Philips Medical Systems North America MX40SH1B4 Users Manual

IntelliVue MX40
Draft Copy
Instructions for Use
Release B.0

Draft Copy
Notice
Proprietary Information
This document contains proprietary information, which is protected by
copyright.
First Edition 2012
4535 643 15721
Copyright
Copyright © 2012 Koninklijke Philips Electronics N.V. All rights reserved.
Reproduction in whole or in part is prohibited without the prior written
consent of the copyright holder. Philips Medical Systems Nederland B.V.
reserves the right to make changes in specifications and/or to discontinue any
products at any time without notice or obligation and will not be liable for any
consequences resulting from the use of this publication.
This product contains software licensed under an open source license. For
acknowledgments, license texts and source code, please refer to the IntelliVue
Information Center iX M3290B Software\References\README.pdf.
Windows ® is a registered trademark of Microsoft Corporation in the United
States and other countries.
EASI is a trademark of Zymed Inc.
OxiCliq ® and OxiMax ® are registered trademarks of Nellcor Incorporated.
Duracell ® is a registered trademark of Procter & Gamble Incorporated.
STERRAD ® is a registered trademark of Advanced Sterilization Products.
GORE-TEX ® is a registered trademark of W.L. Gore & Assoc. Incorporated.
Tone modulation is licensed under US patent 4,653,498 from Nellcor Puritan
Bennett Incorporated.
Manufacturer
Philips Medical Systems
3000 Minuteman Road
Andover, MA 01810-1099
(978) 687-1501
Printed in USA
Document number
ii

Draft Copy
4535 643 15721
Warranty
The information contained in this document is subject to change without
notice. Philips Medical Systems makes no warranty of any kind with regard to
this material, including, but not limited to, the implied warranties or
merchantability and fitness for a particular purpose. Philips Medical Systems
shall not be liable for errors contained herein or for incidental or consequential
damages in connection with the furnishing, performance, or use of this
material.
FCC
This device complies with Part 15 and/or Part 95 of the FCC Rules. Operation
is subject to the following two conditions: (1) these devices may not cause
harmful interference, and (2) these devices must accept any interference
received, including interference that may cause undesired operation.
Changes and modifications not expressly approved by Philips Medical
Systems can void your authority to operate this equipment under Federal
Communications Commission's rules
Protecting Personal Information
It is recommended that customers have policies and procedures for the proper
handling of personal or sensitive information, ePHI (electronic protected
health information) and PHI (protected health information), which will
maintain the confidentiality, integrity, and the availability of these types of
data. Any organization using this product should implement the required
protective means necessary to safeguard personal information consistent with
each applicable country law, code and regulation; and consistent with their
developed and maintained internal policies and procedures.
While handling personal information is outside the scope of this document; in
general, each organization is responsible for identifying:
Who has access to personal data and under what conditions an individual
has authorization to use that data.
What security controls are in place to protect personal and sensitive data.
How the data is stored and the conditions by which it is stored.
How the data is transmitted and the conditions under which that data is
transmitted.
iii

Draft Copy
Protecting personal health information is a primary component of a security
strategy. Personal and sensitive information should be protected according to
the applicable laws, regulations and directives, such as HIPAA, PIPEDA
and/or Council of the European Union security and privacy rules.
Compliance
Uses of the system for purposes other than those intended and expressly
stated by the manufacturer, as well as incorrect use, incorrect operation, or
modifications made to the system without explicit approval from Philips, may
relieve the manufacturer (or his agent) from all or some responsibilities for
resultant noncompliance, damage or injury.
Printing History
New editions of this document will incorporate all material updated since the
previous edition. Update packages may be issued between editions and
contain replacement and additional pages to be merged by a revision date at
the bottom of the page. Note that pages which are rearranged due to changes
on a previous page are not considered revised.
The documentation printing date and part number indicate its current edition.
The printing date changes when a new edition is printed. (Minor corrections
and updates which are incorporated at reprint do not cause the date to
change.) The document part number changes when extensive technical
changes are incorporated.
First Edition February 2012
Document Conventions
In this guide:
Warnings
Warning
A Warning alerts you to a potential serious outcome, adverse event or safety
hazard. Failure to observe a warning may result in death or serious injury to
the user or patient.
iv

Draft Copy
Cautions
Caution
A Caution alerts you to where special care is necessary for the safe and
effective use of the product. Failure to observe a caution may result in minor
or moderate personal injury or damage to the product or other property, and
possibly in a remote risk of more serious injury.
Notes
A Note contains additional information on the product's usage.
v

Draft Copy
vi

Draft Copy
Contents
1. Introducing the IntelliVue MX40 1-1
MX40 Features -------------------------------------------------------------------------- 1-2
MX40 Models ---------------------------------------------------------------------------- 1-3
MX40 Release B.0 Compatibility --------------------------------------------------- 1-4
2. What's New? 2-1
New Features and Enhancements------------------------------------------------- 2-2
3. Product Safety 3-1
General Safety -------------------------------------------------------------------------- 3-2
Safety Symbols & Other Marks ----------------------------------------------------- 3-5
4. Basic Operation 4-1
Controls, Indicators and Connectors ---------------------------------------------- 4-2
MX40 Controls and Indicators -------------------------------------------------- 4-2
Multi-Function Button ----------------------------------------------------------- 4-3
Silence Alarm Button ----------------------------------------------------------- 4-3
SmartKeys Button --------------------------------------------------------------- 4-3
Main Screen Button ------------------------------------------------------------- 4-4
SmartKeys ------------------------------------------------------------------------- 4-4
Alarms Area ----------------------------------------------------------------------- 4-5
Patient Information Area ------------------------------------------------------- 4-6
Paced Status --------------------------------------------------------------------- 4-6
Display Lock ---------------------------------------------------------------------- 4-6
Status Area ------------------------------------------------------------------------ 4-7
Operating and Navigating ------------------------------------------------------------ 4-8
Power-On Self Test --------------------------------------------------------------- 4-8
Navigating --------------------------------------------------------------------------- 4-8
Selecting Display Elements ----------------------------------------------------- 4-8
Locking the Display---------------------------------------------------------------- 4-9
Measurement Area ---------------------------------------------------------------- 4-9
Measurement Area Display Configurations --------------------------------- 4-9
Connecting/Disconnecting the Patient Cable ----------------------------- 4-10
Understanding Settings -------------------------------------------------------------- 4-12
Changing Measurement Settings --------------------------------------------- 4-12
ECG Settings at the MX40 ----------------------------------------------------- 4-12
Waveform Settings at the MX40 ---------------------------------------------- 4-13
Battery Information -------------------------------------------------------------------- 4-14
Battery Safety Information ------------------------------------------------------ 4-14
Lithium-ion Rechargeable Battery Care ------------------------------------ 4-15
Lithium-ion Rechargeable Battery Handling Precautions ------------ 4-15
Lithium-ion Rechargeable Battery Storage ------------------------------ 4-16
Contents 1

Draft Copy
Inserting/Removing Batteries ------------------------------------------------- 4-16
Inserting Batteries ---------------------------------------------------------------- 4-17
Removing the Batteries -------------------------------------------------------- 4-19
Battery Charge Status ---------------------------------------------------------- 4-20
Lithium-ion Rechargeable Battery Charge Status -------------------- 4-20
AA Battery Charge Status --------------------------------------------------- 4-21
Pouch Use ------------------------------------------------------------------------------ 4-22
Securing the Pouch-------------------------------------------------------------- 4-22
Showering -------------------------------------------------------------------------- 4-24
Telemetry Mode Use ---------------------------------------------------------------- 4-26
Monitoring Mode Use ---------------------------------------------------------------- 4-27
Briefing the Patient ------------------------------------------------------------------- 4-28
5. Alarms 5-1
Alarms Overview ------------------------------------------------------------------------ 5-2
Visual Alarm Indicators ----------------------------------------------------------- 5-3
Alarm Message ------------------------------------------------------------------- 5-3
Alarm Indicator ------------------------------------------------------------------- 5-4
Flashing Numeric ---------------------------------------------------------------- 5-4
Audible Alarm Indicators when in Monitoring Mode ---------------------- 5-5
Traditional Audible Alarms (HP/Agilent/Philips/Carenet) -------------- 5-5
ISO/IEC Standard Audible Alarms ------------------------------------------ 5-5
Acknowledging Alarms ----------------------------------------------------------- 5-6
Pausing or Switching Off Alarms----------------------------------------------- 5-6
To Pause All Alarms ------------------------------------------------------------ 5-6
While Alarms are Paused ----------------------------------------------------- 5-7
Restarting Paused Alarms ---------------------------------------------------- 5-7
Alarm Limits ------------------------------------------------------------------------- 5-7
Viewing Individual Alarm Limits ---------------------------------------------- 5-8
Reviewing Alarms ------------------------------------------------------------------ 5-8
Review Alarms Window -------------------------------------------------------- 5-8
Alarm Reminders ---------------------------------------------------------------- 5-8
Latching Alarms -------------------------------------------------------------------- 5-9
Alarm Latching Behavior --------------------------------------------------------- 5-9
Alarm Behavior at Power On ---------------------------------------------------- 5-9
Physiologic Alarms ------------------------------------------------------------------- 5-10
Technical Alarms (INOPs) --------------------------------------------------------- 5-14
6. ECG and Arrhythmia Monitoring 6-1
ECG Safety Information--------------------------------------------------------------- 6-2
For Paced Patients ---------------------------------------------------------------- 6-3
Measuring ECG ------------------------------------------------------------------------- 6-5
Connecting and Positioning ECG Electrodes ----------------------------------- 6-6
2 Contents

Draft Copy
Selecting the Primary and Secondary ECG Leads ---------------------------- 6-8
Checking Paced Status --------------------------------------------------------------- 6-9
Understanding the ECG Display -------------------------------------------------- 6-10
Monitoring Paced Patients ---------------------------------------------------------- 6-11
Optimizing Lead Selection for Paced Patients ---------------------------- 6-11
Changing the Size of the ECG Wave -------------------------------------------- 6-12
Choosing EASI or Standard Lead Placement --------------------------------- 6-13
Derived 12-lead ECG----------------------------------------------------------------- 6-14
Hexad -------------------------------------------------------------------------------- 6-14
EASI ---------------------------------------------------------------------------------- 6-14
ECG Configuration -------------------------------------------------------------------- 6-15
ECG Leads Monitored --------------------------------------------------------------- 6-16
Reconstructed Leads ----------------------------------------------------------------- 6-18
Chest Electrode Placement --------------------------------------------------------- 6-19
3-Wire Placement --------------------------------------------------------------------- 6-20
5-Wire Placement (Standard Mode) ---------------------------------------------- 6-21
5-Wire Placement (EASI Mode) --------------------------------------------------- 6-22
6-Wire Placement --------------------------------------------------------------------- 6-23
Selecting Positions of Va and Vb Chest Leads --------------------------- 6-23
6-Wire Placement (Hexad Mode) ------------------------------------------------- 6-24
Monitoring during Leads Off -------------------------------------------------------- 6-25
ECG Fallback ---------------------------------------------------------------------- 6-25
Relearning -------------------------------------------------------------------------- 6-25
ST/AR Arrhythmia Monitoring ------------------------------------------------------ 6-27
ST/AR Arrhythmia Algorithm --------------------------------------------------- 6-27
Indications for Use ------------------------------------------------------------- 6-27
How the ST/AR Algorithm Works --------------------------------------------- 6-27
Aberrantly-Conducted Beats ------------------------------------------------ 6-28
Atrial Fibrillation Alarm -------------------------------------------------------- 6-28
Intermittent Bundle Branch Block ------------------------------------------ 6-29
ECG and Arrhythmia Alarm Overview --------------------------------------- 6-29
Using ECG Alarms --------------------------------------------------------------- 6-30
Extreme Alarm Limits for Heart Rate -------------------------------------- 6-30
Arrhythmia Alarm Settings --------------------------------------------------- 6-30
Yellow Arrhythmia Alarms ---------------------------------------------------- 6-31
Viewing Arrhythmia Waves -------------------------------------------------- 6-31
Arrhythmia Beat Labels ------------------------------------------------------- 6-32
Enhanced Arrhythmia Chain ------------------------------------------------- 6-33
Basic Arrhythmia Chain ------------------------------------------------------- 6-34
Learning ----------------------------------------------------------------------------- 6-34
Learning Phase ----------------------------------------------------------------- 6-34
Single Lead Analysis ---------------------------------------------------------- 6-35
Multilead Analysis -------------------------------------------------------------- 6-35
Multilead Analysis With Changes in One Lead ------------------------- 6-35
Contents 3

Draft Copy
EASI ECG Monitoring -------------------------------------------------------- 6-36
Initiating Arrhythmia Relearning Manually --------------------------------- 6-36
ST/AR ST Analysis Algorithm ----------------------------------------------------- 6-37
Introduction ------------------------------------------------------------------------ 6-37
The Measurements -------------------------------------------------------------- 6-38
Overview --------------------------------------------------------------------------- 6-38
Turning ST or STE On and Off ----------------------------------------------- 6-38
Displayed ST Data --------------------------------------------------------------- 6-39
ST Lead Groups ------------------------------------------------------------------ 6-39
Derived 12 Lead ECG ---------------------------------------------------------- 6-40
EASI ST Analysis -------------------------------------------------------------- 6-40
HEXAD ST Analysis ---------------------------------------------------------- 6-41
ST Alarms -------------------------------------------------------------------------- 6-41
STE Alarms ------------------------------------------------------------------------ 6-41
QT Interval Monitoring --------------------------------------------------------------- 6-42
Intended Use ---------------------------------------------------------------------- 6-43
How the QT Analysis Algorithm Works ------------------------------------- 6-43
Adjusting QT Settings -------------------------------------------------------- 6-44
Limitations for QT Monitoring ----------------------------------------------- 6-45
7. Monitoring Pulse Rate 7-1
Pulse Rate Measurement ------------------------------------------------------------ 7-2
Displaying the Pulse Rate Measurement at the MX40 ----------------------- 7-3
8. Monitoring Respiration Rate (Resp) 8-1
Respiration Rate Measurement ----------------------------------------------------- 8-2
Resp Safety Information -------------------------------------------------------------- 8-3
Lead Placement for Monitoring Resp --------------------------------------------- 8-4
Optimizing Lead Placement for Resp ----------------------------------------- 8-4
Cardiac Overlay -------------------------------------------------------------------- 8-4
Abdominal Breathing -------------------------------------------------------------- 8-4
Displaying Resp on the MX40 ------------------------------------------------------- 8-5
9. SpO2 Monitoring 9-1
SpO2 Safety Information -------------------------------------------------------------- 9-2
SpO2 Information for the User -------------------------------------------------- 9-3
Pulse Oximetry Measurement ------------------------------------------------------- 9-5
SpO2 Sensors ----------------------------------------------------------------------- 9-6
Selecting an SpO2 Sensor ------------------------------------------------------- 9-6
Sensor Application Safety Information --------------------------------------- 9-7
Applying the Sensor --------------------------------------------------------------- 9-8
Connecting SpO2 Cables -------------------------------------------------------- 9-8
Tone Modulation Indication ------------------------------------------------------ 9-8
Signal Quality Indicator ----------------------------------------------------------- 9-8
Measuring SpO2 -------------------------------------------------------------------- 9-9
4 Contents

Draft Copy
Understanding SpO2 Alarms --------------------------------------------------- 9-10
10. Monitoring with other Assigned Devices 10-1
Assigning Devices --------------------------------------------------------------------- 10-3
Device Assignment at the Information Center ---------------------------- 10-3
Device Assignment at the MX40 ---------------------------------------------- 10-3
Device Assignment at the Patient Monitor --------------------------------- 10-4
Controls Available when Assigned to IntelliVue Cableless Measurements10-6
Controls Available when Assigned to IntelliVue Patient Monitors -------- 10-7
Networked Device Synchronized Settings -------------------------------------- 10-8
MX40 Display when Wirelessly Connected to a Patient Monitor --------- 10-9
11. Monitoring with the MX40 at the Information Center 11-1
MX40 Connection to the Information Center ----------------------------------- 11-2
MX40 Controls in the Patient Window (IIC) ------------------------------------ 11-3
MX40 Controls in the Patient Window (IIC iX) --------------------------------- 11-5
Locating the MX40 (Find Device) ------------------------------------------------- 11-7
Viewing Device Location and Location History (optional) ------------------ 11-8
Using the Device Location Client (optional - IIC only) ----------------------- 11-9
Patient Configurable Settings in Telemetry Setup -------------------------- 11-10
Unit Configurable Settings -------------------------------------------------------- 11-13
12. Operating with Information Center Release L or M 12-1
Display ----------------------------------------------------------------------------------- 12-2
Alarms ------------------------------------------------------------------------------------ 12-3
13. Trends 13-1
Viewing Vital Trend Information --------------------------------------------------- 13-2
14. Maintenance 14-1
Cleaning --------------------------------------------------------------------------------- 14-2
Cleaning Materials for the MX40 --------------------------------------------- 14-3
Disposing of the MX40 --------------------------------------------------------------- 14-5
Label Assignment for Replacement MX40 ------------------------------------- 14-6
Re-assigning an Equipment Label at the IntelliVue Information Center14-6
Re-assigning an Equipment Label at the IntelliVue Information Center
iX -------------------------------------------------------------------------------------- 14-7
Charging Lithium-ion Rechargeable Batteries --------------------------------- 14-8
Battery Power Indicators -------------------------------------------------------- 14-8
Charging Station LEDs -------------------------------------------------------- 14-8
Battery Status on the Charging Station Display ------------------------ 14-9
Battery Lifetime Management ------------------------------------------------- 14-9
Battery Disposal ----------------------------------------------------------------- 14-10
15. Safety Standards & Specifications 15-1
Regulatory Information --------------------------------------------------------------- 15-2
Software Hazard Prevention --------------------------------------------------- 15-2
Contents 5

Draft Copy
AC Power Source ---------------------------------------------------------------- 15-2
Industrie Canada Compliance (Canada) ----------------------------------- 15-2
Safety Standards ----------------------------------------------------------------- 15-2
Intended Use Statement ------------------------------------------------------- 15-3
Indications for Use --------------------------------------------------------------- 15-3
Intended Uses of MX40 -------------------------------------------------------- 15-3
Authorized EU Representative ----------------------------------------------- 15-4
Patient Population --------------------------------------------------------------- 15-4
Rx ------------------------------------------------------------------------------------ 15-4
Essential Performance --------------------------------------------------------- 15-4
Risk Management Considerations ------------------------------------------ 15-5
Electromagnetic Compatibility ----------------------------------------------------- 15-8
Reducing Electromagnetic Interference ------------------------------------ 15-9
Restrictions for Use-------------------------------------------------------------- 15-9
Electromagnetic Compatibility (EMC) Specifications ------------------- 15-9
Accessories Compliant with EMC Standards -------------------------- 15-9
Electromagnetic Emissions --------------------------------------------------- 15-10
Electromagnetic Immunity ---------------------------------------------------- 15-10
Recommended Separation Distance -------------------------------------- 15-11
Electrosurgery Interference/Defibrillation/Electrostatic Discharge15-13
Restart Time ------------------------------------------------------------------- 15-13
Battery Specifications -------------------------------------------------------------- 15-14
Lithium-ion Battery Charge Time ------------------------------------------------ 15-17
Physical Specifications ------------------------------------------------------------- 15-18
MX40 1.4 GHz Smart-Hopping Radio ------------------------------------------ 15-19
MX40 2.4 GHz Smart-Hopping Radio ------------------------------------------ 15-20
MX40 Short-Range Radio --------------------------------------------------------- 15-22
MX40 2.4GHz WLAN Radio ------------------------------------------------------ 15-23
FCC and Industry Canada Radio Compliance -------------------------- 15-24
Environmental Specifications ----------------------------------------------------- 15-25
Measurement Specifications ----------------------------------------------------- 15-26
ECG -------------------------------------------------------------------------------- 15-26
ECG Performance Disclosure/Specifications ---------------------------- 15-27
Respiration ------------------------------------------------------------------------ 15-29
Respiration Alarm------------------------------------------------------------- 15-29
FAST SpO2 ----------------------------------------------------------------------- 15-30
SpO2 Sensor Accuracy -------------------------------------------------------- 15-31
A. Accessories A-1
MX40 Accessories --------------------------------------------------------------------- A-2
Pouches ------------------------------------------------------------------------------ A-2
Miscellaneous ----------------------------------------------------------------------- A-2
ECG Accessories ----------------------------------------------------------------------- A-3
Electrodes ---------------------------------------------------------------------------- A-3
6 Contents

Draft Copy
Leadsets and Patient Cables --------------------------------------------------- A-3
SpO2 Accessories ---------------------------------------------------------------------- A-5
Philips/Nellcor Disposable Sensors ------------------------------------------- A-5
Philips Reusable Sensors ------------------------------------------------------- A-5
Adapter Cables --------------------------------------------------------------------- A-6
B. Default Settings B-1
Alarm Default Settings ---------------------------------------------------------------- B-2
ECG, Arrhythmia, ST and QT Default Settings --------------------------------- B-3
Configuration Default Settings at the MX40 ------------------------------------- B-5
C. Sales and Support Offices C-1
Contents 7

Draft Copy
8 Contents

MX40 Features ........................................................................................... 1-2
MX40 Models ............................................................................................. 1-3
MX40 Release B.0 Compatibility ............................................................. 1-4
Draft Copy
1. Introducing the IntelliVue
MX40
This section introduces the IntelliVue MX40 wearable patient monitor.
Introducing the IntelliVue MX40 1-1

MX40 Features
Draft Copy
MX40 Features
Easy for clinicians to use and comfortable for patients to wear.
2.8" color, touch sensitive display.
Smart, multi-measurement cable system available for use with reusable
and single-patient use supplies.
FAST SpO2 (continuous, automatic or manual measurement).
Standard, EASI or Hexad ECG lead system selection.
Impedance-based Respiration measurement.
6-lead with two V-leads for diagnosing multiple cardiac abnormalities,
including wide-QRS complex tachycardias and acute myocardial
ischemia/infarction.
Local measurement trend/alarm history.
Local alarming for measurements (requires IntelliVue Information
Center Release N or later or IntelliVue Information Center iX).
Integrated radio for connection to an Information Center iX.
Integrated Short-Range Radio (SRR).
Communication with IntelliVue Patient Monitors and Cableless
Measurements via Short-Range Radio connection (MP5/MP5T/MP5SC,
MP2 and X2 monitors only).
Powered by three AA batteries or rechargeable lithium-ion battery
pack.
Note — The WLAN MX40 (Model Number 865352) is powered only by
the rechargeable lithium ion battery pack.
Audio feedback for out-of-range and lost device.
Battery gauge on device and at Information Center.
Alarm suspend and resume from standby at device and Information
Center.
Pouch with clear front that closes securely.
Note — Unlike a traditional bedside monitor which operates on AC power,
the MX40 is powered by battery and provides time-limited screen display
and local alarming.
1-2 Introducing the IntelliVue MX40

MX40 Models
Draft Copy
MX40 Models
The MX40 is available in three models (ECG only, ECG and FAST SpO2, or
ECG and SpO2 Ready (for future upgrade).
Introducing the IntelliVue MX40 1-3

MX40 Release B.0 Compatibility
Draft Copy
MX40 Release B.0 Compatibility
The MX40 is compatible for use with IntelliVue Information Center Release
N and IntelliVue Information Center iX Release A. Limited compatibility is
offered when used with IntelliVue Information Center Release L or M. See
the "Operating with Release L or M" chapter for more information.
The MX40 is compatible for use with IntelliVue Patient Monitors Release G
or later when wirelessly connected.
The MX40 is compatible for use with IntelliVue Cableless Measurements
Release A.1.
The MX40 is compatible for use with Access Point Controller 862147,
Release B.00.19 and Access Point Controller 865346, Release C.00.04.
The MX40 Patient Cable is compatible for use with IntelliVue Patient
Monitor platforms MP2/X2, MP5/MP5T/MP5SC, MP20/30 with MMS or X2,
MP40/50 with MMS or X2, MP60/70 with MMS or X2, MP80/90 with MMS
or X2, and MX800/700/600 with MMS or X2.
1-4 Introducing the IntelliVue MX40

New Features and Enhancements ........................................................... 2-2
Draft Copy
2. What's New?
This section lists the most important new features and improvements to the
MX40 and its user interface. Further information is provided in other
sections of this book.
You might not have all of these features, depending on the MX40
configuration purchased by your hospital.
What's New? 2-1

New Features and Enhancements
Draft Copy
New Features and Enhancements
Compatibility
The MX40 B.0 offers compatibility with the new IntelliVue Information
Center iX
Respiration
The MX40 now offers a Respiration Rate measurement (available with
the IntelliVue Information Center iX only).
Rotating Alarm Presentation
When multiple alarms are active, the MX40 will rotate the display of the
alarm message every three seconds (Only INOPS are displayed with
IntelliVue Information Center Release L or M).
Numeric Only Display
A new display orientation is available showing six numerics only. No
waveforms are shown (available with IntelliVue Information Center iX).
ECG Waveform Size Adjustment
The size of the ECG waveform can now be adjusted by touching the
waveform on the display.
Wireless LAN Availability
The MX40 is now available as a Wireless LAN device for 802.11 a/b/g
communication (for use with IntelliVue Information Center iX only).
ST and QT Measurement Analysis
ST and QT values can be displayed on the MX40 (available with
IntelliVue Information Center iX only).
Hexad
A 12-lead ECG derived from a 6-wire electrode leadset is available to
increase patient comfort and reduce interference (available with
IntelliVue Information Center iX only).
2-2 What's New?

General Safety ............................................................................................ 3-2
Safety Symbols & Other Marks ............................................................... 3-5
Draft Copy
3. Product Safety
This section consolidates the general safety warnings associated with the
IntelliVue MX40. These warnings are repeated throughout the book in
context where relevant.
Safety symbols and other markings on the MX40 are also described here.
Product Safety 3-1

General Safety
Draft Copy
General Safety
Warnings
The MX40 operates exclusively via a wireless network connection,
thereforel, it should not be used for primary monitoring in applications
where momentary loss of the ECG is unacceptable at the Information
Center. It sends ECG and optionally pulse oximetry data to the
Information Center, where the Information Center displays real-time
patient data, provides alarm annunciation, data storage and review
applications. The ECG waveform data, alarms and optionally SpO2 can
always be viewed on the MX40 regardless of the connection to the
Information Center.
A wireless patient monitoring system will never be as reliable as a
patient monitoring system that transmits its signal through a wire, due
to the inherent nature of radio frequency and the many variables that
affect over-the-air communication. This factor should be considered
when electing to monitor patients using wireless technologies. If
occasional loss of ECG monitoring at the Information Center is not
clinically acceptable for certain patients, alternatives must be sought. As
the IntelliVue MX40 does not provide a wired network connection, we
would recommend the use of an IntelliVue patient monitor with a
wired connection to the Information Center for these patients.
For continued safe use of this equipment, it is necessary that the listed
instructions are followed. Instructions in this manual in no way
supersede established medical procedures.
Do not touch the patient, or table, or instruments, during defibrillation.
The battery door must be closed during defibrillation. These steps
protect the clinician from high defibrillator voltage.
This device is not to be used in the vicinity of electrosurgical units
because such use may interrupt or interfere with the transmission of
signals from the MX40.
This equipment is not suitable for use in the presence of a flammable
anesthetic mixture with air, or with oxygen or nitrous oxide.
This equipment is not suitable for use in an MRI environment.
Do not use patient cables with detachable lead wires that have exposed
male pins. Electrocution could result if these pins are plugged into AC
power.
3-2 Product Safety

General Safety
Draft Copy
Do not use patient cables or accessory cables and sensors if prior visual
inspection reveals cable damage or the presence of liquid, lint or dust
inside.
The system is not completely immune from radio interference although
it is designed to minimize interference. Sources of interference that may
be a problem include failing fluorescent lights and construction
equipment. See "Electromagnetic Compatibility p. 15-8". The product
should not be used next to or stacked with other equipment. If you
must stack the product, you must check that normal operation is
possible in the necessary configuration before the product is used on
patients.
Do not rely exclusively on the audible alarm system for patient
monitoring. Adjustment of alarm volume to a low level during patient
monitoring may result in patient danger. Remember that the most
reliable method of patient monitoring combines close personal
surveillance with correct operation of monitoring equipment.
If the MX40 enters a continuous "boot-up" cycle or the main display
does not appear or update, ensure that you are using a freshly charged
lithium-ion battery or new disposable batteries. If the batteries are fresh
and the device reboots or does not update, remove the device from
service and contact your service personnel.
Place the MX40 in a pouch or over clothing, or both, during patient use.
The device should not touch the patient’s skin during use.
Patients should be instructed not to open the battery compartment
while the MX40 is in use.
Failure on the part of the responsible individual hospital or institution
employing the use of this equipment to implement satisfactory
maintenance as needed may cause undue equipment failure and
possible health hazards.
Because the coverage range of Access Points can sometimes overlap,
including different floor levels, the IntelliVue Device Location feature is
not intended for use when attempting to locate a patient.
Product Safety 3-3

General Safety
Draft Copy
Short-range radio connections are subject to interruption due to
interference from other radio sources in the vicinity, including
microwaves, bluetooth devices, and DECT phones. Outside the
frequency band and 5% above and below, i.e. the exclusion band
according to IEC 60601-1-2, the short-range radio connection is immune
up to 3V/m in the frequency range from 80MHz to 2.5 GHz. Depending
on the strength and duration of the interference, the interruption may
occur for an extended period. Any interruption of the signal due to
interference, moving out of range, or for other reasons is indicated with
a Tele Disconnected INOP message on the IntelliVue Patient Monitor.
Caution
Philips recommends that when using a pouch to attach the MX40 to your
patient that you consider your patient's condition and are careful about
placement of the straps as the straps could present a strangulation hazard.
3-4 Product Safety
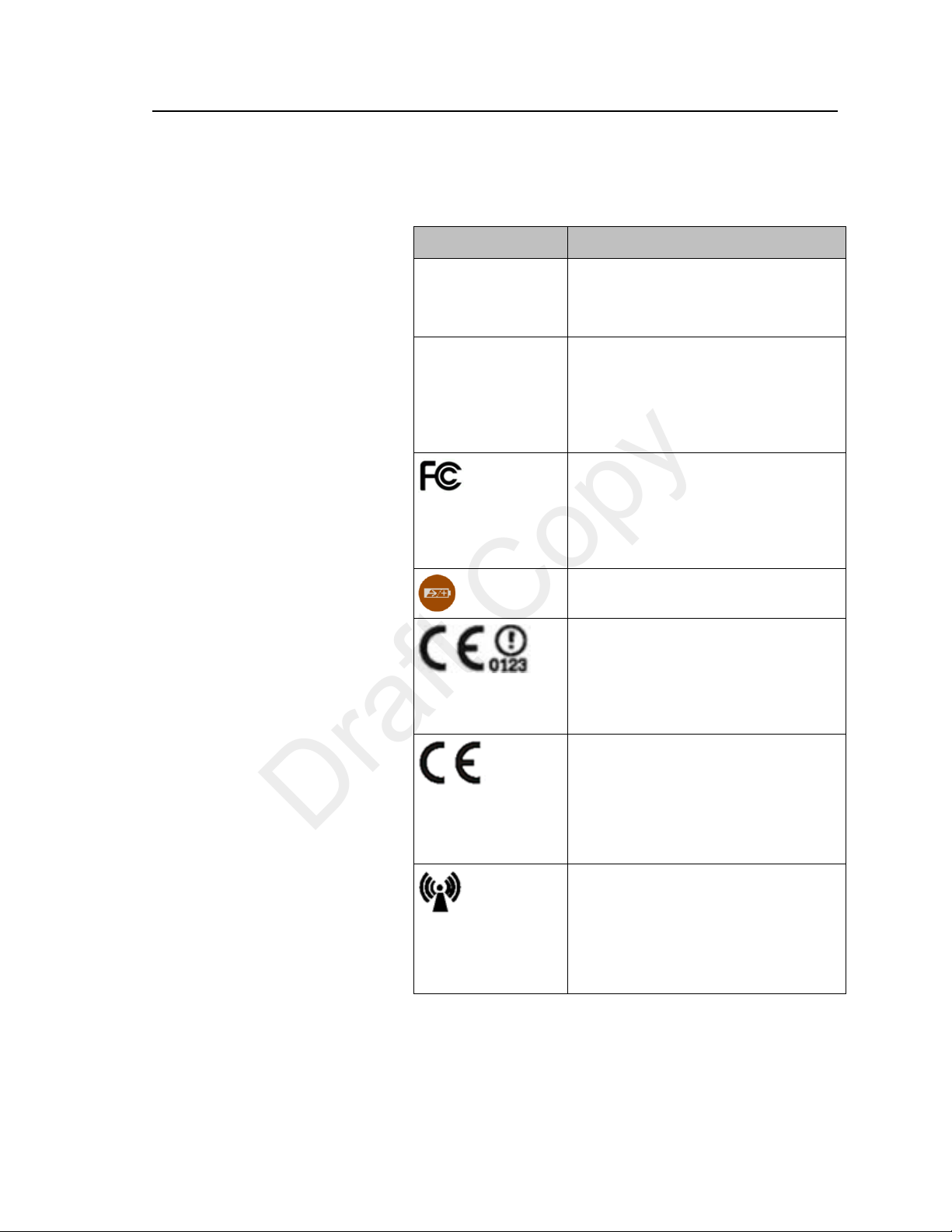
Safety Symbols & Other Marks
Label
Definition
FCC ID:
IC:
Federal Communications Commission
(FCC) ID
Industry Canada Number
GMDN:
Global Medical Device Nomenclature
Federal Communications Commission
(FCC)
Declaration of Conformity
Rechargeable Battery
CE Mark (MX40)
Compliance to Council Directive
93/42/EEC (Medical Device Directive) and
1995/5/EC (Radio Equipment and
Telecommunications Equipment Directive)
Symbol for Class 2 Radio Equipment
CE Mark (Rechargeable Lithium-ion
Battery)
Compliance to Council Directive
2004/108/EC (EMC Directive)
Non-Ionizing Radiation
Interference to electronic equipment may
occur in the vicinity of devices marked with
this symbol.
Draft Copy
Safety Symbols & Other Marks
The table below describes the safety symbols and other markings present on the MX40 and the lithium-ion
battery.
Product Safety 3-5
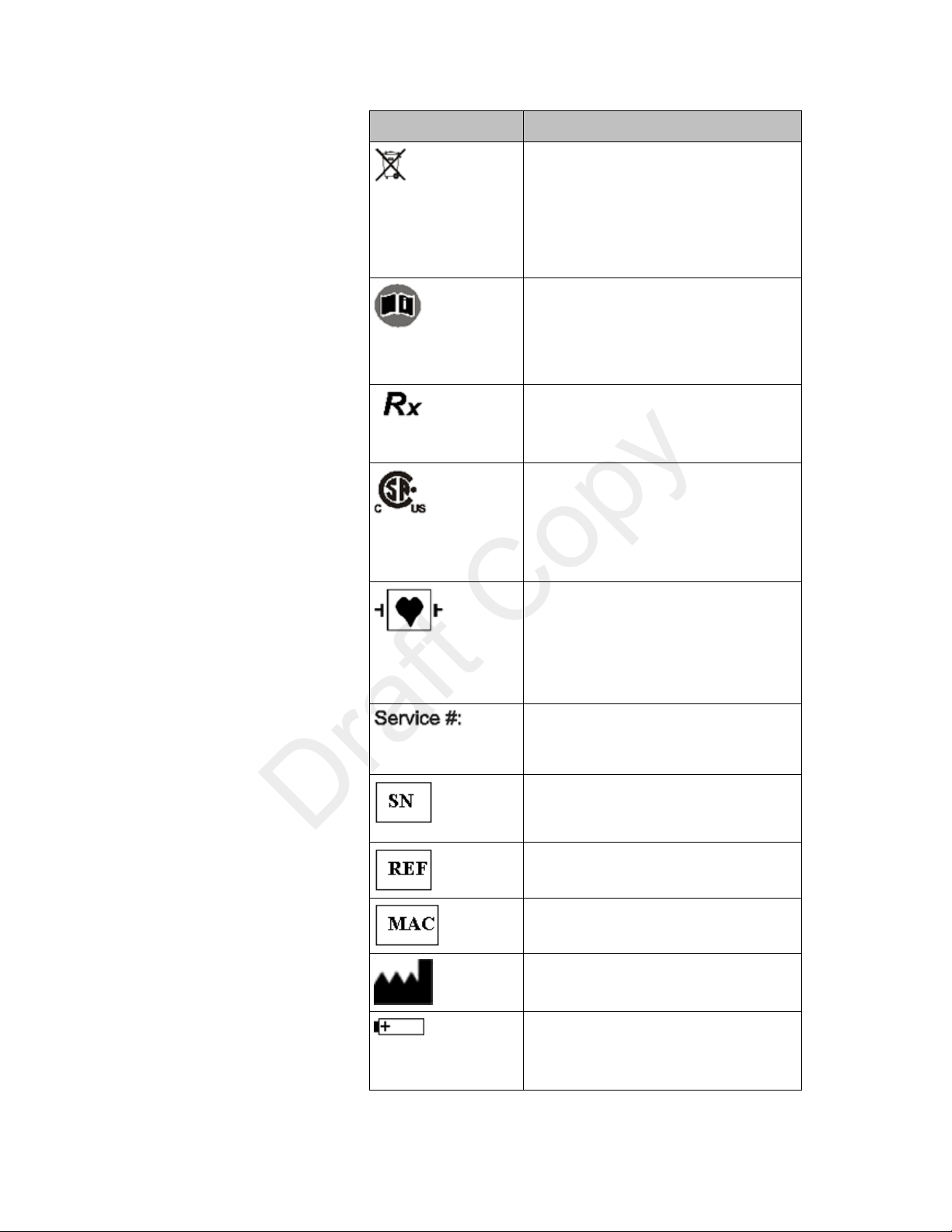
Safety Symbols & Other Marks
Label
Definition
Disposal
Dispose of in accordance with the local
country’s requirements. 2002/96/EC
(Waste Electrical and Electronic
Equipment).
Follow operating instructions.
Prescription Device
CSA Mark for
Certified by CSA to the applicable
Canadian and US standards..
Defibrillation Proof
Patient connections are protected against
defibrillation (DEFIBRILLATION-PROOF)
and are a TYPE CF APPLIED PART.
Service Identification Number
Used to identify the equipment during a
call to Philips Healthcare (Service)
Serial Number
Used to identify the equipment during a
call to the Philips Healthcare (Service).
Reference Number
Indicates Philips Product Number
MAC Address
Manufacturer and Date of Manufacture
Battery Polarity
Draft Copy
3-6 Product Safety

Safety Symbols & Other Marks
Label
Definition
IPX Waterproof Rating
Protected against the effects of temporary
immersion in water.
2D Barcode
UL Listed Device
Listed by Underwriters Laboratories
Attention! See Instructions for Use.
Draft Copy
Product Safety 3-7

Safety Symbols & Other Marks
Draft Copy
3-8 Product Safety

Controls, Indicators and Connectors ...................................................... 4-2
Operating and Navigating ....................................................................... 4-8
Understanding Settings .......................................................................... 4-12
Battery Information ................................................................................. 4-14
Pouch Use ................................................................................................. 4-22
Telemetry Mode Use ............................................................................... 4-26
Monitoring Mode Use ............................................................................. 4-27
Briefing the Patient .................................................................................. 4-28
Draft Copy
4. Basic Operation
This section gives you an overview of the IntelliVue MX40 and its
functions. It tells you how to perform tasks that are common to all
measurements, such as turning a measurement on and off, adjusting wave
size and information in preparation for use.
Familiarize yourself with all instructions including warnings and cautions
before starting to monitor patients. Read and keep the Instructions for Use
that come with any accessories as these contain additional important
information.
Basic Operation 4-1
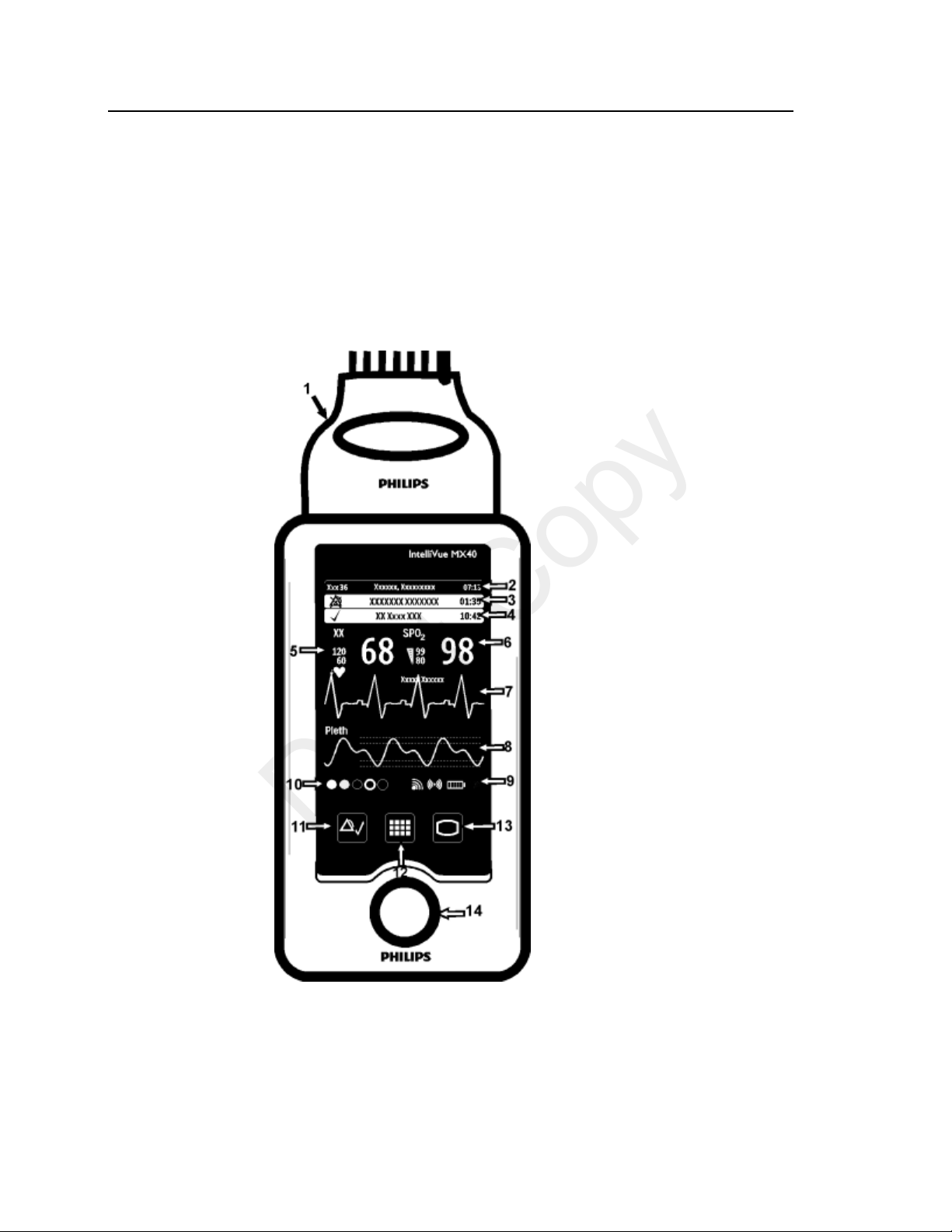
Controls, Indicators and Connectors
1. Patient Cable
2. Patient Information Area
3. Active Alarms Area
4. INOP Area
5. Measurement Area 1
6. Measurement Area 2
7. Waveform 1
8. Waveform 2
9. Radio/Network/Battery Status Area
10. Leads Off Status Area
11. Silence Alarms Button
12. SmartKeys Button
13. Main Screen Button
14. Multi-Function Button
Draft Copy
Controls, Indicators and Connectors
This section describes the clinical controls of the IntelliVue MX40. These
controls include buttons, display icons, visual and auditory indicators,
ports, and safety labeling located on the front and back of the device.
MX40 Controls and Indicators
4-2 Basic Operation
 Loading...
Loading...