Page 1

Philips Fetal Monitors
P
Fetal Monitoring Technology
Application Note
This application note explains how Philips fetal
monitoring technology supports safe and accurate
fetal and maternal monitoring. Four technological
aspects are collected together here:
• Precision Signal Track and Hold
• Cross-Channel Verification
• Fetal Heart Rate Baseline Offset
•Fetal Movement Profile
Precision Signal Track and Hold
This technology allows the monitor to track the fetal
heart rate signal very closely to ensure an accurate
fetal heart rate measurement with almost no gaps.
The signal is monitored with two ultrasound
receiver channels so that two overlapping time
windows can be monitored (ultrasound travelling
time=depth). When the signal is strong in both
windows the optimal monitoring depth is found.
The measurement window stays in this position
while the control window is moved in both
directions to check the strength of the signal at
different depths. When the control window registers
a change in the signal position (due to movement of
the fetus or mother) the measurement window is
moved to the new optimal position. This adaptation
to changing depth happens with each heart beat and
so virtually eliminates gaps in the trace due to lost
signals.
As the signal is so well tracked, the measurement
window around the heart can be made as small as
possible thus reducing the ultrasound energy that
mother and fetus are exposed to.
Ultrasound Crystal Placement for Optimal Geometry
The crystals in the Ultrasound transducer are located
six around the circumference and one in the center.
In this configuration, all lines between crystals are
equidistant. If a line were drawn from crystal to
crystal, a series of equilateral triangles would be
created. This allows the coverage area to be
homogenous, or of equal signal strength throughout.
crystals
A homogenous signal eliminates any "dark spots" in
the beam, which will reduce the number of times the
clinician will need to reposition the transducer. If
there are more than 7 crystals within a transducer
there are no longer equidistant lines between the
crystals and the triangles created are isosceles
triangles. This configuration is unlikely to provide a
homogenous signal, but rather "dark spots" within
the beam.
AD
Page 2
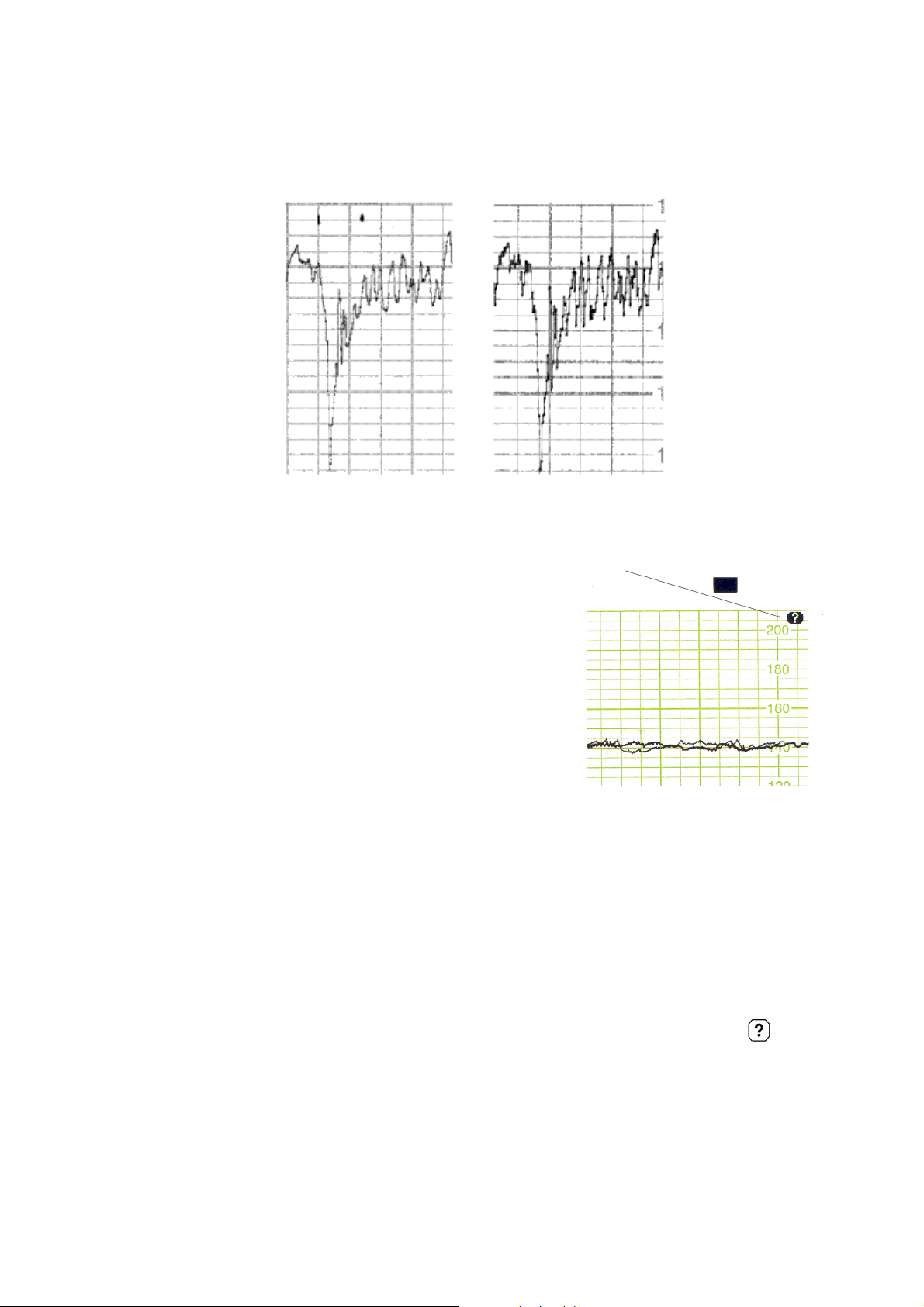
Correlation to Directly Measured FHR
The signal tracking and the homogenous ultrasound
signal from the 7-crystal transducer both contribute
to a fetal heart rate measured by ultrasound which
correlates very highly to the directly measured fetal
heart rate. The degree of smoothing caused by
autocorrelation signal processing is reduced to a
minimum as seen in the following comparison of
traces.
fetal trace from ultrasound
Cross-Channel Verification
Cross-Channel Verification indicates when the same
heart rate is being recorded by different transducers.
When the maternal heart rate and fetal heart rate are
being monitored, Cross-Channel Verification will
alert you when the values are the same. This may be
an indication that the fetus is deceased and the
transducer is picking up a signal from the maternal
heart or a large blood vessel.
Cross-Channel Verification can compare all fetal and
maternal heart rates and indicates when multiple
channels are picking up the same signal. This means
when monitoring multiples and maternal heart rate
simultaneously Cross-Channel Verification will
compare the values from all fetuses and each of these
values with the maternal heart rate.
This technology helps reduce potential legal liability
associated with continuing to monitor an incorrect
heart rate.
When signal “cross-over” occurs, you are alerted
within approximately 60 seconds to check the traces
and potentially reposition the transducers.
Note: Be aware that
a maternal heart rate trace can
exhibit features that are very similar to those of a
fetal heart rate trace, even including
accelerations
and decelerations. Do not rely solely on trace pattern
features to identify a fetal source.
original DECG trace
Figure 1 CCV symbol prints on the trace when two
channels are recording the same heart beat
Cross-Channel Verification Plus (CCV+) indicator
(Series 50 XMO, not available in the USA and Japan)
To warn you if you accidentally record maternal
SpO
instead of fetal SpO2, (because the sensor is
2
facing the uterine wall instead of the fetus) the
monitor compares the heartrate it derives from
DECG on the Cardio 1/Combi channel, (or from
US on the Cardio 2 channel if DECG is not in use)
with the pulse rate it derives from FSpO
. The
2
CCV+ indicator illuminates and is printed on
the trace if the monitor records a pulse rate from
FSpO
and a heart rate from DECG or ultrasound
2
that do not match for more than one minute.
Page 3

FHR Baseline Offset
Fetal Movement Profile
When the baselines of multiple FHR traces are very
similar, independent trend interpretation can be
difficult. To alleviate this, you can offset a baseline
by 20 bpm. You can deactivate the offset feature and
return the FHR trace to its original baseline anytime
you wish.
A section of a twins’ tracing is shown above. It shows
the FHR 1 trace before (Line a) and after (Line b)
the baseline offset feature is activated. To indicate
the recording is in the offset mode, a +20 symbol is
repetitively printed at the top of the trace.
Study finds Philips’ FMP saves clinicians’ and patients’
time, costs, and undue concern
Fetal movement is recognized as an important
indication of fetal condition. Consequently,
recordings of fetal movement are increasingly being
obtained as part of routine antepartum screenings in
obstetricians’ offices, clinics and hospitals.
In use in Europe, the United States and Japan since
1991, Philips Fetal Monitors simultaneously assess
fetal heart rate (FHR), fetal gross body movement
via the Fetal Movement Profile (FMP) parameter,
and uterine activity.
Benefits of the FHR-FMP assessment range from:
• helping clinicians determine the baseline heart
rate - especially in difficult-to-interpret traces, to
• predicting and supervising high risk pregnancies
which involve a number of fetal disorders,
including fetal growth retardation (IUGR).
One of the most important benefits of Philips’ FMP
monitoring is its efficiency and cost effectiveness as
an early screening tool.
Clinical trials confirm that the use of Philips Fetal
Monitors in routine antepartum screenings reduces
the number of patients with “suspicious” FHR test
results, thus eliminating their need for additional
expensive, second-level testing at the hospital. For
the patient, this represents significant savings in
time, cost and concern. It also means cost savings for
the health care system.
Fetal Movement and Fetal Heart Rate
The classic evaluation of fetal movement employs
the mother’s own perception. Clinical trials show
that the Philips Fetal Monitors detect on average 40
percent more movement than perceived by the
mother. Not only can they assure both mother and
clinicians of a more accurate level of fetal movement
detection, but the FMP, recorded simultaneously
with the heart rate, helps in the interpretation of the
FHR trace.
Physicians know that heart rate is directly influenced
by the physical activity of the fetus and they can
assign the baseline heart rate more efficiently with
the Fetal Movement Profile recording.
Furthermore, studies confirm that reduced or lack of
fetal gross body movement often precedes the
change in FHR pattern associated with intrauterine
growth retardation (IUGR)
Page 4

Figure 2 The FMP information provided by the
Philips Fetal Monitors enhances the
clinician’s ability to assign the baseline FHR.
Specifically as shown here (lower trace),
without the assistance of FMP, the baseline
FHR is unclear. With the fetal movement
data (upper trace), the baseline FHR and
accelarations are clearly recognizable.
More Efficient Antepartum Screening
periods are 40 minutes. In 40 to 60 percent of the
tests, acoustic stimulators are used.)
In the 3,500 tests using monitors with FMP, the
“non-reactive” percentage dropped to 3 percent.
After the clinical trials were concluded and the
equipment removed, the reported rate of
“suspicious” nonstress test returned to the higher
level.
Used as a non-invasive tool in nonstress testing,
Philips’ FHR-FMP monitoring employs two of the
five parameters of the Fetal Biophysical Profile
(BPP). This is a second-level testing prescribed for
high-risk pregnancies. These two parameters, along
with the measurement of uterine activity, provide a
more efficient way to assess fetal condition. Clinical
trials have shown a 50 percent reduction in the
number of reported “suspicious” nonstress test
(NST) results.
In a one-year clinical trial conducted at the Women’s
Hospital, University of Southern California School
of Medicine, 3,500 women received antepartum
screening using Philips Series 50 Fetal Monitors.
Normally, 5 to 6 percent of women who undergo the
nonstress FHR test (NST) at Women’s Hospital
present “non-reactive” results; that is, the fetus fails
to show qualifying accelarations of heart rate. (Test
“The fetal Movement Profile is a tool that makes our
testing approach and interpretation better”, states
Dr. Richard H. Paul, Professor of Gynecology at the
University of Southern California School of
Medicine, and Chief of Maternal Fetal Medicine at
the Women’s Hospital. “It has proven to be a great
addition to our antenatal program. Second level
evaluation procedures are expensive and timeconsuming for patients and the hospital alike. With
the FMP monitors, we reduced the number of these
advanced evaluations in half!”
Page 5

Early Detection and Supervision of High-Risk
Pregnancies
Traditionally clinicians have waited until the mother
complains of reduced fetal movement before
initiating further testing such as ultrasound imaging.
However, with routine screening using the Philips
fetal monitors with FMP, fetal movement levels can
be automatically, accurately, and reliably identified.
Patients with FHR-FMP traces indicating little or
no fetal movement can then be referred for fetal BPP
testing, which also assesses fetal tone, fetal breathing
and amniotic fluid volume. This can assist in
identifying potential abnormalities.
With the FHR-FMP test, clinicians can screen
patients for conditions associated with reduced
movement, one of which is intra-uterine growth
retardation (IUGR). Studies show that as many as
50 percent of pregnancies with fetuses below the
10th percentile in weight (considered growth
retarded) go undetected by normal clinical
examination.
Once IUGR is suspected, supervision of the
pregnancy is intensified. FMP fetal monitors can
play an important role in establishing movement
trends during the important growth weeks of
gestation. (IUGR may be associated with
hypertension, antepartum hemorrhage, diabetes,
heavy smoking, chromosomal anomalies and many
other factors.)
At University Women’s clinic in Homburg/Saar,
Germany, Drs W Schmidt and J Gnirs validated the
accuracy of Philips’ FMP parameter. Using a Series
50 fetal monitor, they tracked the test results of 217
patients with normal or pathologic (below the 25th
percentile in fetal weight) pregnancies between 28
and 42 weeks gestation. Comparing them with the
results of independently taken sonographic studies,
they found a 90 percent correlation.
Presented on the following pages are the traces of
one of their patients between gestation weeks 30 and
33. The traces show a steady reduction of
movement, leading to a diagnosis of IUGR.
Sonographical biometry verified the slowing of fetal
growth, showing the fetus’ size falling below the
normal range for its gestation age, beginning in week
22. The baby was delivered by cesarean and required
intensive care.
IUGR Case Study
The following is a case study of a patient monitored
by a Series 50 Fetal Monitor with FMP. Fetal
movement “blocks” and “block clusters” are
automatically recorded on the traces (see highlighted
areas). In weeks 30 to 33, each successive FHR trace
shows a reduction of fetal movement. This fetus was
diagnosed as IUGR. The sonographical biometry
chart verifies the slowing of fetal growth and how
the size of this fetus falls below the normal range for
its gestational age beginning in the 22nd week.
Figure 3 Special thanks to Prof.Dr. W Schmidt and
Dr. J. Gnirs of the University Hospital,
Department of OB/GYN, Homburg/Saar,
Germany for sharing these original traces
with us.
Page 6

Key to German FHR-FMP Traces
UNTERE NORM = Lower than the Normal Value
OLIGOHYDRAMNION = Oligohydramnios
SECTIO CESAREA = Cesarean Section
SSW = Week of Gestation
TOKOGRAMM = Uterine Activity
UNAUFAELLIG = Normal or Inconspicuous
VERLEGUNG NICU = Transfer to NICU
WEIBL. = Female
5.PERZ = 5. PERC
A.ARC (Arteria Arcuata) = Arcuate Artery
ANYDRAMNION = Anydramnios
Page 7

AORTA Fet. = Fetal Aorta
FW = Amniotic Fluid Volume
A.UMB. (Arteria Umbilicalis) = Umbilical Artery
BEL = Breech Presentation
DROHENDE i.u. ASPHYXIE = Fetal Compromise
FET. BEWEGUNGSPROFIL = Fetal Movement
Profile
FHF = FHR
FLOW = Doppler Flow Velocity Analysis
University Women’s
Hospital
Bi-parietal
Diameter
GENETIK = Genetic Diagnosis
INZISUR = Endiastolic Notch
KCTG = FHR with Fetal Movement Profile
parameter
NACH 18 TAGEN EXTUBIERT = Extubation
after 18 days
Breech presentation
transverse abdominal diameter
Gestation
Summary
The fetal Movement Profile, a parameter provided
by Philips in fetal monitors, has been accepted as an
important additional tool for assessing fetal
wellbeing.
Together with the other features described in this
Application Note: Precision Signal Track and Hold,
Cross-Channel Verification and Baseline Offset,
FMP represents a significant contribution to safety
and accuracy in fetal and maternal monitoring.
Date
ultrasound velocity
Page 8

Philips Medical Systems is part of
Royal Philips Electronics
INTERESTED?
Would you like to know more about our
imaginative products? Please do not hesitate to
contact us. We would be happy to provide specific
information about our products and services, or
put you on our mailing list for news about new
product developments, upcoming events or for our
clinical journal, MedicaMundi. We would be glad
to hear from you.
United States:
Philips Medical Systems
Cardiac and Monitoring Systems
3000 Minuteman Road
Andover, MA 01810
(800) 934-7372
Canada:
Philips Medical Systems Canada
281 Hillmount Road
Markham, ON
L6C 2S3
(800) 291-6743
On the web
Contact us through our web site:
www.medical.philips.com
Via e-mail
Our e-mail for all remarks and requests is:
medical@philips.com
By fax
We can be reached at the following fax number:
+31 40 27 64 887
By postal service
Please write to us at the following address:
Philips Medical Systems
Global Information Center
I.B.R.S. / C.C.R.I. Numéro 11088
5600 VC Eindhoven
Pays-Bas / The Netherlands
(no stamp required)
Europe, Middle East and Africa:
Philips Medizin Systeme Böblingen GmbH
Cardiac and Monitoring Systems
Hewlett-Packard Str. 2
71034 Böblingen
Germany
Fax: (+49) 7031 463 1552
Latin America Headquarters:
Philips Medical Systems
1550 Sawgrass Corporate Parkway #300
Sunrise, Fl 33323
Tel: (954) 835-2600
Fax: (954) 835-2626
Asia Pacific Headquarters:
Philips Medical Systems
30/F Hopewell Centre
17 Kennedy Road
Wanc ha i
Hong Kong
Tel: (852) 2821 5888
Fax: (852) 2527 6727
S
© 2002-2005 Koninklijke Philips Electronics N.V.
All rights reserved.
Philips Medizin Systeme Boeblingen GmbH
reserves the right to make changes in specifications or to
discontinue any product at any time without notice or
obligation and will not be liable for any consequences
resulting from the use of this publication.
Published July 2005
4522 962 05621
 Loading...
Loading...