Page 1

PENTAX VIDEO PROCESSOR
EPK-1000
OWNER,S MANUAL
Page 2

Read this manual before operating and save this book for future reference.
This manual describes the recommended procedures for inspecting and preparing the EPK-1000 Video Processor
prior to its use and the care and maintenance after its use. It does not describe how an actual procedure is to be performed,
nor does it attempt to teach the beginner the proper technique or any medical aspects regarding the use of the equipment.
Failure to follow the instructions in this manual may result in damage to and/or malfunction of the equipment.
Do NOT use this device for any other purpose than that for which it has been designed.
If you have any questions regarding any of the information in this manual or concerns pertaining to the safety and/or use
of this equipment, please contact your local PENTAX representative.
CAUTION:
Federal (USA) law restricts this device to sale by, or on the order of a physician or other appropriately licensed medical
professional.
IMPORTANT
このCEマーキングはEC指令への適合宣言マークです。
TheCEmarkingassuresthatthisproductcomplieswiththerequirementsoftheECdirectiveforsafety.
Das CE Zeichen garantiert, daß dieses Produkt die in der EU erforderlichen Sicherheitsbestimmungen
erfüllt.
Le logo CE certifie que ce produit est conforme aux normes de sécurité prévues par la Communauté
Européenne.
IImarchioCEassicurachequestoprodottoè conformealledirettiveCErelativeallasicurezza.
LamarcaCEaseguraqueesteproductocumpletodaslasdirectivasdeseguridaddelaCE.
INTENDED USE:
This electro-medical device (Video Processor) is intended to be used for endoscopic diagnosis and treatment.
Together, this Video Processor and PENTAX video endoscope may provide optical visualization of, and/or therapeutic
access to, various body cavities, organs and canals. Do NOT use this device for any purpose other than that for which it
has been designed.
This device should only be used by physicians who have thoroughly studied all the characteristics of this device and who
are familiar with the proper techniques of endoscopy.
EC REP
Symbol for “MANUFACTURER”
Symbol for “DATE OF MANUFACTURE”
Symbol for “AUTHORISED
REPRESENTATIVE”
Page 3

CONTENTS
1 SAFETY PRECAUTIONS
...............................................................................................................................................................................
1
2 NOMENCLATURE, CONTROLS AND FUNCTIONS
.................................................................................................................................
7
2-1 VIDEO PROCESSOR
.........................................................................................................................................................................
7
(1) MAIN BODY
............................................................................................................................................................................
7
(2) FRONT PANEL
........................................................................................................................................................................
8
(3) REAR PANEL
...........................................................................................................................................................................
9
2-2 WATER BOTTLE ASSEMBLY, MODEL OS-H4
..........................................................................................................................
10
2-3 MONITOR DISPLAY SCREEN
......................................................................................................................................................
11
(1) NORMAL
................................................................................................................................................................................
11
(2) FREEZE (SUB-SCREEN DISPLAY)
.....................................................................................................................................
11
3 PREPARATION AND SAFETY CHECK
.....................................................................................................................................................
12
3-1 PREPARATION
................................................................................................................................................................................
12
(1) SETTING UP THE VIDEO PROCESSOR
............................................................................................................................
12
(2) CONNECTING THE WATER BOTTLE
...............................................................................................................................
12
(3) CONNECTING THE ENDOSCOPE
......................................................................................................................................
13
(4) CONNECTING THE PERIPHERAL EQUIPMENT
.............................................................................................................
13
3-2 PRE-USE SAFETY CHECKLIST
....................................................................................................................................................
14
4 OPERATION
..................................................................................................................................................................................................
16
4-1 PROCESSOR FUNCTIONS
.............................................................................................................................................................
16
(1) MAIN LAMP
...........................................................................................................................................................................
16
(2) AUXILIARY LAMP
...............................................................................................................................................................
16
(3) BRIGHTNESS
.........................................................................................................................................................................
17
(4) COLOR BALANCE
................................................................................................................................................................
17
(5) PUMP
......................................................................................................................................................................................
18
4-2 KEYBOARD FUNCTIONS
..............................................................................................................................................................
19
(1) CONVENTIONAL KEYS
......................................................................................................................................................
19
(2) SPECIAL FUNCTION KEYS
................................................................................................................................................
20
5 MAINTENANCE
...........................................................................................................................................................................................
29
5-1 AFTER EACH PROCEDURE
..........................................................................................................................................................
29
5-2 WATER BOTTLE CLEANING
.......................................................................................................................................................
29
(1) CLEANING
.............................................................................................................................................................................
29
(2) STERILIZATION
....................................................................................................................................................................
30
(3) CARE DURING STORAGE
...................................................................................................................................................
31
5-3 STORAGE
.........................................................................................................................................................................................
31
5-4 CHANGING THE LAMP
.................................................................................................................................................................
31
5-5 RESETING THE BREAKERS
.........................................................................................................................................................
32
6 TROUBLE-SHOOTING GUIDE
...................................................................................................................................................................
33
7 SPECIFICATIONS
.........................................................................................................................................................................................
35
8 ELECTROMAGNETIC COMPATIBILITY
.................................................................................................................................................
36
Page 4
1
1. SAFETY PRECAUTIONS- IMPORTANT
The following precautions should always be exercised with the use of all electro-medical equipment to ensure safety to all
involved parties - user(s), patient(s), etc.
Please carefully read and follow this owner’s manual.
1-1. TRAINING
This equipment should only be used under the supervision of a trained physician in a medical facility. Do NOT use in other
locations or for any other purposes than the intended application.
1-2. INSTALLATION
1. This equipment should NEVER be installed or used in areas where the unit could get wet or be exposed to any environmental
conditions such as high temperature, humidity, direct sunlight, dust, salt, etc., which could adversely affect the equipment.
2. This equipment should NEVER be installed or used in the presence of flammable or explosive gases or chemicals.
3. This equipment should NEVER be installed, used or transported in an inclined position nor should it be subjected to impact
or vibration.
4. For safety reasons, this equipment must be properly grounded. (This equipment should be connected to a three (3) prong
hospital grade receptacle in U.S.A. or Canada.)
5. Ensure that all power requirements are met and conform to those specified on the rating plate located on the rear panel.
6. Do NOT block the air intake vent of this equipment.
7. Do NOT allow the power cord to become twisted, crushed or pulled taut.
8. When using an isolation transformer for any ancillary equipment, ensure that the power requirements of the devices do not
exceed the capacity of the isolation transformer. For further information, contact your local PENTAX distributor.
1-3. PRIOR TO USE
1. Confirm that this equipment functions properly and check the operation of all switches, indicators, etc.
2. To prevent electrical shock when used with endoscopes, this equipment is insulated (type BF electro-medical equipment).
Do NOT allow it to be grounded to other electrical devices being used on the patient. Rubber gloves should always be worn
to prevent grounding through user(s).
3. Confirm that other devices used in conjunction with this equipment function properly and that these other devices will not
adversely affect the operation or safety of this equipment. If any component of the endoscopic system is not properly
functioning, the procedure should not be performed.
4. Check and confirm that all cords or cables are connected correctly and securely.
5. The lamp life when used in this equipment is 400 hours. Prior to use, check the lamp life indicator on the front panel to
ensure the indicator is lit green or yellow. After 400 hours of use, the indicator turns red and the image quality will
deteriorate. Excessive use of the lamp beyond its rated 400 hours (approaching a thousand hours of use or more ) could cause
the lamp to explode resulting in damage to the video processor.
- The lamp life rated at 400 hours, is applicable to the EPK-1000 processor with serial number beginning with UB and
EB.
1-4. DURING USE
1. To prevent electric shock, the endoscope and/or any other ancillary device should NEVER be applied directly to the heart.
2. Make sure that no contact is made between the patient and this equipment.
3. To avoid damage to the luminous display and flat membrane switches, do NOT press any keys with any sharp or pointed
objects.
4. The light emitted by the Xenon lamp is extremely intense. Avoid looking directly at the light exiting the endoscope and/or
this equipment.
NOTE
Page 5
2
5.To protect the users eyes and avoid risk of thermal injury during an endoscopic examination, use only the minimum amount
of brightness required.
6. During clinical procedures, avoid unnecessary prolonged use which could compromise patient/user safety.
7. Continually monitor this equipment and the patient for any signs of irregularities.
8. In the event that some type of irregularity is noted to the patient or this equipment, take the appropriate action to ensure
patient safety.
9. If the operation of any of the components of the endoscopic system fails during the procedure and the visualization of the
procedure is lost or compromised, place the endoscope in the neutral position and slowly withdraw the endoscope.
10. This equipment should only be used according to the instruction and operating conditions described in this manual. Failure
to do so could result in compromised safety, equipment malfunction or instrument damage.
1-5. AFTER USE
1. Refer to the operating instructions supplied with all the components of the endoscopic system to establish the right order in
which components should be turned OFF. Some peripheral devices may have to be turned OFF first to avoid compromising
their operation.
2. Wipe all surfaces clean with gauze slightly dampened with alcohol.
3. Be sure connector interfaces and ventilation ports are not allowed to become wet or splashed with liquids.
1-6. STORAGE
1. This equipment should NEVER be stored in areas where the unit could get wet or be exposed to any environmental
conditions such as high temperature, humidity, direct sunlight, dust, salt, etc., which could adversely affect the equipment.
2. This equipment should NEVER be stored in the presence of flammable or explosive gases or chemicals.
3. This equipment should NEVER be stored or transported in an inclined position, nor should it be subjected to impact or
vibration.
4. Cords, accessories, etc., should be cleaned and neatly stored.
5. This equipment should be maintained in a clean condition during storage and be ready for subsequent use.
1-7. SERVICE
1. Alterations/modifications to the equipment should NEVER be made. Repairs should only be performed by an authorized
PENTAX service facility.
2. When replacing the lamp, use only the lamp recommended by PENTAX and follow all PENTAX instructions provided.
1-8. MAINTENANCE
Periodically this equipment and any applicable accessories should be inspected for operation and safety.
1-9. DISPOSAL
The equipment should be returned for disposal to PENTAX. Contact your local PENTAX representative or service facility.
1-10. FOR THE STATE OF CALIFORNIA, USA ONLY
Perchlorate Material-special handling may apply. See www.dtsc.ca.gov/hazardouswaste/perchlorate.
Perchlorate Material: Lithium battery contains perchlorate.
An information on Disposal for users in the European Union
This product is a medical device. In accordance with European Directive 2002/96/EC on Waste Electrical and Electronic
Equipment, this symbol indicates that the product must not be disposed off as unsorted waste, but should be collected
separately. Contact your local PENTAX distributor for correct disposal and recycling.
By disposing of this product correctly you will help ensure that the waste undergoes the necessary treatment, recovery
and recycling and thus prevent potential negative effects on the environment and human health which could otherwise
arise due to inappropriate waste handling.
Page 6
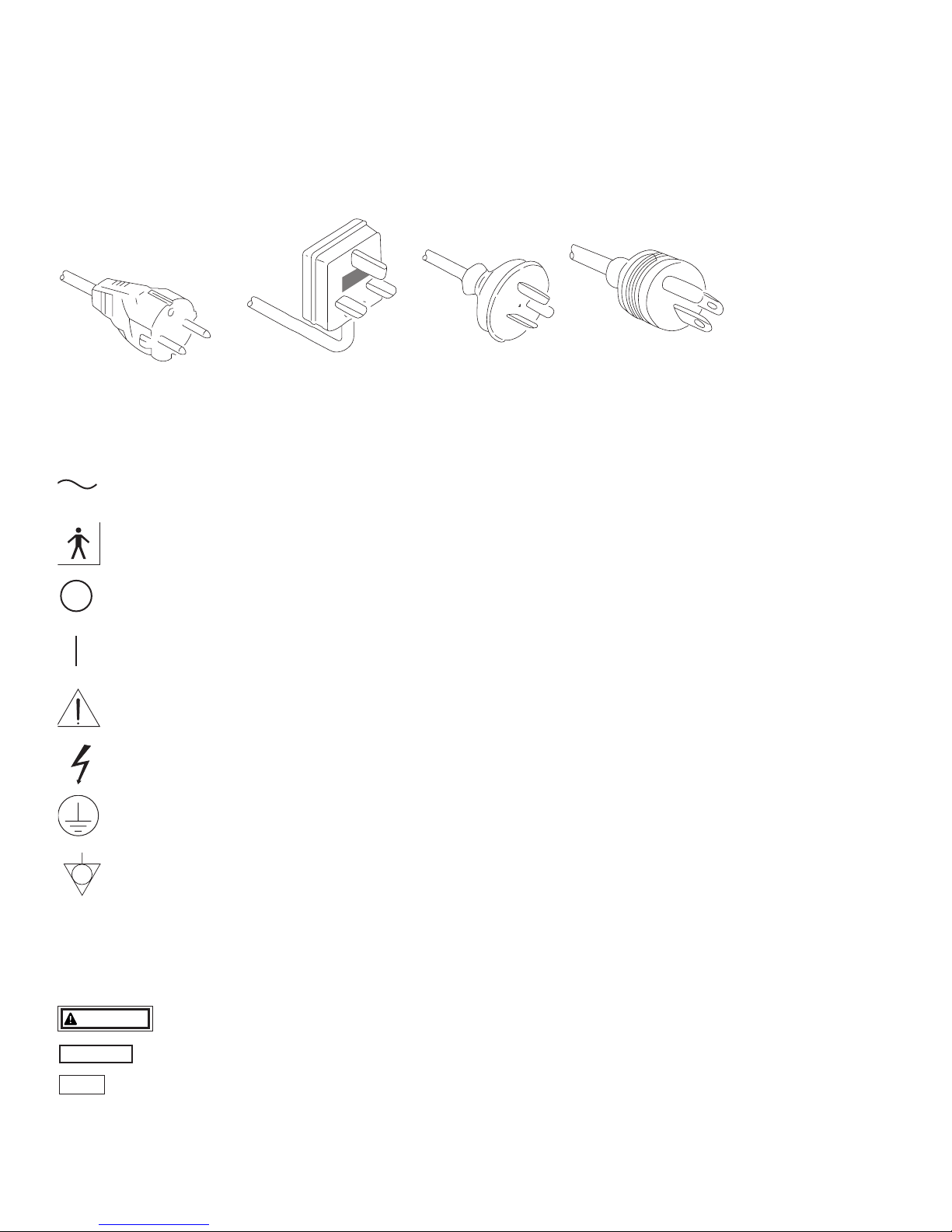
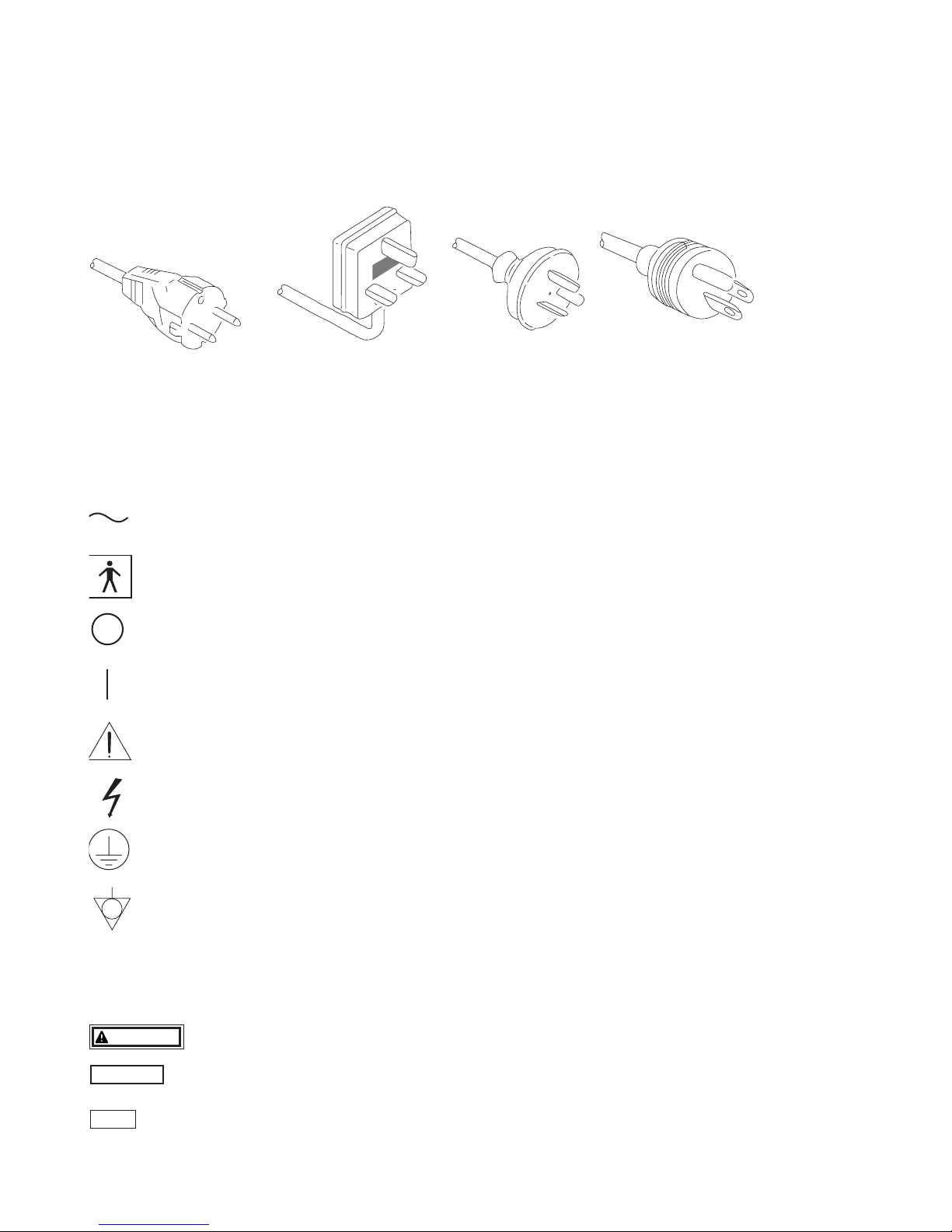
POWER REQUIREMENTS
Check the standard power plug configurations that are used in your country. If the appropriate power cord is not included in your
product, notify your local PENTAX distributor.
SYMBOLS ON MARKING
CONVENTIONS
The following conventions have been established in the text of this manual to aid in the identification of potential hazards of
operation;
: Could result in death or serious injury.
: May result in minor or moderate injury or property-damage.
: May result in property-damage. Also, advises owner/operator about important information on the use of
this equipment.
3
Continental Europe
(Use a SEV approved
plug for Switzerland)
U.K. Australia
and
New Zealand
U.S.A. and
Canada
(Hospital Grade)
Alternating current
Type BF applied part (Safety degree specified by IEC 60601-1)
OFF (Power : disconnection from mains)
ON (Power : connection to the mains)
Attention, consult Owner’s Manual
Dangerous Voltage
CAUTION
NOTE
WARNING
Protective earth (ground)
Equipotentiality
Page 7
4
1. PRECAUTIONS DE SECURITE-IMPORTANT
Les précautions suivantes doivent toujours être obeervées lors de l’utilisation de tout matériel médical électrique susceptible
d’être utilisé avec cet appareil, pour assurer à toutes les personnes concernées (utilisateus, patients, etc...) une sécurité maximale.
Veuillez lire et suivre attentivement les recommandations du manuel d’utilisation.
1-1. FORMATION
L’appareil ne doit être utilisé que sous la surveillance d’un médecin expérimenté, dans un établissement médical. Ne pas utiliser
dans un autre endroit ou pour toute autre application pour laquelle il n’est pas prévu.
1-2. INSTALLATION
1. L’appareil ne doit JAMAIS être placé ou utilisé dans un endroit où il serait mouillé ou exposé à l’humidité, à une température
élevée, à la lumière solaire directe, à la poussière, au sel, etc., qui pourraient l’endommager.
2. L’appareil ne doit JAMAIS être placé ou utilisé en présence de gaz ou de produits chimiques inflammables ou explosifs.
3. L’appareil ne doit JAMAIS être placé, utilisé ou transporté en position inclinée, ni être soumis à des chocs ou des vibrations.
4. Pour des raisons de sécurité, l’appareil doit être correctement relié à la terre (cet appareil doit être branché dans une prise
secteur 3 broches aux normes Hôpital aux U.S.A. et au Canada).
5. Assurez-vous que les spécifications électriques de la prise secteur sont conformes à celles indiquées à l’arrière de l’appareil.
6. Ne pas obturer les orifices de ventilation de l’appareil.
7. Ne pas écraser, plier ou tendre le cordon secteur.
8. Dans le cas ou un transformateur d’isolement est utilisé pour le matériel périphérique, vérifier que la puissance totale de
l’installation ne dépasse pas la capacité du transformateur. Pour de plus amples informations, contacter votre distributeur
PENTAX.
1-3. AVANT UTILISATION
1. Vérifier le fonctionnement de l’appareil et de ses interrupteurs, afficheurs, voyants, etc...
2. Pour prévenir les risques de chocs électriques lorsqu’il est utilisé avec des endoscopes, cet appareil doit être installé comme
“Matériel électrique médical type BF”. Ne pas le relier aux autres appareils électriques utilisés pour le même patient. Les
utilisateurs doivent s’isoler électriquement en portant des gants de caoutchouc.
3. Vérifier le fonctionnement des périphériques utilisés avec l’appareil et s’assurer qu’ils n’en perturbent pas le fonctionnement
et la sécurité. Si l’une des composantes du système endoscopique ne fonctionne pas correctement, interrompre l’utilisation.
4. Vérifier le branchement des différents câbles de liasions (vidéo, secteur, contrôle, etc...).
5. La durée de vie nominale de la lampe est de 400 heures. Avant utilisation, vérifier que le témoin de durée de vie vert ou jaune
est allumé. A partir de 400 heures, le témoin rouge s’allume et la qualité d’image diminue. Une utilisation excessive de la
lampe au-delà de 400 heures (approchant plusieurs milliers d’heures) peut être à l’origine d’une explosion de la lampe
pouvant provoquer des dommages au vidéoprocesseur.
- La duré de vie de la lampe estimée à 400 heures s’applique aux processeurs EPK-1000 dont le numéro de série
commence par UB et EB.
NOTE
Page 8

1-4. PENDANT L’UTILISATION
1. Pour éviter les risques de choc électrique, l’endoscope et/ou tout autre périphérique utilisé conjointement avec l’appareil ne
doivent JAMAIS être placés directement sur le coeur.
2. Ne pas mettre le patient en contact avec l’appareil.
3. Pour conserver l’afficheur et le clavier souple en bon état, ne pas presser les touches du tableau avec un objet pointu ou
tranchant.
4. Eviter de regarder directement la lumière sortant de l’endoscope et/ou de l’appareil du fait de la forte luminosité émise par la
lampe Xénon.
5. Pour protéger l’utilisateur et éviter toute blessure thermique pendant l’examen, régler la luminosité au minimum nécessaire.
6. Eviter une utilisation prologée de l’appareil si elle n’est pas indispensable, pour ne pas compromettre la sécurité du patient
et de l’utilisateur.
7. Surveiller en permanence l’appareil et le patient pour prévenir tout signe de dysfonctionnement.
8. En cas de problème avec le patient ou l’appareil, prendre toutes les mesures nécessaires pour préserver la sécurité du patient.
9. Si un problème de fonctionnement survient sur l’un des appareils du système endoscopique et que l’image est interrompue
ou altéree, placer l’endoscope en position neutre et retirer doucement.
10. Cet appareil doit toujours être utilisé selon les instructions et conditions de fonctionnement décrites dans ce manuel. Ne pas
les suivre peut compromettre la sécurité, le fonctionnement du matériel, ou endommager l’appareil.
1-5. APRES UTILISATION
1. Veuillez vous référer aux instructions fournies avec chaque composante du système endoscopique afin d’éteindre les
composantes dans l’ordre adéquat. Certains périphériques peuvent devoir être éteints d’abord pour ne pas compromettre leur
fonctionnement.
2. Essuyer les appareils avec une compresse légèrement imbibée d’alcool.
3. Vérifier que les connecteurs et les orifices de ventilation sont à l’abris des projections de liquides.
1-6. STOCKAGE
1. L’appareil ne doit JAMAIS être rangé à l’humidité, à température élevée, à la lumière solaire directe, la poussière, le sel, etc.,
qui pourraient l’endommager.
2. L’appareil ne doit JAMAIS être rangé en présence de gaz ou de produits chimiques explosifs.
3. L’appareil ne doit JAMAIS être rangé en position inclinée ni être soumise à des chocs ou des vibrations.
4. Les accessoires et les câbles doivent être nettoyés et rangés correctement.
5. L’appareil doit être maintenu en parfait état de propreté durant le stockage, et tenu prêt pour l’utilisation suivante.
1-7. SERVICE
1. Ne JAMAIS modifier ou altérer l’appareil. Les réparations éventuelles ne doivent être effectuées que par un service aprés-
vente PENTAX.
2. Le remplacement de la lampe ne doit être effectué que par une lampe agréée par PENTAX et en sulvant les instructions
fournies par PENTAX.
1-8. MAINTENANCE
Périodiquement, cet appareil et tous les périphériques associés doivent être vérifiés en fonctionnement et en sécurité.
1-9. ÉLIMINATION
Ce matériel doit être retourné à PENTAX pour élimination. Contacter PENTAX ou votre Agence PENTAX locale.
5
Information concernant l’élimination des produits dans l’Union européenne.
Ce produit est un dispositif médical. En conformité avec la Directive européenne 2002/96/CE relative aux déchets d’équipements électriques et
électroniques, ce symbole indique que le produit ne doit pas être éliminé comme un déchet non trié, mais qu’il doit faire l’objet d’une collecte
sélective. Contactez votre distributeur PENTAX local pour avoir des informations concernant la procédure correcte d’élimination et de recyclage.
En éliminant ce produit correctement, vous contribuerez à garantir que ce déchet est soumis au traitement, à la valorisation et au recyclage
nécessaires, empêchant ainsi les effets négatifs potentiels pour l’environnement et la santé des personnes qui résultent de la gestion
inappropriée des déchets.
Page 9
6
ALIMENTATION NECESSAIRE
Vérifier le type de prise de courant utilisé dans votre pays. Si le cordon secteur approprié n’est pas fourni avec votre appareil,
contactez votre distributeur PENTAX.
SYMBOLES UTILISES:
CONVENTIONS
Les conventions suivantes ont été adoptées dans le texte de ce manuel, afin d’aider á l’identification des risques potentiels liés à
l’utilisation;
: Peut causer la mort ou une blessure grave
: Peut causer une blessure légère à modérée ou des dégâts au materiel
: Peut causer des désgâts au matériel. Donne aussi à l’utilisateur des informations sur les appareils
Europe Continentale Royaume - Uni Australie
et
Nouvelle Zélande
USA et Canada
(Normes Hôpital)
Courant alternatif
Élément type BF (Niveau de sécurité spécifié par la norme IEC60 601-1)
“OFF” (Alimentation : déconnectée du secteur)
“ON” (Alimentation : connectée au secteur)
Attention : consulter le manuel d’utilisation
Voltage dangereux
Mise à la terre de protection
Equipotentialité
(Utiliser une tiche homologuée
SEV pour la Suisse)
CAUTION
NOTE
WARNING
Page 10
7
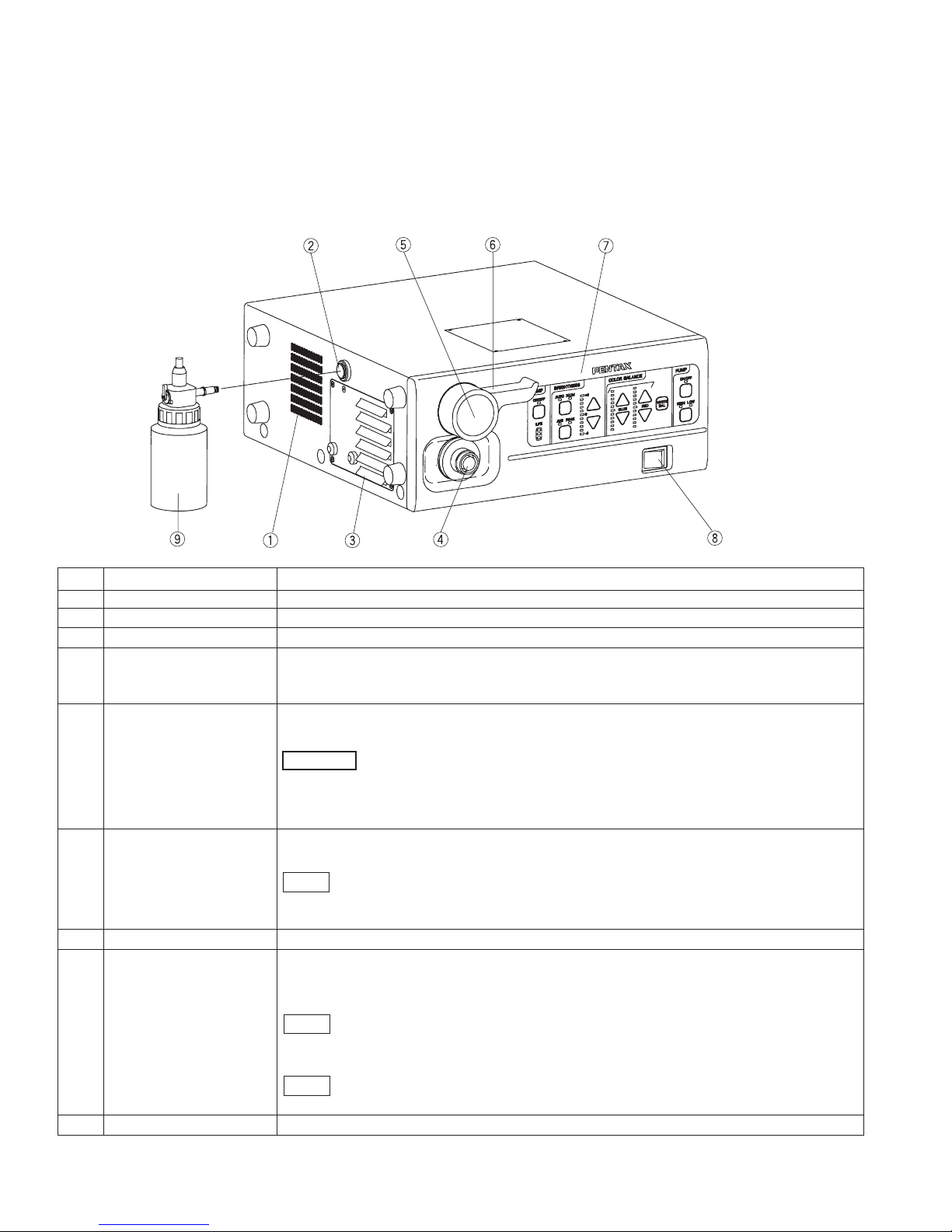
2. NOMENCLATURE, CONTROLS AND FUNCTIONS
2-1 VIDEO PROCESSOR
(1) MAIN BODY
No. NAME FUNCTION
1 Ventilation Grid allows for adequate ventilation and cooling lamp/unit. Do NOT block the grids.
2 Water Bottle Receptacle accepts air pipe from PENTAX water bottle assembly.
3 Lamp Housing Cover provides access to replace lamp cartridge.
4 Light Guide Attachment AE-P1 adapter for standard PENTAX endoscopes. Port accepts video endoscope or
fiberscope light guide. Adapter can be changed for use as light source for other
manufacturer’s endoscopes or for use with fiberscope video adapter module.
5 Endoscope Electrical accepts Color video endoscope electrical connector or fiberscope video adapter module
Connector electrical connector.
- Always turn ON the power switch after connecting an endoscope. Also,
remove the endoscope from the processor after turning OFF the power switch.
- When you take the light guide out of this socket, the sleeve of the light guide might be hot.
Take the light guide out of the socket with caution.
6 Scope Locking Lever Open the lever before setting or removing an endoscope.
After connecting the endoscope to the processor, close the lever.
- After connecting the endoscope to the EPK-1000 video processor, always make
sure that the endoscope is firmly secured to the scope receptacle by turning the locking
lever to the “lock” position.
7 Front Panel See the section 2-1- (2)
8 Power Switch The processor is turned | : ON, or O : OFF.
Switch lights green when switched ON. Switch should not be hit with objects like
endoscope light guides, when being switched ON or OFF.
- Always turn ON the power switch after connecting an endoscope. Also, remove
the endoscope from the processor after turning OFF the power switch.
- Before turning the EPK-1000 power ON, ensure the air flow vents are not obstructed.
-Aside from the pre-use inspection of the equipment, the lamp in the video
processor should be turned OFF when the video system is not clinically used.
9 Water Bottle See the section 2-2.
NOTE
NOTE
NOTE
CAUTION
Page 11
8
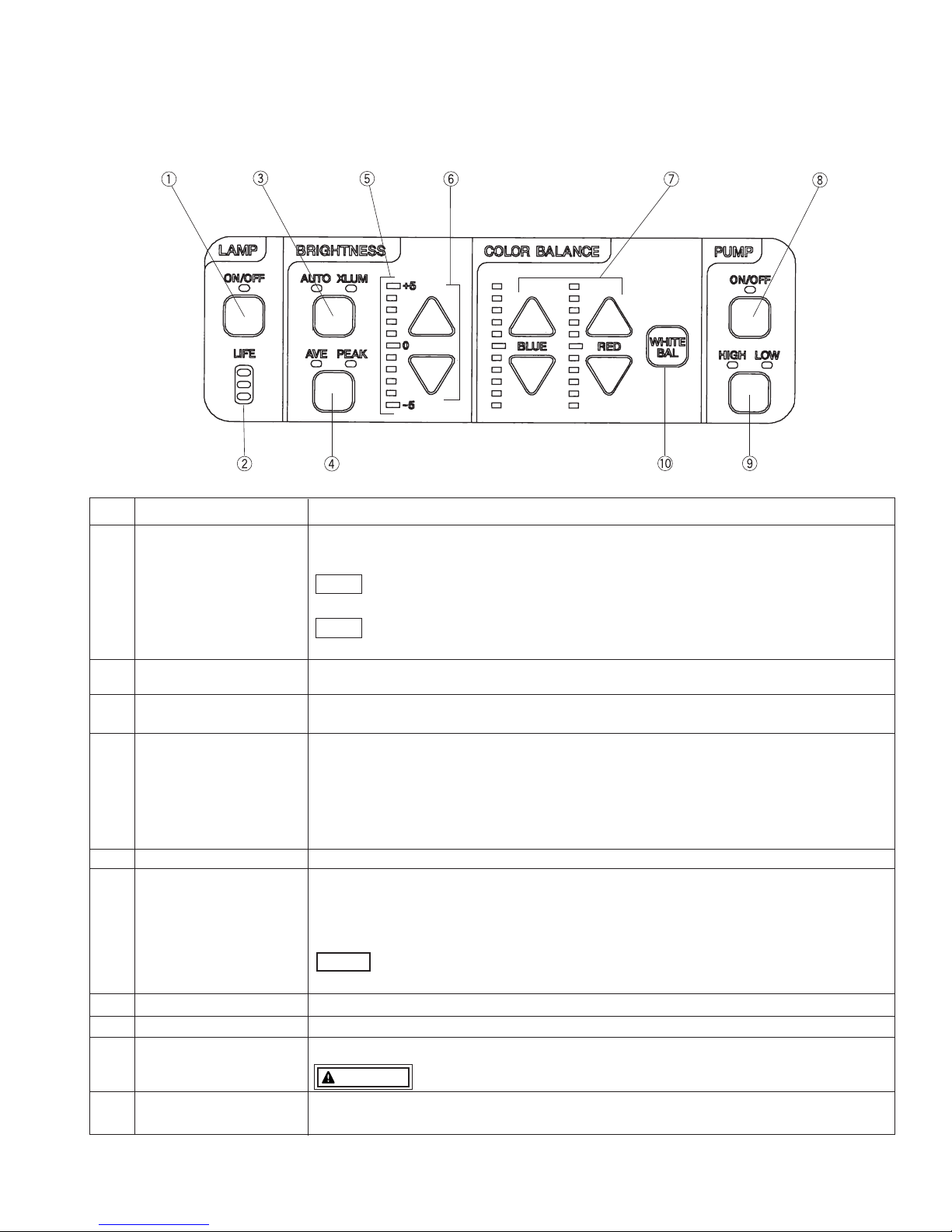
(2) FRONT PANEL
No. NAME FUNCTION
1 Lamp Switch ignites the main lamp. The LED lights green when switched ON. When the main lamp
fails to ignite, the LED flashes. Press the lamp switch again to ignite the auxiliary lamp.
-Aside from the pre-use inspection of the equipment, the lamp in the video
processor should be turned OFF when the video system is not clinically used.
-The auxiliary lamp is incorporated to the processor with serial number beginning
UB and EB.
2 Lamp Life Indicator indicates hours for Xenon lamp installed in video processor. If the indicator lights red, a lamp
should be replaced before beginning the next procedure.
3
AUTO/XLUM Select Switch
selects AUTO (automatic) or XLUM (manual) brightness control mode. AUTO or XLUM
indicators will light to indicate which is selected.
4
AVE/PEAK Select Switch
Selecting AUTO will require selection of light measuring method, AVERAGE or PEAK.
AVE or PEAK indicator lights to indicate which is selected.
AVERAGE: the brightness level is adjusted with respect to an averaging of the brightness
of the video signal.
PEAK: the brightness level is adjusted with respect to the brightness of the peak of
the screen.
5 Brightness Indicator indicates the brightness level settled by the user.
6 Brightness controls the brightness level.
Up or Down button will change the brightness level as shown on the brightness indicator.
Adjustment
△
increases the brightness level.
Switch
▽
descreases the brightness level.
-Minimum reguired brightness should be used at all times to avoid risk of injury
to the patient.
7 Color Balance Switch adjusts the video image color, Blue or Red by +/- 5 steps.
8 Pump on/off controls air pumps on/off. LED on the switch will light when switched ON.
9 Pump high/low LED on the switch indicates the pump output pressure level, High/Low.
WARNING -When selecting HIGH pressure, be careful not to deliver too much air.
10 White Balance Switch adjusts the white balance of the video endoscope. After adjustment, “WB OK!” is
displayed for about 3 seconds.
NOTE
NOTE
NOTE
Page 12
9
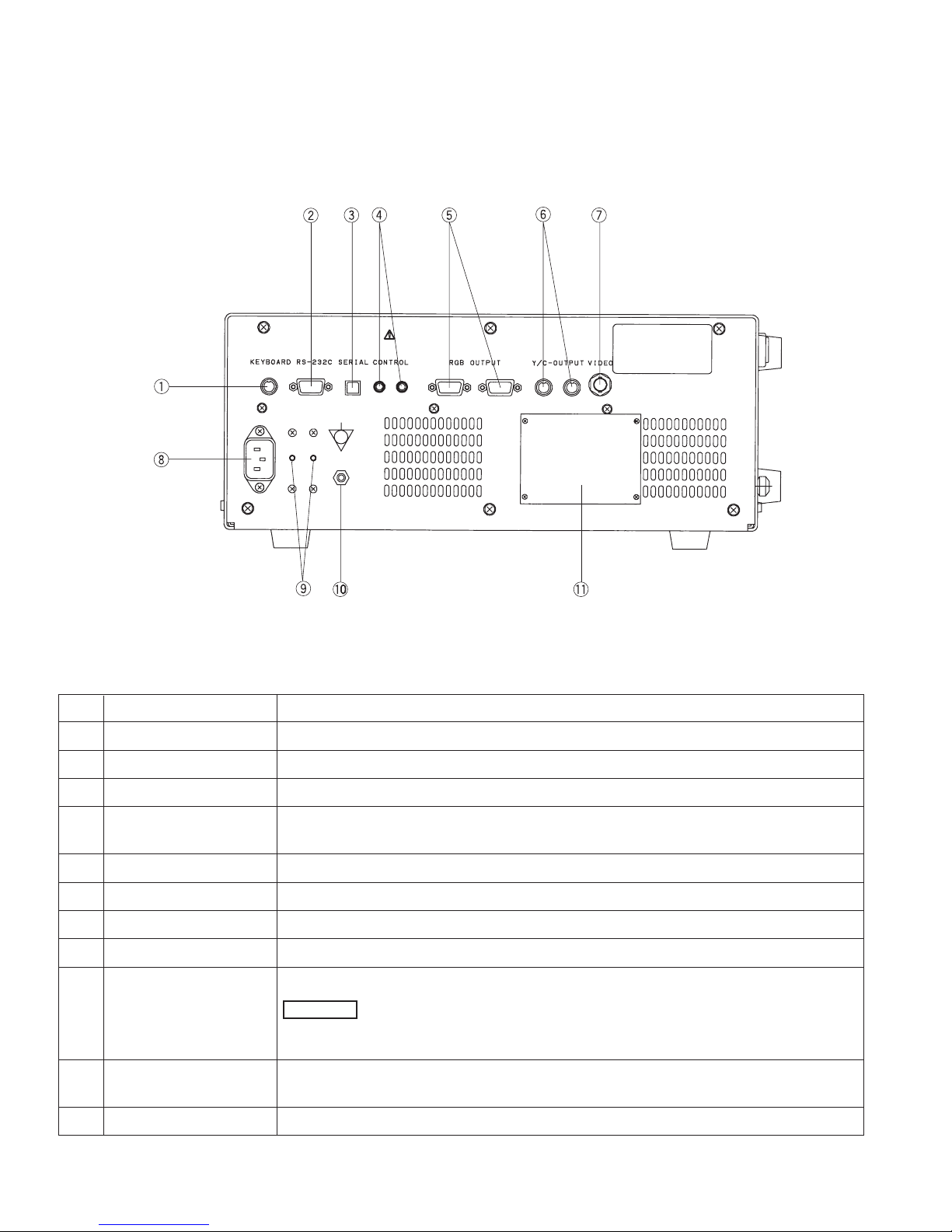
(3) REAR PANEL
No. NAME FUNCTION
1 Keyboard Connector accepts the keyboard supplied by PENTAX.
2 Interface Connector RS-232C serial interface connector.
3 Serial Bus NTSC or PAL Serial Digital Video out connector for a still-frame video image .
4 Control is activated by either the endoscope control buttons (C, V) or the keyboard copy key to
control peripherals.
5 RGB Video Output NTSC or PAL RGB video out connectors, 9-pin D-sub Female connectors.
6 Separated Video Output Y/C video out connector (4-pin S connector)
7 Composite Video Output NTSC or PAL composite video out connector, BNC type connector.
8 Power Input Socket accepts AC power cord.
9 Breaker activates with a red button sticked out when abnormal current flows.
-When the breaker is activated, try to reset first. If the breaker activates
again when the processor is turned ON, do NOT use the processor and return it to
PENTAX.
10
Potential equalization terminal
For safety purposes, this terminal is connected to a potential equalization busbar of the
electrical installation.
11 Rating Plate displays unit model number, serial number and power requirements.
CAUTION
Page 13
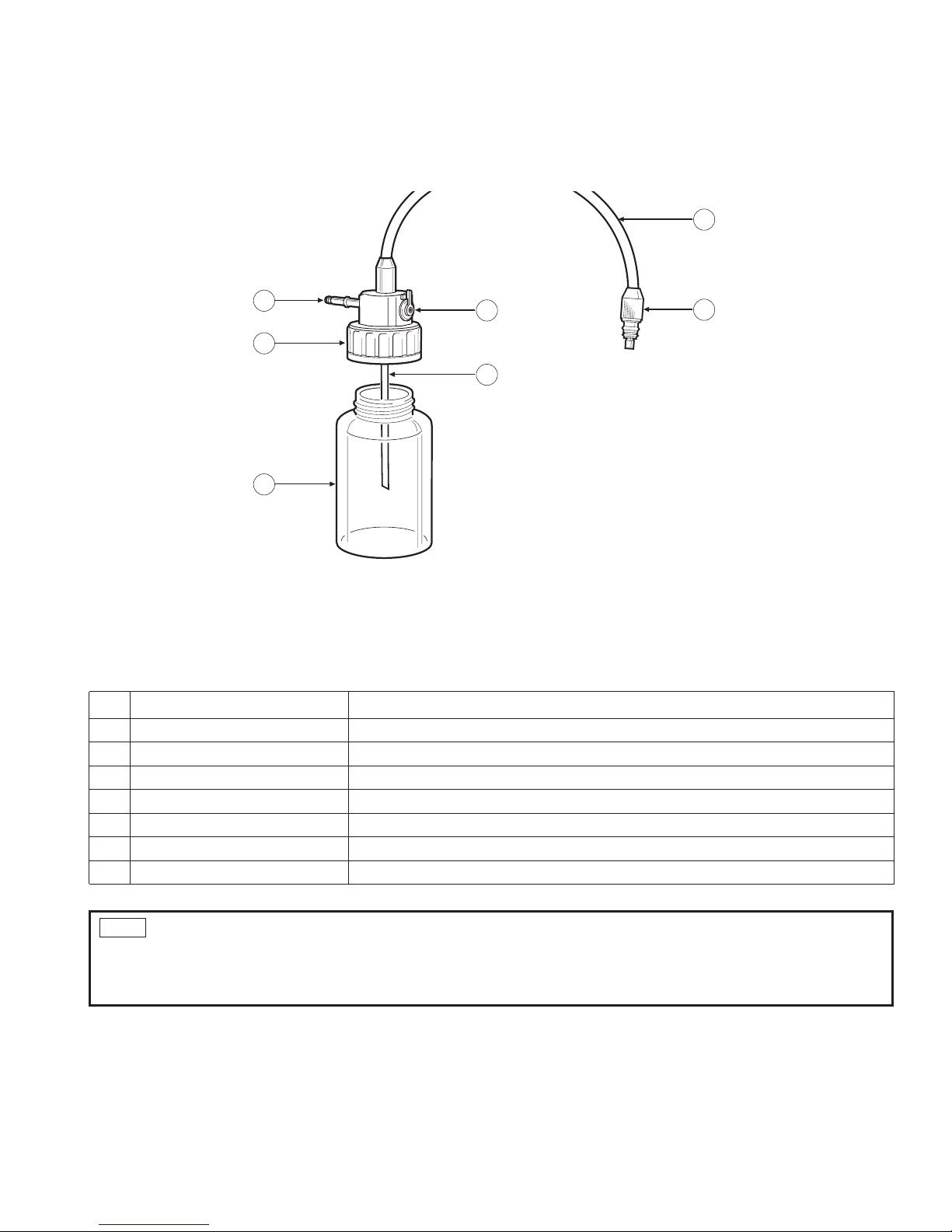
10
2-2 WATER BOTTLE ASSEMBLY, MODEL OS-H4
No. NAME FUNCTION
1 Bottle holds sterile water for procedure. (use up to 2/3’s full)
2 Water Bottle Cap Assembly
must be firmly secured to bottle to prevent air leakage. Do NOT overtighten the bottle cap.
3 Air Pipe Stem inserts into video processor water bottle receptacle.
4 Air/Water Hose contains (2) independent tubes - 1 for air, 1 for water.
5 Air/Water Connector inserts into Air/Water socket of endoscope umbilical connector.
6 Air/Water- Drain Lever must be set to upright (A/W) position for delivery of air and water.
7 Water Feeding Stem channel for water to be displaced from bottle and into scope.
1
2
3
6
7
4
5
- If the water bottle assembly has been handled roughly, the water feeding tube inside the Air/Water hose may be
disconnected at the A/W connector for the endoscope. To test, remove the cap assembly and using a syringe, inject water into
the water feeding stem. If the water comes out of both the center hole of the A/W connector and the series of holes around the
center hole, the water feeding tube is disconnected. Use another water bottle assembly.
NOTE
Page 14

11
2-3 MONITOR DISPLAY SCREEN
(1) NORMAL
(2) FREEZE (SUB-SCREEN DISPLAY)
Endoscopic image will be displayed to this area of the monitor screen when freeze function is activated.
NOTE - Appearance of sub-screen covers Date and Clock.
2
4
5
3
7
10
6
9
8
1
No. NAME FUNCTION
1 NAME Alpha-numeric field, 24 characters long.
2ID Alpha-numeric field, 12 characters long.
3AGE Alpha-numeric field, 3 characters long.
4 SEX Alpha-numeric field, 1 character long.
5 Date Numeric field
6 Clock Military format, Hours: Minutes: Seconds.
7 Doctor’s Name Alpha-numeric field, 12 characters long.
8 Facility Alpha-numeric field, 12 characters long.
9 COMMENT Alpha-numeric field, 40 characters long.
10 Main Screen Endoscopic image will be displayed on the monitor screen.
Sub-Screen Display
Page 15

12
2. Make sure the power switch is OFF.
3. Plug the power cord into the power source
using the three (3) prong plug supplied with the unit.
4. Ensure the keyboard is connected properly.
WARNING
• To reduce the potential for the electric shock, connect the power cord
of the following peripheral components into the medical isolation
transformer supplied.
– Any peripheral components connected to the PENTAX Processor.
– Any peripheral components connected to the PENTAX Endoscope.
– NEVER connect other components to the isolation transformer.
• Check that the total power consumption of all connected devices does
not exceed the isolation transformers’ power rating.
• Make sure that outputs are in compliance with IEC 60601-1-1.
• Make sure that the power cord is connected to the main with a three (3)
prong plug (In the U.S.A use UL 2601-1 rated isolation transformers
and/or power strips only).
(2) CONNECTING THE WATER BOTTLE
1. Fill the water bottle approximately 2/3 full with sterile water.
2. Screw the water bottle cap assembly to the water bottle snugly.
-Do NOT overtighten the water bottle cap
3. Set the Air/Water- Drain lever to A/W position.
4. Insert the water bottle air pipe stem into the video processor water
bottle receptacle and press until the water bottle ‘clicks’ into
position.
-Do NOT press the water bottle too forcefully into the video
processor. Rough handling may cause water to leak onto/into the
video processor.
5. Insert the Air/Water connector into the holder on the water bottle
cap assembly until the endoscope is connected
-Always disconnect the water bottle before moving the
processor into a position not common to normal use.
Always disconnect the water bottle before packing the video
processor for shipment.
EPK-1000
SAT-1300
Peripheral
A/W
DRAIN
NOTE
NOTE
NOTE
●
●
3. PREPARATION AND SAFETY CHECK
3-1 PREPARATION
WARNING - PENTAX video processors are electro-medical devices incorporating delicate
components and sophisticated circuitry that should not be subjected to harsh conditions,
including excessive vibrations and/or severe impact. NEVER drop this equipment or subject it to
severe impact as it could compromise the functionality and /or safety of the unit. Should this
equipment be mishandled or dropped, do NOT use it. Return it to an authorized PENTAX service
facility for inspection and repair.
(1) SETTING UP THE VIDEO PROCESSOR
1. Place the video processor on a stable and level surface (cart, counter, stand,etc..).
• Avoid places where the video processor may be splashed with liquid.
• Absolutely do NOT use in any environment with explosive or flammable gases.
• Avoid places where the unit could be exposed to high temperature, humidity, direct sun light, etc.
• Do NOT install, operate or store electro-medical equipment in a dusty environment.
Accumulation of dust within these units may cause malfunction, smoke, or ignition.
• Do NOT block the venting grids on the sides of the processor.
• When moving the video processor, do NOT hold the scope locking lever.
NOTE
●
Page 16

13
(3) CONNECTING THE ENDOSCOPE
- Always turn ON the processor after connecting an endoscope. Also, remove the endoscope from the processor after
turning OFF the power switch.
1. Check to ensure that the appropriate light guide adapter is mounted to the video processor. The adapter, AE-P1 is already
attached to the video processor.
- Connecting a video endoscope without a light guide adaptor in place will reduce light output at the endoscope distal
end. Attempting to connect a fiberscope without a light guide adapter and/or an appropriate light guide sleeve in place will
damage the fiberscope and the video processor.
2. Check to ensure that the scope locking lever is open.
3. Connect the scope firmly into the processor until it clicks
into position.
4. Close the scope locking lever
- After connecting the endoscope to the EPK-1000
video processor, always make sure that the endoscope is firmly
secured to the scope receptacle by turning the locking lever to
the “lock” position.
- If using the fiberscope video adaptor module, make
sure the eyepiece of the fiberscope is properly connected to the
module (use adapters as required). Connect the electrical
connector of the module to the electrical connector of the video
processor, lining up the red dots on each.
5. Connect the water bottle Air/ Water connector to the
Air/Water receptacle on the endoscope’s umbilical connector.
6. Connect the suction tube of the suction device to the suction
nipple on the umbilical connector of the endoscope.
(4) CONNECTING THE PERIPHERAL EQUIPMENT
1. Referring to the rear panel diagram, connect a TV monitor
and other required equipment such as hard copy equipment,
VCR, etc., to the processor.
- When used in clinical or residential areas near
radio and TV receiver units, this equipment may be subjected
to radio interference.
- To reduce electromagnetic interference, do NOT keep turning
ON the main POWER SWITCH of the equipment while a
videoscope is connected but not ready for use.
- To avoid and resolve adverse electromagnetic effects, do NOT
operate this equipment near the RF energy equipment.
: FOR EUROPE
- This equipment is a Class B Medical Equipment (specified
EN55011) and is intended for hospital or health care districts.
- Use the connection cables as specified below.
Composite Video Cable (1.5m), RGB Video Cable (1.5m), Y/C Video Cable (1.5m), Control (1,2) Cable (1.5m), RS-232C
Cable (1.5m), Serial Bus Cable (1.5m).
- Do NOT connect a printer simultaneously to RS232C and Control terminal located on rear panel of the processor.
- Do NOT connect two or more processors to a computer.
CAUTION
CAUTION
CAUTION
NOTE
NOTE
NOTE
●
●
●
●
CAUTION
CAUTION
Page 17

14
3-2 PRE-USE SAFETY CHECKLIST
WARNING - Before every use the following points should be checked. If any function or device in the video endoscope system
does not perform properly, do NOT perform the endoscopic examination. Contact the manufacturer of the device, your PENTAX
sales representative or a PENTAX service center before using the equipment for an endoscopic examination.
1. Ensure that power switch is OFF.
2. Ensure that video processor is placed in stable and level position.
3. Ensure that water bottle is properly prepared and connected.
4. Ensure that endoscope (and fiberscope video adaptor module if applicable) is connected properly.
- After connecting the endoscope to the EPK-1000 video processor, always make sure that the endoscope is firmly
secured to the scope receptacle by turning the locking lever to the “lock” position.
5. Ensure that keyboard is connected properly.
6. Turn ON monitor and other peripheral devices.
7. Turn ON video processor power switch. Ensure that switch is lighted green and the sound of the ventilation fans can be heard.
WARNING - After 400 hours of use, the image quality will deteriorate. Excessive use of the lamp beyond 400 hours
(approaching a thousand hours of use or more) could cause the lamp to explode resulting in damage to the video processor.
-The lamp life rated at 400 hours, is applicable to the EPK-1000 processor with serial number beginning with UB and EB.
8. Ensure that video processor lamp life indicator lights green or yellow. If red, replace the lamp.
- Always turn ON the processor after connecting an endoscope. Also, remove the endoscope from the processor after
turning OFF the power switch.
-Power function switches should not be activated by contact with objects like endoscope light guides when being switched ON
or OFF. Before turning the video processor power ON, ensure the air flow vents are not obstructed.
9. Turn on the lamp switch to ignite the lamp. If lamp fails to ignite, turn video processor OFF, wait 60 seconds and repeat steps
7 and 9.
-If lamp fails to ignite, do NOT attempt to perform an endoscopic examination, contact your PENTAX service facility.
10. With lamp lit and endoscope connected, check for live endoscopic image on the monitor.
NOTE:
It should be recognized that the use of electro-surgical accessory devices employing high frequency current
may interfere with the normal endoscopic image and this interference is not indicative of a malfunction of the
video endoscope system. PENTAX has developed an earth cable, model OL-Z3 intended to reduce potential
RF interference and electronic noise that may appear in the endoscope image when using electro-surgical
devices. Ensure that cable OL-Z3 is correctly connected between the endoscope and video processor as
described in the instructions provided with the OL-Z3.
NOTE
NOTE
NOTE
NOTE
CAUTION
to endoscope
to video processor
Page 18

15
11. Exercise the endoscope’s automatic iris. Bring the tip of the
endoscope within 1 cm off the palm of your hand and move
it to about 5 cm away from the palm. Watch the image on
the monitor to ensure the brightness at both distances is
similar. Lift the distal end of the endoscope to the room
lights, the light being emitted at the distal end of the scope
should lower significantly (dependent on the ambient light
levels in the room). Return the distal end of the endoscope
to point at the palm and ensure that the light is being emitted
from the distal end of the endoscope.
12. Check the endoscope control buttons positioned on the
control body of the endoscope.
-In combination with PENTAX 70K/72K/80K/81K/85K
series endoscopes, the function of each button can be selected
with the scope buttons key on the keyboard.
13. Select brightness control auto or XLUM. If selecting
AUTO, also select light measurement mode, average or
peak. And exercise the brightness control switches to ensure
that the brightness indicator and controls are functioning.
14. Exercise the color adjustment as described below.
Cup the distal end of the endoscope loosely in your hand. Ensure
that the monitor displays a natural color. Exercise the color adjust
switches (Red Up and Down and Blue Up and Down) to ensure
that the changes are recognizable in the image of your hand.
15. If XLUM is intended to be used during the procedure,
exercise the XLUM function. Upon selecting the switch,
LED will light and maximize the brightness level.
16. Turn ON the air pump on/off switch. The switch LED will
light and the sound of the air pump should be heard.
Select desired level, Low or High.
17. Exercise Air/Water delivery through the endoscope.
Covering air venting hole on top of Air/Water button
lightly should deliver air at the distal end of the endoscope
by submerging the distal end in enough water to cover the
tip, air flow will be demonstrated by a trail of bubbles.
Pressing the button all the way down should deliver water
through the tip of the endoscope.
-Use only “fresh” distilled or sterile water for testing
of the endoscope air/water delivery functions.
If all items above appear to function satisfactorily, then the
endoscopic procedure may be performed. If any functionality
above is compromised, do NOT attempt to perform the
endoscopic procedure.
●
●
●
●
●
Air
Feeding
Water
Feeding
NOTE
NOTE
F
U
L
R
D
Page 19

16
4. OPERATION
4-1 PROCESSOR FUNCTIONS
-Check the lamp life indicator on the front panel. If red, a lamp should be replaced before beginning a procedure.
-Before turning the processor ON, ensure the air flow vents are not obstructed.
WARNING - After 400 hours of use, the image quality will deteriorate. Excessive use of the lamp beyond 400 hours
(approaching a thousand hours of use or more) could cause the lamp to explode resulting in damage to the video processor.
-The lamp life rated at 400 hours, is applicable to the EPK-1000 processor with serial number beginning with UB and EB.
(1) MAIN LAMP
Turn ON the lamp switch to ignite main lamp and the LED on the lamp switch. The light will be emitted from the distal end of
the endoscope. When the procedure is completed, turn OFF the lamp. If the lamp fails to ignite and the LED flashes, do NOT
attempt to perform an endoscopic procedure.
-Aside from the pre-use inspection of the equipment, the lamp in the video processor should be turned OFF when the
video system is not clinically used.
(2) AUXILIARY LAMP
If the LED on the lamp switch is flashing , it indicates that main lamp is burnt out or there is a lamp failure. The message
“PUSH LAMP SWITCH” will be displayed on the monitor screen. While this error message is displayed, all function keys
(except the lamp switch) are deactivated. To ignite the lamp, press the “LAMP SWITCH” - do NOT push the “POWER
SWITCH” - while the LED is flashing. The auxiliary lamp and the LED will light.
Do NOT attempt to perform an endoscopic examination with auxiliary lamp. The auxiliary lamp is intended to assist in the safe
withdrawal of the endoscope in the event that the main lamp fails to ignite during a procedure. After igniting the auxiliary lamp,
return the endoscope to the neutral position and slowly withdraw the endoscope under controlled visualization by the auxiliary lamp.
The auxiliary lamp can be turned OFF by pressing the switch or by turning the processor OFF.
WARNING - The auxiliary lamp is intended to provide sufficient visualization to withdraw the endoscope, should the main
lamp fail. Do NOT attempt to perform an endoscopic examination with the auxiliary lamp.
-The auxiliary lamp is incorporated to the processor with serial number beginning with UB and EB.
- The auxiliary lamp in the EPK-1000 incorporates a LED which is recognized as a class 2 laser product by
IEC60825-1. Emission from the endoscope distal tip into the human body is at a level lower than class 2 as long as the lamp and
all associated finished products are used properly according to provided instructions. To protect user’s eyes, avoid looking
directly at the light exiting the endoscope and/or the processor.
Use of controls or adjustments or performance of procedures other than those specified herein may result in hazardous
radiation exposure.
NOTE
NOTE
CAUTION
NOTE
NOTE
Page 20

17
(3) BRIGHTNESS
Select the desired brightness control mode, AUTO or XLUM, using the brightness control switch.
AUTO = Automatic brightness control, where the video signal of the endoscope will automatically maintain the brightness level
selected by the brightness adjustment switches. The switch will beep when pressed and the AUTO indicator will light when the
AUTO mode is selected.
If the AUTO brightness mode is selected, select the desired brightness level and the light measurement method, AVE or PEAK,
using the light measurement switch on the front panel or the endoscope control buttons.
AVE = The brightness level is automatically adjusted with respect to an averaging of the brightness of the video signal.
PEAK= The brightness level is automatically adjusted with respect to a peak (maximum) value of the brightness of the video signal.
The switches will beep when pressed and the AVE or PEAK label will light when either is selected.
-Halation is defined as the appearance of a halo around an area of extreme brightness. Halation may occur if the AVE mode
and PEAK mode are selected. If halation is observed and if it distorts the endoscopic image, one should reduce the brightness level.
XLUM= Manual brightness control, the user will select the brightness level by using the brightness adjustment switches.
The switch will beep when pressed and the XLUM label will light when manual mode is selected.
WARNING - To protect users eyes and avoid risk of thermal injury, select the Auto mode. When the processor is used as a
light source, select the XLUM mode with the minimum required brightness. When the procedure is suspended for a while, turn
OFF the lamp switch or select the XLUM mode with the lowest brightness.
There are eleven (11) brightness levels. The brightness level will be displayed as a value -5 to +5 on the brightness indicator on
the front panel.
To change the brightness level;
Press the Up switch (△) to increase the level.
Press the Down switch (▽) to decrease the level.
The switches will beep when pressed and the brightness level indicator will change accordingly.
- To protect the users eyes and avoid risk of thermal injury during an endoscopic examination, use only the
minimum amount of brightness required.
-The video processor has a battery backup memory and will retain the last selected brightness value even if the unit is
turned OFF or disconnected from the power outlet.
(4) COLOR BALANCE
There are eleven (11) color levels for both Red and Blue hue.
They will be displayed as a value -5 to +5 on the monitor display at either end of the color bar.
All the switches described above will beep when pressed.
-The video processor has battery backup memory and will retain the last selected color balance levels even if the unit is
turned OFF or disconnected from the power outlet.
The white balance function adjusts the white balance of each endoscope. Proper
white balance requires the use of the white balance adjuster attached to the PENTAX
System Cart. Contact your local PENTAX service center for the details if required.
1) Put the distal end of the video endoscope into the white balance adjuster which
is supplied with the processor.
2) Watching the monitor, adjust the position of the distal end of the scope in the
adjuster so that the circle at the bottom of the white balance adjuster can be
recognized on the full screen.
3) Press the white balance switch while keeping the scope distal end stationary in
the adjuster more than three seconds.
If the white balance is established, “WB OKI” is displayed on the screen.
NOTE
NOTE
+
●
NOTE
CAUTION
Page 21

18
(5) PUMP
To turn the air pump On, press the Air Pump ON/OFF switch. The switch will beep when pressed. Then the LED on the switch
will light and the sound of the air pump will be heard. To turn the air pumps OFF, press the ON/OFF switch again. The switch will
beep and the LED on the switch will turn OFF.
There are two (2) air pump levels, LOW and HIGH. The switches will beep when pressed and the LED on the level switch will light
to indicate the selected level. The air pump level will control the pressure of both the air and water delivery.
- Regardless of pump level setting selected, avoid delivering too much air to minimize the potential for pneumatic
perforation or barotrauma.
-Should debris on the objective lens be difficult to clean, one can temporarily use a higher pump level setting on the
video processor or light source. While doing so, simultaneously activate the air and suction control valves to minimize air
insufflation. After the lens has been cleared return the air pressure to its original setting for routine use. Regardless of pump
level setting selected, avoid delivering too much air.
CAUTION
NOTE
Page 22

19
4-2 KEYBOARD FUNCTIONS
(1) CONVENTIONAL KEYS
No. NAME FUNCTION
1 Function Keys Refer to FUNCTION KEY INDEXES.
Esc (Escape) Key stops data entry or function menu. Returns to the normal mode.
2 Alpha-Numeric Keys include keys for letters, numbers and special characters (brackets, commas, etc.) as well
as command keys (Control, Shift, Enter etc.).
3 Back Space Key moves cursor leftward and delete the character.
4 Caps Lock Key Caps Lock indicator will light to show Caps Lock selected. When Caps Lock is ON, all
alphabet keys will be typed to the monitor screen as capitals.
5 Enter Key moves cursor to the next field or to store selected data.
6 Shift Keys When Caps Lock is OFF, holding the Shift key and pressing alpha - numeric key will
be typed to the monitor screen as a capital letter or the special character pictured on the
key.
7 Ctrl (Control) Keys is used to access function menus.
8 Space Bar gives a space and deletes a previously typed character.
9 Cursor Movement Keys Up, Down, Left and Right arrows move the cursor to each direction.
Page 23

20
(2) SPECIAL FUNCTION KEYS
No. Function Keys Function
1 Patient makes up Patient List
2 New Patient inputs patient information
3 Clear Patient deletes patient data
4 User makes up User List
5 Scope Buttons assign function to each scope control button
6 Scope SW
select two controls (output terminals) on the rear panel
(Ctrl+Scope Buttons)
7 Enhance Level selects enhance level
8 Color Adj
adjust color
(Ctrl+Enhance Level)
9 Shutter Mode changes shutter speed automatically
10 Freeze Mode
selects freeze image Frame or Field
(Ctrl+Shutter Mode)
11 Clear Screen displays items once removed from the screen
12 Color Bar
remove or display color bar
(Ctrl+Clear Screen)
13 Character Off clears all screen information
14 Date
set date and clock
(Ctrl+Character Off)
15 PC selects peripheral equipment to RS232C terminal
16 Init initializes all data
17 Clear Counter initializes the film counter
18 Stopwatch activates stopwatch
19* Multi Picture sets number of split screens on video printer
20* Print Quant sets number of prints with video printer
21* Delete Image clears images currently stored in video printer
22* Print prints data already stored in video printer
23 Pointer shows a small arrow ( pointer ) on the normal screen
24 Editor enables input freely without any format restriction
25 VTR activates output peripheral such as VTR
26 Freeze activates freeze function
27 Copy activates output peripheral such as copy
28 Capture transmits a “frozen” endoscopic image to the PC connected to the processor.
29 Scope Model displays model name and serial number of endoscope.
* applicable only when certain brand and model printers are connected to the PENTAX Video System.
Page 24

21
Operation of Function Keys
1. Patient Key
-If the Enter key is pressed during display of the normal
screen, “Patient Information” is displayed without operation of
the below (1), (2) & (3).
1) Press the key to get “Patient List 1-10” .
2) Press the key repeatedly to scroll the list up to 11-20, 21-30.
3) Press Up/Down arrow key to move the cursor to the desired
patient.
4) Press Right arrow key to get to “Patient Information” of the
selected patient.
4-1) Press Up/Down arrow key to move the cursor to the
desired selection.
4-2) Press alpha-numeric keys to input/change information.
-While Capture function is activated, do NOT use the
following special characters to input patient information such
as patient name, patient ID.
/ . * ? “ < >
4-3) Select ESC and press Enter key ( or ESC key ) to get
back to “Patient List”.
5) Press Up/Down arrow key to move the cursor to the desired
patient.
6) Press Enter key to get back the normal screen of the selected
patient.
2. New Patient Key
1) Press the key to get “Patient Information” .
2) Press Up/Down arrow key to move the cursor to desired
selection.
3) Press alpha-numeric keys to input information.
4) Select ESC and press Enter key ( or ESC key) to get back to
the normal screen of the patient.
3. Clear Patient Key
Function: Delete data on all patients when “Patient List” is
displayed.
Delete data on one particular patient when “Patient
Information” is displayed.
1) Press the key to be asked for confirmation.
2) Press Up/Down arrow key to move the cursor to the desired
selection.
3) Press Enter Key to make selection.
3-1) in case of Patient List
• If Yes, data on all patients is deleted.
• If No, the list retains the previous data.
3-2) in case of Patient Information or normal screen
• If Yes, data on the selected patient is deleted.
• If No, the data on all patients is retained.
4) Select ESC and press Enter key ( or ESC key) to exit the
menu.
●
●
●
●
●
NOTE
NOTE
Page 25

22
4. User Key
1) Press this key to get “User List 1-10”.
2) Press this key to scroll the list up to 11-20... 41-50.
3) Press Up/Down arrow key to move the cursor to the desired
selection.
4) Press Right arrow key to get “User Setup”.
4-1) Press Up/Down arrow key to the desired selection.
4-2) Press alpha-numeric keys to input information.
4-3) Press Right arrow key at “Display Item Setup” to get
“Display Item Setup”.
4-3-1) Press Up/Down arrow key to the desired selection
4-3-2) Press Right/Left arrow key to select On or Off.
4-3-3) Select ESC and press Enter key ( or ESC key ) to
get back User Setup.
4-4) At “User Setup”, select ESC and press Enter key ( or
ESC key) to get back to “User List”.
5) Press Up/Down arrow key to move the cursor to the desired
selection.
6) At “User List”, press the Enter key to return to the original
User List screen selected by the user.
5.
Scope Buttons Key (70K/72K/80K/81K/85K Series Endoscopes)
Function: Assign function to scope control buttons
1) Press this key to get the menu.
2) Press Up/Down arrow key to move the cursor to the desired
button
3) Press Right/Left arrow key to assign function to the each
selected button
• Right arrow key: 0 → 1 → 2 → 3 → 4 → 5 → 6 → 7 →
8 → 0
• Left arrow key: 8 → 7 → 6 → 5 → 4 → 3 → 2 → 1 →
0 → 7 → 8
4) Select ESC and press Enter key ( or ESC key ) to get back to
the normal screen.
●
●
●
●
Page 26

23
6. Scope SW
- NEVER assign a PC or printer using the Scope
Switch menu while a computer or a printer has been disconnected
from the processor
.
1) Press this key to get the menu.
2) Press Up/Down arrow key to move the cursor to the desired
selection
3) Press Right/Left arrow key to assign function to the each
selection
• Film Counter: None
→ Out1 → Out2 → Printer* → None
• Copy Function: None
→ Out1 → Out2 → Out1,2 → PC →
PC & Out1 → PC & Out2 → PC & Out1,2 → Printer* →
Printer & Out1* → Printer & Out2* → Printer & Out1, 2*
→ Printer & PC* → Printer & Out1 & PC* → Printer &
Out2 & PC* → Printer & Out1,2 & PC* → None
• VTR Function: None → Out1 → Out2 → Out1,2 → PC →
PC & Out1 → PC & Out2 → PC & Out1,2 → Printer* →
Printer & Out1* → Printer & Out2* → Printer & Out1, 2*
→ Printer & PC* → Printer & Out1 & PC* → Printer &
Out2 & PC* → Printer & Out1,2 & PC* → None
*Appears only when PC key selects Sony Video Printer UP-50.
• Freeze Release: On → Off → On
• Counter Type: 1 to 99 (1, 2... 98, 99, 1...)
→ 1/2 (1/2, 2/2, 1/2, 2/2... )
→ 1/4 (1/4, 2/4, 3/4, 4/4, 1/4...)
→ 1/8 (1/8, 2/8... 7/8, 8/8, 1/8...)
→ 1/16 (1/16, 2/16... 15/16, 16/16, 1/16...)
→ Off
→ 1 to 99 (1, 2... 98, 99, 1...)
-“Printer followed” is displayed when PC key selects
Sony Video Printer UP-50 and when film counter selects
“printer”.
4) Select ESC and press Enter key ( or ESC key ) to get back to
the normal screen.
7. Enhance Level
1) Press this key to display the message for about 3 seconds.
2) During the display, press the key to move to Off → Low →
Med → High → DFLT → Off.
-DFLT is displayed when special level is previously
assigned to the scope. When the scope is disconnected from the
processor with setting of DFLT , the DFLT remains on the
memory. When the scope is disconnected with setting other than
DFLT , the DFLT will not remain on the memory.
- This menu can only be displayed if an endoscope is
connected to the processor.
8. Color Adjustment
1) Put the distal end of the endoscope into the white balance
adjuster supplied with the video processor.
2) Watching the monitor, adjust the position of the distal end of
the scope in the adjuster so that the grains inside the adjuster
can be recognized on the full screen. If any halation is
recognized on the screen, lower the brightness level so that
the grains inside the adjuster can be recognized clearly.
●
●
●
NOTE
NOTE
NOTE
CAUTION
CAUTION
Page 27

24
3) Press this key to get the menu.
4) Press Up/Down arrow key to move the cursor to “White Balance”.
5) Press Enter key and remove the distal end from the adjuster.
After adjustment, “WB OK!”, is displayed for about 3
seconds.
6) If fine-tuning is necessary, press Up/Down key to the desired
selection, blue or red.
7) At blue or red, press Right/Left arrow key to change the value.
8) Select ESC and press Enter key ( or ESC key ) to exit the
menu.
- This menu can only be displayed if an endoscope
is connected to the processor.
9. Shutter Mode
- When activated (“Shutter On”), the shutter speed is
automatically increased during close viewing to obtain very
sharp “frozen” images. This function applies only to 70K/72K/
80K/81K/85K-series endoscopes.
1) Press this key to display the message for about 3 seconds.
2) During the display, press the key to toggle between Shutter
On and Shutter OFF.
- This menu can only be displayed if an endoscope
is connected to the processor.
10. Freeze Mode
• Field Freeze: for a more stabilized frozen image.
• Frame Freeze: for a higher resolution.
1) Press this key to display for about 3 seconds.
2) During the display, press the key to toggle between Field and
Frame.
11. Clear Screen
1) Press this key to show the data once deleted with setting of
User key.
2) Press the clear screen key again to remove the data
12. Color Bar
1) Press this key to get the color bar on the screen.
2) Press this key to remove the color bar from the screen.
13. Character Off
1) Press this key to remove all screen information. When an
endoscope is not connected, “Scope not connected” is
displayed.
2) Press this key again to get the information back on the
screen.
●
●
●
●
NOTE
NOTE
CAUTION
CAUTION
Page 28

25
14. Date
1) Press this key to get the menu.
2) Press Up/Down arrow key to move the cursor to the desired
selection.
3) Press Enter key to make selection and to get the cursor under
the first field.
4) Input numeric data in each field.
5) Press Right arrow key to advance through a field without
changing the information.
6) Press Enter key or ESC key to exit the menu.
15. PC
1) Press this key to get the menu.
2) Press Up/Down arrow key to move the cursor to the desired
selection.
3) Press Enter key to make selection. The selected one turns
green.
4) Select ESC and press Enter key (or ESC key) to remove the
menu.
- The selection depends upon country and/or local
PENTAX distributor.
16. Init
1) Press this key to be asked for confirmation if “Reset all
processor data? No/Yes”
2) Press Up/Down arrow key to move the cursor to the desired
selection.
3) Press Enter key to make selection
• If Yes: all data is reset.
• If No: the question is removed without resetting any data.
NOTE
●
●
●
●
●
Page 29

26
17. Clear Counter
- This key is not valid when scope SW selects “NONE”
for film counter.
1) Press this key to initialize the value already set with scope
SW.
18. Stopwatch
1) Press this key to get the menu
2) Press Up/Down arrow key to move the cursor to the desired
selection.
3) Press Enter key to make selection
• Start: displays and runs the stopwatch
• Stop: stops the stopwatch and displays the last value
• Restart: restarts the stopwatch from the last value
• Reset: stops the stopwatch and resets the display to
(00:00:00)
• ESC: exit the menu. When it is on “Reset”, the stopwatch
will be removed from the normal screen. In the other
cases, the stopwatch will remain on the normal
19. Multi Picture
- This function is valid on condition that;
• Copy function is activated with scope switch
• PC key selects Sony Video Printer UP-50
• Sony Video Printer UP-50 is connected to RS-232C output
terminal
1) Press this key to display number of split screens already set
for about 3 seconds
2) Press this key repeatedly to change it to 1 → 2 → 4 → 8 →
16 → 1.
- Press this key to start printing provided the video
printer already contains previously stored images (and
provided that before printing, the cursor is not positioned on
the first image). After printing, multi-picture images change
according to the pre-selected setting.
- While menus for other functions are displayed on
the screen, this function key will not work. Return to the live
screen and then press the key to activate this function.
20. Print Quant
- This function is valid only on condition that;
• Copy function is activated with scope switch
• PC key selects Sony Video Printer UP-50
• Sony Video Printer UP-50 is connected to RS-232C output
terminal
1) Press this key to display for three seconds the quantity of
prints required
2) Press the key repeatedly to change it to 1
→ 2 → 3 ... 9 → 1
- While menus for other functions are displayed on
the screen, this function key will not work. Return to the live
screen and then press the key to activate this function.
NOTE
NOTE
NOTE
NOTE
●
●
●
●
●
CAUTION
CAUTION
Page 30

27
21. Delete Image
- This function is valid only on condition that;
• Copy function is activated with scope switch
• PC key selects Sony Video Printer UP-50
• Sony Video Printer UP-50 is connected to RS-232C output
terminal
1) Press this key to display “ Delete Image?” for about 3
seconds.
2) During the display, press this key to delete the images
currently stored.
3) If the monitor is connected to a video printer, “Delete
Image? ” is not displayed. Instead, this cursor moves from a
split screen to another as shown below to delete each image.
- While menus for other functions are displayed on
the screen, this function key will not work. Return to the live
screen and then press the key to activate this function.
22. Print
- This function is valid only on condition that;
• Copy function is activated with scope switch
• PC key selects Sony Video Printer UP-50
• Sony Video Printer UP-50 is connected to RS-232C output
terminal
1) Press this key to display “Print OK?” for about 3 seconds
2) During the display, press the key to start printing
- While menus for other functions are displayed on
the screen, this function key will not work. Return to the live
screen and then press the key to activate this function.
23. Pointer
1) Press this key to call the pointer.
2) Press Up/Down/Right/Left arrow key to change the position.
3) Press ESC key or the pointer key again to remove the pointer
●
●
●
●
NOTE
NOTE
CAUTION
CAUTION
Page 31

28
24. Editor
- Date and clock remain unchanged.
1) Press this key to be asked for confirmation.
2) Press Up/Down arrow key to move cursor to desired
selection.
3) Press Enter key to make selection
• If “No” is selected, data, clock and previous editor data are
displayed.
• If “Yes” is selected, data and clock are displayed ( previous
editor data is deleted.)
4) Press Up/Down/Right/Left arrow key to move cursor to
desired position.
5) Press alpha-numeric key to input ( except date and clock )
6) Press ESC key, Editor key or other function keys to exit the
menu.
25. VTR
- This function is valid only on condition that;
• VTR function is activated with scope switch
• PC key selects Sony Video Printer UP-50
• Sony Video Printer UP-50 is connected to RS-232C output
terminal
1) Press the key to activate output peripheral such as VTR
according to the setting with the scope switch key.
26. Freeze
1) Press this key to get a still endoscopic image frozen on the
main screen and the endoscipic live image on the sub-screen
activate freeze function.
2) Press the key again to release the main screen from the still
image and remove the sub-screen.
27. Copy
- This function is valid only on condition that;
• Copy function is activated with scope switch
• PC key selects Sony Video Printer UP-50
• Sony Video Printer UP-50 is connected to RS-232C output
terminal .
1) Press this key to activate output peripheral such as copy
according to the setting with the scope switch key.
28. Capture
- The PC Capture function will work ONLY when;
The special software, Endoimage OS-I1, is installed onto the
PC connected to the processor.
1) Press this key to transmit a “frozen” endoscopic image to the
PC.
Alternatively, instead of using this button on the keyboard,
the same PC Capture function can be activated via one of the
remote control buttons that have previously been assigned
for this specific function (70K/80K Series Endoscopes only)
29. Scope Model
The model name and serial no. of the scope are displayed for
about 1 minute when pressing any character key during the
normal screen display. Press any character key again to remove
the data.
●
●
sub-screen
●
●
NOTE
NOTE
NOTE
NOTE
Page 32

29
5. MAINTENANCE
5-1 AFTER EACH PROCEDURE
1. Turn OFF the power switch.
-Some peripheral devices may have to be turned OFF
BEFORE the EPK-1000 to avoid compromising their operation.
Refer to the operating instructions supplied with all the components
of the video endoscopy system to establish the right order in which
each component should be turned OFF in due course.
2. Disconnect the power plug, endoscope and water bottle .
-Always turn OFF the processor BEFORE disconnecting
the endoscope.
3. Wipe all surfaces with gauze slightly dampened with alcohol.
-NEVER allow liquids to be splashed on the EPK-1000.
Be sure connector interfaces and ventilation ports are not
allowed to become wet. To avoid processor damage, do NOT
allow harsh chemicals or cleaning agents to contact the front
panel membrane. Wipe with alcohol only.
5-2 WATER BOTTLE CLEANING
-Be careful when handling the water bottle. Do NOT
carry the water bottle by the Air/Water connector or Air/Water
hose. When the cap assembly has been separated from the
bottle, be careful in handling the water feeding stem.
Water bottles should be sterilized at least on a daily basis.
Like all endoscopic accessories, the water bottle assembly must
be thoroughly cleaned. Failure to do so could result in
incomplete or ineffective sterilization.
(1) CLEANING
1. Immediately after use, the entire water bottle assembly
(bottle, cap assembly and tubing) should be washed with
fresh detergent solution and dampened gauze or scrub brush.
Complete immersion in an enzymatic detergent solution
should be used for soiled items.
Internal surfaces of the water bottle assembly may be exposed
to the detergent by injecting the detergent into the air pipe
stem (opposite the A/W-Drain lever on the water bottle cap)
using a syringe. The A/W-Drain lever should be set to the
A/W position to ensure contact with all internal tubes.
2. Ultrasonic cleaning of the entire water bottle assembly is
recommended to access difficult to reach areas. Use an operating
frequency of 44 kHz ± 6% for a period at least 5 minutes.
3. After washing with the cleaning solution, all surface areas of
the water bottle assembly should be thoroughly rinsed and
dried. Use gauze or lint-free cloth to wipe dry most surfaces.
Compressed air and 70% alcohol may be used to facilitate
drying of hard to reach areas.
NOTE
NOTE
NOTE
NOTE
PENTAX
EPK-700
COPE
RED
BLUE
AUTO
MAN
XLUM
SERVICE
AVE
CENT
CONTROL
COLOR BALANCE
BRIGHTNESS
LAMP
FREEZE
+5
-5
0
AIR PUMP
POEWR
O
FF
OFF
ON
ON
12345
6
●
●
Page 33

30
(2) STERILIZATION
Before any attempt has been made to sterilize the water bottle assembly, ensure that the cleaning process above has been completed.
- The sterilization parameters are only valid with sterilization equipment that is properly maintained and calibrated.
Use appropriate heat process indicators and/or biological monitors as recommended by the manufacturer of the sterilizer.
(2-1) STEAM STERILIZATION
1. The OS-H4 water bottle assembly has been designed to withstand high pressure steam sterilization procedures. Use the
parameters below;
2. During steam sterilization, ensure that the cap and tubing section have been removed from the water bottle container. Make
sure that the drain lever on the water bottle cap is set at A/W position (upright).
- Use only the type of packaging material and package configuration as recommended by the manufacturer of the
sterilizer. Use appropriate heat process indicators and/or biological monitors as recommended by the manufacturer of the
sterilizer.
(2-2) ETO STERILIZATION
1. Ethylene Oxide (ETO) gas sterilization can be performed on PENTAX water bottles, provided they have first been properly
cleaned and thoroughly dried.
WARNING - Failure to thoroughly dry all surface areas could result in Incomplete or Ineffective sterilization. Moisture
could prevent contact of the ETO gas with the actual contaminated surfaces.
2. The following parameters for Ethylene Oxide Gas Sterilization are proposed.
Temperature: 55˚C (131˚F)
Relative Humidity: 50%RH
EO Concentration : 600-650mg/L
Gas Exposure Time: 5 Hours
Aeration: 12 Hours at 55˚C (131˚F)
3. During ETO sterilization, ensure that the cap and tubing section have been removed from the water bottle container. Make
sure that the drain lever on the water bottle cap is set at A/W position (upright).
Sterilizer Type : Prevacuum
Temperature : 132 ~ 135°C (270 ~ 275°F)
Time : 5 minutes
NOTE
CAUTION
Page 34

31
5-3 STORAGE
Do NOT store the unit in direct sunlight or where temperature and humidity are high or where it can be exposed to liquids.
For long term storage, take precautions to reduce dust build up within the EPK-1000. Accumulation of dust within these units
may cause malfunction, smoke, or ignition.
5-4 CHANGING THE LAMP
Check the lamp life indicator on the front panel. If red, the old
lamp should be replaced with a new lamp before beginning
another procedure.
Lamp Replacement
WARNING - When lamps require replacement in the EPK-1000, PENTAX strongly recommends the replacement of both
lamp and cartridge as a set. These PENTAX replacement lamp/cartridge assemblies have been developed for optimal
illumination and safety. Only replacement lamp modules, namely PENTAX model OL-X22 for 120V EPK-1000 and OL-X20 for
100V/230V EPK-1000 units should be used.
The use of unauthorized lamps can create a potential for excessive light intensity and/or heat whose impact to patient safety has
not been established.
- During the following procedures, always wear rubber gloves to prevent application of skin oils to the glass. Do
NOT touch the glass surfaces of the new lamp directly with one’s fingers.
A. How to remove the lamp unit.
1. Turn OFF the processor by depressing the power switch and disconnect the plug from the electrical outlet.
2. Using a Phillips screw driver, open the lamp housing cover to expose the lamp unit.
- Immediately after use, the metal lamp cover and
the lamp bulb may be HOT. To avoid burns, do NOT touch
these areas immediately after use.
- Be careful not to lose the four screws removed
from the lamp housing cover.
3. Squeeze both sides of the lamp connector with one’s thumb
and forefinger and pull out the connector from the lamp
socket (Fig.2-1).
4. Turn the lamp fixture knob counter clockwise to loosen it
(Fig.2-2).
- The Lamp fixture knob is wired not to fall out from
the lamp base. NEVER pull it out forcibly.
●
CAUTION
CAUTION
CAUTION
NOTE
Fig.1
Page 35

32
5. Carefully pull out the lamp unit from the lamp base and do
NOT to hit the lamp unit against the fixture knob (Fig.2-3).
6. Follow local regulations for discarding the old lamp. If
unsure of appropriate procedures for lamp disposal, return
the lamp unit to PENTAX after packing it appropriately to
avoid any damage during shipping.
B. How to attach a lamp unit
1. Hold the lamp unit with the nut on the edge of the lamp in
an upward position and carefully align the hook of the lamp
unit with the groove of the lamp base and push the lamp unit
into lamp base (Fig.3-1).
2. Turn the fixture knob clockwise to secure firmly (Fig.3-2).
3. Squeeze both sides of the lamp connector with the V mark
upward as shown in Fig.3 and gently insert the connector
into the socket (Fig.3-3).
4. Attach the lamp housing cover and ensure that the cover
does not make contact with or close upon the cable (Fig.1).
- Contact of the cable with the lamp housing cover
may result in malfunction of ignition.
C. Reset the lamp life indicator.
After replacing the new lamp unit, reset the lamp life indicator
on the front panel of the processor.
1. Plug the power code into the electrical outlet and turn ON
the power.
2. Hold “Ctrl”, “Alt” key and press “Stopwatch” key to display
“Life Meter Set” menu on monitor screen.
3. Press Up/Down arrow key to move the cursor to “Lamp
Reset” and then press “Enter” key. Press “ESC” key to exit
from “Life Meter Set” menu.
4. Turn OFF the power switch and press ON/OFF switch
again. Confirm that the LED on the lamp life indicator lights
in green color.
5-5 RESETTING THE BREAKERS
1. If the video processor is not operational after turning ON the
power, turn the power switch OFF.
2. Check the breaker buttons on the rear panel. If these buttons
are sticked out, press both buttons down until they “click”
into the depressed positions.
3. If operation stops during procedure, first turn the processor
OFF. Wait 10 seconds or more and then press both breaker
buttons down until they click into the depressed positions.
4. After resetting the breakers, if the breakers still activate
when the EPK-1000 is turned ON, turn the power OFF
immediately and disconnect the power cord. Contact
PENTAX service center.
CAUTION
Fig. 2-1 Lamp Connector
Fig. 2-3
Lamp Unit
Fig. 2-2 Fixture Knob
Fig. 3-3 Lamp Connector
Fig. 3-1
Lamp Unit
Fig. 3-2 Fixture Knob
●
Fig. 2 How to remove the lamp unit
Fig. 3 How to attach a lamp unit
Page 36

33
CONDITION CHECK ACTION
Power does not Power cord Ensure proper connection at unit and wall outlet.
come ON
Breaker Ensure that the red button is not protruding out.
Power outlet Move the power cord to a power socket that is known to work.
No display on monitor
Monitor/ other
Ensure that power is ON for all devices.
peripheral devices
Ensure that proper video input is selected for all devices.
Cable connections Ensure that all video cables are connected properly.
Text cannot be Keyboard Ensure that keyboard is properly connected to the processor.
typed to screen
Lamp will not ignite Lamp life indicator If the indicator lights red, a new lamp should be installed before
beginning the next procedure.
No image on main screen Endoscope Ensure that the endoscope is connected properly.
Lamp Ensure that lamp is ignited.
Image on screen Lamp Ensure that lamp is ignited.
is black and white
Unusual characters
on screen and no response
Turn OFF the processor and contact your PENTAX service center.
to any key on the keyboard
Scope Ensure that endoscope is properly connected.
Freeze, Copy, VCR, Test keyboard control buttons. If they activate features and
keyboard, control buttons endoscope control buttons do not, call a service representative.
Cables Ensure that all control cables are properly connected.
Air pump Ensure that air pump is ON.
Water bottle Ensure proper connections at EPK-1000 and endoscope.
Ensure that the A/W-Drain lever is in the A/W position.
A/W valve Ensure that the A/W valve aperture is not clogged, inspect all
O-rings, clean the A/W valve.
Endoscope Check endoscope to confirm that the air channel/nozzle is not
clogged/blocked.
Air pump Ensure that air pump is ON.
Water bottle Ensure proper connections at EPK-1000 and endoscope.
Ensure that water bottle is 2/3 full.
Ensure that the A/W-Drain lever is in the A/W position.
Ensure that the water feeding stem is connected to the cap
Assembly inside the bottle.
A/W valve Ensure that the A/W valve aperture is not clogged, inspect all O-rings,
clean the A/W valve.
Endoscope Check endoscope to confirm that the air channel/nozzle is not
clogged/blocked.
Peripheral devices Ensure that Sony Video Printer, up-50 is connected properly to RS-232C
(up-50) output terminal and turned on.
6. TROUBLE-SHOOTING GUIDE
Endoscope control
buttons do not control
functions
No air delivery
at distal end of
endoscope
“PRINTER NOT
CONNECT” is displayed
on monitor
No water delivery
at distal end of
endoscope
Page 37

34
CONDITION CHECK ACTION
“PC Warning” is
displayed on monitor
Check the amount of
free storage space on
the hard disk drive and
the setup value of
“Alert Disk Size” on
the setting panel of
Endoimage, OS-I1.
To obtain sufficient disk storage space exceeding the value of “Alert
Disk Size”, delete the exceeded files or add more hard disk capacity.
“PC Alert” is displayed
on monitor
(The amount of free
space is less or equal to
1 MByte.)
Check the amount of
free storage space on
the hard disk drive and
the setup value of
“Alert Disk Size” on
the setting panel of
Endoimage, OS-I1.
To obtain sufficient disk storage space exceeding the value of “Alert
Disk Size”, delete the exceeded files or add more hard disk.
“SCP COM ERR” is
displayed on monitor
Ensure that the
endoscope is connected
properly.
First, turn OFF the processor and other peripheral devices per
instructions supplied with the products.
Then remove the endoscope from the interface socket. Next, reconnect
the endoscope to the interface socket. Turn ON all devices per the
instructions.
“PC CAP ERR” is
displayed on monitor
(Internal communication
fault )
Turn OFF the processor and then ON again, or re-enter the patient
name or ID.
Long beep signal. Copy
and VTR function do not
activate.
• Scope Switch menu
• Computer/Peripheral
devices
• Change the selected settings of Copy and VTR by Scope Switch
menu. Do NOT select the device switched OFF or disconnected.
• Ensure that the peripheral devices are properly connected to
computer.
• If Capture function is activated, ensure that Endoimage OS-I1
functions properly.
PRINTER NOT
CONNECTED is
displayed on monitor.
•Printer • Ensure that power switch of the printer is ON.
• Confirm that the cable between the processor and the printer is
securely connected.
• Check the settings of the printer according to the manual supplied
with printer.
PC NOT FOUND is
displayed on monitor.
• Ensure that the PC is
connected prorerly to
the processor.
• Ensure that power
switch of the PC is ON.
• Ensure that Endoimage
is activated.
• First, turn OFF the processor and other peripheral devices per
instructions supplied with the products. Then connect the PC to the
processor.
Next, turn ON all devices per the instructions.
• Turn the power switch ON.
• Confirm that the main screen of Endoimage is appeared on the
monitor.
If not, install the provided installation CD of Endoimage into your
PC as per the manual supplied with the processor.
“LFM COM ERR” is
displayed on the monitor
screen.
Check the lamp life
indicator.
If the LED within the indicator does not light, turn the power OFF the
video processor and contact your local PENTAX service facility.
* If the condition remains, contact your PENTAX service facility.
Page 38

35
7. SPECIFICATIONS
Item Specification FOR USA & CANADA EUROPE & OCEANIA
Power Requirements Voltage 120 VAC (NTSC MODEL) 230VAC (PAL MODEL)
Frequency 50 – 60 Hz
Power consumption 2.0A 1.0A
Voltage fluctuation +/– 10 %
Operating Environment Temperature 10 ~ 40°C
Relative humidity 30 ~ 85 %
Air pressure 700-1060 hPa
Storage/transport Temperature –20 ~ 60°C
Environment Relative humidity 0 ~ 85 %
Air pressure 700 ~ 1060 hPa
Illumination Lamp Xenon Lamp
XBO R 100w/10A
Lamps average life span 400 hours (the processor with serial no. UBxxxx/EBxxxx)
Color temperature ≤ 6,500 K
Lighting format Switching regulator with continuous illumination
Brightness control Selection - Automatic or Manual
Automatic iris Servo type
Scope Compatibility PENTAX color video endoscopes
NTSC MODEL PAL MODEL
PENTAX fiberscopes With use of appropriate fiberscope video adapter module
NTSC MODEL PAL MODEL
Other manufacturers’ fiberscopes With use of appropriate fiberscope video adapter module and
appropriate eyepiece/light guide adapters
Air Feed System Air pump system DC Diaphragm
Pressure setting at flow rate of 0 50 ~ 70 kPa (7.3~10.2 PSI) 40 ~ 62 kPa (5.8~9.0 PSI)
Standard air feed volume at inlet of water bottle
Low: 1.8 ~ 3.2 L/min Low: 3.8 ~ 5.9 L/min
High: 3.3 ~ 5.0 L/min High: 6.0 ~ 9.5 L/min
Water Feed System Water bottle assembly pressurized by pump Bottle capacity = 250mL
Brightness Control System
Automatic Selection - Average or Peak
Manual +/– 5 step adjustment
Color System Color correction Red +/– 5 steps
Blue +/– 5 steps
Freeze Function Live video image provided
when freeze mode activated
Cooling Forced air cooling
Video Outputs 2 sets: RGBS (NTSC or PAL), 9pin D-Sub female connectors
1 set : Composite (NTSC or PAL), BNC connector
2 sets: Separate Video (Y/C), 4-pin female connector
1 set : Computer, 9-pin D-Sub female connector
Classification as Type of protection against electric shock Class I equipment, 3 prong plug
Electro Medical Equipment
Degree of protection against electric shock BF Type (Body Floating), using insulated endoscope.
Use on heart is prohibited
Degree of explosion proofing Do not use in potentially flammable surroundings
Electro Magnetic Compatibility
EN 60601-1-2 (2002) for EU IEC 60601-1-2 (2001) for other countries
Compliance Designed in accordance with
UL 2601-1
IEC 60601-1
Size Dimensions Width = 380 mm Height = 155 mm Depth = 420 mm
Weight Main Body = 15.0 kg
Page 39

36
8. ELECTROMAGNETIC COMPATIBILITY
Guidance and manufacturer’s declaration-electromagnetic emissions
The EPK-1000 is intended for use in the electromagnetic environment specified below. The customer or the user of the EPK-1000
should assure that it is used in such an environment.
Emissions test Compliance Electromagnetic environment-guidance
RF emissions
CISPR 11
Group 1
The EPK-1000 uses RF energy only for its internal function. Therefore, its RF emissions are
very low and are not likely to cause any interference in nearby electronic equipment.
RF emissions
CISPR 11
Class B
Harmonic emissions
IEC 61000-3-2
Class A
Voltage fluctuations/
flicker emissions
IEC 61000-3-3
Complies
The EPK-1000 is suitable for use in all establishments, including domestic establishments
and those directly connected to the public low-voltage power supply network that supplies
buildings used for domestic purposes.
Guidance and manufacturer’s declaration-electromagnetic immunity
The EPK-1000 is intended for use in the electromagnetic environment specified below. The customer or the user of the EPK-1000
should assure that it is used in such an environment.
Immunity test IEC 60601 test level Compliance level Electromagnetic environment-guidance
Electrostatic
discharge (ESD)
IEC 61000-4-2
±(2, 4, 6) kV contact
±(2, 4, 8) kV air
±(2, 4, 6) kV contact
±(2, 4, 8) kV air
Floors should be wood, concrete or ceramic tile, if
floors are covered with synthetic material, the relative
humidity should be at least 30 %.
Electrical fest
transient/burst
IEC 61000-4-4
±2 kV for power supply lines
±1 kV for signal lines
±2 kV for power supply lines
±1 kV for signal lines
Mains power quality should be that of a typical
commercial or hospital environment.
Surge
IEC 61000-4-5
±1 kV differential mode
±2 kV common mode
±1 kV differential mode
±2 kV common mode
Mains power quality should be that of a typical
commercial or hospital environment.
Power frequency
(50/60 Hz)
magnetic field
IEC 61000-4-8
NOTE: UT is the a.c. mains voltage prior to application of the test level.
3A/m 3A/m
Power frequency magnetic fields should be at levels
cheracteristic of a typical location in a typical
commercial or hospital environment.
Voltage dips, short
interruptions and voltage
variations on power
supply input lines
IEC 61000-4-11
<5 %
UT (>95 % dip in UT)
for 0.5 cycle
40 % UT (60 % dip in UT)
for 5 cycles
70 %
UT (30 % dip in UT)
for 25 cycles
<5 %
UT (>95 % dip in UT)
for 5 sec
<5 %
UT (>95 % dip in UT)
for 0.5 cycle
40 % UT (60 % dip in UT)
for 5 cycles
70 %
UT (30 % dip in UT)
for 25 cycles
<5 %
UT (>95 % dip in UT)
for 5 sec
Mains power quality should be that of a typical
commercial or hospital environment, if the user of the
EPK-1000 requires continued operation during power
mains interruptions, it is recommended that the EPK1000 be powered from an uninterruptible power supply.
Page 40

37
Guidance and manufacturer’s declaration-electromagnetic immunity
The EPK-1000 is intended for use in the electromagnetic environment specified below. The customer or the user of the EPK-1000
should assure that it is used in such an environment.
Immunity test IEC 60601 test level Compliance level Electromagnetic environment-guidance
Conducted RF
IEC 61000-4-6
Radiated RF
IEC 61000-4-3
3 Vrms
150 kHz to 80 MHz
3 V/m
80 MHz to 2.5 GHz
3 Vrms
3V/m
Portable and mobile RF communications equipment
should be used no closet to any part of the EPK-1000,
including cables, than the recommended separation
distance calculated from the equation applicable to the
frequency of the transmitter.
Recommended separation distance
d = 1.2 P
d
= 1.2 P 80 MHz to 800 MHz
d = 2.3 P 800 MHz to 2.5 GHz
where
P is the maximum output power rating of the
transmitter in watts (W) according to the transmitter
manufacturer and
d is the recommended separation
distance in meters (m).
Field strengths from fixed RF transmitters, as
determined by an electromagnetic site survey, should be
less than the compliance level in each frequency range
Interferance may occur in the vicinity of equipment
marked with the following symbol:
NOTE 1 At 80 MHz and 800 MHz, the higher frequency range applies.
NOTE 2 These guidelines may not apply in all situations. Electromagnetic propagation is affected by absorption and reflection from
structures, objects and people.
a
Field strengths from fixed transmitters, such as base stations for radio (cellular/cordless) telephones and land mobile radios, amateur
radio, AM and FM radio broadcast and TV broadcast cannot be predicted theoretically with accuracy. To assess the electromagnetic
environment due to fixed RF transmitters, an electromagnetic site survey should be considered. If the measured field strength in the
location in which the EPK-1000 is used exceeds the applicable RF complience level above, the EPK-1000 should be observed to
verify normal operation. If abnormal performance is observed, additional measures may be necessary such as reorlenting or
relocating the EPK-1000.
b
Over the frequency range 150 kHz to 80 MHz, field strengths should be less than 3 V/m.
Page 41

38
Recommended separation distances between
portable and mobile RF communications equipment and the EPK-1000
The EPK-1000 is intended for use in the electromagnetic environment in which radiated RF disturbances are controlled. The customer
or the user of the EPK-1000 can help prevent electromagnetic interference by maintaining a minimum distance between portable and
mobile RF communications equipment (transmitters) and the EPK-1000 as recommended below, according to the maximum output
power of the communications equipment.
Separation distance according to frequency of transmitter
m
150 kHz to 80 MHz 80 MHz to 800 MHz 800 MHz to 2.5 GHz
d = 1.2 Pd= 1.2 Pd= 2.3 P
0.01 0.12 m 0.12 m 0.23 m
0.1 0.38 m 0.38 m 0.73 m
1 1.2 m 1.2 m 2.3 m
10 3.8 m 3.8 m 7.3 m
100 12 m 12 m 23 m
For transmitters rated at a maximum output power not listed above, the recommended separation distance d in meters (m) can be estimated using the
equation applicable to the frequency of the transmitter, where
P is the maximum output power rating of the transmitter in watts (W) according to the
transmitter manufacturer.
NOTE 1 At 80 MHz and 800 MHz, the separation distance for the higher frequency range applies.
NOTE 2 These quidelines may not apply in all situations. Electromagnetic propagation is affected by absorption and reflection from
structures, objects and people.
Rated maximum output power
of transmitter
W
Page 42

39
MEMO
Page 43

Page 44

HOYA Corporation
2-7-5 Naka-Ochiai, Shinjuku-ku, Tokyo 161-8525 Japan
HOYA Corporation PENTAX Tokyo Office
2-36-9 Maeno-cho, Itabashi-ku, Tokyo 174-8639 Japan
Tel: 03-3960-5155
Fax: 03-5392-6724
PENTAX Medical Singapore Pte. Ltd.
60 Alexandra Terrace, #10-08 The Comtech, Singapore 118502
Tel: 65-6271-1669
Fax: 65-6271-1691
PENTAX Medical Company
A Division of PENTAX of America, inc.
102 Chestnut Ridge Road Montvale, New Jersey 07645-1856
Tel: 201-571-2300 Toll Free: (800) 431-5880
Fax: 201-391-4189
PENTAX Canada Inc.
1770 Argentia Road Mississauga, Ontario L5N 3S7
Tel: 905-286-5585
Fax: 905-286-5571
PENTAX Europe GmbH
Julius Vosseler Strasse 104, 22527 Hamburg, Germany
Tel: 040-56-1920
Fax: 040-560-4213
PENTAX U.K. Ltd.
PENTAX House, Heron Drive, Langley SLOUGH SL3 8PN, Great Britain
Tel: 1753-792792
Fax: 1753-792794
● Specifications are subject to change without notice and without
any obligation on the part of the manufacturer. 2009. 01. 6217001 Z446 R13 printed in JAPAN
NOTICE
This equipment is a Class B Medical Equipment (specified EN55011) and is intended for hospital
or health care districts.
When used in clinical or residential areas near radio and TV receiver units, this equipment may be
subjected to radio interference.
To reduce electromagnetic interference do NOT keep turning on the main POWER SWITCH of
the equipment while a videoscope is connected but not ready for use.
To avoid and resolve adverse electromagnetic effects, do NOT operate this equipment near the RF
energy equipment.
Only connection cables and keybord specifled by PENTAX conform to the above standards.
86352
EC REP
0123
 Loading...
Loading...