Page 1

Only For the Americas
INSTRUCTIONS FOR USE
(REPROCESSING)
PENTAX VIDEO BRONCHOSCOPES
EB-1970TK
EB-1170K, EB-1570K
EB-1970K, EB-1575K
EB-1975K, EB-1990i
Page 2

Product Overview
These instruments photograph the subject of observation using a solid-state image sensor located at the endoscope tip under the
light transmitted from the processor/light source. The target of the observation is monitored by the physician using the endoscopic
image displayed on the video monitor. The endoscopic procedure is performed by inserting biopsy forceps and other endoscopic
accessories into the instrument channel inlet on the control body.
The bending section angulates in the intended direction and angle by operating the Angulation Control Lever; and air or fluids can
be suctioned from the distal end of the endoscope by operating the Suction Control Valve.
Indication for Use
The PENTAX Video Bronchoscopes (EB Family) have been designed to be used with a PENTAX Video Processor (including Light
Source), documentation equipment, video monitor, endo-therapy accessories (such as biopsy forceps) and other ancillary
equipment for endoscopy and endoscopic surgery within the airways and tracheobronchial tree.
Application
Medical purpose: Provide images for optical visualization, recording, and/or diagnostic aid.
Patient populations: Adults and lowercase pediatrics who have been determined by the physician to be appropriate candidates for
the use of this instrument.
Intended anatomical area: Airways and tracheobronchial tree
User: Medical doctors (expert approved by the medical safety officer to perform endoscopic examinations at each medical facility)
Place of Use: Medical facillity
Functions Used Frequently
The frequently used functions in these endoscope models are as follows:
Angulation capability using control lever
•
Remote control operation using remote buttons
•
Suctioning function
•
Removable Components
OF-B179
OF-B190
Suction Control Valve
Inlet Seal
Notes
Read this Instructions for Use (IFU) before reprocessing the endoscope, and save this book for future reference. Failure to read and
thoroughly understand the information presented in this IFU, as well as those developed for ancillary endoscopic equipment and
accessories, may result in serious injury, including infection by cross contamination to the patient and/or user. Furthermore, failure
to follow the instructions in this IFU or the companion Instructions for Use (operation) may result in damage to, and/ or malfunction
of, the equipment.
It is the responsibility of each medical facility to ensure that only well hyphen educated and appropriately trained personnel, who are
competent and knowledgeable about the endoscopic equipment, antimicrobial agents/processes, and hospital infection control
protocol be involved in the use and the reprocessing of these medical devices. Known risks and/or potential injuries associated with
flexible endoscopic procedures include, but are not limited to, the following: perforation, infection, hemorrhage, burns, and electric
shock.
This IFU describes the procedures for reprocessing and maintenance of the equipment after its use.
For inspection and preparation prior to its use, please refer to the separate Instructions for Use (Operation).
The text contained in this IFU is common to various types/models of PENTAX endoscopes, and users must carefully follow only
those sections and instructions pertaining to the specific instrument model in question.
If you have any questions regarding any of the information in this IFU or concerns pertaining to the safety and/or use of this
equipment, please contact your local PENTAX representative.
Page 3

Sterility Statement
NOTE
Symbols on Marking
Symboles distinctifs
The endoscopes identified in this IFU are reusable semicritical devices. Since they are packaged non-sterile, they must be highlevel disinfected or sterilized BEFORE inital use. Prior to each subsequent procedure, they must be subjected to appropriate
cleaning and either high-level disinfection or sterilization processes.
Contraindication
Please consult regional and national health authority recommendations and requirements regarding protocols to follow in order to
reprocess and/or destroy endoscopes that will be used or have been determined to have been used (post procedure) on patients
afflicted with Creutzfeldt-Jacob Disease (CJD or vCJD).
Conventions
Throughout this IFU, the following conventions will be used to indicate a potentially hazardous situation which, if not avoided;
WARNING
CAUTION
: could result in death or serious injury.
: may result in minor or moderate injury or property-damage.
: may result in property-damage. Also advises owner/operator about important information on the use of
this equipment.
Prescription Statement
Federal (U.S.A) law restricts this device to sale by or on the order of a physician or other appropriately licensed medical
professional.
Symbols on Marking
Symboles distinctifs
Symbol for “MANUFACTURER”
Symbol for “DATE OF MANUFACTURE”
Symbol for “Authorised Representative in the European Union”
このCEマ ーキ ングは EC指令への適合宣言マークです。
The CE marking assures that this product complies with the requirements of the EC directive for safety.
Das CE Zeichen garantiert, daß dieses Produkt die in der EU erforderlichen Sicherheitsbestimmungen erfüllt.
Le logo CE certie que ce produit est conforme aux normes de sécurité prévues par la Communauté Européenne.
II marchio CE assicura che questo prodotto è conforme alle direttive CE relative alla sicurezza.
La marca CE asegura que este producto cumple todas las directivas de seguridad de la CE.
Attention, consult instructions for use
Attention, consulter le manuel d’utilisation
Type BF applied part (Safety degree specified by IEC 60601-1)
Partie appliquée de type BF (niveau de sécurité spécifié par la norme CEI 60601-1)
Page 4

Page 5

TABLE OF CONTENTS
NOMENCLATURE ............................................................................................................................................ 1
Video Bronchoscopes ..............................................................................................................................1
NOMENCLATURE ............................................................................................................................................ 3
Endoscope Components and Accessories ............................................................................................3
ENDOSCOPE REPROCESSING PROCEDURE FLOW .................................................................................. 5
1 CARE AFTER USE ..................................................................................................................................... 6
1-1. General ............................................................................................................................................... 6
1-1-1. Application ..................................................................................................................6
1-1-2. Important Instructions ................................................................................................ 7
1-1-3. Internal Channels of Video Bronchoscopes ............................................................10
1-1-4. Quick Reference of Injection Volumes for Internal Channel ..................................11
1-1-5. Inspection of Reprocessing Accessories ................................................................12
1-2. Endoscope Reprocessing ................................................................................................................ 14
1-2-1. Pre-Cleaning ................................................................................................................14
1-2-2. Leak Testing ................................................................................................................ 17
1-2-3. Cleaning .......................................................................................................................18
1-2-4. High-Level Disinfection .............................................................................................. 28
1-2-5. Optional Sterilization .................................................................................................. 35
1-3. Endoscope components and accessories .....................................................................................39
1-3-1. Cleaning .......................................................................................................................40
1-3-2. High-Level Disinfection .............................................................................................. 44
1-3-3. Optional sterilization ..................................................................................................47
General
Endoscope
Pre-Cleaning
Leak
Testing
Endoscope
Cleaning
Endoscope
Disinfection
Endoscope
Sterilization
Accessory
Cleaning
2 POST REPROCESSING AND STORAGE ................................................................................................. 50
3 SERVICING ................................................................................................................................................. 51
4 APPENDIX .................................................................................................................................................. 53
4-1. PENTAX Medical Compatible Reprocessing Systems/Agents ..................................................... 53
Accessory
Disinfection
Accessory
Sterilization
Page 6
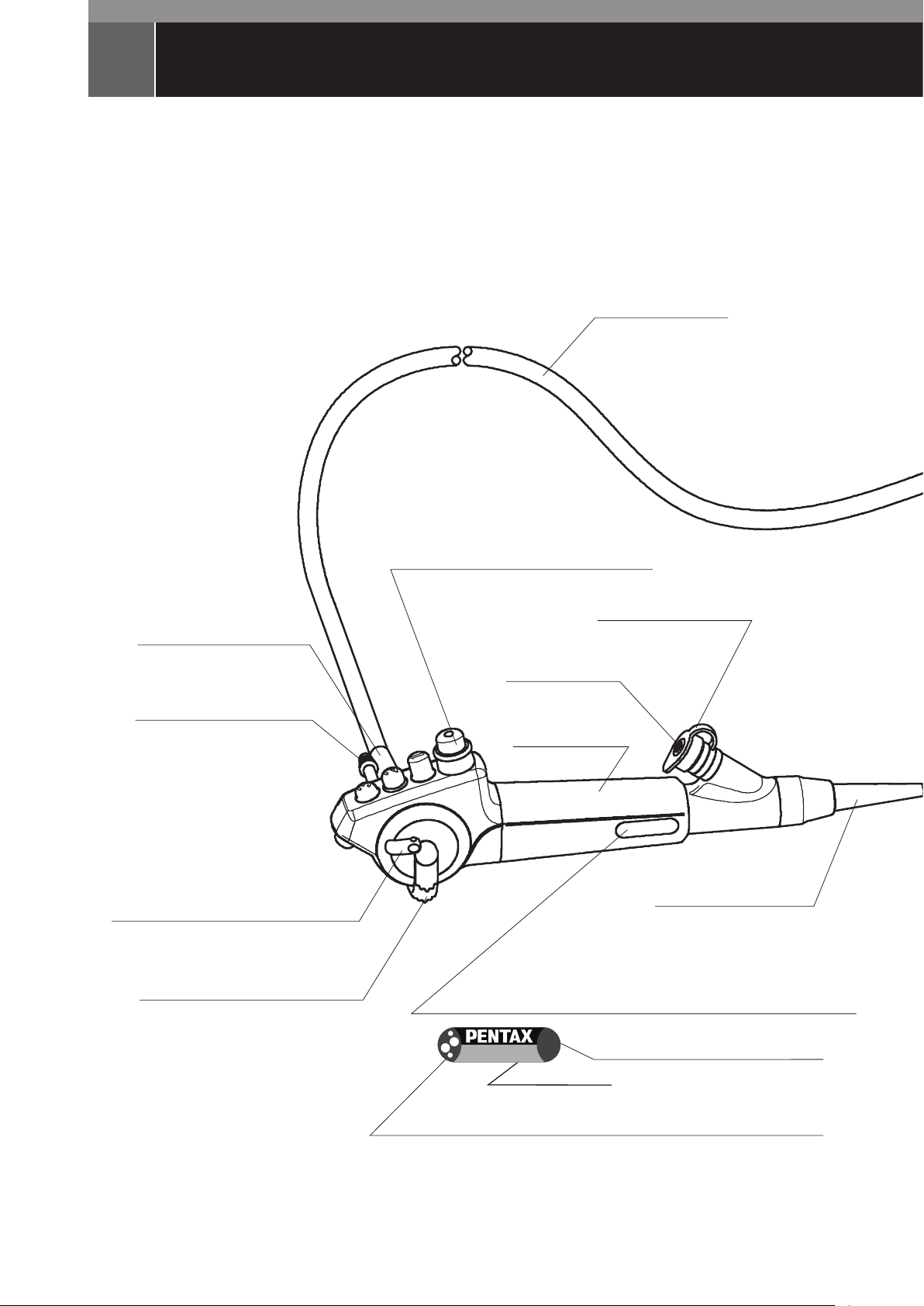
NOMENCLATURE
Video Bronchoscopes
EB-1970TK, EB-1170K, EB-1570K, EB-1970K, EB-1575K,
EB-1975K, EB-1990i
UMBILICAL CABLE
STRAIN RELIEF BOOT
SUCTION NIPPLE
ANGULATION LOCK LEVER
EB-1170K, EB-1570K, EB-1970TK only
ANGULATION CONTROL LEVER
SUCTION CONTROL VALVE (OF–B179)
INLET SEAL (OF–B190)
INSTRUMENT
CHANNEL INLET
CONTROL BODY
STRAIN RELIEF BOOT
MODEL DESIGNATION
STERRAD
This symbol denotes an endoscope model's material compatibility with
the STERRAD
Endoscopes that do not have this symbol are incompatible with
the STERRAD
EB-1970TK)
®
NX™ system material compatibility identification symbol
®
®
– 1 –
Model Name
NX™ system. (EB-1575K, EB-1975K, EB-1990i)
NX™ system. (EB-1170K, EB-1570K, EB-1970K,
Minimum Instrument Channel Width
Page 7
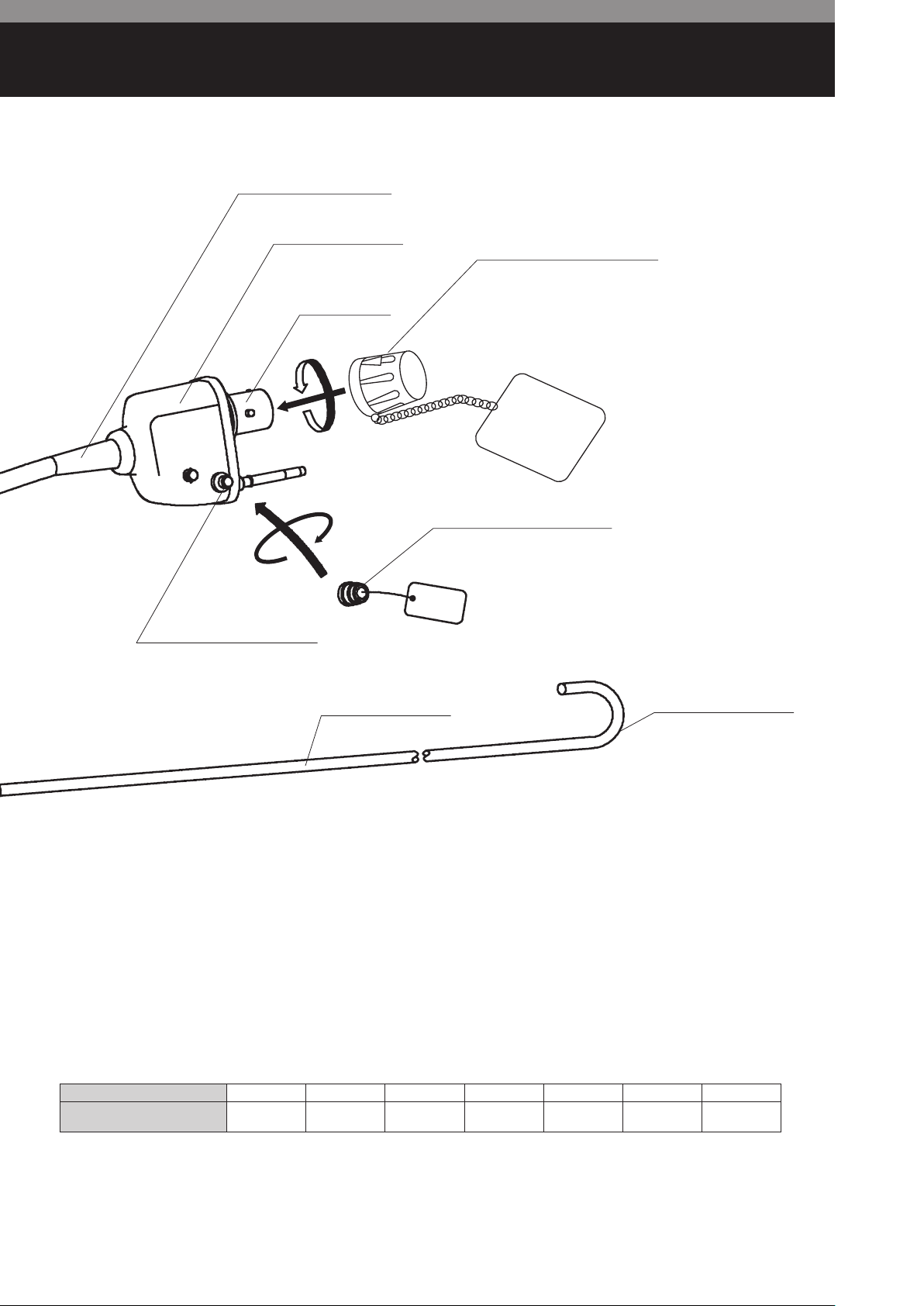
PVE CONNECTOR
STRAIN RELIEF BOOT
VENTING CONNECTOR
PVE SOAKING CAP OE-C9
ELECTRICAL
CONTACTS
VENTILATION CAP OF-C5
INSERTION TUBE
BENDING SECTION
Table of Minimum Instrument Channel Width
Video Bronchoscopes EB-1170K EB-1570K EB-1970K EB-1970TK EB-1575K EB-1975K EB-1990i
Minimum Instrument
Channel Width (I.D.φmm)
1.2 2.0 2.8 3.2 2.0 2.8 1.2
– 2 –
Page 8
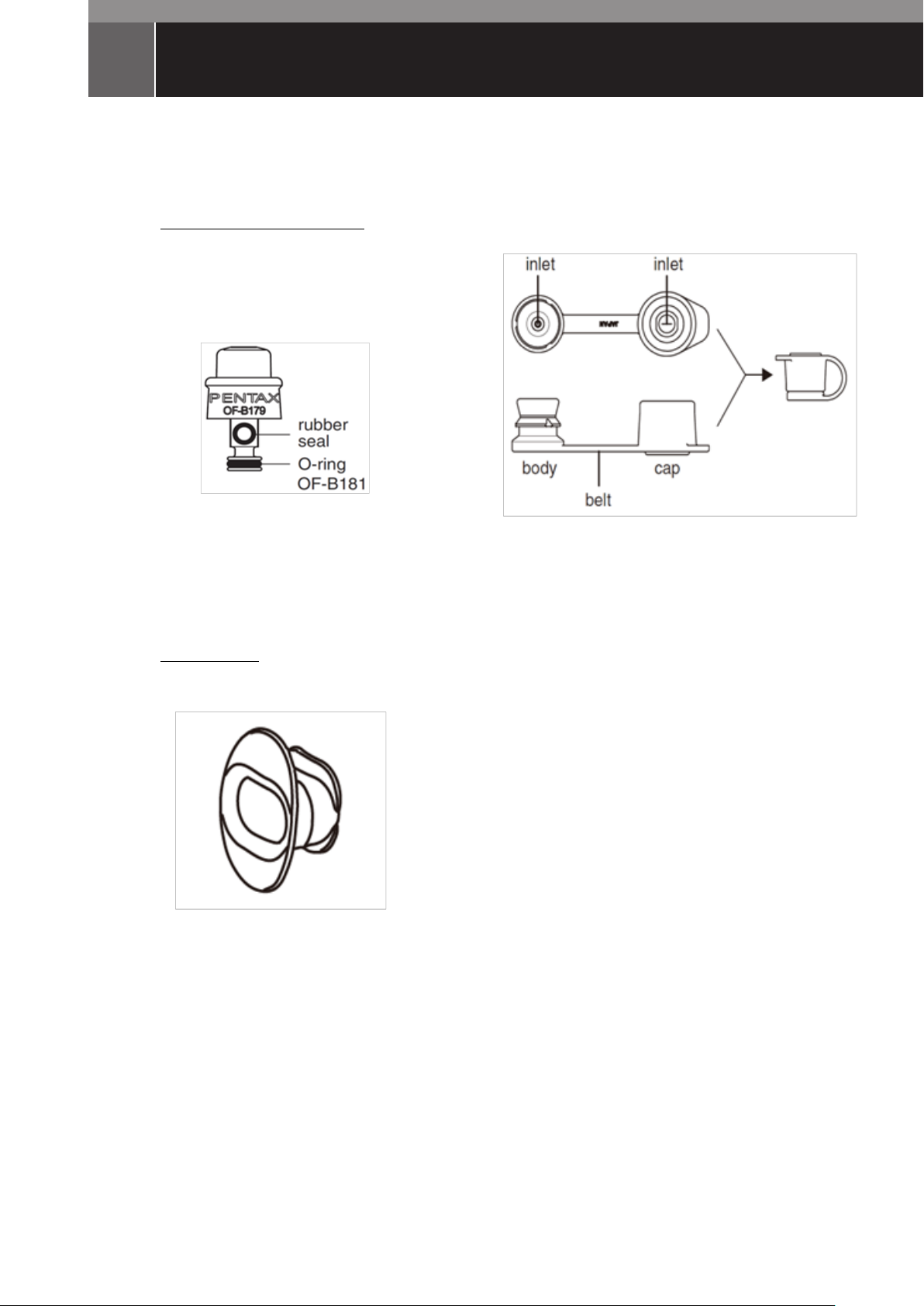
NOMENCLATURE
Endoscope Components
・Suction Valve (OF-B179)
・Inlet Seal (OF-B190)
Accessories
・Bite Bock (OF-Z5)
Endoscope Components and Accessories
– 3 –
Page 9
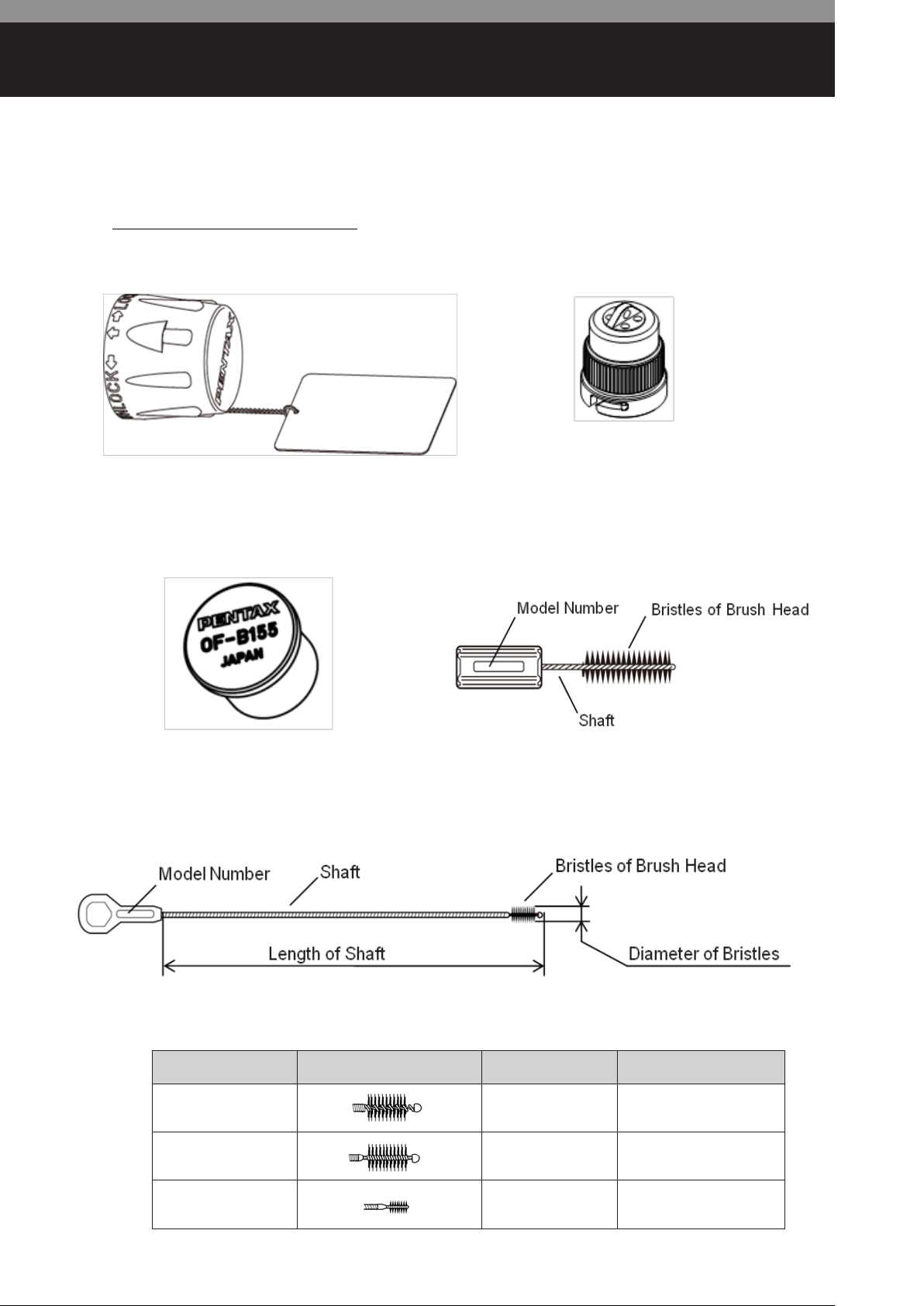
Accessories for Reprocessing
・PVE Soaking Cap (OE-C9)
・Ventilation Cap (OF-C5)
・Cleaning Brush (CS-C3S)
・Cleaning Brush (CS6002SN/CS6015ST/CS3010S)
・Cleaning Adapter (OF-B155)
Model Number Appearance of Bristles Length of Shaft Diameter of Bristles
CS6002SN 20cm
CS6015ST 150cm
CS3010S 100cm
– 4 –
φ
φ
φ
6mm
6mm
3mm
Page 10
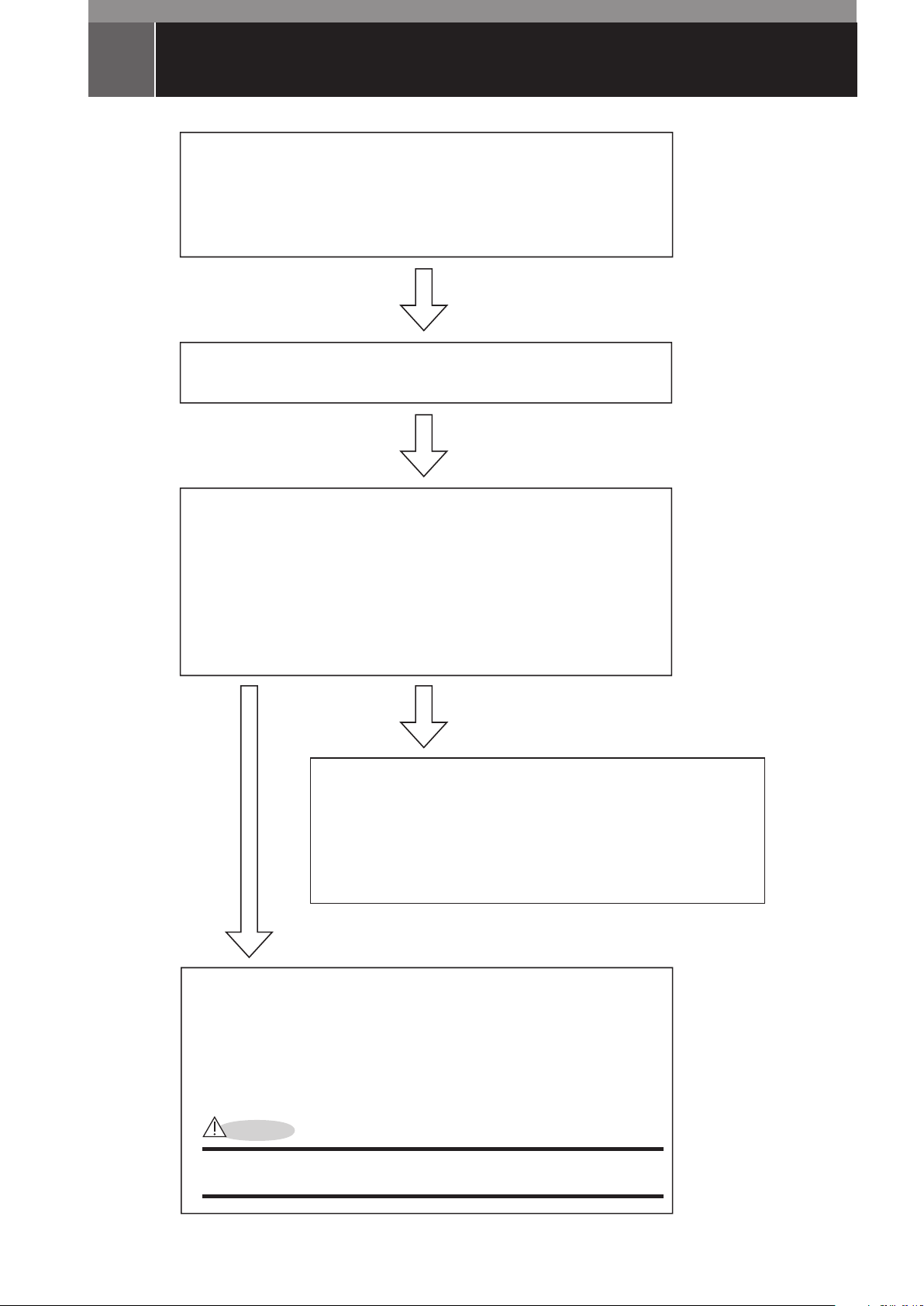
ENDOSCOPE REPROCESSING PROCEDURE FLOW
Pre-Cleaning
• Preparation
• Wiping of insertion tube
• Aspiration of detergent solution through suction channel
• Transport to cleaning room
Leak Testing
Cleaning
• Preparation
• Cleaning of all external surfaces
• Brushing of suction channel
• Filling of detergent solution into suction channel
• Soaking in detergent solution
• Rinsing
• Drying
High-Level Disinfection
• Preparation
• Filling of disinfecting solution into suction channel
• Soaking in disinfecting solution
• Rinsing
• Drying
Optional Sterilization
Sterilization using STERRAD® NX™ system (EB-1575K,
EB-1975K, EB-1990i only)
• Preparation
• Wrapping
• Sterilization Parameter Selection
CAUTION
STERRAD® NX™ Validation data has not been generated for endoscope
models in this manual other than EB-1575K, EB-1975K, and EB-1990i.
– 5 –
Page 11
NOTE
1 CARE AFTER USE
1-1. General
This Instructions for Use (IFU) has been written in accordance with 21CFR
Part801, ISO 17664, and national guidelines on reprocessing of medical
products.
1-1-1. Application
WARNING
Reprocessing may affect device functionality. Prior to use, always inspect the
endoscope, components, and accessories for proper function to determine
that they are appropriate for patient use.
Components and Accessories for Video Bronchoscopes
MODEL Video Bronchoscopes
TYPE
Endoscope
Component
Accessory Bite Block - Adult Size OF-Z5 Y Y
Reprocessing
Accessory
Suction Control Valve OF-B179 Y Y
Inlet Seal OF-B190 Y Y
PVE Soaking Cap OE-C9 Y Y
Ventilation Cap OF-C5 Y Y
Cleaning Adapter OF-B155 Y Y
Cleaning Brush CS6002SN Y Y
Cleaning Brush CS6015ST Y N
Cleaning Brush CS3010S N Y
Cleaning Brush CS-C3S Y Y
Name Number
EB-1570K
EB-1970K
EB-1970TK
EB-1575K
EB-1975K
1
General
EB-1170K
EB-1990i
Y : YES
N : NO
– 6 –
Page 12
1-1-2. Important Instructions
WARNING
1
General
• Reusable Medical Devices that are initially supplied non-sterile require
the end user to disinfect or sterilize them prior to initial use and to
subsequently reprocess them after each subsequent use.
• Proper care of the device after each procedure is extremely important.
Immediately (within one hour) after the completion of a procedure,
the endoscope and its removable components, and accessories should
be both pre-cleaned and mechanically cleaned with detergent solution.
Generally, if these endoscopes and accessories are not precleaned
within 15 minutes and mechanically cleaned within one hour after the
conclusion of the procedure, dried blood, mucus, or other patient debris
may cause damage to the devices or interfere with the ability of the user
to properly reprocess them.
• The use of detergent immediately after each procedure to dissolve and
remove organic contaminants and proteinaceous debris is essential to
the proper care and maintenance of the endoscope. Prior to disinfection
or sterilization, all instruments and components must be meticulously
cleaned. Failure to do so can result in incomplete or ineffective
disinfection or sterilization.
• Always inspect reprocessed endoscopes and accessories prior to use
according to their respective Instructions for Use (IFU).
• During the reprocessing process, always wear protective equipment
(e.g., gloves, gowns, face masks, etc.) to minimize the risk of cross
contamination.
• Contact the manufacturer and follow local regulations regarding safe use,
appropriate handling, and disposal of cleaning and disinfection solutions,
including alcohol and rinse water. Material Safety Data Sheets available
from the cleaning and disinfection solution manufacturer should be
consulted to provide guidance to end users about formulation, hazards,
chemical and physical properties, first aid, handling and storage,
stability, precautions, disposal, etc..
WARNING
Endoscopes are semicritical devices that require cleaning and at least highlevel disinfection. Use only legally marketed solutions and/or automated
endoscope reprocessors (AERs) for which validation testing with PENTAX
products has been performed by their manufacturers. A list of legally
marketed solutions/systems that have been determined to be compatible with
PENTAX brand products is contained in this manual.
– 7 –
Page 13
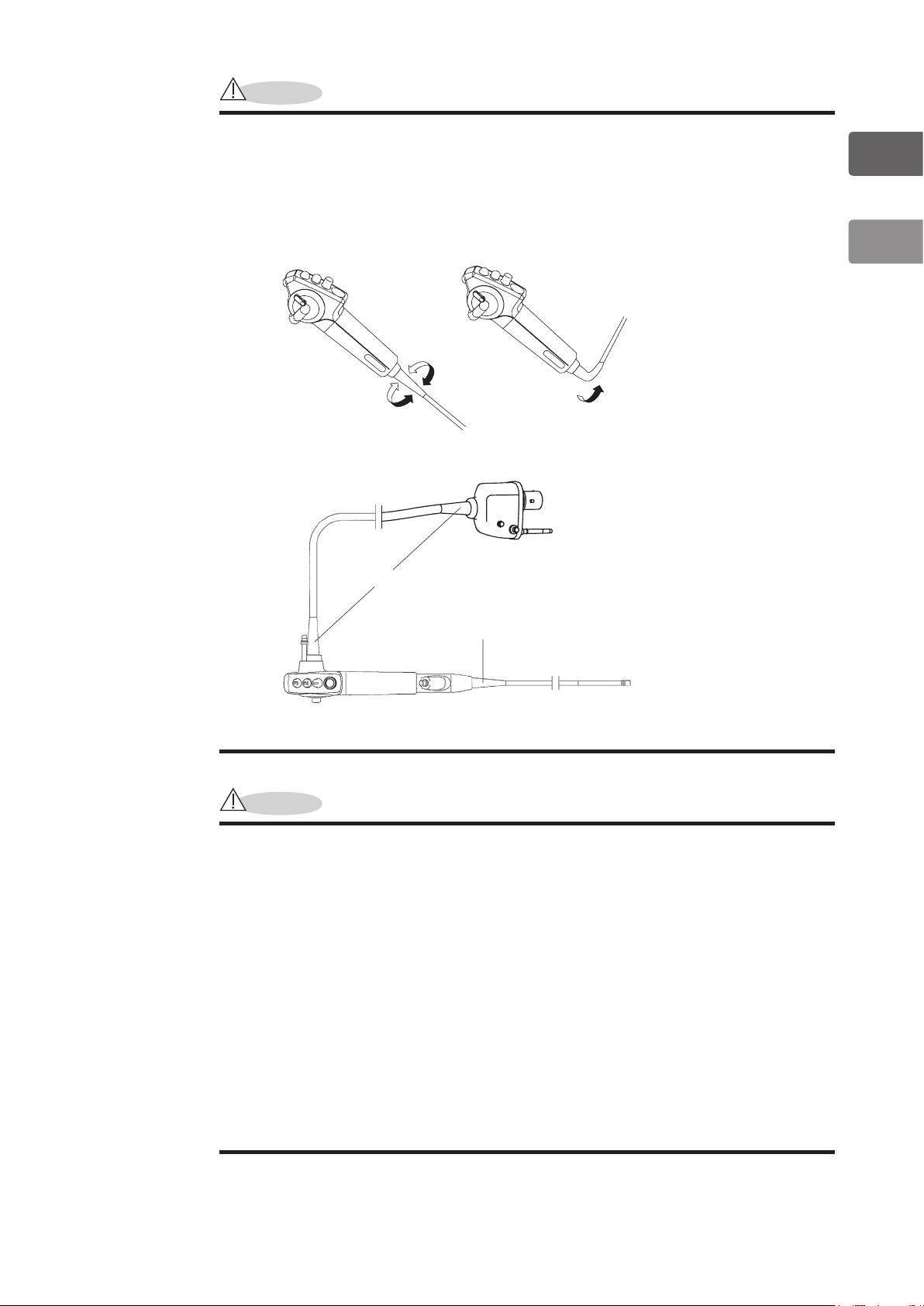
CAUTION
To avoid damaging the endoscope, do NOT twist, rotate or excessively bend
any of the strain reliefs [(1), (2)] during inspection, clinical use, reprocessing,
or any handling activity. Be particularly cautious regarding the insertion tube
strain relief [(1)]. When wiping the insertion tube and the umbilical cable, use
a slow back and forth motion to wipe them along the tube/cable. Never apply
excessive force or torque to these strain reliefs or tubes/cables.
Do NOT Twist or Rotate Do NOT Bend
1
General
(2)
(1)
Figure 1.1
CAUTION
• EB-1575K, EB-1975K, EB-1990i can be sterilized using the STERRAD®
NX™ system. For more detail, refer to sections 1-2-5-1 and 1-3-3-2 of
this instruction for use.
• Video bronchoscopes other than EB-1575K, EB-1975K, EB-1990i are
incompatible with sterilization using STERRAD® NX™ system.
• After every 100 cycles of STERRAD® NX™ exposure, the endoscope
should be returned to an authorized PENTAX service facility for repair.
Replacement of the insertion tube and bending section will be necessary
and other components may also require service.
• Although STERRAD® NX™ compatible endoscopes are generally capable
of withstanding up to 100 cycles of exposure to STERRAD® NX™, repair/
replacement of components might be necessary prior to 100 cycles,
depending upon the condition of the endoscope.
• Be sure to attach the ventilation cap (OF-C5) to the venting connector
before performing STERRAD® NX™ sterilization.
– 8 –
Page 14

1
NOTE
NOTE
NOTE
General
This IFU contains detailed recommendations on the manual reprocessing of
PENTAX endoscopes using PENTAX supplied cleaning/disinfecting adapters.
AERs may also be used to reprocess flexible endoscopes. However, only
those AERs should be used whose manufacturers provide device-specific
instructions and have validation data to support each AER claim with respect
to PENTAX instruments. AER manufacturers should be consulted for their
specic claims including, but not necessarily limited to:
a) the ability of the AER to provide a cleaned and high-level disinfected (or
sterilized) endoscope and endoscope components (e.g., valves),
b) the identification of any special feature (internal channel) or endoscope
component that cannot be reprocessed and therefore requires manual
reprocessing,
c) the microbial quality of the rinse water,
d) the inclusion of an “automated” alcohol rinse cycle,
e) the inclusion of a terminal drying cycle that removes the majority of water
from within endoscope channels,
f) maintenance procedures for water filter replacement and/or
decontamination of the filtration system to ensure water of suitable
quality,
g) compliance with local regulations and/or guidelines.
PENTAX exible endoscopes should not be exposed to temperatures in excess
of 140oF (60oC) during either reprocessing or storage. During reprocessing
depending upon the detergent used, the endoscope may be damaged even if
the temperature does not exceed 140oF (60oC). A list of detergents that are
compatible with PENTAX endoscopes is contained in this manual.
All of the steps in the validated reprocessing protocol described in this
manual are intended to be performed in rapid succession and as a single,
continual procedure. There should be no breaks in between steps of the
protocol that are of sufficient duration to permit the endoscope to dry to
such an extent that dislodged debris and/or microbial contaminants would be
permitted to dry onto any endoscope surface. In the event that drying of the
endoscope occurs due to an excessive break in the reprocessing procedure,
the procedure should be completely repeated, beginning with the rst pre-
cleaning step.
– 9 –
Page 15
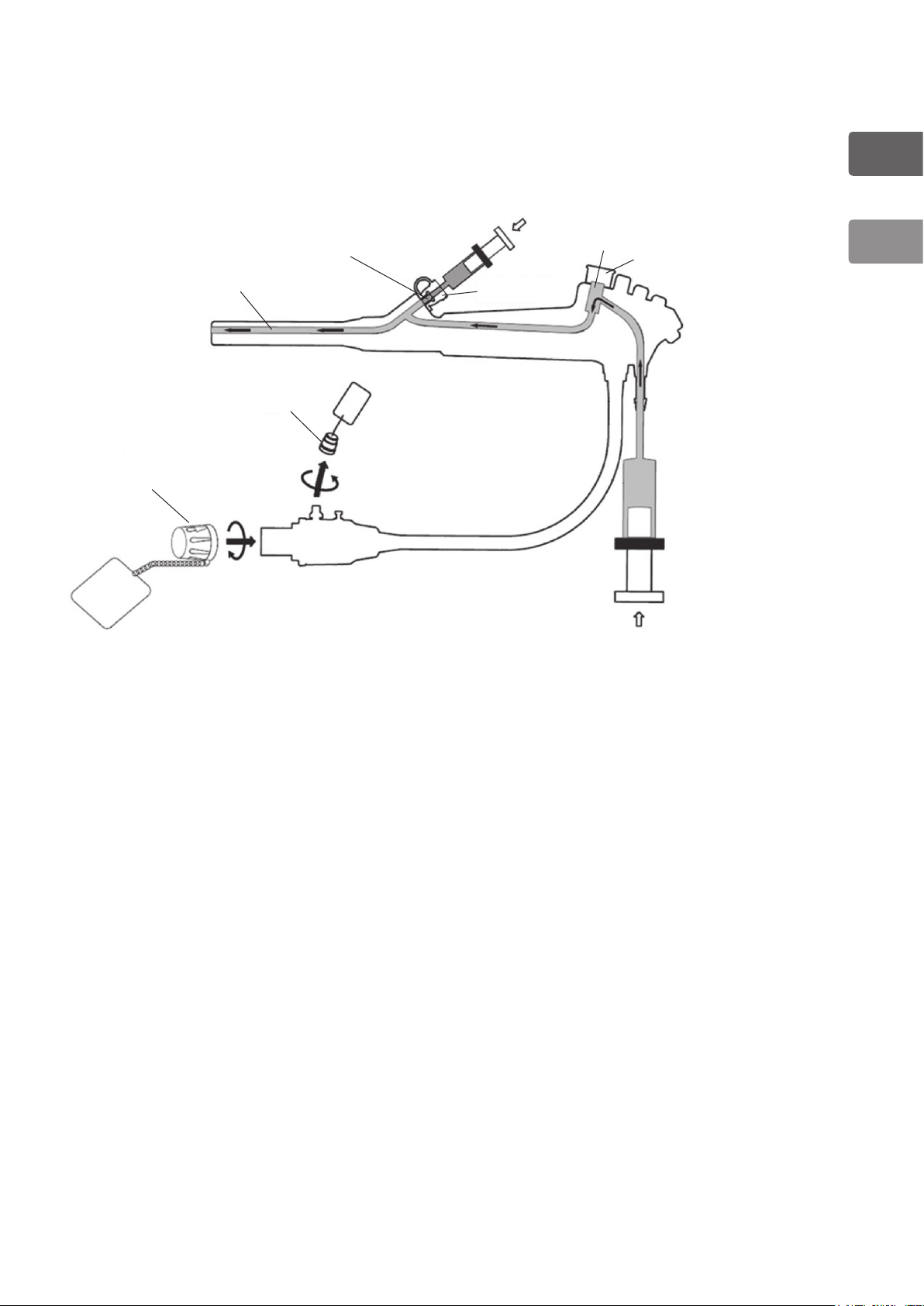
1-1-3. Internal Channels of Video Bronchoscopes
The following internal schematic is designed to help users better understand the intricate
construction of PENTAX endoscopes. Please note that all solution entrance ports and flow
pathways are illustrated below.
(1) Suction Channel
(2) Instrument
(7)
(1)
(6)
(2)
(4)
(5)
(3)
Channel Inlet
(3) Inlet Seal
(OF-B190)
(4) Suction Cylinder
(5) Cleaning Adapter
(OF-B155)
(6) Ventilation Cap
(OE-C5)
(7) PVE Soaking Cap
(OE-C9)
1
General
Figure 1.2
– 10 –
Page 16
1
General
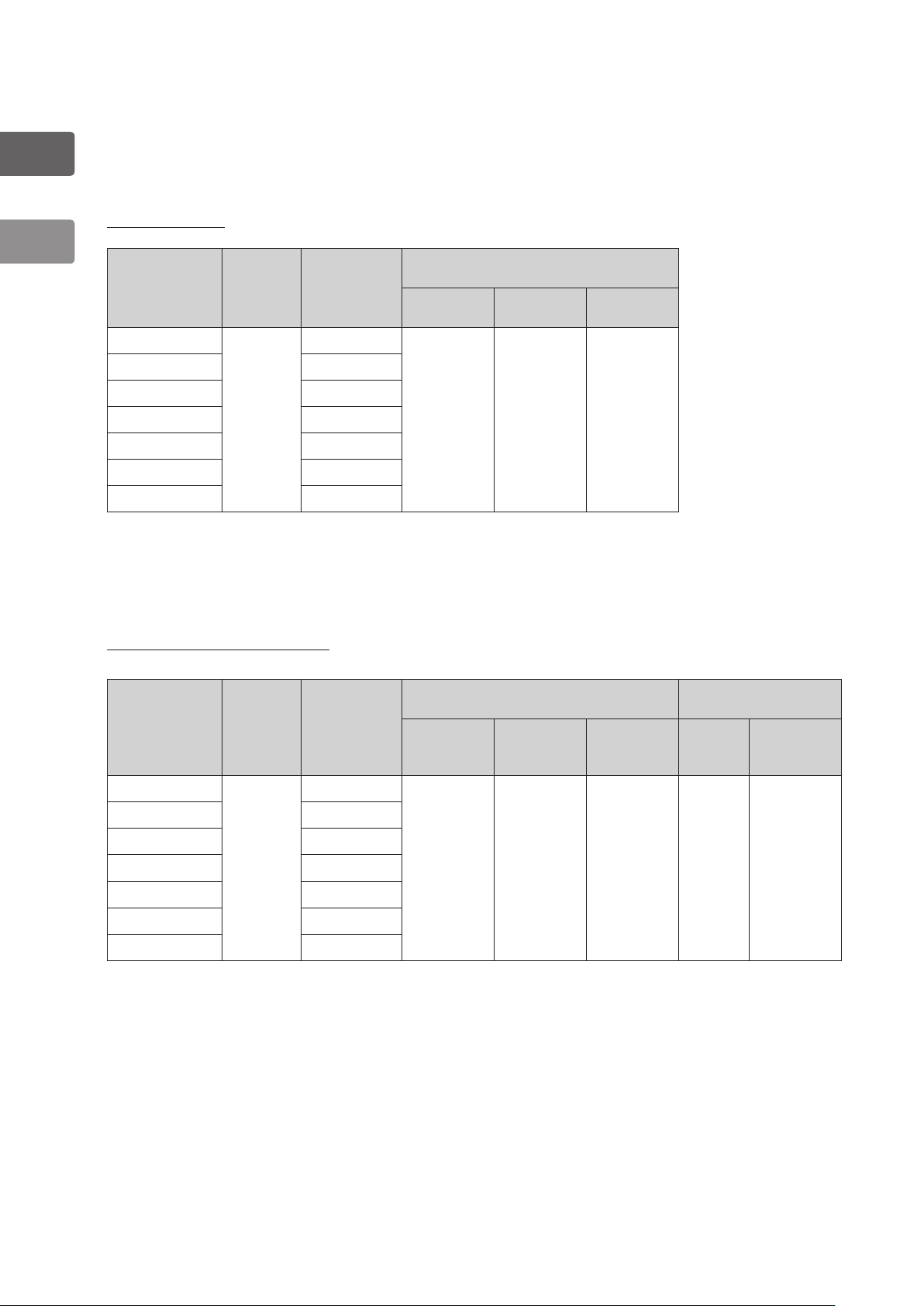
1-1-4. Quick Reference of Injection Volumes for Internal Channel
The following table is designed to to help users better understand the injection volumes for
internal channel of video bronchoscopes.
Cleaning Process
Video
Bronchoscopes
EB-1170K
EB-1570K 6 mL
EB-1970K 8 mL
EB-1970TK 9 mL
EB-1575K 6 mL
EB-1975K 8 mL
EB-1990i 4 mL
①
Injecting from suction nipple
②
Injecting from inlet seal (OF-B190)
Internal
Channel
Suction
Channel
Capacity (mL)
Maximum
Volume
4 mL
Injection Volumes (mL)
Detergent
①
:25 mL
②
:25 mL
Cleaning
Rinse
Water
①
:35 mL
②
:35 mL
①
:35 mL
②
:35 mL
Air
High Level Disinfection Process
Video
Bronchoscopes
EB-1170K
EB-1570K 6 mL
EB-1970K 8 mL
EB-1970TK 9 mL
EB 1575K 6 mL
EB-1975K 8 mL
EB-1990i 4 mL
①
Injecting from suction nipple
②
Injecting from inlet seal (OF-B190)
Internal
Channel
Suction
Channel
Capacity (mL)
Maximum
Volume
4 mL
High Level Disinfection
Injection Volumes (mL)
Disinfectant
①
:25 mL
②
:25 mL
Sterile
Rinse
Water
①
:35 mL
②
:35 mL
Alcohol Rinse
Injection Volumes (mL)
Air Alcohol Air
①
:35 mL
②
:35 mL
①
:15 mL
②
:15 mL
①
:35 mL
②
:35 mL
– 11 –
Page 17
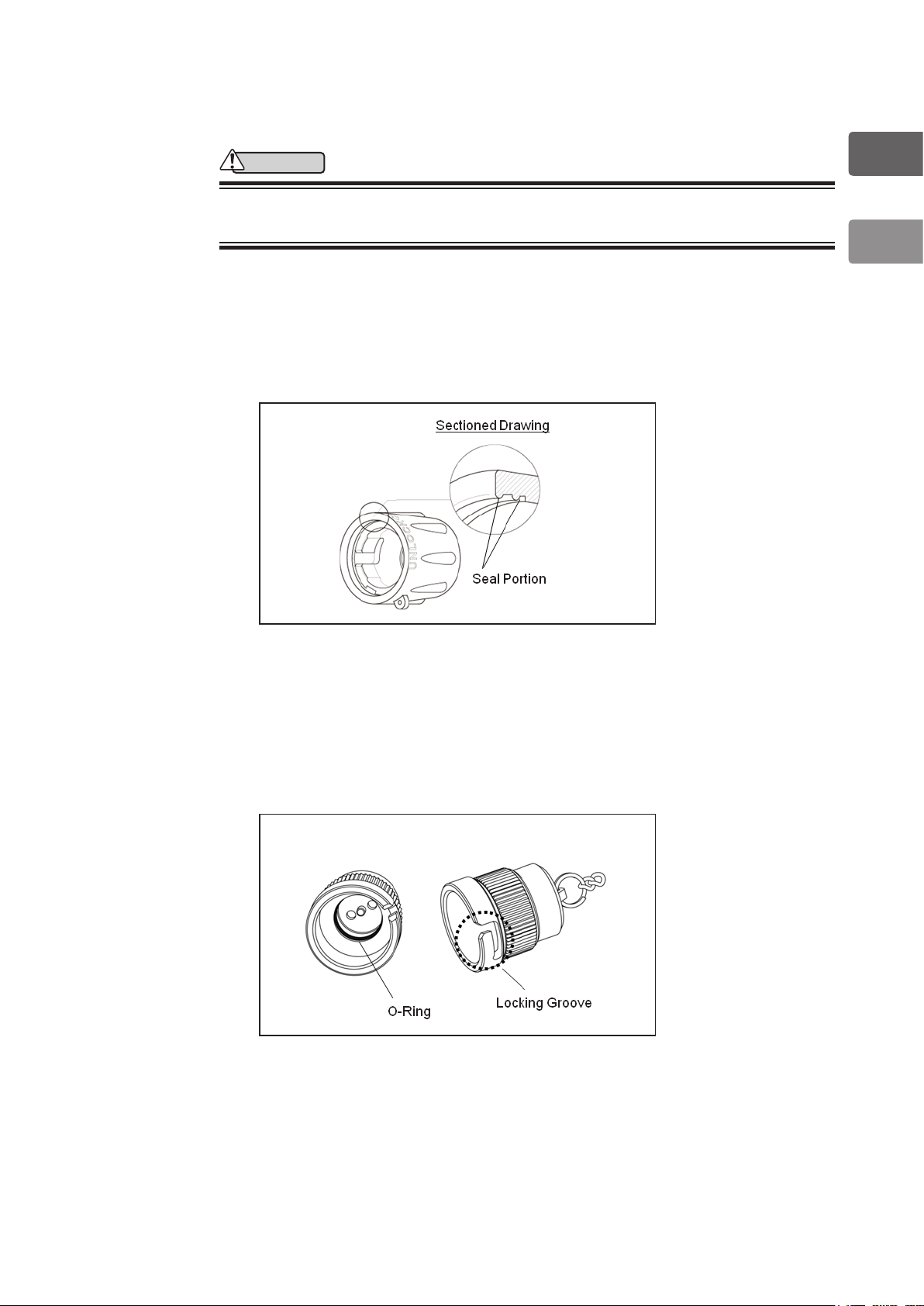
1-1-5. Inspection of Reprocessing Accessories
Before use, inspect reprocessing accessories according to the following procedure.
WARNING
• Replace reprocessing accessory with a new one when inspection of the
device indicates that it is damaged or unable to function properly.
1-1-5-1. Inspection of PVE Soaking Cap (OE-C9)
1) Check that there is no cracking on the outer surface of the PVE Soaking Cap.
2) Check that there are no scratches, cracking, or chipping of the sealing surfaces inside
the PVE Soaking Cap
.
1
General
Figure 1.3
1-1-5-2. Inspection of Ventilation Cap (OF-C5)
1) Make sure that of the Locking Groove Potion of the Ventilation Cap is not deformed.
2) Check that there are no scratches, cracking, or chipping of the O-ring inside the
Ventilation Cap.
Figure 1.4
– 12 –
Page 18
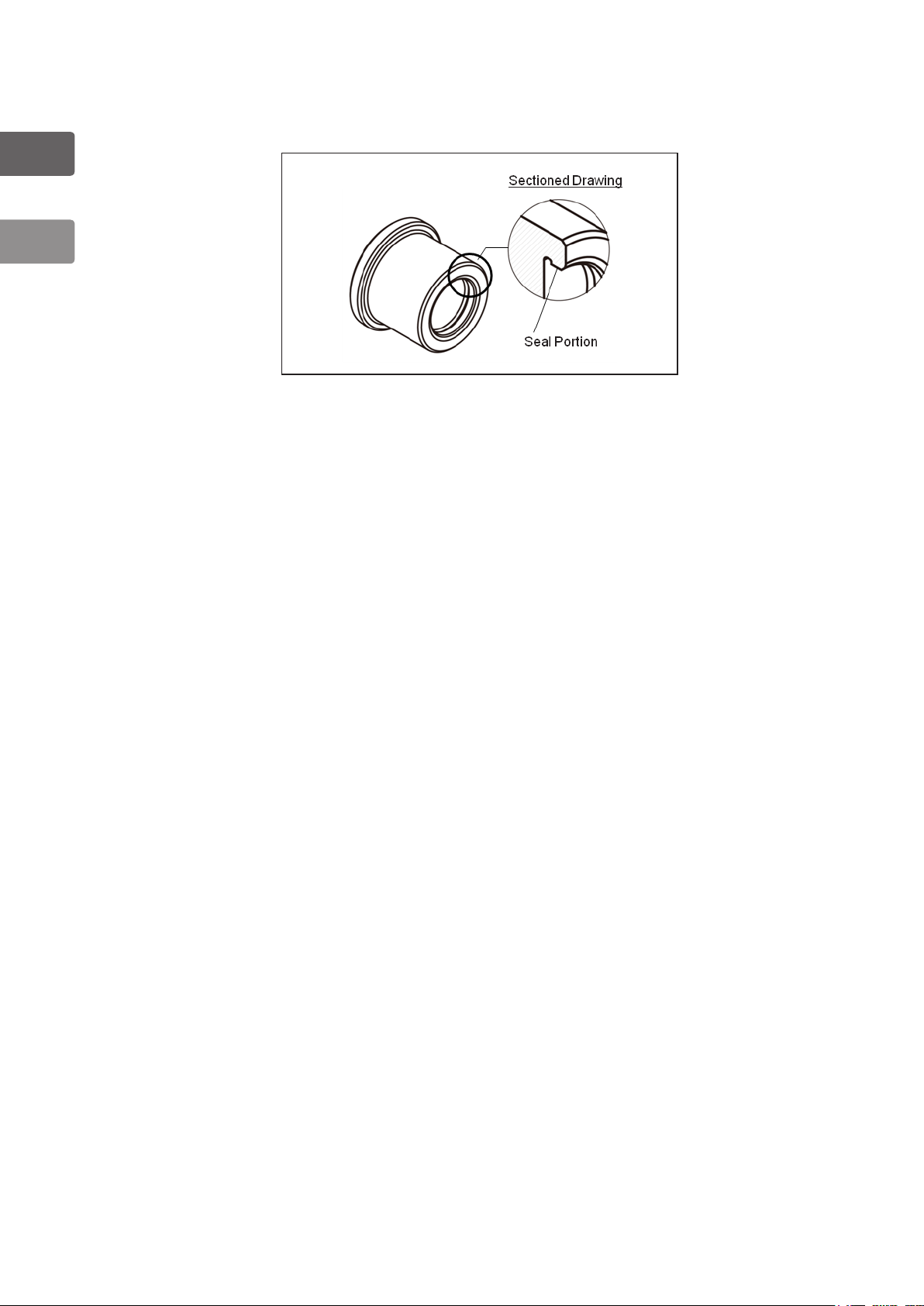
1
General
1-1-5-3. Inspection of Cleaning Adapter (OF-B155)
1) Check that there are no scratches, cracking, or chipping of the sealing surfaces inside
the Cleaning adapter.
Figure 1.5
1-1-5-4. Inspection Cleaning Brushes
(CS-C3S/CS6002SN/CS6015ST/CS3010S)
1) Make sure that there are no missing bristles on Cleaning Brushes.
2) Check that there is no kinking or bending of the Cleaning Brush Shaft.
– 13 –
Page 19

1-2. Endoscope Reprocessing
Video bronchoscopes can be subjected to the following cleaning, disinfection, and optional
sterilization process.
Video
Bronchoscopes
EB-1170K Y N Y N N
EB-1570K Y N Y N N
EB-1970K Y N Y N N
EB-1970TK Y N Y N N
EB-1575K Y N Y N Y
EB-1975K Y N Y N Y
EB-1990i Y N Y N Y
Y : YES
N : NO
Manual Ultrasonic
Cleaning
High-Level
Disinfection
1-2-1. Pre-Cleaning
WARNING
Optional Sterilization
Steam
Sterilization
STERRAD
NX™ system
®
1
Endoscope
Pre-Cleaning
• During reprocessing, always wear protective equipment (e.g., gloves,
gowns, face masks, etc.) to minimize the risk of cross contamination.
• Pre-cleaning is intended to remove visible debris from the endoscope
immediately after its withdrawal from the patient, in order to withdrawal
subsequent cleaning procedure. Endoscopes that are from the patient
are soiled with debris such as blood, tissues, and mucus. When such
debris dries, it cannot be adequately removed in the subsequent cleaning
procedure. It should be noted that pre-cleaning cannot substitute for the
mechanical cleaning process. Always mechanically clean the endoscope
after pre-cleaning.
• During pre-cleaning, never wipe the insertion tube with alcohol or
disinfecting solution. These solutions may x organic contaminants and
proteinaceous debris to the instrument and have an adverse effect on
endoscope functionality and proper reprocessing.
• When using detergent, use only legally marketed brands that have been
tested and found to be compatible by PENTAX. A list of detergents that
are compatible with PENTAX endoscopes is contained in this manual.
CAUTION
• Immediately after use, the metal light guide plug and electrical contacts/
pins of the endoscope may be HOT. To avoid burns, do not touch these
areas immediately after use. For safer handling after a procedure, grasp
the PVE connector housing.
• Prior to pre-cleaning the endoscope, leave the PVE connector attached to
the video processor
• In order to prevent damage to the endoscope, do not place any objects
other than Inlet Seal (OF-B190) and Suction Control Valve (OF-B179)
with the endoscope in the closed container used for transport to the
reprocessing area.
.
– 14 –
Page 20

NOTE
1
Endoscope
Pre-Cleaning
CAUTION
In order to avoid damaging the endoscope, never subject it to suction in
excess of 66kPa
If the use of detergent solution is not permitted in the procedure room,
remove the endoscope from the procedure room and perform pre-cleaning
solution in another location.
.
1-2-1-1. Items required
• Protective equipment such as gloves, gowns, face masks, etc., to minimize the
risk of cross contamination.
• Detergent solution, Endozime (Ruhof Corporation)
• External suction source
• 500 mL basin
• Lint-free gauze
• 30 mL luer lock syringe
• Inlet seal (OF-B190)
• Suction control valve (OF-B179)
1-2-1-2. Preparation
1) Wear personal protective equipment.
2) Prepare a 500 mL basin with detergent solution per manufacturer's instructions
(temperature, concentration). In the case of ENDOZIME, add 30 mL of ENDOZIME
concentrate to 3.8 L (1 gallon) of clean potable water at 20°C~30°C (68°F~86°F).
1-2-1-3. Wiping of the insertion tube
1) Turn off the lamp switch of the video processor.
2) Immediately after removing the endoscope from the patient, gently wipe the entire
length of the insertion tube three times using lint-free gauze soaked with detergent
solution.
Figure 1.6
– 15 –
Page 21

1-2-1-4. Aspiration of detergent solution through the suction channel
1) Connect inlet seal (OF-B190) and suction control valve (OF-B179) to the endoscope.
2) Connect a suction tube from an external suction source to the endoscope suction
nipple.
3) Turn on the external suction source.
4) Place the distal end of the endoscope into a basin, and aspirate detergent solution
through the suction channel by pressing suction control valve (OF-B179) for 10
seconds.
1
(1) OF-B190
(2) OF-B179
(3) External Suction Source
(3)
Figure 1.7
(2)
(1)
5) Take the distal end out of detergent solution, and aspirate air through the suction
channel by pressing suction control valve (OF-B179) for 10 seconds.
6) Turn off the external suction source.
7) Disconnect tubing from the endoscope suction nipple.
1-2-1-5. Transport to cleaning room
1) Turn off the power to the video processor, and detach the PVE connector from the
video processor.
2) Transport the pre-cleaned endoscope to the cleaning room in a closed container.
Endoscope
Pre-Cleaning
– 16 –
Page 22

1
Leak
Testing
1-2-2. Leak Testing
Before reprocessing and/or immersion in any uids, PENTAX endoscopes should be tested for
the loss of integrity in their watertight construction by using a leakage tester.
CAUTION
Various types of manual and automated endoscope leakage testers
exist. Some manual and automated are stand-alone units, and others
may be integrated into an AER. PENTAX does not evaluate non-PENTAX
leakage testers to verify their specific product claims with respect to their
effectiveness to accurately detect leaks and/or their compatibility with
PENTAX endoscopes. Insufficient pressures may reduce the likelihood for
accurate leak detection, especially if the endoscope’s distal bending section
is not flexed during testing. Also, excessive pressures may adversely
affect the endoscope, especially if pressurization occurs during automated
reprocessing at elevated temperatures. PENTAX accepts no responsibility for
use of non-PENTAX Leakage testers. Users should check with the leakage
tester manufacturer and confirm their specific product claims, including
compatibility with PENTAX endoscopes at various temperatures and their
ability to detect leaks with/without uid immersion and with/without exing
of the endoscope’s distal bending section.
– 17 –
Page 23

1-2-3. Cleaning
WARNING
• During reprocessing, always wear protective equipment (e.g., gloves,
gowns, face masks, etc.) to minimize the risk of cross contamination.
• In order to ensure thorough cleaning, be sure to perform all cleaning
steps. The effectiveness of each cleaning step will influence the
effectiveness of subsequent steps. Failure to properly follow the cleaning
steps described may result in incomplete or ineffective cleaning,
disinfection, and/or sterilization of endoscope, and may pose a crossinfection risk.
• Immediately (within one hour) after the completion of a procedure,
the endoscope and its components should be thoroughly and carefully
cleaned with detergent solution. If the endoscope and its components
are left uncleaned for an excessive time after use, dried blood, mucus,
or other patient debris may cause damage or interfere with the ability of
the user to properly reprocess the device.
• For cleaning, use only legally marketed detergents that have been
tested according to the instructions of the manufacturer and found to
be compatible by PENTAX. A list of detergents that are compatible with
PENTAX endoscopes is contained in this manual.
• Fresh detergent solution must be used for each endscope that is
reprocessed.
1
Endoscope
Cleaning
CAUTION
• PVE soaking cap (OE-C9) must be properly secured over the electrical
contacts. Failure to do so could result in water invasion and damage
to the endoscope. If an endoscope is cleaned without the soaking cap
attached, do not use the endoscope, and contact your local PENTAX
service facility or sales representative.
• Ventilation cap (OF-C5) must be taken OFF during reprocessing. Failure
to do so can result in damage to the endoscope. If an endoscope is
cleaned with the ventilation cap attached, do not use the endoscope,
and contact your local PENTAX service facility or sales representative.
• During cleaning, never twist, rotate, or bend the insertion portion and
umbilical cable excessively.
• Never subject the endoscope to ultrasonic cleaning methods.
• In order to prevent damage to the endoscope, do not place any objects
other than the reprocessing accessories listed in section 1-2-3-1 of this
instruction for use with the endoscope when immersing it in a cleaning
basin.
– 18 –
Page 24

1-2-3-1. Items required
Endoscope component
• Inlet seal (OF-B190)
1
Reprocessing accessory
• PVE soaking cap (OE-C9)
• Cleaning brush (CS6002SN)
• Cleaning brush (CS6015ST) for EB-1570K, EB-1970K, EB-1970TK,
EB-1575K and EB-1975K
• Cleaning brush (CS3010S) (EB-1170K and EB-1990i only)
• Cleaning brush (CS-C3S)
• Cleaning adapter (OF-B155)
Other Equipment
Endoscope
Cleaning
• Protective equipment such as gloves, gowns, face masks, etc., to minimize the
risk of cross contamination.
• Detergent solution, Endozime (Ruhof Corporation)
• Clean potable water
• Basin sufcient in size to immerse the entire endoscope (at least 50 cm in width
x 40 cm in depth x 15 cm in height)
• Lint-free gauze
• 30 mL luer slip syringe
1-2-3-2. Preparation
1) Wear personal protective equipment.
2) Attach PVE soaking cap (OE-C9) to the endoscope
3) Detach Ventilation Cap (OF-C5) from the endoscope.
(1)
(1) OE-C9
(2) OF-C5
(2)
Figure 1.8
4) Fill a basin with a sufcient volume of detergent solution to completely immerse the
endoscope. Prepare the detergent in accordance with the manufacturer’s instructions
(temperature, concentration). In the case of ENDOZIME, add 30 mL of ENDOZIME
concentrate to 3.8 L (1 gallon) of clean potable water at 20°C~30°C (68°F~86°F).
– 19 –
Page 25

1-2-3-3. Cleaning of all external surfaces
(1)
CAUTION
• Do not squeeze or severely bend the insertion tube.
• Do not use any abrasive materials.
• Be careful to avoid damage to the distal lenses.
1) Fully immerse the endoscope with the components attached in detergent solution.
2) Detach the inlet seal (OF-B190) and suction control valve (OF-B179) from the
endoscope.
(2)
(1)
Figure 1.9
(1) OF-B190
(2) OF-B179
3) Open the cap of inlet seal (OF-B190) and place it in the detergent solution.
(1) OF-B190
1
Endoscope
Cleaning
Figure 1.10
4) Reprocess suction control valve (OF-B179) separately from the endoscope. (see
Section 1-3 “Endoscope components and accessories”)
5) While still immersed in detergent solution, wash the entire surface of the endoscope
and OF-B190 three times with a lint-free gauze.
6) Visually inspect the entire surface of the endoscope to insure that no soil is present,
paying special attention to areas such as the distal end, and control body, which are
the most likely regions to retain visible soil.
7) If any soil is still present on the endoscope, remove it by wiping the area in question
with lint-free gauze until it has been completely removed.
– 20 –
Page 26

1-2-3-4. Brushing of the suction channel
WARNING
1
Endoscope
Cleaning
• Do not use cleaning brushes other than those that are specied in this
instructions for use. Failure to do so could result in endoscope damage
or incomplete or ineffective cleaning.
• Prior to use, ensure that cleaning brushes are not damaged (e.g., kinked
shaft or bent or missing bristles).
• In order to prevent the dispersal of patient debris that might still be in
the endoscope channel into the environment, always withdraw brushes
slowly.
CAUTION
• In order to avoid damage to the endoscope, never attempt to insert a
cleaning brush into the endoscope distal tip.
• Never apply excessive pressure to introduce or withdraw the brush. This
can result in damage to the endoscope and/or the brush.
Always fully immerse the endoscope during brushing.
1) Insert cleaning brush (CS6002SN), into the opening of the suction nipple, and gently
push it until it appears in the suction cylinder.
(1) CS6002SN
(1)
(2)
(3)
Figure 1.11
(2) Suction Nipple
(3) Suction Cylinder
2) Gently withdraw the brush, and remove from debris from the brush head by rubbing
it with gloved ngers.
3) Repeat steps 1 and 2 three additional times.
4) Insert cleaning brush (CS6002SN) into the opening at bottom of the suction cylinder
and advance it for about 15 cm until resistance is felt. DO NOT USE EXCESSIVE
FORCE.
(1)
(2)
(1) CS6002SN
(2) Suction Cylinder
Figure 1.12
5) Gently withdraw the brush, and remove debris from the brush head by rubbing it
with gloved ngers.
– 21 –
Page 27

6) Repeat steps 4 and 5 three additional times.
7) Insert cleaning brush (CS3010S for EB-1170K and EB-1990i/CS6015ST: for all
other scopes) into the instrument channel inlet, and gently advance it until it exits the
distal end of the endoscope.
(1) CS3010S (for EB-
(1)
(2)
Figure 1.13
1170K and EB-1990i)
CS6015T (for all other
scopes)
(2) Instrument Channel
Inlet
8) Remove debris from the brush head by rubbing it with gloved ngers and then gently
withdraw the brush.
9) Repeat steps 7 and 8 three additional times.
10) Insert cleaning brush (CS-C3S) into the suction cylinder, and rotate it for 30 seconds.
Do not insert the brush excessively.
(1) CS-C3S
(2) Suction Cylinder
(1)
(2)
1
Endoscope
Cleaning
Figure 1.14
11) Withdraw the brush. Remove debris from the brush head by rubbing it with gloved
ngers.
12) Repeat steps 10 and 11 three additional times.
– 22 –
Page 28

1-2-3-5. Filling the suction channel with detergent solution
WARNING
1
Endoscope
Cleaning
• While injecting detergent solution through the channels, avoid the
introduction of air. The presence of air bubbles can prevent contact of
the detergent solution with channel surfaces.
• Always fully immerse the endoscope while flushing detergent solution
into the endoscope channel.
• It is imperative that the cleaning adapter (OF-B155) and Inlet Seal (OF-
B190) be securely attached to the endoscope. Failure to properly mount
and secure the cleaning adapter and inlet seal can result in ineffective
and incomplete reprocessing.
CAUTION
In order to avoid damage to the endoscope, never apply excessive force if
resistance is encountered while ushing detergent solution into channels. Do
not use the endoscope, and contact your local PENTAX service facility or sales
representative.
Always immerse the endoscope, components, and accessories in detergent solution during
cleaning.
Attaching components and accessories to the endoscope
1) Attach inlet seal (OF-B190) and cleaning adapter (OF-B155) to the endoscope.
(2)
(1)
Figure 1.15
(1) OF-B190
(2) OF-B155
Filling the suction channel with detergent solution
2) Attach a 25 mL syringe lled with the detergent solution to the suction nipple, and
inject 25 mL of detergent solution into the suction channel.
(1) Suction Nipple
(1)
Figure 1.16
– 23 –
Page 29

3) Check to conrm that detergent solution ows out from the suction channel opening
(2)
on the distal end of the endoscope.
4) Insert a syringe lled with detergent solution to inlet seal (OF-B190), and inject 25
mL of detergent solution into the suction channel.
(1) OF-B190
(1)
Figure 1.17
5) Detach the inlet seal cleaning adapter inlet seal (OF-B190), and cleaning adapter
(OF-B155) from the endoscope, and leave them to soak along with the endoscope in
detergent solution. Open the cap of inlet seal (OF-B190).
1
Figure 1.18
(1)
(1) OF-B190
(2) OF-B155
Endoscope
Cleaning
– 24 –
Page 30

1-2-3-6. Soaking in detergent solution
WARNING
1
Endoscope
Cleaning
• The detergent solution must remain in contact with ALL internal channels
and external endoscope surfaces for the time period recommended by
the manufacturer of the detergent.
• Adhere to the conditions (temperature, concentration, time) specified
by the detergent manufacturer to accomplish effective and complete
cleaning. Use of the detergent solution under conditions that fall outside
the manufacturer's directions might damage the endoscope. Use of
a timer or audible alarm is recommended in order not to exceed the
recommended soaking time.
• During immersion, detach the cleaning adapter (OF-B155) and inlet
seal (OF-B190) from the endoscope to ensure contact of all endoscope
surfaces with the detergent solution.
CAUTION
Never subject the endoscope to ultrasonic cleaning methods.
1) While fully immersing the endoscope, inlet seal (OF-B190), and cleaning adapter
(OF-B155), ensure that there are no air bubbles on the endoscope surfaces, distal
end, and acessories. If any air bubbles are detected, ush them away with detergent
solution using a 30 mL syringe.
2) Soak the endoscope, inlet seal (OF-B190), and cleaning adapter (OF-B155)
under conditions (temperature, concentration, time) specified by the detergent
manufacturer. In the case of Endozime, the immersion time is 3 minutes.
Figure 1.19
3) After soaking, ultrasonically clean inlet seal (OF-B190), and cleaning adapter (OFB155) according to Section 1-3 “Endoscope components and accessories”.
Purging detergent solution the from suction channel
4) After ultrasonic cleaning, attach inlet seal (OF-B190) and cleaning adapter (OFB155) to the endoscope. (Figure 1.15, p23)
5) Attach a syringe lled with air to the suction nipple, and ush the suction channel
with at least 35 mL of air to purge as much residual detergent solution as possible.
(Figure 1.16, p23)
6) Insert a syringe lled with air to inlet seal (OF-B190), and ush the suction channel
with at least 35 mL of air to purge as much residual detergent solution as possible.
(Figure 1.17, p24)
7) Remove the endoscope (with inlet seal (OF-B190) and cleaning adapter (OF-B155)
attached) from the detergent solution.
– 25 –
Page 31

1-2-3-7. Rinsing
WARNING
It is important that all internal channels, external endoscope surfaces,
and components be thoroughly rinsed with clean water to remove residual
detergent solution. Failure to do so can result in ineffective or incomplete
disinfection and sterilization.
First rinse
1) Place the endoscope with the inlet seal (OF-B190), and cleaning adapter (OF-B155)
attached in a basin of clean water that is of sufcient volume to completely immerse
the endoscope.
2) Detach inlet seal (OF-B190), and cleaning adapter (OF-B155) from the endoscope. Open
the cap of inlet seal (OF-B190). (Figure 1.18, p24)
3) Wipe all exterior surfaces of the endoscope, inlet seal (OF-B190), and cleaning adapter
(OF-B155) one time with a lint-free gauze in order to remove residual detergent solution.
4) While still completely immersed in water, grasp the distal end and control body of the
scope and PVE connector with two hands, and agitate them under the water by moving
them from side to side repeatedly for one minute.
1
Endoscope
Cleaning
Figure 1.20
5) Similarity, agitate inlet seal (OF-B190), and cleaning adapter (OF-B155) under the
water by moving them from side to side repeatedly for at least one minute.
6) Attach inlet seal (OF-B190), and cleaning adapter (OF-B155) to the endoscope.
(Figure 1.15, p23)
7) Attach a syringe lled with water to the suction nipple, and ush the suction channel
with 35 mL of water. (Figure 1.16, p23)
8) Insert a syringe filled with water into inlet seal (OF-B190), and flush the suction
channel with 35 mL of water. (Figure 1.17, p24)
9) Remove the endoscope with inlet seal (OF-B190), and cleaning adapter (OF-B155)
attached from the water.
10) Attach a syringe lled with air to the suction nipple, and ush the suction channel
with 35 mL of air to expel residual water. (Figure 1.16, p23)
11) Insert a syringe lled with air to the inlet seal, and ush the suction channel with 35
mL of air. (Figure 1.17, p24)
– 26 –
Page 32

1
Endoscope
Cleaning
Second rinse
12) Fill a basin with clean water and repeat steps 1-11 to perform a second rinse.
Third rinse
13) Fill a basin with clean water and repeat steps 1-11 to perform a third rinse.
Forth rinse
14) Fill a basin with clean water and repeat steps 1-11 to perform a fourth rinse.
15) Detach inlet seal (OF-B190), and cleaning adapter (OF-B155) from the endoscope.
(Figure 1.18, p24)
1-2-3-8. Drying
1) Gently wipe and dry all external surfaces of the endoscope, inlet seal (OF-B190),
and cleaning adapter (OF-B155) with a new lint-free gauze.
– 27 –
Page 33

1-2-4. High-Level Disinfection
Prior to high-level disinfection, the end user should confirm the minimum effective
concentration (MEC) of reused disinfectant as per the manufacturer’s instructions.
WARNING
• During the reprocessing process, always wear protective equipment
(e.g., gloves, gowns, face masks, etc.) to minimize the risk of cross
contamination.
• Prior to disinfection, it is imperative that any solutions previously used in
the cleaning process be thoroughly rinsed and dried. Failure to do so can
result in ineffective or incomplete disinfection.
• For high-level disinfection, use an appropriate disinfecting solution
according to the instructions of the disinfectant manufacturer
(temperature, concentration, time). Adhere to the instructions to
accomplish effective and complete disinfection. The endoscope may be
damaged if exposed to a disinfectant under conditions other than those
specied by the disinfectant manufacturer.
• Use only a legally marketed disinfectant that has been tested according
to the instructions provided by the manufacturer and found to be
compatible by PENTAX. A list of disinfectants that are compatible with
PENTAX endoscopes is contained in this manual.
• It is imperative that ALL internal channel surfaces be in contact with
the disinfecting solution for the time period recommended by the
manufacturer of the solution.
• Ideally, all final rinses should be performed with sterile water, clean
potable water, or water that meets the requirements of the health care
facility.
• Regardless of the quality of the rinse water used, it is essential to
perform a nal alcohol rinse followed by forced air in order to completely
dry the endoscope channels and prevent bacterial colonization and/or
infections associated with waterborne microorganisms.
• The basin that is used to perform disinfectant immersion must be
thoroughly cleaned prior to lling it with disinfectant solution.
1
Endoscope
Disinfection
– 28 –
Page 34

CAUTION
• Prior to disinfection, attach PVE soaking cap (OE-C9). Failure to do
1
so can result in water invasion and damage to the endoscope. If the
endoscope is disinfected without the soaking cap attached, do not use
the endoscope, and contact your local PENTAX service facility or sales
representative.
• Prior to disinfection, detach the ventilation cap (OF-C5). Failure to do so
can result in damage to the endoscope. If the endoscope is disinfected
with the ventilation cap attached, do not use the endoscope, and contact
your local PENTAX service facility or sales representative.
• During disinfection, never twist, rotate, or bend the insertion tube and
umbilical cable excessively.
• To prevent damaging the endoscope, do not place any objects other
than the reprocessing accessories described in section 1-2-4-1 with the
endoscope when immersing the endoscope in the disinfection basin.
1-2-4-1. Items required
Endoscope component
Endoscope
Disinfection
• Inlet seal (OF-B190)
Reprocessing accessory
• PVE soaking cap (OE-C9)
• Cleaning adapter (OF-B155)
Other equipment
• Protective equipment such as gloves, gowns, face masks, etc., to minimize the
risk of cross contamination.
• Disinfecting solution, Cidex Activated Dialdehyde Solution (Johnson &
Johnson).
• Sterile water (preferred) or clean potable water
• 70-90% medical grade ethyl or isopropyl alcohol
• Basin sufcient in size to immerse the entire endoscope (at least 50 cm in width
x 40 cm in depth x 15 cm in height)
• Sterile gauze
• 30 mL luer slip syringe
– 29 –
Page 35

1-2-4-2. Preparation
1) Wear personal protective equipment.
2) Attach the PVE soaking cap (OE-C9) to the endoscope.
3) Ensure that the ventilation cap (OF-C5) is detached from the endoscope.
(1) OE-C9
(1)
(2)
Figure 1.21
(2) OF-C5
4) Prepare a basin with a sufficient volume of disinfecting solution to completely
immerse the endoscope.
1-2-4-3. Filling the suction channel with disinfecting solution
WARNING
1
• When lling endoscope channels with disinfectant, avoid the introduction
of air. The presence of air bubbles can prevent contact of the disinfectant
with channel surfaces.
• Always immerse the endoscope while filling endoscope channels with
disinfectant.
• It is imperative that cleaning adapter (OF-B155) and Inlet Seal (OF-B190)
be securely attached to the endoscope. Failure to properly mount and
secure the cleaning adapter and inlet seal can result in ineffective and
incomplete reprocessing.
Always fully immerse the endoscope, components, and accessories in disinfecting
solution during disinfection.
Attaching components and accessories to the endoscope
1) Fully immerse the endoscope in disinfecting solution, and attach inlet seal (OF-B190)
and cleaning adapter (OF-B155) to the endoscope.
(2)
(1)
(1) OF-B190
(2) OF-B155
Endoscope
Disinfection
Figure 1.22
– 30 –
Page 36

Filling the suction channel with disinfecting solution
(2)
2) Attach a syringe lled with disinfecting solution to the suction nipple, and inject 25
mL of disinfecting solution into the suction channel.
1
Endoscope
Disinfection
(1) Suction Nipple
(1)
Figure 1.23
3) Conrm that disinfecting solution ows out from the suction channel opening on the
distal end.
4) Insert a syringe lled with disinfecting solution into inlet seal (OF-B190), and inject
25 mL of disinfecting solution into the suction channel.
(1) OF-B190
(1)
Figure 1.24
5) After injecting disinfecting solution into the suction channel, detach inlet seal (OF-
B190), and cleaning adapter (OF-B155) from the endoscope, and leave them with
the endoscope in the disinfecting solution. Open the cap of inlet seal (OF-B190).
(1) OF-B190
(1)
Figure 1.25
(2) OF-B155
– 31 –
Page 37

1-2-4-4. Soaking in disinfecting solution
WARNING
• The disinfecting solution must remain in contact with ALL internal channels
and external endoscope surfaces for the time period recommended by
the disinfectant manufacturer.
• Adhere to the conditions (temperature, concentration, time) specified
by the disinfectant manufacturer to accomplish effective and complete
disinfection. Disinfectant solution use under conditions that fall outside
the manufacturer's directions might damage the endoscope. Use of
a timer or audible alarm is recommended in order not to exceed the
recommended soaking time.
• During immersion, detach the cleaning adapter (OF-B155) and inlet
seal (OF-B190) from the endoscope to ensure contact of all endoscope
surfaces with the disinfecting solution.
1) Fully immerse the endoscope, inlet seal (OF-B190), and cleaning adapter (OF-
B155) to ensure that there are no air bubbles on the endoscope surfaces, distal end,
and accessories. If any air bubbles are detected, ush them away with disinfecting
solution using a syringe.
2) Soak the endoscope, inlet seal (OF-B190), and cleaning adapter (OF-B155) under
the conditions (temperature, concentration, time) specified by the disinfectant
manufacturer. In the case of Cidex Activated Dialdehyde Solution, the immersion
time is 45 minutes at 25°C.
1
Endoscope
Disinfection
Figure 1.26
Purging of disinfecting solution from suction channel
3) After soaking, attach inlet seal (OF-B190) with the cap closed, and cleaning adapter
(OF-B155) to the endoscope. (Figure 1.22, p30)
4) Attach a syringe lled with air to the suction channel, and ush the suction channel
with at least 35 mL of air to purge as much residual disinfecting solution as possible.
(Figure 1.23, p31)
5) Insert a syringe lled with air to inlet seal (OF-B190), and ush the suction channel
with at least 35 mL of air to purge as much residual disinfecting solution as possible.
(Figure 1.24, p31)
6) Remove the endoscope with inlet seal (OF-B190) and cleaning adapter (OF-B155)
attached from the disinfecting solution.
– 32 –
Page 38

1-2-4-5. Rinsing
WARNING
1
Endoscope
Disinfection
• Ideally, all nal rinses should be performed with sterile water. However,
if sterile water is not used, use potable water or the water that meets
the requirements of the health care facility.
• The basin that is used to perform rinsing of the endoscope and
accessories must be thoroughly cleaned prior to lling it with rinse water.
• The rinse volumes recommended for removing residual disinfectant
from channels are sufcient for 14-day glutaraldehydes (Cidex Activated
Dialdehyde Solution). If extended shelf-life glutaraldehydes or other FDAcleared, commercially available high level disinfectants are used, consult
with the disinfectant manufacturer for details regarding recommended
rinse water volumes.
First rinse
1) Place the endoscope with the inlet seal (OF-B190) and cleaning adapter (OF-B155)
attached in a basin of sterile water that is of sufcient volume to completely immerse
the endoscope.
2) Detach inlet seal (OF-B190), and cleaning adapter (OF-B155) from the endoscope.
Open the cap of inlet seal (OF-B190).
3) Wipe all exterior surfaces of the endoscope, inlet seal (OF-B190), and cleaning
adapter (OF-B155) at least two times with a sterile gauze in order to remove residual
disinfecting solution.
4) While still completely immersed in water, grasp the distal end and control body of
the scope and PVE connector with two hands, and agitate them under the water by
moving them from side to side repeatedly for at least one minute.
Figure 1.27
5) Similarity, grasp inlet seal (OF-B190) and cleaning adapter (OF-B155) and agitate
them under water by moving them from side to side for at least one minute.
6) Attach inlet seal (OF-B190) and cleaning adapter (OF-B155) to the endoscope.
(Figure 1.22, p30)
7) Attach a syringe lled with water to the suction nipple, and ush the suction channel
with at least 35 mL of water. (Figure 1.23, p31)
8) Insert a syringe filled with water to inlet seal (OF-B190) and flush the suction
channel with at least 35 mL of water. (Figure 1.24, p31)
– 33 –
Page 39

9) Take the endoscope with inlet seal (OF-B190) and cleaning adapter (OF-B155)
attached out of the water.
10) Attach a syringe lled with air to the suction nipple, and ush the suction channel
with at least 35 mL of air to expel residual water. (Figure 1.23, p31)
11) Insert a syringe lled with air into the inlet seal, and ush the suction channel with
35 mL of air. (Figure 1.24, p31)
Second rinse
12) Fill a basin with sterile water and repeat steps 1-11 in order to perform a second
rinse.
Third rinse
13) Fill a basin with sterile water and repeat steps 1-11 in order to perform a third rinse.
Forth rinse
14) Fill a basin with sterile water and repeat steps 1-11 to perform a fourth rinse.
15) Remove the endoscope and its component from the basin of water and place them on
a clean, dry, lint-free cloth.
1-2-4-6. Drying
1
WARNING
Regardless of the quality of the rinse water used, it is essential to perform
a final alcohol rinse followed by forced air in order to completely dry the
endoscope channels and prevent bacterial colonization and/or infections
associated with waterborne microorganisms.
Flushing the channels with alcohol
1) Attach a syringe lled with 70-90% medical grade ethyl or isopropyl alcohol to the
suction nipple, and ush the suction channel with 15 mL of alcohol. (Figure 1.23,
p31)
2) Insert a syringe lled with 70-90% medical grade ethyl or isopropyl alcohol into the
inlet seal (OF-B190), and ush the suction channel with 15 mL of alcohol. (Figure
1.24, p31)
Purging of alcohol from channel
3) Attach a syringe lled with air to the suction nipple, and ush the suction channel 35
mL of air to expel residual alcohol. (Figure 1.23, p31)
4) Insert a syringe lled with air to inlet seal (OF-B190), and ush the suction channel
with 35 mL of air to expel residual alcohol. (Figure 1.24, p31)
5) Ensure no alcohol exits the endoscope end. If any, repeat steps 3 and 4.
6) Detach inlet seal (OF-B190) and cleaning adapter (OF-B155) from the endoscope.
(Figure 1.25, p31)
Endoscope
Disinfection
Drying of all external surfaces
7) Gently dry all external surfaces of the endoscope, inlet seal (OF-B190), and cleaning
adapter (OF-B155) with a sterile gauze.
– 34 –
Page 40

1-2-5. Optional Sterilization
WARNING
1
Endoscope
Sterilization
• Please note that PENTAX Medical has not validated any sterilization
methods for exible endoscopes othet than STERRAD® NX™ system.
• During the reprocessing process, always wear protective equipment
(e.g., gloves, gowns, face masks, etc.) to minimize the risk of cross
contamination.
• Sterilization efcacy and material compatibility depend on the following
factors:
- thorough cleaning of the device
- load of the devices to be sterilized
- wrapping of the devices to be sterilized
- sterilizer cycle parameters
- quality of rinse water
• Prior to sterilization, clean and dry the endoscope thoroughly. Failure to
do so can result in ineffective or incomplete sterilization.
• Use a chemical indicator (CI) and/or biological indicator (BI) to control
the sterilization process and ensure sterilization efcacy.
• The manufacturer of the sterilizer should be consulted to confirm that
test data exists to substantiate that no harmful levels of any residues
(active/inert ingredients, their by-products or derivatives of the
processed devices) remain on any instrument that may pose a risk to
patients and users.
• After sterilization, ensure that the package is intact. If there are any
signs of abnormalities such as stains, tears, or any other indications that
the packaging has been damaged or opened, repeat the sterilization
process with new packaging.
CAUTION
• Due to the heat sensitive nature and/or the specific biocompatible
materials used in the construction of flexible endoscopes, some
sterilization systems/processes/solutions may have detrimental effects
on flexible endoscopes. To avoid the potential for instrument damage
and/or endoscope failure, confirm the compatibility of such systems/
solutions with your local PENTAX dealer prior to use with any PENTAX
products. Also, confirm the specific claim(s) of any sterilization
methods/processes with the sterilizer manufacturer to ensure that they
have performed microbiological validation studies to support their claims
of achieving sterilization of device specic exible endoscope models and
endoscope components.
• NEVER place the endoscope in a steam sterilizer!
– 35 –
Page 41

1-2-5-1. Sterilization using STERRAD® NX™ System (EB-1575K,
EB-1975K, EB-1990i only)
CAUTION
• PENTAX video bronchoscopes other than EB-1575K, EB-1975K, EB-1990i
are incompatible with the STERRAD® NX™ system.
• When sterilizing the endoscope, do not place any objects other than inlet
seal (OF-B 190) and suction control valve (OF-179) into the sterilization
tray.
• After every 100 cycles of STERRAD® NX™ exposure, the endoscopes
should be returned to an authorized PENTAX service facility for repair.
Replacement of the insertion tube and bending section will be necessary,
and other components may also require repair or replacement.
• Although STERRAD® NX™ compatible endoscopes are capable of
withstanding STERRAD® NX™ sterilization for up to 100 cycles, repair/
replacement of parts might be necessary prior to reaching 100 cycles,
depending upon the condition of the endoscope.
1-2-5-1-1. Items required
• protective garments such as gloves, gowns, face masks, etc. to minimize the risk
of cross contamination.
• APTIMAX Instrument Tray ( 270 mm in width, 576 mm in length, 100 mm in height)
1
• APTIMAX Instrument Tray Mat ( 254 mm in width, 546 mm in length)
• instrument wrap recommended by STERRAD® for use of STERRAD® NX™
system
• tape recommended by STERRAD® for use of STERRAD® NX™ system
• Ventilation cap (OF-C5)
Endoscope
Sterilization
– 36 –
Page 42

1-2-5-1-2. Preparation
WARNING
1
Consult the manufacturer of the sterilization tray regarding its directions for
use.
CAUTION
Prior to the sterilization using STERRAD® NX™ system, ensure that Ventilation
cap (OF-C5) is attached to the scope. Failure to do so can result in bursting of
the bending rubber.
1) Detach cleaning adapter (OF-B155) and the inlet seal (OF-B190) are detached from
the scope.
(1)
(2)
Figure 1.28
(1) OF-B155
(2) OF-B190
Endoscope
Sterilization
2) Detach PVE soaking cap (OE-C9) from the scope.
3) Attach ventilation cap (OF-C5) to the scope
(1) OF-C5
(2) OE-C9
(2)
(1)
Figure 1.29
4) Lay the APTIMAX Instrument Tray Mat on APTIMAX Instrument Tray
5) Place the scope onto the mat as shown below
Figure 1.30
6) Cover the tray with the lid.
– 37 –
Page 43

1-2-5-1-3. Wrapping
WARNING
• Contact the manufacturer regarding directions for use of instrument
wrap and tape.
• Scope components may be wrapped and sterilized together with
the scope. However, do not attach any components other than the
ventilation cap to the scope
• Secure the wrapping with a length of tape that is sufcient to prevent the
wrapping from peeling away from package. Failure to do so can result in
removal of the tape and ineffective sterilization.
1) Wrap the tray containing the scope.
2) Apply the tape lengthwise across the three sides of the wrapped container.
1-2-5-1-4. Sterilization Parameter Selection
WARNING
Contact Johnson & Johnson regarding directions for use of the STERRAD®
NX™ system
1) Set the tray containing the scope into the sterilization chamber.
1
STERRAD® NX™ :
Select "Advanced" cycle and operate the sterilizer according to the instructions supplied
with the STERRAD® NX™.
Endoscope
Sterilization
– 38 –
Page 44

1-3. Endoscope components and accessories
NOTE
Endoscope components and accessories can be subjected to the following cleaning, disinfection,
and sterilization processes.
1
Model Cleaning
High-Level
Name Number Manual Ultrasonic
Endoscope
Component
Accessory Bite Block OF-Z5 Y Y Y Y N
Reprocessing
Accessory
Suction
Control Valve
Inlet Seal OF-B190 Y Y Y Y Y
PVE Soaking
Cap
Ventilation
Cap
Cleaning
Adapter
Cleaning
Brush
Cleaning
Brush
Cleaning
Brush
Cleaning
Brush
OF-B179 Y Y Y Y Y
OE-C9 Y* N Y* N N
OF-C5 N N N N Y**
OF-B155 Y Y Y Y N
CS6002SN Y Y Y Y N
CS6015ST Y Y Y Y N
CS3010S Y Y Y Y N
CS-C3S Y Y Y Y N
Disinfection
Optional Sterilization
Steam
Sterilization
STERRAD®
NX™
system
Accessory
Cleaning
Y : YES
N : NO
* : The PVE Soaking Cap (OE-C9) should be attached to the endoscope during cleaning and
disinfection procedures.
** : The ventilation cap (OF-C5) should be attached to the endoscope during the STERRAD®
sterilization.
Automated Endoscope Reprocessor (AER) manufacturers may not make
specific claims or provide special instructions for reprocessing all of the
removable endoscope components and accessories that are integral to the
safe and effective operation of flexible endoscopes. Therefore, should the
AER manufacturer's instructions not specically address reprocessing of any
particular endoscope component (suction valve, inlet seal, etc.) reprocess
those components manually as described in this manual. Prior to use, check
with the AER manufacturer regarding their specific claims with respect to
reprocessing individual endoscope components.
– 39 –
Page 45

1-3-1. Cleaning
WARNING
• During the reprocessing process, always wear protective equipment
(e.g., gloves, gowns, face masks, etc.) to minimize the risk of cross
contamination.
• Endoscope components and accessories should be thoroughly and
carefully cleaned with detergent solution within one hour after the
conclusion of an endoscopic procedure. If they are left uncleaned for
greater than one hour after use, dried blood, mucus or other patient
debris may cause damage to them or interfere with the ability of the
user to properly reprocess them.
• For cleaning, use only legally marketed detergents which have been
tested and found to be compatible by PENTAX. A list of detergents that
are compatible with PENTAX components and accessories is contained in
this manual.
• Fresh detergent solution must be used for each set of endoscope
components and accessories.
1-3-1-1. Items required
• Protective equipment such as gloves, gowns, face masks, etc., to minimize the
risk of cross contamination.
1
• Detergent solution, Endozime (Ruhof Corporation)
• Clean potable water
• Basin (at least 25 cm in width x 20 cm in depth x 15 cm in height )
• Lint-free gauze
• 10 mL luer slip syringe
• Ultrasonic cleaner (frequency range: 44 kHz +/-6%)
1-3-1-2. Cleaning procedure
Preparation
1) Wear personal protective equipment.
2) Prepare a basin with detergent solution per manufacturer’s instructions (temperature,
concentration). In the case of ENDOZIME, add 30 mL of ENDOZIME concentrate
to 3.8 L (1 gallon) of clean potable water at 20°C~30°C (68°F~86°F).
3) Fully immerse the components and accessories, and keep them immersed in
detergent solution during the following cleaning procedure.
Accessory
Cleaning
– 40 –
Page 46

4) Open the cap of inlet seal (OF-B190) during cleaning.
1
Figure 1.31
Cleaning of all external surfaces
5) Wash all surfaces of components and accessories three times with a lint-free gauze.
Cleaning of brushes
6) Wash the brush heads of cleaning brushes (CS6002SN, CS6015ST, CS3010S, CS-
C3S) by rubbing them with gloved ngers for 30 seconds.
Manipulating of valve mechanisms
7) While fully immersed, manipulate the suction control valve (OF-B179) mechanism
four times.
Accessory
Cleaning
Figure 1.32
Filling of lumens with detergent solution
8) Using a syringe lled with detergent solution, inject 4 mL of the detergent solution
directly into the lumen of the suction control valve (OF-B179).
Figure 1.33
Soaking in detergent solution
9) While fully immersed, ensure there are no air bubbles on the surfaces of components
and accessories. If any bubbles are detected, ush them away with detergent solution
using a syringe.
10) Soak components and accessories in detergent solution according to the instructions
(temperature, concentration, time) specified by the detergent manufacturer. In the
case of Endozime, the immersion time is at 3 minutes
11) Remove the components and accessories from the detergent solution.
– 41 –
Page 47

1-3-1-3. Ultrasonic Cleaning
WARNING
All components and accessories must be ultrasonically cleaned prior to highlevel disinfection or sterilization.
CAUTION
DO NOT use caustic or abrasive solutions in the ultrasonic cleaner.
1) Prepare detergent solution per the manufacturer’s instructions (temperature,
concentration). In the case of ENDOZIME, add 30 mL of ENDOZIME concentrate
to 3.8 L (1 gallon) of clean potable water at 20°C~30°C (68°F~86°F).
2) Immerse the components and accessories in detergent solution.
3) Using a syringe lled with detergent solution, inject detergent solution directly into
each lumen of the suction control valve (OF-B179).
4) Perform ultrasonic cleaning under the following conditions:
Frequency Range: 44 kHz +/- 6 % Time: 5 minutes
5) After completion of the ultrasonic cleaning process, remove the components and
accessories from the ultrasonic cleaner.
1
1-3-1-4. Rinsing
WARNING
All residual detergent solution must be removed from the components and
accessories. Residual detergent solution may interfere with subsequent
disinfection and sterilization processes.
First rinse
1) Prepare a basin with clean water, and fully immerse the components and accessories.
2) Wipe all exterior surfaces of the components and accessories at least two times with
a lint-free gauze in order to remove residual detergent solution.
3) While still completely immersed in water, agitate them under the water by moving
them from side to side for at least one minute.
4) Manipulate the suction control valve (OF-B179) mechanism at least four times in the
water, and using a syringe lled with water, ush the lumen of the valve with at least
5 mL of water. (see Figure 1.32, p41 and Figure 1.33, p41)
5) Discard the water.
Second rinse
6) Fill a basin with clean water and repeat steps 2-5 to perform a second rinse.
Accessory
Cleaning
– 42 –
Page 48

Third rinse
7) Fill a basin with clean water and repeat steps 2-5 to perform a third rinse.
Forth rinse
1
8) Fill a basin with clean water and repeat steps 2-5 to perform a fourth rinse.
9) Remove all components and accessories from the water.
Purging of water from lumens
10) Using a syringe lled with air, ush the lumen of the suction valve (OF-B179) to
purge residual water. (see Figure 1.33, p41)
1-3-1-5. Drying
1) Wipe all surfaces of components and accessories gently with a lint-free gauze.
Accessory
Cleaning
– 43 –
Page 49

1-3-2. High-Level Disinfection
WARNING
• During the reprocessing process, always wear protective equipment
(e.g., gloves, gowns, face masks, etc.) to minimize the risk of cross
contamination.
• Prior to disinfection, all components and accessories must be
meticulously cleaned. Failure to do so can result in incomplete or
ineffective disinfection.
• For high-level disinfection, use disinfecting solution according
to instructions of the disinfectant manufacturer (temperature,
concentration, time). Use only legally marketed disinfecting solutions
that have been tested and found to be compatible by PENTAX. A list of
disinfectant solutions that are compatible with PENTAX components and
accessories is contained in this manual.
• Adhere to the instructions specified by the disinfectant manufacturer
to accomplish effective and complete disinfection. Failure to do so may
result in damage to the endoscope components and accessories.
• Ideally, all nal rinses should be performed with sterile water. However,
if sterile water is not used, use clean potable water that meets the
requirements of the health care facility.
• Regardless of the quality of the rinse water used, it is essential
to perform a final alcohol rinse followed by forced air in order to
completely dry the lumens of components and accessories and prevent
bacteria colonization and/or infections associated with waterborne
microorganisms.
• The basin that is used to perform disinfectant immersion must be
thoroughly cleaned prior to lling it with disinfectant solution.
• Verification that the potency of the liquid chemical germicide is at or
above its Minimum Effective Concentration (MEC) (using recommended
test strips or similar methods) is required to ensure that high-level
disinfection/sterilization can be achieved.
1
1-3-2-1. Items required
• Protective equipment such as gloves, gowns, face masks, etc., to minimize the
risk of cross contamination.
• Disinfecting solution, Cidex Activated Dialdehyde Solution (Johnson &
Johnson)
• Sterile water (preferred) or clean potable water
• 70-90% medical grade ethyl or isopropyl alcohol
• Basin (at least 25 cm in width x 20 cm in depth x 15 cm in height )
• Sterile gauze
• 10 mL luer slip syringe
Accessory
Disinfection
– 44 –
Page 50

1-3-2-2. Disinfection procedure
Preparation
1) Wear personal protective equipment.
1
2) Prepare a basin of disinfecting solution per manufacturer’s instructions (temperature,
concentration).
3) Fully immerse the components and accessories, and keep them immersed in
disinfecting solution during the following disinfection procedure.
4) Open the cap of inlet seal (OF-B190) and fully immerse it in disinfecting solution.
(see Figure 1.31, p41)
Manipulating the valve mechanism
5) While fully immersed, manipulate the suction control valve (OF-B179) mechanism
four times. (see Figure 1.32, p41)
Filling of disinfecting solution into the lumens
6) Using a syringe filled with disinfecting solution, inject at least 4 mL of the
disinfecting solution directly into the lumen of suction control valve (OF-B179). (see
Figure 1.33, p41)
Soaking in disinfecting solution
7) While fully immersed, ensure there are no air bubbles on the surfaces of components
and accessories. If any air bubbles are detected, ush them away with disinfecting
solution using a syringe.
8) Soak them in disinfecting solution according to the in stru c t ion s ( t e m per a t ure ,
concentration, time) specied by the disinfectant manufacturer. In the case of Cidex
Activated Dialdehyde Solution, the immersion time is 45 minutes at 25°C.
9) Remove the components and accessories from the disinfecting solution.
Accessory
Disinfection
1-3-2-3. Rinsing
First rinse
1) Prepare a basin with sterile water and immerse the components and accessories.
2) Wipe all exterior surfaces of the components and accessories at least two times with
a sterile gauze in order to remove residual disinfecting solution.
3) While still completely immersed in water, agitate them under the water by moving
them from side to side for at least one minute.
4) Manipulate the suction control valve (OF-B179) mechanism at least four times in the
water, and using a syringe lled with water, ush the lumen of the valve with at least
5 mL of water. (see Figure 1.32, p41 and Figure 1.33, p41)
5) Discard the water.
Second rinse
6) Fill a basin with clean water and repeat steps 2-5 in order to perform a second rinse.
Third rinse
7) Fill a basin with clean water and repeat steps 2-5 in order to perform a third rinse.
Forth rinse
8) Fill a basin with clean water and repeat steps 2-5 to perform a fourth rinse.
9) Remove all components and accessories from the water.
– 45 –
Page 51

Purging of water from lumens
10) Using a syringe lled with air, ush the lumen of the suction valve (OF-B179) to
purge residual water. (see Figure 1.33, p41)
1-3-2-4. Drying
WARNING
Regardless of the quality of the rinse water used, it is essential to perform a
nal alcohol rinse followed by forced air in order to completely dry the lumens
of components and accessories, and prevent bacterial colonization and/or
infections associated with waterborne microorganisms.
Flushing of lumens with alcohol
1) Using a syringe lled with 70-90% medical grade ethyl or isopropyl alcohol, ush
the lumen of the suction valve (OF-B179) with 2 mL of alcohol. (see Figure 1.33,
p41)
Purging of alcohol from lumens
2) Using a syringe lled with air, ush the lumen of the suction valve (OF-B179) with
air to purge residual alcohol. (see Figure 1.33, p41)
1
Drying of all external surfaces
3) Gently dry all external surfaces of components and accessories with a sterile gauze
moistened with 70-90% medical grade ethyl or isopropyl alcohol.
Accessory
Disinfection
– 46 –
Page 52

1-3-3. Optional sterilization
WARNING
1
• Please note that PENTAX Medical has not validated any sterilization
methods for exible endoscopes other than steam sterilization and the
STERRAD® NX™ system.
• During the reprocessing process, always wear protective equipment
(e.g., gloves, gowns, face masks, etc.) to minimize the risk of cross
contamination.
• Prior to sterilization, all instruments and components must be
meticulously cleaned and dried. Failure to do so can result in incomplete
or ineffective sterilization.
• Sterilization efcacy and material compatibility depend on the following
factors:
- thorough cleaning of the device
- load of the devices to be sterilized
- wrapping of the devices to be sterilized
- sterilization cycle parameters
• Use a chemical indicator (CI) and/or biological indicator (BI)
recommended by the manufacturer of the sterilizer to control the
sterilization process and ensure sterilization efcacy.
• After sterilization, ensure that the package is intact. If there are any
signs of abnormalities such as stains, wetness, tears, or any other
indications that the packaging has been damaged or opened, repeat the
sterilization process with new packaging.
Accessory
Sterilization
1-3-3-1. Steam Sterilization
CAUTION
After sterilization, the components and accessories may be hot. To avoid
burns, wait until they return to room temperature befor handling.
1-3-3-1-1. Preparation
1) Make sure that the cap of the inlet seal (OF-B190) is closed when packaged for
sterilization.
(1)
Figure 1.34
– 47 –
Page 53

1-3-3-1-2. Wrapping
NOTE
1) Prior to steam sterilization, wrap the components and accessories individually with
two layers of one-ply Kimguard KC200 sterilization wrap (Kimberly-Clark) using
sequential wrapping technique.
1-3-3-1-3. Parameters
Steam sterilization can be performed under the following conditions:
Sterilizer Type Temperature Exposure Drying Time
Pre-vacuum
• Validation testing of these parameters was performed using two layers of
one ply Kimberly Clark Wrap KC-200 Sterilization Wrap (Kimberly-Clark).
Sequential wrapping technique was employed.
• Biological and/or chemical indicators used must be appropriate for the
stated sterilization cycle parameters and cleared by FDA.
132°C 4 minutes 30 minutes
135°C 3 minutes 16 minutes
1
1-3-3-2. Sterilization using STERRAD® NX™ system
The suction control valve (OF-B179) and the inlet seal (OF-B190) can be sterilized using
the STERRAD® NX™ sterilization.
1-3-3-2-1. Items required
• protective garments such as gloves, gowns, face masks, etc., to minimize the
risk of cross contamination.
• APTIMAX Instrument Tray (270 mm in width, 576 mm in length, 100 mm in height)
• APTIMAX Instrument Tray Mat (254 mm in width, 546 mm in length)
• instrument wrap recommended by STERRAD® for use of STERRAD® NX™
system
• tape recommended by STERRAD® for use of STERRAD® NX™ system
Accessory
Sterilization
– 48 –
Page 54

1-3-3-2-2. Preparation
WARNING
1
Contact the manufacturer of the tray regarding directions for use of the tray.
1) Lay APTIMAX Instrument Tray Mat on APTIMAX Instrument Tray.
2) Place the suction control valve (OF-B179) and the inlet seal (OF-B190).
3) Cover the tray with the lid.
1-3-3-2-3. Wrapping
WARNING
• Contact the manufacturer regarding directions for use of the wrap and
tape.
• The suction control valve (OF-B179) and the inlet seal (OF-B190) may
be wrapped and sterilized together with the endoscope. In this case, do
not attach any components other than the ventilation cap (OF-C5) to the
endoscope.
• The ventilation cap (OF-C5) must be attached to the endoscope when
performing sterilization using STERRAD® NX™ system.
• Secure the wrapping with a length of tape that is sufcient to prevent
the wrapping from peeling away from package. Failure to do so could
result in removal of the tape and ineffective sterilization.
Accessory
Sterilization
1) Wrap the tray containing the components.
2) Apply the tape lengthwise across the three sides of the container.
1-3-3-2-4. Parameter
WARNING
Contact Johnson & Johnson regarding directions for use of STERRAD® NX™
system.
Set the tray containing the components into the sterilization chamber.
STERRAD®NX™:
Select "Advanced" cycle and operate the sterilizer according to the instructions supplied
with the STERRAD® NX™.
– 49 –
Page 55

2 POST REPROCESSING AND STORAGE
WARNING
• Make sure that all removable components such as the suction control
valve, rubber inlet seal, etc., are detached from the endoscope. This will
allow for better air circulation through the internal channels and permit
thorough drying. All channels should be completely dry before storage.
• Never store the endoscope, its components, or accessories in the
carrying case, as this type of dark, humid, and unventilated environment
is conducive to bacterial colonization and increases the risk of cross
contamination. These cases are intended only for transportation of the
instrument, not storage.
CAUTION
• Never store the endoscope in areas of high humidity, high temperature,
or in direct exposure to sunlight or X-rays.
• Avoid storage of the endoscope in cabinets that have sharp edges,
exposed nails/screws, etc. Contact with sharp objects can puncture,
scratch, or otherwise damage the endoscope.
• When utilizing heated disinfectants for reprocessing PENTAX endoscopes,
the instruments should be allowed to return to room temperature prior
to use and/or further handling.
1) Following reprocessing, the endoscope may either be reused or placed into storage.
2) Prior to storage, ensure that all internal channels, endoscope components, instrument
surfaces, and accessories are thoroughly dry.
3) The endoscope should be hung vertically in a clean, dry, well-ventilated storage
cabinet at room temperature. The insertion tube and light guide cable should be hung
and kept as straight as possible during storage.
4) Prior to reuse, ensure that instrument has been properly inspected and fully prepared
for the next clinical procedure.
22
– 50 –
Page 56

3 SERVICING
WARNING
• Instrument repairs should only be performed by an authorized PENTAX
service facility. PENTAX assumes no liability for any patient/user injury,
instrument damage or malfunction, or reprocessing failure due to repairs
made by unauthorized personnel.
• A list of "compatible" reprocessing agents with PENTAX endoscopes
based upon material compatibility and functionality studies performed
by PENTAX, Japan is contained in this manual. These tests apply only to
genuine PENTAX parts, components, and materials including proprietary
adhesives, sealants, lubricants, etc., specifically selected for use in
PENTAX endoscopes to satisfy their original design criteria. PENTAX
manual reprocessing instructions supplied with each product have been
validated for PENTAX endoscopes utilizing exclusive PENTAX parts/
materials and assembled based upon proprietary PENTAX manufacturing
technologies and/or servicing techniques.
• Plese note that PENTAX does not evaluate non-PENTAX parts,
components, materials, and/or servicing methods. Therefore, questions
regarding material compatibility and/or functionality of PENTAX
instruments repaired with these unauthorized, untested, and unapproved
items, materials, repair/assembly methods must be referred to the third
party service organization and/or device remanufacturer. It is unknown
to PENTAX if serviced or remanufactured instruments (performed by
unauthorized PENTAX entities) which still bear a PENTAX label are
within PENTAX device specifications and/or if unauthorized activities
have signicantly changed the instrument's performance, intended use,
safety, and/or effectiveness.
• Independent Service Organizations should confirm the ability of these
serviced/remanufactured devices to be reprocessed safely and effectively
with reprocessing agents/systems recognized as compatible by PENTAX
for standard PENTAX products. These companies and/or remanufacturers
should be consulted to confirm whether they have performed
reprocessing validation studies on instrument models which they have
serviced (or remanufactured) that support their cleaning, high-level
disinfection, and/or sterilization via the endoscope OEM reprocessing
recommendations, standard AER device-specic instructions, and/or their
own unique reprocessing recommendations.
• Ultimately, owners of these medical devices are responsible for selecting
an appropriate service facility or vendor whose activities will render an
instrument equivalent to the expectations and quality of a nished device
supplied by the endoscope OEM.
CAUTION
Never drop this equipment or subject it to severe impact, as it can
compromise the functionality and/or safety of the unit. Should this equipment
be mishandled or dropped, do not use it. Return it to an authorized PENTAX
3
service facility for inspection or repair.
– 51 –
Page 57

Prior to returning any instrument for repair to PENTAX, the instrument should first undergo
appropriate reprocessing/decontamination procedures for the purpose of infection control. Check
with your local PENTAX service facility for more details.
1) All instruments requiring repair should be shipped in the original carrying case with
appropriate packing along with comments describing the instrument damage and
complaint.
2) A repair purchase order number, contact name, and phone number of the individual
responsible for authorizing repairs, as well as shipping address should be included.
3) The ventilation cap (OF-C5) should be attached to the instrument if it will be shipped
by air freight.
4) Any accessories and/or endoscope components potentially related to the endoscope
damage or complaint should also be returned with the endoscope.
5) PVE soaking cap (OE-C9) should also be returned with the endoscope to check/
conrm the integrity of its watertight seals.
6) After servicing, all endoscopes must be reprocessed prior to patient use.
7) For disposal of instruments, follow local or country regulations.
– 52 –
3
Page 58

4 APPENDIX
4-1. PENTAX Medical Compatible Reprocessing Systems/Agents
The information below is based upon material compatibility and functionality studies performed
by HOYA Corporation- PENTAX Medical Division, Japan. Reference to specific brand
name products is not an endorsement of their efcacy. Tests have shown these solutions to be
compatible with materials used in the construction of PENTAX Medical endoscopes, provided
that the manufacturers’ instructions for use are followed. This document has been prepared by
PENTAX Medical Company for PENTAX Medical customers in the United States, Canada and
Latin America.
Important
PENTAX Medical instructions for use contain detailed recommendations for the manual
reprocessing of PENTAX Medical endoscopes using PENTAX Medical supplied cleaning
accessories. Automated Endoscope Reprocessor (AER) product claims are the responsibility
of the AER manufacturer, including but not limited to cleaning, disinfection, sterilization,
rinsing, drying, biocompatibility, reprocessing instructions, required channel adapters, efcacy
validation studies, and compliance with regulatory requirements and/or professional guidelines.
Prior to reprocessing PENTAX Medical brand endoscopes in a specic model AER machine,
contact the AER manufacturer to conrm the following:
• The AER efficacy claims have been validated for the specific PENTAX Medical model
endoscopes in question.
• Instructions are available for the specific PENTAX Medical model endoscopes and
endoscope components in question.
Enzymatic Detergents
Product Brand Name Manufacturer
Cidezyme® XTRA (used exclusively in EvoTech ECR)
®
Enzol
Endozime
Endozime® AW Plus
Enzy-Clean Care Fusion
MetriZyme
Tergal 800 Custom Ultrasonics
ZymeX™ Enzymatic Cleaner Concentrate Sultan Healthcare
®
®
Advanced Sterilization Products (ASP)
Ruhof Corporation
Metrex Research Corporation
4
– 53 –
Page 59

High Level Disinfectants
The following liquid chemical germicides have received FDA 510(k) clearance for claims of
high level disinfection (HLD). Some HLD products may have multiple label claims and/or may
be FDA-cleared only for use in a legally marketed AER machine that can attain specific use
parameters (e.g., temperature).
Product Brand Name Manufacturer
Cidex® OPA
Cidex® OPA-C (used exclusively in EvoTech ECR)
Cidex® Activated Dialdehyde Solution(14-Day
Glutaraldehyde)
Advanced Sterilization Products
MetriCide® (Glutaraldehyde - may also be marketed
as Omnicide NS or MaxiCide® NS)
Sporicidin® (Glutaraldehyde) Contec Incorporated
Rapicide® (Glutaraldehyde) Medivators Inc.
Wavicide®-01 (Glutaraldehyde) Medical Chemical Corporation
Metrex Research Corporation
Sterilization Agents/Processes
The following agents/processes have received FDA 510(k) clearance for claims of sterilization:
Ethylene Oxide / Carbon Dioxide (80:20 gas mixture)
Ethylene Oxide / Carbon Dioxide (90:10 gas mixture)
– 54 –
4
Page 60

NOTICE
These instruments are used with Class B Medical Equipment (specified CISPR11) and are
intended for Hospitals, Ambulatory Surgery Centers, or Medical Clinics.
Together, these endoscopes and the compatible processor comply with EN 60601-1-2 for EU,
IEC 60601-1-2 for other countries.
When used in clinical or residential areas near radio and TV receiver units, these instruments
may cause radio interference.
To avoid and resolve adverse electromagnetic effects, do NOT operate these instruments near
the radio frequency energy equipment.
HOYA Corporation
2-7-5 Naka-Ochiai, Shinjuku-ku, Tokyo, 161-8525 Japan
PENTAX Medical Company
A Division of PENTAX of America, inc.
3 Paragon Drive Montvale, New Jersey 07645-1782 USA
Tel: +1-201-571-2300 Toll Free: +1-800 431-5880
Fax: +1-201-391-4189
PENTAX Canada Inc.
1770 Argentia Road Mississauga, Ontario L5N 3S7 Canada
Tel: +1-905-286-5585
Fax: +1-905-286-5571
PENTAX Europe GmbH
Julius Vosseler Strasse 104, 22527 Hamburg, Germany
Tel: +49-40-561-920
Fax: +49-40-560-4213
Manufacturing Site
HOYA Corporation, PENTAX Miyagi Factory
30-2 Okada, Aza-Shimomiyano, Tsukidate, Kurihara-shi, Miyagi 987-2203 Japan
• Specicationsaresubjecttochangewithoutnoticeandwithoutanyobligation
on the part of the manufacturer.
88899
2014. 06 6217001 S020 R04 printed in JAPAN
 Loading...
Loading...