Philips Pagewriter Trim Service manual

PageWriter Trim I, II, III, Rx
SERVICE MANUAL

Notice
information to users.
manufacturer.
About This Edition
Published by Philips Medical
Systems
Publication number
453564007071
Edition History
Edition 1, May 2004
Software Revision A.00.00 and
higher
Edition 2, June 2005
Software Revision A.00.02 and
higher
Edition 3, May 2006
Software Revision A.01.01 and
higher
Edition 4, May 2008
Software Revision A.01.03 and
higher
Warranty
Philips Medical Systems reserves
the right to make changes to both
this Service Manual and to the
product that it describes. Product
specifications are subject to change
without notice.
Nothing contained within this
Service Manual is intended as any
offer, warranty, promise, or
contractual condition, and must not
be taken as such.
Copyright
© 2004-2008 Koninklijke Philips
Electronics N.V. All rights are
reserved. All other product names
are the property of their respective
owners.
Reproduction in whole or in part in
any form, or by any means, electrical, mechanical or otherwise, is
prohibited without the written
consent of the copyright holder.
Philips Medical Systems
3000 Minuteman Road
Andover, MA 01810 USA
(978) 687-1501
Unauthorized copying of this publication may not only infringe copyright laws, but may also reduce the
ability of Philips Medical Systems
to provide accurate and current
Compliance
The Philips Medical Systems PageWriter Trim cardiograph complies
with all relevant international and
national standards and laws. Information on compliance will be
supplied on request by a local
Philips Medical Systems representative, or by the manufacturer.
Intended Use of this Service
Manual
This Philips product is intended to
be operated only in accordance with
the safety procedures and operating
instructions provided in this Service
Manual, and in accordance with the
purposes for which it was designed.
Installation, use, and operation of
this product is subject to the laws in
effect in the jurisdiction(s) in which
the product is being used. Users
must only install, use, and operate
this product in such a manner that
does not conflict with applicable
laws or regulations that have the
force of law. Use of this product for
purposes other than the express
intended purpose provided by the
manufacturer, or incorrect use and
operation, may relieve the manufacturer (or agent) from all or some
responsibility for resultant noncompliance, damage, or injury.
United States federal law restricts
this device to use by or on the order
of a physician. THIS PRODUCT IS
NOT INTENDED FOR HOME
USE.
Training
Users of this product must receive
adequate clinical training on its safe
and effective use before attempting
to operate the product as described
in this Service Manual.
Training requirements vary by
country. Users must ensure that
they receive adequate clinical
training in accordance with local
laws or regulations.
For further information on available
training on the use of this product,
please contact a Philips Medical
Systems representative, or the
Medical Device
Directive
The PageWriter Trim Cardiograph
complies with the requirements of
the Medical Device Directive 93/
42/EEC and carries the
mark accordingly.
Authorized EU-representative:
Philips Medizin Systeme
Böblingen GmbH
Hewlett Packard Str. 2
71034 Böblingen
Germany
0123

Contents
Chapter 1 Introduction
Who Should Use this Manual . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 1-2
Safety Summary . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 1-3
Safety Symbols Marked on the Cardiograph . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 1-3
Safety Symbols Marked on the Cardiograph Packaging . . . . . . . . . . . . . . . . . . . . . . . . . . 1-4
Safety and Regulatory Symbols Marked on the Cart. . . . . . . . . . . . . . . . . . . . . . . . . . . . 1-5
Important Patient and Safety Information . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 1-5
The PageWriter Trim Cardiograph . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 1-9
Intended Use. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 1-9
Indications for Use . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 1-10
The Philips 12-Lead Algorithm . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 1-10
Intended Use. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 1-10
Indications for Use . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 1-10
Features and Capabilities . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 1-10
Capabilities . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 1-11
Tour of PageWriter Trim Cardiographs . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 1-12
PageWriter Trim I Cardiograph. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 1-12
PageWriter Trim II, III, and Rx Cardiographs . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 1-14
Using the Cart Wheel Positioners and Brake . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 1-15
Patient Interface Module (PIM) . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 1-17
General Service Information. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 1-17
Installation. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 1-17
Upgrades and Accessories . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 1-18
PageWriter Trim II, III and Rx Token Label . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 1-19
Managing Token Labels. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 1-20
Supplies and Ordering Information. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 1-20
Special Note about Welsh Bulb Electrodes. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 1-21
Preventive Maintenance . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 1-23
Repair Philosophy . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 1-23
Country/Region Options . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 1-23
Philips 12-Lead ECG XML Information and Tools . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 1-26
Using the Philips InCenter Site . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 1-26
About Adobe Acrobat Versions . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 1-26
PageWriter Trim Cardiograph Learning Product Part Numbers . . . . . . . . . . . . . . . . . . . . 1-27
Contacting a Philips Response Center . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 1-32
North America Response Centers . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 1-32
South America Response Centers. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 1-32
Europe Response Centers . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 1-32
Asia Response Centers . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 1-33
Africa and Middle East . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 1-34
Contents-1

Chapter 2 Theory of Operation
Overview. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .2-1
Hardware Logical View . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .2-1
Main Control Board . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .2-1
Display. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .2-4
Patient Interface Module (PIM) . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .2-4
Printer Control (USB) . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .2-4
Battery (Lead-Acid) . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .2-4
Keyboard/Trim Knob (PS/2). . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .2-4
Magnetic Card Reader (PS/2) . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .2-4
Barcode Reader (PS/2) . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .2-5
Smart Card Reader. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .2-5
USB Memory Stick . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .2-5
PCMCIA Storage Card. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .2-5
PCMCIA LAN Card . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .2-5
PCMCIA Wireless LAN Card . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .2-6
PCMCIA Modem Card. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .2-6
High Level ECG Data Flow and Storage. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .2-6
Internal Main Archive . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .2-8
Internal Remote Archive . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .2-8
External PC Card Archives . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .2-8
External USB Flash Memory Stick Archives. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .2-9
Rendered ECG Report Prints . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .2-9
Fax-Rendered ECG Report Print. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .2-10
Power System Overview . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .2-11
Battery . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .2-11
Power Labels. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .2-12
Vin . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .2-12
VB+_T (Battery information). . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .2-12
VO . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .2-12
+3.3V. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .2-13
+3.3VB_P . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .2-13
VDDX . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .2-13
Vcore (+1.86V) . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .2-13
+5VP. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .2-13
USB_VCC. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .2-13
VPH . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .2-13
Power Management. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .2-14
Battery Charging Logic . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .2-14
Battery Gauge. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .2-14
Battery Discharging . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .2-15
Battery Charging. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .2-16
Charge Current . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .2-16
Battery Information . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .2-16
Contents
Contents-2 PageWriter Trim Cardiograph Service Manual

Contents
Chapter 3 Cardiograph Care and Maintenance
Cleaning the Cardiograph . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 3-1
Approved Cleaning Solutions . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 3-1
Cleaning the PIM, Patient Data Cable, and Lead Wires. . . . . . . . . . . . . . . . . . . . . . . . . . . . . 3-2
Cleaning the Print Head . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 3-2
Reusable Electrode Cleaning. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 3-3
Replacing Printer Paper. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 3-3
Battery Maintenance and Care . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 3-6
Caring for the Battery . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 3-6
Storing the Battery . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 3-7
Replacing the Lead Wires in the PIM . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 3-8
Cardiograph and Accessory Disposal. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 3-9
Setting the Date and Time . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 3-9
PageWriter Trim II, III and Rx . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 3-9
PageWriter Trim I . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 3-10
Setting the Paper Size and Lead Settings . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 3-10
Calibrating the Barcode Reader . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 3-11
Removing the Carriage Return . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 3-13
Maintenance Tests for Trim II, III and Rx . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 3-14
Chapter 4 Maintenance Tests
Maintenance Tests (Trim II/III/Rx only) . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 4-1
Patient Interface Module (PIM) Test . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 4-2
Barcode Reader Test . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 4-2
Magnetic Card Reader Test . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 4-3
Printer Test. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 4-3
Network Test . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 4-6
Trim II/III/Rx Diagnostic and Performance Verification Tests . . . . . . . . . . . . . . . . . . . . . . . . 4-6
About the Trim II/III/Rx Biomed Service Utility . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 4-6
Using the Biomed Service Utility for Trim II, III, Rx . . . . . . . . . . . . . . . . . . . . . . . . . . . . 4-7
Launching the Biomed Service Utility for Trim II, III, Rx . . . . . . . . . . . . . . . . . . . . . 4-7
Diagnostic Tests in the Biomed Service Utility . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 4-8
Working with the Diagnostic Tests . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 4-9
Audio Test . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 4-10
Smart Card Reader Test . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 4-10
Magnetic Card Reader Test. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 4-11
Barcode Reader Test . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 4-11
Compact Flash (Archive) Test . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 4-12
Fax/Modem Test . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 4-12
Keyboard Test . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 4-12
Onboard Flash Test . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 4-13
PC Card (PCMCIA Storage) Test . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 4-14
Printer Test . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 4-14
Network Ping Test. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 4-14
Screen Test . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 4-14
PageWriter Trim Cardiograph Service Manual Contents-3

Contents
Serial Loopback Test . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .4-15
Suspend Button Test . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .4-16
Trim Knob Test . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .4-16
Self-Tests . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .4-16
Trim I Diagnostic and Performance Verification Tests . . . . . . . . . . . . . . . . . . . . . . . . . . . . .4-17
Launching the Trim I Biomed Service Program . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .4-17
Diagnostic Tests in the Trim I Biomed Service Program . . . . . . . . . . . . . . . . . . . . . . . .4-18
Battery Test . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .4-19
Keyboard Test . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .4-19
LCD Screen Test . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .4-19
Onboard Flash Test . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .4-20
Options Test . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .4-20
PIM Test . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .4-20
Printer Test . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .4-21
RAM Test . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .4-21
Self-Test . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .4-22
Version Test. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .4-22
Voltage Test . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .4-23
Chapter 5 Troubleshooting
Contacting a Philips Response Center . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .5-1
Power On and Power Off Sequence . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .5-1
Special Note About Software Version A.01.03 . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .5-5
PageWriter Trim I Power Sequence . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .5-5
PageWriter Trim II/III/Rx Power Sequence. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .5-5
Troubleshooting Cardiograph Issues . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .5-6
Display Issues . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .5-6
Keyboard/Trim Knob/Dedicated Key Issues . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .5-8
Signal Acquisition Issues . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .5-10
Real Time Screen Issues . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .5-14
Archive Screen Issues. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .5-15
Configuration Screen Issues . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .5-17
Printer Issues . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .5-18
PC Card/USB Memory Stick Issues . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .5-23
Software Installation Issues . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .5-24
Wireless Troubleshooting . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .5-24
Checking the Remote Site Server Connection . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .5-27
Resolving an Unexplained Reply Received from the Remote Site . . . . . . . . . . . . . . . . .5-30
Checking the Wireless Adapter Association to an Access Point . . . . . . . . . . . . . . . . . .5-31
Restarting the Cardiograph. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .5-33
Contents-4 PageWriter Trim Cardiograph Service Manual

Contents
Chapter 6 Performance Verification and Safety Tests
Required Testing Levels . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 6-1
External Repairs . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 6-1
Internal Repairs . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 6-2
Upgrades . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 6-2
Test and Inspection Matrix . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 6-3
Test Equipment . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 6-4
Performance Verification Tests . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 6-4
Visual Inspection (V) . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 6-4
Power On Test . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 6-5
ECG Simulation (ECG) . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 6-6
Safety Tests. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 6-7
Safety Test S1 - Earth Leakage. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 6-7
Safety Test S2 - Protective Earth Resistance. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 6-7
Safety Test S3 - Leads Leakage Current . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 6-7
Chapter 7 Removing and Replacing Cardiograph Components
About the Cardiograph Components. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 7-1
Removing and Replacing the Battery . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 7-2
Removing the Battery. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 7-2
Replacing the Battery . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 7-3
Removing and Replacing the AC Fuses. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 7-3
Removing and Replacing the Paper Tray . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 7-4
Removing the Paper Tray . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 7-4
Replacing the Paper Tray . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 7-4
Chapter 8 Parts and Accessories
Ordering Replacement Parts . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 8-1
Ordering Supplies and Accessories . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 8-1
Patient Interface Module (PIM) Assembly and Parts . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 8-2
Cart Assembly and Parts. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 8-3
Appendix A Installing PageWriter Trim I Software
Software Upgrades . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . A-1
Upgrading Legacy Software . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . A-1
Special Note about Japanese Localization Option . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . A-1
Software Revision Information. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . A-2
Obtaining Software . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . A-2
Downloading Software Files from Philips InCenter. . . . . . . . . . . . . . . . . . . . . . . . . . . . . A-2
Installing the Software Upgrade . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . A-5
Verifying the Software Installation . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . A-9
Software Version A.01.03 PIM Kernel Revision . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . A-13
Special Note for Software Version A.01.01 . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . A-14
PageWriter Trim Cardiograph Service Manual Contents-5

Contents
Special Note about Software Version A.01.01 Self Test Report . . . . . . . . . . . . . . . . . A-14
Special Note for Software Version A.01.03 . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . A-15
Special Note about PIM Repairs . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . A-15
Appendix B Installing PageWriter Trim II, III, and Rx Software
Software Upgrades . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . B-1
Upgrading Legacy Software . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . B-1
Special Note about Japanese Localization Option. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . B-2
Saving Custom Settings (Software version A.00.03 and lower only). . . . . . . . . . . . . . . . . . . B-2
Obtaining Software . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . B-4
Downloading Software Files from Philips InCenter Site . . . . . . . . . . . . . . . . . . . . . . . . . B-4
Installing the Software Upgrade . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . B-6
Verifying the Software Upgrade . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . B-10
Software Version A.01.03 PIM Kernel Revision . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . B-16
Special Note for Software Version A.01.01 . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . B-17
Special Note about Software Version A.01.01 Self Test Report . . . . . . . . . . . . . . . . . B-17
Special Note for Software Version A.01.03 . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . B-18
Special Note about PIM Repairs . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . B-18
Appendix C Wireless LAN Installation Instructions
Overview. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . C-1
Wireless LAN FAQs. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . C-2
Installing the Wireless LAN Card. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . C-3
Enabling LEAP Credentials . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . C-10
Configuring a TraceMasterVue Remote Site . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . C-10
Appendix D Assembling the Cardiograph Cart and Patient Cable
Arm
Assembling the Cart . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . D-2
Attaching the Cardiograph to the Cart . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . D-4
Using the Cart Wheel Positioners and Brake . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . D-5
Connecting the Patient Data Cable Bracket . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . D-6
Connecting the PIM the Cardiograph. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . D-7
Placing the PIM in the Holder . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . D-7
Assembling the Patient Cable Arm. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . D-8
Appendix E Upgrade Kits
Upgrade Kit Contents. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .E-1
Upgrade Kit Installation . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .E-2
Contents-6 PageWriter Trim Cardiograph Service Manual

Contents
Appendix F Specifications
Technical Specifications. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .F-1
ECG Acquisition . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .F-1
Keyboard. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .F-1
Screen Display . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .F-1
Patient Interface Module . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .F-1
Cardiograph Cart . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .F-2
Signal Processing/Acquisition . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .F-2
Sampling Rate . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .F-2
Auto Frequency Response . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .F-2
Rhythm Frequency Response . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .F-2
Filters . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .F-2
Printer . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .F-2
Printer Resolution . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .F-2
Report Formats . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .F-3
Battery Operation . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .F-3
Capacity . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .F-3
Recharge. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .F-3
Network Connection . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .F-3
FAX Capability (optional). . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .F-3
Modem (optional for USA and Canada) . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .F-4
Barcode Reader (optional) . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .F-4
Magnetic Card Reader (optional). . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .F-4
ECG Storage . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .F-4
ECG File Formats . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .F-4
Power and Environment. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .F-4
Line Power . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .F-4
Environmental Operating Conditions . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .F-5
Environmental Storage Conditions . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .F-5
Cardiograph Dimensions . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .F-5
PageWriter Trim I . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .F-5
PageWriter Trim II/III/Rx. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .F-5
Cardiograph Weight. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .F-5
PageWriter Trim I . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .F-5
PageWriter Trim II/III/Rx. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .F-5
Cardiograph Shipping Container Dimensions . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .F-5
Cardiograph Shipping Container Weight. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .F-5
Cardiograph Cart Dimensions . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .F-5
Cardiograph Cart Weight . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .F-6
Fully Assembled Cardiograph Cart Shipping Container Dimensions . . . . . . . . . . . . . . . .F-6
Partially Assembled Cardiograph Cart Shipping Container Dimensions . . . . . . . . . . . . .F-6
Fully Assembled Cardiograph Cart Shipping Container Weight. . . . . . . . . . . . . . . . . . . .F-6
Partially Assembled Cardiograph Cart Shipping Container Weight . . . . . . . . . . . . . . . . .F-6
Safety and Performance. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .F-6
Electromagnetic Compatibility (EMC) . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .F-6
Reducing Electromagnetic Interference . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .F-7
PageWriter Trim Cardiograph Service Manual Contents-7

Contents
Contents-8 PageWriter Trim Cardiograph Service Manual
1
Chapter 1Introduction
This PageWriter Trim I, II, III, Rx Cardiograph Service Manual provides the information you
need to successfully service the PageWriter Trim cardiographs with software version A.01.03
and higher. The PageWriter Trim Cardiograph product family includes the following four
product models as described in Table 1-1.
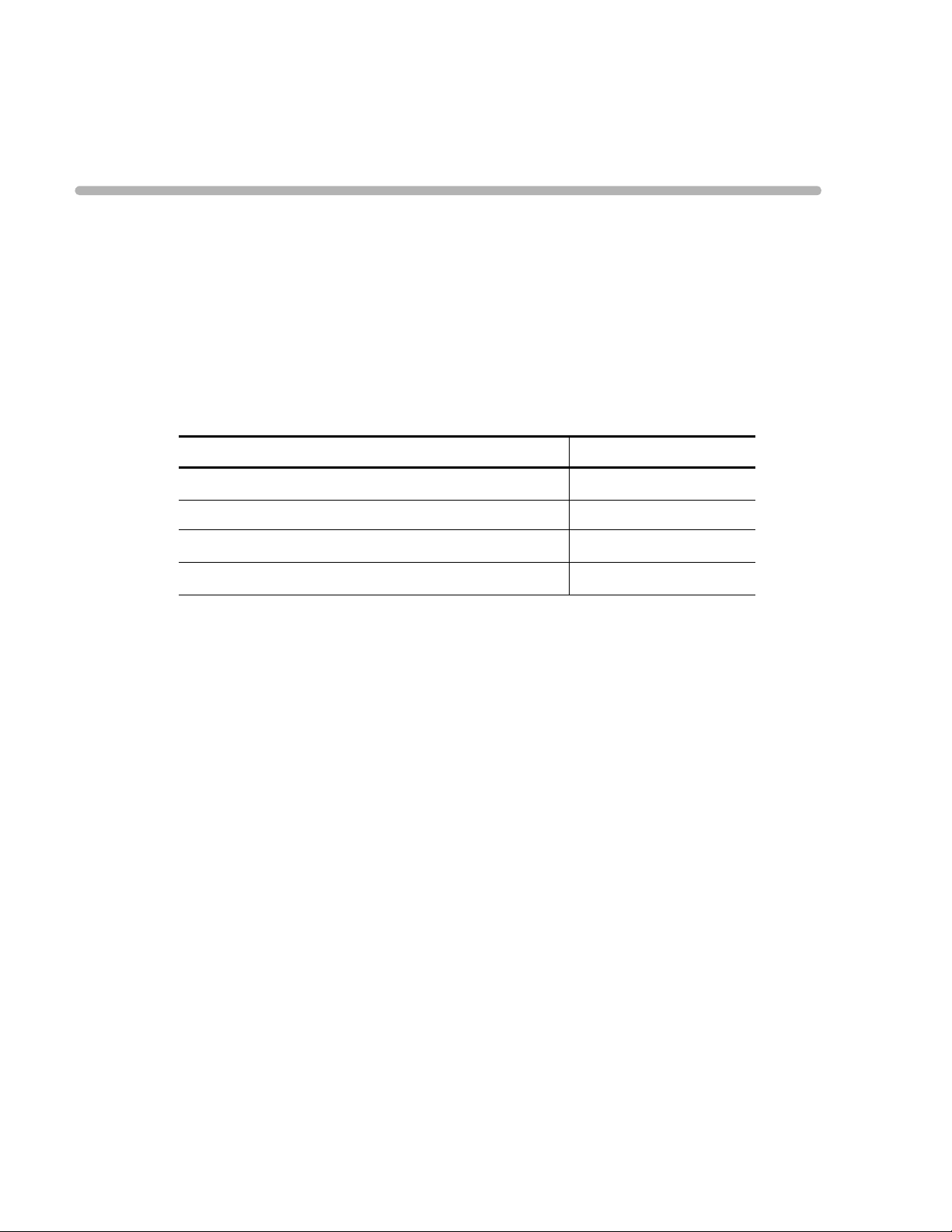
Table 1-1 PageWriter Trim Product Model Information
PageWriter Trim model Philips Part Number
PageWriter Trim III
860286
PageWriter Trim II 860288
PageWriter Trim I
860290
PageWriter Trim Rx 860297
This Service Manual includes information on:
Theory of operation
Maintenance procedures
Performance verification and safety testing
Repairs
Ordering parts and supplies
Specifications
Maintenance tests
Software Installation
Wireless LAN Installation
Upgrade Kit Installation
Before servicing the PageWriter Trim cardiographs, review the PageWriter Trim Instructions
for Use located on the User Documentation CD shipped with the cardiograph, or download the
file from the Philips InCenter site (
incenter.medical.philips.com). For information on accessing
the InCenter site, see “Using the Philips InCenter Site” on page 1-26. This service manual
assumes you are familiar with the controls, basic cardiograph operations, and capabilities of
the device as described in these documents.
1-1
Introduction Who Should Use this Manual
Who Should Use this Manual
This manual is intended for users who handle preventive maintenance, periodic operational
checks, and basic troubleshooting for PageWriter Trim cardiographs.
Before attempting to service the cardiographs, you must review the following documentation
and training materials:
PageWriter Trim Instructions for Use
PageWriter Trim Cardiograph Interactive Training Program
This Service Manual
This PageWriter Trim Cardiograph Service Manual is intended to assist users in the safe and
effective use of the product.
Before attempting to operate this product, read this Service Manual, and note and strictly
observe all Warning and Cautions as described in this document.
Pay special attention to all of the safety information provided in the Safety Summary section.
For more information, see page 1-5.
The following conventions are used in this document.
WARNING Warning statements describe conditions or actions that may result in a potentially
serious outcome, adverse event, or a safety hazard. Failure to follow a Warning may
result in death or serious injury to the user or to the patient.
CAUTION Caution statements describe when special care is necessary for the safe and effective use of the
product. Failure to follow a caution may result in minor to moderate personal injury or damage to the
product or other property, a remote risk of more serious injury, or may cause environmental
pollution.
NOTE Notes contain additional important information about a topic.
TIP A Tip contains suggested information on using a particular feature.
Menu items and button names appear in bold no-serif font. Example: Touch the Config button.
Internal software components or file directories appear in regular no-serif font. Example:
ECGs are stored to the
RubyArchiveInternal directory.
1-2 PageWriter Trim Cardiograph Service Manual
Introduction Safety Summary
Safety Summary
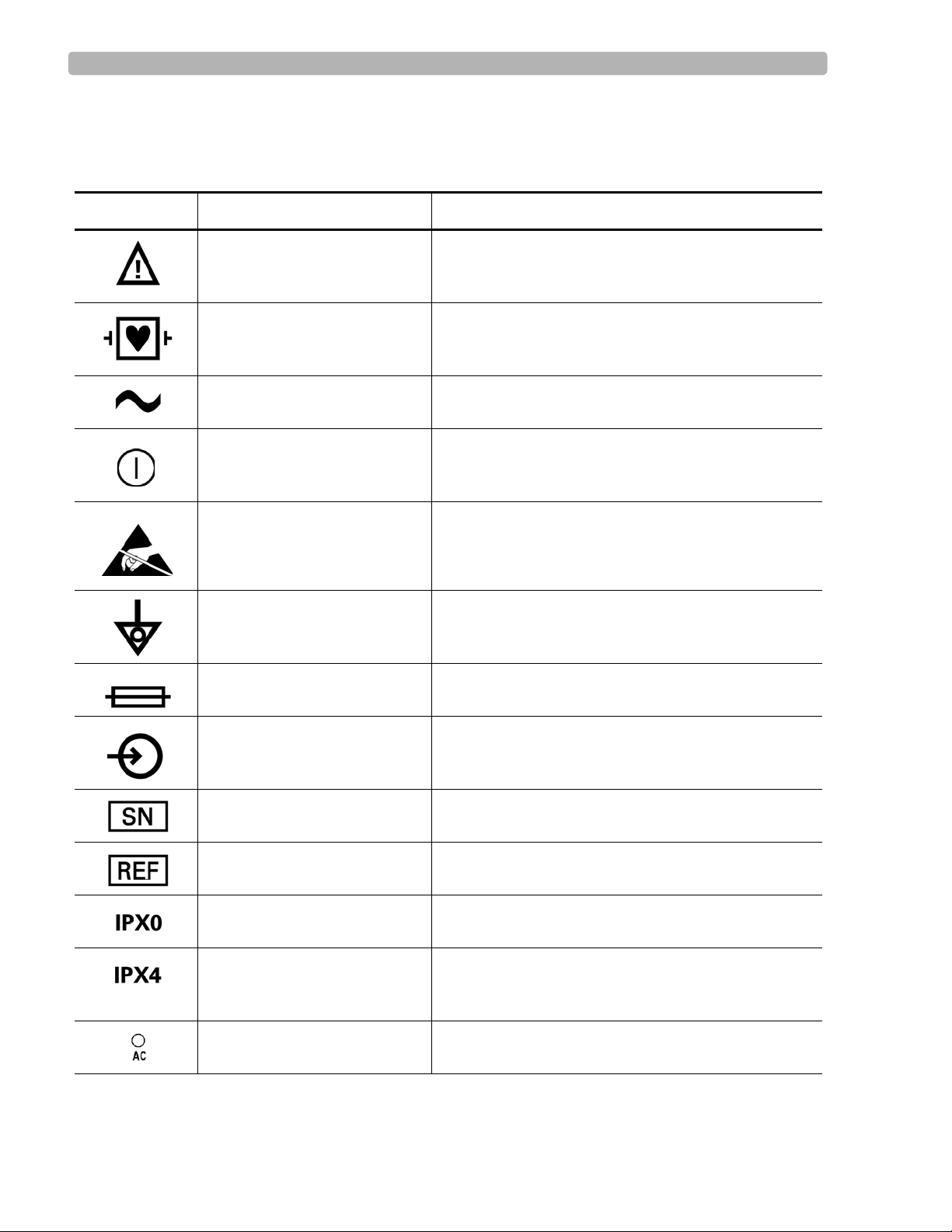
Safety Symbols Marked on the Cardiograph
Symbol
Type CF ECG physio isolation is type CF, defibrillator proof.
Alternating current Indicates that the cardiograph is receiving alternating
Name Description
Attention See PageWriter Trim Instructions for Use for
information.
Electrical leakage current is suitable for all patient
applications including direct cardiac application.
currents.
On/Standby Pressing the button with this symbol on it turns on the
cardiograph or puts the cardiograph into Standby
(power saving mode).
Do not touch exposed pins. Touching exposed pins can
Electrostatic Discharge
Equipotential grounding post Equipotential grounding post used for establishing
cause electrostatic discharge that can damage the
cardiograph.
common ground between instruments.
Fuse Cardiograph contains a 1.5 amp (250V) time-delay
fuse.
Input The connector near this symbol receives an incoming
signal.
Serial Number The number next to this symbol is the serial number of
the cardiograph.
Product model number The number next to this symbol is the product model
number of the cardiograph
Entry of liquids The cardiograph is not protected against splashing
water.
Entry of liquids The PIM (Patient Interface Module) is protected
against splashing water. Water splashed against the
PIM from any direction shall have no harmful effect.
AC power indicator light When lit, indicates that AC power is on. The battery is
charging when inserted into the cardiograph.
PageWriter Trim Cardiograph Service Manual 1-3
Introduction Safety Summary
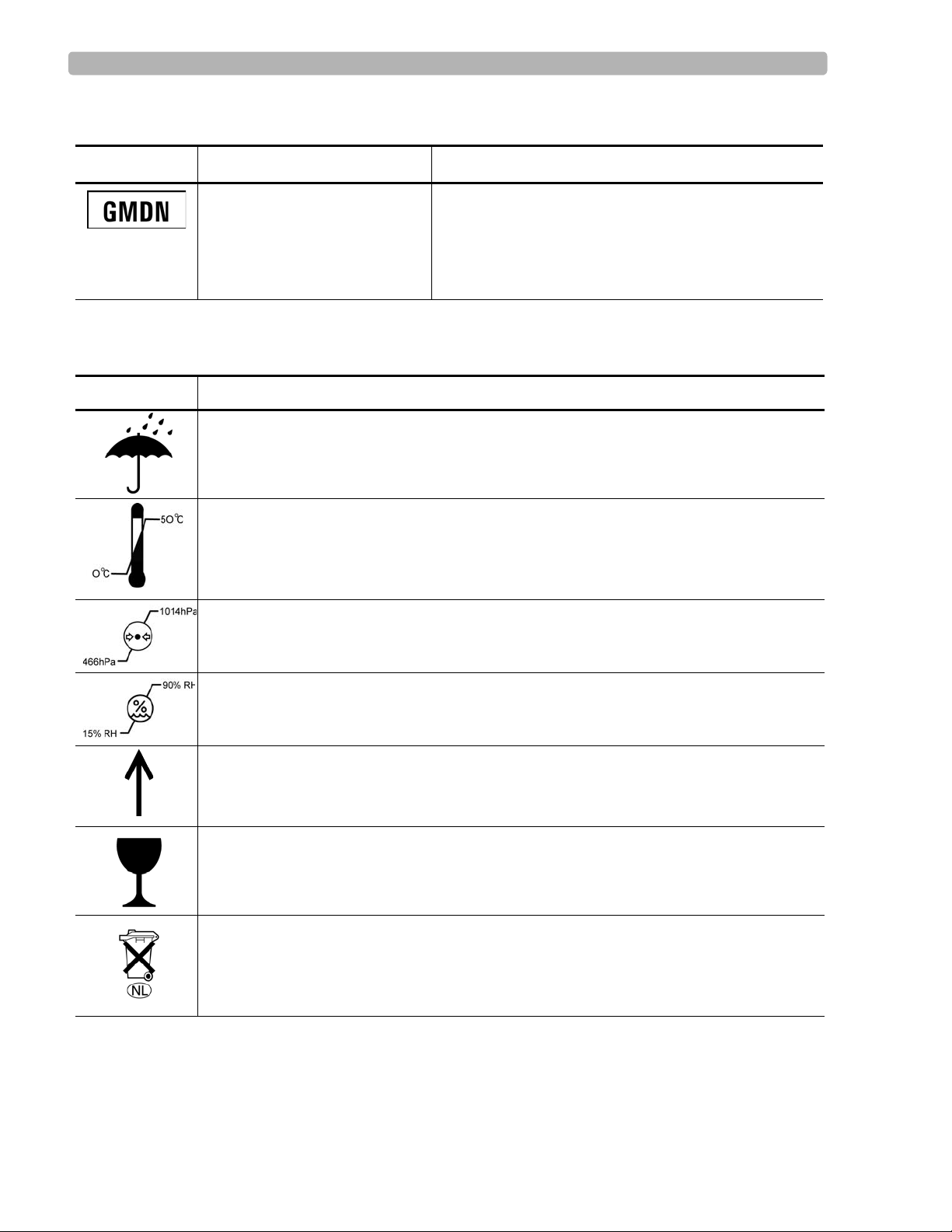
Safety Symbols Marked on the Cardiograph (continued)
Symbol
Name Description
Global Medical Device
Nomenclature Code
Global Medical Device Nomenclature Code is a 5-digit
code providing a brief description of the device, as
defined by EN ISO 15225.
Safety Symbols Marked on the Cardiograph Packaging
Symbol Description
Keep dry.
o
Ambient temperature range of 0
transport and storage.
C (32o.F) to 50 oC (122o F) (non-condensing) for
Atmospheric pressure range of 466 hPa to 1014 hPa for transport and storage.
Relative humidity range of 15% to 90% (non-condensing) for transport and storage.
Move and store packaging this end up.
Fragile.
Sealed lead acid battery . Do not dispose of in trash. Follow local regulations for di sposing
of as small chemical waste.
1-4 PageWriter Trim Cardiograph Service Manual
Introduction Important Patient and Safety Information
Safety Symbols Marked on the Cardiograph Packaging (continued)
Symbol Description
Dispose of in accordance with the requirements of your country.
This product consists of devices that may contain mercury, which must be recycled or
disposed of in accordance with local, state, or federal laws. (Within this system, the
backlight lamps in the monitor display contain mercury.)
Safety and Regulatory Symbols Marked on the Cart
Symbol Name Description
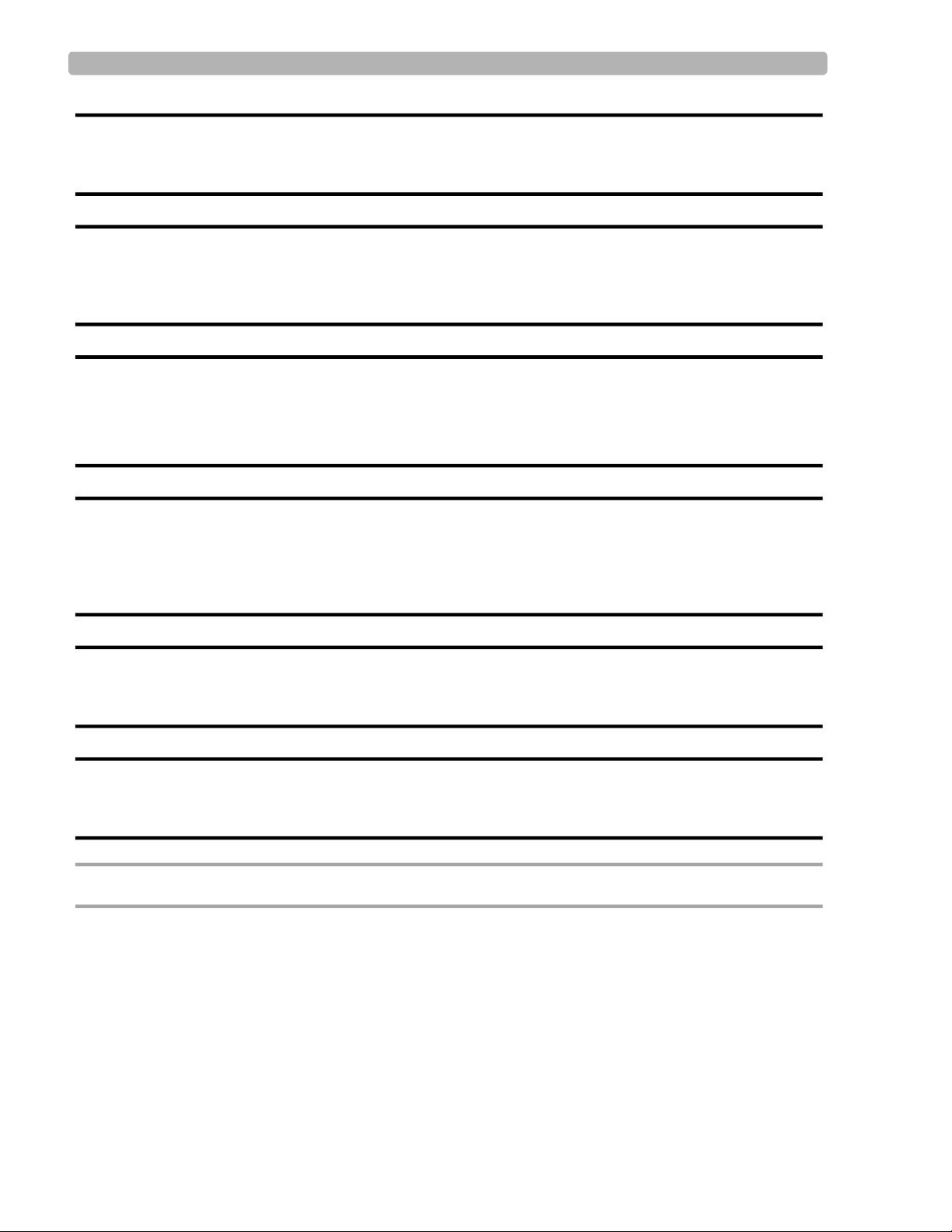
Cart Transport Use care when moving the cardiograph cart. Pushing the cart
over bumps without holding onto the cardiograph may cause the
cart to tip.
Cart Storage Bin
Weight Limit
Do not place more than 3 kilograms or 6.6 pounds of weight
into the cart storage bin.
Important Patient and Safety Information
The PageWriter Trim cardiograph isolates all connections to the patient from electrical ground
and all other conductive circuits in the cardiograph. This reduces the possibility of hazardous
currents passing from the cardiograph through the patient’s heart to ground , and from other
equipment connected to the patient passing through the leads into the cardiograph to ground.
WARNING Failure to follow these warnings could affect both patient and operator safety.
PageWriter Trim Cardiograph Service Manual 1-5
Introduction Important Patient and Safety Information
WARNING The Welsh bulb electrodes (available as an accessory for the cardiograph) do not meet
the requirements of IEC 60601-2-25 for defibrillation recovery time, and cannot be
reliably used for immediate patient diagnosis following defibrillation.
WARNING The PageWriter Trim I cardiograph is not recommended for diagnostic cardiograph use
during defibrillation. It does not provide real-time data in less than 10 seconds. Reusable
electrodes should not be used during defibrillation for diagnostic purposes as ECG
recovery will be greater than ten seconds.
WARNING Do not touch accessible connector pins and the patient simultaneously.
Electrical shock hazard. Keep cardiograph, Patient Interface Module (PIM) and all
cardiograph accessories away from liquids. Do not immerse cardiograph, PIM, or other
accessories in any liquids.
WARNING When using additional peripheral equipment powered from an electrical source other
than the cardiograph, the combination is considered to be a medical system. It is the
responsibility of the operator to comply with IEC 60601-1-1 and test the medical system
according to the requirements. For additional information contact Philips Medical
Systems.
WARNING Do not use non-medical peripherals within 1.83 meters or 6 feet of a patient unless the
non-medical peripherals receive power from the cardiograph or from an isolation
transformer that meets medical safety standards.
WARNING Always clean and disinfect reusable electrodes before patient use. Failure to properly
clean and disinfect reusable electrodes before patient use may cause infectious materials
to be transferred between patients.
CAUTION The Welsh bulb electrodes contain natural rubber latex which may cause allergic reactions.
When operating the cardiograph on AC power, ensure that the cardiograph and all other
electrical equipment connected to or near the patient are effectively grounded.
Use only grounded power cords (three-wire power cords with grounded plugs) and
grounded electrical outlets.
removing the ground prong. Use the equipotential post when redundant earth ground is
necessary according to IEC 60601-1-1.
If a safe ground connection is not ensured, operate the cardiograph on battery power only.
1-6 PageWriter Trim Cardiograph Service Manual
Never adapt a grounded plug to fit an ungrounded outlet by

Introduction Important Patient and Safety Information
The use of equipment that applies high frequency voltages to the patient (including
electrosurgical equipment and some respiration transducers) is not supported and may
produce undesired results. Disconnect the patient data cable from the cardiograph, or detach
the leads from the patient prior to performing any procedure that uses high frequency
surgical equipment.
Do not perform ST analysis on the R/T ECG screen display or on Rhythm reports when the
0.5 Hz Baseline Wander filter is applied.
If abnormal ECG data appears on the printed report, and the abno rmal data d oes no t have a
physiological origin, perform the printer diagnostic test to assess printer performance.
When printing a Rhythm report, there may be a slight delay before the Rhythm report
begins to print on the cardiograph. Rhythm printing is not completed in real-time.
Pace pulse tick marks will not print on an Auto ECG that uses simultaneous acquisition.
Periodically inspect the patient data cable, lead wires, and AC power cord for any worn or
cracked insulation to ensure that no inner conductive material is exposed. Discard worn
accessories and replace them only with Philips Medical Systems accessories (see page 1-
20).
Keep the patient data cable away from power cords and any other electrical equipment.
Failure to do so can result in AC power line frequency interference on the ECG trace.
The Philips Medical Systems patient data cable (supplied with cardiograph) is an integral
part of the cardiograph safety features. Use of any other patient data cable may compromise
defibrillation protection, degrade cardiograph performance, and may result in distorted
ECG data.
Only qualified personnel may service the cardiograph or may op en the cardiograp h housing
to access internal cardiograph components. Do not open any covers on the cardiograph.
There are no internal cardiograph components that are serviced by the operator.
Do not use this cardiograph near flammable anesthetics. It is not intended for use in
explosive environments or in operating rooms.
Do not touch the patient, the patient data cable, any unused patient leads, or the cardiograph
during defibrillation. Death or injury may occur from the electrical shock delivered by the
defibrillator.
Always use electrode gel with reusable electrodes during defibrillation as ECG recovery
will be greater than 10 seconds. Philips Medical Systems recommends the use of disposable
electrodes at all times.
Ensure that the electrodes or lead wires do not come in contact with any other conductive
materials (including earth-grounded materials) especially when connecting or
disconnecting electrodes to or from a patient.
Connecting multiple medical electrical equipment to the same patient may pose a safety
hazard due to the summation of leakage currents. Any combination of instruments should
be evaluated by local safety personnel before being put into service.
PageWriter Trim Cardiograph Service Manual 1-7

Introduction Important Patient and Safety Information
Portable medical equipment such as X-rays and MRI may produce electromagnetic
interference that produces noise in the ECG signal. Move the cardiograph away from these
potential sources of electromagnetic interference.
Do not pull on the paper while an ECG report is being printed. This can cause distortion of
the waveform and can lead to potential misdiagnosis.
Only use the Philips Medical Systems AC power cord supplied with the cardiograph.
Periodically inspect the AC power cord and AC power connector (rear of cardiograph, see
page 1-15) to ensure that both are in a safe and operable condition. If the AC power cord or
AC power connector is not in a safe or operable condition, operate the cardiograph on
battery power and contact Philips Medical Systems for service.
The cardiograph has been safety tested with the recommended accessories, peripherals, and
leads, and no hazard was found when the cardiograph is operated with cardiac pacemakers
or other stimulators.
Do not connect any equipment or accessories to the cardiograph that are not manufactured
or approved by Philips Medical Systems or that are not IEC 60601-1 approved. The
operation or use of non-approved equipment or accessories with the cardiograph is not
tested or supported, and cardiograph operation and safety are not guaranteed.
The list of cables and other accessories with which Philips claims compliance with the
emissions and immunity requirements of IEC standard 60601-1-2 are listed in “Supplies
and Ordering Information” on page 1-20.
Only install Philips Medical Systems software on the cardiograph. The installation or use of
software not approved by Philips Medical Systems is strictly prohibited and cardiograph
safety and performance are not guaranteed.
Only use Philips Medical Systems replacement parts and supplies with the cardiograph. The
use of non-approved replacement parts and supplies with the cardiograph is strictly
prohibited. Cardiograph safety and performance are not guaranteed when non-approved
replacement parts and supplies are used with the cardiograph.
Manual measurements of ECG intervals and magnitudes should be performed on printe d
ECG reports only . Do not make manual measurements of ECG intervals and magnitudes on
the R/T ECG display since these ECG representations are scaled.
Only use patient electrodes that are approved by Philips Medical Systems. The use of non-
approved patient electrodes may degrade cardiograph performance.
The Philips Medical Systems warranty is applicable only if you use Philips Medical
Systems approved accessories and replacement parts. See “Supplies and Ordering
Information” on page 1-20 for more information.
Before using the Patient Cable Arm with the cardiograph cart, properly install the counter
weight on the cardiograph base.
Only use the shielded LAN cable provided with the PageWriter Trim cardiograph, Philips
part number 989803138021. Do not use any other LAN cables with the Pa geWriter Trim
cardiograph. Use of unapproved LAN cables may result in radiated emissions that exceed
the limit specified by CISPR11 Class B.
1-8 PageWriter Trim Cardiograph Service Manual

Introduction The PageWriter Trim Cardiograph
The combined maximum weight that can be placed on the cardiograph cart shelf and the top
surface of the cart cannot exceed 20 kg (44 lbs). Do not place more than the specified
weight on the cardiograph top surface and shelf.
Do not connect any device to the RS-232 port on the rear of the cardiograph when the
patient data cable is connected to a patient.
There are no cardiograph parts that can be sterilized.
The cardiograph is not intended for direct, or invasive cardiac monitoring purposes.
Excessive, repetitive use of the cardiograph keyboard and the cardiograph Trim Knob may
result in a risk of developing carpal tunnel syndrome.
Ensure that the patient data cable is tucked away from the cardiograph cart wheels when
transporting the cardiograph. Ensure that the patient data cable does not present a hazard
when pushing the cardiograph cart.
For information on the standard IEC 60601-2-51, please see the document on the
PageWr iter Trim Cardiograph User Documentation CD, or go to the Philips InCenter web
site (
incenter.medical.philips.com). For information on using the Philips InCenter site, see
page 1-26.
The combined maximum weight that can be placed on the cardiograph cart shelf and the top
surface of the cart cannot exceed 20 kilograms (44 pounds). Do not place more than the
specified weight on the cardiograph top surface and shelf.
Ensure that the patient data cable is tucked away from the cardiograph cart wheels when
transporting the cardiograph. Ensure that the patient data cable does not present a hazard
when pushing the cardiograph cart.
The PageWriter Trim Cardiograph
Intended Use
The intended use of the cardiograph is to acquire multi-channel ECG signals from adult and
pediatric patients from body surface ECG electrodes and to record, display, analyze, and store
these ECG signals for review by the user. The cardiograph is to be used in healthcare facilities
by trained healthcare professionals. Analysis of the ECG signals is accomplished with
algorithms that provide measurements, data presentations, graphical presentations, and
interpretations for review by the user.
The interpreted ECG with measurements and interpretive statements is offered to the clinician
on an advisory basis only. It is to be used in conjunction with the clinician's knowledge of the
patient, the results of the physical examination, the ECG tracings, and other clinical findings.
A qualified physician is asked to overread and validate (or change) the computer-generated
ECG interpretation.
PageWriter Trim Cardiograph Service Manual 1-9

Introduction The Philips 12-Lead Algorithm
Indications for Use
The cardiograph is to be used where the clinician decides to evaluate the electrocardiogram of
adult and pediatric patients as part of decisions regarding possible diagnosis, potential
treatment, effectiveness of treatment, or to rule out causes for symptoms.
The Philips 12-Lead Algorithm
The PageWriter Trim Cardiograph software uses the Philips 12-Lead Algorithm. The
algorithm in the software analyzes the morphology and rhythm on each of the 12 leads and
summarizes the results. The set of summarized measurements is then analyzed by the
clinically-proven ECG Analysis Program.
12-lead Reports may include or exclude ECG measurements, reasons, or analysis statements.
Intended Use
The intended use of the Philips 12-Lead Algorithm is to analyze multi-channel ECG signals
from adult and pediatric patients with algorithms that provide measurements, data
presentations, graphical presentations, and interpretations for review by the user.
The interpreted ECG with measurements and interpretive statements is offered to the clinician
on an advisory basis only. It is to be used in conjunction with the clinician's knowledge of the
patient, the results of the physical examination, the ECG tracings, and other clinical findings.
A qualified physician is asked to overread and validate (or change) the computer-generated
ECG interpretation.
Indications for Use
The Philips 12-Lead Algorithm is to be used where the clinician decides to evaluate the
electrocardiogram of adult and pediatric patients as part of decisions regarding possible
diagnosis, potential treatment, effectiveness of treatment, or to rule out causes for symptoms.
Features and Capabilities
The Philips PageWriter Trim family of cardiographs includes four product models:
PageWriter Trim Rx, PageWriter Trim III, PageWriter Trim II, and PageWriter Trim I. Each
cardiograph is designed to be economical, interpretive, and lightweight, and includes a remote
digital patient module. The cardiograph contains the controls, the printer, and all the
processing circuitry.
The features of the PageWriter Trim cardiographs include:
Battery or AC operated
Remote digital acquisition module with replaceable patient leads
Capability for up to 12 leads
1-10 PageWriter Trim Cardiograph Service Manual
Introduction Features and Capabilities
Color or monochrome display as described in Table 1-2:
Table 1-2 PageWriter Trim Cardiograph Display by model
Model Display
PageWriter Trim I 40 x 2 character LCD
PageWriter Trim II 640 x 480 monochrome LCD
PageWriter Trim III 640 x 480 color LCD
PageWriter Trim Rx 640 x 480 color LCD
Export and import of ECG data in XML format to a TraceMasterVue ECG Management
System by modem transmission, or by LAN or wireless LAN connection as described in
Table 1-3:
Table 1-3 PageWriter Trim ECG data transmission options by model
Model Modem LAN WLAN
PageWriter Trim I
PageWriter Trim II **
PageWriter Trim III
**
PageWriter Trim Rx
**
** Optional feature
Optional cart with convenient storage areas for supplies
Capabilities
Downloads patient data from HIS with a barcode, magnetic card swipe, or Smart Card
swipe
Stores ECGs on a removable PCMCIA card or USB memory stick
Transmits ECGs by FAX, PCMCIA-modem, LAN, or wireless LAN
PageWriter Trim Cardiograph Service Manual 1-11
Introduction Tour of PageWriter Trim Cardiographs
A
B
D
E
F
H
G
C
I
J
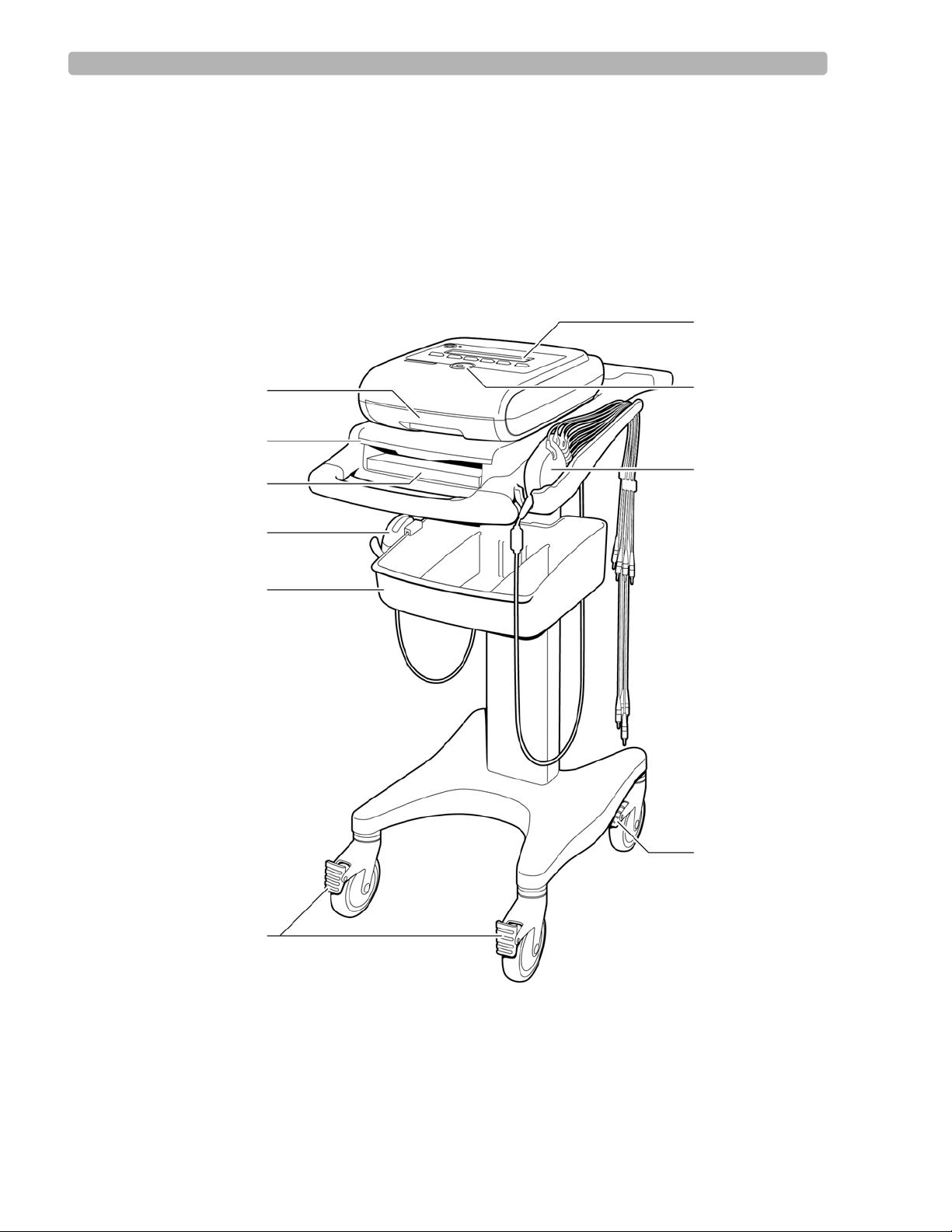
Tour of PageWriter Trim Cardiographs
This section gives an overview of the exterior of the cardiograph, as well as the Patient
Interface Module (PIM). For more information, see the PageWriter Trim Instructions for Use.
PageWriter Trim I Cardiograph
The following section shows front and rear views of the PageWriter Trim I cardiograph.
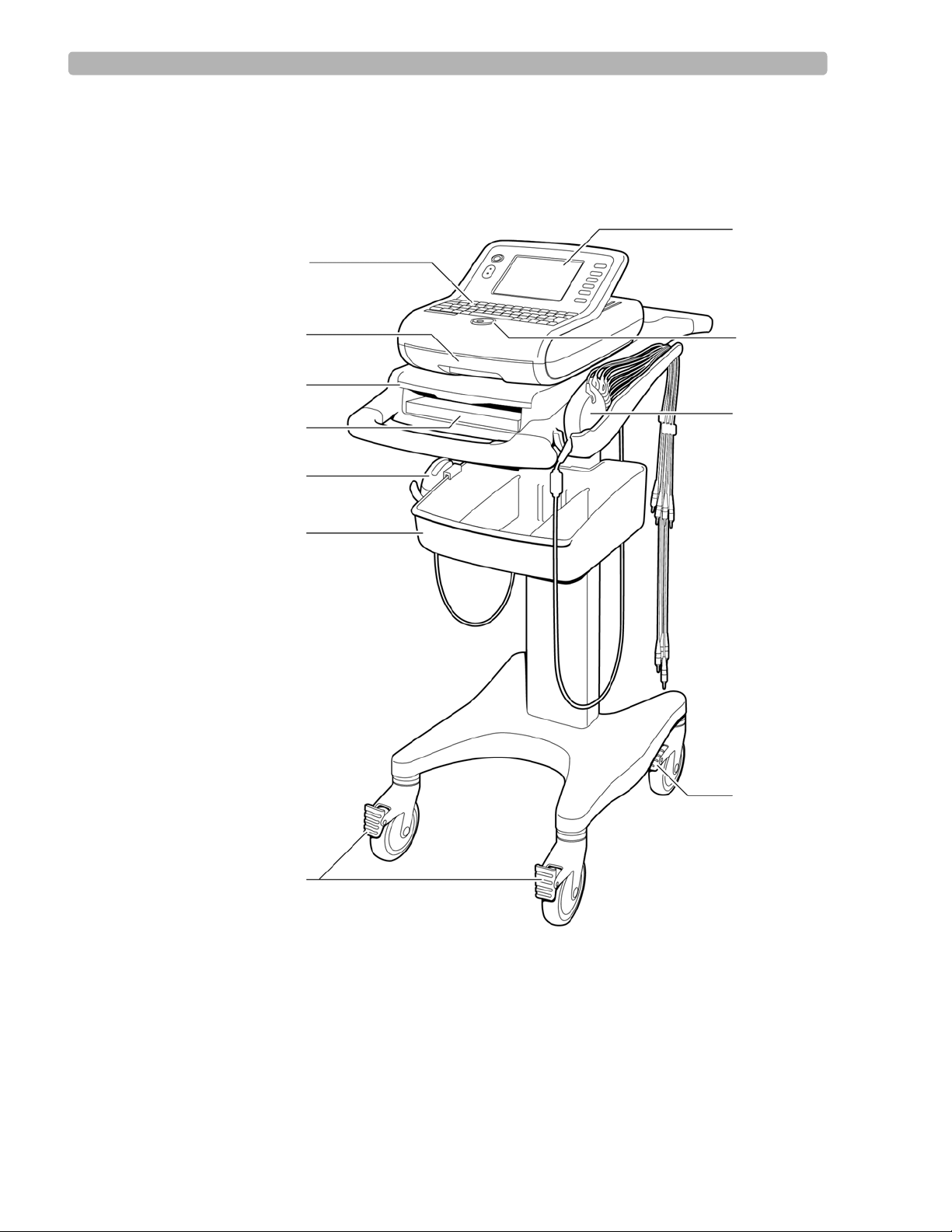
Figure 1-1 PageWriter Trim I Cardiograph and Cart (Front View)
A Printer paper drawer F Wheel positioners
B Slide out shelf G Control panel
C Printer paper/report storage slot H Trim Knob
D Optional barcode reader in holder I Patient Interface Module (PIM)
E Storage bin J Wheel brake
1-12 PageWriter Trim Cardiograph Service Manual
Introduction Tour of PageWriter Trim Cardiographs
M
NQ
PO
KL
CAUTION Always lock the wheel brake (J) when the cart is not in use. Press down on the wheel brake to set or
to release the wheel brake.
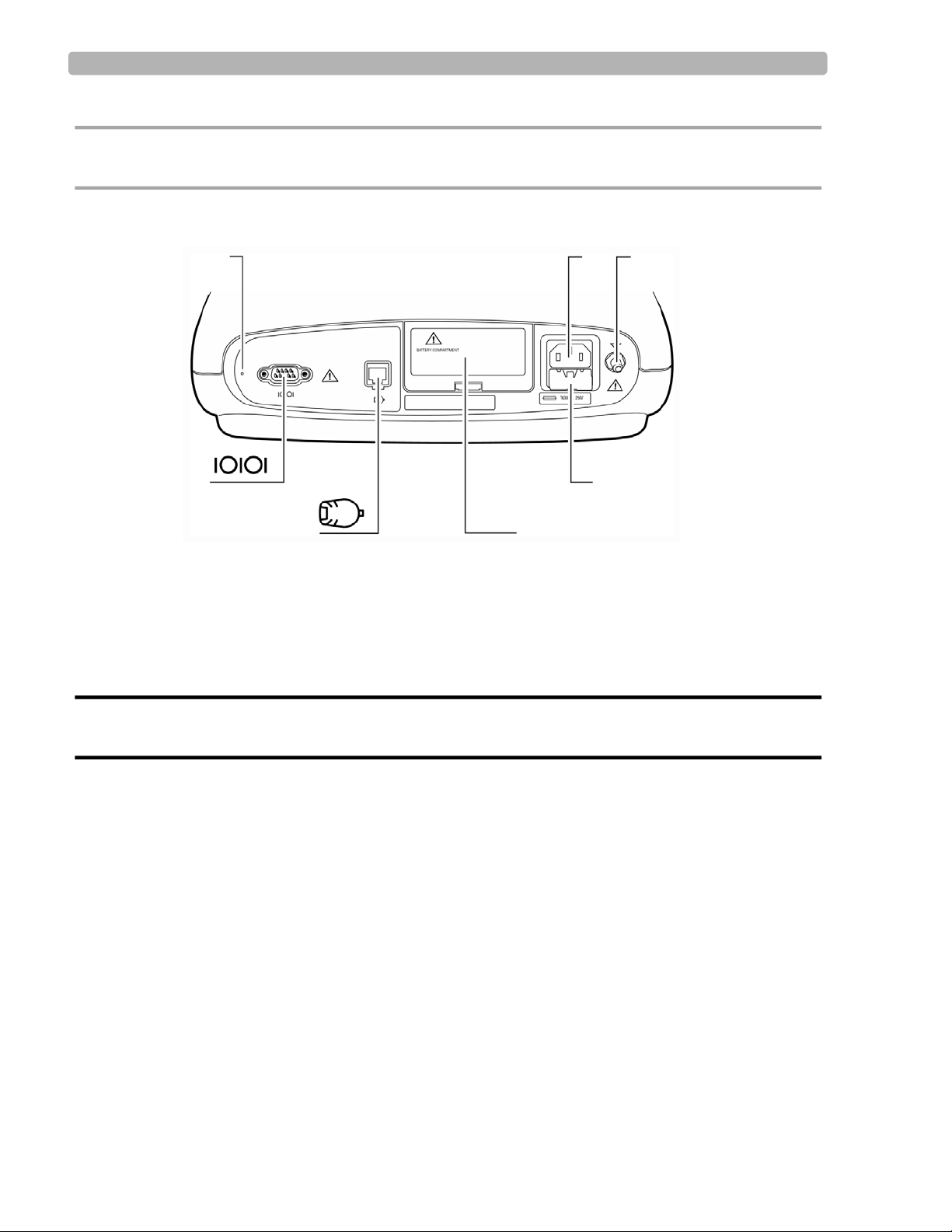
Figure 1-2 PageWriter Trim I Cardiograph (Rear View)
K Reset button O Battery door
L AC power cord connector P PIM connector
M Equipotential post Q Serial connector (not supported)
N Fuse door
WARNING Do not connect a LAN cable connector to the PIM connector.
Do not plug a telephone connector into the PIM connector.
PageWriter Trim Cardiograph Service Manual 1-13
Introduction Tour of PageWriter Trim Cardiographs
A
B
D
E
F
H
I
J
G
C
K
PageWriter Trim II, III, and Rx Cardiographs
The following section shows front and rear views of the PageWriter Trim II, III, and Rx
cardiographs.
Figure 1-3 PageWriter Trim II, III, and Rx Cardiograph and Cart (Front View)
A Keyboard G Wheel positioners
B Printer paper drawer H Display screen
C Slide out shelf I Trim Knob
D Printer paper/report storage slot J Patient Interface Module (PIM)
E Optional barcode reader in holder K Wheel brake
F Storage bin
1-14 PageWriter Trim Cardiograph Service Manual
Introduction Tour of PageWriter Trim Cardiographs
P
Q
S
O
R
T
U
MN
V
L
CAUTION Always lock the wheel brake (K) when the cart is not in use. Press down on the wheel brake to set or
to release the wheel brake.
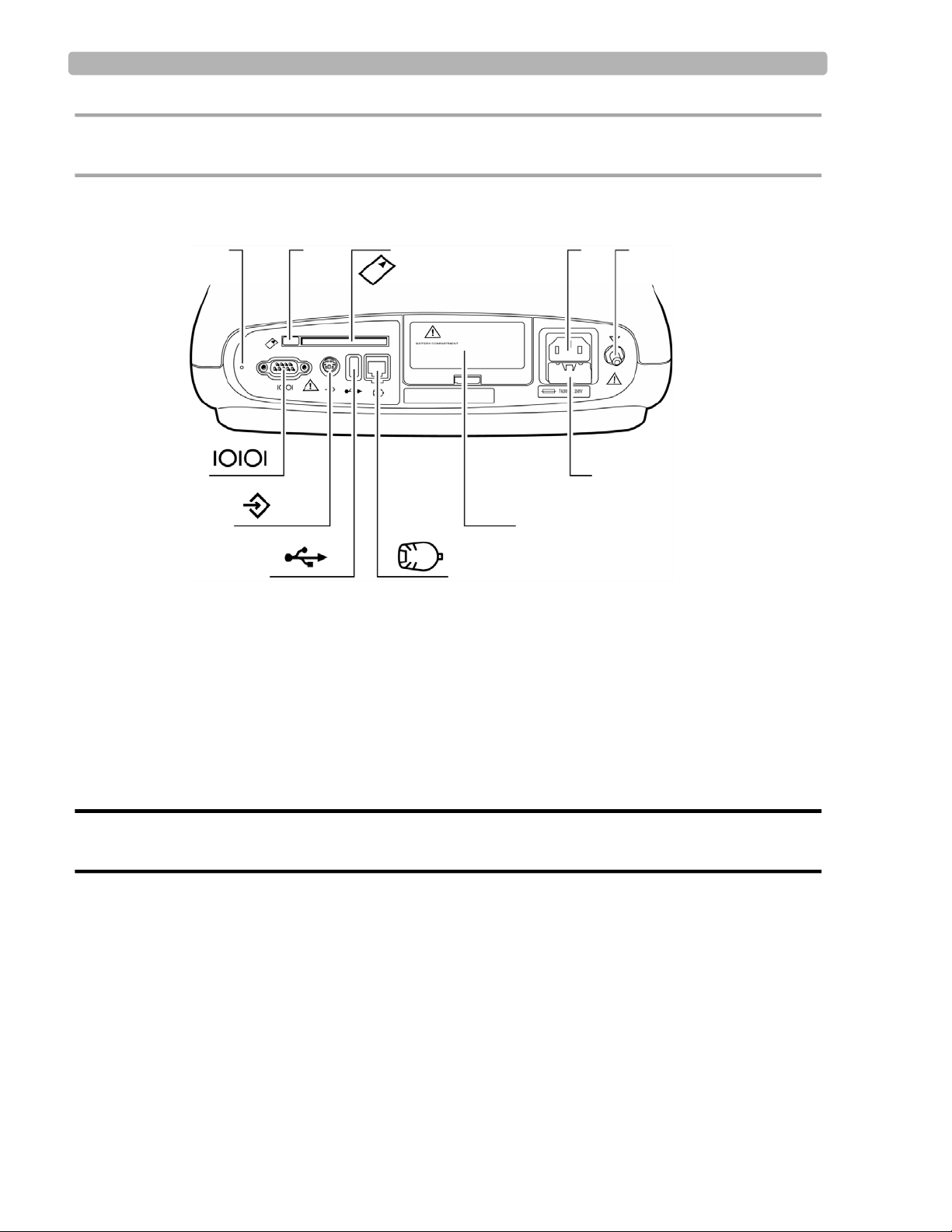
Figure 1-4 PageWriter Trim II, III, and Rx Cardiograph (Rear View)
L Reset button R Battery door
M PC card eject button S PIM connector
N PC card slot T Smart Card Reader or USB memory stick connector
O AC power connector U Barcode reader or magnetic card reader connector
P Equipotential post V Serial connector (not supported)
Q Fuse door
WARNING Do not connect the LAN cable connector into the PIM connector.
Do not plug a telephone connector into the PIM connector.
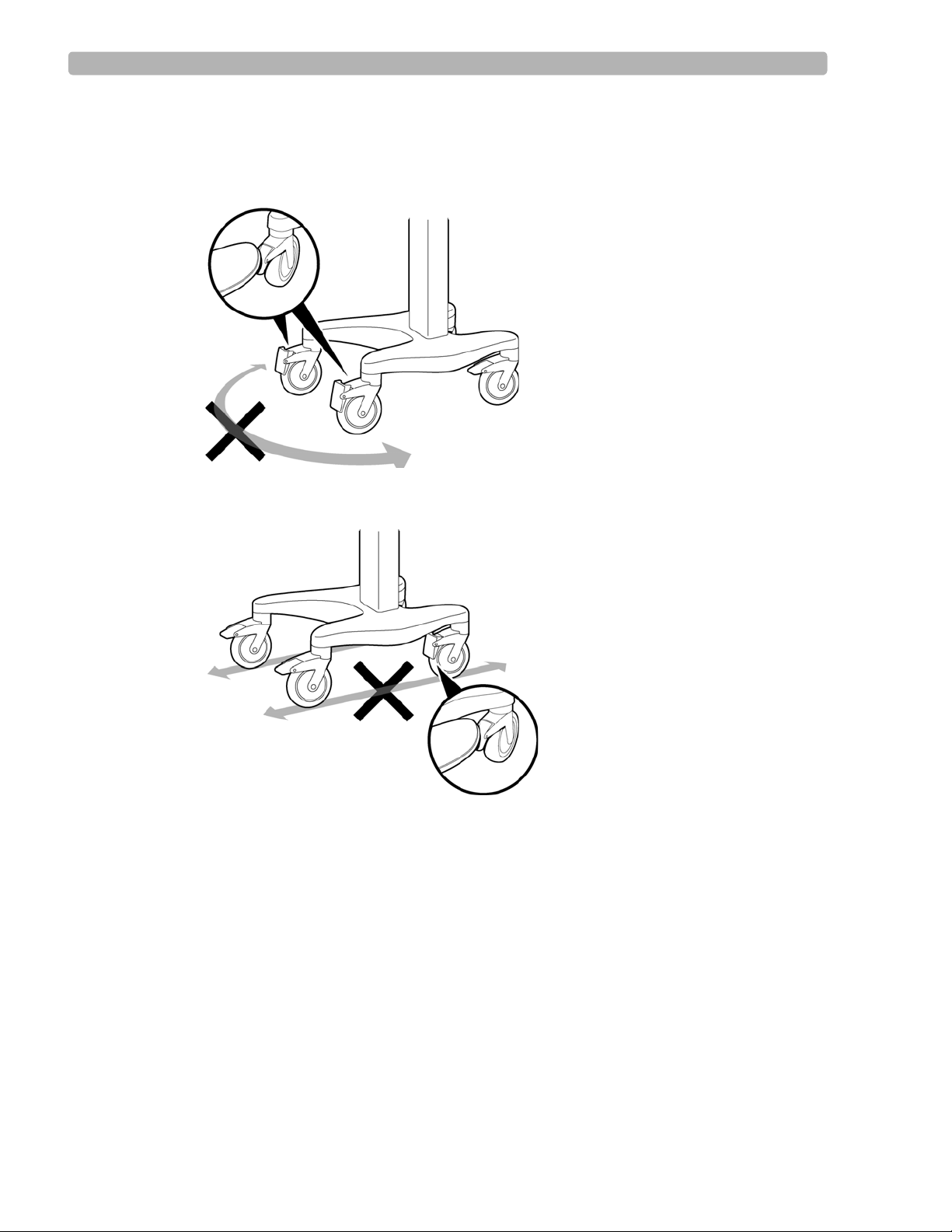
Using the Cart Wheel Positioners and Brake
The cart includes one wheel brake and two wheel positioners. Lock the wheel positioners at all
times when using the cart. The wheel positioners keep the cart straight when moving forward
or backward, or when turning corners. The wheel positioners also help the cart maneuver in
tight spaces.
PageWriter Trim Cardiograph Service Manual 1-15
Introduction Tour of PageWriter Trim Cardiographs
To use the cart wheel positioners and brake:
1
Align the front wheels so that they are straight. Step on both wheel positioners. Move the
cart forward until the wheels lock into position. The cart will move forward or backward
in a straight line.
2 Step on the gray rear wheel brake to lock the cart wheels. The cart will not move. Step on
the wheel brake again to unlock the wheels.
1-16 PageWriter Trim Cardiograph Service Manual
Introduction General Service Information
A
CD
B
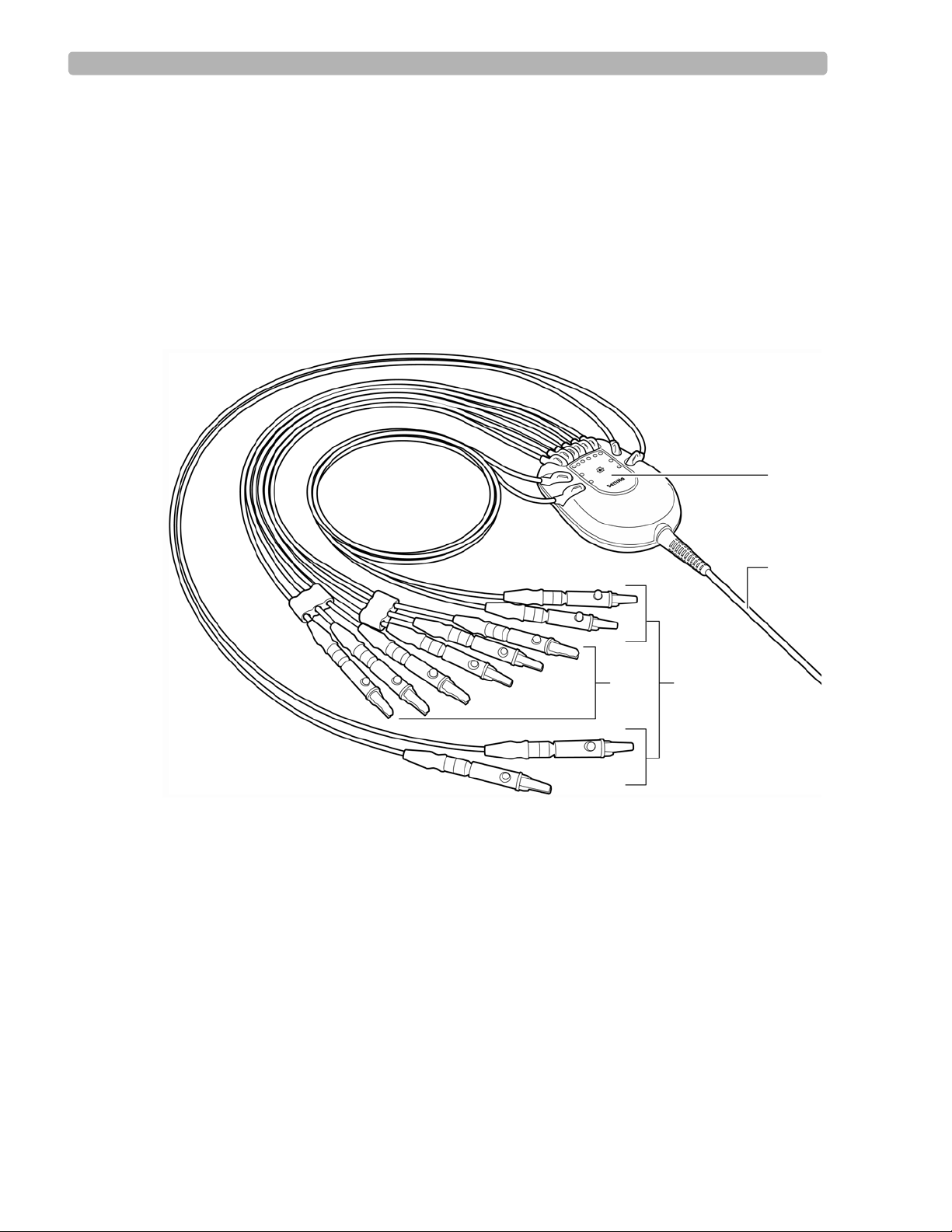
Patient Interface Module (PIM)
The Patient Interface Module (PIM) is a hand-held device that connects to the cardiograph.
The lead wires on the PIM attach to the electrodes placed on the patient. The exterior of the
PIM is labeled for quick and easy lead identification.
The PIM connects to the patient data cable and to the lead wires attached to the patient. See
Figure 1-5 on the following page.
For details about connecting the lead wires to the PIM, see the PageWriter Trim Instructions
for Use.
Figure 1-5 Patient Interface Module
A Lead wire labeling C Limb lead wires
B Patient data cable D Precordial (chest) lead wires
General Service Information
Keep the following points in mind when servicing this product.
Installation
The PageWriter Trim cardiographs do not require installation by Philips field personnel. The
cardiograph can be installed by the customer. See Chapter 1 “Getting Started” and 2
“Configuration” of the PageWriter Trim Instructions for Use or the PageWriter Trim Rx
PageWriter Trim Cardiograph Service Manual 1-17
Introduction Upgrades and Accessories
Instructions for Use for information on the proper setup and configuration of the cardiograph
and cart system.
NOTE There are no configurable features for the PageWriter Trim I cardiograph.
Upgrades and Accessories
Upgrades and cardiograph accessories are available to add specific functionality to the device.
The standard upgrades and available accessories are listed in Table 1-4 and in Table 1-5.
Table 1-4 PageWriter Trim II, III, and Rx Cardiograph Accessories
Part Number Description
989803129931 Barcode Reader
989803129941 Magnetic Card Reader
989803127331 PC Card
989803129961 Smart Card Reader
989803142041 Wireless LAN Card
989803129951 LAN PCMCIA Network Card
989803145331 USB Memory Stick
989803149571 Patient Cable Arm for Cardiograph Cart
989803127461 Modem Card (USA and Canada only)
989803138021 LAN Cable
Table 1-5 PageWriter Trim Cardiograph Upgrades
Part Number Option
Description
860320 PageWriter Trim Cardiograph Cart
860302 PageWriter Trim II Cardiograph Upgrade Options
B01 ECG Interpretation Upgrade Option
B02 LAN Connectivity Upgrade Option
860303 PageWriter Trim III Cardiograph Upgrade Options
B02 LAN Connectivity Upgrade Option
B03 Wireless LAN Connectivity Options (802.11b compliant)
860299 PageWriter Trim Rx Cardiograph Upgrade Options
B02 LAN Connectivity Upgrade Options
1-18 PageWriter Trim Cardiograph Service Manual

Introduction PageWriter Trim II, III and Rx Token Label
Table 1-5 PageWriter Trim Cardiograph Upgrades (continued)
Part Number Option
Description
B03 Wireless LAN Connectivity Options (802.11b compliant)
860304 PageWriter Trim Cardiograph External Battery Charger
AXX Localization Code (refer to Table 1-11, “PageWriter Trim I, II,
III and Rx Country and Region Options,” on page 1-23 for the
correct localization option code for your country or region)
B01 Additional Battery for the PageWriter Trim Cardiograph
(any model)
Consult your sales representative, dealer, or distributor for the latest details.
PageWriter Trim II, III and Rx Token Label
Each cardiograph manufactured after January of 2006 has a token number assigned to it, and is
shipped from the factory with a token label installed on the unit. See Figure 1-7 on page 1-20
for the location of the token label on the cardiograph. And, any cardiograph with installed
software version A.01.00 or higher (regardless of manufacture date) must have an active token
number assigned to it in order to operate. If upgrading from software version A.00.03 to
A.01.01 or higher, or following a repair that necessitates upgrading the cardi ogra ph software
to version A.01.01 or higher, a token number is required in order to complete the software
upgrade or repair procedure. For information on obtaining a token number in order to
complete a software upgrade procedure, contact the nearest Philips Response Center. See
“Contacting a Philips Response Center” on page 1-32 for a listing of contact telephone
numbers.
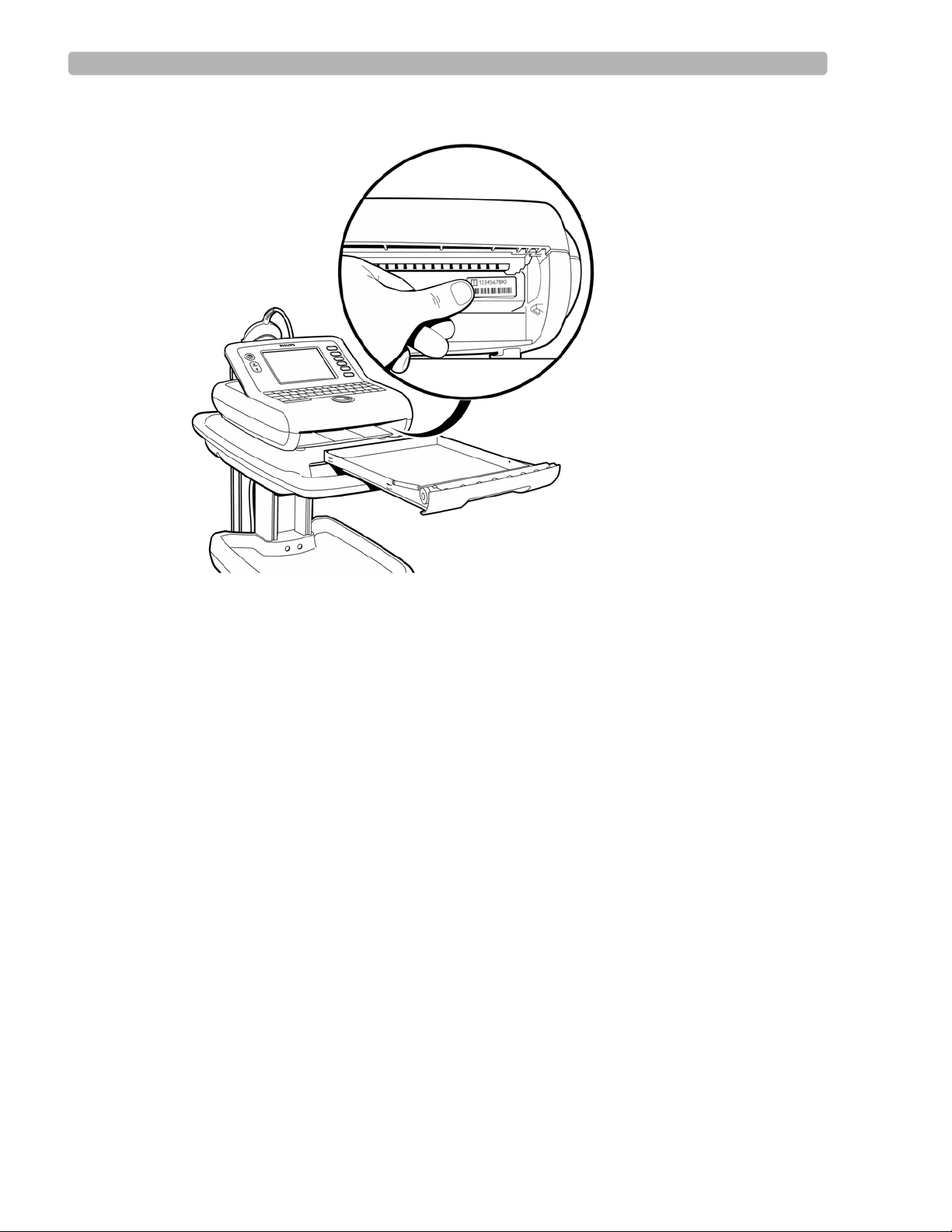
The token label contains the unique token number assigned to the cardiograph, and is affixed
to the inside of the paper tray. To locate the token label, remove the paper tray from the
cardiograph. The label is located on the far right side of the metal housing. Always ensure that
the current token label is affixed to the cardiograph to help facilitate the servicing or
troubleshooting of the unit.
NOTE There are no token numbers assigned to the PageWriter Trim I cardiograph.
Figure 1-6 PageWriter Trim II, III, Rx Cardiograph Token Label
PageWriter Trim Cardiograph Service Manual 1-19
Introduction Supplies and Ordering Information
Figure 1-7 Location of Token Label on Cardiograph
Managing Token Labels
Each upgrade option available for purchase is enabled by a unique token number that is
provided with the upgrade kit. Each time that a new option is purchased for the cardiograph,
the new token number must be entered on the cardiograph, and the new token lab el must be
affixed to the cardiograph in the specified location to facilitate future servicing and
troubleshooting. For information on enabling and installing an optional upgrade, see “Upgrade
Kit Installation” on page E-2.
Supplies and Ordering Information
The part numbers for all supplies for the PageWriter Trim I/II/III/Rx cardiographs are listed in
this section.
You can order all supplies on the World Wide Web at
http://shop.medical.philips.com
1-20 PageWriter Trim Cardiograph Service Manual
 Loading...
Loading...