Page 1
1
2
234
DL8760
56789
2 - 3 cm
0.8" - 1.2"
10111213141516
171819
Specifications are subject to change without notice.
© 2015 Koninklijke Philips N.V.
All rights reserved
Philips Consumer Lifestyle BV
Tussendiepen 4, 9206AD Drachten, Netherlands
Fax +31 (0)512594316
4222.100.4492.2 (10/2015)
>75% recycled paper
>75% papier recyclé
Page 2

English
1 2 3
6
7
13
5
8
10
9
11
14
15
12
4
1
Introduction
Congratulations on your purchase and welcome to
Philips! To fully benefit from the support that
Philips offers, register your product at
www.philips.com/welcome.
General
The Philips upper arm blood pressure monitor with
Bluetooth® enables you to perform blood pressure
measurements, heart rate (pulse) measurements,
transmit data via Bluetooth® to your mobile device
and display your personal measurement results in
the Philips HealthSuite health app. The device can
also be used as a standalone device.
This user manual contains important safety infor
mation and provides step-by-step instructions for
using the blood pressure monitor.
Read this information carefully before you use the
device and save it for future reference.
Features:
- 86.5mm×24mm display with white backlight
- Measure-during-inflation technology
- Supports 2 users
Intended use
The Philips upper arm blood pressure monitor is a
digital monitor intended for use in measuring blood
pressure and heart rate in adult patient population
with arm circumference ranging from 22cm to
42cm (about 9-17 inches). The device is intended
to be used in a home environment.
The warning signs and symbols are essential to
ensure that you use this product safely and cor
rectly and to protect you and others from injury.
Below you find the meaning of the warning signs
and symbols on the label and in the user manual.
Symbol for 'follow instructions for use'.
This symbol means that the part of the
device that comes into physical contact
with the user (also known as the applied
part) is of type BF (Body Floating) accord
ing to IEC 60601-1. The applied part is the
cuff.
Symbol for 'the device complies with
European Medical Device Directive
93/42/EEC requirements'. 0344 refers to
the notified body.
Symbol for WEEE, waste electrical and
electronical equipment. Electrical waste
products should not be disposed of with
household waste. Please recycle where
facilities exist. Check with your local
authority or retailer for recycling advice
and see chapter 'Battery recycling'.
This symbol means that this product con
tains batteries which shall not be disposed
of with normal household waste
(2006/66/EC).
Indicates the manufacturer, as defined in
EU Directives 93/42/EEC.
Indicates manufacturing date.
Symbol for 'direct current'.
Symbol for the 'Bluetooth Combination
mark'. The device uses Bluetooth for com
munication.
Indicates the manufacturer's batch code.
Indicates the manufacturer's serial num
ber so that a specific medical device can
be identified.
Fuse T1A/250V Φ3.6*10CCC.
Symbol for 'Class II Equipment'. The
adapter is double insulated (Class II) and
complies with IEC 60601-1.
Symbol for indoor use only.
Page 3

Symbol for 'Including RF transmitter'. This
–20ºC
60ºC
means that this device emits non-ionizing
radiation. All devices with RF transmitters
or that use RF electromagnetic energy
must have a label with this symbol.
Indicates caution.The user should consult
the instructions for use for important cau
tionary information such as warnings and
precautions that cannot, for a variety of
reasons, be presented on the medical
device itself.
This symbol on the device means: pro
tected against access to hazardous parts
with a finger and against vertically falling
water drops when tilted up to 15 degrees.
Indicates the storage and transportation
temperature limits to which the medical
device can be safely exposed: -20°C to
60°C.
Symbol for the 2 year Philips guarantee.
The Green Dot ('Der Grüne Punkt' in Ger
man) is the license symbol of a European
network of industry-funded systems for
recycling the packaging materials of con
sumer goods.
This symbol on the DC charger indicates
that it is TUV certified.
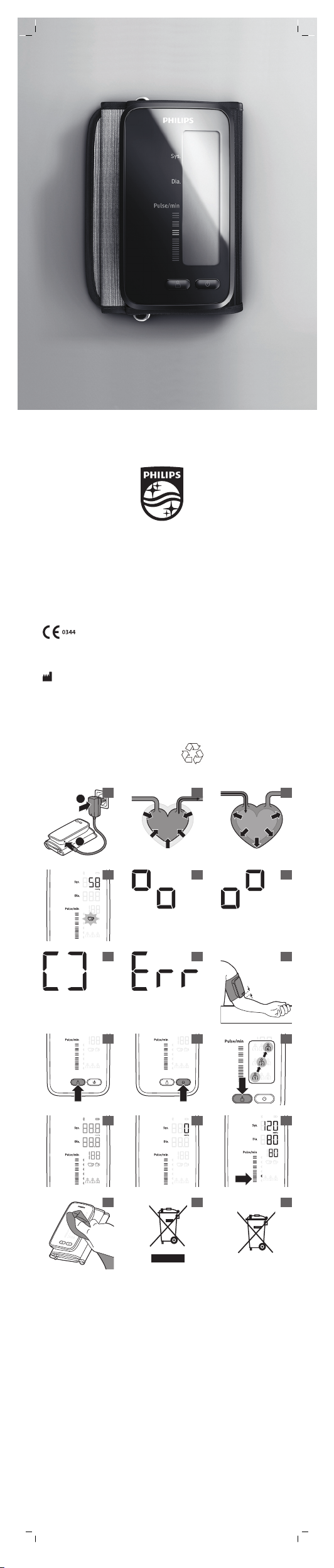
General description (Fig. 1)
1 Socket for DC charger plug
2 Bluetooth® symbol
3 Battery symbol
4 Systolic blood pressure
5 Diastolic blood pressure
6 Heart rate
7 Movement detector
8 Heart rate/irregular heart rate detector
9 User IDs
10 Cuff
11 On button
12 User ID button
13 Blood pressure classification
14 DC plug
15 DC charger
Important safety information
Read this important information carefully before
you use the device and save it for future reference.
Warning
- Please keep the unit out of reach of infants,
children or pets, since inhalation or swallowing
of small parts can be dangerous or even fatal.
- The device is not suitable for measuring the
blood pressure of children.
- The device is not suitable for persons who have
electrical implants.
- Do not use this blood pressure monitor on any
arm where intravascular access or therapy
(such as an intravenous drip or a blood transfu
sion), or an arterio-venous shunt (A-V shunt) is
present. The temporary interference to blood
flow by the blood pressure measurement could
result in injury.
- If you had a mastectomy (breast amputation)
do not use this blood pressure monitor on the
arm on the side of the mastectomy. The inflat
ing cuff can lead to pain, trauma and further
injury in the arm on the side of the mastectomy.
- Consult your doctor if your suffer from illnesses
prior to using the device.
- No modifications of this equipment are allowed.
This may result in increased emissions or
decreased immunity of the device.
- Do not use the blood pressure monitor during
charging as this can cause injury.
- Do not touch the output of the adapter as this
can cause injury.
- Do not dispose of batteries in fire. Battery may
explode or leak.
- If you experience discomfort during a measure
ment, such as pain in the arm or other com
plaints, press the 'on' button to release the air
immediately from the cuff. Loosen the cuff and
remove it from your arm.
- On the rare occasion of a fault causing the cuff
to remain fully inflated during measurement,
open the cuff immediately. Prolonged high
pressure (cuff pressure >300mmHg or constant
pressure >15mmHg for more than 3 minutes)
applied to the arm, may lead to bruises (ecchy
mosis).
- Too frequent and consecutive measurements
could cause disturbances in blood circulation
and injuries.
- Beware of strangulation, particularly for children
and infants due to cables.
- Do not connect the tube to other medical
equipment, as this could lead to dangerous
injuries.
- This device is not intended for patients outside
a home environment.
- Never use any accessories or parts from other
manufacturers or that Philips does not specifi
cally recommend. Using such accessories or
parts could cause a hazardous situation for the
user or damage to the device.
- Only use the accessories and detachable parts
authorized by the manufacturer. The use of
unauthorized parts or accessories may cause
damage to the device or injury to the user.
Caution
- Always check the device before you use it. Do
not use the device if it is damaged, as this may
cause injury.
- The effectiveness of this blood pressure moni
tor has not been established in pregnant
(including pre-eclamptic) women.
Page 4

- Common arrhythmias (such as atrial or ventric
ular premature beats or atrial fibrillation) and
peripheral artery disease / arteriosclerosis can
affect the performance (accuracy) of this blood
pressure monitor. Please consult your doctor
how to best use this blood pressure monitor if
you suffer from any of these conditions.
- Only use this device for its intended purpose as
described in this user manual.
- Do not confuse self-monitoring with self-diag
nosis. This device allows you to monitor your
blood pressure. Do not begin or end medical
treatment based on the measurement results.
Always consult your doctor for treatment
advice.
- Do not take any therapeutic measures on the
basis of a self-measurement. Never change
prescribed medication without consulting your
doctor. Consult your doctor if you have any
questions about your blood pressure.
- If you are taking medication, consult your
physician to determine the most appropriate
time to measure your blood pressure.
- This device is not intended for use on extremi
ties other than the arm or for functions other
than obtaining a blood pressure measurement.
- If the cuff pressure exceeds 40kPa (300mmHg),
the unit will deflate automatically. If the cuff
does not deflate when pressures exceeds
40kPa (300mmHg), detach the cuff from the
arm and press the 'on' button to stop inflation.
- Do not attach the cuff on the same arm on
which other monitoring medical electrical
equipment is attached simultaneously, because
this could cause temporary loss of function of
those simultaneously-used monitoring medical
electrical equipment.
- Never attach the cuff on injured skin, an injured
arm or an arm under medical treatment as this
can cause further injury.
- Do not use the device in case of existing poly
ester or nylon material allergies.
- This device is not washable. Never immerse the
device in water and do not rinse it under the
tap.
- This device is not suitable for continuous moni
toring during medical emergencies or opera
tions.
- This device cannot be used with HF (High Fre
quency) surgical equipment at the same time.
- Never use compressed air, scouring pads, abra
sive cleaning agents or aggressive liquids such
as petrol or acetone to clean the device.
- Do not use the adapter in or near wall sockets
that contain or have contained an electric air
freshener to prevent irreparable damage to the
adapter.
- If the battery can no longer be recharged or
when the device does not function properly
anymore (see 'Specifications'), please contact
the Philips Consumer Care center in your coun
try.
- Keep the device away from fire and heat
sources, as the battery can overheat, causing
fire or bursting. The battery could explode
causing injury or death.
- After charging, remove the small plug from the
device and remove the adapter from the wall
socket.
- The equipment is not AP/APG equipment and
is not suitable for use in the presence of a flam
mable anesthetic mixture with air, with oxygen
or nitrous oxide.
- To avoid measurement errors, do not use the
device near strong electrical or magnetic fields,
for example magnets, radio transmitters,
microwave ovens.
- To avoid measurement errors, do not use the
device near a strong electromagnetic field radi
ated interference signal or electrical fast tran
sient/burst signal.
- Use this device under the right environmental
conditions as indicated in this user manual. If
not, this could affect the performance, lifetime
of the device and measurement results.
- Use a soft cloth to clean the whole unit. Do not
use any abrasive or volatile cleaners.
- Only use the DC charger supplied (model: AC
power adapter KH0601000EW) to charge the
device.
- The device does not need to be calibrated for
two years of normal use. After two years the
measurements may become less accurate.
- If you have any problems with this device, such
as setting up, malfunction, maintaining or using,
please contact Philips Consumer Care.
- Do not open, disassemble or repair the device
yourself. Modification of the device voids your
warranty.
- Report to Philips Consumer Care if any unex
pected operation or events occur.
- Dispose of accessories, detachable parts, and
the ME equipment according to the local guide
lines.
- Do not attempt to replace your blood pressure
monitor's battery. It is built-in and not change
able.
- Avoid charging your blood pressure monitor in
extremely high or low temperatures (see 'Speci
fications').
- Do not clean the blood pressure monitor when
it is being charged. Always unplug the charger
first before cleaning the blood pressure moni
tor.
Compliance with standards
- The device meets the relevant standards for this
type of Class IIa electrical medical equipment
for home use.
- This Philips device complies with all applicable
standards and regulations regarding exposure
to electromagnetic fields and complies with EN
60601-1-2.
Display
Sy
Description Explanantion
m
bol
Page 5

Systolic
+
blood pres
sure
Maximum blood pressure,
see also section systolic and
diastolic pressure.
Diastolic
blood pres
sure
Heart rate Number of heartbeats per
Battery sta
tus
Measure
ment unit
Irregular
heart rate
detector
User IDs Start measurement for
Movement
detector
Blood pres
sure classifi
cation
Bluetooth®
Smart sym
bol
Heart rate
detection
Minimum blood pressure, see
also section systolic and
diastolic pressure.
minute (pulse is typically
equivalent to heart rate).
Indicates status of battery
during charging.
Measurement unit of blood
pressure.
Irregular heart rate detection
during the measurement.
selected user, and transmit
the measurement result.
Moving during the measure
ment will result in an inaccu
rate result.
Classification of measured
blood pressure following
WHO system (see 'Blood
pressure classification').
The device uses Bluetooth
for communication.
Heart rate detection during
the measurement.
Battery status indications
Battery
symbol
Battery status
The battery is almost empty.
The battery is empty.
When you measure 3 times a day starting with a
fully charged battery, the device can be used for
about 20 days until a recharge is needed. In case
of normal use, the battery can be charged around
300 times.
Note: Data will be lost when the battery is com
pletely empty.
Charging
The battery of this device is a built-in rechargeable
li-polymer battery with a capacity of 1000 mAh.
Use the original DC charger supplied to charge the
battery.
When the battery is empty, it takes approx. 2 hours
to fully charge the battery of the device.
1 Put the small plug in the socket of the device
(Fig. 2).
2 Put the adapter in the wall socket.
Battery charging indications
Battery
symbol
Battery charging indication
Battery charging: half full
Battery charging: almost full
Battery fully charged
Using the blood pressure
monitor
This tubeless device uses the oscillometric method
to measure blood pressure and heart rate.
Before every measurement, the unit establishes a
“zero point” equivalent to the atmospheric pres
sure. Then it starts inflating the cuff. During the
measurement, the device detects the pressure
oscillations in the blood vessels generated by the
heart pumping blood through the body. These
pressure oscillations are used to determine systolic
and diastolic blood pressure as well as heart rate.
While measuring heart rate, the device also deter
mines the small variations between the individual
heartbeats. If these variations exceed a predefined threshold, the irregular heart rate detector
symbol lights up.
Systolic and diastolic pressure
The heart consists of two large chambers - the
ventricles - and two smaller chambers - the atria.
The ventricles collect blood from the atria and
expel it towards the peripheral beds of blood ves
sels within the body and the lungs. The atria collect
blood from these peripheral beds and prime the
ventricles.
When the ventricles contract and pump blood out
of the heart, the blood pressure reaches its maxi
mum value in the cycle, which is called systolic
pressure (Fig. 3).
When the ventricles relax and are filled again with
blood, the blood pressure reaches its minimum
value in the cycle, which is called diastolic pressure
(Fig. 4).
Blood pressure classification
Consult a doctor in case of questions about your
blood pressure. Your doctor can inform you:
- About your normal blood pressure range.
- If your measuring result falls out of the range.
- Whether your blood pressure has reached a
dangerous level.
The following table shows the classification system
for the blood pressure measurements used in this
device. This system follows the classification sys
tem of the World Health Organisation (WHO).
Blood pressure classification according to
WHO system*
Page 6

Systolic
pressure
mmHg
Diastolic
pressure
mmHg
Blood
pressure
indicator
³180 ³110
160 - 179 100 - 109 moderate
140 - 159 90 - 99 mild hyper
130 - 139 85 - 89 high to nor
120 - 129 80 - 84 normal
< 120 < 80 optimal
< 100 < 60 low blood
*Source: Chalmers J et al. WHO-ISH Hypertension
Guidelines Committee. 1999 World Health Organi
zation - International Society of Hypertension
Guidelines for the Management of Hypertension. J
Hypertens, 1999, 17:151-185.
severe
hypertension
hypertension
tension
mal blood
pressure
blood pres
sure
blood pres
sure
pressure
red
orange
yellow
green
green
green
green
Irregular heart rate detector
The device is equipped with an irregular heart rate
detector. An irregular heart rate is detected when
the heart rhythm varies above a pre-defined level
while the device is measuring the systolic and
diastolic blood pressure. During each measure
ment, this device records the heartbeat intervals
and calculates the standard deviation. If the stan
dard deviation exceeds a pre-defined threshold,
the irregular heart rate detector symbol lights up
when the measurement results are displayed (Fig.
5).
Caution:The appearance of the irregular heart rate
detector symbol indicates that a heart rate irregu
larity was detected during measurement. Usually
this is not a cause for concern. Due to the irregu
larity in your heart rate the blood pressure mea
surement might not be accurate, i.e. it might not
reflect the 'real' situation in your body. However,
if the symbol appears often, we recommend that
you seek medical advice. Please note that the
device does not replace a cardiac examination.
Preparing for use
Pairing the blood pressure monitor to your mobile device
Note: To switch on the device for the first time,
press the 'on' button for 3 seconds.
Note: Before you use the device for the first time,
remove the protective foil from the display.
The blood pressure monitor is equipped with Blue
tooth Smart. You can receive your personal health
data on a mobile device that is equipped with the
Bluetooth Smart function. Download the Philips
HealthSuite health app from the App store or
Google Play. Use the search term 'Philips Health
Suite health app'. The app is available for iOS 8.0+
and Android 4.4+.
Note: You can only use the Philips HealthSuite
health app to communicate with the device. It is
not possible to use third party applications.
1 Download the Philips HealthSuite health app
on your mobile device, start the Setup wizard
and follow the steps to create a user profile and
add the blood pressure monitor.
2 Make sure the app is active and Bluetooth is on
when pairing is in progress.
Keep the mobile device and the blood pres
-
sure monitor within transmission range (no
more than 5 meters from each other, in the
same room).
3 With the device turned off, press the 'on' button
for 3 seconds, until it turns on in pairing mode.
These symbols are shown on the display
-
alternatively, indicating that the connection
is being established: (Fig. 6) and (Fig. 7).
4 When pairing is successful, the display shows
this symbol: (Fig. 8). The app shows which user
profile is assigned to you.
If the connection fails, the display shows this
-
symbol: (Fig. 9).
The blood pressure monitor has 2 user pro
-
files. If both user profiles are in use, choose
an existing profile to overwrite.
You can also delete both user profiles by
-
pressing and holding the user ID button for
approx. 10 seconds. The display of the
device shows 'del'. All stored date is deleted
and you have to follow step 1-4 to pair and
add a new user.
5 The blood pressure monitor shows the Blue
tooth icon on the display as soon the connec
tion has been established and switches off
automatically after a few seconds.
When the blood pressure monitor is successfully
paired with your mobile device, the blood pressure
monitor automatically transmits your personal
health data to your mobile device via Bluetooth
Smart.
Note: Only when the Philips HealthSuite health
app is active, your personal health data can be
transmitted.
Measuring blood pressure
Tips for proper measurement
- Rest for 5 minutes before you measure your
blood pressure.
- Wait at least 3 minutes between measurements.
This allows your blood circulation to recover.
- For a meaningful comparison, try to measure
under similar conditions. For example, take dai
ly measurements at approximately the same
time, on the same arm, or as directed by your
doctor.
Page 7

- For a good Bluetooth connection between the
blood pressure monitor and your mobile
device, make sure the two are in close distance
and there are no obstacles between the two
devices. We recommend not to have the two
devices further than 5 meters apart.
We do not advise you to take a measure
ment under the following circumstances, as this
may cause inaccurate measurements:
- Within 1 hour after eating or drinking
- Immediately after smoking
- Within 20 minutes after taking a bath
- While you are talking or moving your arm, hand
or fingers
- In a very cold environment
- When you need to urinate
Attaching the cuff
1 Remove all jewelry, such as watches and
bracelets from your left arm.
Note: If your doctor has diagnosed you with
poor circulation in your left arm, use your right
arm.
2 Roll or push up your sleeve to expose the
skin. Make sure your sleeve is not too tight.
3 Hold your arm with your palm facing up and
slide the cuff onto your left upper arm (Fig. 10).
4 Position the lower edge of the cuff about 2-3cm
above the crease of the elbow.
5 Fasten the cuff around your arm, leaving no
extra room between the cuff and your skin. If
the cuff is too loose, the measurement will not
be accurate.
The cuff will not cause any potential sensiti
-
zation or irritation of the skin. The materials
of the cuff have been tested and found to
comply with requirements of ISO
10993-5:2009, ISO 10993-1:2009 and ISO
10993-10:2010.
6 Correct posture for measurement:
Make sure you do not wear tight clothing
-
during measurement.
Sit comfortably with legs uncrossed, feet flat
-
on the floor. Make sure that you sit upright
with your back straight.
The center of the cuff should be at the same
-
level as the heart.
Relax your wrist and hand. Do not bend your
-
wrist back, clench your fist, or bend your
wrist forward.
Start measurement
1 Press the user ID button (Fig. 11) or 'on' button
(Fig. 12) once, to switch on the device. The
device automatically selects the previous user.
To change the user profile, press the user
-
ID button (Fig. 11) and select a different user
(Fig. 13). Make sure the correct user is select
ed, so the measurement data is properly
transmitted and stored. It is not possible to
change a user profile after a measurement.
When the health app is open, the app auto
-
matically selects the correct user profile. In
this case, the user profile can be changed by
either closing the app and reopening it again
with the correct user profile, or by closing
the app and using the user ID button.
Also a guest user can be selected. The guest
-
user is for performing a measurement on
other persons without a user profile in the
health app. Measurements performed when
using the guest user are not stored in the
memory nor transmitted to the app.
2 Attach the cuff to your arm (see 'Attaching the
cuff') and make sure your posture is correct (see
'Tips for proper measurement').
3 Press the 'on' button to start the measurement
(Fig. 12). All display characters are briefly shown
on the display (Fig. 14). The device is ready for
measurement and the number 0 appears (Fig.
15). Inflation of the cuff starts automatically.
During inflation, the unit determines the sys
-
tolic pressure and diastolic pressure as well
as heart rate. This is shown by the heart rate
detection symbol.
The movement detector will light up when
-
movement is detected. This may result in
inaccurate measurement results.
4 When the measurement is finalized, the cuff
deflates and the measurement results are
shown on the display (Fig. 16). To transmit the
measurement results to the app, see section
'Transmit and store personal health data in the
app'.
5 Press the 'on' button to switch off the device.
Note: after 1 minute, the device will turn off
automatically
If, after finishing the first measurement, another
measurement is required, press the user ID button
to select the correct user profile and follow steps
2-7.
Note: Wait at least 3 minutes between measure
ments. This allows your blood circulation to recov
er.
The device can store results of 60 blood pressure
measurements for both user 1 and 2.
Transmit and store personal health data in the app
Note: Your personal measurement data is only
stored and displayed in the Philips HealthSuite
health app.
1 Activate the Philips HealthSuite health app and
Bluetooth on your mobile device directly after a
measurement.
Keep the mobile device and the blood pres
-
sure monitor at transmission distance (no
more than 5 meters from each other, in the
same room).
2 Once successfully connected, the measurement
results are being transmitted to the health app
and the Bluetooth symbol lights up.
- If the data transmission is successful, the mea
surement results are displayed in the health
app.
Page 8

- If the data transmission fails, the Bluetooth
symbol together with 'Err' is shown. The pend
ing measurement data will be transmitted to
your mobile device the next time it connects
with your blood pressure monitor. You can also
try to resend the data:
Activate the health app on your mobile
-
device.
Press the user ID button or 'on' button to
-
switch on the blood pressure monitor.
The measurement results will be automati
-
cally sent to your mobile device if a user
profile has been connected.
When the blood pressure monitor connects
-
via Bluetooth to the app of a user, the
device will automatically select that user and
measurements can only be done for that
user.
Cleaning and storage
Caution: This device is not washable. Never
immerse the device in water and do not rinse it
under running water.
Caution: Avoid violent movements and hard con
tacts with objects.
1 Switch off the device and unplug the adapter
from the wall socket.
2 Use a slightly damp or dry cloth to wipe the sur
face of the display (Fig. 17).
3 Store the device in a cool, dry, and ventilated
environment.. For further information please
refer to the transport and storage specifications
detailed in this manual.
Ordering accessories
To buy accessories or spare parts, visit
www.shop.philips.com/service or go to your
Philips dealer. You can also contact the Philips
Consumer Care Centre in your country (see the
worldwide guarantee leaflet for contact details).
Recycling
- This symbol means that this product shall not
be disposed of with normal household waste
(2012/19/EU) (Fig. 18).
- This symbol means that this product contains a
built-in rechargeable battery which shall not be
disposed of with normal household waste (Fig.
19) (2006/66/EC). We strongly advise you to
take your product to an official collection point
or a Philips service centre to have a profession
al remove the rechargeable battery.
- Follow your country’s rules for the separate
collection of electrical and electronic products
and rechargeable batteries. Correct disposal
helps prevent negative consequences for the
environment and human health.
Removing the rechargeable
battery
Warning: This procedure is irreversible. You
cannot use the device anymore after this pro
cedure.
Note: We strongly advise you to take your product
to an official collection point or a Philips service
centre to have a professional remove the battery.
Caution: Observe basic safety precautions when
you follow the procedure described below. Be
sure to protect your eyes, hands, fingers, and the
surface on which you work.
1 Make sure the rechargeable battery is empty.
2 Open the device.
3 Remove the battery with appropriate tools.
Guarantee and support
Recalibration can be carried out by an appropriate
authority or authorized service center. This calibra
tion will be charged for by said authority.
If you need information or support, please visit
www.philips.com/support or read the separate
worldwide guarantee leaflet.
If you need more information about the app,
please visit www.philips.com/healthprograms
Troubleshooting
This chapter summarises the most common prob
lems you could encounter with the device. If you
are unable to solve the problem with the informa
tion below, visit www.philips.com/support for a list
of frequently asked questions or contact the Con
sumer Care Centre in your country.
Troubleshooting
Problem Possible
cause
Solution
My blood
pressure
fluctuates
throughout
the day.
Fluctuations
Your mea
surement
position, the
conditions
under which
you measure
or the time of
measure
ment, are dif
ferent during
each mea
surement.
of blood
pressure dur
ing the day
are normal.
For a meaningful
comparison, try to
measure under
similar conditions.
For example, take
daily measure
ments at approxi
mately the same
time, on the same
arm, or as directed
by a doctor.
Blood pressure
fluctuates from
minute to minute
and normally
shows a circadian
rhythm over a
24-hour period,
with highest read
ings in the after
noons and lowest
readings at night.
That is why, for
comparable mea
surements, the
measurements
should be taken at
approx. the same
time of day.
Page 9

Problem Possible
cause
Solution
You per
My blood
pressure
measure
ment from
the hospital
is different
from the
measure
ment at
home.
You are using
medication.
formed multi
ple measure
ments direct
ly after each
other.
Multiple vari
ables may
affect your
blood pres
sure such as
the weath
er, emotions
and exercise.
The variations in
blood pressure can
be greater if you
are using medica
tion.
Wait at least 3
minutes between
measurements.
This allows your
blood circulation to
recover.
Pay attention when
you measure your
blood pressure at
home. Check for
instance:
If the cuff is
attached properly.
If the cuff is not too
tight or too loose.
If the cuff is
attached on the
upper arm.
If you feel anxious
or stressed, try to
relax. Take a deep
breath 2-3 times
before you start a
measurement.
Advice: Rest for 5
minutes before you
measure your
blood pressure..
The result is
different
when I per
form mea
surements
on my right
arm.
The blood
pressure
monitor
does not
work when I
press the
'on' button
The light of
the display
dims and a
battery
symbol+Lo
is showing
The display
shows Err
The display
shows E3
The display
shows E10
or E11
The blood
pressure
monitor is
suitable to be
used on both
arms, but the
measurement
results on the
right arm and
left arm will
differ.
The
rechargeable
battery is
empty.
The battery is
low.
Communica
tion error.
The cuff is
not properly
secured.
The device
detected
motion, talk
ing or the
heart rate is
too weak
during the
measure
ment.
For a meaningful
comparison, try to
measure under
similar conditions
and measure on
the same arm
every time.
Recharge the bat
tery (see 'Charg
ing').
Charge the battery
(see 'Charging').
Check if the app is
on and try data
transmission again.
Refasten the cuff,
wait 3 minutes and
then measure
again.
Wait for 3
minutes and then
measure again. Do
not move during
measurement.
The display
shows E20
The display
show E21
The display
shows EExx
Data trans
mission or
pairing
failed.
The device
does not
detect the
heart rate
signal.
The mea
surement
failed.
A system
error
occurred.
Bluetooth is
off.
The Philips
HealthSuite
health app is
off.
The blood
pressure
monitor and
mobile
device are
more than 5
meters away
from each
other.
Make sure the
device is in contact
with the skin.
Loosen the cloth
ing on the arm and
measure again.
Wait for 3 minutes
and then measure
again.
Retake the mea
surement. If the
problem per
sists, contact the
Philips Consumer
Care Center in your
country.
Turn on Bluetooth
on your mobile
device.
Press the icon on
your mobile device
to activate the
health app.
Place your mobile
device closer to the
blood pressure
monitor.
You selected
the wrong
profile on the
blood pres
sure monitor.
Select the correct
user profile on the
blood pressure
monitor before
your measurement.
Otherwise the data
cannot be trans
mitted to your app.
Repeat the mea
surement with the
correct profile
selected
Specifications
Power supply 3.7V 1000mAH built-in
rechargeable li-polymer
battery, 6V 1A DC charger
Page 10

Display Display with white
Measurement method Oscillometric method
LED backlight
Visible area = 86.1mm (L)
x 24mm (W)
Measurement range Rated cuff pressure:
Accuracy Pressure: 5°C-40°C with
Normal operating
condition
Storage and trans
portation conditions
Measurement perime
ter of the upper arm
Net weight Approx. 265g
External dimensions Approx.
Accessories DC charger, user manual
0kPa-40kPa
(0mmHg-300mmHg).
Measurement pressure:
5.3kPa-30.7kPa
(40mmHg-230mmHg).
Heart rate: 40-199 beats
per minute
in ±0.4kPa (3mmHg)
heart rate: ±5% of mea
surement result on dis
play
Temperature: 5°C to
40°C. Relative humidity:
≤85%RH. Atmospheric
pressure: 86kPa to
106kPa
Temperature: -20°C to
60°C. Relative humidity:
10% to 93%. Atmospheric
pressure: 50kPa to
106kPa
About 22cm-42cm
130.9mm×73mm×29.4m
m
Mode of operation Continuous operation
Degree of protection Type BF applied part
Protection against
ingress of water
Device classification Battery Powered Mode:
Caution: No modification of this equipment is
allowed.
IP22, This means: pro
tected against access to
hazardous parts with a
finger and against verti
cally falling water drops
when tilted up to 15
degrees.
Internally Powered ME
Equipment. DC charger
charged mode: Class II
ME Equipment
Electromagnetic emissions
and immunity
The device is approved according to EMC safety
standard EN 60601-1-2. It is designed to be used in
typical domestic environments.
EMC Guidance
- The Blood Pressure Monitor needs special pre
cautions regarding EMC and needs to be
installed and put into service according to the
EMC information provided in the accompanying
documents.
- Wireless communications equipment such as
wireless home network devices, mobile phones,
cordless telephones and their base stations,
walkie-talkies can affect this equipment and
should be kept at least a distance d = 3.3 m
away from the equipment.
Note: As indicated in IEC 60601-1-2:2007 for ME
equipment, a typical cell phone with a maximum
output power of 2 W yields d = 3.3 m at an immuni
ty level of 3V/m.
Guidance and manufacturer's declara
tion – electromagnetic emissions - for
all ME equipment and ME systems
Guidance and manufacturer’s declaration – elec
tromagnetic emissions
The device is intended for use in the electromag
netic environment specified below. The customer
or the user of the device should assure that it is
used in such an environment.
Emissions test Com
pliance
Electromagnetic
environment - guid
ance
RF emissions
CISPR 11
RF emissions
CISPR 11
Harmonic emis
sions IEC
61000-3-2
Voltage fluctua
tions/flicker
emissions IEC
61000-3-3
Group2The device must emit
Class B
Not
applica
ble
Not
applica
ble
electromagnetic
energy in order to
perform its intended
function. Nearby
electronic equipment
may be affected.
Guidance and manufacturer's declara
tion – electromagnetic immunity – for
all ME equipment and ME systems
Guidance and manufacturer’s declaration – elec
tromagnetic immunity
The device is intended for use in the electromag
netic environment specified below. The customer
or the user of the device should assure that it is
used in such an environment.
Page 11

Immu
nity test
IEC
60601
test
level
Com
pliance
level
Electromagnetic
environment guidance
Electro
static
dis
charge
(ESD)
IEC
61000-
4-2
Electri
cal fast
tran
sient/b
urst IEC
61000-
4-4
Surge
IEC
61000-
4-5
Voltage
dips,
short
interrup
tions
and
voltage
varia
tions on
power
supply
input
lines IEC
61000-
4-11
±6 kV
contact
±8 kV air
±2 kV for
power
supply
lines
±1 kV for
input/o
utput
lines
±1 kV
line(s) to
line(s)
±2 kV
line(s) to
earth
<5% UT
(>95%
dip in
UT) for
0.5
cycle
40% UT
(60%
dip in UT
) for 5
cycles
70% UT
(30% dip
in UT )
for 25
cycles
<5% UT
(>95%
dip in UT
) for 5 s
±6 kV
contact
±8 kV
air
±2 kV
for
power
supply
lines
±1 kV
line(s)
to
line(s)
<5% UT
(>95%
dip in
UT) for
0.5
cycle
40% UT
(60%
dip in
UT ) for
5
cycles
70% UT
(30%
dip in
UT ) for
25
cycles
<5% UT
(>95%
dip in
UT ) for
5 s
Floors should be
wood, concrete or
ceramic tile. If
floors are covered
with synthetic
material, the rela
tive humidity
should be at least
30%.
Mains power qual
ity should be that
of a typical com
mercial or hospital
environment.
Mains power qual
ity should be that
of a typical com
mercial or hospital
environment.
Mains power qual
ity should be that
of a typical com
mercial or hospital
environment. If the
user of the device
requires continued
operation during
power mains
interruptions, it is
recommended
that the device be
powered from an
uninterruptible
power supply or a
battery.
Power
fre
quency
(50/60
Hz)
magnet
ic field
IEC
61000-
4-8
Note: UT is the AC mains voltage prior to applica
tion of the test level.
3A/m 3A/m Power frequency
magnetic fields
should be at levels
characteristic of a
typical location in
a typical commer
cial or hospital
environment.
Guidance and manufacturer's declara
tion – electromagnetic immunity –for
ME equipment and ME systems that
are not life supporting
Guidance and manufacturer’s declaration – elec
tromagnetic immunity
The device is intended for use in the electromag
netic environment specified below. The customer
or the user of the device should assure that it is
used in such an environment.
IMMUNI
TY test
Conduct
ed RF
IEC
61000-4
-6
Radiated
RF
IEC
61000-4
-3
IEC
60
601
TES
T
LEV
EL
3
Vrms
150
kHz
to
80
MHz
3
V/m
80
MHz
to
2.5
GHz
Com
Electromagnetic
pli
environment - guid
anc
ance
e
lev
el
3
Portable and mobile
Vrms
RF communications
equipment should be
used no closer to any
part of the device,
including cables, than
the recommended
separation distance
calculated from the
equation applicable to
the frequency of the
transmitter.
3
V/m
Recommended sepa
ration distance:
d = 1.167 ÖP
d = 1.167 ÖP 80 MHz to
2.5 GHz
d = 2.333 ÖP 800 MHz
to 2.5 GHz
where P is the maxi
mum output power
rating of the transmit
ter in watts (W)
according to the trans
mitter manufacturer
and d is the recom
mended separation
distance in metres (m).
Field strengths from
fixed RF transmitters,
as determined by an
electromagnetic site
survey (a), should be
less than the compli
ance level in each fre
quency range (b).
Interference may occur
in the vicinity of equip
ment marked with the
following symbol:
NOTE 1 At 80 MHz and 800 MHz, the higher fre
quency range applies.
Page 12

NOTE 2 These guidelines may not apply in all situ
ations. Electromagnetic propagation is affected by
absorption and reflection from structures, objects
and people.
(a) Field strengths from fixed transmitters, such as
base stations for radio (cellular/cordless) tele
phones and land mobile radios, amateur radio, AM
and FM radio broadcast and TV broadcast cannot
be predicted theoretically with accuracy. To assess
the electromagnetic environment due to fixed RF
transmitters, an electromagnetic site survey should
be considered. If the measured field strength in the
location in which the device is used exceeds the
applicable RF compliance level above, the device
should be observed to verify normal operation. If
abnormal performance is observed, additional
measures may be necessary, such as re-orienting
or relocating the device.
(b) Over the frequency range 150 kHz to 80 MHz,
field strengths should be less than 3V/m.
Recommended separation distances between portable and mobile RF communications equipment and the ME equipment or ME system – for ME equipment and ME systems that are not life supporting
Recommended separation distances between
portable and mobile RF communications equip
ment and the device.
The device is intended for use in an electromag
netic environment in which radiated RF distur
bances are controlled. The customer or the user of
the device can help prevent electromagnetic inter
ference by maintaining a minimum distance
between portable and mobile RF communications
equipment (transmittters) and the device as rec
ommended below, according to the maximum out
put power of the communications equipment.
Separation distance according to
frequency of transmitter (m)
Rated
maximum
output
power of
transmitter
(W)
0.01 0.117 0.117 0.233
0.1 0.369 0.369 0.738
1 1.167 1.167 2.333
10 3.690 3.690 7.378
100 11.67 11.67 23.33
For transmitters rated at a maximum output power
not listed above, the recommended separation
distance d in metres (m) can be estimated using
the equation applicable to the frequency of the
transmitter, where P is the maximum output power
rating of the transmitter in watts (W) according to
the transmitter manufacturer.
NOTE 1 At 80MHz and 800MHz, the separation
distance for the higher frequency range applies.
NOTE 2 These guidelines may not apply in all situ
ations. Electromagnetic propagation is affected by
absorption and reflection from structures, objects
and people.
150 kHz to
80 MHz
d = 1.167 Ö
P
80 MHz
to 800
MHz
d = 1.167 Ö
P
800 MHz
to 2.5 GHz
d = 1.167 Ö
P
 Loading...
Loading...