertragung erneut
versuchen.
• Bei fortbestehendem Problem den
technischen Kundendienst verständigen.
©2013 Physio-Control, Inc. 41
Page 46

Reinigung des Geräts
Reinigung des Geräts
Das TrueCPR-Gerät muss nach jedem Gebrauch gereinigt und überprüft werden.
1 Das Gerät mit einem Schwamm oder Lappen reinigen, der mit einem der
folgenden Reinigungsmittel befeuchtet wurde:
• Quartäre Ammoniumverbindungen
• Wasserstoffperoxid, 3%-Lösung
• Natriumdichloroisocyanurat (NaDCC), Lösung von 3000 ppm
• Chlorbleiche, 1:10 verdünnt mit Wasser
2 Das Gerät und Kabel auf Abnutzung und Beschädigung überprüfen. Bei
Beschädigung außer Betrieb nehmen.
3 Nach dem Reinigen mit sauberem, feuchten Lappen abwischen.
WICHTIG! Brustsensor und Rückenplatte nicht in Flüssigkeiten eintauchen oder
einlegen.
Aufbewahrung des Geräts
Brustsensor auf Rückenplatte legen. So in Tragetasche aufbewahren, dass
Batteriebereitschaftsanzeige sichtbar ist.
VORSICHT
MÖGLICHE GERÄTESCHÄDEN: Wenn das TrueCPR-Gerät für längere Zeit
gelagert wird, die Batterien herausnehmen, um Schäden durch Auslaufen der
Batterien zu vermeiden.
Wartung und Recycling des Geräts
Wartung
Das TrueCPR-Gerät und das Kabel müssen regelmäßig im Rahmen der
routinemäßigen Geräteinspektionen überprüft werden. Eine Anleitung finden Sie
in der „Bedienerprüfliste“ auf Seite 39.
42 Gebrauchsanweisung für TrueCPR-Gerät
Page 47

German/Deutsch
Auswechseln der Batterien
Für zuverlässige Leistung nur Duracell DL123 Batterien verwenden.
WARNHINWEIS
MÖGLICHE AUSLAUF-, BRAND- ODER EXPLOSIONSGEFAHR:
• Die Batterien nicht auseinandernehmen, durchstechen, zerdrücken,
über 100 °C erhitzen oder anzünden.
• Im TrueCPR-Gerät keine wiederaufladbaren Batterien verwenden. Nur
nicht aufladbare Duracell DL123 Batterien verwenden.
• Beide Batterien gleichzeitig durch neue Duracell DL123 Batterien
ersetzen. Teilweise entladene und neue Batterien nicht zusammen
verwenden.
Zum Austauschen der Batterien die Batteriefachabdeckung mit einer Münze oder
einem ähnlichen Gegenstand öffnen. Die Batterien herausnehmen und nach 30
Sekunden die neuen Batterien wie im Diagramm auf der Batteriefachabdeckung
gezeigt einsetzen. Die Batteriefachabdeckung ordnungsgemäß schließen.
Hinweis: Wenn nach dem Austauschen der Batterien die
Batteriebereitschaftsanzeige nicht blinkt, die folgenden Abhilfemaßnahmen
ergreifen.
• Überprüfen, ob Batterien ordnungsgemäß eingelegt sind.
• Batterien herausnehmen und nach 30 Sekunden erneut einlegen.
• Das Gerät einschalten und auf dem Bildschirm die Anzeige für niedrigen
Batteriestand suchen. Wenn die Anzeige für niedrigen Batteriestand nicht
erscheint, ist die Batteriebereitschaftsanzeige möglicherweise defekt.
Wenden Sie sich zur Unterstützung an den technischen Kundendienst
oder an einen Vertreter von Physio-Control vor Ort.
Service und Reparatur
Das TrueCPR-Gerät enthält keine zu wartenden Teile. Wenn das Gerät nicht
ordnungsgemäß funktioniert, wenden Sie sich an einen Vertreter von
Physio-Control vor Ort.
Lebensdauer
Das TrueCPR-Coaching-Gerät wird während der Produktentwicklung strengen
Lebensdauertests unterzogen. Jedoch wird die Nutzungsdauer aller modernen
medizinischen Geräte durch schnelle technologische Änderungen und die
Verfügbarkeit von Ersatzteilen begrenzt. Das in diesem Handbuch empfohlene
Programm für die regelmäßige Reinigung und Inspektion trägt zu einem
zuverlässigen Betrieb bei.
©2013 Physio-Control, Inc. 43
Page 48

Symbole
IP55
Recycling des Geräts und der Batterien
Das Gerät und die Batterien entsprechend den nationalen und lokalen
Vorschriften und Gesetzen dem Recycling zuführen. Eine Anleitung zur
Entsorgung dieses Produkts erhalten Sie unter
www.physio-control.com/recycling oder vom zuständigen Physio-ControlVertreter.
Symbole
Die folgenden Symbole können auf dem TrueCPR-Gerät oder der Verpackung
vorhanden sein.
SYMBOL BESCHREIBUNG
Netztaste
Ton-Aus-Taste
Atemweg-Taste
Gerät erfordert zwei Duracell DL123 Batterien (nicht aufladbar)
Betriebstemperatur 0 °C bis 40 °C
Defibrillationssicheres Teil zur Anwendung am Patienten vom Typ BF
Achtung, Produktunterlagen zu Rate ziehen. (Der Hintergrund des
Symbols ist blau, das grafische Symbol weiß.)
Nicht auf das Gerät treten.
Nicht auf das Gerät setzen.
Dieses Produkt darf nicht im unsortierten Hausmüll entsorgt werden.
Das Produkt gemäß den lokalen Bestimmungen entsorgen.
Anweisungen zur Entsorgung dieses Produkts finden Sie unter
www.physio-control.com/recycling.
Gehäuseschutzart gemäß IEC 60529. Diese Schutzart bietet einen
bestimmten Schutz vor dem Eintreten von Fremdkörpern und dem
Eindringen von Wasser.
Gerät enthält HF-Sender.
CE-Prüfsiegel nach der EU-Richtlinie für medizinische Geräte
93/42/EWG
Prüfsiegel gemäß kanadischen und US-amerikanischen Normen
Prüfsiegel gemäß ACA-Normen
44 Gebrauchsanweisung für TrueCPR-Gerät
Page 49

German/Deutsch
oder
oder
YYYY
SYMBOL BESCHREIBUNG
Alarmsymbol für ein Gerät der Klasse 2 gemäß R&TTE-Richtlinie
(Radio and Telecommunications Terminal Equipment)
Herstellerkennnummer (Teilenummer)
Katalognummer
Seriennummer
Herstellungsdatum
Hersteller
Autorisierte EU-Vertretung
Technische Daten zum Produkt
SPEZIFIKATION BESCHREIBUNG
Leistungsbedarf Batterien: 2 nicht aufladbare Duracell DL123 Batterien
Betriebszeit 180 Minuten bei Raumtemperatur mit neuen Batterien
Betriebstemperatur Gerät kann bei 0 bis 40 °C verwendet werden.
Bei längerem Betrieb mit einer Umgebungstemperatur von 40 °C
können die am Patienten angewendeten Teile eine Temperatur
von 43 °C erreichen.
Verwendbarkeitsdauer Bei Inbetriebnahme mit neuen Batterien nach 24 Monaten
Lagertemperatur -30 bis 70°C
Relative Luftfeuchtigkeit 5 bis 90%, nicht kondensierend
Höhe -382 bis 4.572 m
Schutz vor dem
Eindringen von
Objekten und Wasser
Gewicht Mit installierten Batterien unter 0,75 kg
Aufforderungen zur
Beatmung
Kompressionstiefe Zieltiefe von 5 bis 6 cm
Metronomfrequenz 104.4
Modell 80596-000003
Lagerung bei 25 °C werden 30 Minuten HLW-Feedback geliefert.
IP55 nach IEC 60529 und EN 1789
Im Modus „Kein Atemweg“ alle 30 Kompressionen 2
Aufforderungen zur Beatmung.
Im Modus „Atemweg“ keine Aufforderungen zur Beatmung.
± 1 Kompression pro Minute gemäß AHA- und ERC-
Richtinien.
©2013 Physio-Control, Inc. 45
Page 50

Sicherheitsklassifikationen
Sicherheitsklassifikationen
KATEGORIE KLASSIFIKATION
Schutz vor Stromschlag Medizinisches elektrisches Gerät mit interner
Stromversorgung.
Defibrillationssichere Teile zur Anwendung am
Patienten vom Typ BF.*
Schutz vor dem Eindringen von
Festkörpern und Flüssigkeiten
Betriebsart Dauerbetrieb
* Defibrillationssichere Teile zur Anwendung am Patienten vom Typ BF sind die Unterseite
des Brustsensors und die Oberseite der Rückenplatte. Symbolabbildung siehe Seite 44.
IP55
Hinweise zur elektromagnetischen Verträglichkeit
Elektromagnetische Emissionen
Tabelle 1 Hinweise und Herstellerklärung - Elektromagnetische Emissionen
Das TrueCPR-Coaching-Gerät, Modell 80596-000003, ist für die Verwendung in der unten
beschriebenen elektromagnetischen Umgebung vorgesehen. Der Kunde oder Benutzer
muss gewährleisten, dass das TrueCPR-Gerät in einer solchen Umgebung eingesetzt
wird.
Emissionstest Konformität
HF-Emissionen
CISPR 11
HF-Emissionen
CISPR 11
Oberwellenaussendung
IEC 61000-3-2
Spannungsschwankungen/
Flicker
IEC 61000-3-3
Gruppe 1 Das TrueCPR-Gerät verwendet HF-Energie
Klasse B Das TrueCPR-Gerät eignet sich für den
Nicht zutreffend
Nicht zutreffend
Elektromagnetische Umgebung Hinweise
nur für interne Funktionen. Daher sind
seine HF-Emissionen sehr gering und es ist
unwahrscheinlich, dass sie irgendwelche
Störungen bei elektronischen Geräten in
der Nähe verursachen.
Einsatz in allen Einrichtungen einschließlich
Wohnungen und solche Einrichtungen, die
direkt an das öffentliche
Niederspannungsnetz angeschlossen sind,
über das Gebäude versorgt werden, die für
Wohnzwecke genutzt werden.
Das TrueCPR-Gerät ist batteriebetrieben.
Das TrueCPR-Gerät gibt ein kontinuierliches Wellensignal bei 12 ± 0.1 kHz aus. Die
maximalen Werte für „Effective Isotropic Radiated Power“ (EIRP) und „Effective Radiated
Power“ (ERP) betragen -40 dBm, gemessen in einer Entfernung von 3 m.
46 Gebrauchsanweisung für TrueCPR-Gerät
Page 51

German/Deutsch
Wesentliche Leistungsfähigkeit
Das TrueCPR-Gerät bewahrt eine sichere und effektive Leistungsfähigkeit (genaue Tiefe),
wenn es innerhalb der elektromagnetischen Umgebung gemäß Tabelle 2 bis Tabelle 4
betrieben wird.
Elektromagnetische Störfestigkeit
Tabelle 2 Hinweise und Herstellerklärung - Elektromagnetische Störfestigkeit
Das TrueCPR-Gerät, Modell 80596-000003, ist für die Verwendung in der unten
beschriebenen elektromagnetischen Umgebung vorgesehen. Der Kunde oder Benutzer
muss gewährleisten, dass das TrueCPR-Gerät in einer solchen Umgebung eingesetzt
wird.
Störfestigkeits-
prüfung
Elektrostatische
Entladung (ESD)
IEC 61000-4-2
Schnelle
transiente
elektrische
Störgrößen/
Burst
IEC 61000-4-4
Spitzenstrom
IEC 61000-4-5
Spannungseinbr
üche, kurze
Unterbrechunge
n und
Spannungsschw
ankungen in
Stromversorgun
gsleitungen
IEC 61000-4-11
IEC 60601
Prüfpegel
±6 kV Kontakt
±8 kV Luft
±2 kV für
Stromversorgungs
leitungen
±1 kV für
Eingangs-/
Ausgangsleitunge
n
±1 kV Leitung(en)
an Leitung(en)
±2 kV Leitung(en)
an Erde
<5% U
(>95%
T
Einbruch in U
T
) für
0,5 Zyklus
40% U
(60%
T
Einbruch in U
5 Zyklen
70% U
Einbruch in U
25 Zyklen
<5% U
Einbruch in U
(30%
T
(>95%
T
T
T
T
) für
) für
) für
5 s
Konformitätsstufe
±6 kV Kontakt
±8 kV Luft
Nicht zutreffend Das TrueCPR-Gerät ist
Nicht zutreffend Das TrueCPR-Gerät ist
Nicht zutreffend Das TrueCPR-Gerät ist
Elektromagnetische
Umgebung - Hinweise
Böden müssen aus Holz,
Beton oder Keramikfliesen
bestehen. Wenn Böden mit
synthetischem Material
ausgelegt sind, muss die
relative Feuchtigkeit
mindestens 30% betragen.
batteriebetrieben.
batteriebetrieben.
batteriebetrieben.
©2013 Physio-Control, Inc. 47
Page 52

Hinweise zur elektromagnetischen Verträglichkeit
Tabelle 2 Hinweise und Herstellerklärung - Elektromagnetische Störfestigkeit (Fortsetzung)
Das TrueCPR-Gerät, Modell 80596-000003, ist für die Verwendung in der unten
beschriebenen elektromagnetischen Umgebung vorgesehen. Der Kunde oder Benutzer
muss gewährleisten, dass das TrueCPR-Gerät in einer solchen Umgebung eingesetzt
wird.
Störfestigkeits-
prüfung
Netzfrequenz
(50/60 Hz)
Magnetfeld
IEC 61000-4-8
Hinweis: U
ist die Wechselstrom-Netzspannung vor Anwendung auf den Prüfpegel.
T
IEC 60601
Prüfpegel
3 A/m 3 A/m Netzfrequenz-Magnetfelder
Konformitätsstufe
Elektromagnetische
Umgebung - Hinweise
müssen sich auf einem für
einen typischen Ort in einer
typischen Geschäfts- oder
Krankenhausumgebung
charakteristischen Pegel
befinden.
48 Gebrauchsanweisung für TrueCPR-Gerät
Page 53

German/Deutsch
d 1.2 P=
d 1.2 P=
d 2.3 P=
Tabelle 3 Hinweise und Herstellerklärung - Elektromagnetische Störfestigkeit
Das TrueCPR-Gerät, Modell 80596-000003, ist für die Verwendung in der unten
beschriebenen elektromagnetischen Umgebung vorgesehen. Der Kunde oder Benutzer
muss gewährleisten, dass das TrueCPR-Gerät in einer solchen Umgebung eingesetzt wird.
Störfestigkeits-
prüfung
Geleitete HF
IEC 61000-4-6
Abgestrahlte HF
IEC 61000-4-3
IEC 60601
Prüfpegel
3 Vrms
150 kHz bis
80 MHz
3 V/m
80 MHz bis
2,5 GHz
Konformitäts-
Elektromagnetische Umgebung -
stufe
Tragbare und mobile HFKommunikationsgeräte sollten zu
keinem Teil des TrueCPR-Geräts,
einschließlich der Kabel, einen
geringeren Abstand als den
empfohlenen Abstand haben, der durch
die Gleichung für die Frequenz des
Senders berechnet wurde.
Empfohlener Schutzabstand
3 Vrms
3 V/m
Hinweise
80 MHz bis 800 MHz
800 MHz bis 2,5 GHz
Wobei P die maximale Nennleistung
des Senders in Watt (W) gemäß
Angaben des Herstellers und d der
empfohlene Schutzabstand in Metern
(m) ist.
Feldstärken von festen HF-Sendern,
wie sie durch eine elektromagnetische
Untersuchung vor Ort festgestellt
a
wurden,
Konformitätsstufe in jedem
Frequenzbereich.
sollten kleiner sein als die
b
Störungen können in der Nähe von
Geräten auftreten, die mit folgendem
Symbol gekennzeichnet sind:
Hinweis 1: Bei 80 MHz und 800 MHz gilt der höhere Frequenzbereich.
Hinweis 2: Diese Leitlinien treffen eventuell nicht in allen Situationen zu. Die Ausbreitung
elektromagnetischer Wellen wird durch Absorption und Reflexion von Gebäuden,
Gegenständen und Menschen beeinflusst.
©2013 Physio-Control, Inc. 49
Page 54

Hinweise zur elektromagnetischen Verträglichkeit
PPP
Tabelle 3 Hinweise und Herstellerklärung - Elektromagnetische Störfestigkeit
(Fortsetzung)
a Feldstärken von stationären Sendern wie Basisstationen für Funktelefone (Handys/schnurlose
Telefone) und mobile Funkgeräte, Amateurfunk, AM- und FM-Rundfunksender und Fernsehsender
können theoretisch nicht genau vorhergesagt werden. Um die elektromagnetische Umgebung
hinsichtlich der stationären Sender zu ermitteln, sollte eine Studie des Standorts erwogen werden.
Wenn die gemessene Feldstärke an dem Standort, an dem das TrueCPR-Gerät benutzt wird, die
geltenden HF-Grenzwerte überschreitet, sollte das TrueCPR-Gerät auf normalen Betrieb überprüft
werden. Wenn ungewöhnliche Leistungsmerkmale beobachtet werden, können zusätzliche
Maßnahmen erforderlich sein, zum Beispiel die Neuorientierung oder Umsetzung des TrueCPRGeräts.
b Über den Frequenzbereich 150 kHz bis 80 MHz sollten die Feldstärken unter 3 V/m liegen.
Schutzabstände
Tabelle 4 Empfohlene Schutzabstände zwischen tragbaren und mobilen HFKommunikationsgeräten und dem TrueCPR-Gerät, Modell 80596-000003.
Das TrueCPR-Gerät ist für den Betrieb in einer elektromagnetischen Umgebung
vorgesehen, in der abgestrahlte HF-Störungen kontrolliert werden. Der Kunde oder
Benutzer des TrueCPR-Geräts kann zur Vermeidung elektromagnetischer Störungen
beitragen, indem wie nachfolgend empfohlen ein Mindestabstand zwischen tragbaren und
mobilen HF-Kommunikationsgeräten (Sendern) und dem TrueCPR-Gerät eingehalten wird,
welcher der maximalen Ausgangsleistung der Kommunikationsgeräte entspricht.
Maximale Nennleistung
des Senders,
W
0,01 0,12 0,12 0,23
0,1 0,38 0,38 0,73
1 1,2 1,2 2,3
10 3,8 3,8 7,3
100 12 12 23
Für Sender mit einer maximalen Ausgangsleistung, die nicht oben aufgeführt ist, kann der
empfohlene Abstand d in Metern (m) mit Hilfe der Gleichung für die Frequenz des Senders
ermittelt werden, wobei P die maximale Nennleistung des Senders in Watt (W) gemäß den
Angaben des Herstellers ist.
Hinweis 1: Bei 80 MHz und 800 MHz gilt der Schutzabstand für den höheren
Frequenzbereich.
Hinweis 2: Diese Leitlinien treffen eventuell nicht in allen Situationen zu. Die Ausbreitung
elektromagnetischer Wellen wird durch Absorption und Reflexion von Gebäuden,
Gegenständen und Menschen beeinflusst.
Schutzabstand entsprechend der Frequenz des Senders,
m
150 kHz bis
80 MHz
d = 1,2
80 MHz bis
800 MHz
d = 1,2
800 MHz bis 2,5 GHz
d = 2,3
50 Gebrauchsanweisung für TrueCPR-Gerät
Page 55

German/Deutsch
Konformitätserklärung
Konformität mit der „Federal Communications Commission“ (FCC)
Dieses Gerät entspricht Teil 15 der FCC-Vorschriften. Der Betrieb unterliegt den folgenden
zwei Bedingungen: (1) Dieses Gerät darf keine schädlichen Störungen verursachen und (2)
dieses Gerät muss jede empfangene Störung akzeptieren, einschließlich Störungen, die
einen unerwünschten Betrieb verursachen.
WARNUNG
Durch Änderungen oder Modifikationen, die nicht ausdrücklich von der für die
Konformität verantwortlichen Partei genehmigt wurden, kann die Berechtigung des
Benutzers zum Betrieb des Geräts erlöschen.
Konformität mit der Richtlinie „Radio and Telecommunications Terminal Equipment“ (R&TTE)
Dieses Gerät ist ein Klasse 2-Gerät gemäß R&TTE-Richtlinie:
BE BG CZ DK DE
FR ES EL IE EE
IT CY LV LT LU
PL AT NL MT HU
PT RO SI SK FI
SE UK
©2013 Physio-Control, Inc. 51
Page 56

Page 57

Introduction
French/Français
Le dispositif d’assistance Physio-Control TrueCPR donne aux secouristes des
informations en temps réel sur les compressions thoraciques pratiquées pendant
la réanimation cardio-pulmonaire (RCP) conformément aux directives actuelles
en matière de RCP.
IMPORTANT ! Lire attentivement ces instructions avant d’utiliser l’appareil, et
les conserver pour référence ultérieure.
Ce mode d’emploi détaille les étapes de la procédure à suivre pour utiliser
correctement l’appareil TrueCPR, et peut être utilisé à des fins de formation. Les
utilisateurs doivent lire ce mode d’emploi et parfaitement se familiariser avec
l’appareil avant de l’utiliser.
Utilisation prévue
L’appareil TrueCPR vise à fournir des renseignements aux secouristes pour les
aider à pratiquer la RCP. Les secouristes doivent être formés à la pratique de la
RCP et à l’utilisation de l’appareil.
L’appareil TrueCPR est conçu pour être utilisé chez les patients âgés de 8 ans ou
plus.
Indications
L’utilisation de l’appareil TrueCPR est indiquée chez les patients en arrêt
cardiaque (inconscients, sans pouls, ne respirant pas normalement).
Principe de fonctionnement
L’appareil TrueCPR se compose d’un module thoracique, placé sur le sternum,
et d’un module dorsal, placé sous le patient. L’appareil détermine la profondeur
des compressions thoraciques pendant la RCP en mesurant la distance entre le
module thoracique et le module dorsal. L’appareil mesure la distance par l’envoi
et la réception de signaux électromagnétiques entre le module thoracique et le
module dorsal.
Informations relatives à la sécurité
Les termes suivants sont utilisés dans ce mode d’emploi :
Avertissement : Dangers ou méthodes peu sûres pouvant entraîner des
blessures graves ou la mort.
©2013 Physio-Control, Inc. 53
Page 58

Informations relatives à la sécurité
Attention : Dangers ou méthodes peu sûres pouvant entraîner des blessures
non graves, des dommages à l’appareil ou au matériel.
AVERTISSEMENTS
RISQUE DE BLESSURE POUR LE PATIENT :
• L’appareil TrueCPR n’est pas destiné à être utilisé chez l’enfant ou le
nourrisson. Utiliser uniquement l’appareil sur des patients âgés de 8 ans
ou plus.
RISQUE DE RÉANIMATION CARDIO-PULMONAIRE NON ADÉQUATE :
• L'appareil TrueCPR n'indique pas la décompression complète. Veiller à
relâcher complètement chaque compression.
• Après utilisation, contrôler l’appareil et le câble pour rechercher
d’éventuels signes d’usure ou de dommages. En cas de dommages,
retirer l’appareil du service.
• Ne pas utiliser l’appareil TrueCPR sur de grandes surfaces métalliques.
Pratiquer plutôt la RCP sans l’appareil.*
• L’appareil TrueCPR risque de fournir des informations erronées s’il n’est
pas correctement positionné.
• Vérifier la position du module dorsal et du module thoracique après un
mouvement du patient et pendant le transport.
• Si le patient est étendu sur un matelas, glisser une planche dorsale
sous le patient, conformément aux protocoles standards. Insérer
ensuite le module dorsal entre le patient et la planche dorsale.
• S’il est impossible de placer correctement l’appareil sur le patient ou
si l’appareil ne fonctionne pas, retirer l’appareil et pratiquer une RCP
sans l’appareil.
RISQUE D’ÉCHEC DE L’APPAREIL : Ne pas altérer l’appareil TrueCPR.
RISQUE D’INTERFÉRENCE ÉLECTRIQUE AVEC LA PERFORMANCE DE
L’APPAREIL : Tout équipement en fonctionnement à proximité de l’appareil
risque d’émettre de fortes interférences électromagnétiques ou des
radiofréquences (RFI) qui pourraient affecter la performance de cet appareil.
Éviter d’utiliser l’appareil à proximité de matériel de cautérisation,
d’équipements de diathermie ou de tout autre appareil de communication RF
portable ou cellulaire. Consulter la section « Distances de séparation » sur la
page 76 pour connaître les distances recommandées de l’appareil. Contacter
le service technique de Physio-Control en cas de besoin.
RISQUE D’EXPLOSION : Ne pas utiliser cet appareil en présence de gaz
inflammables ou de produits anesthésiques.
* L’appareil TrueCPR a été conçu pour une utilisation sur des lits d’hôpitaux,
des brancards, des civières et dans des ambulances. L’appareil est
compatible avec les métaux couramment portés par les patients ou
implantés, comme les bijoux, les DAI ou les stimulateurs cardiaques.
54 Instructions d’utilisation de l’appareil TrueCPR
Page 59

French/Français
5
1
2
4
3
11
6
7
8
10
12
9
IMPORTANT ! Contrôler régulièrement l’indicateur d’état de la batterie. Si
l’indicateur ne clignote pas, remplacer immédiatement les batteries.
Indications de base
Module thoracique Écran
N° Description
Bouton d’alimentation : Appuyer sur le bouton vert pour mettre
1
l’appareil sous tension. Appuyer et maintenir enfoncé pendant 2
secondes pour éteindre l’appareil.
Bouton Muet : Appuyer pour activer ou désactiver le métronome.
2
En mode Examen des évènements, appuyer pour basculer d’un écran à
l’autre.
3 Plaque de paume : Endroit où placer les mains.
Bouton Voies aériennes : Appuyer pour faire basculer le métronome
4
entre le mode Voies aériennes (compressions continues) et le mode
©2013 Physio-Control, Inc. 55
Absence de voies aérienne.
Écran : Affiche les informations de retour sur les compressions, ainsi
5
que d’autres indicateurs.
Rappel de relâchement : Se souvenir de bien relâcher complètement
6
chaque compression. L'appareil n'indique pas le relâchement complet.
L'icône est toujours présent durant le retour de compression.
Indicateur de batterie faible : Apparaît lorsque l’autonomie restante de
7
la batterie est inférieure à 25 minutes de fonctionnement.
Page 60

Indications de base
1
2
3
N° Description
Dispositif de mesure de la fréquence de compression : Affiche la
fréquence de compression en tant que nombre de compressions par
8
minute. Bascule sur Compteur d’inactivité lorsque les compressions
sont interrompues.
Compteur de temps écoulé : Affiche le temps écoulé depuis le début
9
de l’évènement.
Indicateur Voies aériennes : Apparaît lorsque l’appareil est en mode
10
Voies aériennes.
Zone de compression cible : Passe de vert clair à vert foncé lorsque la
11
compression atteint la profondeur cible de 5-6 cm.
Historique de la profondeur de compression : Un segment vert foncé
12
ou gris dans le cercle extérieur indique la profondeur de la compression
précédente. Chaque marque représente 0,5 cm de profondeur.
Module dorsal
N° Description
Indicateur d’état de la batterie : Une DEL clignotante sur la poignée du
module dorsal indique que l’autonomie de la batterie est suffisante pour
1
au moins 25 minutes de fonctionnement.
Remarque : La DEL clignote environ une fois toutes les 4 secondes.
Couvercle du port USB : Donne accès au port USB. Utiliser une pièce
2
de monnaie ou un objet similaire pour l’ouvrir.
Compartiment des batteries : Contient deux batteries Duracell
non-rechargeables. Utiliser une pièce de monnaie ou un objet similaire
3
®
DL123
pour l’ouvrir.
56 Instructions d’utilisation de l’appareil TrueCPR
Page 61

French/Français
ET
Formation recommandée
Avant d'utiliser l'appareil TrueCPR sur un patient, l'utilisateur doit être formé à la
pratique de la RCP avec l'appareil TrueCPR. Nous recommandons l'inclusion
des mesures de performance RCP dans votre programme de formation pour
l'appareil TrueCPR.
Utilisation de l’appareil TrueCPR
Pratiquer une RCP
Pour utiliser l’appareil TrueCPR :
1 Confirmer que le patient est en arrêt cardiaque.
2 Sortir l’appareil de sa sacoche de transport.
3 Séparer le module thoracique du module dorsal.
4 Appuyer sur le bouton (ALIMENTATION) pour allumer l’appareil. L’écran
d’étalonnage apparaît.
IMPORTANT ! Ne pas appuyer sur le module thoracique jusqu’à ce que
l’étalonnage soit terminé et que l’écran de retour apparaisse (environ 3
secondes).
5 Placer le module dorsal sous le patient comme indiqué (Figure 1). Cela
permet au module dorsal de rester en place. Le module dorsal peut être placé
de n’importe quel côté du patient.
6 Essuyer la poitrine, si nécessaire, pour éviter que le module thoracique ne
glisse.
7 Placer le module thoracique de façon à ce que la plaque de paume se trouve
au milieu de la poitrine, sur la partie inférieure du sternum.
Figure 1 Position recommandée de l’appareil
Module dorsal en position verticale
©2013 Physio-Control, Inc. 57
Module thoracique en position
verticale
Page 62

Utilisation de l’appareil TrueCPR
ET
Remarques :
• Si l’indicateur d’alerte « Corriger la position du module dorsal » apparaît,
ajuster la position du module dorsal. Pour plus d’informations, consulter
la section « Indicateurs d’alerte » sur la page 64. Si l’alerte « Corriger la
position du module dorsal » persiste, pratiquer la RCP sans l’appareil.
• S’il est impossible de placer l’appareil dans la position recommandée, il
est possible de le placer dans la position alternative présentée à la Figure
2. Lorsque le module dorsal est placé en position latérale, le module
thoracique doit être tourné de 180°. Maintenir la plaque de paume au
milieu de la poitrine sur la partie inférieure du sternum. S’il est impossible
de placer l’appareil correctement, pratiquer la RCP sans l’appareil.
Figure 2 Position alternative de l’appareil
Module dorsal en position latérale
Module thoracique en position
tournée
8 Placer la paume de votre main sur la plaque de paume et commencer les
compressions au rythme du métronome. Veiller à garder les mains dans la
bonne position adaptée à la RCP.
IMPORTANT! Veiller à relâcher complètement chaque compression.
L'appareil indique la profondeur et la fréquence de compression. Il n'indique
pas le relâchement.
58 Instructions d’utilisation de l’appareil TrueCPR
Page 63

French/Français
Compteur d’inactivité
AVERTISSEMENT
RISQUE DE RÉANIMATION CARDIO-PULMONAIRE NON ADÉQUATE :
Si l’écran de l’appareil TrueCPR est illisible, ne répond pas ou est visiblement
endommagé, ne pas utiliser l’appareil. Pratiquer la RCP sans l’appareil.
9 Suivre le métronome de l’appareil pour garder votre rythme de compression.
Remarque : Pour couper le son du métronome, appuyer sur le bouton
(MUET).
10 Observer l’écran pour consulter les informations de profondeur et d’éventuels
indicateurs d’alerte. Ajuster votre technique en fonction du retour affiché.
Consulter les illustrations d’écran à partir de la page 61 pour une description
des informations affichées.
11 Pratiquer des insufflations lorsque l’écran et les tonalités de ventilation vous
le demandent. Trois tonalités courtes vous indiquent quand vous préparer à
ventiler le patient. Deux tonalités plus longues vous indiquent quand ventiler
le patient. Reprendre immédiatement les compressions thoraciques après les
deux tonalités longues.
Remarque : En mode Absence de voies aériennes, l’appareil émet des
invites de ventilation selon le ratio de 30 compressions pour 2 ventilations
(30:2). En mode Voies aériennes, aucune invite de ventilation n’est émise. Voir
« Utilisation de l’appareil TrueCPR avec Voies aériennes protégées » sur la
page 60 pour en savoir plus.
12 Continuer la RCP aussi longtemps que vos protocoles locaux l’indiquent. À
l’interruption des compressions, le compteur d’inactivité s’affiche à la place
du dispositif de mesure de la fréquence de compression. Si les compressions
reprennent dans les 10 minutes, les informations relatives aux compressions
réapparaissent à l’écran. L’appareil s’éteint automatiquement après 10
minutes d’inactivité.
13 Pour éteindre l’appareil, appuyer et maintenir enfoncé le bouton
(ALIMENTATION) pendant 2 secondes.
Utilisation de l’appareil TrueCPR sur un matelas
Si le patient est étendu sur un matelas, placer une planche dorsale sous le
patient, conformément aux protocoles standards. Glisser ensuite le module
dorsal de l’appareil TrueCPR entre le patient et la planche dorsale.
©2013 Physio-Control, Inc. 59
Page 64

Utilisation de l’appareil TrueCPR
Indicateur voies
aériennes
Utilisation de l’appareil TrueCPR avec Voies aériennes protégées
Lorsque l’appareil TrueCPR s’allume, il se met en mode Absence de voies
aériennes par défaut. Des invites de ventilation sont émises selon le ratio de 30
compressions pour 2 ventilations (30:2). En présence d’une canule, appuyer sur
le bouton (VOIES AÉRIENNES) pour faire basculer l’appareil en mode Voies
aériennes (compressions continues).
Lorsque l’appareil est basculé en mode Voies aériennes, les deux changements
suivants se produisent.
• L’indicateur voies aériennes apparaît sur l’écran.
• Aucune invite de ventilation n’est émise.
À chaque appui sur le bouton VOIES AÉRIENNES, l’appareil bascule entre le mode
Voies aériennes et le mode Absence de voies aériennes.
Utilisation de l’appareil TrueCPR lors d’une défibrillation
Il est envisageable de laisser l’appareil TrueCPR sur le patient lors d’une
défibrillation. Observer les précautions suivantes :
• Placer le module thoracique et les électrodes de
défibrillation comme indiqué.
• Ne pas toucher l’appareil TrueCPR lors de la
défibrillation.
• Ne pas appliquer les électrodes de défibrillation sur le
câble de l’appareil TrueCPR.
Utilisation de l’appareil TrueCPR sur des patients présentant des incisions sternales
Établir et suivre les protocoles locaux pour utiliser l’appareil TrueCPR sur des
patients présentant des incisions ou des lésions sternales.
Utilisation de l’appareil TrueCPR sur des patients porteurs de dispositifs médicaux implantables
Les champs magnétiques utilisés par l’appareil TrueCPR ont passé tous les tests
d’interaction et répondent à toutes les normes de sécurité en vigueur concernant
les stimulateurs et les dispositifs cardiaques implantables.
60 Instructions d’utilisation de l’appareil TrueCPR
Page 65

French/Français
Utilisation de l’appareil TrueCPR à des fins de formation
L’appareil TrueCPR peut être utilisé sur un mannequin à des fins de formation. Il
sera peut-être nécessaire de mettre à jour certains mannequins pour atteindre
les profondeurs de compression de 5 à 6 cm, conformes aux recommandations
de l’AHA et de l’ERC. Il est possible d’utiliser un mannequin qui ne permet pas
d’atteindre ces profondeurs, mais l’appareil TrueCPR n’atteindra pas la zone de
compression cible.
Remarque : L’indicateur Corriger la position du module dorsal (voir page 64)
peut apparaître si le mannequin contient de grandes plaques métalliques.
Illustrations d’écran
Écran d’étalonnage
Lorsque l’appareil TrueCPR est mis sous tension, l’écran d’étalonnage apparaît.
Étalonnage en cours.
• Ne pas appuyer sur le
module thoracique.
• Veiller à séparer le
module thoracique du
module dorsal pendant
l’étalonnage.
©2013 Physio-Control, Inc. 61
Étalonnage terminé.
Commencer les
compressions
thoraciques.
Page 66

Utilisation de l’appareil TrueCPR
Indicateurs de compression
Pendant les compressions, l’éventail gris oscille entre la zone de référence et la
zone de compression comme illustré.
Compression en cours :
L’éventail gris
représentant la
profondeur de
compression se déplace
sur l’écran. L'indication
de profondeur débute
lorsque la profondeur de
compression atteint
1,5 cm, et se déplace sur
l'écran à mesure que la
profondeur augmente.
Profondeur suffisante :
Tranche vert foncé
dans la zone de
compression cible.
Indicateurs de compressions incorrecte
Compression
superficielle :
Une flèche apparaît.
Compression > 6 cm :
Une tranche orange
apparaît en dehors de la
zone de compression cible.
Rappel de relâchement :
Continuez de décoller
vos mains de la poitrine
du patient après la
disparition de l'éventail
gris. L'appareil n'indique
pas le relâchement
complet.
62 Instructions d’utilisation de l’appareil TrueCPR
Page 67

French/Français
Indicateurs et tonalités d’insufflations
En mode Absence de voies aériennes, un compte à rebours s’affiche à l’écran, et
l’appareil émet des tonalités vous indiquant quand vous préparer à pratiquer des
insufflations. Le compte à rebours est suivi de deux invites de ventilation. Le ratio
compressions/insufflations est de 30:2.
Compte à rebours avant invites de ventilation. Trois tonalités courtes sont
émises.
Invites de ventilation. Deux tonalités longues
indiquent quand ventiler le patient. Reprendre
immédiatement les compressions thoraciques
après les deux tonalités.
©2013 Physio-Control, Inc. 63
Page 68

Utilisation de l’appareil TrueCPR
Indicateurs d’alerte
Du bruit électronique
provoque des
interférences avec
l’appareil. Localiser et
éliminer la source des
interférences. Si
l’indicateur persiste,
pratiquer la RCP sans
L’appareil ne peut pas
donner de retour.
Éteindre, puis rallumer
l’appareil. Si l’indicateur
persiste, pratiquer la RCP
sans l’appareil.
La distance séparant le
module dorsal du module
thoracique est incorrecte.
Corriger la position du
module dorsal. Si
l’indicateur persiste,
pratiquer la RCP sans
l’appareil.
l’appareil.
Écran de fermeture
Une barre de progression apparaît à la
fermeture.
Remarque : La fermeture peut prendre
quelques minutes. Ne pas retirer les batteries
en cours de fermeture.
Écrans d’examen des évènements
Deux écrans d’examen des évènements affichent des données relatives à la
dernière utilisation de l’appareil. Pour afficher l’écran d’examen des évènements,
l’appareil doit être éteint. Appuyer simultanément sur les boutons (VOIES
AÉRIENNES) et (ALIMENTATION) pour allumer l’appareil en mode Examen des
évènements. Pour basculer d’un écran à l’autre, appuyer sur le bouton
(MUET).
Pourcentage de compressions où la
profondeur de > 5 cm a été atteinte.
Fréquence de compression moyenne
(compressions par minute).
64 Instructions d’utilisation de l’appareil TrueCPR
Page 69

French/Français
Durée totale de l’évènement.
Pourcentage total de l’évènement avec
compressions.
Conseils de dépannage
Ces conseils de dépannage proposent des actions correctives immédiates pour
résoudre d’éventuels problèmes. Pour obtenir de l’aide, contacter votre
représentant local Physio-Control.
OBSERVATION ACTION CORRECTIVE
Métronome inaudible. • Utiliser le dispositif de mesure de la
fréquence de compression pour vous aider.
L’appareil ne fonctionne pas. • Utiliser uniquement l’appareil dans la plage
de température de fonctionnement
spécifiée, entre 0° et 40°C.
• Enlever les batteries, attendre 30 secondes,
puis remettre les batteries.
• Pratiquer la RCP sans l’appareil.
L’écran est visible, mais ne
fournit pas de retour sur les
compressions.
L’indicateur d’alerte persiste. • Éteindre, puis rallumer l’appareil. Si
• Éteindre, puis rallumer l’appareil. Ne pas
appuyer sur le module thoracique jusqu’à
ce que l’étalonnage soit terminé et que
l’écran de retour apparaisse.
• Pratiquer la RCP sans l’appareil.
l’indicateur persiste, pratiquer la RCP sans
l’appareil.
• Pour obtenir de l’aide, contacter le service
technique ou votre représentant local
Physio-Control.
©2013 Physio-Control, Inc. 65
Page 70

™
Année : ______Numéro de série de l’appareil : ______________
Emplacement : ________________
Date
Initiales
DL123 non-
®
Liste de contrôle de l’utilisateur
Liste de contrôle de l’utilisateur du dispositif d’assistance TrueCPR
Utiliser cette liste de contrôle pour contrôler l’état de l’appareil TrueCPR. La reproduction de ce formulaire est autorisée.
Instructions et action corrective recommandée Cocher chaque case après avoir effectué l’action
Vérifier que l’indicateur d’état de la batterie sur le
module dorsal clignote.
Si ce n’est pas le cas, remplacer les batteries par
deux batteries neuves Duracell
rechargeables.
Contrôler l’appareil et le câble pour rechercher
d’éventuels signes d’usure ou de dommages.
En cas d’usure ou de dommages, retirer l’appareil du
service et contacter votre représentant Physio-
Control pour obtenir de l’aide.
Page 71

French/Français
Gestion des données
L’appareil TrueCPR peut conserver des données de compression pendant trois
séances de 60 minutes, ou jusqu’à six séances pour un total de 180 minutes.
Lorsque tout l’espace disponible a été utilisé, les données de la plus ancienne
utilisation sont écrasées automatiquement.
Les données peuvent être transférées vers un ordinateur grâce à un logiciel
compatible avec l’appareil TrueCPR vis une connexion USB. Il est possible
d’imprimer les rapports d’évènements directement à partir du logiciel. Pour
obtenir des informations sur les rapports créés par l’appareil TrueCPR et
l’ensemble des fonctionnalités d’examen des évènements, contacter votre
représentant local Physio-Control.
AVERTISSEMENTS
RISQUE DE CHOC ÉLECTRIQUE :
• Pour éviter le risque de choc électrique, tout appareil relié à l'appareil
TrueCPR via le port USB doit fonctionner sur batterie ou être certifié IEC
60601-1.
• Ne pas utiliser le connecteur USB si l’appareil TrueCPR est endommagé.
RISQUE D’INTERFÉRENCE ÉLECTRIQUE : L’utilisation de câbles ou
d’accessoires n’ayant pas été conçus pour être utilisés avec l’appareil
TrueCPR peut entraîner une augmentation des émissions ou une diminution
de l’immunité aux interférences électromagnétiques ou radio (RFI) de cet
appareil. Cela peut affecter la performance de l’appareil TrueCPR ou de
l’équipement se trouvant à proximité. Utiliser uniquement les câbles et les
accessoires spécifiés dans ce mode d’emploi.
Pour lancer le transfert de données :
1 Brancher un câble USB pour relier l’appareil TrueCPR à un ordinateur équipé
d’un logiciel compatible.
Remarque : Utiliser uniquement un câble USB correspondant aux
spécifications suivantes : Câble USB 2.0 de type A mâle à mini B 5 mâle à 5
broches, 28/24 AWG avec noyau de ferrite (plaqué or), 1 m.
2 Mettre l’appareil TrueCPR sous tension. Si le logiciel présent sur l’ordinateur
ne se lance pas automatiquement, ouvrir le programme manuellement.
3 Suivre les instructions du logiciel sur l’ordinateur pour sélectionner l’appareil
TrueCPR et lancer le transfert de données. Le transfert de données peut
©2013 Physio-Control, Inc. 67
Page 72

Nettoyage de l’appareil
prendre jusqu’à 5 minutes si la mémoire est pleine. Les écrans suivants
peuvent apparaître sur l’appareil TrueCPR :
Transfert de données
en cours.
Transfert de données
terminé.
Échec du transfert de
données.
Conseils de dépannage pour la gestion de données
OBSERVATION ACTION CORRECTIVE
L’écran de retour apparaît sur
l’appareil TrueCPR, et le
métronome fonctionne.
L’écran Échec du transfert de
données apparaît.
• Vérifier que le câble USB soit bien branché.
• Vérifier que l’ordinateur soit bien allumé.
• Remplacer le câble USB.
• Utiliser le câble USB recommandé
(consulter l’étape 1 sur la page 67).
• Éteindre l’appareil TrueCPR et vérifier les
branchements. Rallumer l’appareil et
relancer le transfert de données.
• Si le problème persiste, contacter le service
technique.
Nettoyage de l’appareil
L’appareil TrueCPR doit être nettoyé et contrôlé après chaque utilisation.
1 Nettoyer l’appareil à l’aide d’une éponge ou d’un chiffon imbibé de l’un des
produits de nettoyage suivants :
• Composés d’ammonium quaternaire
• Peroxyde d’hydrogène, solution à 3 %
• Dichloroisocyanurate de sodium (NaDCC), solution à 3 000 ppm
• Eau de javel chlorée, 1:10 diluée dans l’eau
68 Instructions d’utilisation de l’appareil TrueCPR
Page 73

French/Français
2 Contrôler l’appareil et le câble pour rechercher d’éventuels signes d’usure ou
de dommages. En cas de dommages, retirer l’appareil du service.
3 Après avoir nettoyé l’appareil, essuyer la solution avec un chiffon humide
propre.
IMPORTANT ! Ne pas immerger ou laisser tremper le module thoracique ou le
module dorsal.
Stockage de l’appareil
Placer le module thoracique sur le module dorsal. Ranger le tout dans la sacoche
de transport de manière telle que l’indicateur d’état de la batterie est visible.
ATTENTION
RISQUE D’ENDOMMAGEMENT DE L’APPAREIL : Si l’appareil TrueCPR
doit être rangé pour une longue période de temps, retirer les batteries pour
éviter tout risque d’endommagement dû à une fuite des batteries.
Maintenance et recyclage de l’appareil
Maintenance
L’appareil TrueCPR et le câble doivent être contrôlés régulièrement dans le cadre
de vos inspections de routine de l’équipement. Pour savoir comment procéder,
consulter la « Liste de contrôle de l’utilisateur » sur la page 66.
Remplacement des batteries
Utiliser uniquement des batteries Duracell DL123 pour obtenir des performances
fiables.
AVERTISSEMENT
RISQUE DE FUITE, D’INCENDIE OU D’EXPLOSION :
• Ne pas démonter, percer, écraser, chauffer à plus de 100°C, ou incinérer
les batteries.
• Ne pas utiliser de batteries rechargeables dans l’appareil TrueCPR.
Utiliser uniquement des batteries Duracell DL123 non-rechargeables.
• Remplacer les deux batteries en même temps par des batteries Duracell
DL123 neuves. Ne pas mélanger des batteries partiellement déchargées
avec des batteries neuves.
©2013 Physio-Control, Inc. 69
Page 74

Symboles
Pour remplacer les batteries, utiliser une pièce de monnaie ou un outil similaire
pour ouvrir le couvercle du compartiment à batteries. Retirer les batteries
usagées, attendre 30 secondes, puis insérer des batteries neuves comme
indiqué dans le schéma sur le couvercle du compartiment à batteries. Bien
refermer le couvercle du compartiment à batteries.
Remarque : Si l’indicateur d’état de la batterie ne clignote pas après le
remplacement des batteries, effectuer les actions correctives suivantes.
• Vérifier que les batteries sont installées correctement.
• Enlever les batteries, attendre 30 secondes, puis remettre les batteries.
• Allumer l’appareil et rechercher l’indicateur de batterie faible sur l’écran.
Si l’indicateur de batterie faible n’apparaît pas, l’indicateur d’état de la
batterie est peut-être défectueux. Pour obtenir de l’aide, contacter le
service technique ou votre représentant local Physio-Control.
Entretien et réparations
L’appareil TrueCPR ne contient aucune pièce réparable. Si l’appareil ne
fonctionne pas correctement, contacter votre représentant local Physio-Control
pour obtenir de l’aide.
Durée de vie
Durant le développement du produit, l'appareil d'accompagnement TrueCPR est
sujet à des tests rigoureux de durée de vie. Cependant, les progrès
technologiques rapides et la disponibilité des pièces de rechange limitent la
durée de vie utile de tous les appareils médicaux modernes. Le nettoyage de
routine et le programme d'inspection recommandés dans ce manuel aideront à la
fiabilité du fonctionnement.
Recyclage de l’appareil et des batteries
Recycler l’appareil et les batteries conformément aux réglementations locales et
nationales. Pour savoir comment mettre ce produit au rebut, consulter
www.physio-control.com/recycling ou contacter votre représentant local
Physio-Control.
Symboles
Les symboles suivants peuvent figurer sur l’appareil TrueCPR ou sur son
emballage.
SYMBOLE DESCRIPTION
Bouton d’alimentation
Bouton Muet
70 Instructions d’utilisation de l’appareil TrueCPR
Page 75

SYMBOLE DESCRIPTION
IP55
ou
o
AAAA
Bouton Voies aériennes
L’appareil nécessite deux batteries Duracell DL123 (nonrechargeables)
Température de fonctionnement 0° à 40°C
Pièce appliquée de type BF, protégée contre la défibrillation
Attention, consulter la documentation jointe. (Le symbole est blanc sur
fond bleu).
Ne pas marcher sur l’appareil.
Ne pas s’asseoir sur l’appareil.
Ne pas éliminer ce produit dans le flux des ordures ménagères non
sujettes au tri sélectif. Mettre ce produit au rebut selon les
règlementations locales. Pour savoir comment mettre ce produit au
rebut, consulter www.physio-control.com/recycling.
Indice de protection du boîtier selon la norme IEC 60529. Cet indice
prévoit un niveau de protection spécifique contre l’intrusion de
poussière et d’eau aux effets nuisibles.
L’appareil comprend un émetteur RF.
French/Français
Marquage de conformité selon la directive européenne
93/42/CEE relative aux dispositifs médicaux
Marquage de conformité selon les normes canadiennes et américaines
Marquage de conformité selon les normes ACA
Signe d’alerte associé à un appareil de Classe 2 selon la directive
R&TTE (équipements radio et équipements terminaux de
télécommunication)
Numéro d’identification du fabricant (numéro de pièce)
Numéro de catalogue
Numéro de série
Date de fabrication
Fabricant
Représentant européen agréé
©2013 Physio-Control, Inc. 71
Page 76

Spécifications du produit
Spécifications du produit
CARACTÉRISTIQUE DESCRIPTION
Alimentation Batteries : 2 accumulateurs Duracell DL123 non-rechargeables
Durée de
fonctionnement
Température de
fonctionnement
Durée de conservation Fournit 30 minutes de rétroaction sur la RCP après 24 mois
Température de
stockage
Humidité relative 5 à 90 %, sans condensation
Altitude de -382 à 4 572 m
Résistance aux chocs et
à l’eau
Poids Moins de 0,75 kg, batteries incluses
Invites de ventilation 2 invites de ventilation toutes les 30 compressions en mode
Profondeur de
compression
Fréquence du
métronome
Modèle 80596-000003
180 minutes à température ambiante avec des batteries neuves
L’appareil peut être utilisé entre 0° et 40°C.
La température de la pièce appliquée peut atteindre 43°C en cas
d’utilisation prolongée à une température ambiante de 40°C.
d’inutilisation à 25°C, avec des batteries neuves.
de -30° à 70°C
IP55 selon IEC 60529 et EN 1789
Absence de voies aériennes.
Aucune invite de ventilation en mode Voies aériennes.
Plage de compression cible de 5 à 6 cm
104.4
± 1 compression par minute, conformément aux
recommandations de l’AHA et de l’ERC.
Classifications de sécurité
CATÉGORIE CLASSIFICATION
Protection contre les chocs électriques Appareil électromédical à alimentation interne.
Protection contre les chocs et la
pénétration de liquides
Mode de fonctionnement Fonctionnement continu
* Les pièces appliquées de type BF, protégées contre la défibrillation sont la face inférieure
du module thoracique et la face supérieure du module dorsal. Consulter la page 70 pour
voir une illustration des symboles.
72 Instructions d’utilisation de l’appareil TrueCPR
Pièces appliquées de type BF, protégées contre
la défibrillation.*
IP55
Page 77

French/Français
Guide de compatibilité électromagnétique
Émissions électromagnétiques
Tableau 1 Guide et déclaration du fabricant - Émissions électromagnétiques
Le dispositif d’assistance TrueCPR, modèle 80596-000003, est prévu pour être utilisé
dans l’environnement électromagnétique décrit ci-dessous. Le client ou l’utilisateur doit
veiller à ce que l’appareil TrueCPR soit utilisé dans un tel environnement.
Test d’émissions Conformité Environnement électromagnétique - Guide
Émissions
radioélectriques
CISPR 11
Émissions
radioélectriques
CISPR 11
Émissions
harmoniques
IEC 61000-3-2
Fluctuations de
voltage/émissions
fluctuantes
IEC 61000-3-3
Groupe 1 L’appareil TrueCPR utilise de l’énergie RF pour ses
Classe B L’appareil TrueCPR Plus peut être utilisé dans tous
Non
applicable
Non
applicable
fonctions internes uniquement. Par conséquent, le
niveau de ses émissions radioélectriques est très
faible et il n’est pas susceptible de provoquer des
interférences avec des équipements électroniques
voisins.
les établissements, dont les établissements
domestiques et ceux qui sont directement reliés au
réseau public de fourniture d’énergie basse tension
utilisé à des fins domestiques.
L’appareil TrueCPR fonctionne sur batteries.
L’appareil TrueCPR émet un signal à ondes continues à 12 ± 0.1 kHz. Les niveaux
maximum de puissance isotrope rayonnée équivalente (EIRP) et de puissance rayonnée
équivalente (ERP) atteignent -40 dBm, mesure prise à une distance de 3 m.
Performances essentielles
L’appareil TrueCPR conserve des performances sûres et efficaces (exactitude de la
profondeur) lorsqu’il est utilisé dans l’environnement électromagnétique précisé du Tableau
2 au Tableau 4.
©2013 Physio-Control, Inc. 73
Page 78

Guide de compatibilité électromagnétique
Immunité électromagnétique
Tableau 2 Guide et déclaration du fabricant - Immunité aux interférences
électromagnétiques
Le dispositif TrueCPR, modèle 80596-000003, est prévu pour être utilisé dans
l’environnement électromagnétique décrit ci-dessous. Le client ou l’utilisateur doit veiller à
ce que l’appareil TrueCPR soit utilisé dans un tel environnement.
Test
d’immunité
Décharges
électrostatiques
IEC 61000-4-2
Transitoires
électriques
rapides / en
salve
IEC 61000-4-4
Surtension
IEC 61000-4-5
Baisses de
tension, brèves
coupures et
variations de
tension sur les
lignes d’entrée
de l’alimentation
IEC 61000-4-11
Champ
magnétique
avec fréquence
du secteur (50/
60 Hz).
IEC 61000-4-8
Remarque : U
Niveau de test
IEC 60601
Contact ±6 kV
Air ±8 kV
Niveau de
conformité
Contact ±6 kV
Air ±8 kV
Environnement
électromagnétique - Guide
Les sols doivent être en bois, en
béton ou recouverts de dalles en
céramique. Si les sols sont
recouverts de matériaux
synthétiques, le niveau
d’humidité relative doit être d’au
moins 30 %.
±2 kV pour les
lignes
Non applicable L’appareil TrueCPR fonctionne
sur batteries.
d’alimentation
électrique
±1 kV pour les
lignes d’entrée/de
sortie
±1 kV de ligne(s) à
ligne(s)
Non applicable L’appareil TrueCPR fonctionne
sur batteries.
±2 kV de ligne(s) à
terre
<5% U
de baisse en U
(>95%
T
pour 0,5 cycle
40% U
(60% de
T
baisse en U
Non applicable L’appareil TrueCPR fonctionne
sur batteries.
) pour
T
)
T
5 cycles
70% U
baisse en U
25 cycles
<5% U
de baisse en U
pour 5 sec
(30% de
T
) pour
T
(>95%
T
)
T
3 A/m 3 A/m Les niveaux de fréquence des
champs magnétiques doivent
correspondre aux niveaux
courants d’un environnement
commercial ou hospitalier
standard.
correspond à la tension CA avant l’application du niveau de test.
T
74 Instructions d’utilisation de l’appareil TrueCPR
Page 79

French/Français
d 1.2 P=
d 1.2 P=
d 2.3 P=
Tableau 3 Guide et déclaration du fabricant - Immunité aux interférences
électromagnétiques
Le dispositif TrueCPR, modèle 80596-000003, est prévu pour être utilisé dans
l’environnement électromagnétique décrit ci-dessous. Le client ou l’utilisateur doit veiller à
ce que l’appareil TrueCPR soit utilisé dans un tel environnement.
Test
d’immunité
Énergie
radioélectrique
par conduction
IEC 61000-4-6
Énergie
radioélectrique
par émission
IEC 61000-4-3
Niveau de
test IEC
60601
3 Vrms
150 kHz à
80 MHz
3 V/m
80 MHz à
2,5 GHz
Niveau de
Environnement électromagnétique -
conformité
La distance séparant les équipements
de communication RF mobiles et
portables et les pièces du dispositif
TrueCPR (câbles compris) ne doit pas
être inférieure à la distance de
séparation recommandée et calculée à
partir de l’équation applicable pour la
fréquence du transmetteur.
Distance de séparation
recommandée
3 Vrms
3 V/m
Guide
80 MHz à 800 MHz
800 MHz à 2,5 GHz
Où P est la puissance de sortie
maximale de l’émetteur en watts (w)
selon le fabricant de l’émetteur et d est
la distance de séparation
recommandée en mètres (m).
Les intensités de champs provenant
d’émetteurs radioélectriques fixes,
établies après une reconnaissance des
sites électromagnétiques,
inférieures au niveau de conformité de
chaque bande de fréquences.
a
doivent être
b
Des interférences sont possibles à
proximité d’un appareil portant le
symbole suivant :
Remarque 1 : À 80 MHz et 800 MHz, la bande de fréquences maximale s’applique.
Remarque 2 : Ces recommandations ne s’appliquent pas dans toutes les situations. La
propagation électromagnétique est affectée par l’absorption et la réflexion des structures,
des objets et des personnes.
©2013 Physio-Control, Inc. 75
Page 80

Guide de compatibilité électromagnétique
PPP
Tableau 3 Guide et déclaration du fabricant - Immunité aux interférences
électromagnétiques (suite)
a Les intensités de champs provenant d’émetteurs fixes tels que stations de base pour téléphones
sans fil et radios terrestres mobiles, radio amateur, radiodiffusions AM et FM, diffusions TV, etc., ne
peuvent pas être théoriquement prévues de manière précise. Pour évaluer l’environnement
électromagnétique engendré par les transmetteurs RF fixes, une étude de site électromagnétique
devrait être envisagée. Si l’intensité de champ mesurée sur le site dans lequel le dispositif TrueCPR
est utilisé dépasse le niveau de conformité RF applicable susmentionné, il conviendra de vérifier le
bon fonctionnement du dispositif TrueCPR. En cas de fonctionnement anormal, des mesures
complémentaires pourraient être nécessaires, comme par exemple la réorientation ou la
relocalisation du dispositif TrueCPR.
b Avec une bande de fréquences comprise entre 150 kHz et 80 MHz, les intensités de champs doivent
être inférieures à 3 V/m.
Distances de séparation
Tableau 4 Distances de séparation recommandées entre les équipements de
communication RF portables et mobiles et le dispositif TrueCPR, modèle 80596-000003.
Le dispositif TrueCPR est conçu pour être utilisé dans un environnement
électromagnétique dans lequel les nuisances RF émises sont contrôlées. Le client ou
l’utilisateur du dispositif TrueCPR peut prévenir les interférences électromagnétiques en
conservant une distance minimale entre l’équipement de communication RF portable
(transmetteurs) et le dispositif TrueCPR, comme recommandé ci-dessous, selon la
puissance maximale de l’équipement de communication.
Puissance de sortie
nominale maximale
de l’émetteur,
W
150 kHz à
80 MHz
d = 1,2
Distance de séparation en fonction
de la fréquence de l’émetteur,
m
80 MHz à
800 MHz
d = 1,2
800 MHz à 2,5 GHz
d = 2,3
0,01 0,12 0,12 0,23
0,1 0,38 0,38 0,73
1 1,2 1,2 2,3
10 3,8 3,8 7,3
100 12 12 23
Pour les émetteurs dont la puissance de sortie nominale maximale n’est pas répertoriée
ci-dessus, la distance de séparation recommandée d en mètres (m) peut être déterminée
par l’équation applicable à la fréquence de l’émetteur, où P désigne la puissance de sortie
maximale de l’émetteur en watts (W) selon le fabricant.
Remarque 1 : À 80 MHz et 800 MHz, la distance de séparation des bandes de fréquences
maximales s’applique.
Remarque 2 : Ces recommandations ne s’appliquent pas dans toutes les situations. La
propagation électromagnétique est affectée par l’absorption et la réflexion des structures,
des objets et des personnes.
76 Instructions d’utilisation de l’appareil TrueCPR
Page 81

French/Français
Informations de conformité règlementaire
Déclaration de conformité de la Commission fédérale des communications (FCC)
Cet appareil est conforme à la section 15 des règles de la FCC. Son utilisation est soumise
aux deux conditions suivantes : (1) Cet appareil ne doit pas causer d’interférences
nuisibles, et (2) il doit accepter toute interférence reçue, y compris les interférences
pouvant entraîner un fonctionnement non désiré.
AVERTISSEMENT
Tout changement ou modification non expressément approuvé par la partie
responsable de la conformité pourrait annuler l’autorisation de l’utilisateur à utiliser
l’appareil.
Conformité avec la directive sur les équipements radio et équipements terminaux de télécommunication (R&TTE)
Cet appareil est un appareil de Classe 2 selon la directive R&TTE :
BE BG CZ DK DE
FR ES EL IE EE
IT CY LV LT LU
PL AT NL MT HU
PT RO SI SK FI
SE UK
©2013 Physio-Control, Inc. 77
Page 82

Page 83

Page 84

Physio-Control, Inc.
11811 Willows Road NE
Redmond, WA 98052 USA
Telephone: 425.867.4000
Toll Free (USA only):
800.442.1142
Fax: 425.867.4121
www.physio-control.com
Physio-Control, Inc., 11811 Willows Road NE,
Redmond, WA 98052 USA
Physio-Control Operations Netherlands B.V.
Keizersgracht 125-127, 1015 CJ Amsterdam
©2013 Physio-Control, Inc.
TrueCPR is a trademark of Physio-Control, Inc.
Duracell is a registered trademark of Procter & Gamble Company.
Publication date: 03/2013 3312954-901
Physio-Control Australia Pty Ltd
Suite 4.01
15 Orion Road
Lane Cove
NSW 2066
Australia
 Loading...
Loading...