Philips 862474, 862478 User Manual

Philips C3 Patient Monitor
C3 Service Guide
Models: 862474, 862478
989803129451
Part Number 989803129451
Printed in the U.S.A. March 2003
Edition 1

About this Manual
Proprietary Information
This document contains proprietary information, which is protected by copyright. All Rights
Reserved. Reproduction, adaptation, or translation without prior written permission is
prohibited, except as allowed under the copyright laws.
Philips Medical Systems
3000 Minuteman Road
Andover, MA 01810-1085
(978) 687-1501
Publication number
989803129451
Printed in USA
Warranty The information contained in this document is subject to change without notice.
Philips Medical Systems makes no warranty of any kind with regard to this material,
including, but not limited to, the implied warranties or merchantability and fitness for Philips
Medical Systems shall not be liable for errors contained herein or for incidental or
consequential damages in connection with the furnishing, performance, or use of this material.
Copyright Copyright © 2003 Philips Electronics North America Corporation
Microstream
®
is a registered trademark of Oridion Medical Inc.
Printing History
Additional Documents
New editions of this document incorporate all material updated since the previous edition.
Update packages may be issued between editions and contain replacement and additional
pages to be merged by a revision date at the bottom of the page. Pages that are rearranged due
to changes on a previous page are not considered revised.
The documentation printing date and part number indicate its current edition. The printing
date changes when a new edition is printed. (Minor corrections and updates that are
incorporated at reprint do not cause the date to change.) The document part number changes
when extensive technical changes are incorporated.
First Edition............................................................... March 2003
Use this document in conjunction with the C3 Instructions for Use guide (pn
989803228691).
ii
Safety
All warnings, precautions and notes are located in the chapters that follow. You must read all
of this safety information before you begin monitoring with your C3 patient monitor.
Safety Standards
CE Marking
Europe The following products and accessories from Philips Medical Systems carry the CE mark
The C3 Patient Monitor is compliant with the following safety standards:
• UL 2601-1
• CAN/CSA C22.2 No. 601.1-M90
• EN60601-1
• EN60601-2-27
• EN60601-2-30
• EN60601-2-49
•EN865
to Council Directive 93/42/EEC.
0123
C3 Products: - 862474 - 862478
Accessories: - 21075A - 21076A - 21078A - 21090A
- M1837A - M4820A - M4818A
The following accessories from Philips Medical Systems carry one of the following CE
marks: or to Council Directive 93/42/EEC.
Accessories: - M1191A - M1192A - M1194A - M1941A
- 40493D - 40493E - M1980A - M1981A
- M1510A - M1550C - M1611A - M1613A
- M1615A - M1879A - M1878A - M1877A
- M1876A - M1875A - M1874A - M1576A
- M1575A - M1574A - M1573A - M1572A
- M1571A - M1577A - M1578A - M1579A
- 40401E - 40401D - 40401C - 40401B
- 40401A - M1598B - M1599B - M2526A
- M2525A - M2524A - M2528A - M2522A
- M2521A - M2520A - M1920A - M1922A
- M1921A - M4816A - M4817A
0366
Accessories from companies other than Philips Medical Systems carry CE markings
appropriate to the accessory.
Additional accessories not identified above fall outside the definition of a medical device.
The Former Agilent Technologies’ Healthcare Solutions Group is now a part of Philips
Medical Systems. Some accessories may still be branded with the Agilent name.
iii

Authorized EU-representative: Philips Medizinsystems Böblingen GmbH, Hewlett Packard
Str., 71034, Böblingen Germany
Note
United States
Canada This ISM device complies with Canadian ICES-001.
United States Federal Law restricts this device to sale by or on the order of a physician.
Cet appareil ISM est conforme a la norme NMB-001 du Canada.
Philips Software License Terms
ATTENTION: USE OF THE SOFTWARE IS SUBJECT TO THE PHILIPS SOFTWARE LICENSE
TERMS SET FORTH BELOW. USING THE SOFTWARE INDICATES YOUR
ACCEPTANCE OF THESE LICENSE TERMS. IF YOU DO NOT ACCEPT THESE
LICENSE TERMS, YOU MAY RETURN THE SOFTWARE FOR A FULL REFUND. IF
THE SOFTWARE IS BUNDLED WITH ANOTHER PRODUCT, YOU MAY RETURN
THE ENTIRE UNUSED PRODUCT FOR A FULL REFUND.
PHILIPS SOFTWARE LICENSE TERMS
The following License Terms govern your use of the accompanying Software unless you have
a separate signed agreement with Philips Medical Systems.
License Grant. Philips Medical Systems grants you a license to Use one copy of the
Software. "Use" means storing, loading, installing, executing or displaying the Software. You
may not modify the Software or disable any licensing or control features of the Software. If
the Software is licensed for "concurrent use", you may not allow more than the maximum
number of authorized users to Use the Software concurrently.
Ownership. The Software is owned and copyrighted by Philips or its third party suppliers.
Your license confers no title to, or ownership in, the Software and is not a sale of any rights in
the Software. Philips’ third party suppliers may protect their rights in the event of any
violation of these License Terms.
Copies and Adaptations. You may only make copies or adaptations of the Software for
archival purposes or when copying or adaptation is an essential step in the authorized Use of
the Software. You must reproduce all copyright notices in the original Software on all copies
or adaptations. You may not copy the Software onto any public network.
No Disassembly or Decryption. You may not disassemble or decompile the Software unless
Philips prior written consent is obtained. In some jurisdictions, Philips consent may not be
required for limited disassembly or decompilation. Upon request, you will provide Philips
with reasonably detailed information regarding any disassembly or decompilation. You may
not decrypt the Software unless decryption is a necessary part of the operation of the
Software.
iv

Transfer. Your license will automatically terminate upon any transfer of the Software. Upon
transfer, you must deliver the Software, including any copies and related documentation, to
the transferee. The transferee must accept these License Terms as a condition to the transfer.
Termination. Philips Medical Systems may terminate your license upon notice for failure to
comply with any of these License Terms. Upon termination, you must immediately destroy
the Software, together with all copies, adaptations and merged portions in any form.
Export Requirements. You may not export or re-export the Software or any copy or
adaptation in violation of any applicable laws or regulations.
U.S. Government Restricted Rights. The Software and any accompanying documentation
have been developed entirely at private expense. They are delivered and licensed as
"commercial computer software" as defined in DFARS 252.227-7013 (Oct. 1988), DFARS
252.211-7015 (May 1991) or DFARS 252.227-7014 (Jun. 1995), as a "commercial item" as
defined in FAR 2.101(a), or as "Restricted computer software" as defined in FAR 52.227-19
(Jun. 1987)(or any equivalent agency regulation or contract clause), whichever is applicable.
You have only those rights provided for such Software and any accompanying documentation
by the applicable FAR or DFARS clause or the Philips standard software agreement for the
product involved.
v

Text
Conventions
The following conventions for Notes, Cautions, and Warnings are used in this manual.
WarningWarning
A Warning calls attention to a condition or possible situation that could cause injury to
the user and/or patient.
Caution
A Caution calls attention to a condition or possible situation that could damage or destroy the
product or the user’s work.
Note
A Note calls attention to an important point in the text.
Explanation of Symbols
Symbols on products and packaging mean the following. All symbols found in this section are
used this Instructions For Use guide.
Reference Number
Serial Number
0123
5% to 95% RH
CE Marking
Humidity
vi
Temperature Limits
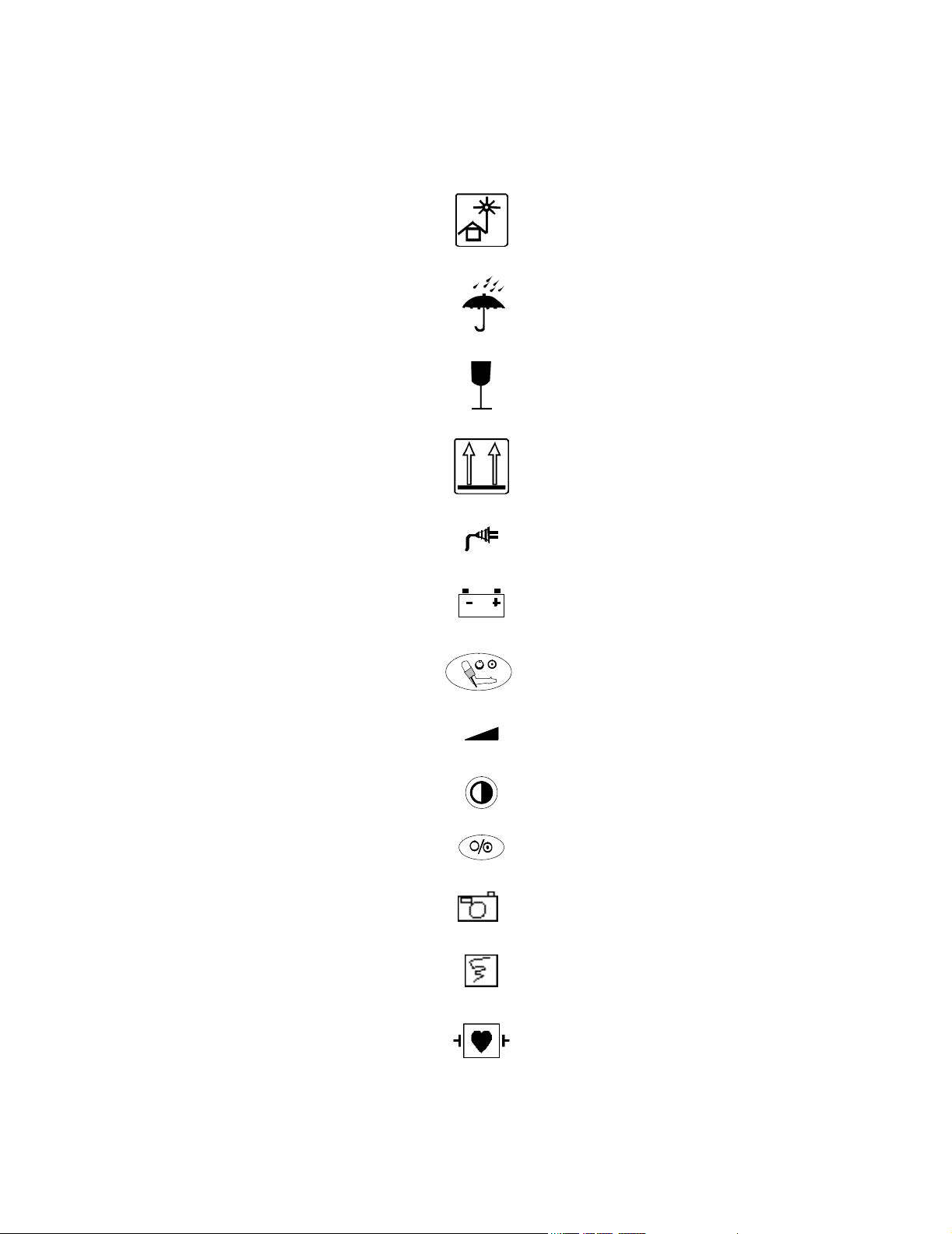
Keep out of Sun
Keep Dry
Fragile
Keep Upright
AC LED
Battery LED
NBP
Volume
Contrast
On/Standby
Snapshot
Continuous
Defibrillator-proof type CF equipment
vii
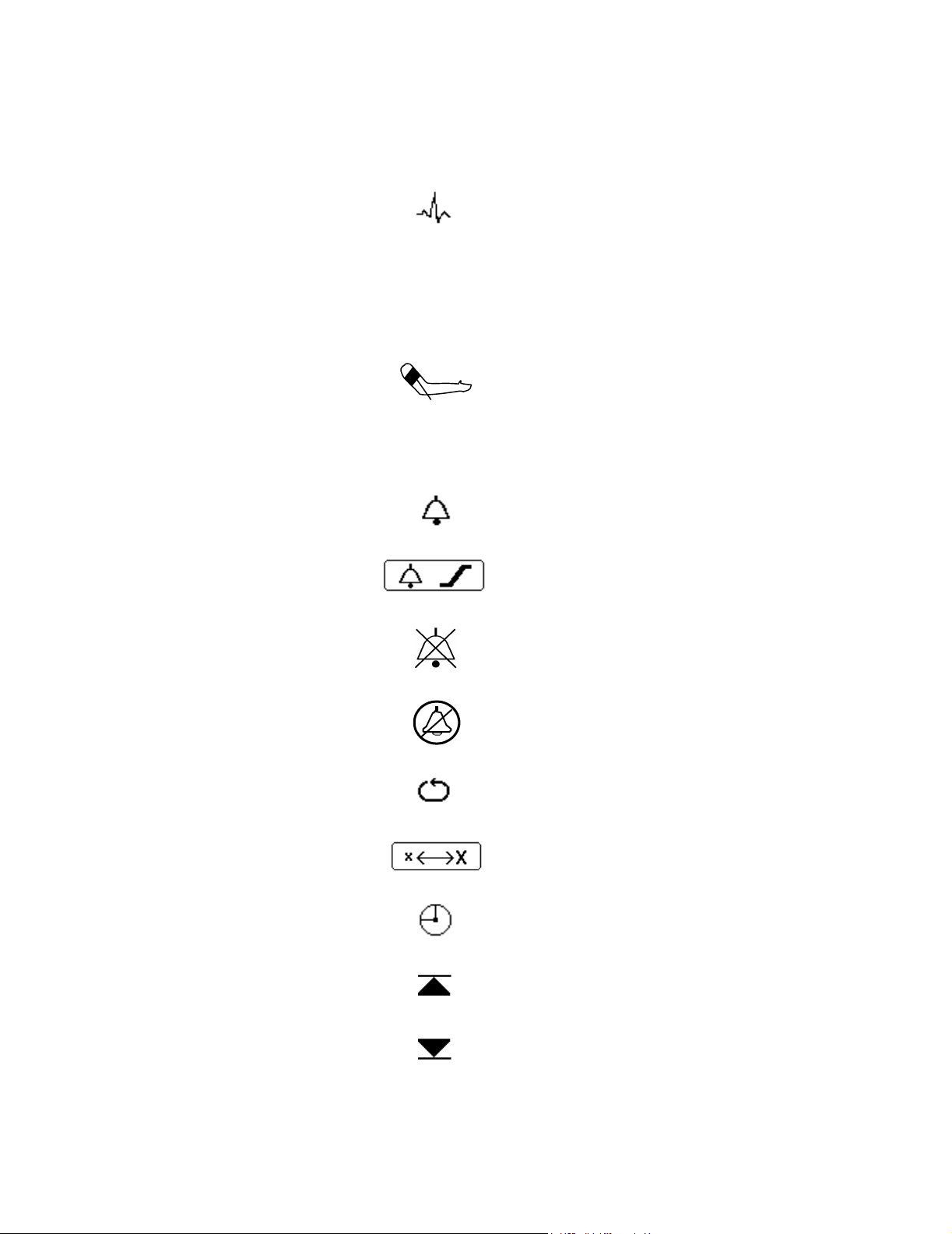
ECG
T
SpO
CO
2
2
Temperature
SpO
2
NBP
Carbon Dioxide
Alarm
Alarm Limits Menu
Audio Off
Silence/Reset
viii
NBP Automatic Interval Mode
Big Numbers
Clock
Up Alarm Arrow Limit
Down Alarm Arrow Limit

Empty Battery
Perfusion Indicator Bar
Heart Rate
Powering Down
Heart Rate taken from ECG
Heart Rate taken from NBP
Heart Rate taken from SpO
Respiration
Stat Mode
Audio Pause
Setup Menu
ECG Size Bar
RF Interference
2
ix
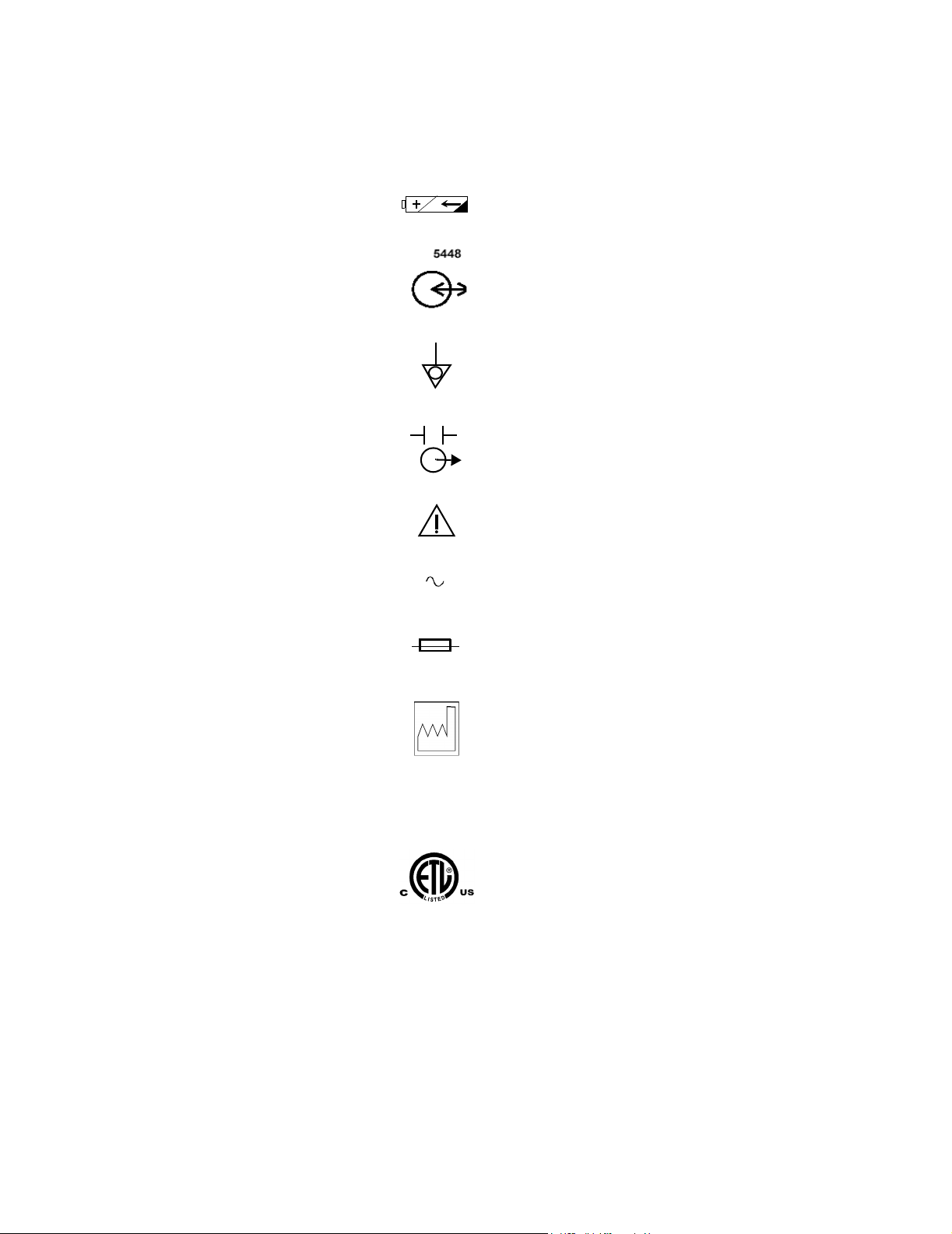
T1AL 250V
Battery Compartment
RS-232 I/O
Equipotential Grounding Post
Defib Sync
Attention, consult accompanying documents
Alternating Current
Fuse Type
x
YYYY-XX
IPX1
Date of Manufacture (Y= year; X = Month)
Drip-proof
ETL Mark

Sales and Support Offices
Please call your local Philips Medical Systems sales office listed in your telephone directory,
or a Philips Medical Systems regional office listed below for the location of your nearest sales
office or for information on how to contact the Philips Response Center.
CORPORATE HEADQUARTERS:
Philips Medical Systems
Netherlands B.V.
Postbus 10.000
5680 DA Best
Netherlands
CORPORATE HEADQUARTERS:
Philips Medical Systems
3000 Minuteman Road
Andover, MA 01810
United States
(800) 934-7372
CANADA:
Philips Medical Systems Canada
281 Hillmount Road
Markham, ON
L6C 2S3
(800) 291-6743
EUROPE, MIDDLE EAST AND AFRICA:
Philips Medizinsysteme Böblingen GmbH
Cardiac and Monitoring Systems
Hewlett-Packard Str. 2
71034 Böblingen
Germany
Fax: (+49) 7031 463 1552
xi

LATIN AMERICA HEADQUARTERS:
Philips Medical Systems
1550 Sawgrass Corporate Parkway #300
Sunrise, FL 33323
Telephone: 954-835 2600
Fax: 954-835-2626
ASIA PACIFIC HEADQUARTERS:
Philips Medical Systems
30/F Hopewell Centre
17 Kennedy Road
Wanchai
Hong Kong
Tel: (852) 2821 5888
Fax: (852) 2527 6727
xii

Contents
1. Overview . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 1-1
General Safety Information . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .1-2
Introduction . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .1-3
C3 Patient Monitor Description . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .1-3
Features and Options . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .1-4
Front Panel Description . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .1-4
Front of Monitor . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .1-5
Rear of Monitor . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .1-6
Screen Display . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .1-7
Additional Documentation . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .1-8
2. Site Preparations. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 2-1
General Site Preparation Safety Information . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .2-2
Site Preparation Guidelines . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .2-3
Warning, Cautions and Safety Precautions . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .2-3
Patient Safety . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .2-3
Patient Leakage Current . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .2-3
Preparing to Use the Monitor. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .2-4
Power Source Requirements. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .2-4
Protecting Against Electrical Shock . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .2-4
Equipotential Grounding. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .2-4
Combining Equipment . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .2-4
Disposing of the Monitor . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .2-5
Unpacking the Monitor . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .2-5
Checking the Shipment . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .2-5
Returning System Components . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .2-6
Mounting . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .2-7
Warnings, Cautions, and Safety Precautions Relating to Wall Mount Installation . . . . . . . . . . . .2-8
Mounting the GCX Wall Channel . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .2-9
Attaching the Mounting Plate to the Monitor . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .2-9
Attaching the Monitor to the Wall Channel. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .2-10
Contents-1

3. Maintaining the Monitor . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 3-1
Maintenance Safety Information. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .3-2
Objectives . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 3-3
Concepts . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 3-3
Light Maintenance . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 3-3
Battery Conditioning . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 3-3
Recommendations for Maintenance Frequency . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 3-4
Maintenance Checklist . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 3-5
Inspecting the Monitor . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 3-6
Inspecting the Cables and Cords . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 3-6
Cleaning . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 3-7
Battery Maintenance . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 3-7
About the Battery . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 3-7
Battery Guidelines . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 3-8
Checking the Battery Status. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 3-8
Identifying Battery Strength. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 3-9
Charging the Battery . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 3-9
Conditioning a Battery . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 3-10
Why is Battery Conditioning Necessary?. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 3-10
When Should Battery Conditioning be Performed? . . . . . . . . . . . . . . . . . . . . . . . . . . . 3-10
How to Condition a Battery . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 3-10
Battery INOP Messages . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 3-11
4. Testing the Monitor . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 4-1
Testing Safety Information . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 4-2
Objectives . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 4-3
Concepts . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 4-3
Functionality Assurance. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .4-3
Preventative Maintenance . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 4-3
Performance and Safety Tests . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 4-3
Testing Checklist. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 4-4
Test Reporting. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 4-5
Recommendations for Testing Frequency. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 4-6
Test Map . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 4-7
Equipment . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 4-8
Serial Numbers . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 4-9
Passwords . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 4-9
Visual Test. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 4-10
Power On Test. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 4-10
Functionality Assurance Tests . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .4-11
Contents-2

Performance Assurance Test . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .4-11
Power-On Self-Test . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .4-11
Alarm Test . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .4-12
Volume Control Test . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .4-12
Preventative Maintenance Tests. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .4-14
Documenting NBP Test Results . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .4-15
Pneumatic System Functionality . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .4-16
To Zero the Simulator . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .4-16
Pressure Transducer Accuracy Test . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .4-17
Pneumatic Leakage Test . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .4-17
Inflation Rate Test . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .4-18
Over-Pressure Test . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .4-19
Deflation Rate Test . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .4-20
Basic Pneumatic Leakage (BPL) Test . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .4-21
Performance Procedures . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .4-22
Battery Performance Test . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .4-22
Temperature Test . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .4-23
ECG/Respiration Performance . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .4-25
ECG Test. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .4-25
Respiration Test . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .4-26
SpO2 Performance . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .4-27
Dynamic Operating Range Test . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .4-27
LED Excitation Test . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .4-28
CO2 Performance . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .4-29
Documenting CO2 Test Results . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .4-30
Barometric Pressure Check and Calibration . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .4-31
Leakage Check. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .4-32
Pump Check. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .4-33
Flow Rate Check and Calibration . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .4-33
CO2 Gas Measurement Calibration Check. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .4-34
Calibration Verification . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .4-35
Reset Pump Operating Time Counters . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .4-35
Serial Interface and Nurse Call Signal Test . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .4-36
ECG Sync Test . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .4-38
Patient Safety Tests . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .4-39
Ground Integrity . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .4-39
Electrical Leakage. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .4-41
Earth Leakage Current . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .4-41
Enclosure Leakage Current . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .4-42
Patient Leakage Current . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 4-43
Contents-3

Patient Leakage Current, with Mains Voltage on the Applied Part . . . . . . . . . . . . . . . 4-44
5. Configuring the Power-up Defaults Menu . . . . . . . . . . . . . . . . . . . . . . . . . . . . 5-1
General Safety Information. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 5-2
Power-Up Defaults Menu . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 5-3
Menu Options. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 5-4
Diagnostic Mode . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 5-5
Error Codes . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 5-6
System Information . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 5-6
System A/D Values . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .5-8
NBP Test . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 5-10
CO2 Test . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 5-12
Restoring Factory Settings . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 5-14
6. Troubleshooting . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 6-1
General Troubleshooting Safety Information . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 6-2
Objectives . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 6-3
Concepts . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 6-3
Trouble-shooting . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 6-3
Error Codes . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 6-3
Part 1 Troubleshooting Checklist . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 6-4
Checks for Obvious Problems . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 6-4
Checks Before Opening the Monitor . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 6-4
Part 2 Isolating and Solving Monitor Problems . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 6-6
INOP Messages . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 6-6
Isolating the Defective Component . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 6-8
Part 3 Using Support Functions . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .6-11
Serviceable Hardware Error Codes . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 6-11
Error Code Categories . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 6-12
Other Error Codes . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .6-13
7. Disassembly . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 7-1
Introduction . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 7-2
Tools Required . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 7-2
Disassembly Safety Information . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .7-3
Disassembly Procedures . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 7-4
Closed Case Disassembly Procedures . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 7-4
Removing the Battery . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 7-4
Removing the Navigation Wheel. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 7-5
Removing the Keypad on the Front Panel . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 7-6
Contents-4

Removing the Optional, External Recorder . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .7-6
Separating the Front from the Rear Case . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .7-7
Front Case Disassembly . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .7-8
Replacing the Speaker . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .7-9
Replacing the Backlight Tube . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .7-9
Rear Case Disassembly . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .7-10
Removing the Main PCB Module . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .7-12
Removing the Patient Monitoring I/O Module . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .7-13
Removing the SpO2 Module . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .7-14
Removing the CO2 Module . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .7-15
Removing the NBP Pump . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .7-16
Removing the Power Supply Module . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .7-17
8. Spare Parts . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 8-1
Small Parts Kit . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .8-2
C3 Top Level Assembly . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .8-3
Front Panel Assembly . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .8-4
Rear Panel Assembly . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .8-6
Power Cords . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .8-9
Exchange Unit Part Numbers . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .8-10
9. Packing for Shipment . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 9-1
General Instructions . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .9-2
Repacking the Original Carton . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .9-2
Repacking in a Different Carton . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .9-2
10. RS-232 Interface . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 10-1
General RS-232 Safety Information . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .10-2
About the RS-232 Interface . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .10-3
Cable Connections . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .10-3
Nurse-Call . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .10-4
Contents-5

11. Training . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 11-1
Introduction . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 11-2
Levels of Involvement . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 11-2
Preventative Maintenance Only . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 11-2
Phone Support or Service . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 11-2
Training Materials . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 11-3
Essential Materials . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 11-3
Optional Materials . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 11-3
Overview . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 11-3
Support Strategies . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 11-4
Unit Exchange . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 11-4
Bench Repair . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 11-4
Theory of Operation and System Architecture . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 11-5
System Overview . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 11-5
Block Diagram . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 11-5
Isolated Front End . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 11-7
NBP Front End . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 11-7
Power System . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 11-7
Micro-processor, Memory and Control . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 11-7
Display . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 11-7
Keypad . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 11-7
Navigation Wheel . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 11-8
RS-232 I/O . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 11-8
Defib Sync Pulse . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 11-8
Speaker . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 11-8
Recorder . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 11-9
ECG Processing . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 11-9
Respiration Processing . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 11-9
NBP Processing . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .11-10
SpO2 Processing . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 11-10
CO2 Processing . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .11-10
Temperature Processing . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 11-10
Troubleshooting . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 11-11
Disassembly . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 11-12
Monitor Applications and Algorithms . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 11-13
Contents-6

12. Specifications. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 12-1
Hardware Specifications . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .12-2
Safety Standards . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .12-2
Electrical . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .12-3
Environmental . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .12-3
Measuring and Displaying Parameters . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .12-4
ECG . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .12-4
ECG Standards . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .12-5
Respiration . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .12-6
NBP (Non-Invasive Blood Pressure) . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .12-7
Temperature . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .12-7
SpO2 . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .12-8
CO2 . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .12-9
Trends . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .12-10
Contents-7

Contents-8

1
Overview
This chapter provides a brief overview of the C3 patient monitor. It also provides a list of the
monitor’s features and parameters.
Overview 1-1
General Safety Information
General Safety Information
WarningWarning
This monitor is not intended for neonates.
If you connect the monitor to any instrument, verify proper operation before clinical
use. Refer to that instrument’s Instructions for Use guide for full
instructions.
Accessory equipment connected to the monitor’s data interface must be certified
according to IEC Standard 60950 for data-processing equipment or
IEC Standard 60601-1 for electromedical equipment. All combinations
of equipment must be in compliance with IEC Standard 60601-1-1
systems requirements.
Anyone who connects additional equipment to the signal input port or signal output port
configures a medical system and is therefore responsible to ensure that
the system complies with the requirements of system standard IEC
Standard 60601-1-1. If in doubt, contact the Philips’ Response Center
or your local Philips representative.
Note
The monitor and its accessories must be tested by qualified service personnel at regular
intervals to verify proper operation, according to the procedures of the user’s institution.
Other important safety information is located in this Service Guide where appropriate.
1-2 Overview
Introduction
This manual contains information for servicing the C3 patient monitor, subsequently referred
to as "the monitor" throughout this manual. Only qualified service personnel should service
this product. Before servicing the monitor, carefully read the C3 Instructions for Use guide
for a thorough understanding of operation.
C3 Patient Monitor Description
The purpose and function of the C3 family of patient monitors is to monitor ECG, heart rate,
non-invasive blood pressure (NBP), functional arterial oxygen saturation (SpO
rate, temperature and carbon dioxide
and hospital-type facilities, such as clinics.The monitors may be used by clinical users during
hospital transport.
The intended users of the C3 patient monitors are clinicians and nursing staffs within hospital,
out-patient, and ambulatory settings. This monitor is not intended for helicopter transport or
home use.
Introduction
), respiration
1
(CO2) for adult and pediatric patients in hospital areas
2
The physical and operational characteristics of the monitor are described in the C3
Instructions for Use guide.
1. Carbon Dioxide monitoring is available within the C3 Sedation monitors only.
Overview
1-3

Features and Options
Features and Options
The measurement parameters and features for each model are indicated below.
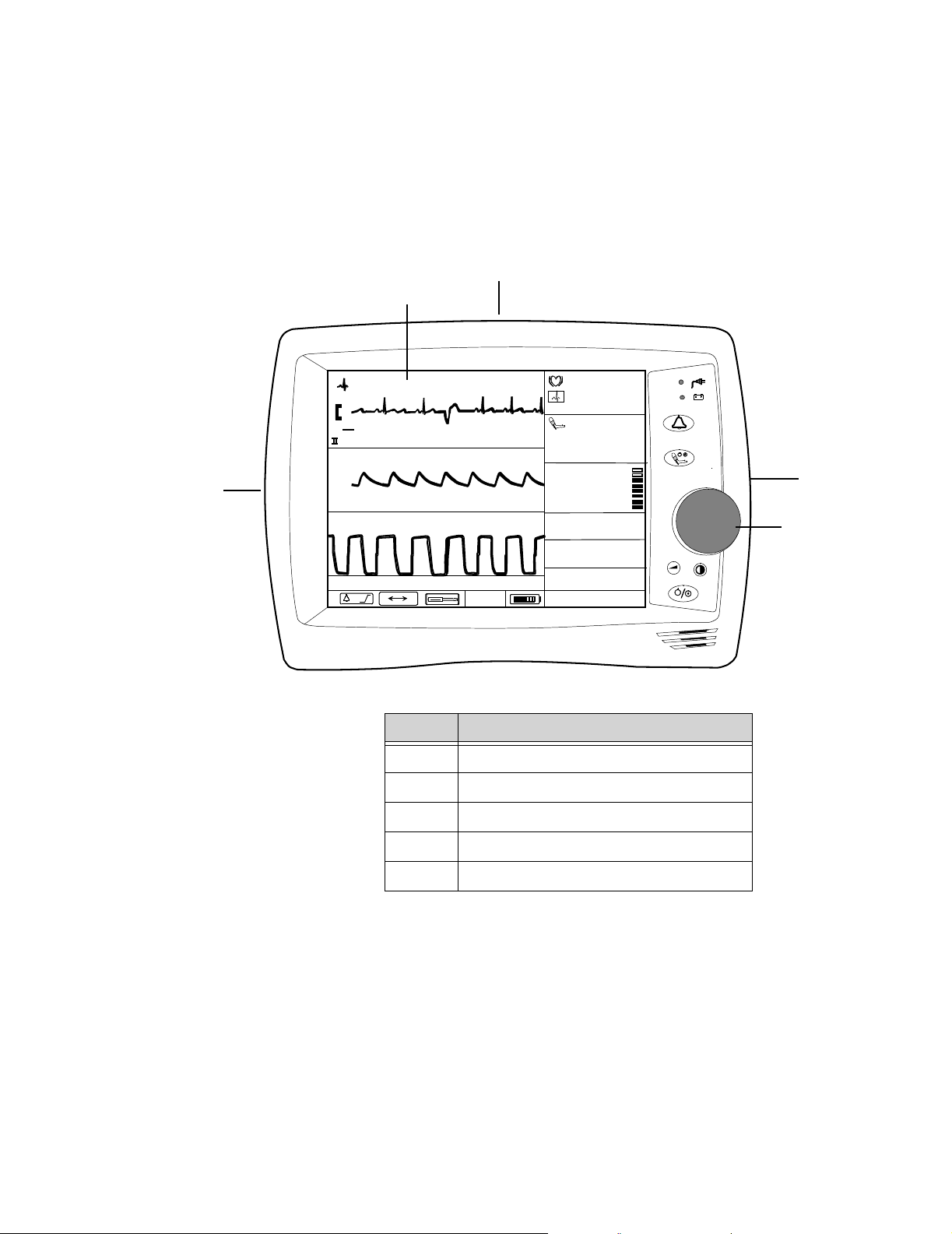
Front Panel Description
Order
Number
Description Display Measurements Printout
Color ECG NBP Temp SpO2CO2Recorder
862474 Standard Color ••••• (optional)
862478 Sedation Color ••••••(optional)
The following diagram illustrates the controls located on the front panel of your C3.
A
B
C
D
1-4 Overview
E
Callout Button/LED
A. AC LED
B. Battery LED
C. Audio Alarm Control button
D. NBP button
E. Volume button
F. Contrast button
G. On/Standby button
F
G
Features and Options
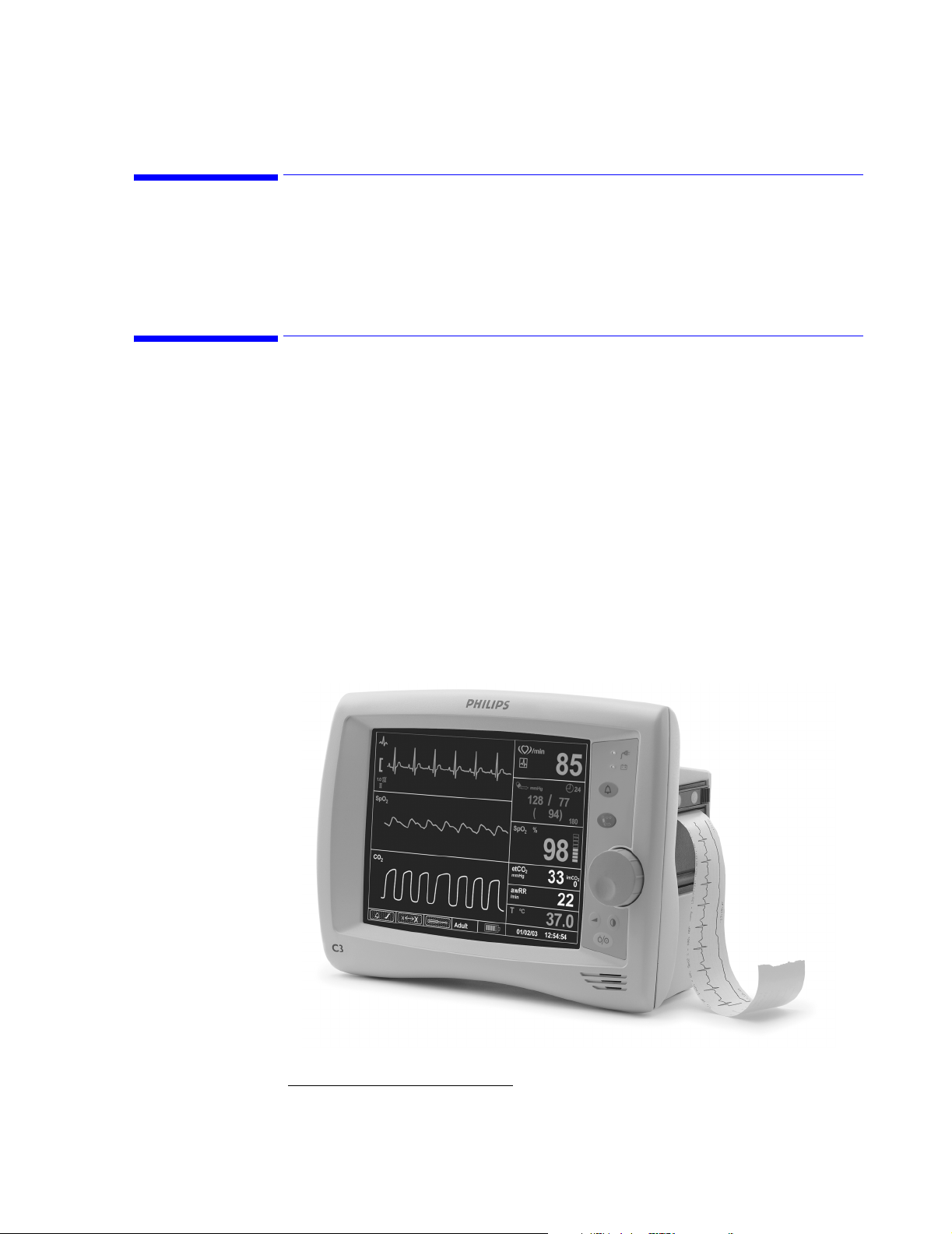
Front of Monitor
Use the following diagram to familiarize yourself with the main features of your C3 monitor.
The recorder module and all patient input connectors are located on the monitor’s side panels.
C
B
/min
85
mV
1.0
cm
SpO
2
A
CO
2
x
X
Adult
mmHg
183/ 107
(149 )
SpO
%
2
93
etCO
2
mmHg
33
awRR
/min
23
°
T °C
37.8
01/06/03 01:09:17
180
imCO
1
D
2
E
Callout Description
A. Patient Monitoring Input Connectors
B. Main Monitoring Screen
C. Handle
D. Recorder (optional. Not available in all models)
E. Navigation Wheel
Overview
1-5
Features and Options
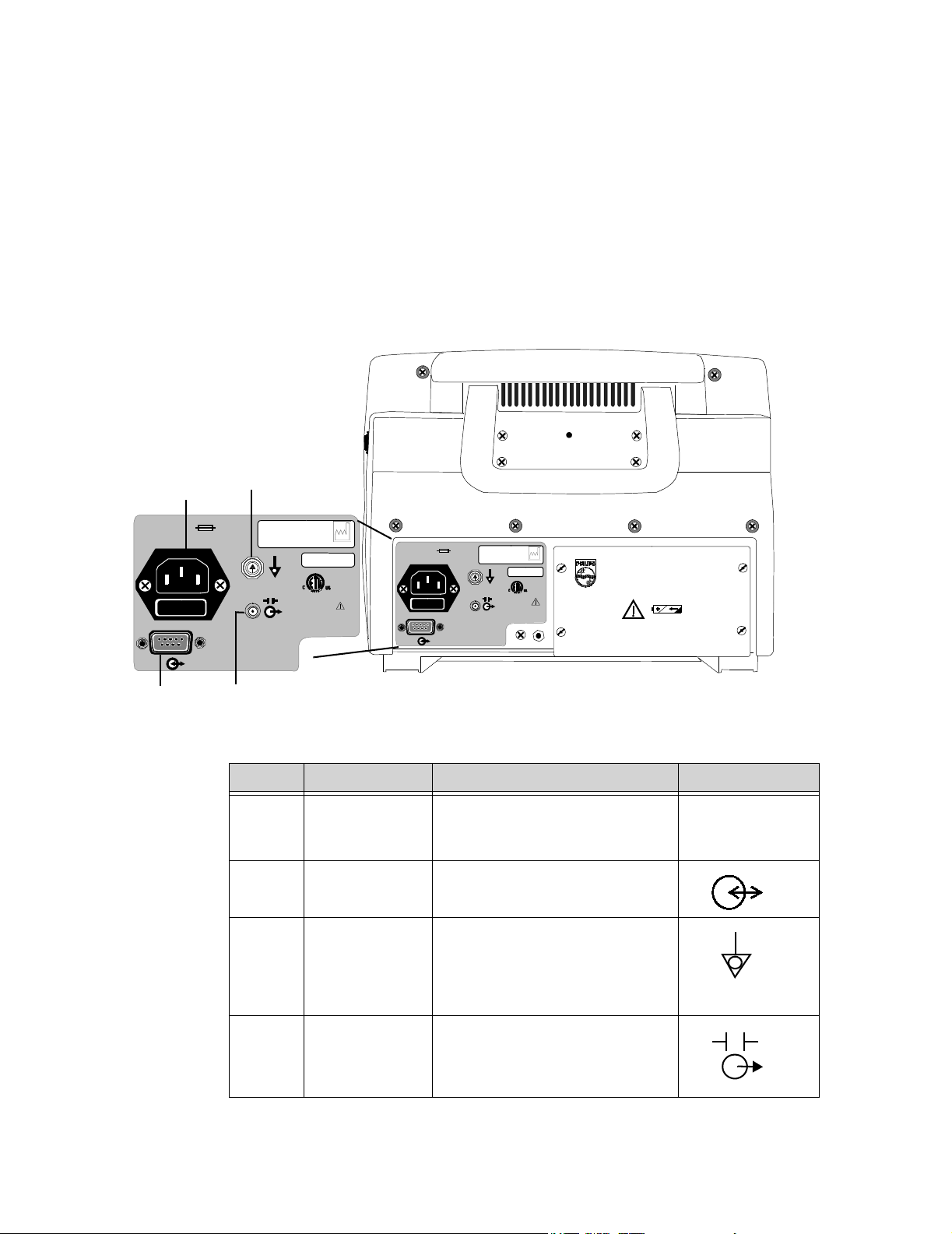
Rear of Monitor
100-230V~
50-60 Hz. 1.0A
The following diagram is of the rear panel. For an explanation of the symbols located on this
panel, see “Explanation of Symbols” on page vi.
The C3 has four connections on its rear panel. The diagram below is of the rear panel. It
shows you how to make the four possible connections (Defib Sync, Equipotential, AC Input,
and RS-232 I/O).
A
T1AL 250VT1AL 250V
C
9700859
IPX1
YYYY-XX
CE
0123
100-230V~
50-60 Hz. 1.0A
T1AL 250VT1AL 250V
970085
YYYY-XX
CE
0123
IPX1
PHILIPS C3 PATIENT MONITOR
Manufactured for Philips for
Philips Medical Systems
3000 Minuteman Road
Andover, MA 01810
Made in U.S.A.
B
D
Callout Connector Connector Type Label
A. AC Input 3-line connector IEC 320 receptacle
100 - 240V ~
50 - 60Hz
1A
B. RS-232 I/O DB-9 (male)
C. Equipotential
Equipotential
Grounding Post
The equipotential grounding post
may be used by facilities as required
by their procedures.
D. Defib Sync 2.5mm subminiature phone jack
1-6 Overview
Features and Options
Screen Display
The LCD displays parameter values, real-time waveforms, alarm messages and screen
selection icons.
• Numeric frames displays icons and numeric values of
real-time physiologic parameter.
• Waveform frames displays real-time waveforms, graphical
trend, or tabular trend data.
• Message and Icon frames displays alarm messages and screen
selection icons.
Standard Model
/min
85
%
SpO
2
93
mmHg
183 / 107
(149 )
/min
14
Numeric
Frames
180
Waveform
Frames
1.0
SpO
mV
cm
2
Message
and Icon
Frames
T °C
37.8
x
X
Adult
01/06/03 01:09:17
Overview
1-7
Additional Documentation
Frames
Message
and Icon
Frames
1.0
SpO
CO
mV
Sedation Model
/min
85
cm
2
2
x
X
Adult
mmHg
183/107
SpO
(149 )
%
2
180
93
etCO
2
mmHg
33
awRR
/min
T °C
01/06/03 01:09:17
23
37.8
imCO
1
Numeric
Frames
2
Additional Documentation
To perform test and troubleshooting procedures and to understand the principles of operation
and circuit analysis sections of this manual, you must know how to operate the monitor. Refer
to the C3 Instructions For Use guide to understand the various sensors, ECG leads, blood
pressure cuffs, CO
monitor. Instructions for cleaning and caring for the accessories can also be found in the
individual Directions for Use that accompany these accessories.
2
parameters and accessories and temperature probes that work with the
1-8 Overview

Site Preparations
This chapter describes how to perform site preparation and how to comply with safety
guidelines and requirements.
2
Site Preparations 2-1
General Site Preparation Safety Information
General Site Preparation Safety Information
WarningWarning
To avoid contaminating or infecting personnel, the service environment or other
equipment, make sure that equipment which has been used before has
been appropriately disinfected and decontaminated.
Disconnect the monitor from the AC source by unplugging the power cable from the AC
power connector located on the rear of the monitor. The On/Standby
button does not disconnect the monitor from the AC mains supply
Accessory equipment connected to the monitor’s data interface must be certified
according to IEC Standard 60950 for data-processing equipment or
IEC Standard 60601-1 for electromedical equipment. All combinations
of equipment must be in compliance with IEC Standard 60601-1-1
systems requirements.
Explosion Hazard. Equipment not suitable for use in the presence of a flammable
anaesthetic mixture with air, or with oxygen or nitrous oxide.
Make sure that you have read all applicable instructions before attempting to install the
wall mount.
Wall mounts that are intended to support monitors must be capable of supporting 4
times the weight of the monitor when properly installed.
Do not mount any portion of a monitoring instrument over a patient’s bed.
Do not exceed the maximum rated load specified for each wall mount.
Ensure that no electrical wiring, utilities, or piping interfere with the selected wall
mounting location.
Note
Do not return sensors, patient cables, NBP tubing and cuff, or the power cord.
2-2 Site Preparations
 Loading...
Loading...