Philips Medical Systems North America M2601B Instructions for Use

Philips Telemetry System
with the M2601B Transmitter and Telemetry Functions
at the IntelliVue Information Center
Notice
Operation of this equipment requires the prior coordination with a frequency coordinator designated by the FCC for the Wireless Medical Telemetry Service.
Instructions for Use
Part Number: M2600-92201
Printed in the U.S.A. November 2004
First Edition

Notice
Equipment specifications are subject to alteration without notice. All changes will be in compliance with regulations governing manufacture of medical equipment.
Printed in the USA.
Document number: M2600-92201
© Copyright 2004 Koninklijke Philips Electronics N.V. All Rights Reserved. OxiCliq and OxiMax are registered trademarks of Nellcor Incorporated.
Printing History
New editions of this document incorporate all material updated since the previous edition. Update packages may be issued between editions and contain replacement and additional pages to be merged by a revision date at the bottom of the page. Pages that are rearranged due to changes on a previous page are not considered revised.
The documentation printing date and part number indicate its current edition. The printing date changes when a new edition is printed. (Minor corrections and updates which are incorporated at reprint do not cause the date to change.) The document part number changes when extensive technical changes are incorporated.
First Edition................................................................ |
November 2004 |
Philips Telemetry System, model M2600B is compatible with:
M2604A Philips Mainframe, #01D or #0EU, revision E.00.19
Philips Information Center, revisions F.00, E.01, E.00, D.01, D.00
M2636B TeleMon Companion Monitor, Options A02/A03
Philips Transmitter, model M2601A
Philips Transmitter, model M1400A/B/J
Note—Some features are not available on all products.
ii

About this Book
About this Book
This book contains operating instructions for use of the M2601B Transmitter, a part of the Philips Telemetry System. It also includes operational information for the telemetry functions of the IntelliVue Information Center. The intended audience is the clinician who uses and/or teaches others to use the equipment in a healthcare environment. For operating information on other functionality of the Information Center, see the IntelliVue Information Center Instructions for Use (order number M3150-9001F). For preventive maintenance, repair, and test methods for verification of device performance, refer to the M2600B Philips Telemetry System Service Guide in the M2600B Documentation Kit, shipped with the product (order number M2600-90323).
This book does not address Philips IntelliVue TRx transceivers or the Philips IntelliVue Telemetry System. For information on those products, refer to the manual Philips IntelliVue Telemetry System Instructions for Use (order number M4841-91001).
Note—Standard and EASI M2601A Transmitters can be used with the Philips Telemetry System and can operate simultaneously with M2601B Transmitters. “What’s New” on page 1-2 summarizes the differences between the M2601B and M2601A transmitters.
iii

About this Book
Document Procedures
Conventions Procedures are indicated in text by the heading Task Summary followed by the following table:
Step Action
1
2
3
Bold Typeface
Objects of actions in procedures appear in bold typeface. Note the following example:
Click the Standby button.
Warnings
Warning
Warnings are information you should know to avoid injuring patients and personnel.
Cautions
Caution
Cautions are information you should know to avoid damaging your equipment and software.
Notes
Note—Notes contain additional information on Philips Telemetry System usage.
iv

Product Safety Information
Product Safety Information
The warnings below refer to the following devices:
•Philips M2601B Transmitter
•Philips Telemetry System
•IntelliVue Information Center
Warning
For continued safe use of this equipment, it is necessary that the listed instructions are followed. Instructions in this manual in no way supersede established medical procedures.
Warning
Do not touch the patient, or table, or instruments during defibrillation. The battery door must be closed during defibrillation. These steps protect the clinician from high defibrillator voltage.
Warning
This device is not to be used in the vicinity of electrosurgery units because use may interrupt or interfere with the transmission of signals from the transmitter.
Warning
This equipment is not suitable for use in the presence of a flammable anesthetic mixture with air or with oxygen or nitrous oxide.
v

Product Safety Information
Warning
Do not use patient cables with detachable lead wires that have exposed male pins. Electrocution could result if these pins are plugged into AC power.
Warning
Use of product accessories (e.g., ECG lead sets, SpO2 sensors) other than those prescribed by Philips could lead to patient injury.
Warning
Strangulation Hazard! Under no circumstances should any pouch be tied solely around a patient’s neck.
Warning
ECG SAFETY FOR ALL PATIENTS
Always confirm Information Center observations with clinical observation of the patient before administering interventions.
Every lead must be secured to an electrode on the patient.
Conductive parts of electrodes must not contact earth or other conductive parts.
Philips recommends that you change the lead label only to reflect the physical placement of electrodes. This will ensure a match between the monitored lead and the label, and prevent any possible confusion.
When switching between EASI and Standard monitoring, there is a loss of data for 30 seconds.
vi

Product Safety Information
Warning
ECG SAFETY FOR PACED PATIENTS
The output power of the M2601B Transmitter and other sources of radio frequency energy, when used in the proximity of a pacemaker, can be sufficient to interfere with pacemaker performance. Due to the shielding effects of the body, internal pacemakers are somewhat less vulnerable than external pacemakers. However, caution should be exercised when monitoring any paced patient.
In order to minimize the possibility of interference, position electrodes, electrode wires, and the transmitter as far away from the pacemaker as possible.
Consult the pacemaker manufacturer for information on the RF susceptibility of their products and the use of their products with the Philips Telemetry System. See the IntelliVue Information Center Instructions for Use for additional information on monitoring paced patients.
vii

Product Safety Information
Warning
ST/AR ARRHYTHMIA SAFETY FOR ALL PATIENTS
During complete heart block or pacemaker failure (to pace or capture), tall P-waves (greater than 1/5 of the average R-wave height) can be erroneously counted by the arrhythmia algorithm, resulting in missed detection of cardiac arrest.
Learning/Relearning
--If you initiate learning during ventricular rhythm, the ectopics can be incorrectly learned as the normal QRS complex. This can result in missed detection of subsequent events of V-Tach and V-Fib.
--When using EASI ECG monitoring, Relearn happens automatically when there is a LEADS OFF technical alarm. If learning takes place during ventricular rhythm, the ectopics can be incorrectly learned as the normal QRS complex. This can result in missed detection of subsequent events of V-Tach and V-Fib. Be sure to check the beat labels and initiate a relearn to correct. Therefore, when a technical alarm is generated:
1.Respond to the technical alarm [for example, reconnect the electrode(s)].
2.Ensure that the arrhythmia algorithm is labeling beats correctly.
viii

Product Safety Information
Warning
ST/AR ARRHYTHMIA SAFETY FOR PACED PATIENTS
It is possible that pacemaker pulses will not be detected when the ECG analog output of a defibrillator or telemetry unit is plugged into a bedside monitor. This can result in the arrhythmia algorithm’s failure to detect pacemaker non-capture or asystole.
Some pace pulses can be difficult to reject. When this happens, the pulses are counted as a QRS complex, and could result in an incorrect HR and failure to detect cardiac arrest or some arrhythmias. Keep pacemaker patients under close observation.
--During complete heart block or pacemaker failure (to pace or capture), tall P-waves (greater than 1/5 of the average R-wave height) can be erroneously counted by the arrhythmia algorithm, resulting in missed detection of cardiac arrest.
--When arrhythmia monitoring paced patients who exhibit only intrinsic rhythm, the monitor can erroneously count pace pulses as QRS complexes when the algorithm first encounters them, resulting in missed detection of cardiac arrest.
For patients who exhibit intrinsic rhythm only, the risk of missing cardiac arrest can be reduced by monitoring these patients with the low heart rate limit at or slightly above the basic/demand pacemaker rate. A low heart rate alarm alerts you when the patient begins pacing. Proper detection and classification of the paced rhythm can then be determined.
-- When an external pacemaker is being used on a patient, arrhythmia monitoring is severely compromised due to the high energy level in the pacer pulse. This can result in the arrhythmia algorithm’s failure to detect pacemaker non-capture or asystole.
ix
Product Safety Information
x

Contents
1. Introduction to the Philips Telemetry System. . . . . . . . . . . . . . . . . . . . . . . . . 1-1
What’s New. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 1-2 New Transmitter . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 1-2 Connection to TeleMon . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 1-4 Own Bed Overview . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 1-4 Indications for Use . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 1-5 Regulatory Information . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 1-6 System Overview . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 1-7 Transmitters. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 1-9 M2601B Transmitter Features . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 1-10 Transmitter Controls - Front . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 1-11 Transmitter Controls - Back. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 1-15 Sounds . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 1-18 Transmitter Safety Information . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 1-18 Briefing the Patient . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 1-19 Pouch Use . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 1-19 Securing the Pouch. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 1-20 Showering . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 1-21 Making Monitoring Adjustments . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 1-22 Turning the Transmitter On/Off. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 1-22 Turning Telemetry Monitoring On/Off . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 1-23 Transmitter Auto Shutoff . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 1-23 Turning Nurse Call On/Off . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 1-23 Standby Mode . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 1-24 Use with TeleMon B, Options A02/A03. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 1-25 Operation with TeleMon . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 1-25 Testing the Transmitter Functionality. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 1-27 Self Test . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 1-27 Status Check. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 1-28 Battery Information. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 1-29 Battery Safety Information. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 1-29 Inserting/Removing Batteries . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 1-31 Checking the Battery Power Level . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 1-32 Receiver Module . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 1-34 Receiver Mainframe . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 1-35 Antenna System. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 1-36
Contents-1
2. Alarms . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 2-1
Alarm Indicators . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 2-2 Pause/Suspend Alarms . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 2-2 Alarm Behavior with Own Bed Overview . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 2-3 Physiologic (Patient) Alarms . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 2-6 Technical Alarms (INOPs) . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 2-11
3. ECG & ST/AR Measurement . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 3-1
Measuring ECG. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 3-2 EASI ECG . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 3-2 ECG Lead Sets . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 3-2 ECG Leads Monitored . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 3-4 Setting Up for ECG Monitoring . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 3-7 Positioning ECG Electrodes. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 3-8 Connecting the ECG Cable . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 3-14 Verifying Electrode Connections . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 3-17 Making ECG Adjustments . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 3-18 Changing Lead/Label. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 3-18 Adjusting Wave Size . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 3-19 Monitoring During Leads Off . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 3-19 Lead Fallback. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 3-19 Extended Monitoring . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 3-20 Relearning . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 3-20 Using EASI Leads to Troubleshoot . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 3-21 Optimizing System Performance . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 3-22 The Telemetry Signal. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 3-22 Troubleshooting Signal Disturbance . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 3-23 Dropouts. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 3-23 Muscle and Movement Artifact . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 3-24 ECG Safety Information . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 3-27 ST/AR Arrhythmia Analysis. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 3-28 ECG and ST/AR Alarms . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 3-30 ST/AR Arrhythmia Safety Information . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 3-31
4. ST/AR ST Segment Monitoring. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 4-1
ST/AR ST Algorithm . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 4-2
Intended Use . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 4-2
Patient Population . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 4-2
The Measurement. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 4-3
How the Algorithm Works. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 4-4
Contents-2
Displayed ST Data . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .4-4
EASI ST Analysis. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .4-4
Adjusting Measurement Points . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .4-5
Establishing ST Reference Beats (Baseline) . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .4-7
Turning ST On/Off. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .4-7
ST Alarms . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .4-8
ST Alarm Adjustments . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .4-9
5. SpO2 Monitoring. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 5-1
About the Pulse Oximetry Measurement . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .5-2 Pulse Indication. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .5-3 SpO2 Information for the User . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .5-4
Preparing for Telemetry SpO2 Monitoring . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .5-7 SpO2 Sensors . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .5-8 Disposable Sensors . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .5-8
Reusable Sensors . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .5-8 Selecting an SpO2 Sensor . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .5-9 Applying the Sensor. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .5-12 Sensor Application Safety Information . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .5-12 Adult Finger Sensor (M1191A) . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .5-14 Small Adult/Pediatric Finger Sensor (M1192A) . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .5-15 Ear Clip Sensor (M1194A) . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .5-16 Connecting the SpO2 Cable. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .5-17
Making SpO2 Measurements . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .5-18 SpO2Measurement when Connected to TeleMon . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .5-18 Making a Spot Check Measurement. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .5-19
Monitoring SpO2 Continuously . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .5-20 Turning SpO2 Monitoring Off . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .5-20 Turning the SpO2 Parameter On/Off . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .5-21
SpO2 Parameter Auto ON. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .5-22 Turning SpO2 Alarms On/Off. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .5-22 Turning the Pulse Parameter On/Off . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .5-23
Measurement Limitations . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .5-24 Optimizing Sensor Performance . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .5-25 SpO2 Alarms and Technical Alarms . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .5-25
Contents-3
6. Maintenance and Configuration . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 6-1
Troubleshooting . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 6-2 Basic Troubleshooting . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 6-2 Testing Alarms. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 6-2 Maintenance . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 6-3 Transmitter Cleaning, Disinfection, & Cross-Infection Prevention . . . . . . . . . . . . . . . . . . . . . . . 6-4 Cleaning the Transmitter . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 6-4 Disinfecting the Transmitter . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 6-5 Cross-Infection Prevention for the Transmitter . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 6-7 Receiver Mainframe Cleaning . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 6-15 Configuration . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 6-16 Configuration Settings. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 6-17 M2604A Mainframe . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 6-17 Philips M2601B Transmitter . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 6-19 Changing the Configuration. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 6-19
7. System Safety and Specifications. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 7-1
Product Safety . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 7-2
. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 7-2 System Classification. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 7-3 Essential Performance . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 7-4 Philips Telemetry System Warnings . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 7-4 Electromagnetic Compatibility . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 7-5 M2600B Philips Telemetry System Testing . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 7-5 FCC Compliance (USA only) . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 7-7 Canadian Radio Equipment Compliance (Canada Only) . . . . . . . . . . . . . . . . . . . . . . . . . . . 7-7 System Symbols . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 7-9 Type CF Defibrillation Proof. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 7-14 Installation and Maintenance Safety . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 7-14 Installation . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 7-14 Preventive Maintenance. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 7-18 End of Life . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 7-18 Additional Safety Information . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 7-19 System Specifications . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 7-20 Battery Life Specifications. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 7-20 Environmental Specifications . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 7-21 Electrical Power Specifications . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 7-22 Antenna System Specifications . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 7-26 Measurement Specifications . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 7-30
Contents-4
A. Optional Patient Monitor/Holter Interface (Analog Output) . . . . . . . . . . . A-1
Overview . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . A-2 Correct Labeling . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . A-2 Analog Output Bedside Monitor Cables . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . A-3 Lead Placement and Selection . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . A-5 Using Non-Standard Lead Placement. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . A-5 Controls for Telemetry Setup. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . A-6 Functionality with Paced Waves . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . A-6 Technical Alarms (Inoperative Conditions). . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . A-7 Holter Interface . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . A-8
B. Accessory List . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . B-1
Accessory Safety . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . B-1
ECG Accessories . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . B-2
SpO2 Accessories . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . B-4
C. Sales and Support Offices . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . C-1
Contents-5
Contents-6
1 Introduction to the Philips Telemetry System
This chapter introduces the Philips Telemetry System. It includes the following sections:
• What’s New. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 1-2
• Indications for Use . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 1-5
• System Overview . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 1-7
• Transmitters. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 1-9
• Briefing the Patient . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 1-19
• Making Monitoring Adjustments . . . . . . . . . . . . . . . . . . . . . . . . . . . . 1-22
• Use with TeleMon B, Options A02/A03. . . . . . . . . . . . . . . . . . . . . . . 1-25
• Testing the Transmitter Functionality. . . . . . . . . . . . . . . . . . . . . . . . . 1-27
• Battery Information. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 1-29
• Receiver Module . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 1-34
• Receiver Mainframe . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 1-35
• Antenna System . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 1-36
Introduction to the Philips Telemetry System 1-1

What’s New
What’s New
|
This section highlights the differences between the M2600B Philips Telemetry |
|
|
System, utilizing the M2601B Transmitter and the Philips M2600A Telemetry |
|
|
System, Release C, utilizing the M2601A Transmitter. |
|
New |
The main difference between the two systems is the introduction of the new |
|
Transmitter |
M2601B transmitter. |
|
Differences |
The following table summarizes the differences between the two transmitters. |
|
Between |
|
|
Transmitters |
|
|
|
|
|
|
M2601B |
M2601A |
|
|
|
Function |
One transmitter for both Standard and |
Separate Standard or EASI versions of |
|
EASI monitoring -- clinician simply |
transmitter |
|
changes the ECG lead set position |
|
|
|
|
|
FAST (Fourier Artifact Suppression |
Traditional (not motion tolerant) SpO2 |
|
Technology) SpO2 algorithm |
algorithm |
|
Continuous and Spot Check (Manual) |
Continuous, Spot Check (Manual), and |
|
SpO2 measurements |
Intermittent (1- and 5-minute) SpO2 |
|
|
measurements |
|
|
|
1-2 Introduction to the Philips Telemetry System
|
|
What’s New |
|
|
|
|
M2601B |
M2601A |
|
|
|
Controls & |
Spot Check SpO2 initiated by inserting |
Manual measurement initiated by |
Indicators |
sensor cable |
button push on transmitter |
|
|
|
|
Auditory feedback for Spot Check and |
N/A |
|
self test |
|
|
|
|
|
Two electrode placement diagrams |
One electrode placement diagram |
|
show both Standard and EASI |
appropriate for the transmitter: either |
|
placement |
Standard or EASI placement |
|
|
|
|
Check button for verifying transmitter |
N/A |
|
status: lead set type, battery level, |
|
|
EASI indicator (if in use) |
|
|
|
|
|
Battery gauge to indicate power level |
N/A |
|
|
|
|
Audible volume/mute configurations |
N/A |
|
|
|
|
Audible pulse detection during Spot |
N/A |
|
Check measurement |
|
|
|
|
|
Unit designator label on battery |
N/A |
|
compartment |
|
|
|
|
Physical |
Smaller and lighter ECG-only |
One-size transmitter (ECG-only or |
|
transmitter |
ECG/SpO2) |
|
ECG/SpO2 transmitter approximately |
|
|
same size as the M2601A |
|
|
|
|
Power Source |
Battery Type: 2 AA Alkaline |
Battery Types: 1 9-volt Alkaline, |
|
|
Lithium, Zinc Air |
|
|
|
|
No Support for Battery Extender |
Compatible with Battery Extender |
|
|
|
Accessories |
New 5-wire lead sets, with color- |
|
|
coded lead wires available |
|
|
|
|
Introduction to the Philips Telemetry System 1-3
What’s New |
|
Connection |
The M2601B Transmitter also has a different method of connecting to the |
to TeleMon |
TeleMon Companion Monitor: |
|
• M2601B: Transmitter is connected to the outside of TeleMon via a 3- |
|
meter tether cable. |
|
• M2601A: Transmitter is docked in TeleMon. |
Own Bed |
The system supports the concept of Own Bed Overview, the pairing of a |
Overview |
telemetry bed and an IntelliVue Patient Monitor (Release B.1 or higher) for a |
|
single patient. Own Bed Overview provides the telemetry-monitor data |
|
(waveforms, parameters, and alarms) in an integrated form both on the bedside |
|
monitor and at the IntelliVue Information Center. |
|
Own Bed Overview is available with both the M2601B and M2601A |
|
transmitters. |
|
Information on Own Bed Overview can be found in the IntelliVue Patient |
|
Monitor Instructions for Use and the IntelliVue Information Center Instructions |
|
for Use. In this book, “Alarm Behavior with Own Bed Overview” on page 2-3 |
|
summarizes alarm functionality with Own Bed Overview. |
1-4 Introduction to the Philips Telemetry System

Indications for Use
Indications for Use
The paragraphs below are the elements of the indications for use statement for the Philips Telemetry System.
Condition The licensed clinician decides that the Philips Telemetry System should be used to monitor the patient.
Prescription The Philips Telemetry System is a prescription device.
Versus Over- the-Counter
Part of the The ECG signal is obtained from accessory electrodes in contact with the Body or Type patient’s skin. The SpO2 signal is obtained from an accessory sensor in contact of Tissue with with the patient’s skin.
which the Device Interacts
Frequency of As prescribed by a licensed physician.
Use
Physiological To monitor the ECG and SpO2 of patients on the order of a licensed physician.
Purpose
Patient Adult and pediatric patients.
Population
Introduction to the Philips Telemetry System 1-5

Indications for Use
Intended Use The Philips Telemetry System is a comprehensive ambulatory system solution for the intermediate care unit for adult and pediatric patients. The foundation of the system is a transmitter that can capture and transmit ECG signals and SpO2 values (if available) that are then processed and displayed on the IntelliVue Information Center. The Information Center generates alarms and recordings, thus notifying clinicians of changes in patients' conditions. The Telemetry System communicates with other devices via the Philips patient care system.
Warning
United States law restricts this device to sale by or on the order of a physician. This product is intended for use in health care facilities by trained health care professionals. It is not intended for home use.
Regulatory This device is not for use with infant or neonatal patients.
Information
The transmitter and related accessories are in compliance with the relevant requirements of EN ISO 10993-1 for Biocompatibility. The transmitter is not designed for direct contact with the patient’s skin. The accompanying pouch is the appropriate means for holding the transmitter.
Use of the transmitter is restricted to one patient at a time.
The system is not intended to be connected to public mains as defined in
CISPR 11.
1-6 Introduction to the Philips Telemetry System

System Overview
System Overview
The Philips Telemetry System is used with the IntelliVue Information Center to provide multi-parameter measurements for transitional care and other ambulatory monitoring environments for adult and pediatric patients. The system:
•Enunciates patient monitoring alarms.
•Monitors adult and pediatric patients’ ECG.
•Provides ST/AR arrhythmia detection.
•Measures pulsatile arterial oxygen saturation (SpO2) and pulse rate, if available.
•Enables viewing of ECG and SpO2 measurements and waveforms at the patient’s side when connected to the TeleMon Companion Monitor.
•Makes ST segment measurements.
The Philips Telemetry System consists of:
•A transmitter for each patient.
•An antenna system.
•A receiver for each transmitter.
•A mainframe housing up to eight receivers.
Other possible items include:
•The TeleMon Companion Monitor: TeleMon can be used to view
waveforms and heart rate and SpO2 numerics as well as measure NBP. For more information see the Philips TeleMon B A02/A03 Companion Monitor Instructions for Use.
See the Philips Telemetry System Service Guide or your local trained service professional for assistance.
Introduction to the Philips Telemetry System 1-7
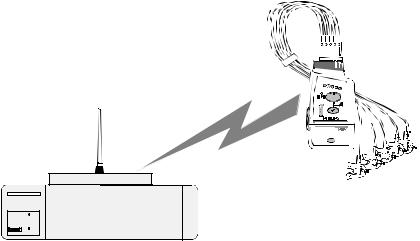
System Overview
M2601B
EASI, 3 5
Philips Telemetry System
Dual-Band The Philips Telemetry System (M2600B) can operate in both the 406-480 MHz Operation and 590-614 MHz ranges. The exact operating frequency for each transmitter/
receiver pair is set so as to meet specific customer needs, while maintaining compliance with local and international radio regulations.
For United States operation, the M2600B will operate only in the protected, dedicated Wireless Medical Telemetry Service (WMTS) band (608-614 MHz).
1-8 Introduction to the Philips Telemetry System
Transmitters
Transmitters
The following Philips transmitters can be used with the Philips Telemetry
System:
•ECG-only transmitter
•ECG/SpO2 transmitter
Standard and EASI M2601A transmitters can also be used. These transmitters can operate simultaneously with M2601B transmitters. For operating information, refer to the Instructions for Use for the Philips Telemetry System (part number M2600-9001C).
Note—“What’s New” on page 1-2 summarizes the differences between the M2601B and M2601A transmitters.
The M2601B Transmitter models are illustrated on the following pages in this chapter. Subsequent tables describe the controls, indicators, markings, and audible sounds respectively.
If your hospital uses both the M2601B Transmitter and IntelliVue
TRx devices
The M2601B Transmitter and M4841A TRx Transceiver are similar in appearance. You can distinguish between them by:
•Name on the front of the device
•Label color (dark gray for M2601B and pale gray for TRx)
Introduction to the Philips Telemetry System 1-9

Transmitters |
|
|
|
|
|
|
M2601B |
• Clinician-selectable Standard or EASI leads in same transmitter, at the |
|||||
Transmitter |
bedside. |
|||||
Features |
• Powered by two AA Alkaline batteries. |
|||||
|
• Spot Check SpO2 without using any control buttons. |
|||||
|
• FAST-SpO2 (Fourier Artifact Suppression Technology) for improved |
|||||
|
motion artifact rejection and low-perfusion performance. |
|||||
|
• Simultaneous operation in system with M2601A Transmitter. |
|||||
|
• Two sizes - smaller ECG-only version and larger ECG-SpO2 version. |
|||||
|
• Battery gauge on transmitter. |
|||||
|
• Designed to be ergonomic and comfortable for patients to wear. |
|||||
|
• Colored labels provide clinical unit identifiers. |
|||||
|
• Lead sets are optimized for ambulating patients, with a cable length of |
|||||
|
79 cm (30 in). |
|||||
|
• Gunk guards prevent dirt from accessing unused ECG and SpO2 cable |
|||||
|
ports and the unused TeleMon/Service port, thus simplifying cleaning. |
|||||
|
• New pouches with clear front and flaps. |
|||||
|
. |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
M2601B
EASI, 3 5
EASI S
|
|
|
|
|
|
|
|
|
|
|
|
I |
A |
||
|
|
|
|
|
|
|
|
|
|
|
|
|
E |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
FCCID: XXXXXXXX |
! |
||
|
|
|
|
|
|
|
|
|
|
|
|
||||
|
|
|
|
|
|
|
|
|
|
|
|
||||
|
|
|
|
|
|
|
|
|
|
|
|
CANADA IC: XXXX |
0123 |
||
|
|
|
|
|
|
|
|
|
|||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
i |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Front View Back View
M2601B Transmitter - ECG only
1-10 Introduction to the Philips Telemetry System
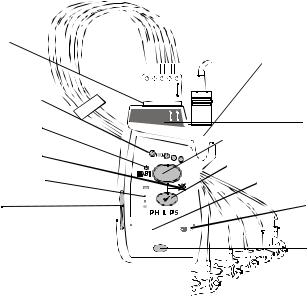
Transmitters
Transmitter
Controls -
Front
i
|
|
|
|
ii |
a |
|
|
|
|
b |
M2601B |
|
|
1 |
EASI, 3 |
5 |
A |
||
c |
|
|
|
|
|
|
B |
|
|
d |
|
|
C |
|
|
|
|
iii |
2 |
||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
3
ECG/SpO2 Transmitter - Front View
The labeled items in the diagram above include:
•Transmitter controls (A-C)
•Indicators (a-d)
•Labels (1-3)
•Ports (i-iii)
These items are defined on subsequent tables.
Introduction to the Philips Telemetry System |
1-11 |

Transmitters
Controls
Callout |
Control |
Definition |
|
|
|
A |
|
Telemetry Button: Depending on the |
|
|
configuration, this multi-function button directs |
|
|
the Information Center to generate a Nurse Call, |
|
|
central recording, both, or none. |
|
|
If desired, you can turn Nurse Call off for |
|
|
individual patients at the Information Center by |
|
|
using the Telemetry Setup Window. See |
|
|
“Turning Nurse Call On/Off” on page 1-23 for |
|
|
additional information. |
|
|
Note—Recordings generated by the telemetry |
|
|
button are stored in Alarm Review at the |
|
|
Information Center. |
|
|
Note—If the installation includes a paging |
|
|
system and if the Information Center is |
|
|
configured for paging upon receipt of Nurse |
|
|
Call, a Nurse Page signal will be initiated. |
|
|
|
B |
|
Check Button. Checks the status of the |
|
|
transmitter. When pressed, the battery gauge, |
|
|
lead set type, and EASI (if in use) indicators |
|
|
illuminate. |
|
|
|
C |
Power On/Off |
Battery Compartment. Battery insertion turns |
|
|
power on; battery removal turns power off. |
|
|
|
1-12 Introduction to the Philips Telemetry System

Transmitters
Indicators
Callout |
Indicator |
Definition |
|
|
|
a |
|
Lead Indicator. |
|
|
• Lights momentarily to display leads |
|
|
attached when lead set is inserted or when |
|
|
the Check button is pressed. |
|
|
• When a Leads Off condition occurs, the |
|
|
light(s) indicate the lead(s) that need to be |
|
|
reapplied. The light(s) remain on until the |
|
|
Leads Off condition ends. |
|
|
Note—The 6th indicator (left-most LED) is not |
|
|
used for the M2601B Transmitter. |
|
|
|
b |
|
EASI Indicator. Illuminates momentarily upon |
|
|
insertion of lead set in EASI position. Lit by |
|
EASI |
Check button when EASI is in use. |
|
|
|
c |
|
Alarms Pause/Suspend Indicator. Inactive. |
|
|
Note—If the transmitter is connected to the |
|
|
TeleMon Companion Monitor this indicator is |
|
|
lit during 3 minute alarm pause period initiated |
|
|
at TeleMon. |
|
|
|
d |
|
Battery Gauge. When the Check button is |
|
|
pressed, indicates the amount of power |
|
|
remaining in the batteries. Valid only for |
|
|
recommended battery type. |
|
|
Note—See “Checking the Battery Power Level” |
|
|
on page 1-32. |
|
|
|
Introduction to the Philips Telemetry System |
1-13 |

Transmitters
Front Labels
Callout |
Label |
Definition |
||||||
|
|
|
|
|
|
|
|
|
1 |
|
|
|
|
|
|
|
Lead Set Insertion Guide. See |
|
M2601B |
“Connecting the ECG Cable” on page 3- |
||||||
|
|
|
|
EASI, 3 5 |
14. |
|||
|
|
|
|
|
|
|
|
|
2 |
|
|
|
|
|
|
|
Device Identification Label |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
3 |
|
|
|
|
|
|
|
Unit Identification Label. (one of |
|
|
|
|
|
|
|
|
seven colors). Color-coded sticker. |
|
|
|
|
|
|
|
|
|
Ports
Callout |
Definition |
|
|
i |
ECG Lead Set Port. Connection for 3-wire or 5-wire lead set. |
|
|
ii |
SpO2 Sensor Port. Connection for SpO2 sensor. |
|
|
iii |
TeleMon/Service Port. Connection for cable to TeleMon |
|
Companion Monitor or to Service Tool. |
|
|
1-14 Introduction to the Philips Telemetry System
 Loading...
Loading...