Page 1
PAD M2703A Software Revision D.0 Data Sheet
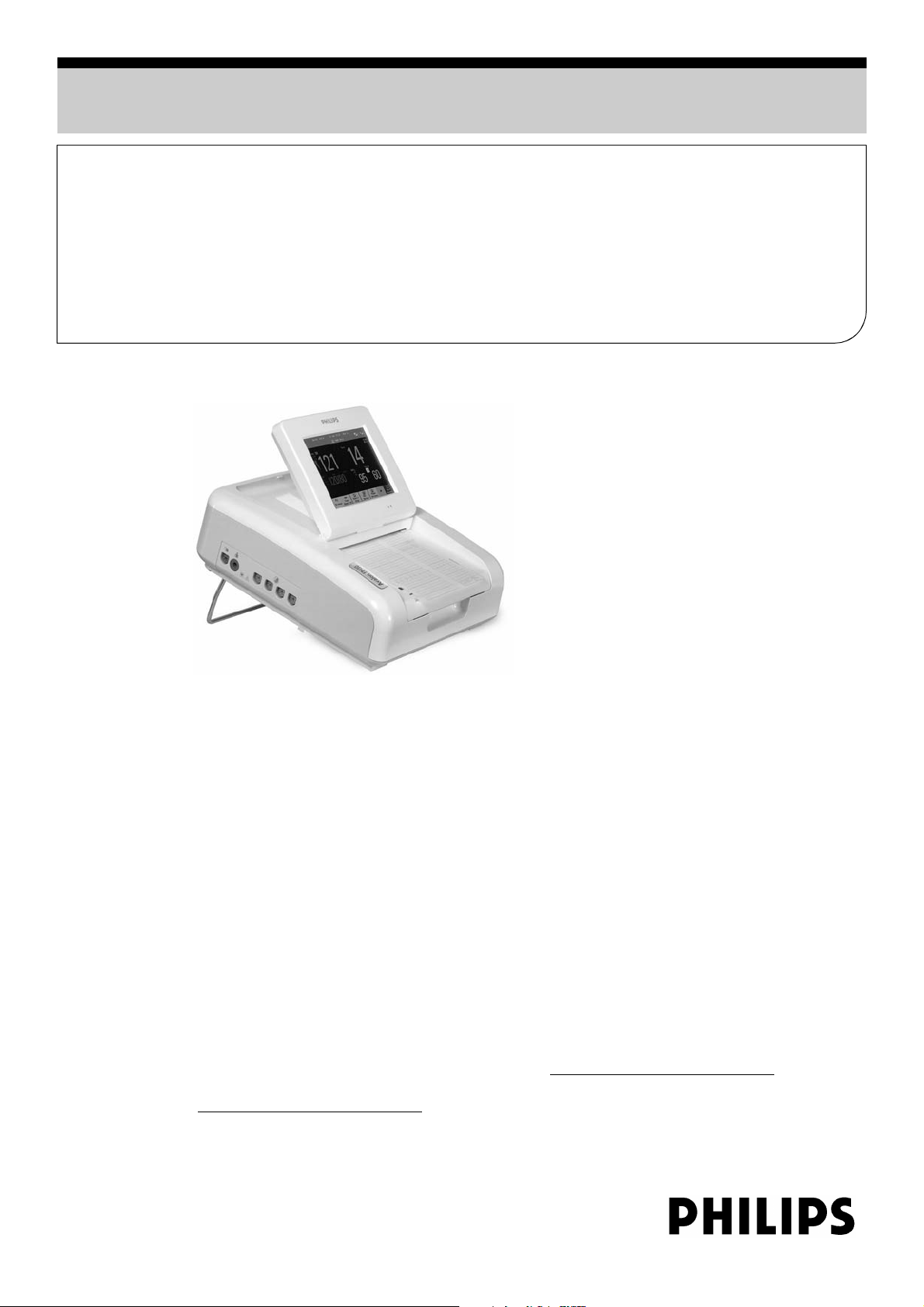
Avalon FM30
Fetal / Maternal Monitor
• 6 second maternal ECG strip, with a choice of
real-time recording or “snapshot” together with
the fetal trace
• Maternal blood pressure and pulse oximetry
(optional)
Features
• Touchscreen for intuitive, easy operation
• Tilt and fold 6.5-inch TFT color display with a
wide viewing angle and large numerics
• Built-in 6-inch recorder for automatic printing of
fetal and maternal parameters on the trace
• Intelligent transducers are waterproof, light,
comfortable to wear and easy to clean
• Automatic channel management lets you connect
any fetal transducer, patient module or remote
The Philips Avalon FM30 fetal/maternal monitor
offers a state-of-the-art solution for all external and
internal fetal monitoring measurements in doctors’
practices, clinics and hospitals, during labor and
delivery and for antepartum monitoring of high-risk
patients.
With a cutting-edge feature set, including advanced
transducers, high quality signal processing, and a
touchscreen interface, the Avalon FM30 sets new
standards for performance, flexibility, convenience,
and ease of use.
Measurements
The Avalon FM30 lets you monitor and document:
• Up to three
ultrasound
• DECG via combined Toco/ECG “Toco
transducer (also can measure MECG)
• Uterine activity externally, or intrauterine pressure
• Maternal heart rate with ECG waveform display
1. Triplets monitoring is not currently available in the USA.
1
fetal heart rates externally using
+
”
event marker to any fetal sensor socket
• Cross-Channel Verification between all fetal and
maternal heart rates
• The blue transducer Finder LED lets you identify
at a glance which transducer is monitoring which
measurement
• Transmission of fetal and maternal parameters to
an obstetrical surveillance system
2
• Network connectivity to an OB TraceVue
via LAN connection using the optional system
interface, where OB TraceVue controls all patient
admit/discharge events
• Internal backup memory allows recovery of up to
one hour of trace data, either to paper or to an OB
Tr a c e V u e
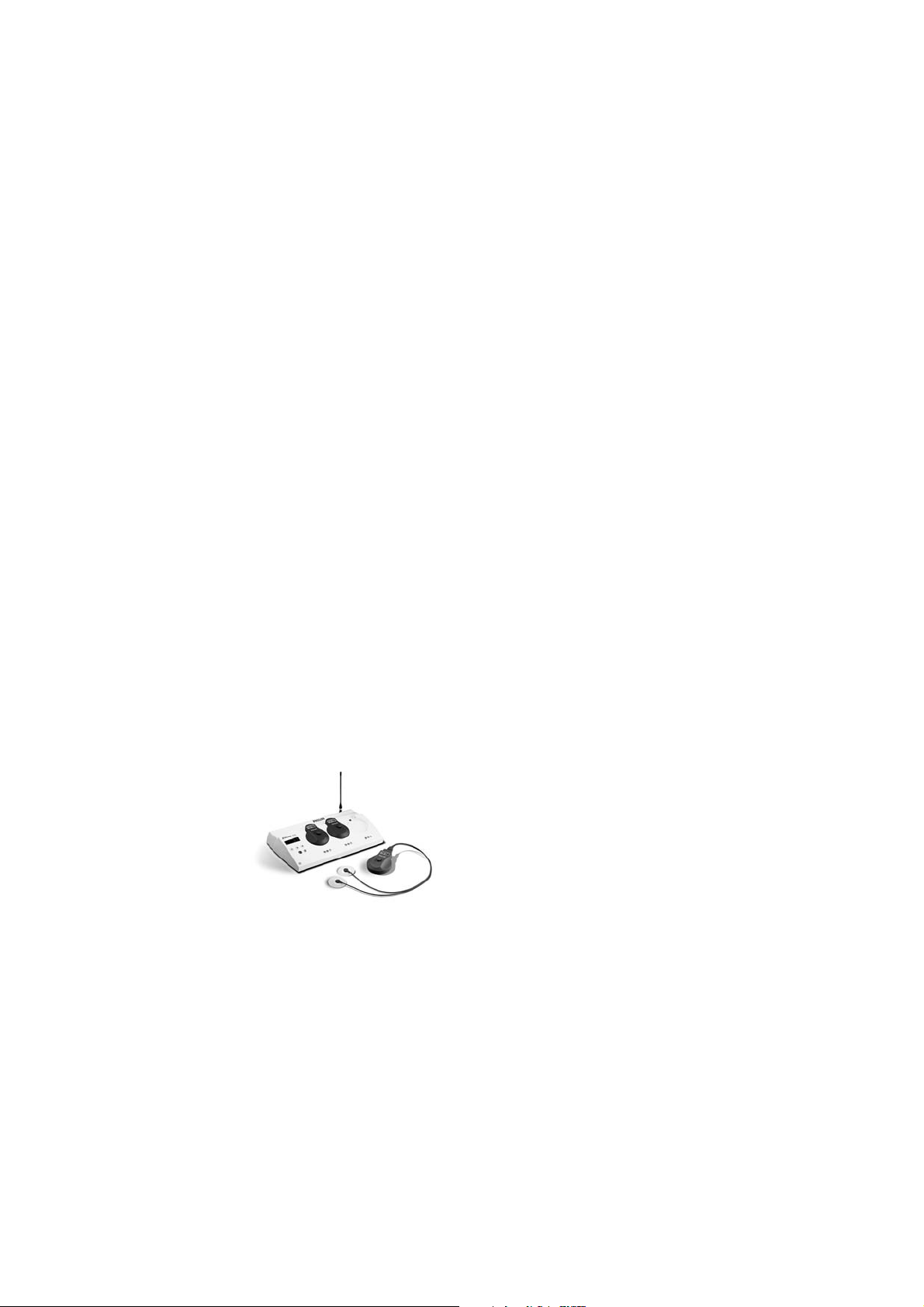
• Cordless monitoring using the award-winning
Avalon CTS Cordless Fetal Transducer System
• Compact and light, with built-in carrying handle,
and stand for angled stance on any flat surface
• Comprehensive patient data presentation,
including at-a-glance patient identification, and
2. OB TraceVue Revision E.00.00 or later.
3. Requires Avalon CTS interface cable M2731-60001 (see Avalon
CTS data sheet for details), and monitor software revision D.00.00
or higher.
2
system over a LAN connection
system
3
Page 2

the choice to add user-configurable notes
4
• Automatic screen layouts optimize size and
presentation of measurements
• Fetal Movement Profile (FMP) detects and
automatically records gross fetal body movements
for antepartum applications
• NST timer for antepartum applications
Description of Main Components
TFT Color Display
The monitor has an integrated, tiltable color TFT
display, with a wide viewing angle, providing high
resolution data presentation. Fetal and maternal
measurements are displayed as large, easy-to-read
numerics.
Information such as patient identification (including
the bed label when connected to a surveillance
system such as OB TraceVue), date and time, alarm
information, icons for recorder status, and prompt
and error messages are also clearly shown.
Touchscreen User Interface
•Fetal Movement
• Uterine Activity
The monitor prints the time, the date, trace
identification symbols, the monitoring mode and
the paper speed when first switched on, every ten
minutes after, and whenever the monitoring modes
change.
Maternal heart rate and the maternal pulse oximetry
value is printed every 5 minutes. In the case of
maternal noninvasive blood pressure measurement,
the annotation is made at the end of the
measurement.
Depending on the geography, there are two possible
paper scales:
•FHR Scale:
- Red paper: 30 to 240 bpm @ 30 bpm/cm
- Green paper: 50 to 210 bpm @ 20 bpm/cm
•Labor Scale:
- 0 to 100 units @ 25 units/cm
Alarms
When an alarm limit is exceeded, it is signalled by
the monitor in the following ways:
• An alarm tone sounds
• An alarm message is shown on the screen
• The numeric of the alarming measurement flashes
on the screen
The monitor is operated using the touchscreen. All
user functions (apart from the On/Off switch) are
controlled directly from the screen, and the intuitive,
color, graphical user interface not only makes it
quick and easy to learn and use, but the lack of
mechanical controls also makes cleaning the Avalon
fetal monitor much quicker and simpler.
A number of SmartKeys at the bottom of the main
screen allow fast access to functions, for starting a
recording, or resetting a Toco baseline, for instance.
Heart rate and alarm volume adjustment, with an
indication of the current setting, are always available
at the top of the screen.
Integrated Recorder
The Avalon fetal monitor has an integrated, high
resolution thermal array recorder, with paper-out/end detection.
Traces are recorded at either 1, 2 or 3 cm/min.
Continuous traces are made for:
• Fetal Heart Rate(s)
• Maternal Heart Rate
4. Using standard Roman-8 character sets.
Alarm categories:
• Physiological Alarms for fetal and maternal
parameters (to indicate vital sign alarm limit
violations, for instance)
• Tec h nic a l Al a r m s ( I NO PS)
are triggered by signal
quality problems, equipment malfunction or
equipment disconnect.
Alarm modes:
• INOP only: only INOPs are enabled, with audible
and visual indication active. This is the default.
• All: patient alarms and INOPs are enabled, with
audible and visual indicators active.
Alarm suspension:
• Alarms Off/Pause Alarms function
• Alarm pause period: indefinite, or one, two, or
three minutes.
Alarm latching/non-latching:
•Visual
•Audible
• Visual and audible
Interfaces
LAN/RS-232 System Interface
The monitor has an optional LAN/RS-232 interface
board with two fully-isolated ports:
- The LAN connection is for connecting the
monitor to an OB TraceVue obstetrical
2
Page 3
information and surveillance system on a
network, or to a PC for configuration or
upgrade using the Support Tool
- The RS232 connection can be used for
connecting the monitor to an obstetrical
information and surveillance system, such as OB
Tr a c e V u e
Input Device Interface (Dual PS/2 Interface)
This optional interface provides two PS/2 ports to
enable the monitor to be connected to off-the-shelf,
“plug-and-play” input devices:
- Mouse: any specified PS/2 mouse or trackball
may be used for navigation and data entry.
- Computer keyboard: a PS/2 computer keyboard
can be used for data entry instead of the onscreen pop-up keyboard.
Service Features
• The Support Tool helps technical personnel to
– carry out configuration, upgrades and
troubleshooting on an individual monitor.
– share configuration settings between monitors.
– back up the monitor settings.
• The Service Mode is password-protected and
ensures that only trained staff can access service
tests and tasks.
• The Configuration Mode is password-protected
and allows trained users to customize the monitor
configuration.
• The Demo Mode is password-protected and is
intended for training and educational purposes.
Related Products
The monitor is in conformity with the essential
requirements of the European Medical Devices
Directive 93/42/EEC and the following major
international standards:
Safety, Performance
• EN 60601-1:1990+A1:1993+A2:1995/IEC
60601-1:1988+A1:1991+A2:1995
• EN 60601-1-1:2001/IEC 60601-1-1:2000
• EN/IEC 60601-2-27:1994
• EN/ISO 9919:2005
• EN 60601-2-30:2000/IEC 60601-2-30:1999
• EN/IEC 60601-2-37:2001+A1:2004
• EN 60601-2-49:2001/IEC 60601-2-49:2001
• UL 60601-1:2003
• CAN/CSA C22.2#601.1-M90
• AS/NZS 3200.1.0-1998
The possibility of hazards arising from hardware and
software errors was minimized in compliance with
ISO 14971:2000+A1:2003, EN60601-14:1996+A1:1999 and IEC 60601-14:1996+A1:1999.
Alarm sounds are compliant with the draft ISO/
IEC 9703-2 Standard.
Electromagnetic Compatibility
• EN/IEC 60601-1-2:2001+A1:2004
• ICES-001:1988
The Avalon fetal monitor is classified as
electromagnetic emissions Class B, except whenever
used with the IUP/ECG patient module M2738A,
when it is classified as Class A.
This ISM device complies with Canadian ICES-
001. Cet appareil ISM est conforme a la norme
NMB-001 du Canada.
Monitors with software revision D.00.00 (or higher)
are compatible with the Avalon CTS Cordless Fetal
Transducer System (M2720A), allowing expectant
mothers to be continuously monitored without
wires, whether in the bath or shower, in the bed, or
while ambulating. Monitors with earlier software
revisions can be upgraded to the lastest software
version (see “Upgrade Options” on page 14). Refer
to the Avalon CTS Data Sheet for the required
Avalon CTS interface cable (M2731-60001) and
further information.
Standards Compliance
US Federal Law restricts this device to sale by or on
the order of a physician.
3
Page 4

Environmental Specifications
The monitor may not meet the given performance specifications if stored and used outside the specified
temperature and humidity ranges.
FM30 Monitor
Temperature Range Operating 0°C to 45°C (32°F to 113°F)
Storage
Humidity Range Operating <95% relative humidity @ 40°C/104°F
Storage
Altitude Range Operating -500 to 3000 m/-1640 to 9840 ft.
Storage
Transducers (M2734A/M2735A/M2736A/M2738A)
Temperature Range Operating 0°C to 40°C (32°F to 104°F)
Storage
Humidity Range Operating <95% relative humidity @ 40°C/104°F
Storage
Altitude Range Operating -500 to 3000 m/-1640 to 9840 ft.
Storage
SpO2 Sensors
Operating Temperature Range 0°C to 37°C (32°F to 98.6°F)
-20°C to 60°C (-4°F to 140°F)
<90% relative humidity @ 60°C/140°F
-500 to 13100 m/-1640 to 43000 ft.
-20°C to 60°C (-4°F to 140°F)
<90% relative humidity @ 60°C/140°F
-500 to 13100 m/-1640 to 43000 ft.
Physical Specifications
FM30 Monitor
Power Supply Voltages 100 VAC to 240 VAC ± 10%
Supply Frequency Range
Power consumption (maximum)
Dimensions and Weight Size mm/(in) ±1%: width x height x depth
Degree of Protection Against
Electrical Shock
Electrical Class Class II equipment
Mode of Operation Continuous operation
Startup Time Time taken from switching on the monitor to
(without options)
Weight
Type CF
seeing the first parameter labels
Transducers (M2734A/M2735A/M2736A)
Shock Resistance Withstands ten 1m drops to concrete surface with possible cosmetic damage only
Water Ingress Protection Code M2734/35/36A IP 68 (immersion up to 1 m water depth for 5 hours)
M2738A IP 67 (immersion up to 0.5 m water depth for 30 minutes)
Dimensions and Weight M2734/35/36A Size (diameter) 83 mm/3.27 in
Weight (without cable)
M2738A Maximum size mm/(in):
width x height x depth
Cable length
Weight
Water Ingress Protection Code IP 68 (immersion up to 1m water depth of for 5 hours)
Degree of Protection Against
Electrical Shock
Type CF
50 Hz to 60 Hz
60 VA
286 x 133 x 335 (11.3 x 5.2 x 13.2 in)
< 5.1 kg/11.2 lbs
< 30 seconds
< 220 g/7.8 oz.
50 x 28 x 135 (2.0 x 1.1 x 5.3 in)
2.5 m
< 150 g/5.3 oz.
4
Page 5

Performance Specifications
Complies with EN/IEC EN 60601-2-37:2001+A1:2004. ECG measurement follows EN/IEC 60601-227:1994.
Fetal / Maternal
Fetal/Maternal Performance Specifications
Ultrasound Measurement Method Ultrasound Pulsed Doppler
Measurement Range US 50 to 240 bpm
Resolution Display 1 bpm
Printer 1/4 bpm
Jitter @ 200 bpm ≤ 3 bpm
Display Update Rate 1 / second
US Intensity Average output power P = (4.3 ± 0.4) mW
Peak-negative acoustic pressure p_ = (33.9 ± 3.6) kPa
Output beam intensity (Iob)
(= spatial average - temporal average intensity)
Spatial-peak temporal average intensity I
Effective radiating area @ -6 dB 1.81 cm
Signal Quality
Indication
Beat to Beat Change (max.) for Ultrasound 28 bpm
US Frequency 1 MHz ± 100 Hz
US Signal range 3.5 μVpp to 350 μVpp @ 200 Hz
US Burst Repetition Rate 3.0 kHz
US LF Frequency Passband @ -3dB 100 to 500 Hz ± 20%
FMP Signal Range @ 33 Hz 200 μVpp to 40 mVpp
FMP Frequency Passband @ -3dB 10 to 100 Hz
Toco Measurement Method Strain Gauge Sensor Element
Sensitivity 1 unit = 2.5 g
Resolution Display 1 unit
Measurement Range 400 units
Signal Range 0 to 127 Units
Maximum Offset Range -300 units
Baseline Setting 20 units
Update Rate Display 1 / second
Auto Offset Correction 3 seconds after connecting the
Auto Zero Adjust TOCO value is set to zero following a
IUP Measurement Method Passive Resistive Strain Gauge Elements
Measurement Range -100 to +300 mmHg
Signal Range -99 to 127 mmHg
Resolution Display 1 mmHg
Sensitivity 5 μV/V/mmHg
Offset Compensation +100 to -200 mmHg
Baseline Setting 0 mmHg
Accuracy (not including sensor accuracy) ± 0.5% per 100 mmHg
Update Rate Display 1 / second
Auto Offset Correction 3 seconds after connecting the
Poor Empty
Acceptable Two-thirds full
Good Full
Duration ≤ 100 μs
Printer 1/4 unit
Printer ~4 / second
Printer 1/4 mmHg
Printer ~4 / second
I
(2.38 ± 0.75) mW/cm
sata =
= (10.3 ± 2.2) mW/cm
spta
2
transducer, the TOCO value is set to 20
units
negative measurement value for 5
seconds
transducer, the IUP value is set to 0
mmHg
2
2
5
Page 6

Fetal/Maternal Performance Specifications
Direct ECG
and
Maternal ECG
Type DECG Single Lead ECG (derived from Fetal
MECG Single Lead ECG (derived from RA and
Measurement Range 30 to 240 bpm
Resolution Display 1 bpm
Recorder 1/4 bpm
Accuracy ± 1 bpm or 1%, whichever is greater
Filter Bandwidth 0.8 to 80 Hz
Inop Auxiliary Current (Leads Off Detection) < 100 μA
Input Signal Range DECG 20 μVpp to 6 mVpp
MECG 150 μVpp to 6 mVpp
Defibrillator Protection None
ESU Protection None
Fetal Heart Rate (Ultrasound/DECG) Alarm Specifications
FHR Alarm Limits Range Bradycardia
(low limit)
Tachycardia
(high limit)
FHR Alarm Delay Range Bradycardia
(low limit) Delay
Tachycardia
(high limit) Delay
Signal Loss Delay 10 to 300 seconds
Scalp Electrode)
LA electrodes)
60 to 200 bpm
adjustable in 10 bpm steps
Default: 110 bpm
60 to 210 bpm
adjustable in 10 bpm steps
Default: 170 bpm
10 to 300 seconds in steps of 10 s
Default: 240 s
10 to 300 seconds
in steps of 10 s
Default: 300 s
in steps of 10 s
MECG Alarm
Range Adjustment
Specifications
MECG Alarm Limits High Range: 31 to 240
Default: 120 bpm
Low Range: 30 to 235
Default: 50 bpm
Tachycardia Difference to high limit: 0 to 50 bpm
Default: 20 bpm
Clamping at: 150 to 240 bpm
Default: 200 bpm
Bradycardia Difference to low limit: 0 to 50 bpm
Default: 20 bpm
Clamping at: 30 to 100 bpm
Default: 40 bpm
1 bpm steps (30 to 40 bpm)
5 bpm steps (40 to 240 bpm)
5 bpm steps
5 bpm steps
5 bpm steps
5 bpm steps
Noninvasive Blood Pressure
Measurement Validation: In adult mode, the blood pressure measurements determined with this device comply with the American National
Standard for Electronic or Automated Sphygmomanometers (ANSI/AAMI SP10 - 1992) in relation to mean error and standard deviation,
when compared to intra-arterial or auscultatory measurements (depending on the configuration) in a representative patient population. For the
auscultatory reference the 5th Korotkoff sound was used to determine the diastolic pressure.
Complies with IEC 60601-2-30:1999/EN60601-2-30:2000.
6
Page 7

Noninvasive Blood Pressure Performance Specifications
Measurement Ranges Systolic 30 to 270 mmHg (4 to 36 kPa)
Diastolic
Mean
Accuracy Max. Std. Deviation: 8 mmHg (1.1 kPa)
Pulse Rate Range 40 to 300 bpm
Accuracy
(average over
noninvasive blood
pressure measurement
cycle)
Measurement Time Typical at HR > 60bpm
Cuff Inflation Time Typical for normal adult cuff: Less than 10 seconds
Initial Cuff Inflation Pressure 165 ±15 mmHg
Auto Mode Repetition Times 1, 2, 2.5, 3, 5, 10, 15, 20, 30, 45, 60 or 120 minutes
Venipuncture Mode Inflation
Inflation Pressure 20 to 120 mmHg (3 to 16 kPa)
Automatic deflation after 170 seconds
10 to 245 mmHg (1.5 to 32 kPa)
20 to 255 mmHg (2.5 to 34 kPa)
Max. Mean Error: ±5 mmHg (±0.7 kPa)
40 to 100 bpm: ±5 bpm
101 to 200 bpm: ±5% of reading
201 to 300 bpm: ±10% of reading
Auto/manual: 30 seconds (adult)
Maximum time: 180 seconds (adult)
Alarm Specifications Range Adjustment
Systolic Adult: 30 to 270 mmHg (4 to 36 kPa) 10 to 30 mmHg: 2 mmHg (0.5 kPa)
Diastolic Adult: 10 to 245 mmHg (1.5 to 32 kPa)
Mean Adult: 20 to 255 mmHg (2.5 to 34 kPa)
> 30 mmHg: 5 mmHg (1kPa)
Overpressure Settings Adjustment
> 300 mmHg (40 kPa) > 2 sec not user adjustable
SpO
2
Maternal pulse oximetry provides numerics and pulse trace for oxygen saturation (SpO2) of functional
maternal arterial hemoglobin. Heart rate is measures from maternal ECG electrodes (if connected).
Otherwise, the pulse rate is derived from pulse oximetry.
Complies with EN/ISO 9919:2005 (except alarm system; alarm system complies with IEC 60601-249:2001).
Measurement Validation: The SpO2 accuracy has been validated in human studies against arterial blood sample reference measured with a
CO-oximeter. Pulse oximeter measurements are statistically distributed, only about two-thirds of the measurements can be expected to fall
within the specified accuracy compared to CO-oximeter measurements. Display Update Period: Typical: 2 seconds, Maximum: 30 seconds.
Max. with noninvasive blood pressure INOP suppression on: 60 seconds.
SpO2 Performance Specifications
SpO
2
The specified accuracy is
the root-mean-square
(RMS) difference
between the measured
values and the reference
values
Pulse Range 30 to 300 bpm
Range
Accuracy
Resolution
Accuracy
Resolution
0 to 100%
Philips Reusable Sensors:
M1191A, M1191AL, M1191ANL, M1192A, M1192AN= 2% (70% to 100%)
M1191T, M1192T, M1194A, M1194AN = 3% (70% to 100%)
Philips Disposable Sensors with M1943A(L):
M1131A, M1901B, M1903B, M1904B = 3% (70% to 100%)
®
NellcorPB
MAX-A, MAX-AL, MAX-P, MAX-N, D-25, D-20, N-25, OxiCliq A, P, N = 3% (70% to
100%)
1%
±2% or 1 bpm, whichever is greater
1 bpm
Sensors with M1943A(L):
7
Page 8

SpO2 Performance Specifications
Sensors Wavelength range 500 to 1000 nm.
Emitted Light Energy
Pulse Oximeter Calibration Range 70% - 100%
Information about the wavelength range can be especially useful to clinicians (for
instance, when photodynamic therapy is performed).
≤ 15mW
SpO2 Alarm
Range Adjustment Delay
Specifications
SpO
2
Desat 50 to low alarm limit 1% steps
Pulse 30 to 300 bpm 1 bpm steps (30 to 40 bpm)
Tachycardia Difference to high limit 0 to 50 bpm 5 bpm steps max. 14 seconds
Bradycardia Difference to low limit 0 to 50 bpm 5 bpm steps max. 14 seconds
50 to 100% 1% steps (0, 1, 2, 3,... 30) + 4
5 bpm steps (40 to 300 bpm)
Clamping at 150 to 300 bpm
Clamping at 30 to 100 bpm
5 bpm steps
5 bpm steps
seconds
max. 14 seconds
Recorder Specifications
Built-in Thermal Array Fetal Trace Recorder
Mechanism Thermal Array Recorder
Paper & Printing Type Standard Z-fold paper
Standard Speeds (real-time traces) 3 cm/min, 2 cm/min, 1cm/min
ECG Wave Recording
(not real-time)
Paper Advance Up to 20 mm/s
Sensing Optical Reflex Sensor for black page marks
Accuracy
@ 3 cm/min, 2 cm/min, 1 cm/min
Usable Print Width 128 mm
Resolution 8 dots/mm (200 dpi)
Time Delay to see trace on paper <30s @ 1 cm/min
Trace Separation Offset for FHR
(Ultrasound and DECG)
±5 mm/page
Twin FHR2 +20 bpm
Triplet FHR2 +20 bpm
Emulated 25 mm/s
Print speed is variable up to 20 mm/s and
depends on the print load
FHR3 -20 bpm
Ordering Guide and Accessory Options
You can order the Avalon FM30 fetal monitor under the product number M2703A. To order, precede the
required option number with M2703A (for example, M2703A #B71 adds the noninvasive blood pressure
measurement). “K” options let you modify your order. Below you will find tables giving you a quick overview
of the monitor’s standard capablities, and what is available as options. Refer to “Standard Accessories
Included” on page 10 for a list of items shipped as part of the standard configuration. Start-up quantities of
fetal supplies are available in five convenient kits.
All features listed as options can be added at a later time (see “Upgrade Options” on page 14).
8
Page 9

Standard Measurements
Type Description
Standard Fetal
Measurements:
Standard Maternal
Measurements:
*We recommend to switch off FMP during labor.
**You can measure MECG using the “Toco
Accessories” on page 11).
Fetal Heart Rate (FHR) via ultrasound -
Twins monitoring via ultrasound K02
Fetal Movement Profile* (FMP) -
Toco -
FHR via Direct ECG (DECG) -
Intrauterine Pressure (IUP) K03
Maternal ECG** and Heart Rate via MECG electrodes, including MECG
waveform
+
” transducer if you are not already using it to measure DECG. Order M1363A separately (see “MECG
Optional Measurements
Type Description Required Option
Optional Fetal
Measurements:
Optional Maternal
Measurements:
Triple FHR via ultrasound
(Not currently available in USA)
Noninvasive Blood Pressure (NIBP) with
Pulse Rate
Pulse Oximetry (maternal SpO2) together
with NIBP with Pulse Rate
C73 K02
B71 K31
B73 K31 + K91
Recommended
Accessory Option
(See “Accessory Options” on
page 9)
K03
Recommended
Accessory Option(s)
(See “Accessory Options” on
page 9)
Optional Interfaces
Description Required Option
Dual PS/2 Interface
for keyboard and mouse connection
System Interface
(1 x RS232 port and 1 x LAN port)
J22 M8024A #A01)
J70 -
Accessory Options
Option Number Description
K01
K02
K03
K04
K31
K91
The Avalon FM30 comes with one ultrasound transducer. To add a second M2736A ultrasound transducer,
order option K01.
The Avalon FM30 comes with one ultrasound transducer. To add two additional M2736A ultrasound
transducers, order option K02 (only in conjunction with C73).
Order ECG/IUP Patient Module M2738A (includes MECG adapter cable M1363A), using option K03.
Order the Remote Event Marker (9898 031 43411) using option K04.
Order Antimicrobial NIBP Cuff assortment kit with 3 m (10 ft) connection tubing (M1599B) using option
K31:
small adult - M4554A; adult - M4555A; large adult - M4557A
Order reusable Adult Finger SpO2 sensor (M1191AL), cable length 3 m (10 ft), using option K91.
Recommended
Accessory Option
(See “Input Devices” on
page 13)
Paper Scaling Options
Option Number Description
P01
P02
50-210 bpm paper scaling
30-240 bpm paper scaling
9
Page 10

Fetal Supplies Starter Kits
Kit Number Description Contents
862214
862215
862216
862217
862218
Standard Antepartum Kit (North America) Paper (30-240), belts, gel, belts buttons, belt
clips
Standard Antepartum Kit (Europe, Latin America, Asia) Paper (50-210), belts, gel, belts buttons, belt
Standard Antepartum Kit (Japan) Paper (50-210), belts, gel, belts buttons, belt
Intrapartum DECG Kit (single spiral) Fetal scalp electrodes, fetal adhesive pad
Intrapartum DECG Kit (double spiral).
Europe only. Not for USA
clips
clips
electrodes
Fetal scalp electrodes, fetal adhesive pad
electrodes
Standard Accessories Included
Description Quantity
“Toco+” transducer for Toco, DECG, MECG or IUP monitoring 1
Ultrasound transducer 1
Butterfly Belt Kit 2
Fetal paper pack (country-specific, installed) 1
Power cable 1
DECG adapter cable 9898 031 37651 1
Instructions for Use 1
Documentation CD-ROM (includes Service Guide and Instructions for Use) 1
Transducers
Transducer Part Number
Ultrasound transducer M2736A
Toco transducer for monitoring external Toco M2734A
“Toco+” transducer for monitoring Toco, DECG, MECG or IUP M2735A
ECG Patient Module for monitoring maternal heart rate or IUP M2738A
Fetal Accessories
Fetal Accessories Part Number
Belt
(reusable, gray, water
resistant)
Belt
(reusable, brown, contains
latex)
Belt
(disposable, yellow, water
resistant)
Ultrasound gel 12 Bottles 40483A
Belt buttons, pack of 5 M1569A
Butterfly belt clip (pack of 6) 9898 031 43401
DECG Accessories:
New Philips DECG Solution
32 mm wide, 15 m roll M4601A
60 mm wide, 5 belts M4602A
60 mm wide, 15 m roll M4603A
50 mm wide, 5 belts M1562B
50 mm wide, 5 belts M1562A
60 mm wide, 5 belts 1500-0642
60 mm wide, 15 m roll 1500-0643
60 mm wide, pack of 100 M2208A
5 liter refill (with dispenser) for 40483A
Shelf life: 24 months max.
DECG reusable legplate adapter cable (with flushing port) 9898 031 37651
DECG leg attachment electrode for DECG legplate adapter cable 9898 031 39771
DECG fetal scalp electrode: single spiral, worldwide availability 9898 031 37631
DECG fetal scalp electrode: double spiral, Europe only. Not for USA 9898 031 37641
40483B
10
Page 11
Fetal Accessories Part Number
DECG Accessories:
QwikConnect Plus™
Solution
Disposable Koala IUP catheter M1333A
Reusable Koala IUP adapter cable 9898 031 43931
External Marker 9898 031 43411
ECG reusable legplate adapter cable (QwikConnect Plus™) M1362B
ECG leg attachment electrode for DECG legplate adapter cable M1349A
DECG fetal scalp electrode: single spiral, worldwide availability 15133E
DECG fetal scalp electrode: double spiral, Europe only. Not for USA 15133D
Recorder Paper
Supplied in cases of 40 packs. Each pack has 150 numbered pages.
Product
Number
M1910A USA/Canada and Asia 30 - 240 Red/Orange mmHg Yes
M1911A Europe/Japan 50 - 210 Green mmHg and kPa No
M1913A Japan 50 - 210 Green mmHg Yes
M1913J Japan 50 - 210 Green* mmHg Yes
*Normal bradycardia and tachycardia alarm ranges are yellow; severe bradycardia and tachycardia ranges are red.
Geography FHR Scale Grid Color Scale
Units
Highlighted
3cm Lines?
MECG Accessories
Maternal Heart Rate Accessories Part Number
MECG adapter cable M1363A
Foam ECG electrodes, snap-fit, for MECG adapter cable
40493D/E
Noninvasive Blood Pressure Accessories
Adult/Pediatric Multi-Patient Comfort Cuffs and Disposable Cuffs
Patient Category Limb
Circumference
(cm)
Adult (Thigh) 42.0 - 54.0 20.0 M1879A M1576A M1598B (1.5 m)
Large Adult 34.0 - 43.0 16.0 M1878A M1575A
Adult 27.0 - 35.0 13.0 M1877A M1574A
Small Adult 20.5 - 28.0 10.5 M1876A M1573A
Bladder
Width
(cm)
Disposable cuff
Part No.
Reusable cuff
Part No.
Tubing
or
M1599B (3.0 m)
Adult Antimicrobial Coated Reusable cuffs
Patient Category (color) Limb
Circumference
(cm)
Adult Thigh (grey) 45.0 - 56.5 21.0 M4559A M1598B (1.5 m)
Large Adult X-Long (burgundy) 35.5 - 46.0 17.0 M4558A
Large Adult (burgundy) 35.5 - 46.0 17.0 M4557A
Adult X-Long (navy blue) 27.5 - 36.5 13.5 M4556A
Adult (navy blue) 27.5 - 36.5 13.5 M4555A
Small Adult (royal blue) 20.5 - 28.5 10.6 M4554A
Bladder Width
(cm)
Part No. Tubing
or
M1599B (3.0 m)
11
Page 12

Adult Soft Single Patient Single-Hose Disposable Cuffs
Patient Category Limb Circumference
(cm)
Adult (Thigh) 45.0 - 56.5 20.4 M4579A M1598B (1.5 m)
Large Adult X-Long 35.5 - 46.0 16.4 M4578A
Large Adult 35.5 - 46.0 16.4 M4577A
Adult X-Long 27.5 - 36.5 13.1 M4576A
Adult 27.5 - 36.5 13.1 M4575A
Small Adult 20.5 - 28.5 10.4 M4574A
Bladder
Width (cm)
Part No. Tubing
or
M1599B (3.0 m)
SpO2 Accessories
All listed sensors operate without risk of exceeding 41°C on the skin if ambient temperature is below 37°C.
Product
Number
Philips reusable sensors
M1191A Adult sensor (2.0 m cable), for patients over 50 kg. Any finger, except
M1191AL M1191A with longer cable (3.0 m)
M1192A Small adult, pediatric sensor (1.5m cable) for patients between 15 kg and
M1194A Ear sensor (1.5m cable) for patients more than 40 kg.
M1191T Adult sensor (0.45 m), for patients over 50 kg. Any finger, except thumb. Requires M1943A (1.0 m) or M1943AL
M1192T Small adult, pediatric sensor (0.45m cable) for patients between 15 kg and
M1191ANL Special Edition (SE)
M1192AN Special Edition (SE)
M1194AN Special Edition (SE)
Philips disposable sensors. Not available in the USA.
M1904B Identical to OxiMax MAX-A Requires M1943A (1.0 m) or M1943AL
M1903B Identical to OxiMax MAX-P
M1901B Identical to OxiMax MAX-N
Philips disposable sensors. Available worldwide.
M1131A Adult/Pediatric finger sensor (0.45 m cable)
NELLCOR disposable sensors (must be ordered from Nellcor)
OxiMax
MAX-A
OxiMax
MAX-AL
OxiMax
MAX-P
OxiMax
MAX-N
Description Comments
thumb.
50 kg. Any finger except thumb.
Use only on adult patients with FM30
Use only on adult patients with FM30
50 kg. Any finger except thumb.
Use only on adult patients with FM30
Adult sensor (3m cable), for patients over 50 kg. Any finger, except
thumb.
Small adult, pediatric sensor (1.5m cable) for patients between 15 kg and
50 kg. Any finger except thumb.
Use only on adult patients with FM30
Ear sensor (1.5m cable) for patients more than 40 kg.
Use only on adult patients with FM30
Adult finger sensor (patient size >30kg) Requires M1943A (1.0 m) or M1943AL
OxiMax MAX-A with long cable
Pediatric foot/hand sensor (patient size 10-50 kg)
Use only on adult patients with FM30
Adult finger or neonatal foot/hand sensor
(patient size >40 kg or <3 kg)
Use only on adult patients with FM30
No adapter cable required.
(3.0 m) adapter cable.
No adapter cable required.
SE sensors work with FM30, as well as
with OxiMax-compatible SpO
of other Philips monitors.
(3.0 m) adapter cable
Requires M1943A (1.0 m) or M1943AL
(3.0 m) adapter cable
(3.0 m) adapter cable.
versions
2
12
Page 13

Product
Description Comments
Number
Oxisensor II
D-25
Oxisensor II
D-20
Oxisensor II
N-25
OxiCliq A See OxiMax MAX-A Requires M1943A (1.0 m) or M1943AL
OxiCliq P See OxiMax MAX-P
OxiCliq N See OxiMax MAX-N
Extension / Adapter Cables
M1941A
M1943A
M1943AL
OC 3 Adapter Cable for OxiCliq sensors Available from Nellcor.
a. Do not use more than one extension cable with any sensors or adapter cables. Do not use an extension cable with Philips reusable sensors or adapter
a
a
cables with part numbers ending in -L (indicates “Long” version).
Adult sensor (patient size >30kg) Requires M1943A (1.0 m) or M1943AL
Pediatric sensor (patient size 10-50 kg)
Use only on adult patients with FM30
Neonatal/Adult sensor (patient size <3 kg or >40 kg
Use only on adult patients with FM30
Use only on adult patients with FM30
Use only on adult patients with FM30
Extension cable (2 m) For use with Philips reusable sensors
Adapter cable (1.1 m cable) Adapter cable for Philips/Nellcor
a
Adapter cable (3 m cable)
(3.0 m) adapter cable.
(3.0 m) adapter cable together with
OC3 adapter cable.
and adapter cables.
disposable sensors.
Input Devices
Description Option
Slimline keyboard with integrated trackball (includes spill cover) M8024A #A01
Optical mouse
Trackball
Wireless trackball
Hand-Track
M8024A #B01
M8024A #C01
M8024A #C02
M8024A #C03
Mounting Hardware
Mounts, Carts and Rollstands
M2740A #A01 M2740A #A05 M2740A #C01 M2740A #R01 M2740A #U01 M2740A #W01
Flush Wall Mount
for flat wall
mounting
Wall Mount (Arm) Cart with fixed
angle mount and
two drawers
Rollstand with tray Mounting Kit for
Avalon CTS for use
with M2740A #C01
Wall Channel
required for wall
mounts A01 and
A05
13
Page 14

Upgrade Options
Upgrade options are prefixed with M2703AU. For example, to add a sytem interface upgrade, order
M2703AU Option J70.
Upgrade Options
Option Number Option Adds
B71 Noninvasive Blood Pressure. No supplies included.
B72 SpO
B73 Noninvasive Blood Pressure and SpO
C73 Triplets monitoring capability.
J22 Dual PS/2 Interface for connecting a keyboard and mouse.
J70 System Interface: 1 x RS232 port and 1 x LAN port.
SD0 Rel. D.0 Software Upgrade.
(only for monitors that already have Noninvasive Blood Pressure installed). No supplies included.
2
. No supplies included.
2
(Not currently available in USA.)
Required for monitors with Rel. C.0 software for compatibility with Avalon CTS and OB TraceVue via LAN
connection.
14
Page 15

15
Page 16

Philips Medical Systems is part of
Royal Philips Electronics
Interested?
Would you like to know more about our
imaginative products? Please do not hesitate to
contact us. We would be glad to hear from you.
Asia
Tel: +852 2821 5888
Europe, Middle East, Africa
Tel: +31 40 27 63005
Latin America
Tel: +55 11 2125 0764
On the web
www.medical.philips.com
Via e-mail
medical@philips.com
By fax
+31 40 27 64 887
By postal service
Philips Medical Systems
Global Information Center
P.O Box 1168
5602 BD Eindhoven
The Netherlands
North America
Tel: +1 800 229 6417
© 2003-2006 Koninklijke Philips Electronics N.V.
All Rights Reserved.
Printed in The Netherlands.
4522 962 14531/862 * JUN 2006
0366
M2703A complies with the requirements of the
Council Directive 93/42/EEC (Medical Device
Directive).
S
 Loading...
Loading...